Abstract
This review covers recent advances in sampling fluid from the extracellular space of brain tissue by electroosmosis (EO). Two techniques, EO sampling with a single fused-silica capillary and EO push–pull perfusion, have been developed. These tools were used to investigate the function of membrane-bound enzymes with outward-facing active sites, or ectoenzymes, in modulating the activity of the neuropeptides leu-enkephalin and galanin in organotypic-hippocampal-slice cultures (OHSCs). In addition, the approach was used to determine the endogenous concentration of a thiol, cysteamine, in OHSCs. We have also investigated the degradation of coenzyme A in the extracellular space. The approach provides information on ectoenzyme activity, including Michaelis constants, in tissue, which, as far as we are aware, has not been done before. On the basis of computational evidence, EO push–pull perfusion can distinguish ectoenzyme activity with a ~100 µm spatial resolution, which is important for studies of enzyme kinetics in adjacent regions of the rat hippocampus.
Keywords: Organotypic hippocampal slice cultures, membrane-bound enzymes, enzyme activity, electroosmotic sampling, neuropeptides, thiols
Introduction
Obtaining samples of the extracellular space
Tools for measuring extracellular concentrations of chemicals in the brain have been around for more than half a century. MacIntosh and Oborin introduced the cortical cup in 1953 [1], Gaddum the push–pull cannula in 1961 [2], and Delgado the chemitrode [3] and the dialytrode [4] in 1962 and 1970, respectively [5]. Improvements in push–pull perfusion [6–10] were followed by microdialysis, the most widely used method for sampling the extracellular space [11]. These tools were motivated by the need to establish a relationship between behavior and regional chemistry of the brain and to monitor changes in brain chemistry in response to specific disease states. These early sampling methods were an advance, but they were not optimal when considering criteria including damage radius and degree, mechanical obstruction by tissue, spatial and temporal resolution, and solute recovery. Several new tools for probing neurochemistry, in particular low-flow push–pull perfusion, direct sampling, and work from our laboratory on electroosmotic (EO) sampling and EO push–pull perfusion, promise better spatial resolution, less damage, and the ability to make quantitative measurements of ectoenzyme activity (specific citations are found below).
In microdialysis, a membrane permeable to water and small molecules separates the brain from a fluid perfusing the luminal volume inside the membrane. The membrane enables transport of water and small molecules (the size depending on characteristics of the cylindrical membrane) across the membrane into the lumen. The luminal volume is connected to inlet and outlet ports. Solute exchange occurs by diffusion. The perfusion fluid’s velocity affects the mass transport by its effect on the concentration gradient during diffusion [12], although with high-molecular-weight (MW)-cutoff membranes, flow through the membrane into the tissue can occur [13]. Recent improvements include Stenken’s use of affinity-based recovery enhancement via molecular receptors including cyclodextrins (for nonspecific binding to low-MW molecules, for example YGGFL and YGGFM), antibody-immobilized beads (for binding to cytokines and endocrine hormones), heparin (for binding to human cytokines), and bovine-serum-albumin–heparin conjugates (for cytokine and tumor necrosis factor-α recovery) [14–16]. Membranes with larger MW cutoffs are available for detection of large molecules, for example some peptides and proteins [14, 15, 17, 18]. Microdialysis has also been coupled to flow segmentation to achieve temporal resolution as low as 15 seconds [19].
In recent years, the Shippy group revisited the push–pull cannula and developed a novel method called low-flow push–pull-perfusion sampling (LFPS). In this concentric design, flow is directed from outer tubing into the inner tubing, generating a low flow rate (10–50 nL min−1) while reliably producing 70–80 % recovery in vitro. It also provides better spatial resolution than microdialysis. The Shippy group successfully used LFPS to quantify the basal level of glutamate in the rat striatum [20], determine the relative levels of amino acids in vitreous humor and the vitreoretinal interface of the eye [21], and study glutamate release in the lateral hypothalamus during feeding and drinking [22]. When coupled to capillary electrophoresis (CE), LFPS can be used to measure basal and K+-stimulated ascorbate levels at the rat vitreoretinal interface [23]. The Kennedy group coupled LFPS to flow segmentation to achieve 7 s temporal resolution at 50 nL min−1 flow and, after further miniaturizing the probe inlet, achieved 200 ms temporal resolution at 30 nL min−1 flow [24]. Kennedy et al. also investigated the method of direct sampling. In this technique, a fused-silica capillary tube with 90 µm outer diameter (o.d.) was used for sampling extracellular levels of glutamate, aspartate, glycine, phosphoethanolamine, and γ-aminobutyric acid in rat striatum. It yielded flows of 1–50 nL min−1, 90 s temporal resolution, and 500 times better spatial resolution than microdialysis [25].
We have developed sampling approaches based on EO flow in tissue that address some of the limitations of the foregoing. Specifically, we wanted a method that could be applied equally well to small tissue cultures and potentially in vivo, that had useful and controllable spatial resolution, and that could be used for determining endogenous compounds and measuring the biochemical fate of exogenously applied compounds while causing minimal damage. In particular, there is a dearth of methods designed to determine ectoenzyme activity in intact tissue. The EO methods described below are well-suited to this problem. In addition, EO flow is much less affected by the size of the fluid paths in a porous medium than pressure is. As a result, the fluid flow generated electroosmotically will be more homogenous (in space) than pressure flow. Before providing a background on electroosmotic flow and describing its use in sampling, it is appropriate first to review a few important facts about ectoenzymes.
Ectoenzymes
Ectoenzymes comprise a family of membrane-bound proteins whose catalytic domains face the extracellular space (ECS). It is a diverse family of enzymes, with substrates that include both nucleotides and peptides. Their function is usually believed to be clearing active species from the ECS [26]. However, more recent studies suggest that ectoenzymes are also capable of more subtle biomolecular modulation, as will be described below.
Receptors for nucleotides are expressed on nearly all cell types [27]. Ectoenzymes that cleave nucleotides in the ECS, called ectonucleotidases, were first discovered in the 1940s. Since then several families of these enzymes have been identified on the basis of spatial distribution and functional differences. Ectonucleotidases are involved in the salvage and/or hydrolysis of adenosine monophosophate (AMP) and adenosine triphosphate (ATP), thereby having a function in purinergic transmission and energy metabolism [27–35]. The importance of these enzymes is further illustrated by their functions in a variety of physiological processes, including angiogenesis, immune response, and neurogenesis [27]. In addition, a specific family of ectonucleotidase, CD38/157 (EC 3.2.2.5), has a function in NAD+ metabolism and chronic lymphocytic leukemia [36–39].
Similar to their nucleotide-hydrolyzing counterparts, ectopeptidases not only activate and inactivate [40] but in some cases can regulate the bioactivity of peptides in a variety of physiological processes, including growth, cell survival, and stress responses [41–45]. Dipeptidyl peptidase IV (DP-IV, DPP-IV, DPP-4, or CD26, EC 3.4.14.5) cleaves hormones that regulate insulin release in the gastrointestinal system and cleaves neuropeptide Y (NPY) into the fragment NPY(3–36), which is a selective agonist for the Y2 class of NPY receptors [46–48]. Another ectopeptidase, membrane alanyl-aminopeptidase (aminopeptidase N, APN, CD13, or mAAP, EC 3.4.11.2), is a type II integral zinc-dependent metallopeptidase that hydrolyzes angiotensin III and other hormones and the neuropeptide family of enkephalins and endorphins [49–51]. As evidenced by these substrates, there is evolving support for the theory that APN is involved in the regulation of arterial blood pressure and pain [49, 51, 52]. Neutral endopeptidase (NEP, neprilysin, endopeptidase, enkephalinase, EC 3.4.24.11) [53] degrades toxic Aβ-amyloid peptides that are associated with Alzheimer’s disease [54]. It also hydrolyzes enkephalins and neuropeptide Y, suggesting that it has functions in pain and in energy metabolism [46, 51, 55]. As a result of and as evidence for their importance, DPIV, APN, and NEP are all therapeutic targets for pharmacological treatment of specific disease states [40, 51, 56]. Additionally, with angiotensin-converting enzyme (ACE, EC 3.4.15.1) and disintegrin-metalloproteinases (EC 3.4.24.81), these three ectopeptidases are involved in tumor development and metastasis [57].
Recently, it was discovered that the activity of neurolysin (endopeptidase 24.16, microsomal endopeptidase, mitochondrial oligopeptidase, neurotensin-degrading enzyme, EC 3.4.24.16) is altered in stroke [58]. This endopeptidase is expressed in neurons and astrocytes. In neurons, but not astrocytes, approximately 10 % of the enzymatic activity is membrane-bound [59]. Wangler et al. [60] report that “membrane- anchoring mechanism (s)” are not understood. Clearly, analytical techniques with the objective of measuring membrane-bound (ecto) enzyme activity would be useful.
Conventionally, ectoenzyme activity in tissue or cell culture is determined by adding the substrate to medium containing whole cells or membrane fractions isolated after homogenization. Products in the supernatant can be quantified using a colorimetric method, radiochemical assay, or HPLC analysis after further treatment. The reaction rate and the ectoenzyme activity can then be estimated [50, 61–67]. It is also informative to examine changes in translational precursors (mRNAs) to determine changes in ectoenzyme synthesis, using reverse-transcription polymerase chain reaction (RT-PCR), northern blot, and in-situ hybridization coupled to immunohistochemistry [68–70]. We are not aware of methods other than ours for determining ectoenzyme levels in intact tissue.
EO-flow basics
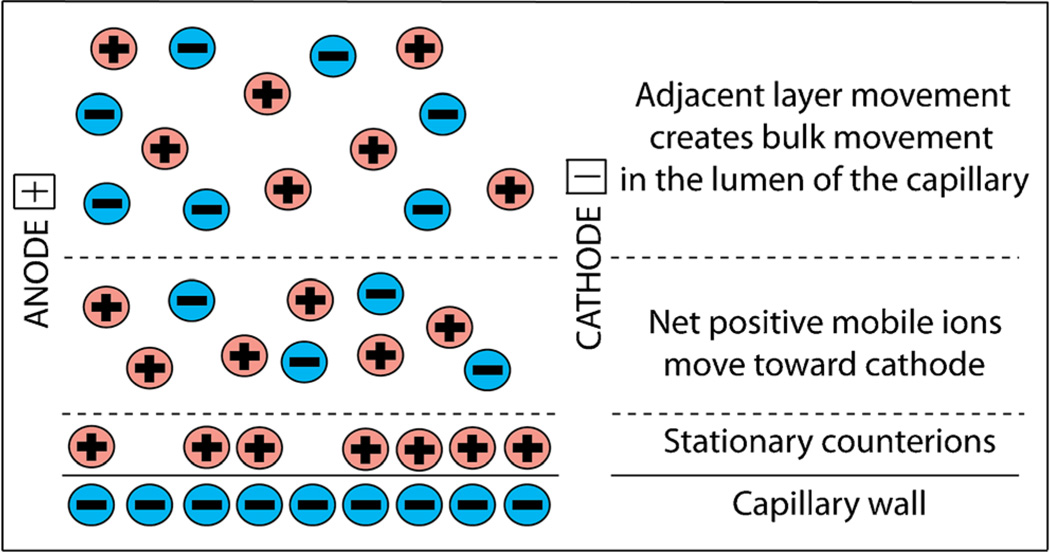
Bulk fluid movement called electroosmosis results when an electric current is passed through electrolyte-filled channels with charged walls, ranging from fused-silica capillaries to soil. We have found that electroosmosis occurs in brain tissue [71, 72]. Electroosmosis is the result of the presence of an excess of mobile counterions near fixed surface charges. Because of electrostatic and entropic factors, counterions in the electrolyte solution form a double layer adjacent to the charged surface of walls (e.g. in a capillary). There may be strongly adsorbed counterions reducing the surface charge. A more diffuse layer extending some distance away contains the remainder of the counterions required for charge neutrality (Fig. 1). The diffuse layer is therefore charged and moves under the influence of an externally applied electric field parallel to the interface. There is an easily measured but difficult to define property of surfaces that support electroosmotic flow: the ζ-potential. There exists a shear plane somewhere within the double layer separating surface-bound water (solvent) from water (solvent) moving under the electroosmotic force. The electrostatic potential at this shear plane with respect to a point far outside the double layer where the electrolyte is locally electroneutral is the ζ-potential. The application of an external electric field parallel to the wall surface induces movement of the diffuse layer, which, in the steady state, “carries” the rest of the macroscopically neutral, bulk solution with it via the diffusion of momentum. It is important to note that the system as a whole, the surface plus the surrounding electrolyte, must be electroneutral. The net surface charge density, defined as the fixed charge density minus adsorbed counterion density, is related to the ζ-potential. Equation 1 reveals that the ζ-potential can be determined experimentally by measuring the interstitial velocity, veo, of an electrolyte solution with a specific permittivity, εw, and dynamic viscosity, η, in a local field with magnitude E:
| (1) |
Fig. 1.
Schematic diagram illustrating the distribution of ions in the electrical double layer near a charged wall that leads to electroosmosis when a field is applied parallel to the surface
It is convenient to express the electroosmotic flow (volume/time) in terms of the applied current when working with systems having a series of connected but independent conducting elements including an electrolyte-filled capillary and a tissue slice. This relationship is summarized in the following equation:
| (2) |
where Ueo is the electroosmotic flow rate, i is the current, and σel is the bulk conductivity of the electrolyte solution in the absence of a porous medium [73]. The electroosmotic mobility, µeo, is implicitly defined in Eq. 2. We note that the physical properties of the porous medium, namely porosity and tortuosity, are accounted for in the derivation of Eq. 2 despite the fact that they are not explicitly seen in the equation. For example, a reduction in a medium’s porosity increases the electric field across the medium (at constant current), resulting in an increase in the electroosmotic velocity so that the flow rate is maintained.
Fused-silica capillaries have a negative ζ-potential. Our capillaries, conditioned with sodium-hydroxide solution, have a ζ-potential of −46.5±1.2 mV (one standard deviation) at pH 7.4, which is consistent with the literature [74]. Therefore, electrolyte solutions can be driven through fused-silica capillaries by electroosmotic flow. We have found that rat organotypic-hippocampal-slice cultures (OHSCs) also have a significant ζ-potential [71, 72]. The tissue is a porous medium, a bed of nonconducting particles (cells) (under non-electroporating conditions [75]). The surfaces of these “particles” are functionalized with proteins, anionic carbohydrates, and phospholipids that act as stationary negative “wall” charges. The ζ-potential of OHSCs is −22.8±0.8 mV [71, 72]. Thus, passing a current through this brain tissue is accompanied by electroosmotic flow.
It is worthwhile to compare fluid flow and solute transport in a porous space by electroosmosis to that by pressure. Darcy’s law relates a pressure gradient to the superficial fluid velocity, vp, in a porous medium, by Eq. 3:
| (3) |
The superficial velocity is the flow rate divided by the total (i.e., pores and obstructions) cross-sectional area of the porous medium. The analogous expression for electroosmotic velocity (superficial) is given by Eq. 4:
| (4) |
In Eq. 3, the pressure difference, ΔP, over distance, L, drives fluid through a medium with a hydraulic permeability κ, (in units of m2). For a cylindrical open tube of radius a, κ is a2/8. Substitution of this value for the hydraulic permeability of a cylindrical open tube into Eq. 3 leads to the familiar Hagen–Poiseuille law. Thus, the hydraulic permeability relates to the cross-sectional area of individual channels or interstitial spaces containing the fluid in a porous medium. The hydraulic permeability for gray matter in the brain is approximately 10−15 m2, but it is anisotropic and significantly larger for white matter: as much as three orders of magnitude larger along axonal tracts and two orders of magnitude larger across tracts [76, 77]. As a consequence, fluid flow induced by pressure in the brain travels most readily along white matter tracts and in perivascular spaces [78]. In addition, brain tissue can undergo deformation, meaning physical perturbation caused by pressure can lead to alteration of the tissue structure. This in turn has a significant effect on the hydraulic permeability [77].
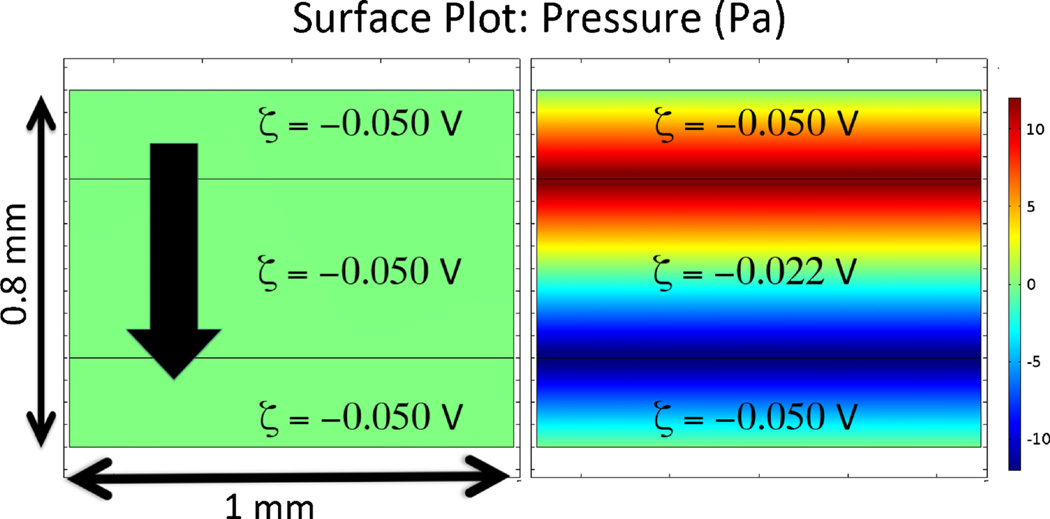
Fluid flow driven purely by an electric field (current flowing in electrolyte solution) through porous media of uniform ζ-potential is not accompanied by a pressure drop. Thus, variability in the hydraulic permeability has little to no effect on the pattern of electroosmotic flow in a complex porous medium. Because pressure is not created, there is also no deformation of tissue. The situation is different when the flow or current passes through two regions with different ζ-potentials, however. Pressure gradients are created in such systems [79]. Figure 2 shows a pair of surface plots that illustrate this principle. Other than the ζ-potential, all properties of the adjacent porous media are identical for the two panels. The downward arrow shows the direction of the flow path. Because the fluid is virtually incompressible, the fluid flow rate must be the same in each of the three serial sections of the material. However, because of the ζ-potential differences in the domains (Fig. 2, right panel), flow rate in the top and bottom domains is faster than that in the middle domain. Thus, to satisfy the law of conservation of mass, a flow-equalizing intersegmental pressure results at the domain interfaces. In general, it is difficult to determine the flow rate (or the velocity) without simulations in cases with intersegmental pressure because the magnitude of the pressure gradient depends on many factors, including hydraulic permeability, electric field strength, and differences in ζ-potential and in the lengths of the individual segments. (Fig. 3)
Fig. 2.
Pressure created by variation of ζ-potential along the direction of the current flow. A field of 1000 V m−1 was impressed across the vertical dimension. ζ-potential values are indicated in their respective domains. Porous properties are the same for all domains and are defined as follows: porosity (ε)=0.4; tortuosity (λ)=1.4; conductivity (σ)=1.43 S m−1; density (ρ)=1×103 kg m−3; permeability (κ, calculated using the Kozeny–Carman equation for a packed bed of 10 µm particles)=5×10−14 m2; dynamic viscosity (η)=8.9×10−4 Pa s. The pressure at the top and bottom boundaries is set to the arbitrary value of zero so that the net pressure drop applied across each 0.8 mm-thick object is zero. Calculations were performed with COMSOL Multiphysics v4.4
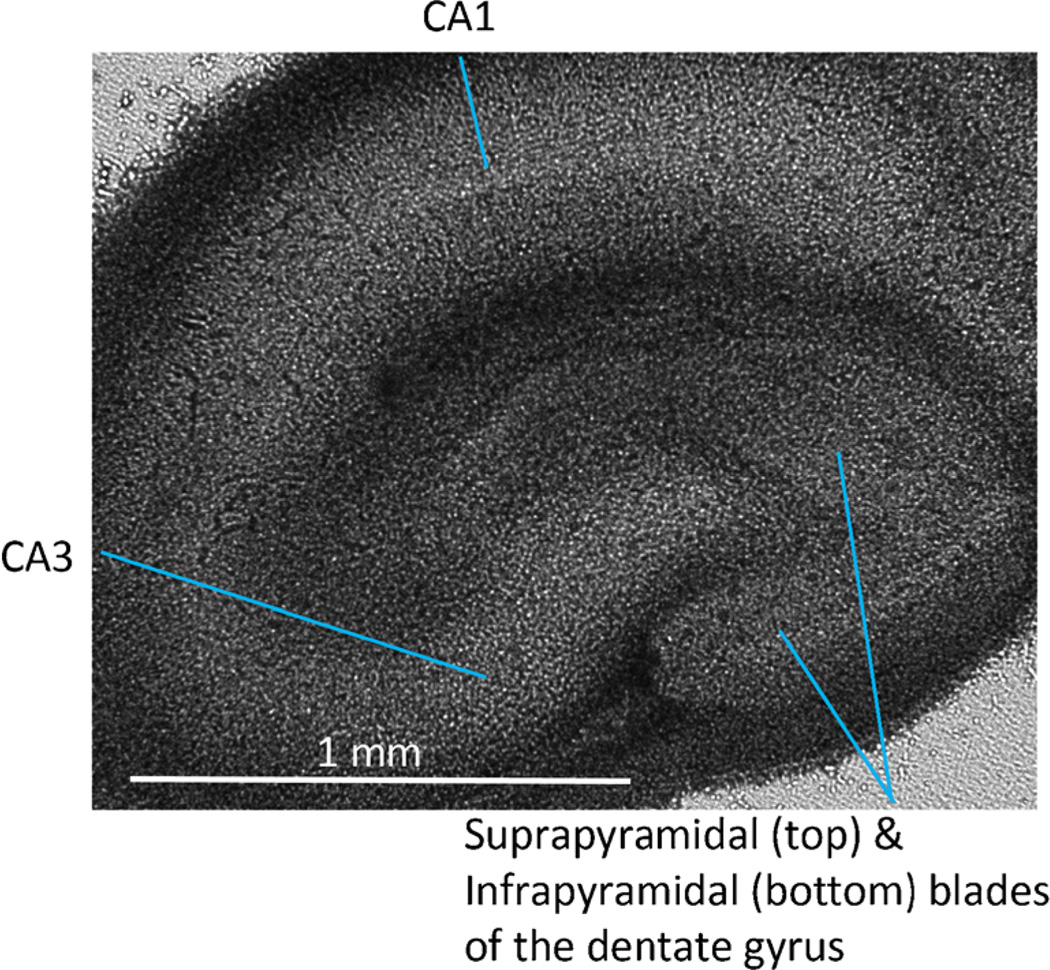
Fig. 3.
Bright field image of organotypic-hippocampal-slice culture (4 × 0.16 NA objective, IX-71 inverted microscope, Olympus, Melville, NY).
The objective of both pressure and electroosmotic flow is the transport of solutes out of the sample to an instrument or sensor. The effectiveness of solute capture depends on the ratio of the advective transport to diffusive transport. This is embodied in the Péclet number:
| (5) |
Here, v is the velocity of the fluid, a is a characteristic distance, and D is the diffusion coefficient of the solute. Thus, for example, the probability that a molecule is captured in a sampling process based on fluid flow in the tissue is proportional to the fluid velocity and a characteristic distance over which the molecule must diffuse to escape the flow field, but inversely proportional to the diffusion coefficient of the solute. The characteristic distance (a) is related to the inner diameter (i.d.) of the sampling capillary.
EO perfusion and sampling with a single capillary
Investigations of physiological and molecular processes require appropriate in-vitro models that maintain both functional and anatomical integrity. We use organotypic-hippocampalslice culture (OHSC). OHSC has been widely used for studying brain function and disease, including neurogenesis [80–82], neurotoxicity and/or cell death [83–89], synaptic plasticity [80, 84], and neuroprotection [80, 86, 90, 91]. Preparations of OHSCs from 6–7-day-old (p6–7) rat pups were based on modifications of the original technique by Stoppini, and can be cultured for up to 2 months in vitro [92, 93].
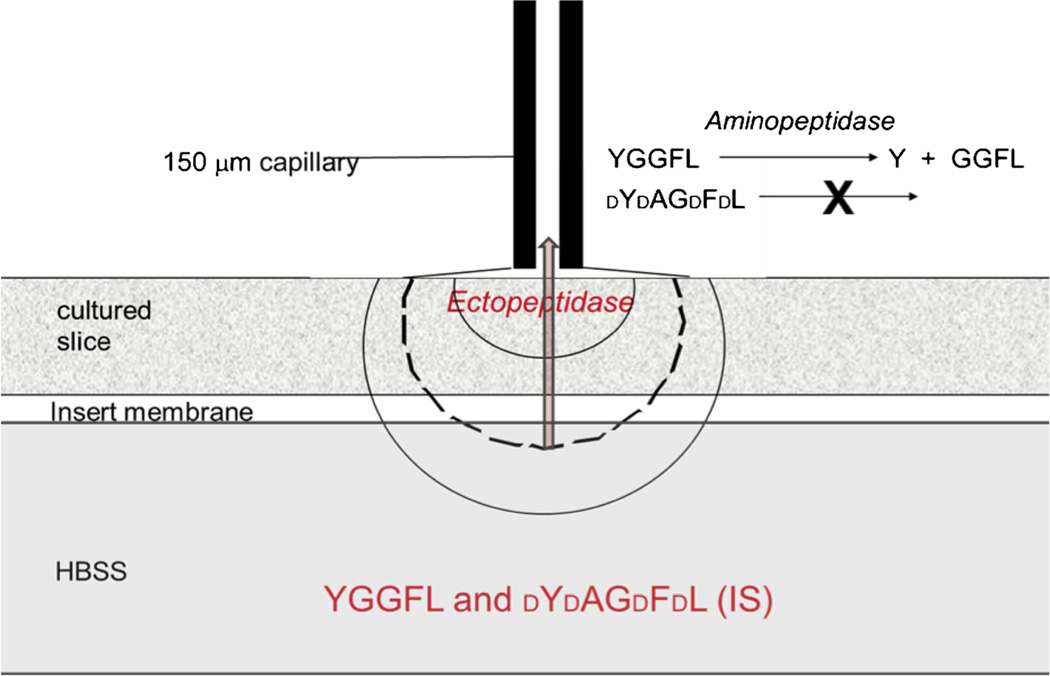
The cultures grow on a porous “insert” membrane that rests on growth medium or buffer. The cultures obtain nutrients by capillary action through the membrane and oxygen from the 95 % air–5 % CO2 environment in the incubator. Figure 4 shows how the peptide is drawn through the tissue electroosmotically in the single-probe approach. A fused-silica capillary is placed in contact with the tissue through a thin electrolyte layer. An electrical circuit is established through the electrolyte or tissue. The application of a voltage induces electroosmotic flow from the peptide-filled bath, through the tissue, and into the sampling capillary.
Fig. 4.
Schematic portrayal of the single-probe system. An electrolyte-filled fused-silica capillary is placed perpendicular to the tissue culture and separated from it by a thin, liquid layer (25–50 µm). An electrode and the distal end of the capillary are placed in electrolyte. The other electrode is placed in the HBSS. Peptide substrate and a d-amino-acid peptide are dissolved in the HBSS. The approximately-semicircular lines indicate isopotentials
The flow rate of the fluid is not directly measurable. Furthermore, although the flow rate is proportional to current, the arrangement shown in Fig. 1 does not permit direct application of Eq. 2 because a single ζ-potential does not characterize the system. In cases where there is a change in ζ-potential along the fluid path, a flow-equalizing intersegmental pressure develops at the interfaces between domains of different ζ- potential, as seen in Fig. 2. As aforementioned, qualitatively, the ζ-potential in the capillary is more negative than that in the tissue, so the inherent electroosmotic velocity of the capillary is larger than that of the tissue. The tissue’s hydraulic permeability does not enable the capillary’s fluid to flow at its inherent electroosmotic velocity, because pulling fluid through the dense tissue requires (negative) pressure. In the final analysis, depending on the two ζ-potentials and the pressure–velocity relationships in the two regions of differing ζ-potential (e.g., Darcy’s law or the Hagen–Poiseuille equation), the overall velocity and flow rate is intermediate between the two extremes [73, 94].
The spatial resolution of the technique is, in theory, dictated mostly by the current path, the diameter of the capillary, and the diffusion coefficient of the solute. In the tissue (Fig. 4) the current density (current/area) decreases with the depth into the tissue. Current density is directly proportional to electric field, which dictates the electroosmotic velocity. Therefore, the electroosmotic velocity (distance/time) decreases away from the capillary tip. It is convenient to consider the Péclet number (Pe) defined above in Eq. 5. When Pe is greater than one, the deterministic velocity is dominant. When Pe is less than one, diffusion dominates. In the latter case, solute can escape the influence of the fluid flow. A dashed line in Fig. 4 shows a hypothetical Pe of unity. Note that the sampling volume is much larger than the diameter of the capillary lumen. The spatial resolution of the EO sampling is therefore good, but it cannot be assumed that it is as small as the capillary-lumen diameter.
Hydrolysis of leu-enkephalin by ectoenzymes in CA3
Enkephalins are opioid pentapeptides that are involved in pain and immune response [51, 50] among other things. Studies have revealed that an endogenous blocker of enkephalin-hydrolyzing ectopeptidases has potent therapeutic properties [95]. We used our EO sampling method to study ectopeptidase activity in the OHSC. Figure 4 shows that the substrate-peptide leu-enkephalin, YGGFL, was in the bath below the tissue. We also added a d-amino-acid peptide dYdAGdFdL to the bath to act as an internal standard (IS). To determine the flow rate experimentally, we measured the number of moles of IS collected from the bath below the tissue in a fixed time. The flow rate depends on the applied current. For typical conditions (application of 23–45 µA current, or 3000–6000 V m−1 field, across a 30 cm, 150 µm i.d. capillary) the flow is in the range of 60–150 nL min−1.
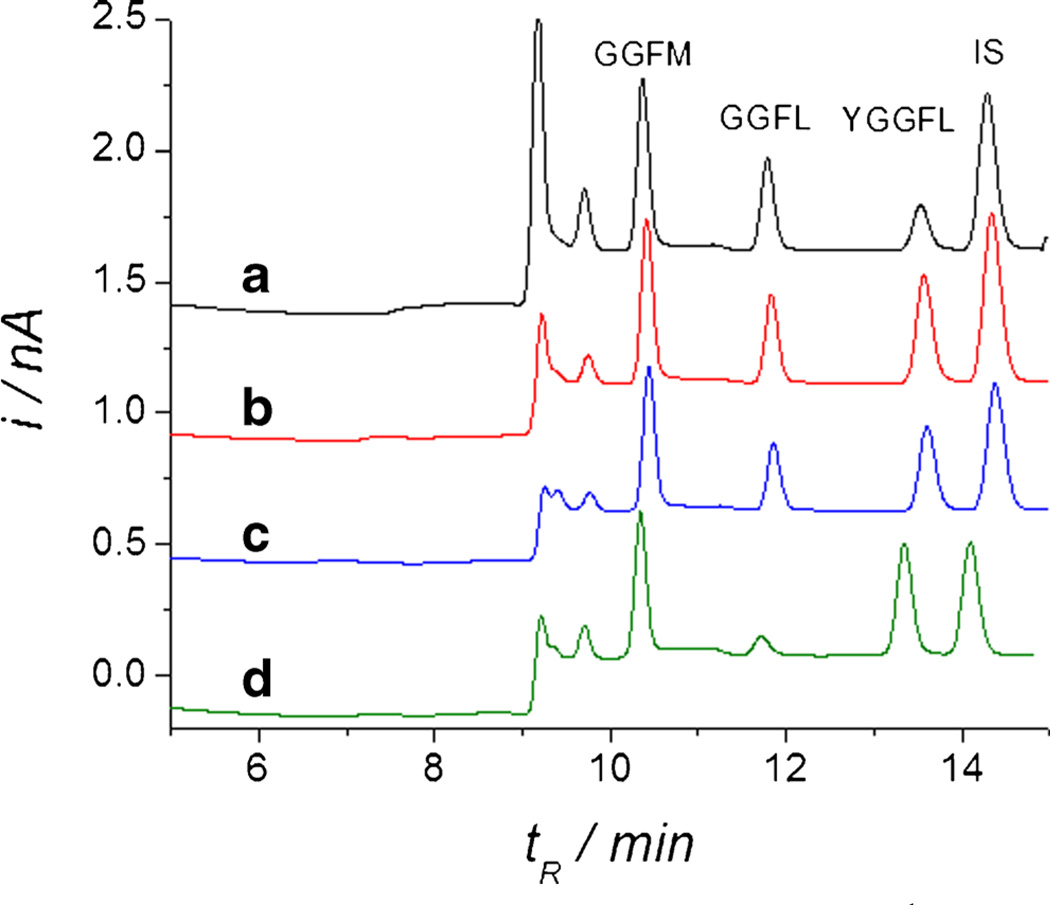
Cleavage of the N-terminal tyrosine from enkephalins inactivates them [96]. Thus we developed a capillary chromatographic separation of the substrate peptide, the IS, GGFL, and an external standard in the injected fluid, GGFM. Using EO perfusion we pulled YGGFL-containing solution through the extracellular space of the CA3 region of OHSCs. GGFL was determined by capillary liquid chromatography with a copper-tartrate postcolumn reagent and electrochemical detection [97–99] (Fig. 5). Quantitative measurement of the GGFL peak enabled the determination of the rate of hydrolysis of the YGGFL to GGFL. Experiments with a variety of inhibitors enabled Xu et al. to determine that the ectopeptidase responsible for YGGFL hydrolysis to GGFL in the CA3 region is a bestatin-sensitive aminopeptidase, for example APN. Xu also determined the Michaelis constant, KM, for the ectoenzyme hydrolyzing YGGFL. The value of KM agreed with literature estimates for bestatin-sensitive aminopeptidase. As far as we are aware, this was the first measurement of variables for enzyme kinetics in tissue. Tissue measurements, although more difficult, are preferred. Ectoenzymes solubilized by surfactants and the same enzymes residing in membranes may have different activities [100]. The physical structure of the extracellular space and the existence of other enzymes in the in-vivo environment more closely reflect the accessibility and reaction rate of the substrate with the ectoenzyme in vivo.
Fig. 5.
Five-minute sampling through CA3 at 5000 V m−1 (38 µA current). The bath solution is HBSS with 150 µmol L−1 YGGFL and IS with or without inhibitors as follows: Experiment A, no inhibitors; B, includes inhibitors (µmol L−1): thiorphan (15), GEMSA (210), captopril (25); C, as B plus puromycin (20); D, as C plus bestatin (100). Reprinted with permission from Xu et al. (2010) Electroosmotic Sampling. Application to Determination of Ectopeptidase Activity in Organotypic Hippocampal Slice Cultures. Analytical Chemistry 82 (15):6377–6383. Copyright 2010 American Chemical Society
Catabolism of coenzyme A by ectoenzymes
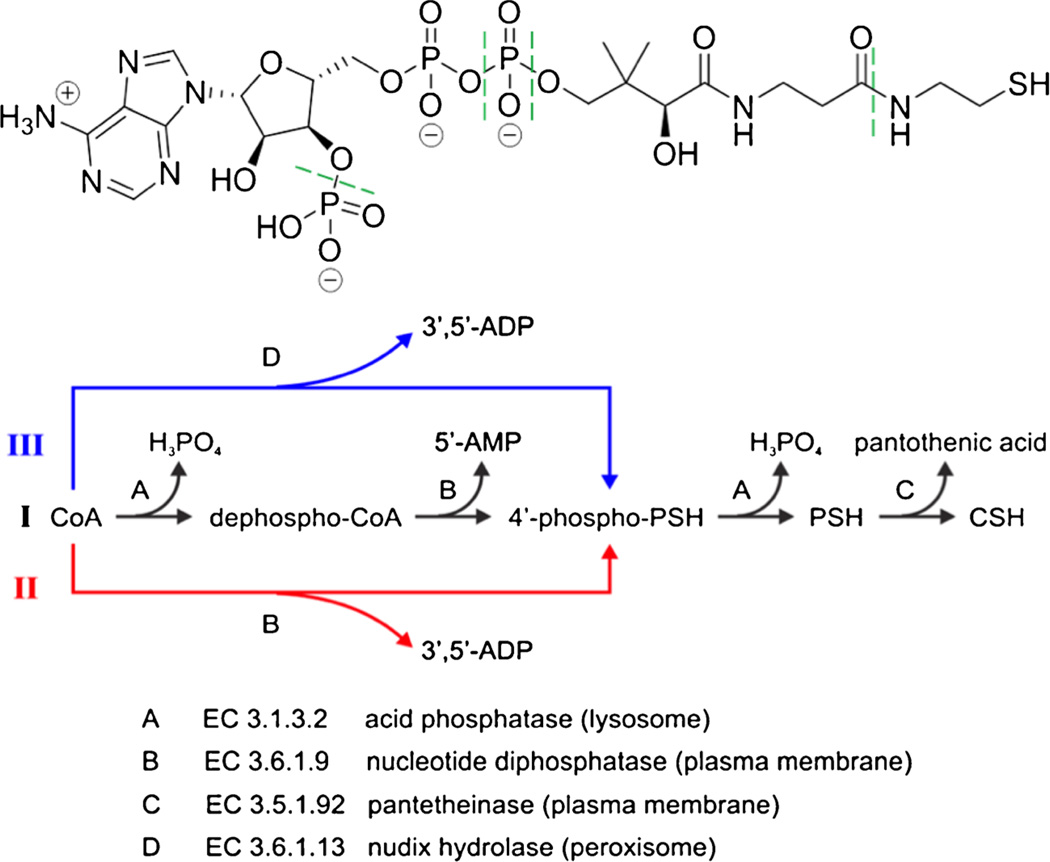
Cytosolic biosynthesis of coenzyme A (CoA) is accomplished through the action of multiple cytosolic enzymes in five well-established steps [101–104]. Cysteine, pantothenic acid, and ATP are the three basic substrates for this biosynthesis. The degradation of CoA, however, is not as well understood [101–103, 105]. Stipanuk et al. proposed an integrated mechanism of cysteine and CoA metabolic pathways [105]. Interestingly, an ectoenzyme is involved in the degradation (Fig. 6): the degradation of pantetheine (PSH) to pantothenic acid and cysteamine (CSH), the last step in the CoA-degradation pathway, is catalyzed by the ectoenzyme pantetheine hydrolase (EC 3.5.1.92) [101, 103, 105].
Fig. 6.
Proposed biodegradation pathways of CoA. CoA is depicted at the top. Enzymatic hydrolysis occurs at the bonds indicated by green dashed lines. Note that potential pathB involves an ectonucleotide pyrophosphatase. PathC also involves an ectoenzyme, pantotheinase. Reprinted with permission from Wu et al. (2013) Integrated Electroosmotic Perfusion of Tissue with Online Microfluidic Analysis to Track the Metabolism of Cystamine, Pantethine, and Coenzyme A. Analytical Chemistry 85 (24):12020–12027. Copyright 2013 American Chemical Society Electroosmotic perfusion of tissue
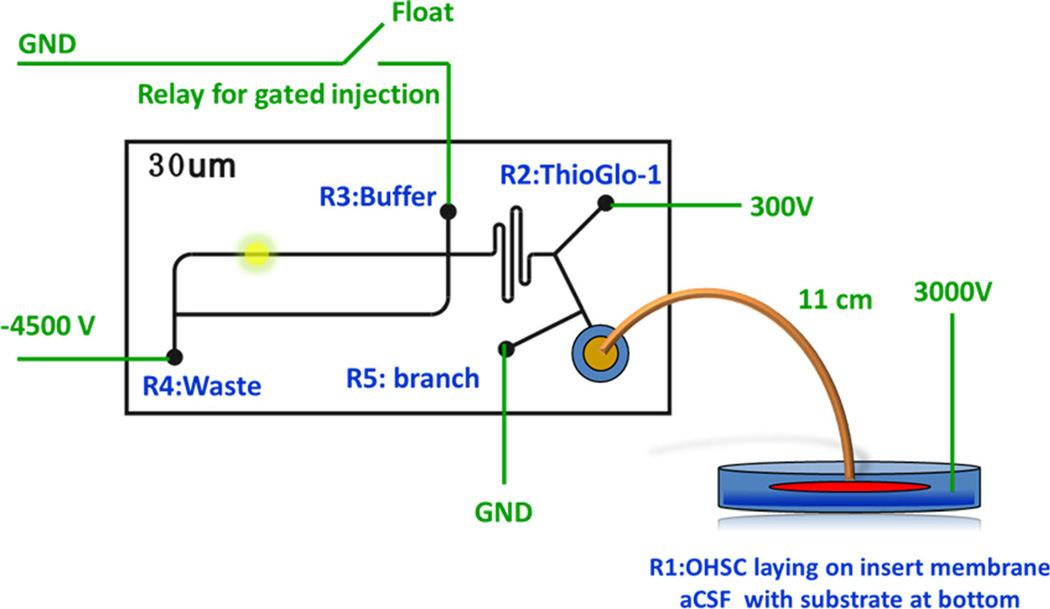
To study this and other problems involving extracellular processing of thiols, we built an “all electric” microfluidic system for EO sampling coupled to a fluorogenic reaction, electrophoretic separation, and laser-induced-fluorescence detection [106–108]. As illustrated in Fig. 7, the tissue is situated as in Fig. 4. Reservoirs R1 and R3 are the main drivers of fluid flow. Reservoir R4 sinks most of the current from the sample stream coming from the culture dish. Reservoir R2 is used for gated injection. Perfusate from the culture is transported to the chip and thiols are derivatized using a fluorogenic maleimide reagent. Derivatized thiols are injected and separated by electrophoresis. Reservoir R4 serves as an isolator for the sampling and derivatizing steps. This reservoir is necessary for controlling the electric field across the sampling capillary without greatly affecting the following on-chip currents and flow rates. Moreover, it expands the usable potential range that can be applied to the sampling capillary and provides the option of splitting the sample flow to R4.
Fig. 7.
Integration of the electroosmotic sampling with online microfluidic analysis. The fused-silica capillary is 11 cm (length)× 50 µm (i.d.), 360 µm (o.d.). Adapted with permission from Wu et al. (2013) An in Situ Measurement of Extracellular Cysteamine, Homocysteine, and Cysteine Concentrations in Organotopic Hippocampal Slice Cultures by Integration of Electroosmotic Sampling and Microfluidic Analysis. Analytical Chemistry 85 (6):3095–3103. Copyright 2013 American Chemical Society
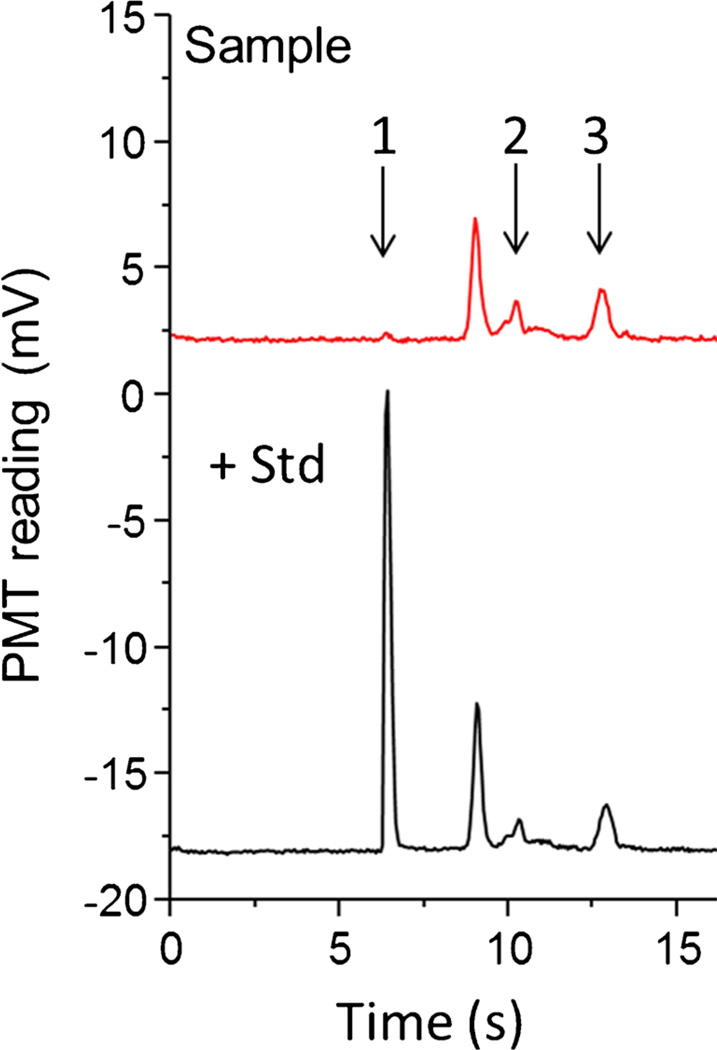
Coupling EO sampling to an EO-driven microfluidic system posed some challenges. One was the high conductivity of biological samples. Buffers used in microfluidic analysis typically have a much lower conductivity (~0.14 S m−1 for 20 mmol L−1 Tris–HCl at pH 7.5) than the buffer used to perfuse the tissue (1.54 S m−1 for artificial cerebrospinal fluid (ACSF)). ACSF was necessary for maintaining osmolarity and simulating an in-vivo environment for OHSCs. Other than the well-known problems resulting from high conductivity, namely Joule heating, the formation of gas bubbles, and rapid electrolysis of buffer, high-conductivity samples can cause gated injection to fail. A direct solution was to find a low-conductivity substitute for normal ACSF. We replaced the major contributor to high conductivity in ACSF, NaCl, with the dipeptide GG, which effectively reduced the conductivity of the original buffer to 0.96 S m−1. Propidium-iodide cell-death assays revealed that the GG-modified ACSF has minimal effects on the tissue in the sampling time scale. Furthermore, to prevent the failure of the gated injection caused by the mismatch of conductivity between the sample stream coming from the reaction channel and the running buffer from the gated reservoir, the running buffer was augmented with NaCl to a conductivity of 0.42 S m−1. Using this set-up we successfully measured the basal concentrations of cysteamine, homocysteine, and cysteine in the extracellular space of the OHSCs (Fig. 8), which were 10.6±1.0 nmol L−1, 0.18± 0.01 µmol L−1, and 11.1±1.2 µmol L−1, respectively [107]. It is important to point out that GG is a substrate for γ-glutamyl transpeptidase (γ-GT), another ectoenzyme of interest in the regulation thiols (specifically in the regulation of glutathione, GSH), and is consequently a poor choice as a buffer component for studies involving that specific enzyme and determination of extracellular GSH concentrations.
Fig. 8.
Detection of aminothiols in the extracellular fluid of OHSCs. The culture cell dish contains (red line) GG-modified aCSF only; or (black line) GG-modified aCSF with added cysteamine. At the beginning of the experiment, R1 contains 12.7 µmol L−1 ThioGlo-1 in a 20 mmol L−1 Tris–HCl buffer (pH 7.5); R2 and R3 contain 40 mmol L−1 Bis–Tris propane buffer with 15 mmol L−1 NaCl (pH 8.50); R4 and the sampling capillary contain 20 mmol L−1 Tris–HCl augmented with 60 mmol L−1 NaCl (pH 7.5). +3000 V, +300 V, and +4500 V are applied at the cell dish, R1, and R3, respectively. R2 is switched between ground (24.5 s) and floating (0.5 s); R4 is connected to ground. There is a six-minute presampling step before each experiment, during which +3000 V is applied to the culture dish and R4 is grounded. All other reservoirs are floating. Electroosmotic sampling is performed in the CA3 region of the OHSCs. Adapted with permission from Wu et al. (2013) An in Situ Measurement of Extracellular Cysteamine, Homocysteine, and Cysteine Concentrations in Organotopic Hippocampal Slice Cultures by Integration of Electroosmotic Sampling and Microfluidic Analysis. Analytical Chemistry 85 (6):3095–3103. Copyright 2013 American Chemical Society Electroosmotic perfusion of tissue
For measurements of substrate processing in the extracellular space, the substrate of the enzyme reaction or the drug of interest is incorporated into the culture dish. The tissue absorbs the fluid at the beginning of the experiment. On application of the electric field, the compounds of interest pass through the extracellular space of OHSCs with the sampling buffer, driven by the electroosmotic flow in the extracellular space. Each experiment results in a series of electropherograms in time. The signals from components of the sample (i.e., peaks in the electropherograms) reach a steady state.
On the basis of this idea, we obtained the apparent Michaelis constant (16±4 µmol L−1) and maximum reaction rate (7.1±0.5 nmol L−1 s−1) for the sequential degradation of CoA in the extracellular space of OHSCs. Using this method, we also successfully correlated the effectiveness and toxicity of two drug compounds to the distinct differences in their conversion rates to the active molecule, cysteamine. The disulfides cystamine and pantethine, the two drug compounds that treat cystinosis, yielded conversation rates of 91±4%and 0.01–0.03 %, respectively. The tissue-based measurements provide a direct way to monitor and evaluate drug metabolism in tissue and are potentially valuable for facilitating drug discovery.
It is important to understand that extracellular concentrations of solutes are not at equilibrium but at steady-state. The basal thiol concentrations in the tissue were determined by standard addition. This procedure is typically used in analytical determinations where so-called “matrix effects” occur. For the standard-additions approach to work, the interference must be proportional to the signal. For example, a matrix component may reduce a compound’s fluorescence by 10 %, e.g., dynamic quenching by oxygen. In such a case, standard additions are effective at providing a quantitative determination despite the interference. We note that first-order reactions occurring over a fixed time period also have an effect that is proportional to the ambient concentration of a compound. Thus, to the degree that the reactions, including uptake by cells, are first order, the standard-additions procedure will work for measurements of steady-state concentrations of solutes in the extracellular space of tissues.
Two probes: EO push–pull perfusion
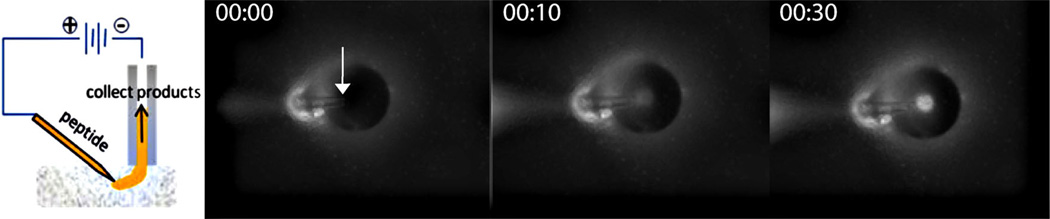
Several neuropeptides have protective behavior in the hippocampus, a region highly susceptible to excitotoxic and inflammatory damage [109–113]. One such peptide, galanin (GWTLN SAGYL LGPHA IDNHR SFSDK HGLT-NH2), reduces glutamatergic synaptic transmissions and, as a result, protects neurons against excitotoxic conditions that result from anoxia [114]. It also binds to galanin receptors that inhibit the production of pro-inflammatory cytokines including interleukin 1 (IL-1) and tumor necrosis factor-α (TNF-α) [115]. We are generally interested in how neuropeptides including enkephalins and galanin promote the survival of hippocampal neurons after an insult, for example oxygen–glucose deprivation (OGD), an experimental model for stroke [82]. The susceptibility of neurons to injury after OGD varies among regions within the hippocampus. In the rat and mouse, there are significant anatomical and functional differences seen on the ~100 µm distance scale or smaller. To perform experiments on intact OHSCs that examine specific regions, better spatial resolution than that shown in Fig. 4 is required. Thus we developed a method, EO push–pull perfusion (EOPPP), using a pair of fused-silica capillary probes. Figure 9 illustrates the concept. The sampling capillary (analogous to a “pull” in push–pull; labeled “collect products” in Fig. 9) is positioned as in Fig. 4. However, the source (“push”) is a capillary pulled to approximately 20 µm in diameter at the tip (labeled “peptide” in Fig. 9). Fluorescence-microscopy images of an EOPPP experiment performed with a Texas- Red-labeled 3 kDa dextran (TR3) in the push capillary reveal the trajectory of the probe molecule from the source tip to the collection capillary. A plume of dye is evident after 30 s. The volume of tissue interrogated is thus the region between the probes, which has been confirmed by simulation.
Fig. 9.
Left: Schematic diagram of EOPPP. A continuous voltage is applied across the push capillary (labeled as “peptide”), the tissue, and the pull capillary (labeled as “collect products”). The resulting field drives electroosmotic flow of the peptide solution through the system from the push to the pull capillary. Right: Image of sampling scheme taken from below the tissue using an inverted microscope. The tip of the push probe in the tissue is indicated with an arrow. The tip of the sampling capillary (perpendicular to the tissue) can be seen as a dark circle outlined by a halo in all three micrographs (at t=0, 10, and 30 s, left-to-right). The flow of the dye from the push to the pull capillary can be seen as the increasing fluorescence of the lumen. The push tip is approximately 60 µm below the pull capillary, which has an i.d. of 75 µm
Using COMSOL Multiphysics (V4.3a), we determined that the flow-rate-to-current ratio for EOPPP is 1.51 nL min−1 µA−1 in the sampling capillary. For the range of currents that we applied in our sampling experiments (7–16 µA), this would translate to a flow-rate range of 10–25 nL min−1. From the simulations, we have also determined that there is flow from the underlying buffer solution during sampling. This suggests that some dilution occurs as a result of this drawing of buffer from the solution underneath. Comparing the volume of internal standard (d-YAGFL) collected experimentally to that predicted by numerical calculations (COMSOL Multiphysics), we calculated a sampling efficiency in tissue of 20 % at the 16 µA current applied. Here, we define sampling efficiency as the ratio of moles collected to moles delivered into the tissue. The sampling efficiency increases with current. In addition, the sampling efficiency was higher in tissue than in a gel (25 % (w/w) acrylic acid) with comparable ζ-potential [73, 116]. The increase in sampling efficiency with increasing current and the reduction in sampling efficiency in the gel can both be explained qualitatively using the Péclet number. In the former case, increasing the current increases the velocity while the diffusion coefficient and length scale remain unchanged, thereby increasing Pe and the dominance of advective over diffusive flux. In the latter case, the reduced tortuosity of the medium increases the diffusion coefficient and the increased porosity enables a greater diffusive flux away from the sampling path, reducing Pe.
We have applied this sampling technique to the quantitative determination of ectopeptidase activity acting on the 29-amino-acid neuropeptide galanin. Our sampling studies in the CA1 and CA3 of OHSCs reveal significant spatial differences in collection of galanin and galanin hydrolysis products, as revealed by capillary LC followed by offline MALDI-TOF. At high currents, the probability of having both short and long galanin fragments with intact carboxy termini (suggesting activity of an aminopeptidase) was higher in the CA3 (p<0.001) than in the CA1. At low currents, the same trend was observed for short galanin fragments but not for long fragments (short: p=0.032; long: p=0.503). It is well known that the CA1 is more susceptible to ischemic stress than the CA3 [87, 89]. Our results suggest that the function of galanin, and the ectoenzyme activity that modulates this peptide, may also differ in two regions. This is the subject of our ongoing studies [73].
EO and damage
Measurements of extracellular processes can only be performed accurately in intact tissue. They are therefore among the most demanding and informative measurements that can be made. However, most if not all measurements in tissue are somewhat invasive. Measurements, including the ones presented here, that use probes, sensors, electrodes, etc. must be performed with an understanding of the potential effect of the probe itself or of the fluid flow initiated in the process on the measurement itself. For our work, a concern is the effect of the electric current or field on the tissue. From earlier work on electroporation [75, 117–120] we know that an electric field of approximately 6.7×103 V m−1 is required to electroporate a cell of typical dimensions, approximately 20–30 µm in diameter. This is in line with the observation that a cell can be electroporated if it experiences a transmembrane potential difference of approximately 200 mV. When the field diverges, as it does in our electroosmotic approaches, the situation is more complex. The cells closest to the probe(s) will experience the highest fields.
In practice, we have investigated the effect of single [121] and two-probe [122] experiments on cell death. These investigations involved changing the capillary dimensions and positions and the current or field to find conditions that were acceptable. Our determination of cell death was on the basis of propidium-iodide fluorescence 18–24 h after the procedure. The fluorescence measurements were sensitive to loss of any type of cell, neuron, astrocyte, or microglial cell. Unfortunately, the rules of thumb developed for the two procedures were not the same. Nonetheless, in both cases there are conditions that give rise to healthy flow rates and to minimum damage of less than 10 % cell death, compared with fully dead tissues. It is worth pointing out that our typical measurements span a long time, five or ten minutes. If in the process of acquiring a sample from the extracellular space a cell membrane was compromised, its picoliter contents would be collected but quite diluted thus having little effect on the measurement. It is possible that enzymes produced by cellular damage could affect results because the substrate peptide and the released enzyme would co-exist in the sampling capillary. We have looked for this and not found any degradation in the capillary [94]. In addition, in studies on galanin [73] we did not see significant cleavage at the carboxy side of proline, the favored cleavage site of one of the most abundant intracellular peptidases, prolyl endopeptidase (EC 3.4.21.26). The measurements of ectopeptidase activity are thus likely to reflect the natural processes within the tissue culture.
Conclusions
Our research group has developed two new tissue-perfusion methods based on electroosmotic flow under the influence of an external electric field. As far as we are aware, before our work published in 2010 there were no reports of tissue-based ectoenzyme kinetic measurements based on any method. Our methods result in minimal to no damage to the tissues themselves. Measuring kinetic variables of the ectoenzyme in tissue cultures can provide metabolic information more relevant to the biological organism than that obtained from currently practiced in-vitro experiments, in which homogenization results in loss of any spatial and temporal information.
Acknowledgements
This work has been funded by the National Institutes of Health (Grants R01 GM044842 and GM066018) and a Dietrich School of Arts and Sciences Fellowship to Y.O. from the Kenneth P. Dietrich School of Arts and Sciences at the University of Pittsburgh.
Contributor Information
Yangguang Ou, Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA.
Juanfang Wu, Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA.
Mats Sandberg, Department of Medical Biochemistry and Cell Biology, University of Gothenburg, 40530 Gothenburg, Sweden.
Stephen G. Weber, Email: sweber@pitt.edu, Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA.
References
- 1.MacIntosh FC, Oborin PE. Release of acetylcholine from intact cerebral cortex. Proc XIX Int Cong Physiol. 1953:580–581. [Google Scholar]
- 2.Gaddum JH. Push-pull cannulae. J Physiol Lond. 1961;155:1P–2P. [Google Scholar]
- 3.Delgado JMR, Simhadri P, Apelbaum J. Chronic implantation of chemitrodes in the monkey brain. Proc XXII Int Union Physiol Sci. 1962;2:1090. [Google Scholar]
- 4.Delgado JMR, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch Int Pharmacodyn. 1972;198:9–21. [PubMed] [Google Scholar]
- 5.Di Chiara G. In-vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci. 1990;11(3):116–121. doi: 10.1016/0165-6147(90)90197-g. [DOI] [PubMed] [Google Scholar]
- 6.Redgrave P. A modified push-pull system for the localised perfusion of brain tissue. Pharmacol Biochem Behav. 1977;6(4):471–474. doi: 10.1016/0091-3057(77)90187-3. [DOI] [PubMed] [Google Scholar]
- 7.Dluzen DE, Ramirez VD. A miniaturized push-pull cannula for use in conscious, unrestrained animals. Pharmacol Biochem Behav. 1986;24(1):147–150. doi: 10.1016/0091-3057(86)90059-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Myers RD, Wooles WR. New triple microbore cannula system for push-pull perfusion of brain nuclei of the rat. J Neurosci Methods. 1990;32(2):93–104. doi: 10.1016/0165-0270(90)90164-b. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wulfert E, Hanin I. Development of a sensitive and inexpensive micropush—pull technique for the continuous analysis of brain neurotransmitters and metabolites in vivo. J Neurosci Methods. 1992;42(1–2):139–147. doi: 10.1016/0165-0270(92)90144-3. [DOI] [PubMed] [Google Scholar]
- 10.Myers RD, Adell A, Lankford MF. Simultaneous comparison of cerebral dialysis and push–pull perfusion in the brain of rats: a critical review. Neurosci Biobehav Rev. 1998;22(3):371–387. doi: 10.1016/s0149-7634(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 11.Ungerstedt U. Measurement of neurotransmitter release by intercranial dialysis. In: Marsden CA, editor. Measurement of neurotransmitter release in vivo. New York: Wiley; 1984. pp. 81–105. [Google Scholar]
- 12.Westerink BHC. Monitoring molecules in the conscious brain by microdialysis. TrAC Trends Anal Chem. 1992;11(5):182–186. [Google Scholar]
- 13.Bungay PM, Sumbria RK, Bickel U. Unifying the mathematical modeling of in vivo and in vitro microdialysis. J Pharm Biomed Anal. 2011;55(1):54–63. doi: 10.1016/j.jpba.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duo J, Fletcher H, Stenken JA. Natural and synthetic affinity agents as microdialysis sampling mass transport enhancers: Current progress and future perspectives. Biosens Bioelectron. 2006;22(3):449–457. doi: 10.1016/j.bios.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Stenken JA. Affinity-based microdialysis sampling using heparin for in vitro collection of human cytokines. Anal Chim Acta. 2009;651(1):105–111. doi: 10.1016/j.aca.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher HJ, Stenken JA. An in vitro comparison of microdialysis relative recovery of Met- and Leu-enkephalin using cyclodextrins and antibodies as affinity agents. Anal Chim Acta. 2008;620(1–2):170–175. doi: 10.1016/j.aca.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trickler WJ, Miller DW. Use of osmotic agents in microdialysis studies to improve the recovery of macromolecules. J Pharm Sci. 2003;92(7):1419–1427. doi: 10.1002/jps.10410. [DOI] [PubMed] [Google Scholar]
- 18.Takeda S, Sato N, Ikimura K, Nishino H, Rakugi H, Morishita R. Novel microdialysis method to assess neuropeptides and large molecules in free-moving mouse. Neuroscience. 2011;186:110–119. doi: 10.1016/j.neuroscience.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Improved Temporal Resolution for in Vivo Microdialysis by Using Segmented Flow. Anal Chem. 2008;80(14):5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottegoda S, Shaik I, Shippy SA. Demonstration of low flow push–pull perfusion. J Neurosci Methods. 2002;121(1):93–101. doi: 10.1016/s0165-0270(02)00245-5. [DOI] [PubMed] [Google Scholar]
- 21.Kottegoda S, Pulido JS, Thongkhao-on K, Shippy SA. Demonstration and use of nanoliter sampling of in vivo rat vitreous and vitroretinal interface. Mol Vis. 2007;13:2073–2082. [PubMed] [Google Scholar]
- 22.Thongkhao-on K, Wirtshafter D, Shippy SA. Feeding specific glutamate surge in the rat lateral hypothalamus revealed by low-flow push–pull perfusion. Pharmacol Biochem Behav. 2008;89(4):591–597. doi: 10.1016/j.pbb.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson EE, II, Pritchett JS, Shippy SA. High temporal resolution coupling of low-flow push-pull perfusion to capillary electrophoresis for ascorbate analysis at the rat vitreoretinal interface. Analyst (Cambridge, United Kingdom) 2009;134(2):401–406. doi: 10.1039/b813887g. [DOI] [PubMed] [Google Scholar]
- 24.Slaney TR, Nie J, Hershey ND, Thwar PK, Linderman J, Burns MA, Kennedy RT. Push–Pull Perfusion Sampling with Segmented Flow for High Temporal and Spatial Resolution in Vivo Chemical Monitoring. Anal Chem. 2011;83(13):5207–5213. doi: 10.1021/ac2003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy RT, Thompson JE, Vickroy TW. In vivo monitoring of amino acids by direct sampling of brain extracellular fluid at ultralow flow rates and capillary electrophoresis. J Neurosci Methods. 2002;114(1):39–49. doi: 10.1016/s0165-0270(01)00506-4. [DOI] [PubMed] [Google Scholar]
- 26.Malavasi F, Deaglio S, Zaccarello G, Horenstein AL, Chillemi A, Audrito V, Serra S, Gandione M, Zitella A, Tizzani A. The hidden life of NAD+-consuming ectoenzymes in the endocrine system. J Mol Endocrinol. 2010;45(3,4):183–191. doi: 10.1677/JME-10-0082. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann H. Ectonucleotidases in the nervous system. Novartis Foundation Symposium. 2006;276:113–130. (Purinergic Signalling in Neuron-Glia Interactions) [PubMed] [Google Scholar]
- 28.Bogan KL, Brenner C. 5'-Nucleotidases and their new roles in NAD + and phosphate metabolism. New J Chem. 2010;34(5):845–853. [Google Scholar]
- 29.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 31.Fausther M, Gonzales E, Dranoff JA. Role of purinergic P2X receptors in the control of liver homeostasis. Wiley Interdiscip Rev: Membr Transp Signal. 2012;1(3):341–348. doi: 10.1002/wmts.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer-Fernandes JR, Cosentino-Gomes D, Vieira DP, Lopes AH. Ecto-nucleoside triphosphate diphosphohydrolase activities in trypanosomatids: possible roles in infection, virulence and purine recycling. Open Parasitol J. 2010;4:116–119. [Google Scholar]
- 33.Mitchell CH, Reigada D. Purinergic signaling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signal. 2008;4(2):101–107. doi: 10.1007/s11302-007-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta, Mol Cell Res. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Kukulski F, Levesque SA, Sevigny J. Impact of ectoenzymes on P2 and P1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 36.Deaglio S, Aydin S, Vaisitti T, Bergui L, Malavasi F. CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med. 2008;14(5):210–218. doi: 10.1016/j.molmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Funaro A, Ortolan E, Bovino P, Lo BN, Nacci G, Parrotta R, Ferrero E, Malavasi F. Ectoenzymes and innate immunity: the role of human CD157 in leukocyte trafficking. Front Biosci, Landmark Ed. 2009;14(3):929–943. doi: 10.2741/3287. [DOI] [PubMed] [Google Scholar]
- 38.Stein LR, S-i I. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23(9):420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaisitti T, Audrito V, Serra S, Bologna C, Brusa D, Malavasi F, Deaglio S. NAD + -metabolizing ecto-enzymes shape tumor-host interactions: The chronic lymphocytic leukemia model. FEBS Lett. 2011;585(11):1514–1520. doi: 10.1016/j.febslet.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Mentlein R. Cell-surface peptidases. Int Rev Cytol. 2004;235:165–213. doi: 10.1016/S0074-7696(04)35004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antczak C, De Meester I, Bauvois B. Ectopeptidases in pathophysiology. BioEssays. 2001;23(3):251–260. doi: 10.1002/1521-1878(200103)23:3<251::AID-BIES1035>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooper NM, Kenny AJ, Turner AJ. The metabolism of neuropeptides. Neurokinin A (substance K) is a substrate for endopeptidase-24.11 but not for peptidyl dipeptidase A (angiotensin-converting enzyme) Biochem J. 1985;231(2):357–361. doi: 10.1042/bj2310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson K, Sharma H, Nyberg F. Chromatographic characterization of substance P endopeptidase in the rat brain reveals affected enzyme activity following heat stress. Biomed Chromatogr. 2006;20(1):77–82. doi: 10.1002/bmc.531. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez J, Segarra AB, Ramirez M, Banegas I, de Gasparo M, Alba F, Vives F, Duran R, Prieto I. Stress Influences Brain Enkephalinase, Oxytocinase and Angiotensinase Activities: A New Hypothesis. Neuropsychobiology. 2009;59(3):184–189. doi: 10.1159/000219306. [DOI] [PubMed] [Google Scholar]
- 46.Dos Santos Medeiros M, Turner AJ. Metabolism and functions of neuropeptide Y. Neurochem Res. 1996;21(9):1125–1132. doi: 10.1007/BF02532423. [DOI] [PubMed] [Google Scholar]
- 47.Fadini GP, Avogaro A. Dipeptidyl peptidase-4 inhibition and vascular repair by mobilization of endogenous stem cells in diabetes and beyond. Atherosclerosis (Amsterdam, Neth) 2013;229(1):23–29. doi: 10.1016/j.atherosclerosis.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59(4):464–471. doi: 10.1016/j.toxicon.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Danziger R. Aminopeptidase N in arterial hypertension. Heart Fail Rev. 2008;13(3):293–298. doi: 10.1007/s10741-007-9061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller BC, Ackroyd A, Hersh LB, Cottam GL. Methionine enkephalin metabolism by murine macrophage ectopeptidase(s) Regul Pept. 1994;50(1):87–98. doi: 10.1016/0167-0115(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 51.Roques BP, Fournie-Zaluski M-C, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discovery. 2012;11(4):292–310. doi: 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- 52.Toth F, Toth G, Benyhe S, Rougeot C, Wollemann M. Opiorphin highly improves the specific binding and affinity of MERF and MEGY to rat brain opioid receptors. Regul Pept. 2012;178(1–3):71–75. doi: 10.1016/j.regpep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Turner AJ, Nalivaeva NN. Peptide degradation (neprilysin and other regulatory peptidases) In: Kastin A, editor. Handbook of Biologically Active Peptides. Elsevier Inc.; 2013. pp. 1757–1764. [Google Scholar]
- 54.Bauer C, Pardossi-Piquard R, Dunys J, Roy M, Checler F. γ-Secretase-Mediated Regulation of Neprilysin: Influence of Cell Density and Aging and Modulation by Imatinib. J Alzheimers Dis. 2011;27(3):511–520. doi: 10.3233/JAD-2011-110746. [DOI] [PubMed] [Google Scholar]
- 55.Malfroy B, Schofield PR, Kuang WJ, Seeburg PH, Mason AJ, Henzel WJ. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- 56.Thielitz A, Ansorge S, Bank U, Tager M, Wrenger S, Gollnick H, Reinhold D. The ectopeptidases dipeptidyl peptidase IV (DP IV) and aminopeptidase N (APN) and their related enzymes as possible targets in the treatment of skin diseases. Front Biosci. 2008;13:2364–2375. doi: 10.2741/2850. [DOI] [PubMed] [Google Scholar]
- 57.Carl-McGrath S, Lendeckel U, Ebert M, Roecken C. Ectopeptidases in tumour biology: a review. Histol Histopathol. 2006;21(10,11 and 12):1339–1353. doi: 10.14670/HH-21.1339. [DOI] [PubMed] [Google Scholar]
- 58.Rashid M, Wangler NJ, Yang L, Shah K, Arumugam TV, Abbruscato TJ, Karamyan VT. Functional up-regulation of endopeptidase neurolysin during post-acute and early recovery phases of experimental stroke in mouse brain. J Neurochem. 2014;129(1):179–189. doi: 10.1111/jnc.12513. [DOI] [PubMed] [Google Scholar]
- 59.Vincent B, Beaudet A, Dauch P, Vincent J-P, Checler F. Distinct Properties of Neuronal and Astrocytic Endopeptidase 3.4.24.16: A Study on Differentiation, Subcellular Distribution, and Secretion Processes. J Neurosci. 1996;16(16):5049–5059. doi: 10.1523/JNEUROSCI.16-16-05049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wangler NJ, Santos KL, Schadock I, Hagen FK, Escher E, Bader M, Speth RC, Karamyan VT. Identification of Membrane-bound Variant of Metalloendopeptidase Neurolysin (EC 3.4.24.16) as the Non-angiotensin Type 1 (Non-AT1), Non-AT2 Angiotensin Binding Site. J Biol Chem. 2012;287:114–122. doi: 10.1074/jbc.M111.273052. Copyright (C) 2014 American Chemical Society (ACS). All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad S, Okine L, Wood R, Aljian J, Vistica DT. γ-glutamyltranspeptidase (γ-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987;131(2):240–246. doi: 10.1002/jcp.1041310214. [DOI] [PubMed] [Google Scholar]
- 62.Picher M, Boucher RC. Metabolism of extracellular nucleotides in human airways by a multienzyme system. Drug Dev Res. 2001;52(1/2):66–75. [Google Scholar]
- 63.Henz SL, Ribeiro CG, Rosa A, Chiarelli RA, Casali EA, Sarkis JJF. Kinetic characterization of ATP diphosphohydrolase and 5'-nucleotidase activities in cells cultured from submandibular salivary glands of rats. Cell Biol Int. 2006;30(3):214–220. doi: 10.1016/j.cellbi.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 64.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997;272(33):20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- 65.Mawrin C, Wolke C, Haase D, Krueger S, Firsching R, Keilhoff G, Paulus W, Gutmann DH, Lal A, Lendeckel U. Reduced activity of CD13/aminopeptidase N (APN) in aggressive meningiomas is associated with increased levels of SPARC. Brain Pathol. 2010;20:200–210. doi: 10.1111/j.1750-3639.2009.00267.x. Copyright (C) 2013 American Chemical Society (ACS). All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vargas MA, Cisneros M, Joseph-Bravo P, Charli JL. Thyrotropin-releasing hormone-induced down-regulation of pyroglutamyl aminopeptidase II activity involves L-type calcium channels and CaM kinase activities in cultures of adenohypophyseal cells. J Neuroendocrinol. 2002;14(3):184–193. doi: 10.1046/j.0007-1331.2001.00755.x. [DOI] [PubMed] [Google Scholar]
- 67.Bauer K. Purification and characterization of the thyrotropin-releasing hormone degrading enzyme. Eur J Biochem. 1994;224(2):387–396. doi: 10.1111/j.1432-1033.1994.00387.x. [DOI] [PubMed] [Google Scholar]
- 68.De Gortari P, Vargas MA, Martinez A, Garcia-Vazquez AI, Uribe RM, Chavez-Gutierrez L, Magdaleno V, Boileau G, Charli J-L, Joseph-Bravo P. Stage-specific modulation of neprilysin and aminopeptidase N in the limbic system during kindling progression. J Mol Neurosci. 2007;33(3):252–261. doi: 10.1007/s12031-007-0020-9. [DOI] [PubMed] [Google Scholar]
- 69.Dringen R, Gutterer JM, Gros C, Hirrlinger J. Aminopeptidase N mediates the utilization of the GSH precursor CysGly by cultured neurons. J Neurosci Res. 2001;66(5):1003–1008. doi: 10.1002/jnr.10042. [DOI] [PubMed] [Google Scholar]
- 70.Heuer H, Schafer MK, Bauer K. The thyrotropin-releasing hormone-degrading ectoenzyme: the third element of the thyrotropin-releasing hormone-signaling system. Thyroid. 1998;8(10):915–920. doi: 10.1089/thy.1998.8.915. [DOI] [PubMed] [Google Scholar]
- 71.Guy Y, Muha RJ, Sandberg M, Weber SG. Determination of ζ-Potential and Tortuosity in Rat Organotypic Hippocampal Cultures from Electroosmotic Velocity Measurements under Feedback Control. Anal Chem. 2009;81(8):3001–3007. doi: 10.1021/ac802631e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guy Y, Sandberg M, Weber SG. Determination of ζ-potential in rat organotypic hippocampal cultures. Biophys J. 2008;94:4561–4569. doi: 10.1529/biophysj.107.112722. Copyright (C) 2014 American Chemical Society (ACS). All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rupert AE, Ou Y, Sandberg M, Weber SG. Electroosmotic Push–Pull Perfusion: Description and Application to Qualitative Analysis of the Hydrolysis of Exogenous Galanin in Organotypic Hippocampal Slice Cultures. ACS Chem Neurosci. 2013;4(5):838–848. doi: 10.1021/cn400082d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirby BJ, Hasselbrink EF., Jr Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis. 2004;25(2):187–202. doi: 10.1002/elps.200305754. [DOI] [PubMed] [Google Scholar]
- 75.Nolkrantz K, Farre C, Brederlau A, Karlsson RID, Brennan C, Eriksson PS, Weber SG, Sandberg M, Orwar O. Electroporation of Single Cells and Tissues with an Electrolyte-filled Capillary. Anal Chem. 2001;73(18):4469–4477. doi: 10.1021/ac010403x. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Astary GW, Kantorovich S, Mareci TH, Carney PR, Sarntinoranont M. Voxelized computational model for convection-enhanced delivery in the rat ventral hippocampus: comparison with in vivo MR experimental studies. Ann Biomed Eng. 2012;40(9):2043–2058. doi: 10.1007/s10439-012-0566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoverud KH, Darcis M, Helmig R, Hassanizadeh SM. Modeling Concentration Distribution and Deformation During Convection-Enhanced Drug Delivery into Brain Tissue. Transp Porous Media. 2012;92(1):119–143. [Google Scholar]
- 78.Foley CP, Nishimura N, Neeves KB, Schaffer CB, Olbricht WL. Real-time imaging of perivascular transport of nanoparticles during convection-enhanced delivery in the rat cortex. Ann Biomed Eng. 2012;40(2):292–303. doi: 10.1007/s10439-011-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rathore AS, Horvath C. Axial Nonuniformities and Flow in Columns for Capillary Electrochromatography. Anal Chem. 1998;70:3069–3077. doi: 10.1021/ac971260a. Copyright (C) 2014 American Chemical Society (ACS). All Rights Reserved. [DOI] [PubMed] [Google Scholar]
- 80.Holopainen I. Organotypic Hippocampal Slice Cultures: A Model System to Study Basic Cellular and Molecular Mechanisms of Neuronal Cell Death, Neuroprotection, and Synaptic Plasticity. Neurochem Res. 2005;30(12):1521–1528. doi: 10.1007/s11064-005-8829-5. [DOI] [PubMed] [Google Scholar]
- 81.Rom Poulsen F, Blaabjerg M, Montero M, Zimmer J. Glutamate receptor antagonists and growth factorsmodulate dentate granule cell neurogenesis in organotypic, rat hippocampal slice cultures. Brain Res. 2005;1051(1–2):35–49. doi: 10.1016/j.brainres.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 82.Ziemka-Nalecz M, Stanaszek L, Zalewska T. Oxygen-glucose deprivation promotes gliogenesis and microglia activation in organotypic hippocampal slice culture: involvement of metalloproteinases. Acta Neurobiol Exp (Wars) 2013;73(1):130–142. doi: 10.55782/ane-2013-1927. [DOI] [PubMed] [Google Scholar]
- 83.Lindroos MM, Soini SL, Kukko-Lukjanov T-K, Korpi ER, Lovinger D, Holopainen IE. Maturation of cultured hippocampal slices results in increased excitability in granule cells. Int J Dev Neurosci. 2005;23(1):65–73. doi: 10.1016/j.ijdevneu.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 84.Lushnikova I, Skibo G, Muller D, Nikonenko I. Excitatory synaptic activity is associated with a rapid structural plasticity of inhibitory synapses on hippocampal CA1 pyramidal cells. Neuropharmacology. 2011;60(5):757–764. doi: 10.1016/j.neuropharm.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi M, Liou SY, Kunihara M. Ca(2+)- and Cl(−)-dependent, NMDA receptor-mediated neuronal death induced by depolarization in rat hippocampal organotypic cultures. Brain Res. 1995;675(1–2):249–256. doi: 10.1016/0006-8993(95)00078-5. [DOI] [PubMed] [Google Scholar]
- 86.Won R, Lee KH, Lee BH. Coenzyme Q10 protects neurons against neurotoxicity in hippocampal slice culture. NeuroReport. 2011;22(14):721–726. doi: 10.1097/WNR.0b013e32834acb8d. [DOI] [PubMed] [Google Scholar]
- 87.Vornov JJ, Park J, Thomas AG. Regional Vulnerability to Endogenous and Exogenous Oxidative Stress in Organotypic Hippocampal Culture. Exp Neurol. 1998;149(1):109–122. doi: 10.1006/exnr.1997.6673. [DOI] [PubMed] [Google Scholar]
- 88.Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Pauly JR, Mulholland PJ, Prendergast MA. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-d-asparate-type glutamate receptors. Neuroscience. 2010;165(2):525–534. doi: 10.1016/j.neuroscience.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tasker RC, Coyle JT, Vornov JJ. The regional vulnerability to hypoglycemia-induced neurotoxicity in organotypic hippocampal culture: protection by early tetrodotoxin or delayed MK-801. J Neurosci. 1992;12(11):4298–4308. doi: 10.1523/JNEUROSCI.12-11-04298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simão F, Zamin LL, Frozza R, Nassif M, Horn AP, Salbego CG. Protective profile of oxcarbazepine against oxygen–glucose deprivation in organotypic hippocampal slice culture could involve PI3K cell signaling pathway. Neurol Res. 2009;31(10):1044–1048. doi: 10.1179/174313209X385671. [DOI] [PubMed] [Google Scholar]
- 91.Liu J, Solway K, Messing RO, Sharp FR. Increased Neurogenesis in the Dentate Gyrus After Transient Global Ischemia in Gerbils. J Neurosci. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 93.Caroni P, De Paola V, Galimberti I, Gogolla N. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165+. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- 94.Xu H, Guy Y, Hamsher A, Shi G, Sandberg M, Weber SG. Electroosmotic Sampling. Application to Determination of Ectopeptidase Activity in Organotypic Hippocampal Slice Cultures. Anal Chem. 2010;82(15):6377–6383. doi: 10.1021/ac1012706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wisner A, Dufour E, Messaoudi M, Nejdi A, Marcel A, Ungeeheuer M-N, Rougeot C. Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc Natl Acad Sci U S A. 2006;103(47):17979–17984. doi: 10.1073/pnas.0605865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barclay RK, Phillipps MA. Inhibition of the enzymatic degradation of leu-enkephalin by puromycin. Biochem Biophys Res Commun. 1978;81(4):1119–1123. doi: 10.1016/0006-291x(78)91252-4. [DOI] [PubMed] [Google Scholar]
- 97.Chen J-G, Weber SG. Detection of bioactive oligopeptides aftermicrobore HPLC with electrochemical detection of their Cu(II) complexes: effect of operating parameters on sensitivity and selectivity. Anal Chem. 1995;67(19):3596–3604. doi: 10.1021/ac00115a033. [DOI] [PubMed] [Google Scholar]
- 98.Chen J-G, Woltman SJ, Weber SG. Sensitivity and selectivity of the electrochemical detection of the copper(II) complexes of bioactive peptides, and comparison to model studies by rotating ring-disc electrode. J Chromatogr A. 1995;691(1–2):301–315. doi: 10.1016/0021-9673(94)00843-x. [DOI] [PubMed] [Google Scholar]
- 99.Woltman SJ, Chen J-G, Weber SG, Tolley JO. Determination of the pharmaceutical peptide TP9201 by post-column reaction with copper(II) followed by electrochemical detection. J Pharm Biomed Anal. 1995;14(1/2):155–164. doi: 10.1016/0731-7085(95)01623-6. [DOI] [PubMed] [Google Scholar]
- 100.Müller H, Neufang H, Knobloch K. Kinetic studies on the membrane-bound and the purified coupling factor-ATPase from Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1983;224(1):283–289. doi: 10.1016/0003-9861(83)90211-4. [DOI] [PubMed] [Google Scholar]
- 101.Robishaw JD, Neely JR. Coenzyme A metabolism. Am J Physiol Endocrinol Metab. 1985;248(1):E1–E9. doi: 10.1152/ajpendo.1985.248.1.E1. [DOI] [PubMed] [Google Scholar]
- 102.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 103.Leonardi R, Zhang YM, Rock CO, Jackowski S. Coenzyme A: Back in action. Prog Lipid Res. 2005;44(2–3):125–153. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Spry C, Kirk K, Saliba KJ. Coenzyme A biosynthesis: An antimicrobial drug target. Fems Microbiol Rev. 2008;32(1):56–106. doi: 10.1111/j.1574-6976.2007.00093.x. [DOI] [PubMed] [Google Scholar]
- 105.Stipanuk MH, Dominy JE, Lee J-I, Coloso RM. Mammalian cysteine metabolism: New insights into regulation of cysteine metabolism. J Nutr. 2006;136(6):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 106.Wu J, Ferrance JP, Landers JP, Weber SG. Integration of a Precolumn Fluorogenic Reaction, Separation, and Detection of Reduced Glutathione. Anal Chem. 2010;82(17):7267–7273. doi: 10.1021/ac101182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu J, Xu K, Landers JP, Weber SG. An in Situ Measurement of Extracellular Cysteamine, Homocysteine, and Cysteine Concentrations in Organotypic Hippocampal Slice Cultures by Integration of Electroosmotic Sampling and Microfluidic Analysis. Anal Chem. 2013;85(6):3095–3103. doi: 10.1021/ac302676q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu J, Sandberg M, Weber SG. Integrated Electroosmotic Perfusion of Tissue with Online Microfluidic Analysis to Track the Metabolism of Cystamine, Pantethine, and Coenzyme A. Anal Chem. 2013;85(24):12020–12027. doi: 10.1021/ac403005z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fox MW, Anderson RE, Meyer FB. Neuroprotection by corticotropin releasing factor during hypoxia in rat brain. Stroke. 1993;24(7):1072–1075. doi: 10.1161/01.str.24.7.1072. [DOI] [PubMed] [Google Scholar]
- 110.Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744(1):166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 111.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27(4):415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shishido Y, Furushiro M, Tanabe S, Shibata S, Hashimoto S, Yokokura T. Effects of prolyl endopeptidase inhibitors and neuropeptides on delayed neuronal death in rats. Eur J Pharmacol. 1999;372(2):135–142. doi: 10.1016/s0014-2999(99)00185-5. [DOI] [PubMed] [Google Scholar]
- 113.Malva JO, Xapelli S, Baptista S, Valero J, Agasse F, Ferreira R, Silva AP. Multifaces of neuropeptide Y in the brain – Neuroprotection, neurogenesis and neuroinflammation. Neuropeptides. 2012;46(6):299–308. doi: 10.1016/j.npep.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Ben-Ari Y. Galanin and GlibenclamideModulate the Anoxic Release of Glutamate in Rat CA3 Hippocampal Neurons. Eur J Neurosci. 1990;2(1):62–68. doi: 10.1111/j.1460-9568.1990.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 115.Hwang IK, Yoo K-Y, Kim DS, Do S-G, Oh Y-S, Kang T-C, Han BH, Kim JS, Won MH. Expression and changes of galanin in neurons and microglia in the hippocampus after transient forebrain ischemia in gerbils. Brain Res. 2004;1023(2):193–199. doi: 10.1016/j.brainres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 116.Faraji AH, Cui JJ, Guy Y, Li L, Gavigan CA, Strein TG, Weber SG. Synthesis and Characterization of a Hydrogel with Controllable Electroosmosis: A Potential Brain Tissue Surrogate for Electrokinetic Transport. Langmuir. 2011;27:13635–13642. doi: 10.1021/la202198k. Copyright (C) 2014 American Chemical Society (ACS). All Rights Reserved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olofsson J, Nolkrantz K, Ryttsen F, Lambie BA, Weber SG, Orwar O. Single-cell electroporation. Curr Opin Biotechnol. 2003;14(29–34) doi: 10.1016/s0958-1669(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 118.Olofsson J, Levin M, Stroemberg A, Weber SG, Ryttsen F, Orwar O. Generation of focused electric field patterns at dielectric surfaces. Anal Chem. 2005;77:4667–4672. doi: 10.1021/ac0502302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olofsson J, Levin M, Stroemberg A, Weber SG, Ryttsen F, Orwar O. Scanning electroporation of selected areas of adherent cell cultures. Anal Chem. 2007;79:4410–4418. doi: 10.1021/ac062140i. [DOI] [PubMed] [Google Scholar]
- 120.Zudans I, Agarwal A, Orwar O, Weber SG. Numerical calculations of single-cell electroporation with an electrolyte-filled capillary. Biophys J. 2007;92:3696–3705. doi: 10.1529/biophysj.106.097683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamsher AE, Xu H, Guy Y, Sandberg M, Weber SG. Minimizing Tissue Damage in Electroosmotic Sampling. Anal Chem. 2010;82(15):6370–6376. doi: 10.1021/ac101271r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rupert AE, Ou Y, Sandberg M, Weber SG. Assessment of Tissue Viability Following Electroosmotic Push–Pull Perfusion from Organotypic Hippocampal Slice Cultures. ACS Chem Neurosci. 2013;4(5):849–857. doi: 10.1021/cn4000814. [DOI] [PMC free article] [PubMed] [Google Scholar]