Abstract
The present study reports an efficient in vitro micropropagation protocol for a medicinally important tree, Terminalia bellerica Roxb. from nodal segments of a 30 years old tree. Nodal segments taken from the mature tree in March-April and cultured on half strength MS medium gave the best shoot bud proliferation response. Combinations of serial transfer technique (ST) and incorporation of antioxidants (AO) [polyvinylpyrrolidone, PVP (50 mg l−1) + ascorbic acid (100 mg l−1) + citric acid (10 mg l−1)] in the culture medium aided to minimize browning and improve explant survival during shoot bud induction. Highest multiplication of shoots was achieved on medium supplemented with 6-benzyladenine (BA, 8.8 μM) and α-naphthalene acetic acid (NAA, 2.6 μM) in addition to antioxidants. Shoot elongation was obtained on MS medium containing BA (4.4 μM) + phloroglucinol (PG, 3.9 μM). Elongated shoots were transferred to half strength MS medium containing indole-3-butyric acid (IBA, 2.5 μM) for root development. The acclimatization of plantlets was carried out under greenhouse conditions. The genetic fidelity of the regenerated plants was checked using inter simple sequence repeats (ISSR) and randomly amplified polymorphic DNA (RAPD) analysis. Comparison of the bands among the regenerants and mother plant confirmed true-to-type clonal plants.
Keywords: Antioxidants, ISSR, Mature node, Phloroglucinol, RAPD, Terminalia bellerica
Introduction
Terminalia bellerica (Family: Combretaceae) known as belleric myrobalan is a large deciduous tree, widely found in different parts of India (Mushke et al. 2014). The fruits are rich source of commercial myrobalan which is used in pharmaceutical industries. It is secondary host of tasar silkworm (Anonymous 1976). The fruit is also one of the three ingredients of “Triphala” an Ayurvedic herbal formulation, which is recognized with various useful properties like antibacterial, antiviral, antifungal, antioxidant and immunostimulant activities (Sumathi and Parvathi 2010).
Commercial exploitation of Terminalia by various industries along with losses due to indiscriminate felling of trees, conversion of forest land into agriculture land and human settlements have all led to rapid depletion of genetic resources of T. bellerica (Dangi et al. 2012a). There is an increasing need for the preservation of these plants through systematic cultivation for germplasm conservation, selection of desired genotypes and mass propagation of superior clones. Conventional methods of T. bellerica propagation are insufficient because of poor seed germination and low survival rates of stem cuttings (Roy et al. 1987). Therefore, alternative methods for propagation of selected trees are needed. Tissue culture has proved to be a promising technique for conservation and large-scale multiplication of several woody species (Giri et al. 2004). Earlier reports on micropropagtion of T. bellerica used juvenile cotyledonary node as explant (Ramesh et al. 2005; Rathore et al. 2008; Dangi et al. 2012b). Phulwaria et al. (2012) reported a protocol by taking explants from 10 year old plants. In spite of several protocols for regeneration being reported in T. bellerica, the regeneration efficiency has been shown to be compromised by phenolic exudation, basal callusing, vitrification and shoot tip necrosis. Also, clonal fidelity of regenerated plants is a major consideration in commercial micropropagation of trees. Plant cell culture results in high frequency of variation in regenerated plants (Larkin and Scowcroft 1981). Owing to this variation, the resulting plant may not possess the same properties as that of the parent plant. Molecular marker techniques like Random Amplified Polymorphic DNA (RAPD) and Inter Simple Sequence Repeats (ISSR) are rapid, sensitive and reliable in analysis of genetic stability of in vitro regenerants. These two marker systems have been successfully applied to detect the somaclonal variations in a number of species such as Cymbopogon martini (Bhattacharya et al. 2010), Phoenix dactylifera (Kumar et al. 2010) and Simmondsia chinensis (Kumar et al. 2011).
Considering the above facts, the present study was undertaken to develop a more efficient protocol for micropropagation of selected mature trees of T. bellerica from nodal explants and assessment of clonal fidelity of regenerated plants by use of molecular markers.
Material and methods
Plant regeneration
Nodal explants from 30 years-old Terminalia bellerica tree growing in the Grassfarm nursery, Jaipur, India were excised from 1st to 5th node of side branches. The shoots were thoroughly washed with 20 % (v/v) Extran® (Merck, India) for 5–7 min followed by surface-sterilization with a 0.1 % HgCl2 (Merck, India) solution (w/v) for 7 min and then rinsed three times with sterile distilled water. After sterilization, the shoots were cut into segments (1.0–1.5 cm) with each piece having a node in leaf axil.
Murashige and Skoog (1962) basal medium supplemented with 3 % (w/v) sucrose, solidified with 0.8 % agar (Merck, India) and adjusted to pH 5.8 before autoclaving at 1.06 kg cm−2 (121 °C) for 20 min was used in all the experiments. Cultures were maintained at 26 ± 1 °C under a 16 h photoperiod with 25 μmol m−2 s−1 photosynthetic photon flux density provided by white fluorescent tubes (40 W; Philips, India). The medium was dispensed in Erlenmeyer flasks (100 ml, Borosil, India) each containing 30 ml of medium and sealed with non-absorbent cotton plugs.
The surface sterilized nodes were inoculated on medium containing different concentrations of plant growth regulators (Sigma, India) viz. BA (2.2, 4.4, 8.8, 13.2, 22.2 μM), kinetin (Kn; 2.3, 4.6, 9.2, 13.9 and 23.2 μM) alone or in a combination of BA with indole-3-acetic acid (IAA) or α-naphthaleneacetic acid (NAA) for axillary shoot bud proliferation. The experiments were also conducted to circumvent media browning for improved survival of explants. Nodal explants were cultured on nutrient media containing various additives; PVP (50 mg l−1), activated charcoal (500 mg l−1) and ascorbic acid (50 mg l−1) alone or in combination. Also, a combination of serial transfers with antioxidant solution (ST + AO) was checked. Antioxidant solution (AO) contains PVP (50 mg l−1), ascorbic acid (100 mg l−1) and citric acid (10 mg l−1) (Purohit et al. 1995). In serial transfer technique, explants were cultured on MS medium supplemented with BA (8.9 μM) + NAA (2.6 μM) + AO followed by repeated transfer of the explants onto fresh medium at an interval of 48 h for 2–3 times.
In another experiment, the nodal explants were inoculated on full and ½ MS (half strength of major inorganic salts) and Woody Plant Medium (WPM) (Llyod and McCown 1980) fortified with BA (8.9 μM) + NAA (2.6 μM). To study the influence of explant harvest period on induction, nodal explants were collected during January–February, March–April, May–June, July–August, September–October and November–December.
After 4 weeks, the shoot buds induced on half MS medium containing BA (8.8 μM) + NAA (2.6 μM) were sectored into pieces and subcultured on the same medium for proliferation. Shoots were then cultured on MS medium containing lower level of BA (4.4 μM) with PG (3.9, 7.9 μM) or gibberellic acid (GA3; 0.57, 1.44, 2.88 μM) for elongation. Some of the in vitro produced shoots were used to establish new cultures and rest were placed on ½ MS medium supplemented with different concentrations of IBA (0.98, 2.5, 4.9 μM) for rooting. Cultures were evaluated after 4 weeks. Plantlets with well developed roots were thoroughly washed with running tap water to remove the adhering agar and transferred to pots containing garden soil and placed in greenhouse for hardening. Successfully acclimatized plantlets were transferred to field conditions. The relative humidity and temperature of the greenhouse was 70 % and 30 ± 2 °C respectively.
Statistical analysis
Each experiment of in vitro propagation study consisted of 15 replicates, and all experiments were repeated 3 times. Data for explant establishment, shoot proliferation, and rooting of micro-shoots were collected after 4 weeks. Percent response for bud break and rooting experiments was quantified as: % response = (Total number of shoots responded in bud break or rooting/Total number of replicates) × 100. Percent survival was calculated as: (Total number of explants survived after inoculation/Total number of replicates) × 100. Results were expressed as mean values ± standard error (S.E.). Data were analyzed for significance using analysis of variance (ANOVA) and the differences contrasted using a Duncan’s multiple range test (DMRT).
Molecular analysis
ISSR and RAPD markers were used to evaluate the genetic fidelity of micropropagated plants of T. bellerica. DNA was extracted from the leaves of 14 randomly selected regenerated plants and from field grown mother plant (Mp, mature tree). Each sample was powdered in liquid nitrogen (−196 °C) and stored at –20 °C until used for DNA extraction by CTAB method (Doyle and Doyle 1990). A total of 10 arbitrary RAPD primers and 10 ISSR primers were screened to determine their potential for clear polymorphism and reproducibility. RAPD and ISSR amplification reactions were performed in 20 μl reactions each containing 200 μM of each dNTPs, 0.8 μM of primer, 1X PCR buffer, 0.3 U (for RAPD) or 0.6 U (for ISSR) of Taq DNA polymerase (Genei, Bengalore, India), 0.5 mM of MgCl2 (only for ISSR) and 20 ng of genomic DNA. PCR reactions were performed in a Thermal Cycler (BioRad, UK). For RAPD, amplification conditions were an initial denaturation at 94 °C for 3 min followed by 45 cycles at 94 °C for 45 s, 36 °C for 45 s and 72 °C for 2 min with a final extension at 72 °C for 7 min. ISSR reactions were performed with initial denaturation at 94 °C for 4 min followed by 35 cycles at 92 °C for 30 s, 1 min at annealing temperature (depending upon the primer) and 72 °C for 2 min with a final extension at 72 °C for 7 min. Amplified products were separated on 1.5 % or 1.8 % agarose gel for RAPD and ISSR reactions respectively in 1x TBE buffer by electrophoresis at 100 V, visualized with ethidium bromide staining and photographed using Gel Documentation System (BioRad, UK). The size of the amplification products was determined by 1Kb ladder as a molecular standard.
Results and discussion
The present investigation described the clonal propagation of T. bellerica using nodal segments of a mature tree. In T. bellerica regeneration of plants from explants derived from seedlings (Ramesh et al. 2005; Rathore et al. 2008; Dangi et al. 2012b) and 10 year-old plant (Phulwaria et al. 2012) have been reported. It was suggested that the disadvantage of using juvenile rather than adult specimen for propagation is that full genetic developmental potential of the former is less known than that of adult tree. Besides, tissue culture method developed for cloning juvenile plant may not be applicable for adult one as latter show recalcitrance in culture (Phulwaria et al. 2011).
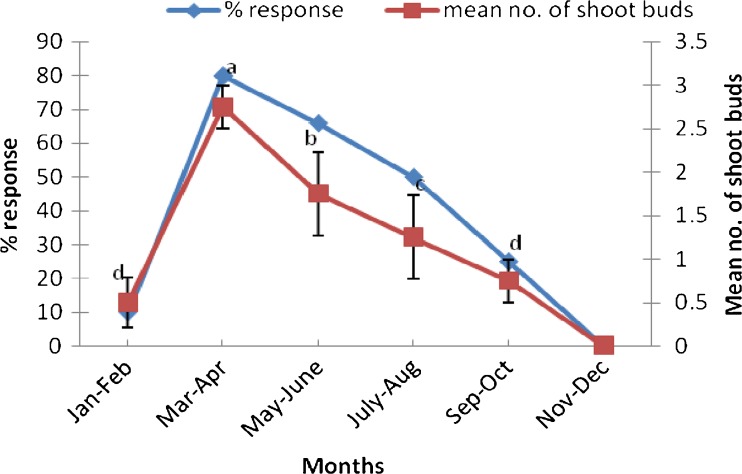
It was observed that time of collection of explants influenced shoot bud induction (Fig. 1). The cultures initiated during March–April exhibited best induction response (80 %). Explants collected during May–June resulted into 66 % of aseptic culture establishment. Nodal explants collected during January–February responded poorly in cultures. The induction response fell to zero in the months of November and December. It was also observed that maximum browning in the medium occurred in July–August and least during March–April. The better response of bud break and sprouting initiated from nodal explants could be attributed to the active vegetative growth phase of T. bellerica trees during that period. Explants harvested during other periods were found to be less responsive and with high infection percentage. The effect of season on bud sprouting has also been reported previously in Crataeva adansonii (Sharma et al. 2003), Terminalia arjuna (Pandey et al. 2006), Salvadora persica (Phulwaria et al. 2011) and Dalbergia sissoo (Vibha et al. 2013).
Fig. 1.
Effect of different seasons on regeneration frequency of mature nodal explants of Terminalia bellerica. Each treatment consisted of 15 replicates and experiment was repeated thrice. The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
The mature nodal explants exhibited bud break on MS medium containing cytokinins (BA or Kn) after 2–3 weeks of culture. BA was found to be more favorable for the production of shoots (Table 1). Superiority of BA in shoot bud induction has been reported to be due to ability of plant tissues to absorb and metabolize BA readily than other synthetic growth regulators (Zhang et al. 2010; Malik et al. 2005).
Table 1.
Effect of various concentrations of cytokinins on bud break from cultured nodal segments of Terminalia bellerica
| PGR (μM) | % response | Shoot Buds [explants]−1 Mean ± S.E. |
|---|---|---|
| BAP | ||
| 2.2 | NR | NR |
| 4.4 | 26.6 | 0.7 ± 0.2e |
| 8.8 | 53.3 | 2.5 ± 0.2a |
| 13.2 | 20.0 | 1.7 ± 0.2b |
| 22.2 | 20.0 | 1.2 ± 0.2d |
| Kn | ||
| 2.3 | NR | NR |
| 4.6 | NR | NR |
| 9.2 | 26.6 | 1.7 ± 0.3b |
| 13.9 | 33.3 | 1.4 ± 0.2c |
| 23.2 | 13.3 | 1.7 ± 0.2b |
NR No Response
Each treatment consisted of 15 replicates and experiment was repeated thrice
The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
Combination of BA (8.8 μM) and NAA (2.6 μM) could increase the morphogenic response both in terms of number of shoots and percent response of explants (Table 2). Further increase in concentration of NAA showed decline in shoot induction and percent response. Organogenic differentiation depends on a delicate balance between the concentration of auxin and cytokinin in the nutrient medium. The significant role of low concentration of auxin in combination with cytokinin on shoot regeneration has previously been accepted in many trees (Sharma et al. 2003; Ahmad and Anis 2007; Husain and Anis 2009).
Table 2.
Effect of BA and auxins on shoot bud proliferation from cultured mature nodal segments of Terminalia bellerica
| PGRs (μM) | % response | Shoot buds [explant]−1 Mean ± S.E. | ||
|---|---|---|---|---|
| BAP | NAA | IAA | ||
| 4.4 | 0.5 | 13.3 | 0.8 ± 0.2f | |
| 2.6 | 33.3 | 2.0 ± 0.3c | ||
| 5.3 | NR | NR | ||
| 8.8 | 0.5 | 60.0 | 2.9 ± 0.3b | |
| 2.6 | 73.3 | 3.8 ± 0.3a | ||
| 5.3 | 10.0 | 1.4 ± 0.2de | ||
| 4.4 | 0.5 | NR | NR | |
| 2.8 | 13.3 | 1.2 ± 0.2e | ||
| 5.7 | 26.6 | 1.5 ± 0.2d | ||
| 8.8 | 0.5 | NR | NR | |
| 2.8 | 66.6 | 2.2 ± 0.3c | ||
| 5.7 | 60.0 | 2.0 ± 0.3c | ||
NR No Response
Each treatment consisted of 15 replicates and experiment was repeated thrice
The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
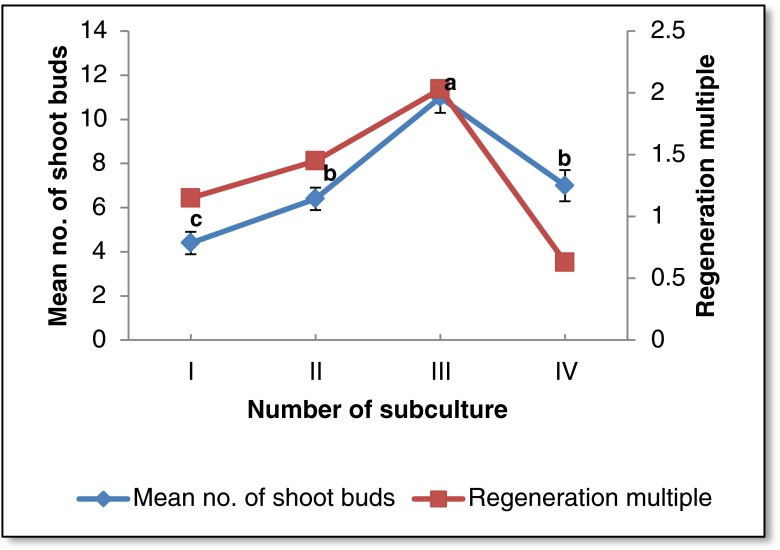
Multiplication of in vitro regenerated shoots was achieved by repeated transfer of the shoot clumps (with 2–3 shoots) on the medium containing 8.8 μM BA + 2.6 μM NAA after every 4 weeks of culture. Maximum number of shoots (11 ± 0.7) per explant was observed after third passage of culture (Fig. 2) which is comparable to the previous reports on the same plant species (Phulwaria et al. 2012). A prolific shoot culture was established by repeated subculturing of nodal segments from newly formed shoots. Repeated subculturing has been reported as a method for rejuvenation of mature explants for clonal propagation of several woody plants (Phulwaria et al. 2011, Goyal et al. 2012).
Fig. 2.
Effect of subculture stages on shoot proliferation. Each treatment consisted of 15 replicates and experiment was repeated thrice. The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
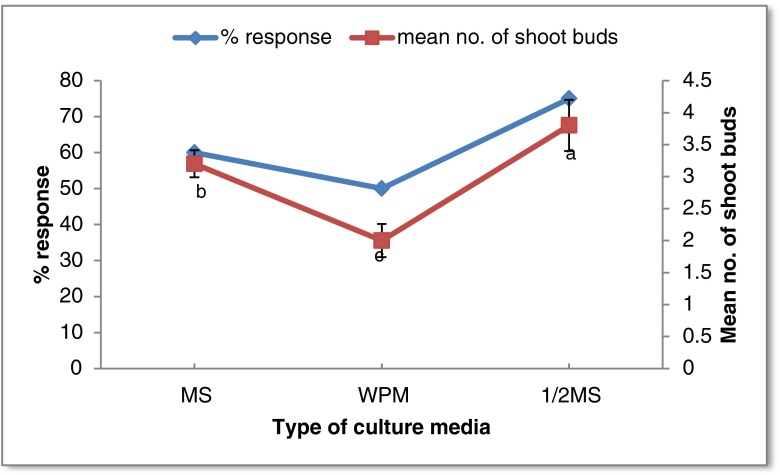
Influence of media type (MS, WPM and ½ MS) was studied after determining the optimum growth regulator level for shoot bud induction from nodal segments. Of the different basal media tested, the medium having a low salt concentration (½ MS medium) induced maximum shoots (Fig. 3). On WPM, less in number but healthy shoots were formed. Shoot tip necrosis was observed in shoots induced on full MS after 2 weeks of incubation. Hence, ½ MS medium was used for establishing primary cultures from nodal segments. Similar to the present observation, low salt concentration was reported to be superior for shoot proliferation in Terminalia arjuna (Pandey et al. 2006) and Azadirachta indica (Chaturvedi et al. 2004).
Fig. 3.
Response of nodal segments of Terminalia bellerica on various types of culture media supplemented with BA (8.8 μM) and NAA (2.6 μM). Each treatment consisted of 15 replicates and experiment was repeated thrice. The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
The excessive phenolic exudation in the medium limits establishment of cultures from mature nodal explants and causes deterioration of explants. Preliminary trial had resulted in severe leaching of phenolic compounds from cut end of nodal explants into medium (Fig. 4a). Only 10 % of explants survived after inoculation. Various treatments were employed to avoid injurious effects of phenolic exudates on explants survival. Charcoal (500 mg l−1) increased percent survival up to 70 but number of shoots induced was low (Fig. 4b). Addition of PVP (50 mg l−1) resulted in better induction response. However, combination of serial transfers at an interval of 48 h with incorporation of antioxidants [PVP (50 mg l−1) + Ascorbic acid (100 mg l−1) + Citric acid (10 mg l−1)] in culture medium proved effective for minimizing phenolic exudation and improving explants survival. About 55 % of nodal explants survived using this treatment and number of shoots induced also increased in the presence of antioxidants (Table 3, Fig. 4c.). The serial transfer technique have been found to be beneficial in reducing phenolic leaching and improving explants survival in Teak (Tiwari et al. 2002) and Pterocarpus santalinus (Prakash et al. 2006). Antioxidant mixture containing PVP, ascorbic acid and citric acid has been previously used to minimize phenolic exudation and for healthy growth of cultures in Boswellia serrata (Purohit et al. 1995). In the present study, the combination of antioxidants has proved to be beneficial for explant survival.
Fig. 4.
Micropropagation of T. bellerica from nodal explants of mature tree (a) Induction of shoot buds on nodal explants cultured on ½ MS + BA (8.8 μM) + NAA (2.6 μM) (b) Induction of shoot buds on nodal explants cultured on ½ MS + BA (8.8 μM) + NAA (2.6 μM) + Charcoal (500 mg l−1) (c) Shoot multiplication on nodal explants cultured on ½ MS + BA (8.8 μM) + NAA (2.6 μM) + Antioxidant solution and periodical serial transfer of explant (d) Proliferation of shoots on MS + BAP (8.8 μM) + NAA (2.6 μM) + Antioxidant solution after 4 weeks of third subculture (e) Elongation of shoots on MS + BAP (4.4 μM) + PG (3.9 μM) (f) Rooting of regenerated shoot on ½ MS medium supplemented with IBA (2.5 μM) (g) Regenerated plant transferred in pot PCR amplification products obtained with (h) RAPD primer OPJ-19 (i) ISSR primer ISSR-808 Lanes designated as M Molecular weight marker; mp Donor plant; 1–14 Regenerated plants
Table 3.
Effect of various antioxidants on survival and multiplication of shoots from mature nodal segments of Terminalia bellerica cultured on medium supplemented with BA (4.4 μM)
| Antioxidants | % survival | Shoot buds [explant]−1 Mean ± S.E. |
|---|---|---|
| Control | 10 | 3.0 ± 0.2b |
| Charcoal (500 mg l−1) | 70 | 1.6 ± 0.2d |
| Ascorbic acid (50 mg l−1) | 15 | 1.8 ± 0.3d |
| PVP (50 mg l−1) | 20 | 2.5 ± 0.2c |
| Serial Transfer + AO* | 55 | 4.7 ± 0.3a |
*AO = PVP (50 mg l−1) + Ascorbic acid (100 mg l−1) + Citric acid (10 mg l−1)
Each treatment consisted of 15 replicates and experiment was repeated thrice
The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
Explant browning and its subsequent death, generally attributed to phenolic compounds, was a major problem in the culture establishment from mature nodes of T. bellerica. Antioxidants are often incorporated in the culture medium to reduce the inhibitory phenolics released by the cultured tissue. In the present study, three different antioxidants were added to the medium in order to prevent the phenolic exudation of explants. Antioxidants may significantly delay or prevent oxidation of its oxidizable substrate when present at low concentrations compared to those of its substrate, thus preventing phenolic oxidation (Dan 2008). It has also been recommended that certain antioxidants like ascorbic acid, citric acid and PVP can both reduce tissue browning and promote organogenesis and shoot growth from buds during micropropagation across different plant species (Dan 2008).
Multiple shoot bud clusters obtained from nodal explants remained stunted and only few elongated on proliferation medium (Fig. 4d). To avoid suppressive effect of BA on shoot elongation, sub culturing of shoots on lowered concentration of BA in combination with GA3 or PG was tested. Shoot buds (70 %) attained the height of 2.8 cm when BA (4.4 μM) was conjugated with PG (3.9 μM) (Table 4, Fig. 4e). Therefore, the shoots obtained from nodal explants were elongated by repeated subculture at 2-week interval on medium containing BA (4.4 μM) and PG (3.9 μM). Similar effect of PG has been reported by Sarkar and Naik (2000), Feeney et al. (2007) and Jain et al. (2011).
Table 4.
Elongation response of shoot buds proliferated from mature nodal segments of Terminalia bellerica cultured on MS + BAP (4.4 μM) containing various plant growth regulators
| Plant growth regulators | ||
|---|---|---|
| (μM) | % response | Shoot Length (cm) |
| Mean ± S.D. | ||
| GA3 | ||
| 0.57 | 20 | 1.3 ± 0.3bc |
| 1.44 | 33.3 | 1.2 ± 0.4c |
| 2.88 | 10 | 0.8 ± 0.2d |
| PG | ||
| 3.9 | 70 | 2.8 ± 0.5a |
| 7.9 | 40 | 1.5 ± 0.5b |
Each treatment consisted of 15 replicates and experiment was repeated thrice
The mean values marked with the same letter (s) do not differ significantly at P ≤ 0.05 according to Duncan’s multiple range test
The shoots (>2.5 cm) were cultured on ½ MS medium supplemented with various concentration of IBA (0.98, 2.5, 4.9 μM) for rooting. IBA (2.5 μM) was found to be optimum for root formation (Fig. 4f). Optimum rooting response using IBA has been reported for many trees including T. arjuna (Pandey et al. 2006), Azadirachta indica (Chaturvedi et al. 2004) and T. chebula (Shyamkumar et al. 2004). Plantlets with well-developed shoot and roots were taken out and washed gently to remove traces of agar and transferred to earthen pots containing garden soil and organic manure in 1:1 ratio. These pots were initially covered by polythene bags to maintain humidity and kept at 26 ± 2 °C. Small perforations were made in the polythene bag at 5 days interval to acclimatize the plantlet to outer environment. Plants were initially kept in shade in greenhouse and after 8–10 days they were transferred to natural light (Fig. 4g).
Clonal fidelity of the mature node regenerated plants was accessed using RAPD and ISSR markers. Fingerprinting profiles of the culture regenerants and the donor plants was generated using a total of 10 primers (RAPD and ISSR) which produced distinct, reproducible amplified products. Total of 645 bands were produced that could be scored, ranging from 300–2,200 bp in size. The number of amplified fragments ranged from 3 (OPF-12, OPT-05, OPJ-20) to 6 (OPJ-19) with an average of 4.3 bands per primer (Table 5). Overall, no changes in the amplified fragments were detected among the plants derived from newly differentiated shoots with reference to the donor plant, which confirmed the genetic fidelity of plantlets derived in vitro. The RAPD and ISSR profiles of the regenerants along with the mother plant are depicted in Fig. 4h, i. Corresponding results were also observed in almond tree (Martins et al. 2004) and Capparis decidua (Tyagi et al. 2010). RAPD and ISSR markers were chosen in the present studies because they are very simple, fast, cost-effective, highly discriminative and reliable. They require only a small quantity of DNA sample and do not need any prior sequence information to design the primer. Martins et al. (2004) suggested the use of more than one marker, which amplifies different regions of the genome, allows better chances for the identification of genetic variations in the clones.
Table 5.
List of RAPD and ISSR primers used in the detection of genetic stability in micropropagated plants of T. bellerica
| Primer | Sequence (5′–3′) | Number of scorable bands per primer | Size Range (bp) |
|---|---|---|---|
| OPF-12 | ACGGTACCAG | 3 | 300–800 |
| OPT-05 | GGGTTTGGCA | 3 | 600–1,000 |
| OPJ-19 | GGACACCACT | 6 | 300–1,000 |
| OPJ-20 | AAGCGGCCTC | 3 | 500–1,100 |
| OPF-07 | CCGATATCCC | 4 | 100–1,400 |
| OPT-09 | CACCCCTGAG | 4 | 300–1,700 |
| ISSR-808 | AGAGAGAGAGAGAGAGC | 6 | 600–1,200 |
| ISSR-814 | CTCTCTCTCTCTCTCTA | 6 | 400–2,000 |
| ISSR-815 | CTCTCTCTCTCTCTCTG | 4 | 100–2,200 |
| ISSR-816 | CACACACACACACACAA | 4 | 600–1,100 |
However, there are several reports on tissue culture of T. bellerica but this is the only report that is confirming the clonal fidelity of T. bellerica plantlets obtained using nodal segments. The protocol developed here is reproducible and high rate of shoot multiplication was achieved. As the regeneration system developed from nodal explants in the present study is direct, it is appropriate for mass multiplication of T. bellerica and can be used for conservation of elite germplasm.
Acknowledgments
Senior Research Fellowship to Bhawna Dangi awarded by the Council of Scientific and Industrial Research (CSIR), New Delhi, India is gratefully acknowledged. Dr. Varsha Khurana-Kaul thanks DBT, India for providing financial support under IPLS program.
References
- Ahmad N, Anis M. Rapid clonal multiplication of a woody tree, Vitex negundo L. through axillary shoots proliferation. Agroforest Syst. 2007;71:195–200. doi: 10.1007/s10457-007-9078-1. [DOI] [Google Scholar]
- Anonymous . Wealth of India. Volume X (Combretaceae). Plant Systematic and Evolution, (Rh-Z) New Delhi: Directorate CSIR; 1976. p. 177. [Google Scholar]
- Bhattacharya S, Bandopadhyay TK, Ghosh PD. High frequency clonal propagation of Cymbopogon martinii var motia (palmarosa) through rhizome culture and true to type assessment using ISSR marker. J Plant Biochem Biotech. 2010;19:27–274. doi: 10.1007/BF03263355. [DOI] [Google Scholar]
- Chaturvedi R, Razdan MK, Bhojwani SS. In vitro clonal propagation of an adult tree of neem Azadirachta indica A. Juss. by forced axillary branching. Plant Sci. 2004;166:501–506. doi: 10.1016/j.plantsci.2003.10.021. [DOI] [Google Scholar]
- Dan Y. Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Biol Plant. 2008;44:149–161. doi: 10.1007/s11627-008-9110-9. [DOI] [Google Scholar]
- Dangi B, Jain R, Kachhwaha S, Kothari SL. Assessment of diversity in Terminalia bellerica Roxb. using morphological, phytochemical and molecular markers. Natl Acad Sci Lett. 2012;35:27–35. doi: 10.1007/s40009-011-0005-7. [DOI] [Google Scholar]
- Dangi B, Kachhwaha S, Kothari SL. Regeneration and Agrobacterium-mediated genetic transformation of Terminalia bellerica Roxb.: a multipurpose tree species. In Vitro Cell Dev Biol Plant. 2012;48:304–312. doi: 10.1007/s11627-012-9436-1. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Feeney M, Bhagwat B, Mitchel JS, Lane WD. Shoot regeneration from organogenic callus of sweet cherry (Prunus avium L.) Plant Cell Tissue Org Cult. 2007;90:201–214. doi: 10.1007/s11240-007-9252-1. [DOI] [Google Scholar]
- Giri CC, Shyamkumar B, Anjaneyulu C. Progress in tissue culture, genetic transformation and applications of biotechnology to trees: an overview. Trees. 2004;18:115–135. doi: 10.1007/s00468-003-0287-6. [DOI] [Google Scholar]
- Goyal P, Kachhwaha S, Kothari SL. Micropropagation of Pithecellobium dulce (Roxb.) benth- a multipurpose leguminous tree and assessment of genetic fidelity of micropropagated plants using molecular markers. Physiol Mol Biol Plant. 2012;18:169–176. doi: 10.1007/s12298-012-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MK, Anis M. Rapid in vitro multiplication of Melia azedarach L. (a multipurpose woody tree) Acta Physiol Plant. 2009;31:765–772. doi: 10.1007/s11738-009-0290-7. [DOI] [Google Scholar]
- Jain R, Sinha A, Jain D, Kachhwaha S, Kothari SL. Adventitious shoot regeneration and in vitro biosynthesis of steroidal lactones in Withania coagulans (stocks) dunal. Plant Cell Tiss Organ Cult. 2011;105:135–140. doi: 10.1007/s11240-010-9840-3. [DOI] [Google Scholar]
- Kumar N, Modi AR, Singh AS, Gajera BB, Patel AR, Patel MP, Subhash N. Assessment of genetic fidelity of micropropagated date palm (Phoenix dactylifera L.) plants by RAPD and ISSR markers assay. Physiol Mol Biol Plants. 2010;16:207–213. doi: 10.1007/s12298-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Mangal M, Dhawan AK, Singh N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (link) schneider using RAPD and ISSR markers. Acta Physiol Plant. 2011;33:2541–2545. doi: 10.1007/s11738-011-0767-z. [DOI] [Google Scholar]
- Larkin PJ, Scowcroft WR. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor Appl Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Llyod G, McCown B. Commercial feasible micropropagation of mountain laurel Kalmia latifolia by use of shoot tip culture. Comb Proc Int Plant Propagators Soc. 1980;30:421–427. [Google Scholar]
- Malik SK, Chaudhary R, Kalia RK. Rapid in vitro multiplication and conservation of Garcinia indica: a tropical medicinal tree species. Sci Horti. 2005;106:539–553. doi: 10.1016/j.scienta.2005.05.002. [DOI] [Google Scholar]
- Martins M, Sarmento D, Oliveira MM. Genetic stability of micropropagated almond plantlets as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004;23:492–496. doi: 10.1007/s00299-004-0870-3. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog FA. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Mushke R, Yarra R, Kokkirala VR, Abbagani S. Cell, tissue culture and gene transfer techniques for tasar (wild) sericulture plants-introspect and prospect. J Sustain For. 2014;33:173–183. doi: 10.1080/10549811.2013.836719. [DOI] [Google Scholar]
- Pandey S, Singh M, Jaiswal U, Jaiswal VS. Shoot initiation and multiplication from a mature tree of Terminalia arjuna Roxb. In Vitro Cell Dev Biol Plant. 2006;42:389–393. doi: 10.1079/IVP2006790. [DOI] [Google Scholar]
- Phulwaria M, Ram K, Gahlot P, Shekhawat NS. Micropropagation of Salvadora persica-a tree of arid horticulture and forestry. New For. 2011;42:317–327. doi: 10.1007/s11056-011-9254-z. [DOI] [Google Scholar]
- Phulwaria M, Rai MK, Harish GAK, Khetaram SNS. An improved micropropagation of Terminalia bellirica from nodal explants of mature tree. Acta Physiol Plant. 2012;34:299–305. doi: 10.1007/s11738-011-0828-3. [DOI] [Google Scholar]
- Prakash E, Khan P, Rao T, Meru ES. Micropropagation of red sanders (Pterocarpus santalinus L.) using mature nodal explants. J For Res. 2006;11:329–335. doi: 10.1007/s10310-006-0230-y. [DOI] [Google Scholar]
- Purohit SD, Tak K, Kukda G. In vitro propagation of Boswellia serrata Roxb. Biol Plant. 1995;37:335–340. doi: 10.1007/BF02913975. [DOI] [Google Scholar]
- Ramesh M, Umate P, Rao KV, Sadanandam A. Micropropagation of Terminalia bellirica Roxb.- a sericulture and medicinal plant. In Vitro Cell Dev Biol Plant. 2005;41:320–323. doi: 10.1079/IVP2004626. [DOI] [Google Scholar]
- Rathore P, Suthar R, Purohit SD. Micropropagation of Terminalia bellerica Roxb. from juvenile explants. Indian J Biotechnol. 2008;7:246–249. [Google Scholar]
- Roy SK, Pal PK, Das AK. Propagation of a timber tree Terminalia bellerica Roxb. by tissue culture. Bangladesh J Bot. 1987;16:125–130. [Google Scholar]
- Sarkar D, Naik PS. Phloroglucinol enhances growth and rate of axillary shoot proliferation in potato shoot tip cultures in vitro. Plant Cell Tissue Org Cult. 2000;60:139–149. doi: 10.1023/A:1006419805980. [DOI] [Google Scholar]
- Sharma PK, Tyagi P, Sharma KC, Kothari SL. Clonal micropropagation of Crataeva adansonii (DC.) Prodr.: a multipurpose tree. In vitro Cell Dev Biol Plant. 2003;39:156–160. doi: 10.1079/IVP2002384. [DOI] [Google Scholar]
- Shyamkumar B, Anjaneyulu C, Giri CC. Multiple shoot induction from cotyledonary node explants of Terminalia chebula. Biol Plant. 2004;47:585–588. doi: 10.1023/B:BIOP.0000041066.78766.34. [DOI] [Google Scholar]
- Sumathi P, Parvathi A. Antibacterial potential of the three medicinal fruits used in triphala: an ayurvedic formulation. J Med Plants Res. 2010;4:1682–1685. [Google Scholar]
- Tiwari SK, Tiwari KP, Siril EA. An improved micropropagation protocol for teak. Plant Cell Tiss Organ Cult. 2002;71:1–6. doi: 10.1023/A:1016570000846. [DOI] [Google Scholar]
- Tyagi P, Khanduja S, Kothari SL. In vitro culture of Capparis decidua and assessment of clonal fidelity of the regenerated plants. Biol Plant. 2010;54:126–130. doi: 10.1007/s10535-010-0019-x. [DOI] [Google Scholar]
- Vibha JB, Shekhawat NS, Mehandru P, Dinesh R. Rapid multiplication of Dalbergia sissoo Roxb.: a timber yielding tree legume through axillary shoot proliferation and ex vitro rooting. Physiol Mol Biol Plants. 2013 doi: 10.1007/s12298-013-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Horgan KJ, Reynolds PH, Jameson PE. 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiata buds in vitro. Tree Physiol. 2010;30:514–526. doi: 10.1093/treephys/tpp130. [DOI] [PubMed] [Google Scholar]