Abstract
The central pathways subserving the feline pupillary light reflex were examined by defining retinal input to the olivary pretectal nucleus (OPt), the midbrain projections of this nucleus, and the premotor neurons within it. Unilateral intravitreal wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) injections revealed differences in the pattern of retinal OPt termination on the two sides. Injections of WGA-HRP into OPt labeled terminals bilaterally in the anteromedian nucleus, and to a lesser extent in the supraoculomotor area, centrally projecting Edinger-Westphal nucleus and nucleus of the posterior commissure. Labeled terminals, as well as retrogradely labeled multipolar cells, were present in the contralateral OPt, indicating a commissural pathway. Injections of WGA-HRP into the anteromedian nucleus labeled fusiform premotor neurons within the OPt, as well as multipolar cells in the nucleus of the posterior commissure. Connections between retinal terminals and the pretectal premotor neurons were characterized by combining vitreous chamber and anteromedian nucleus injections of WGA-HRP in the same animal. Fusiform shaped, retrogradely labeled cells fell within the anterogradely labeled retinal terminal field in OPt. Ultrastructural analysis revealed labeled retinal terminals containing clear spherical vesicles. They contacted labeled pretectal premotor neurons via asymmetric synaptic densities. These results provide an anatomical substrate for the pupillary light reflex in the cat. Pretectal premotor neurons receive direct retinal input via synapses suggestive of an excitatory drive, and project directly to nuclei containing preganglionic motoneurons. These projections are concentrated in the anteromedian nucleus, indicating its involvement in the pupillary light reflex.
Keywords: Pretectum, Edinger-Westphal, Luminance, Parasympathetic, Oculomotor
Introduction
The pupillary light reflex ensures optimal visual perception by accurately adjusting pupil diameter in response to variable ambient illumination. This reflex activates the constrictor pupillae muscle in order to reduce pupil diameter and protect the visual pigments of retinal photoreceptors from bleaching. It thus reduces light adaptation, in preparation for the animal's return to a darkened environment (Campbell and Woodhouse, 1975; Loewenfeld, 1993) and supplements biochemical and vascular adaptive changes occurring at the retinal level in response to illumination changes (Dowling, 1987; Fitzgerald et al., 1990). In frontal-eyed species that have been tested, pupillary constriction occurs in both eyes, even if only one is exposed to illumination. The response in the illuminated eye is the direct response, whereas that in the other eye is the indirect, or consensual, response. In addition to its actions in the light reflex, the pupil can be constricted to increase the depth of focus as part of the near triad (Lowenstein and Loewenfeld, 1969; McDougal and Gamlin, 2008). Constriction also reduces both spherical and chromatic aberrations to enhance visual acuity (Lowenstein and Loewenfeld, 1969; Barr 1989). The constrictor pupillae muscle is controlled by preganglionic parasympathetic motoneurons whose axons exit with the oculomotor nerve to access postganglionic motoneurons in the ciliary ganglion. Thus, examination of pupil size and its response to light can be useful in gauging optic nerve, oculomotor nerve and midbrain function. Pupil size is also modulated by sympathetic input to the dilator pupillae muscle, so pupillary size under even illumination conditions can be utilized as an indicator of autonomic nervous system tone.
Despite its physiological importance and clinical relevance, the anatomical substrate for the pupillary light reflex has not been directly demonstrated in mammals. It is believed to be mediated by a four-neuron arc: retinal ganglion cells → olivary pretectal nucleus (OPt) → Edinger-Westphal nucleus → ciliary ganglion. However, the specific cells in each of these structures that subserve the pupillary light reflex have not been definitively characterized. Furthermore, the critical central connections in the pathway are still a matter of dispute (see Simpson et al., 1988; Gamlin, 2006; McDougal and Gamlin, 2008 for reviews), in part because of species differences that led to confusion concerning the location of the preganglionic and centrally projecting divisions of the Edinger-Westphal nucleus (EWpg and EWcp, respectively)(see Kozicz et al., 2011, for review). For example, in the cat, the preganglionic motoneurons are spread in an arc around the oculomotor nucleus (III). They lie in the supraoculomotor area (SOA), extend around the rostral end of III into the anteromedian nucleus (AM) and then continue caudally, ventral to III, amongst the exiting rootlets of the oculomotor nerve. Few preganglionic motoneurons are found in the EWcp (Erichsen and May, 2002; May et al., 2008; Sun and May, 2014).
In fact, the connection between OPt and the preganglionic motoneurons in Edinger-Westphal nucleus has not been anatomically verified, and there is some evidence that other intermediaries may be involved. Specifically, while there are several lines of evidence indicating a direct projection from the OPt nucleus to preganglionic motoneurons (monkey: Pierson and Carpenter, 1974; Benevento et al., 1977; Steiger and Büttner-Ennever, 1979; cat: Graybiel, 1974; Itoh, 1977; Distler and Hoffmann, 1989b; rat: Itaya and Van Hoesen, 1982; Campbell and Lieberman, 1985), there is also evidence showing otherwise (monkey: Tokunaga et al., 1981; tree shrew: Weber and Harting, 1980; cat: Graybiel and Hartwieg, 1974; Berman, 1977; Weber and Harting, 1980). Some of the latter authorities have suggested that the pretectum and preganglionic motoneurons are instead connected via a relay in the nucleus of the posterior commissure (NPC) (Graybiel and Hartwieg, 1974; Berman, 1977; Weber and Harting, 1980; Breen et al., 1983).
The neuronal basis of the consensual pupillary response has also not been completely clarified. Due to a partial decussation in the optic chiasm in frontal-eyed mammals, retinal ganglion cells synapse bilaterally in the OPt (cat: Laties and Sprague, 1966; Kanaseki and Sprague, 1974; Berman, 1977; Koontz et al., 1985; Distler and Hoffmann, 1989b; monkey: Campos-Ortega and Glees, 1967; Giolli and Tigges, 1970; Pierson and Carpenter, 1974; Tigges et al., 1977; Benevento and Standage, 1983; Hutchins and Weber, 1985). This comprises a primary neuroanatomical basis for the consensual pupillary light reflex. Other neuronal connections, such as reciprocal connections between the two OPt and bilateral projections of the OPt neurons to preganglionic motoneurons (Magoun et al., 1936; Lowenstein and Loewenfeld, 1969; Carpenter and Pierson, 1973; Gamlin et al., 1984; Distler and Hoffmann, 1989b) have been suggested to play a role in determining the level of the consensual response, but these connections remain to be proven in a frontal-eyed species.
The primary purpose of the present study was to define the neuronal populations and connections mediating the direct and consensual pupillary light reflex in a frontal-eyed, visual mammal. This study utilized transport of wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) and examined the results at the light microscopic (LM) and electron microscopic (EM) level. Cats were used because they are a frontal-eyed species with well documented lens accommodation and pupillary constriction capabilities (Loewenfeld, 1993). First, the pattern of the retinal termination in the pretectum was determined by vitreous injections of WGA-HRP. Next, the midbrain targets of OPt were identified. Finally, the distribution and morphology of premotor neurons in the pretectum and their relationship with retinal terminals was revealed. The resultant data allowed us to gain more insight into the anatomical substrate for feline pupillary control.
Materials and Methods
General animal procedures
A total of 10 adult cats, both male and female, 1.5 - 3.5 kg in body weight, were used in the present study. Animal procedures were performed in accordance with NIH guidelines and institutional regulations for animal care and use, using IACUC approved protocols. Prior to surgery, animals received an injection of steroid (dexamethasone, 2.5 mg/kg, SC) to limit tissue swelling and atropine (0.1 mg/kg, SC) to reduce respiratory secretions. Animals were anesthetized either by intramuscular administration of a mixture of ketamine hydrochloride (22 mg/kg) and xylazine (0.25 mg/kg) or by intraperitoneal administration or intravenous infusion of sodium pentobarbital (35 mg/kg). Anesthesia was supplemented, as necessary, to maintain an adequate surgical level throughout the operations. An analgesic (butorphanol, 0.3 mg/kg, or buprenex, 0.01mg/kg) was administrated to alleviate postoperative discomfort. After a 24 hour survival period for transport of tracers, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) before transcardial perfusion. Administration of sodium nitrite (1.0 ml, 1.0%, IV) to facilitate vascular dilation was followed by perfusion with 1.0 liter of 0.1 M phosphate buffered saline (pH 7.2). Next, 2.0 liters of fixative containing 1-3% paraformaldehyde and 1-2% glutaraldehyde in 0.1 M phosphate buffer (PB) (pH 7.2) were perfused. The brains were blocked in the stereotaxic plane, postfixed in the same fixative solution, and then stored in 0.1 M PB (pH 7.2) at 4 ° C overnight. The appropriate blocks were sectioned with a vibratome (Technical Products International). For light microscopic observations, 50 µm sections were used, while 100 µm sections were used for electron microscopic procedures.
Tracer injections
Vitreous chamber injection (n=2)
This injection was used for anterogradely labeling the retinal terminals in the pretectum. Topical analgesic (propararcaine HCl 0.5%) and antibiotic (gentamicin ophthalmic drops) were applied to the cornea and conjunctiva. The eye was rotated medially to expose the sclera and a monocular intravitreous injection of 50 µl of 1.0% WGA-HRP (Sigma) and 10% HRP (Sigma) was made using a 100 µl Hamilton microsyringe fitted with a 27 gauge needle to inject the vitreal chamber.
Olivary pretectal nucleus injection (n=3)
This injection was intended to reveal the course of projections from OPt and localize their terminals. It also retrogradely labeled cells projecting to OPt. Following a midline skin incision to expose the skull, a craniotomy was made over the injection target. The midbrain was visualized by aspirating the medial part of the occipital and parietal lobes, exposing the surface of the superior colliculus and the caudal pole of the pulvinar. Pretectal injection sites were chosen at the pulvinar-tectum junction. They were located 2.5 - 2.8 mm lateral to the midline, and 0.8 - 1.2 mm in depth from the visualized brainstem surface. Small amounts of tracer, 0.01 µl of 1% WGA-HRP, were injected by use of a 1.0 µl Hamilton microsyringe.
Anteromedian nucleus injection (n=5)
This injection was intended to reveal the distribution of pretectal neurons projecting to the AM. Three of these same animals also received vitreal injections to identify the pattern of retinal terminations. Animals were prepared for surgery according to the procedures outlined above. The surgical approach was the same as for Opt injections. The midbrain was visualized by aspirating the medial part of the occipital and parietal lobes, exposing the third ventricle and the posterior commissure. To avoid overlying structures, a syringe needle angled 10° tip caudal in the sagittal plane was advanced immediately in front of the posterior commissure to penetrate the midbrain at the base of the entrance to the cerebral aqueduct. Injections were made at 5.0 - 6.0 mm beneath the commissure surface using a 1.0 µl Hamilton microsyringe. A bolus of 0.015 µl of 1.0% WGA-HRP and 10% HRP was injected into the rostral part of the oculomotor complex over 10-15 minutes.
Histochemical procedures and analysis
WGA-HRP Protocol
Labeling with the tracers WGA-HRP and HRP was demonstrated by using either of two tetramethylbenzidine (TMB)(Sigma) protocols. One is a modification of the Mesulam (1978) method (see May et al., 1990 for details). The other is a modification of Olucha and colleagues (1985) method, in which sections are preincubated in 0.1 M PB (pH 6.0) that contains 0.25% ammonium molybdate and 0.005% TMB. Next, 0.3% hydrogen peroxide is added to drive the reaction. The incubation is extended overnight at 4°C, followed by a stabilization step in a 5.0% ammonium molybdate solution in 0.1M PB (pH 6.0). Those sections used for light microscopic study were rinsed, mounted onto gelatinized slides, air dried, counterstained with cresyl violet or neutral red, dehydrated, cleared, and coverslipped. The pattern of labeling was charted by use of an Olympus BH-2 microscope equipped with a drawing tube. Areas containing anterograde and retrograde label were photographed by use of a camera attached to the same instrument, while utilizing crossed polarizers to reveal the TMB crystals. Photomicrographic prints were digitized on a scanner, and the brightness and contrast were adjusted in Photoshop (Adobe) to mimic the appearance of the original material as closely as possible.
Electron microscopy procedure
The 100 µm sections that had undergone the Olucha reaction method were further processed to protect the reaction product during EM procedures. They were placed in 0.05% diaminobenzidine HCl (DAB) (Sigma) in 0.1M PB (pH 7.2). The reaction was initiated by adding hydrogen peroxide to achieve a concentration of 0.003 %. After PB rinses, the sections were examined using a stereoscope (Olympus SZ40). Areas in the pretectum containing retrogradely labeled cells were cut out of the section and collected. The samples were rinsed in 0.1 M, pH 7.2 phosphate buffer with 7.0% dextrose. They were then reacted in 1.0% osmium tetroxide in the same buffer, followed by en block staining with a 2.0% uranyl acetate solution in acetate buffer (0.1M, pH 5.2). They were dehydrated through a graded series of ethanols, followed by propylene oxide. Finally, samples were infiltrated first with a 1:1 propylene oxide/TAAB resin mixture overnight, then with straight TAAB resin, before being embedded in TAAB EPOXY 812 resin with 1.0% EM Corp internal lubricant. The blocks were trimmed and 1.0 µm semithin sections were cut with a glass knife and counterstained with toluidine blue for inspection. After further trimming, ultrathin (70-90 nm) sections were cut with a glass or diamond knife, collected on either copper mesh grids or slot formvar grids, and stained with lead citrate following standard procedures. The ultrathin sections were examined and labeled profiles were photographed using a Zeiss 10C transmission electron microscope.
Results
Retinal termination within the pretectum
This experiment confirmed and extended descriptions of the distribution of retinal terminals in the pretectum. As shown in figure 1, intravitreous injection of WGA-HRP into the left eye produced bilateral anterograde labeling of retinal terminals in the pretectum, along with terminal labeling in the dorsal lateral geniculate nucleus and superior colliculus (not illustrated). The pretectal label was more prominent on the side contralateral to the injection site. A dense terminal field filled the cytoarchtectonically defined OPt nucleus. It extended laterally to distribute within the nucleus of the optic tract (NOT). The terminal label also extended ventrally into an area that is included within the posterior pretectal nucleus by some authors (Kanaseki and Sprague, 1974; Avendano and Juretschke, 1980). The contralateral field in OPt showed a core region with few terminals (Fig. 1D). Moreover, the terminal field extended densely to the surface. However, on the ipsilateral side, the terminal field was homogeneously dense in the core of the nucleus, but was much sparser near the surface (Fig. 1C). The extent and absolute distribution of terminal label varied somewhat between cases, most likely because the tracer was concentrated in different portions of the retina.
Figure 1.
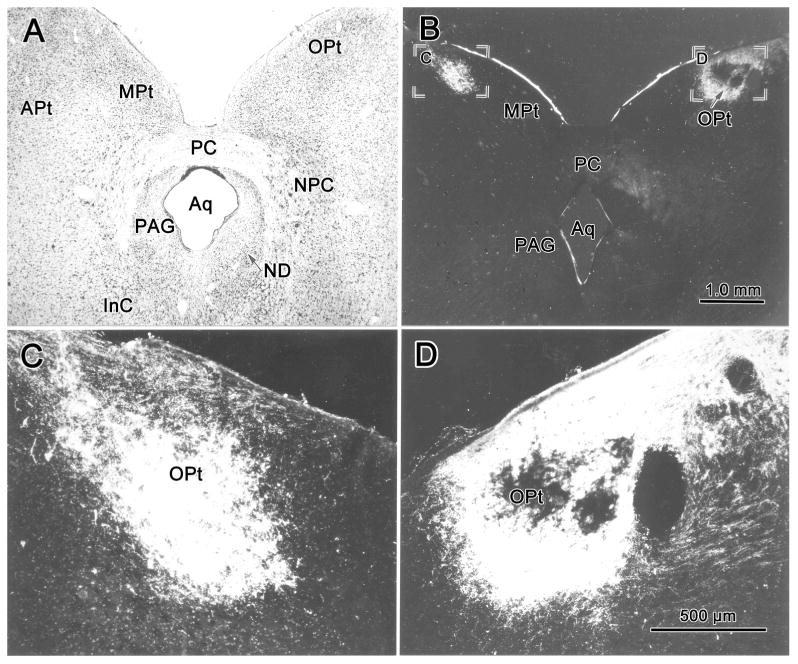
Brightfield (A) and darkfield (B-D) photomicrographs demonstrating the olivary pretectal nucleus (OPt) and the pattern of labeling in this nucleus following a WGA-HRP injection of the left vitreous chamber. Anterogradely labeled retinal terminal fields were heavier on the right (contralateral) side (B&D) than the left (ipsilateral) side (B&C). A Nissl-stained section (A) at about the same level as B shows the cytoarchitecture of OPt and adjacent structures for reference. Scale in A=B, C=D.
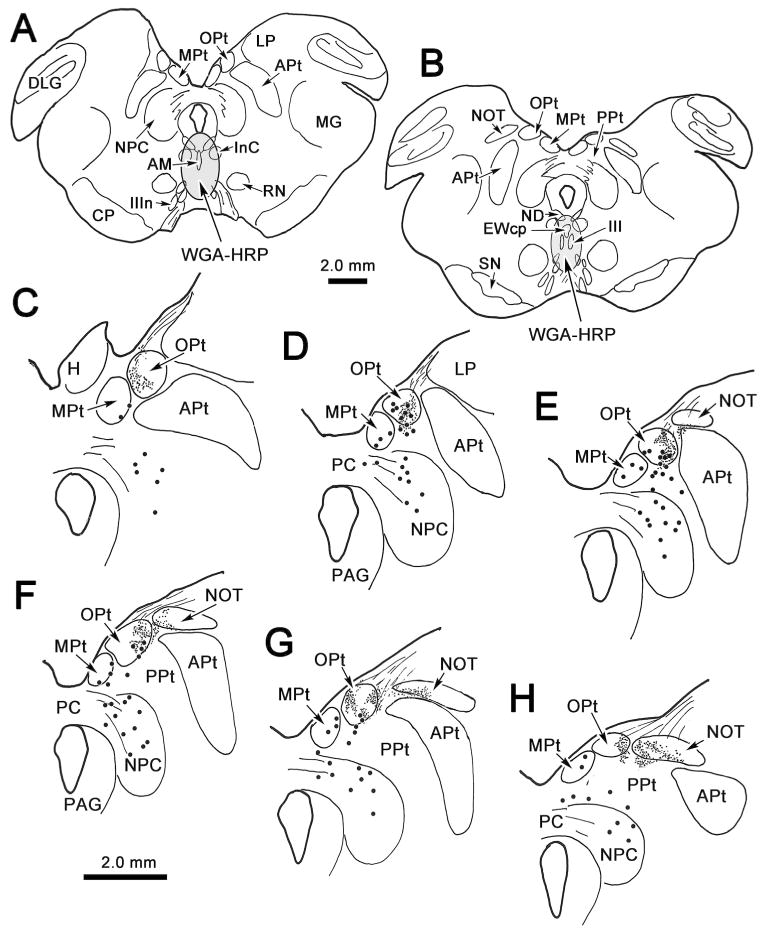
Projections of the olivary pretectal nucleus
The OPt was injected with WGA-HRP in order to demonstrate its projections within the midbrain. In the example shown in figure 2, the injection site was centered in OPt (Fig. 2 B-D). It included adjacent areas of the NOT, the medial pretectal nucleus (MPt) and anterior pretectal nucleus (APt) (Fig. 2B&C). Ipsilaterally, labeled axonal fibers (lines) were observed traveling through the nucleus of the posterior commissure (NPC) and extending ventromedially within the periaqueductal gray (PAG) toward the oculomotor nucleus (III) (Fig. 2B&C). Other fibers crossed in the posterior commissure (PC) and extended either toward the contralateral OPt or into (Fig. 2B-D), and through (Fig. 2C), the NPC. A portion of these uncrossed and crossed axons terminated (stipple) in and ventrolateral to AM (Fig. 2A-D). Terminals were also observed more caudally (Fig. 2E&F) between the rostral poles of III, in the supraoculomotor area (SOA) and in the rostral end of the centrally projecting Edinger-Westphal nucleus (EWcp). Slightly more labeled terminals were found on the contralateral than the ipsilateral side. Little anterograde label was observed at more caudal levels of the oculomotor complex (Fig. 2F). Labeled terminals were also present in the nucleus of Darkschewitsch (ND) (Fig 2A-E), in the contralateral OPt, and in the NPC (Fig. 2B-D). Terminals in the contralateral OPt were denser ventrally. In addition, retrogradely labeled cells (dots) were observed in the contralateral pretectum, including in OPt and in the adjacent medial pretectal and anterior pretectal nuclei (Fig. 2B-D). The labeled cells in OPt tended to be dorsally located. Labeled cells were also found in the contralateral NPC, mainly in the medial part of the nucleus (Fig. 2B-D). The darkfield photomicrographs in figure 3A&B show the fine labeled OPt axons supplying terminals within AM. They run dorsoventrally, matching the orientation of the nucleus. The appearance of the retrogradely labeled contralateral OPt neurons is also shown (Fig. 3C-D). An additional discrete cluster of labeled cells was located within the territory of the anterior pretectal nucleus (Fig. 3C). These multipolar neurons were presumably labeled due to extension of the injection site into the anterior pretectal nucleus. In a case in which the NPC was included in the injection site (not illustrated), more retrogradely labeled cells were present in the contralateral NPC. The pattern of anterogradely labeled axonal fibers in this second case was similar to the case described above. However, terminal labeling was denser in and lateral to AM on the side ipsilateral to the injection site.
Figure 2.
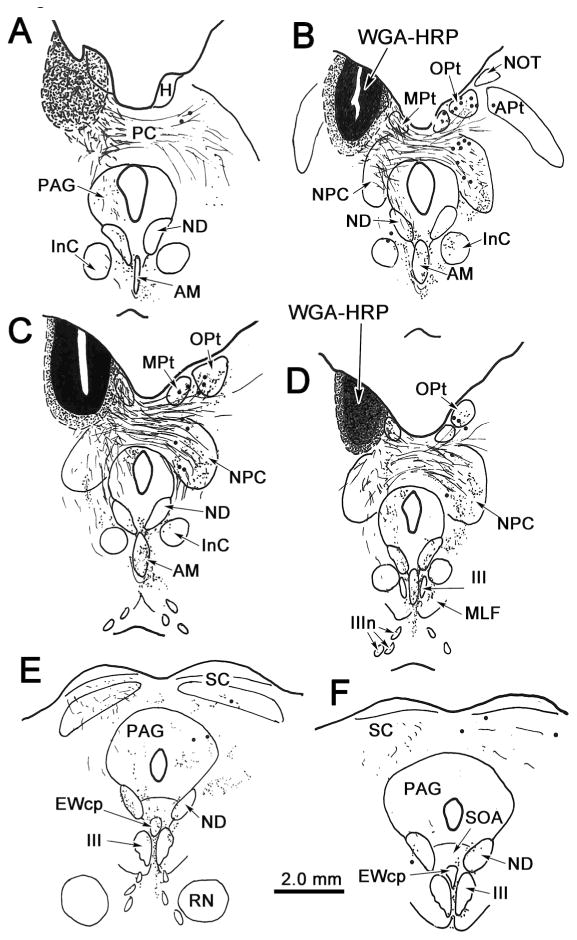
Distribution of labeled cells and terminals in the midbrain following an injection of WGA-HRP into the left olivary pretectal nucleus (OPt). In this and all subsequent chartings, sections are arranged from rostral (A) to caudal (F), retrogradely labeled cells are indicated by dots, anterogradely labeled terminals are indicated by fine stipple and fine lines represent labeled axon fibers. Injection site and tracer spread area are indicated by black and shaded areas, respectively (A-D). The injection site was centered in OPt and spread slightly into adjacent parts of the medial pretectal nucleus (MPt), the nucleus of the optic tract (NOT), and the anterior pretectal nucleus (APt). Terminals are present bilaterally in the anteromedian nucleus (AM) (A-D), and to a lesser extent in the centrally projecting Edinger-Westphal nucleus (EWcp), supraoculomotor area (SOA) and between the oculomotor nuclei (III). Terminals are also present in the ipsilateral periaqueductal gray (PAG)(A-E), both nuclei of Darkschewitsch (ND)(A-F), and contralaterally in OPt (B-D), MPt (B-D), the nucleus of the posterior commissure (NPC)(B-D) and the interstitial nucleus of Cajal (INC)(B&C). Labeled cells are present in contralateral OPt, MPt, APt and NPC (B-D).
Figure 3.
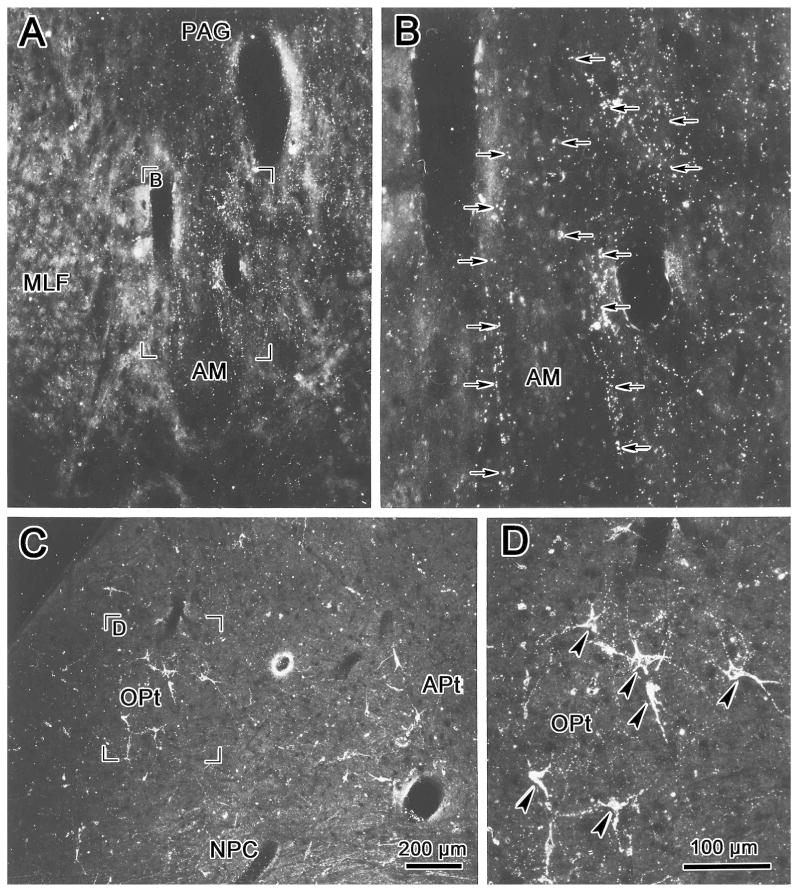
Darkfield micrographs of WGA-HRP labeled terminals in the anteromedian nucleus (AM) (A, B) and cells and terminals in the contralateral pretectum (C, D) following an olivary pretectal nucleus injection like that shown in figure 2. Examples of labeled terminals are indicated by arrows (B) and labeled cells are indicated by arrowheads (D). Scale in A=C, B=D.
Pretectal premotor neurons
In light of the pattern of termination following OPt injections and due to the fact that preganglionic motoneurons in the cat are most densely distributed rostrally, we targeted AM with retrograde tracer in order to identify putative pretectal premotor neurons. An example of an injection of WGA-HRP into AM is shown in figure 4A-H. This injection site included most of AM, but spread more extensively to the right side, lateral and dorsal to AM, to include parts of the ND, the interstitial nucleus of Cajal (InC) and the periaqueductal gray (Fig. 4A-F). It extended caudally to involve the rostral pole of III and the adjacent area ventral to III (Fig. 4G&H). Despite this injection site spread, pretectal labeling was quite discrete. At the most rostral level, a few labeled cells appeared dorsolateral to the posterior commissure (Fig. 4A&B). This is more rostral than the conventionally described rostral pole of OPt (Berman, 1977; Hutchins and Weber, 1985). At more caudal levels, labeled cells were present within the boundaries of OPt (Fig. 4C-G), although they failed to fill the nucleus. Labeled cells in OPt tended to be more ventrally located than the distribution of OPt cells projecting to the contralateral OPt, which was described above. In this study, the boundaries of OPt are provisionally defined by the extent of the retinal terminal field, which extends somewhat ventral to the cytoarchitectural boundaries of the cat OPt (Kanaseki and Sprague, 1974; Avendano and Juretschke, 1980; Hutchins and Weber, 1985; Koontz et al., 1985). Labeled cells were continuously distributed throughout most of the rostral-caudal extent of OPt (Fig. 4C-G), with more cells found on the left. The more ventrally placed cells fell within the territory sometimes ascribed to the posterior pretectal nucleus (Fig. 4D-G).
Figure 4.
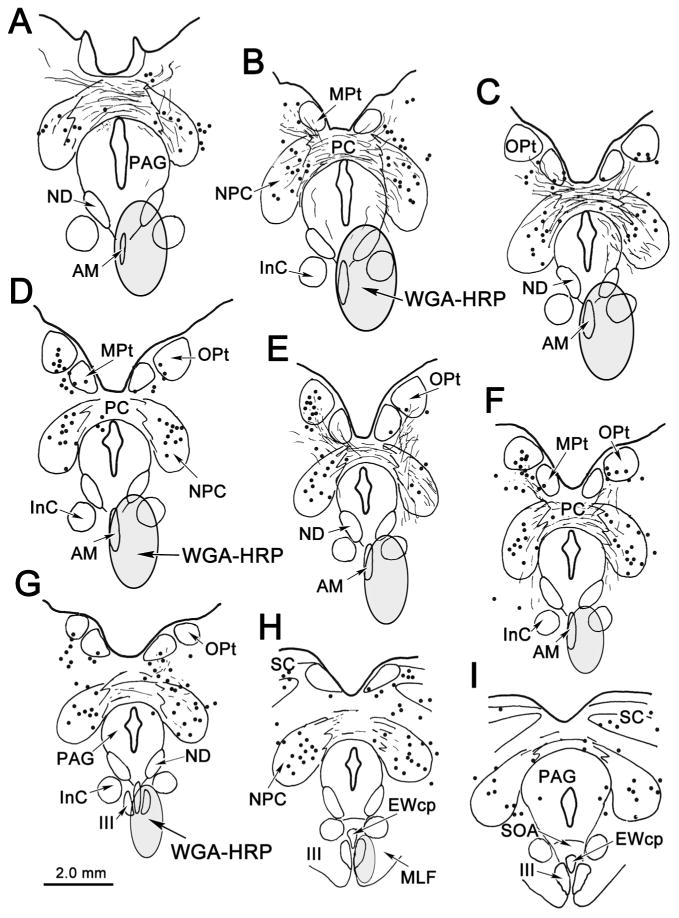
Distribution of labeled cells and terminals in midbrain following a WGA-HRP injection into the anteromedian nucleus (AM). Note labeled cells (dots) in and ventral to the olivary pretectal nucleus (OPt) (E, F). Labeled cells were also present in the nucleus of the posterior commissure (NPC)(A-I).
Labeled cells were also continuously present throughout the rostral-caudal extent of NPC (Fig. 4A-I). These labeled cells were distributed in both the medial (magnocellular) and lateral (parvocellular) parts of the NPC, with no obvious pattern. A few labeled cells were also present in the medial pretectal nucleus and in the superior colliculus and periaqueductal gray (Fig. 4H&I). Additional areas of cell labeling were observed outside the midbrain (not illustrated). A discrete group of retrogradely labeled cells was found bilaterally in the anterior hypothalamic area, immediately dorsal to the optic chiasm, and labeled cells were also dispersed within the lateral hypothalamic area at more caudal levels. In the cerebellum, a few retrogradely labeled cells were observed contralaterally in the dentate nucleus and bilaterally in the caudal fastigial nucleus.
The morphology of retrogradely labeled cells in the pretectum is demonstrated in figure 5. Most labeled cells within OPt and immediately ventral to it were fusiform shaped, with their long axes orientated from dorsolateral to ventromedial (Fig. 5A&B). This differentiates them from the multipolar OPt cells that project to the contralateral OPt (compare Fig. 3C&D to 5A&B). The tightly clustered labeled neurons in the NPC (Fig. 5C) also displayed a different morphology from the OPt premotor neurons. They were multipolar neurons with no obvious orientation (Fig. 5D).
Figure 5.
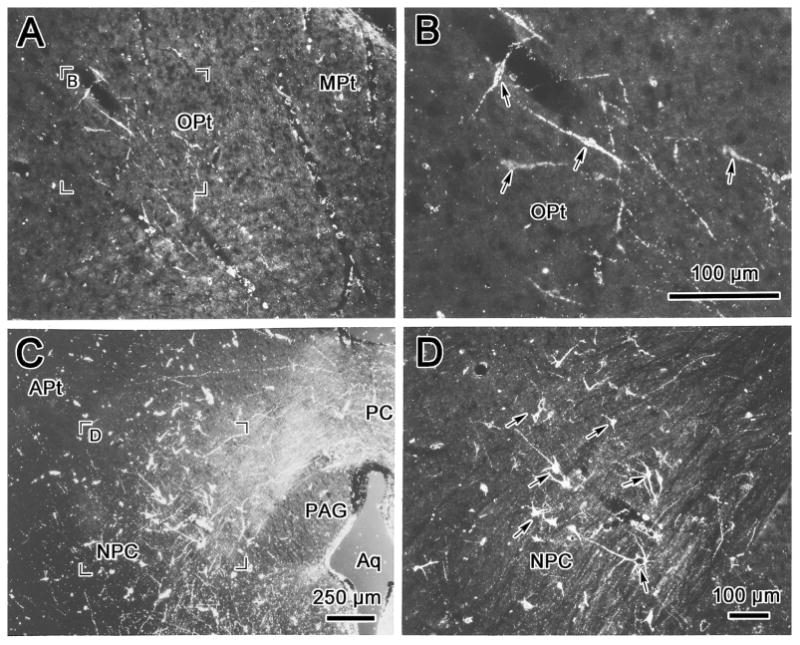
Darkfield photomicrographs showing retrogradely labeled cells in the olivary pretectal nucleus (OPt)(A, B), and in the nucleus of the posterior commissure (NPC)(C, D) following a WGA-HRP injection into the anteromedian nucleus (AM) like that shown in figure 4. Examples of the labeled cells are indicated by arrows (B,D&F). The region selected for the high magnification views (B&D) is indicated in the low magnification views (A&C). Scale in A=D.
Retinal input to premotoneurons
The relationship between retinal terminals and the OPt projection neurons was directly investigated by combined injections of WGA-HRP into the left vitreous and into AM. The latter injection is illustrated in figure 6A&B. It covered AM and extended to include portions of the INC. The halo of the injection included the rostral pole of III and adjacent structures. Most of the retrogradely labeled cells (dots) in OPt were found within the region of the nucleus containing retinal terminals (stipple)(Fig. 6D-G). However, areas were present in OPt that contained retinal terminals, but no labeled cells (Fig. 6C, G, and I), even though the retinal terminal field was sparsely labeled by comparison to other cases. The labeled cell distribution extended ventromedially, beyond the limits of the retinal terminals that define the OPt ventral boundary (Fig. 6D-G). Labeled retinal terminals were present in the NOT, but labeled cells were not found there (Fig 6F-H). In contrast, labeled cells were present in the NPC, but retinal terminals were not found there (Fig. 6D-H). The relationship between the labeled elements is further demonstrated by use of crossed polarizers in figure 7. Anterogradely labeled retinal terminal fields extended from dorsolateral to ventromedial within the left (Fig. 7A) and right (Fig. 7B) OPt. The retrogradely labeled cells located within the terminal field can be better observed at higher magnification (Fig. 7C&D). The cells intermingled with the labeled terminals and their dendrites were oriented in the same axis as the retinal terminal field.
Figure 6.
Distribution of labeling in the pretectum following injections of WGA-HRP into left vitreous chamber and into the anteromedian nucleus (AM)(A&B). Anterogradely labeled retinal terminals (stipple) are present in the olivary pretectal nucleus (OPt)(A-F) and in the nucleus of the optic tract (NOT)(C-F). Retrogradely labeled cells (dots) from the AM injection are present in OPt (B-E). Their distribution overlaps with that of the retinal terminals. Labeled cells, which are outside of the retinal terminal field, are also present in the medial pretectal nucleus (MPt)(A-F), in the area ventral to OPt (B-E), in the nucleus of the posterior commissure (NPC) (A-F), and in the posterior pretectal nucleus (PPt)(F).
Figure 7.
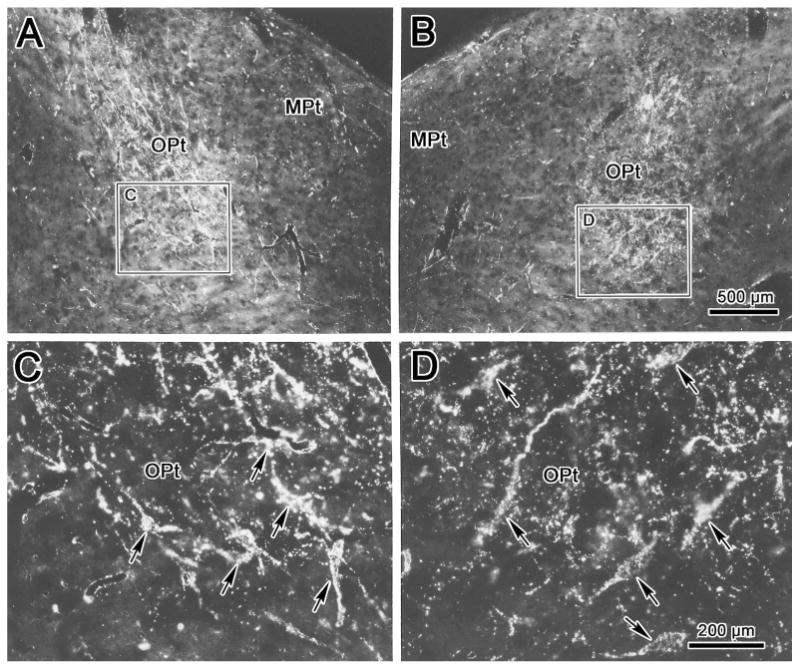
Darkfield photomicrographs showing labeling in the olivary pretectal nucleus (OPt) following combined injections of WGA-HRP into the left vitreous chamber and into the anteromedian nucleus. Lower magnification (A&B) and higher magnification (C&D) views from regions indicated by boxes reveal distribution of label in the left (A&C) and right (B&D) OPt. Premotor neuons retrogradely labeled from the AM nucleus overlaped with anterogradely labeled retinal terminals. Examples of these labeled cells are indicated by arrows. Scale in A=B. C=D.
Electron microscopic analysis of the double injection cases revealed synaptic contacts between the anterogradely labeled retinal terminals and retrogradely labeled OPt projection neurons (Fig. 8). The TMB crystals appeared as flocculent electron-dense material in retrogradely labeled somata (Soma*, Fig. 8A&B) and dendrites (Den*, Fig. 8C&D), and in anterogradely labeled axon terminals (At*, Fig. 8A-D). This reaction product sometimes hid the underlying ultrastructure (Fig. 8B). In other cases, the labeled retinal terminals were observed to contain clear spherical vesicles and several mitochondria (Fig. 8C&D). They were found adjacent to both the somata (Fig. 8A&B) and dendrites (Fig. 8C&D) of retrogradely labeled neurons. The synaptic densities observed (arrowhead) between the labeled elements were asymmetric, but did not display a prominent postsynaptic contribution (Fig. 8C&D). The presence of the labeled terminals in contact with labeled cells in this experiment indicates that retinal afferents have direct access to pretectal premotor neurons.
Figure 8.
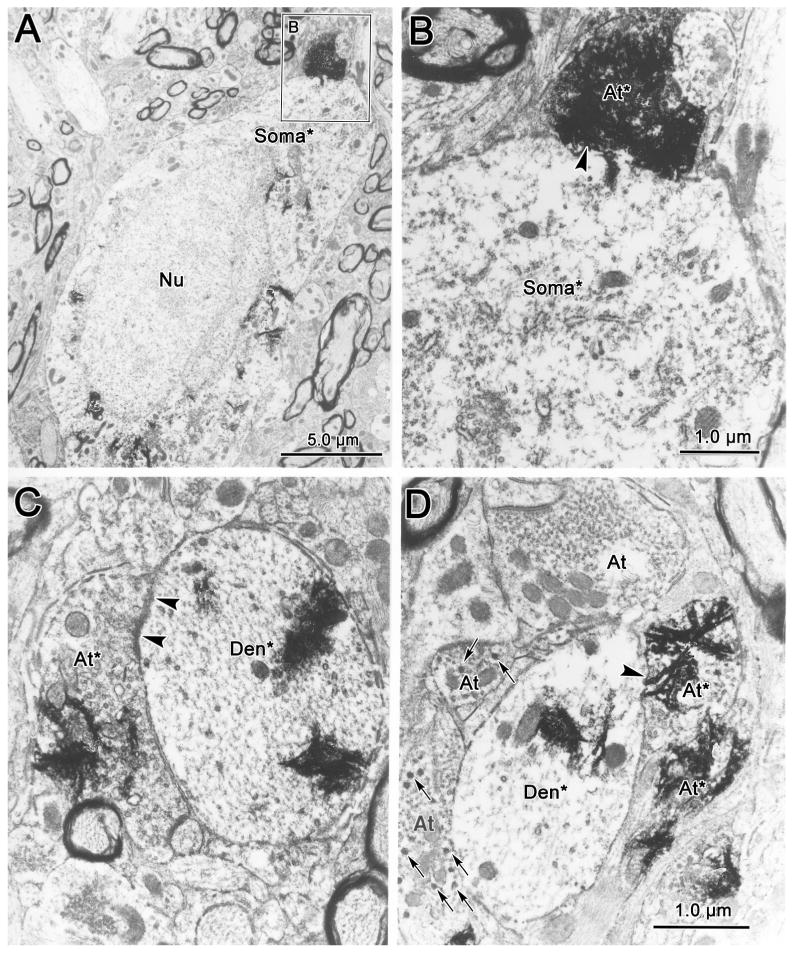
Electron micrographs of WGA-HRP labeled retinal terminals and WGA-HRP labeled premotor neurons in the olivary pretectal nucleus. Anterogradely labeled retinal terminals (At*) can be distinguished from unlabeled terminal profiles (At) by the presence of electron dense, flocculent reaction product. The labeled terminals contained clear spherical vesicles, but generally lacked dense-cored vesicles (arrows). The labeled terminals synaptically contacted (arrowheads) either retrogradely labeled somata (Soma*)(A&B) or dendrites (Den*)(C&D), which also contained the reaction product. Scale in C=D.
Discussion
The pupillary light reflex produces bilateral compensatory pupillary constriction in response to increased retinal illumination. This protects the retinal photoreceptors and ensures optimal visual perception under shifting illumination levels. It has long been presumed that this reflex is mediated by a four-neuron arc. However, the specific neurons, nuclei and connections have not been completely characterized in mammals. This study has delineated part of the anatomical substrate for the central portion of the pupillary light reflex in cats. Specifically, it has defined the location and morphology of the premotor neurons of OPt and their relationship to the retinal terminals. Furthermore, the results demonstrate the ipsi- and contralateral course of the premotor axons, and suggest that the preganglionic targets of this OPt projection lie in the more rostral component of the preganglionic motoneuron population: in and around AM. Thus, the findings represent an important step toward specifying the central components of the pupillary light reflex pathway in frontal-eyed mammals.
The results allow us to draw certain conclusions with respect to the central pathways controlling the two pupils, as shown schematically in Figure 9. First, this study confirms that there is a bilateral retinal projection to OPt, with a contralateral predominance in the cat. Second, fusiform cells within OPt project to AM and its vicinity. Thus, these OPt premotor neurons receive a monosynaptic retinal input, and their projection is bilaterally distributed, with a slight contralateral predominance. Third, there is a pretectal projection to the contralateral OPt from multipolar neurons in OPt that may help balance the responses in the two eyes. Finally, the OPt also projects to the NPC, which contains multipolar neurons that were labeled bilaterally following the AM injections. This may provide an additional, multisynaptic pathway to serve the reflex.
Figure 9.
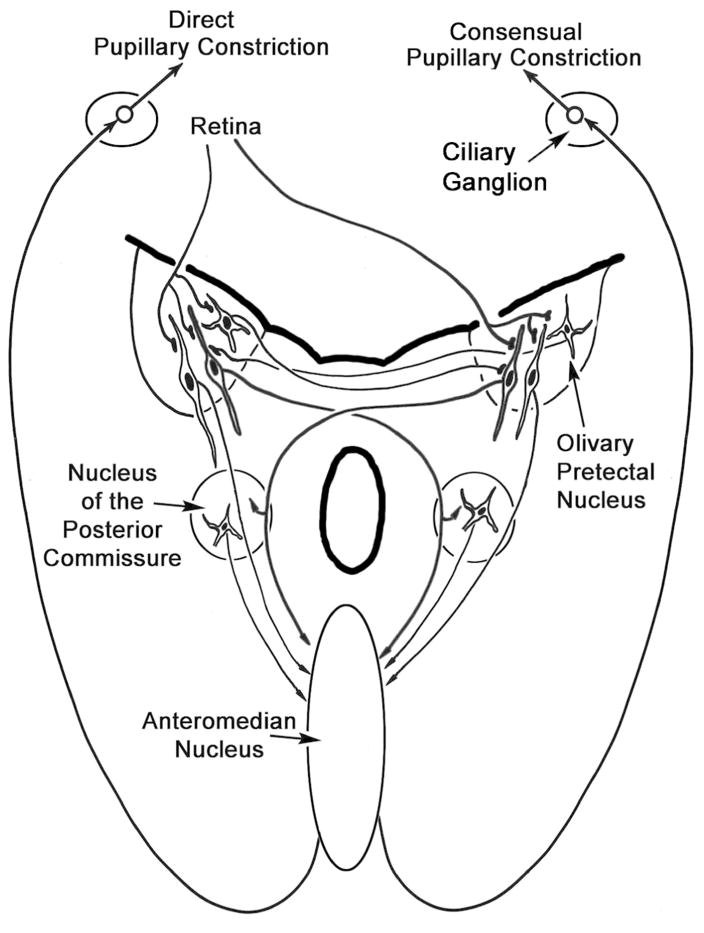
Schematic diagram summarizing the results of this study. Retinal terminals contact fusiform premotor cells in the olivary pretectal nucleus, with a contralateral predominance. Both ipsilateral and contralateral projections to the anteromedian nucleus are present, with a slight contralateral predominance. The anteromedian nucleus is the source of the preganglionic output that underlies the direct and consensual pupillary response. The olivary nucleus also contains a population of multipolar commissural neurons. In addition to targeting the anteromedian nucleus, the olivary pretectal nucleus also projects to the contralateral nucleus of the posterior commissure, which may provide an indirect pathway to preganglionic motoneurons in the anteromedian nucleus.
Technical considerations
These experimental results must be considered in light of the two major technical difficulties with tracer experiments: spread of tracer into adjoining structures and uptake of tracer by fibers of passage. The cat OPt does not contain extensive fibers of passage, so it seems unlikely that this was a major confound for OPt injections. Numerous axons pass around AM, so some of the retrogradely labeled cells observed following AM injections might have been labeled because of fiber uptake. However, since OPt injections do label terminals in AM, we can assume that a significant portion of the OPt neurons labeled after AM injections do indeed terminate there. The major findings in the present study are strengthened by the fact they were determined by both anterograde and retrograde tracing experiments. It should be noted, however, that OPt injections also labeled terminals in ND and INC, and that the AM injections spread into these nuclei, so it is possible that the neurons labeled following AM injections do not all represent premotor neurons. Looking across AM injection cases, we noted that the involvement of these two nuclei was variable, but this variability did not change the parameters of the OPt labeling, suggesting that these OPt cells are indeed premotor neurons, and that the projections to ND and INC may arise from other pretectal sources.
The other critical finding is that OPt premotor neurons receive direct retinal input. The retinal axons terminate not only in OPt, but also in other nuclei of the pretectum (Fig. 5). However, the pattern of retrograde label following AM injections confirms the function of OPt by showing that it is the only pretectal site at which cells labeled retrogradely from AM intermingle with retinal terminals. Electron microscopic analysis confirmed the direct synaptic connection between these two. Since there is no evidence of a projection to AM from the other retinal recipient pretectal nuclei, the role of OPt in this circuit seems well supported. One potential problem of the combined technique used in the present study is the possibility of trans-synaptic transport by WGA-HRP in retinal terminals (Spencer et al., 1982; Baker and Spencer, 1986). However, according to these reports, trans-synaptic transport of WGA-HRP usually occurs only after 48 hours. Survival time in the present study was limited to 24 hours, making trans-synaptic transport unlikely. Furthermore, our ultrastructural analysis showed cells that received multiple labeled retinal synaptic contacts, but were not themselves labeled, indicating that trans-synaptic transport had not occurred.
The olivary pretectal nucleus
The cat OPt is a more poorly defined than that of the monkey. It lacks clear cytoarchitectural boundaries in conventional histological staining. Previous investigators have suggested that the patterns of retinal terminal distribution in the pretectum help to define each individual pretectal nucleus (Hutchins and Weber, 1985; Koontz et al., 1985; Simpson et al., 1988; Nabors and Mize, 1991). In the present study, the ventral extent of the retinal terminal field has been used to define the ventral border of OPt. We chose this approach because it fits with the connectional argument of the study, and seemed more valid than including portions of this region in the posterior pretectal nucleus based on cell packing density alone.
The results from combined anterograde and retrograde labeling in the pretectum clearly delineated the location of premotor neurons in OPt that were monosynaptically targeted by the retina. The ultrastructure of WGA-HRP labeled terminals in the present study is similar to that of retinal terminals described in rat OPt (Campbell and Lieberman, 1985; Klooster and Vrensen, 1997). However, the distribution of these premotor neurons extends slightly beyond the cytoarchitectonic borders of OPt (Kanaseki and Sprague, 1974; Avendano and Juretschke, 1980; Koontz et al., 1985). Nevertheless, since these cells receive direct retinal input, it seems safe to include them within OPt. Additional retrogradely labeled cells located between OPt and the NPC may send their dendrites up into OPt to receive retinal luminance input. These cells are also slender fusiform cells oriented from dorsolateral to ventromedial like those observed in OPt. The morphology and location of the labeled premotor neurons are largely in agreement with a previous report in cats (Distler and Hoffmann, 1989b) and in rats (Klooster and Vrensen, 1997). However, the fusiform premotor neurons observed in the cat do not readily fit into the categories of Golgi-stained OPt cells described in the rat (Gregory, 1985).
It should be noted that a population of neurons with vergence-related activity has been reported in the area of the pretectum of monkeys (Judge and Cumming, 1986; Zhang et al., 1991). In addition, OPt cells have been reported to target extraocular motoneurons supplying multiply innervated fibers (Büttner-Ennever et al., 1996; Wasicky et al., 2004; Ugolini et al., 2006). Thus, it is possible that the labeled cells ventral to OPt are not pupillary premotor neurons, but are instead midbrain near-response neurons that were labeled because they also project to III. If this is the case, then these near triad cells may be responsible for a portion of the terminals observed in the vicinity of III following the OPt injections. However, the anatomical location of this physiologically determined vergence population has not been precisely defined in monkeys, and OPt cells in monkeys respond to luminance, but do not modulate their activity during the near response (Zhang et al., 1996). Whether these vergence-related cells are present in the cat pretectum is unknown.
OPt neurons projecting to the AM nucleus seem to represent only a portion of the total number of the neurons in OPt. This suggests that the OPt is involved in functions in addition to mediation of the pupillary light reflex. In fact, there is anatomical evidence that cat OPt projects to several other targets. These include the facial motor nucleus (Itoh et al., 1983; Holstege et al., 1986; Fort et al., 1989), a pathway which presumably activates squinting in bright light and the visual-triggered blink reflex. Other targets of the OPt are the pulvinar (Graybiel, 1972; Berman, 1977; Itoh, 1977; Berson and Graybiel, 1978; Roberson et al., 1983; Weber and Hutchins, 1986), and dorsal lateral geniculate nucleus (Berman, 1977; Kubota et al., 1987, 1988; Cucchiaro et al., 1991), where OPt input may supply baseline luminance information necessary to interpret the meaning of firing rate changes in neurons subserving visual sensation. Finally, as shown in the present cat study, the OPt neurons project to the contralateral OPt nucleus and bilaterally to the NPC. In this regard, the cat OPt projections are similar to those of the bird and rat (McDougal and Gamlin, 2008; Young and Lund, 1994; Klooster and Vrensen, 1997).
Central pupillary light reflex pathways
The role of the OPt as the first relay in the pupillary light reflex has been extensively studied. Destruction of the OPt abolishes or diminishes the pupillary light reflex (cat: Magoun and Ranson, 1935; monkey: Carpenter and Pierson, 1973; pigeon: Gamlin et al., 1984; rat Young and Lund, 1994), and stimulation of the OPt elicits pupillary constriction (cat: Ranson and Magoun, 1933; Hultborn et al., 1978; Distler and Hoffmann, 1989a; monkey: Magoun et al., 1936; pigeon: Gamlin et al., 1984). Early suggestions as to the type of retinal ganglion cells that project to the pretectum included M-type (magnocellular or Y-type), and P-type (parvocellular or X-type) and K-type (koniocellular or W-type) ganglion cells (Schoppmann and Hoffmann, 1979; Koontz et al., 1985). However, based on the properties of the OPt neurons, such as their broad receptive fields, graded response to luminance levels (rat Clarke and Ikeda, 1981; 1985; Trejo and Cicerone, 1984; monkey: Clarke et al., 2003), and the slow conduction velocity from retina (Distler and Hoffmann, 1989a), a K-type input seems most likely. In fact, there is recent evidence in primates that melanopsin-containing K-type retinal ganglion cells are the predominant cell type supplying OPt with luminance information (Gamlin et al., 2007; McDougal and Gamlin, 2010) and that this nucleus is connected to circadian rhythm circuits (Klooster et al., 1995; Baver et al., 2008).
A bilateral retinal projection to OPt was clearly evident in the present study (Fig. 1). This projection is heavier on the contralateral than ipsilateral side, and it showed side-specific heterogeneity in its density, as previously reported (Hutchins and Weber, 1985; Koontz et al., 1985; Simpson et al., 1988; Distler and Hoffmann, 1989b; Nabors and Mize, 1991). The pattern of retinal termination does not completely correlate with the location of the pretectal neurons projecting to AM. Nevertheless, retinal terminals were found wherever the OPt premotor neurons lay, and the ultrastructural evidence presented here proves the existence of a direct connection between these two.
In the present study, OPt injections resulted in more labeled terminals in AM, than caudally in the EWcp or SOA. This supports the contention there is a functional segregation of cat preganglionic motoneurons along the rostrocaudal axis, as proposed by Erichsen and May (2002). Specifically, the cat preganglionic motoneurons controlling the pupillary constrictor are located in and around AM, while those controlling lens accommodation are distributed more caudally, scattered in areas dorsal to III in the SOA, and to a lesser extent in EWcp, between the oculomotor nuclei, and ventral to III amongst the exiting oculomotor nerve fibers (Erichsen and May, 2002; May et al., 2008; Kozicz et al., 2011; Sun and May, 2014). In fact, there is evidence from single unit recording studies that accommodation-related motoneurons in the cat are located in SOA (Bando et al., 1981, 1984). This hypothesis is investigated further in a companion paper where projections to EWpg motoneurons are defined in relation to OPt projections (Sun and May, 2014).
The results from injections into AM indicated that preganglionic motoneurons may also receive input from NPC. This is in agreement with some reports (Graybiel and Hartwieg,1974, Klooster et al., 1995; 1998 Breen et al., 1983), but this pathway was not observed by Distler and Hoffmann (1989b). This retrograde labeling might be due to spread of tracer into INC, since this nucleus is known to receive input from the contralateral NPC (Berman, 1977). However, the fact that a greater density of anterogradely labeled terminals was found in and around AM following a combined OPt and NPC injection supports the contention that NPC projects to AM (although it remains possible that this increase in label was due to labeling fibers of passage from the contralateral OPt nucleus). Furthermore, OPt injections labeled terminals in NPC (Fig. 8, B-D), and injection of the NPC retrogradely labeled cells in the contralateral OPt nucleus (data not shown). These results seem to support the view that the NPC may provide a longer, secondary pathway for the pupillary light reflex, by acting as a relay between OPt and preganglionic motoneurons (cat: Graybiel and Hartwieg, 1974; Berman, 1977; Breen et al., 1983; tree shrew: Weber and Harting , 1980; monkey: Carpenter and Pierson, 1973). Unfortunately, there is, to our knowledge, no physiological evidence from studies of the NPC to support or refute this view. In fact, the connections of the NPC suggest it may be involved with control of vertical gaze, for it is interconnected with the rostral interstitial nucleus of the medial longitudinal fasciculus and INC (Chen and May, 2007; Schmidtke and Büttner-Ennever, 1992). Moreover, the NPC terminal fields near III do not show any preference for AM, and are, if anything, denser in SOA (Chen and May, 2007, personal observation). So, while luminance information may be provided by OPt to NPC, this may be related to the fact that upward and downward eye movements are generally associated with changes in retinal illumination.
Direct and consensual pathways
The characteristics of cat pupillary light reflex can be explained through consideration of the connectional pattern. Pupillary constriction in the cat is unequal when only one eye is exposed to illumination. Specifically, the consensual response in cats is always less intense than the direct response, a phenomena called the imperfect consensual light reflex (Loewenfeld, 1993). Since the contralateral retinal projection to OPt is heavier than the ipsilateral, it has been suggested that direct pathway predominance must be due to the OPt projection to the preganglionic motoneurons being more heavily weighted to the contralateral side, bringing the predominant response back across the midline to the illuminated side (Fig. 9). Indeed, we observed somewhat heavier terminal and retrograde labeling contralaterally in this study.
Based on the above argument, it appears that the degree of ipsilateral/contralateral projections both to and from the OPt output cells determines the symmetry of the pupillary constriction. In birds and rodents, both retinal and OPt projections are completely contralateral (Scalia, 1972; Reiner et al., 1983; Gamlin et al., 1984). Consequently, given a crossed projection by OPt to preganglionic motoneurons, the consensual pupillary light reflex is reported to be absent or very limited in these species (Loewenfeld, 1993). The consensual light reflex develops in parallel with the presence of increased ipsilateral retinal-pretectal projections. The development of the consensual light reflex may then be an important adaptive step for the development of binocular vision in frontal-eye animals. In most primate species tested, including humans, the direct and consensual pupillary light reflexes are equal. This is presumably due to a symmetrically bilateral retinal-pretectal input. There are conflicting reports that primates show either an exclusively contralateral pretectal-preganglionic motoneuron projection (Steiger and Büttner-Ennever, 1979) or a bilaterally equal one (Pierson and Carpenter, 1974; Benevento et al., 1977). However, if the two olivary nuclei are equally activated by the retina, either pretectal projection pattern would result in symmetric pupillary responses. This suggests an evolutionary scenario in which more and more retinal ganglion cell axons turned into the ipsilateral optic tract at the chiasm, as the degree of binocular overlap increased in frontal-eyed species, until in primates, the OPt on both sides were equally innervated, producing balanced direct and consensual responses. The pattern described here in the cat appears to be an intermediate one.
Other anatomical characteristics may play roles in the cat consensual light reflex. We observed reciprocal connections between the OPt (Fig. 9). These commissural cells lie in the retinal recipient zone dorsal to the premotor neurons and they differ morphologically from premotor neurons. A similar population has been reported in the rat (Klooster et al. 1995). Thus, projections to the preganglionic motoneurons and to the contralateral OPt arise from separate populations, and are not axon collaterals, as has been previously proposed (Gamlin et. al., 1984). The commissural terminal field is densest where the premotor OPt cells are located. This reciprocal projection might play a compensatory role, helping to balance the gain between the cat's asymmetric retinal inputs to OPt premotor cells, since contralateral retinal terminations are more intense in this dorsal region. In monkeys, reciprocal connections between the OPt have not been reported (Baleydier et al., 1990; Mustari et al., 1994), but they may be unnecessary in light of the balanced retinal input present in these species. On the other hand, the consensual light reflex is not present in the lateral eyed birds that have been tested, but the reciprocal OPt projections are reported (Gamlin et al., 1984). This would appear to argue against a role for this crossed projection in the consensual pupillary light reflex. The roles of the non-premotor populations in OPt clearly deserve further study.
Acknowledgments
We are grateful to Malinda Danielson for her help with the histology and Glenn Hoskins for his assistance with preparing the material for EM examination.
Grant Support: NEI EY07166 and EY014263
Abbreviations
- AM
anteromedian nucleus
- APt
anterior pretectal nucleus
- At
axonal terminal
- At*
anterogradely labeled axon terminal
- Aq
cerebral aqueduct
- CP
cerebral peduncle
- Den*
retrogradely labeled dendrite
- DLG
dorsal lateral geniculate nucleus
- EWcp
centrally projecting Edinger-Westphal nucleus
- EWpg
preganglionic Edinger-Westphal nucleus
- H
habenula
- III
oculomotor nucleus
- IIIn
oculomotor nerve
- INC
interstitial nucleus of Cajal
- LP
lateral posterior nucleus
- MG
medial geniculate nucleus
- MLF
medial longitudinal fasciculus
- MPt
medial pretectal nucleus
- ND
nucleus of Darkschewitsch
- NOT
nucleus of optic tract
- NPC
nucleus of posterior commissure
- Nu
nucleus
- Opt
olivary pretectal nucleus
- PAG
periaqueductal gray
- PC
posterior commissure
- RB
retroflex bundle
- RN
red nucleus
- SC
superior colliculus
- SN
substantia nigra
- SOA
supraoculomotor area
- Soma*
retrogradely labeled cell body
- WGA-HRP
wheat germ agglutinin conjugated horseradish peroxidase
Footnotes
Conflict of Interest: Neither of the authors has any known or potential conflicts of interest to declare with respect to the publication of this work.
Role of the Authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: PJM. Acquisition of data: WS & PJM. Analysis and interpretation of data: WS & PJM. Drafting of the manuscript: WS. Critical revision of the manuscript for important intellectual content: WS & PJM. Statistical analysis: WS & PJM. Obtained funding: PJM. Study supervision: PJM.
Literature Cited
- Avendano C, Juretschke MA. The pretectal region of the cat: A structural and topographical study with stereotaxic coordinates. J Comp Neurol. 1980;193:69–88. doi: 10.1002/cne.901930106. [DOI] [PubMed] [Google Scholar]
- Baker H, Spencer RF. Transneuronal transport of peroxidase conjugated wheat germ agglutinin (WGA) from the olfactory epithelium to the brain of the adult rat. Exp Brain Res. 1986;63:461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Magnin M, Cooper HM. Macaque accessory optic system: II. Connections with the pretectum. J Comp Neurol. 1990;302:405–416. doi: 10.1002/cne.903020216. [DOI] [PubMed] [Google Scholar]
- Bando T, Tsukuda K, Yamamoto N, Maeda J, Tsukahara N. Mesencephalic neurons controlling lens accommodation in the cat. Brain Res. 1981;213:201–204. doi: 10.1016/0006-8993(81)91262-2. [DOI] [PubMed] [Google Scholar]
- Bando T, Tsukuda K, Yamamoto N, Maeda J, Tsukahara N. Physiological identification of midbrain neurons related to lens accommodation in cats. J Neurophysiol. 1984;52:870–878. doi: 10.1152/jn.1984.52.5.870. [DOI] [PubMed] [Google Scholar]
- Barr L. Photomechanical coupling in the vertebrate sphincter pupillae. Crit Rev Neurobiol. 1989;4:325–366. [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalmic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Rezak M, Santos-Anderson R. An autoradiographic study of the projections of the pretectum in the rhesus monkey (Macaca mulatta): evidence for sensorimotor links to the thalamus and oculomotor nuclei. Brain Res. 1977;127:197–218. doi: 10.1016/0006-8993(77)90536-4. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Standage GP. The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J Comp Neurol. 1983;217:307–336. doi: 10.1002/cne.902170307. [DOI] [PubMed] [Google Scholar]
- Berman N. Connections of the pretectum in the cat. J Comp Neurol. 1977;174:227–254. doi: 10.1002/cne.901740204. [DOI] [PubMed] [Google Scholar]
- Berson DA, Graybiel AM. Parallel thalamic zones in the LP-pulvinar complex of the cat identified by their afferent and efferent connections. Brain Res. 1978;147:139–148. doi: 10.1016/0006-8993(78)90778-3. [DOI] [PubMed] [Google Scholar]
- Breen LA, Burde RM, Loewy AD. Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res. 1983;261:303–306. doi: 10.1016/0006-8993(83)90633-9. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AKE, Resine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Campbell G, Lieberman AR. The olivary pretectal nucleus: Experimental anatomical studies in the rat. Phil Trans R Soc Lond B. 1985;310:573–609. doi: 10.1098/rstb.1985.0132. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Woodhouse JM. The role of the pupil light reflex in dark adaptation. J Physiol (Lond) 1975;245:111–112. [PubMed] [Google Scholar]
- Campos-Ortega JA, Glees P. The subcortical distribution of the optic fibers in Saimiri sciureus (squirrel monkey) J Comp Neurol. 1967;131:131–142. doi: 10.1002/cne.901310205. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Pierson RJ. Pretectal region and the pupillary light reflex. An anatomical analysis in the monkey. J Comp Neurol. 1973;149:271–300. doi: 10.1002/cne.901490302. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor control of eyelid movements in conjunction with vertical saccades in the cat: The interstitial nucleus of Cajal. J Comp Neurol. 2007;500:676–692. doi: 10.1002/cne.21203. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Ikeda H. Pupillary responses and luminance and darkness detector neurons in the pretectum of the rat. In: Maffei L, editor. Pathophysiology of the visual system, documenta ophthalmologia proceedings series. Vol. 30. Dr W Junk Publishers; The Hague: 1981. pp. 53–61. [Google Scholar]
- Clarke RJ, Ikeda H. Luminance detectors in the olivary pretectal nucleus and their relationship to the pupillary light reflex in the rat. II. Studies using sinusoidal light. Exp Brain Res. 1985;59:83–90. doi: 10.1007/BF00237669. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Zhang HY, Gamlin PDR. Primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. J Neurophysiol. 2003;89:3168–3178. doi: 10.1152/jn.01130.2002. [DOI] [PubMed] [Google Scholar]
- Cucchiaro JB, Bickford ME, Sherman SM. A GABAergic projection from the pretectum to the dorsal lateral geniculate nucleus in the cat. Neuroscience. 1991;41:213–226. doi: 10.1016/0306-4522(91)90211-6. [DOI] [PubMed] [Google Scholar]
- Distler C, Hoffmann KP. The pupillary light reflex in normal and innate microstrabismic cats, I: Behavior and receptive-field analysis in the nucleus praetectalis olivaris. Vis Neurosci. 1989a;3:127–138. doi: 10.1017/s0952523800004442. [DOI] [PubMed] [Google Scholar]
- Distler C, Hoffmann KP. The pupillary light reflex in normal and innate microstrabismic cats, II: retinal and cortical input to the nucleus praetectalis olivaris. Vis Neurosci. 1989b;3:139–153. doi: 10.1017/s0952523800004454. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Harvard University Press; Cambridge: 1987. pp. 187–223. [Google Scholar]
- Erichsen JT, May PJ. The pupillary and cilliary components of the cat Edinger-Westphal nucleus: A transsynaptic transport investigation. Visual Neurosci. 2002;19:15–29. doi: 10.1017/s0952523801191029. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Vanna BA, Reiner Control of choroidal blood flow by the nucleus of Edinger-Westphal in pigeons: a laser Doppler study. Invest Ophthalmol Vis Sci. 1990;31:2483–2492. [PubMed] [Google Scholar]
- Fort P, Sakai K, Luppi PH, Salvert D, Jouvet M. Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. J Comp Neurol. 1989;283:285–302. doi: 10.1002/cne.902830209. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Reiner A, Erichsen JT, Karten HJ, Cohen DP. The neural substrate for the pupillary light reflex in the pigeon (Columba livia) J Comp Neurol. 1984;226:523–543. doi: 10.1002/cne.902260407. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, McDougal DH, Pokorny J, Smith VC, Yao KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giolli RA, Tigges JH. The primary optic pathways and nuclei of primates. In: Noback C, Montagna W, editors. The Primate Brain. Appleton-Century-Crofts; New York: 1970. pp. 29–54. [Google Scholar]
- Graybiel AM. Some extrageniculate visual pathways in the cat. Invest Opthalmol. 1972;11:322–332. [PubMed] [Google Scholar]
- Graybiel AM. Some efferents of the pretectal nuclei in the cat. Anat Rec. 1974;178:365. [Google Scholar]
- Graybiel AM, Hartwieg E. Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res. 1974;81:543–551. doi: 10.1016/0006-8993(74)90850-6. [DOI] [PubMed] [Google Scholar]
- Gregory KM. The dendritic architecture of the visual pretectal nuclei of the rat: a study with the Golgi-Cox method. J Comp Neurol. 1985;234:122–135. doi: 10.1002/cne.902340110. [DOI] [PubMed] [Google Scholar]
- Holstege G, Van Ham JJ, Tan J. Afferent projections to the orbicularis oculi motoneuronal cell group: An autoradiographic tracing study in the cat. Brain Res. 1986;374:306–320. doi: 10.1016/0006-8993(86)90425-7. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Mori K, Tsukahara N. The neuronal pathway subserving the pupillary light reflex. Brain Res. 1978;159:255–267. doi: 10.1016/0006-8993(78)90533-4. [DOI] [PubMed] [Google Scholar]
- Hutchins B, Weber J. The olivary pretectal nucleus of the cat: evidence for a two-tailed structure. Brain Res. 1985;331:150–154. doi: 10.1016/0006-8993(85)90725-5. [DOI] [PubMed] [Google Scholar]
- Itaya SK, Van Hoesen GHC. WGA-HRP as trans-neuronal marker in the visual pathways of monkey and rat. Brain Res. 1982;236:199–204. doi: 10.1016/0006-8993(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Itoh K. Efferent projections of the pretectum in the cat. Exp Brain Res. 1977;30:89–105. doi: 10.1007/BF00237861. [DOI] [PubMed] [Google Scholar]
- Itoh K, Takada M, Yasui Y, Mizuno N. A pretectofacial projection in the cat: a possible link in the visual-triggered blink reflex pathways. Brain Res. 1983;275:332–335. doi: 10.1016/0006-8993(83)90713-8. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Kanaseki T, Sprague JM. Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol. 1974;158:319–338. doi: 10.1002/cne.901580307. [DOI] [PubMed] [Google Scholar]
- Klooster J, Vrensen G, Müller LJ, Van der Want JJL. Efferent projections of the olivary pretectal nucleus in the albino rat subserving the pupillary light reflex and related reflexes. A light microscopic tracing study. Brain Res. 1995;688:34–46. doi: 10.1016/0006-8993(95)00497-e. [DOI] [PubMed] [Google Scholar]
- Klooster J, Vrensen GFJM. The ultrastructure of the olivary pretectal nucleus in rats. A tracing and GABA immunohistochemical study. Exp Brain Res. 1997;114:51–62. doi: 10.1007/pl00005623. [DOI] [PubMed] [Google Scholar]
- Klooster J, Vrensen GFJM. New indirect pathways subserving the pupillary light reflex: projections of the accessory oculomotor nuclei and the periaqueductal gray to the Edinger-Westphal nucleus and thoracic spinal cord. Anat Embryol. 1998;198:123–132. doi: 10.1007/s004290050170. [DOI] [PubMed] [Google Scholar]
- Koontz MA, Rodieck RW, Farmer SG. The retinal projection to the cat pretectum. J Comp Neurol. 1985;236:42–59. doi: 10.1002/cne.902360105. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PDR, Palkovits M, Horn AKE, Toledo CAB, Ryabinin AE. The Edinger-Westphal nucleus: A historical, structural and functional perspective on a dichotomous terminology. J Comp Neurol. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Morimoto M, Kanaseki T, Inomata H. Projection from the pretectal nuclei to the dorsal lateral geniculate nucleus in the cat: a wheatgerm agglutinin-horseradish peroxidase study. Brain Res. 1987;421:30–40. doi: 10.1016/0006-8993(87)91271-6. [DOI] [PubMed] [Google Scholar]
- Kubota T, Morimoto M, Kanaseki T, Inomata H. Visual pretectal neurons projecting to the dorsal lateral geniculate nucleus and pulvinar nucleus in the cat. Brain Res Bull. 1988;20:573–579. doi: 10.1016/0361-9230(88)90216-x. [DOI] [PubMed] [Google Scholar]
- Laties AM, Sprague JM. The projection of the optic fibers to the visual centers in the cat. J Comp Neurol. 1966;127:35–70. doi: 10.1002/cne.901270104. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. The light reflex In: The Pupil, Anatomy, Physiology, and Clinical Applications. Iowa State University Press; Ames: 1993. pp. 83–274. [Google Scholar]
- Lowenstein O, Loewenfeld IE. The pupil. In: Davison H, editor. The Eye. Vol. 3. Academic Press; New York: 1969. pp. 255–337. [Google Scholar]
- Magoun HW, Ranson SW. The central path of the light reflex: A study of the effect of lesions. Arch Ophthalmol. 1935;13:791–811. [Google Scholar]
- Magoun HW, Ranson SW, Mayer LL. The pupillary light reflex after lesions of the posterior commissure in the cat. Am J Ophthalmol. 1936;18:624–630. [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: Implications for collicular generation of saccades. Neuroscience. 1990;36:305–324. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal DH, Gamlin PDR. Pupil control pathways. In: Masland R, Albright TD, editors. The Senses: A Comprehensive Reference. Vol. 1. San Diego: Academic Press; 2008. pp. 521–536. Vision I. [Google Scholar]
- McDougal DH, Gamlin PDR. The influence of intrinsically photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50:72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: A non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF, Kaneko CRS, Robinson FR. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol. 1994;349:111–128. doi: 10.1002/cne.903490108. [DOI] [PubMed] [Google Scholar]
- Nabors LB, Mize RR. A unique neuronal organization in the cat pretectum revealed by antibodies to the calcium-binding protein calbindin-D 28K. J Neurosci. 1991;11:2460–2476. doi: 10.1523/JNEUROSCI.11-08-02460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olucha F, Martinez-Garcia F, Lopez-Garcia C. A new stabilizing agent for the TMB reaction product in the histochemical detection of horseradish peroxidase. J Neurosci Meth. 1985;13:131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Pierson RJ, Carpenter MB. Anatomical analysis of the pupillary light reflex pathways in the rhesus monkey. J Comp Neurol. 1974;158:121–144. doi: 10.1002/cne.901580202. [DOI] [PubMed] [Google Scholar]
- Ranson SW, Magoun HW. The central path of the pupillo-constrictor response to light. Arch Neurol Psychiat. 1933;30:1193–1204. [Google Scholar]
- Reiner A, Karten HJ, Gamlin PDR, Erichsen JT. Parasympathetic ocular control-functional subdivisions and circuitry of the avian nucleus of Edinger-Westphal. Trends Neurosci. 1983;6:140–145. [Google Scholar]
- Roberson RT, Thompson SM, Kaitz SS. Projections from the pretectal complex to the thalamic lateral dorsal nucleus of the cat. Exp Brain Res. 1983;51:157–171. doi: 10.1007/BF00237191. [DOI] [PubMed] [Google Scholar]
- Scalia F. The termination of retinal axons in the pretectal region of mammals. J Comp Neurol. 1972;145:223–258. doi: 10.1002/cne.901450208. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Büttner-Ennever JA. Nervous control of eyelid function: A review of clinical, experimental and pathological data. J Comp Neurol. 1992;181:745–761. doi: 10.1093/brain/115.1.227. [DOI] [PubMed] [Google Scholar]
- Schoppmann A, Hoffmann KP. A comparison of visual responses in two pretectal nuclei and in the superior colliculus of the cat. Exp Brain Res. 1979;35:495–510. doi: 10.1007/BF00236767. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Giolli RA, Blanks RHI. The pretectal nuclear complex and the accessory optic system. In: Büttner-Ennever JA, editor. Neuroanatomy of the Oculomotor System. Elsevier; New York: 1988. pp. 335–364. [PubMed] [Google Scholar]
- Spencer RF, Baker H, Baker R. Evaluation of wheat germ agglutinin immunohistochemistry as a neuroanatomical method for retrograde, anterograde, and anterograde transsynaptic labeling in the cat visual and oculomotor systems. Soc Neurosci Abst. 1982;8:785. [Google Scholar]
- Steiger HJ, Büttner-Ennever JA. Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res. 1979;160:1–15. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- Sun W, May PJ. Central pupillary light reflex circuits in the cat: Morphology, ultrastructure and inputs of preganglionic motoneurons. J Comp Neurol (Submitted) 2014 doi: 10.1002/cne.23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Bos J, Tigges M. An autoradiographic investigation of the subcortical visual system in chimpanzee. J Comp Neurol. 1977;172:367–380. doi: 10.1002/cne.901720211. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Akert K, Garey LJ, Otaui K. Primary and secondary subcortical projections to the monkey visual system: An autoradiographic study. Brain Res. 1981;214:137–143. doi: 10.1016/0006-8993(81)90444-3. [DOI] [PubMed] [Google Scholar]
- Trejo LJ, Cicerone CM. Cells in the olivary pretectal nucleus are in the pathway for the direct light reflex of the pupil in the rat. Brain Res. 1984;300:49–62. doi: 10.1016/0006-8993(84)91340-4. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Dans MD, Dubayle D, Brandi AM, Büttner-Ennever J, Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: Differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol. 2006;498:762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- Warwick R. The ocular parasympathetic nerve supply and its mesencepahalic sources. J Anat. 1954;88:71–93. [PMC free article] [PubMed] [Google Scholar]
- Wasicky R, Horn AK, Büttner-Ennever JA. Twitch and nontwitch motoneuron subgroups in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–29. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Weber JT, Harting JK. The efferent projections of the pretectal complex: an autoradiographic and horseradish peroxidase analysis. Brain Res. 1980;194:1–28. doi: 10.1016/0006-8993(80)91315-3. [DOI] [PubMed] [Google Scholar]
- Weber JT, Hutchins B. The pretectal complex of the cat: cells of origin of projections to the pulvinar nucleus. Brain Res. 1986;397:389–394. doi: 10.1016/0006-8993(86)90645-1. [DOI] [PubMed] [Google Scholar]
- Young MJ, Lund RD. The anatomical substrates subserving the pupillary light reflex in rats: Origin of the consensual pupillary response. Neurosci. 1994;62:481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gamlin PDR, Mays LE. Antidromic identification of midbrain near response cells projecting to the oculomotor nucleus. Exp Brain Res. 1991;84:525–528. doi: 10.1007/BF00230964. [DOI] [PubMed] [Google Scholar]
- Zhang H, Clarke RJ, Gamlin PDR. Behavior of luminance neurons in the pretectal olivary nucleus during the pupillary near response. Exp Brain Res. 1996;112:158–162. doi: 10.1007/BF00227189. [DOI] [PubMed] [Google Scholar]


