Abstract
Mesenchymal stem cells (MSCs) are primary candidates in cell therapy and tissue engineering and are being tested in clinical trials for a wide range of diseases. Originally isolated and expanded as plastic adherent cells, MSCs have intriguing properties of in vitro self-assembly into three-dimensional (3D) aggregates reminiscent of skeletal condensation in vivo. Recent studies have shown that MSC 3D aggregation improved a range of biological properties, including multilineage potential, secretion of therapeutic factors, and resistance against ischemic condition. Hence, the formation of 3D MSC aggregates has been explored as a novel strategy to improve cell delivery, functional activation, and in vivo retention to enhance therapeutic outcomes. This article summarizes recent reports of MSC aggregate self-assembly, characterization of biological properties, and their applications in preclinical models. The cellular and molecular mechanisms underlying MSC aggregate formation and functional activation are discussed, and the areas that warrant further investigation are highlighted. These analyses are combined to provide perspectives for identifying the controlling mechanisms and refining the methods of aggregate fabrication and expansion for clinical applications.
Introduction
In recent years, mesenchymal stem cells (MSCs) have emerged as a primary candidate in cell-based therapies owing to their unique properties.1 To date, over 320 clinical trials in a broad range of diseases making use of MSCs have been reported (www.clinicaltrials.org). The clinical promise of human MSCs is supported by their ability to differentiate and mature into specific phenotypes, their immune-suppressive properties, and their distinct migratory and potent trophic effects during tissue repair and regeneration.2–6 Initially isolated from bone marrow (BM),7 MSCs are usually defined as plastic adherent cells, displaying fibroblastic shape and expressing nonspecific surface markers.8 MSCs are capable of forming discrete colonies and possess multipotentiality in adipogenic, osteogenic, and chondrogenic lineages.8 Based on these in vitro criteria, MSCs have been extracted from many connective tissues,9 including bone marrow (BM-MSC), adipose tissue (A-MSC), Wharthon jelly (WJ-MSC), umbilical cord (UC-MSC), cartilage tissue (C-MSC), and gingiva (G-MSC).10–12 While whether these MSCs share the same traits as BM-MSCs is still being debated,13,14 the vast majority of clinical trials under development have been using BM-MSCs, which comprise only ∼1 in 105 BM mononuclear cells.15
Recent clinical studies have shown that manufactured BM-MSCs after extensive ex vivo expansion have altered immune properties and low survival rate post-transplantation, failing to meet the clinical endpoint compared to minimally expanded BM-MSCs.16 While initially selected and defined as plastic adherent cells, it was progressively realized that plastic two-dimensional (2D) cultures alter the native phenotype of MSCs.1,17 Recently, self-assembly of MSCs into tightly packed clusters with 500–10,000 cells in each aggregate has been shown to create an “in vivo-like” microenvironment and better preserve MSC phenotype and innate properties.5,6 Indeed, the formation of three-dimensional (3D) cellular aggregates, even short-term post-expansion, enhanced the regenerative capacity of MSCs by promoting the secretion of anti-inflammatory cytokines, proangiogenic, and chemotaxic factors.5,18,19 The benefits of cell aggregation has also been shown in preclinical studies, in which intramyocardial transplantation of the 3D A-MSC and BM-MSC aggregates in a porcine model improved cell retention, survival, and integration.20,21 Similarly, transplantation of synovial MSC aggregates promoted cartilage tissue regeneration in a rabbit model and enhanced meniscus tissue regeneration effectively in rats.22,23 Thus, there is a growing interest to better understand the mechanism of MSC 3D aggregation, its impact on cell properties, and its feasibility in therapeutic applications.
Cellular aggregates have long been used as model systems to understand tumor biology and embryonic development24–26 and are reported to represent a physiologically relevant environment to mimic in vivo behavior.27,28 For neural stem cells, assembly of cells into 3D neurospheres has been found to revert the progenitor cells to an early phenotype.29 For MSCs, the pellet (i.e., a forced cell self-assembly by centrifugation) or micromass (formed by high-density cell suspension) cultures have long been used in chondrogenic differentiation.30–32 Recently, in vitro MSC self-assembly as 3D aggregates has been suggested to recapitulate the in vivo mesenchymal condensation events that influence MSC properties beyond chondrogenic lineage.5,33,34 MSC 3D aggregation is thought to be mediated through intrinsic cell–cell contacts and cell–extracellular matrix (ECM) interactions, which enables the localization of endogenous growth factors and enhances MSC therapeutic potential.24,35,36 Additionally, the formation of MSC aggregates activated anti-inflammatory protein expression, had high resistance to ischemic stress, better preserved the multilineage potential, and enhanced the expression of migratory cytokine receptor, such as C-X-C chemokine receptor type 4 (CXCR4).5,37,38 Finally, the formation of MSC aggregates could also recreate histotypic structures that serve as building blocks in tissue engineering to create 3D complex tissues.39,40
Hence, it becomes evident that self-assembly of MSCs into aggregates has significant implication in MSC's applications in cell therapy and tissue regeneration. This review seeks at understanding and evaluating the potential mechanism underlying the property enhancement associated with MSC aggregation. To the practical point of view, this work also discussed the methods suitable for the generation of MSC aggregates and expansion in bioreactors. Finally, the application of MSC aggregates in various diseases and the prospects for their clinical application are also discussed.
Formation of 3D MSC Aggregates
Hypothesis of MSC aggregation and self-assembly
Self-assembly of a dispersed cell population occurs during embryogenesis, morphogenesis, and organogenesis in vivo and is thought to arise from intracellular adhesiveness and energy minimization.41–44 During skeletal development, a pivotal stage is the condensation of mesenchymal progenitor cells with the formation of dense cell–cell contacts via adhesion molecules.45 At cellular level, the closely packed cells are the fundamental cellular units of morphological changes during prenatal organogenesis, and their initiation, size, boundaries, and differentiation are tightly regulated by a set of genes and gene products of cell adhesion molecules (i.e., N-CAM and N-cadherin).46 Although the precise in vivo origin of MSC has yet to be defined and whether MSCs in culture are bona fide in vitro counterparts of the mesenchymal progenitors is still being debated,13 MSCs have many unique properties and have been used as in vitro models to recapitulate condensation events.47 Indeed, extensive studies have shown that MSCs have the tendency for self-assembly and spontaneously form 3D aggregates in vitro in the absence of adherent surface, under mechanical forces, or within confined spaces, reminiscent of their in vivo properties of aggregation.24,35,48–50 However, the mechanisms by which MSCs organize into the aggregates and the impact of such structure on cell behaviors are just beginning to be investigated.
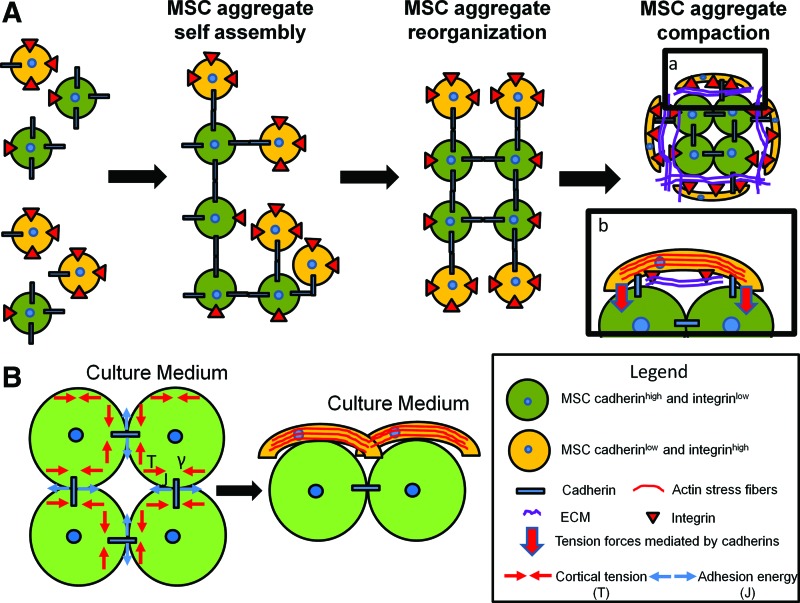
Differential adhesion hypothesis (DAH) proposed by Steinberg suggests that cells exhibit cell-type dependent “affinity” and that cell-to-cell adhesion and cell-sorting behavior in embryonic tissues are driven by the need to minimize interfacial energy (Fig. 1A).44,51 According to DAH, the differential expressions of cell adhesion molecules influence cell surface tension such that cell populations displaying high cadherin expression were found at the interior, whereas cells with high integrin expression at the exterior of the spheroids.43 However, the formation and renewal of cell adhesion bonds and junctions require changes in cell morphology, which is also determined by cell contractility and cortical tension.27 Thus, both interfacial adhesion among contacting cells and cortical tension of the individual cells are important factors that influence the progression of cell aggregation and the resulted heterogeneous aggregate structure (Fig. 1B).52–54 While a direct link between integrin density and intracellular tension is consistent with the spindle shape of MSCs at the surface of aggregates,6,32,55 whether cell morphology correlates with the expression of cell adhesion molecules in the aggregates is yet to be determined. Further studies are also needed to elucidate mechanistic connection between cortical forces and the aggregates' structural and biomechanical properties.
FIG. 1.
Proposed mechanisms for mesenchymal stem cell (MSC) aggregation and ultrastructure organization. (A) MSCs are heterogeneous population containing cells expressing various levels of integrins and cadherins. Initial MSC aggregation is mediated by cadherin–cadherin interactions. Minimizing the free energy, cadherin low/integrin high MSCs sort out at the edge of the aggregates.43 Mechanical polarization and the increased aggregate compactness are mediated through cadherins. Cadherins act as anchoring point for the cytoskeleton and mediate the increased cortical tension at the medium-aggregate interface.53 As a consequence of increased cortical tension and integrin–extracellular matrix (ECM) interactions, MSCs at the edge of aggregate display spindle shape and organize the actin as stress fibers. (B) In homotypic cell-to-cell contacts, the interfacial tension (γ) is increased by the cortical tension (T) and decreased by the adhesion energy (J). Since the cells at the interface with the medium are not associated with cell–cell adhesion complexes, the interfacial tension (γ) is equal to the cortical tension (T). As a consequence, the surface tension is increased by cell–cortex tension (T), leading to increase actin polymerization at the edge of the aggregates. Color images available online at www.liebertpub.com/teb
In vitro, a three-step model that involves initial cell–cell contacts, cadherin accumulation, and aggregate compaction has been proposed.24,56 Although this simplified model could not solely explain the heterogeneity of aggregate architecture arising during development, it depicts a sequence of cellular events occurring during aggregate formation in vitro.43 After the establishment of cell–cell contacts under physical forces or spatial proximity, the 3D aggregate self-assembly involves the adapted homophilic cadherin interaction and/or integrin binding to the ECM proteins, enabling the formation of bridges between cells (Fig. 1).24 The specific involvement of cadherin and/or integrin/ECM depends on both cell type and the specific fabrication method.57 N-cadherin and cadherin-11 are the most expressed cadherins in BM-MSCs,58 whereas E-cadherin expression was dominant during UC-MSC aggregation.59 The contribution of cadherin-mediated self-assembly in aggregate formation is demonstrated in the studies that showed EDTA was able to disturb BM-MSC and UC-MSC spheroid formation49,59 and that A-MSCs cultivated in serum-free medium without exogenous adhesion molecules were able to form spheroids.60,61 ECM proteins also play important roles in MSC aggregations and influence aggregate stabilization and compactness. During the early stage of aggregate formation, the ECM (i.e., collagen type I) was more diffuse, while cell–cell adherent junctions were the dominating force in initial aggregate formation.32 However, binding to exogenous ECM proteins has been shown to stabilize and compact tumor aggregates and the incorporation of gelatin microparticles in BM-MSC spheroids increased their stiffness, suggesting the ECM involvement in aggregate stabilization.57,62 In the aggregates of Chinese hamster ovary cells with varying integrin α5β1 receptor density, the interplay between soluble fibronectin and integrin α5β1 was found to contribute significantly to the tissue cohesion and mediated a shift from liquid to elastic behavior.63 Thus, the ECMs in the aggregates not only function as signaling molecules in cell adhesion but also play a biomechanical role that influences the force balance and biomechanical signal transduction between intracellular cytoskeleton and extracellular microenvironment.64–66 The ECM's biomechanical role in MSC aggregates is yet to be elucidated.
Compared to adherent culture, MSC aggregates display temporospatial heterogeneity owing to the differences in cellular and biomechanical microenvironments based on their intrinsic characteristics (e.g., integrin and cadherin expressions as well as cortical tension) (Fig. 1). For example, MSCs at the interior of the aggregates show round shape with low intracellular tension, whereas cells at the exterior display high degree of spreading and intracellular tension (Fig. 1).62,67 The MSC aggregates also undergo considerable compaction in culture, and the reduction in diameter from 632 to 353 μm over 21 days has been reported.5,50 These morphological changes require effective rearrangement and adaptation of cytoskeleton network, which is known to influence MSC fate in 2D cultures.68 Understanding the role of cytoskeleton in MSC aggregate formation and its involvement in aggregate compaction and structural organization is fundamental to better understand the aggregate property and to refine aggregate fabrication and expansion methods.
Methods to generate 3D MSC aggregates
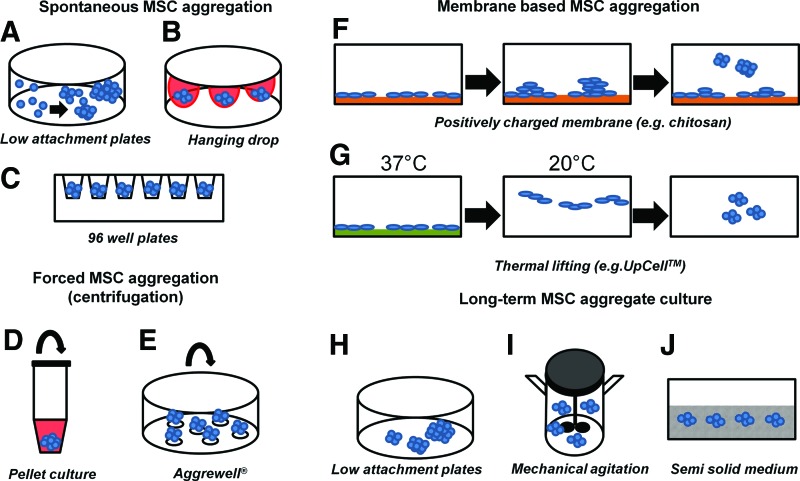
Various methods have been used to generate 3D MSC aggregates, including self-assembly on nonadherent surface, hanging drop, forced aggregation, surface treatment, and microfabrication (Table 1 and Fig. 2).5,24,35,69 Similar to their use in embryoid body formation, these methods utilize spatial confinement or mechanical forces to increase cell–cell contacts to precisely control aggregate size and cellular composition.26,70 Suspension culture on ultra-low attachment surfaces is easy to implement in the laboratory but leads to the formation of aggregates with large variations in size with low viability and low efficiency.50 Various surface modification methods have been explored to increase aggregate-forming efficiency and modulate composition of the aggregates. On the polycationic chitosan membrane, A-MSCs aggregated through noncontrolled cell motility and readily merged to form aggregates, but the size distribution displayed high variability.71,72 Nanoculture and poly(ethylene glycol)-micropatterned plates promoted homogeneity and increased the viability of BM-MSC aggregates.34,73 To enhance initial aggregate size homogeneity and cell viability, thermal lifting surface incorporating with endogenous ECMs was also applied to form BM-MSC aggregates.49
Table 1.
Fabrication Methods and the Properties of MSC Aggregates
| Methods | MSC source | Starting cell density | Initial cell number per aggregate | Initial aggregate size | Aggregate size after long-term culture | Long-term viability | Proliferation | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nonadhesive 96-well plate | Human bone marrow | 1×105 cells/mL | 1×105 | 632±35 μm | 353±28 μm at day 21 | +++ (at day 17) | No proliferation during a 21-day culture period | 50 |
| Nonadhesive 96-well plate | Human umbilical cord | 1×104–1.8×105 cells/mL | 1×104–1.8×105 | 150 μm to 1.0 mm | ND | ND | ND | 36 |
| Low attachment plate | Human umbilical cord | ND | ND | 40–100 μm | ND | ND | Increased proliferation compared to 2D at day 1 (cell cycle analysis) | 59 |
| Low attachment plate | Human gingiva | 2×105 cells/mL | 20–100 | 20–100 μm | ND | ++ (at day 3) | ND | 12 |
| Low attachment plate+agitation | Human bone marrow | 2×104 cells/mL | ND | 32–100 μm (day 7) | No change in bioreactor cultures | ++ (at day 7) | Similar to monolayer at day 7 (cell cycle analysis) | 6 |
| Hanging drop+static semisolid | Human bone marrow | 5×103 cells/mL | ND | ND | 159±19 μm at day 17 | ++ (at day 17) | ND | 50 |
| Polypropylene tube+suspension | Human bone marrow | 2×104 cells/mL | 2×104 | ND | 360±60 μm at day 17 | ++ (at day 17) | ND | 50 |
| Thermal lifting | Human bone marrow | 3×103 cells/cm2 | 10 | 89±32 μm | 100–250 μm at day 2 | ++ | Increased proliferation compared to self-assembly (BrdU assay at day 1) | 49 |
| Forced aggregation | Human bone marrow | 2.5×105 cells | 2.5×105 | ±1 mm after 1 week | No change after 4 weeks | ++ (at day 21) | No proliferation after 7 days (cell cycle analysis) | 77 |
| Forced aggregation +agitation | Mouse bone marrow | 1.8–6×106 cells/mL | 300–1000 | 100 μm (day 7) | No change in stirred culture at day 7 | ++ (up to day 7) | Similar to monolayer (BrdU assay at day 7) | 35 |
| PEG-micropatterned surface | Human bone marrow and UET-13 line | 5×104 cells/cm2 | 50 | 100 μm | ND | + (day 5–15) | Decreased proliferation compared to 2D | 34,73 |
| Chitoson membrane | Human adipose tissue | 1×103–5×104 cells/cm2 | ND | 22–54 μm | Increased size, 77–195 μm day 7 | + | No proliferation after 7 days (Alamar blue) | 71,72 |
MSC, mesenchymal stem cell; PEG, poly(ethylene glycol); BrDU, 5-bromo-2-deoxyuridine; 2D, two-dimensional; ND, not determined.
FIG. 2.
Methods to generate and cultivate MSC aggregates. MSC aggregates can be generated by (A) spontaneous self-assembly; (B) gravity-induced aggregation in hanging drops; or in (C) confined space of 96-well plates. Applied centrifugal forces induce MSC aggregation in (D) tubes or (E) AggreWell. (F) MSC aggregates form on chitosan membranes through the increased cell motility. (G) Thermal lifting liberates MSC-ECM complexes to form aggregates. Long-term culture of MSC aggregates in (H) low attachment plates, (I) stirred bioreactors, or (J) semisolid gels. Color images available online at www.liebertpub.com/teb
Efforts have also focused on improving aggregate-forming efficiency, size distribution, and scalability. High throughput methods that combine spatial confinement and mechanical forces have been developed and shown to enhance aggregate-forming efficiency, size distribution, and cell viability.35 The use of rotary orbital shakers or spinner flask minimized aggregate fusion, improved nutrient delivery, and increased MSC viability.6,50 The formation of MSC aggregates in AggreWell™ also promoted cell viability in a high throughput manner through the tight control of initial aggregate size and minimizing the probability of aggregate fusion.35 Hanging drop method in semisolid gel limited aggregate contacts and enhanced cell viability.50 Although spatial confinement during aggregate formation and culture is a common approach to obtain aggregates of uniform size distribution, the addition of surfactant such as Pluronic F-68 (PF-68) has also been found to effectively reduce aggregate coalescing.49 As a nonionic surfactant, PF-68 has been widely used as a culture additive to reduce cell damage associated with mechanical stress and to prevent cell re-aggregation in bioreactors, which are advantageous in scalability compared to spatial confinement.74
Biological Properties of 3D MSC Aggregates
Enhanced multilineage differentiation and secretion of trophic factors
The formation of 3D pellet has long been used to induce chondrogenic differentiation from MSCs,75,76 but recent studies have shown that MSC aggregation enhances the differentiation potential to other lineages as well (Table 2). Three-dimensional pellet culture of BM-MSC has been shown to mimic osteochondral bone formation with spatially segregated chondrogenesis and osteogeneis in the aggregates.77 Direct replating or after dissociation of BM-MSC and A-MSC aggregates showed increased differentiation efficiency toward adipogenic, osteogenic, and potentially epithelial-like or neuronal-like phenotype, suggesting that 3D aggregation could be used as a preconditioning strategy to enhance MSC function.36,71,73,78 It is commonly believed that the formation of a hypoxic core in the aggregate is responsible for the enhanced differentiation potential, although direct experimental evidence is lacking.
Table 2.
Aggregation Enhances MSC Differentiation Potential Compared to 2D Monolayer
| MSC source | Method to generate aggregates | Cell organization | Differentiation potential | Ref. |
|---|---|---|---|---|
| Osteogenic differentiation | ||||
| Human bone marrow | Pellet culture | Aggregates | Qualitative Alzarin red, collagen type I, and bone sialoprotein staining (no comparison with 2D) | 77 |
| Human bone marrow | Modified AggreWell™ | Aggregates | 2-fold increase in ALP, 2- to 5-fold increase in osteocalcin, osteopontin, ALP, and RUNX2, 14-fold increase collagen I | 89 |
| Human bone marrow | Low attachment plates | Aggregates | Two to eightfold increase in osteocalcin, osteopontin | 6 |
| Mouse bone marrow | AggreWell | Dissociated aggregates | Fourfold increase in Alzarin red staining | 35 |
| Rabbit bone marrow | 3D cell sheet | 3D cell sheet | Twofold increase in ALP, osteocalcin, osteopontin, collagen type I | 141 |
| Human adipose tissue | PEG micropatterned surface | Aggregates | Qualitative Alzarin red, fourfold increase in RUNX2 | 34 |
| Human adipose tissue | Chitosan membrane | Aggregates | Qualitative Alzarin red, two to threefold increase in RUNX2 | 71 |
| Rhesus monkey bone marrow | Hanging drop | Dissociated aggregates | 25% increase in ALP, twofold increase calcification and 20% increase in osteocalcin expression | 78 |
| Chondrogenic differentiation | ||||
| Rabbit and human bone marrow | Pellet culture | Aggregates | Collagen type II expression (no comparison with 2D) | 75,77 |
| Human synovial membrane | Hanging drop and pellet culture | Aggregates | 10%–20% increase in collagen type II, aggrecan, and SOX9 of hanging drop compared to pellet cultures | 22 |
| Adipogenic differentiation | ||||
| Mouse bone marrow | Forced aggregation | Dissociated aggregates | Threefold increase in Oil red O staining | 35 |
| Human bone marrow | Low attachment plates | Aggregates | Qualitative Oil red O staining, two to threefold increase in PPAR-γ, LPL, C/EBP-α, and aP2 | 6 |
| Human adipose tissue | PEG micropatterned surface | Aggregates | Qualitative Nile Red staining, increase PPAR-γ expression (14-fold), increased expression of LRP2, FABP4, LPL | 34 |
| Human adipose tissue | Nanoculture plates | Aggregates | Twofold increase in triglycerides accumulation | 73 |
| Human gingiva | Low attachment plates | Aggregates | Increased Oil Red O staining | 12 |
| Rhesus monkey adipose tissue | Hanging drop | Dissociated aggregates | 10%–15% increase in Oil red O and LPL staining | 78 |
ALP, alkaline phosphatase; RUNX2, Runt-related transcription factor 2; PPAR-γ, peroxisome proliferator-activated receptor gamma; LPL, lipoprotein lipase; C/EBP-α, CAAT/enhancer binding protein α; aP2, adipocyte protein 2; LRP2, low-density lipoprotein-related protein 2; FABP4, fatty acid binding protein 4; 3D, three-dimensional.
The enhanced secretion of anti-inflammatory, proangiogenic, and promitotic factors by aggregation has fueled strong interest in MSC aggregates because of MSC's therapeutic potential (e.g., in ischemic cerebral and cardiovascular injuries) (Table 3). For example, the increased secretion of anti-inflammatory cytokine stimulated gene/protein 6 (TSG-6) by BM-MSC aggregates was suggested as the major mechanism for the beneficial effects of MSCs in mice with myocardial infarcts.5 Further study has shown that MSCs in the aggregates are self-activated to increase prostaglandin E2 (PGE2) secretion, which in turn modulates macrophage responses.18 Interleukin-24 (IL-24) secretion was promoted upon cultivation of BM-MSCs as aggregates in spinner flasks, which significantly reduced the viability of cancer cells.6 BM-MSC, A-MSC, and UC-MSC aggregates also showed the elevated secretion of angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), CXCR4, MCP-3, RANTES, EGF, SDF-1, and angiogenin.12,37,38,59,79,80 Additionally, an increase in ECM expression such as collagen, fibronectin, and laminin was observed in BM-MSC and A-MSC aggregates compared to monolayers,38,48,79 which enhanced cell survival and therapeutic potential in wound healing and cardiac diseases (Table 4). The increased secretion of endogenous growth factors and ECM proteins may regulate the differentiation potentials of MSC aggregates. For instance, endogenous FGF-2 secretion promoted BM-MSC proliferation and osteogenic differentiation.81,82 The elevated secretion of collagen I in BM-MSC aggregates may be a contributing factor to the enhanced osteogenic differentiation, while the increased collagen III expression may promote adipogenesis.48,83,84
Table 3.
Aggregation Enhanced Secretion of Trophic Factors Compared to 2D Monolayer
| Type of secreted factors | MSC source | Method for aggregation | Trophic factor detected | Ref. |
|---|---|---|---|---|
| Anti-inflammatory | Human bone marrow | Hanging drop | 500- to 1000-fold increase in TSG-6, and 300-fold PGE2 expression | 5,18 |
| Human adipose tissue | Hanging drop | Fivefold increase in SDF-1 expression | 79 | |
| Tumor suppressor | Human bone marrow | Low attachment plates | 75-fold increase in IL-24 expression | 6 |
| Chemotactic receptor | Human bone marrow | Hanging drop | 35% increase in CXCR4 expression | 37 |
| Human gingiva | Low attachment plates | 35% increase in CXCR4 expression compared to 2D | 12 | |
| Proangiogenic | Human umbilical cord | Low attachment plates | Threefold increase in VEGF | 59 |
| Human gingiva | Low attachment plates | Three to fivefold increase in MCP-2, MCP-3, RANTES, M-CSF, EGF, VEGF, SDF-1, and angiogenin expression | 12 | |
| Human adipose tissue | Spinner flask | 5- to 10-fold increase in FGF-2, HGF, and VEGF expression compared to dissociated cells | 38 | |
| Human adipose tissue | Hanging drop | 23-fold increase in angiopoietin-like 2 expression | 79 |
TSG-6, TNF-stimulated gene 6 protein; PGE2, prostaglandin E2; IL-24, interleukin-24; CXCR4, C-X-C chemokine receptor type 4; VEGF, vascular endothelial growth factor; MCP-2, monocyte chemotactic protein 2; RANTES, regulated on activation, normal T cell expressed and secreted; M-CSF, macrophage colony-stimulating factor; EGF, epithelial growth factor; SDF-1, stromal cell-derived factor 1; FGF-2, fibroblast growth factor 2; HGF, hepatocyte growth factor.
Table 4.
Aggregation Enhanced ECM Secretion of MSCs Compared to 2D Monolayer
| Type of ECM molecule | MSC source | Method for aggregation | Fold of the increased expression | Ref. |
|---|---|---|---|---|
| Fibronectin and laminin | Rat bone marrow | Low attachment plates | Qualitative increase assessed by immunostaining at 0 h to day 2 | 48 |
| Fibronectin | Human adipose tissue | Chitosan membrane | 3.6-fold increase at day 7 | 71 |
| Fibronectin | Human adipose tissue | Spinner flask | 90% increase compared to trypsinized cells | 38 |
| Fibronectin | Human adipose tissue | Hanging drop | Twofold increase at day 6 | 79 |
| Laminin | Human adipose tissue | Chitosan membrane | 51-fold increase at day 7 | 71 |
| Laminin | Human adipose tissue | Hanging drop | Sixfold increase at day 6 | 79 |
| Glycoaminoglycans: biglycan and decorin | Human adipose tissue | Hanging drop | Respectively 21- and 1.3-fold increase at day 6 | 79 |
| Collagens | Human adipose tissue | Hanging drop | 2- to 80-fold increase at day 6 | 79 |
| Elastin | 1.6-fold increase at day 6 | |||
| Tenascin C | 13-fold increase at day 6 | |||
| Matrix remodeling proteins (MMPs, TIMPs) | Human adipose tissue | Hanging drop | 2- to 30-fold increase at day 6 | 79 |
MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinases; ECM, extracellular matrix.
Mechanisms of functional enhancement of 3D MSC aggregates
Compared to the adherent cultures, the formation of 3D aggregates leads to a multitude of changes in both MSC and their immediate microenvironment. Chief among these factors are changes in MSC morphology, mechanical stress, cell–cell and cell–ECM contacts, and the gradients of oxygen and nutrients. Although these factors in concert regulate MSC properties, studies have provided important insight into their respective roles in regulating MSC fate.
Effects of morphology and cytoskeleton organization
Compared to adherent MSCs on surface, MSCs in aggregates have drastic changes in morphology, adhesion structure, and cytoskeleton organization and tension, which are known to regulate MSC properties and contribute to functional enhancement.85 Morphologically, a flattened cell layer was formed at the surface of MSC aggregates, which spread efficiently with less distinct boundaries among cells.6,71 Conversely, MSCs at the interior of the aggregate displayed a more irregular and round morphology.6 Cell shape is known to regulate MSC fate and preferentially direct lineage-specific differentiation through reorganization of actin cytoskeleton modulated by Rho/ROCK pathways.86,87 Analysis of actin organization indicates a combined presence of stress fibers and disorganized actin in the BM-MSC aggregates,62 which are compatible with the enhanced osteogenic and adipogenic differentiation.85 Indeed, at the interior of BM-MSC aggregates, it was observed that cells exposed to less cortical tension more easily became adipocytes,88 whereas cells at the exterior of the aggregates were exposed to higher contractile forces and more prone to osteoblastic differentiation.88 Cell morphological changes are also closely coupled with chondrogenic differentiation in micromass culture, where chondrocyte-like cells congregated in the aggregate center.32 Additionally, sequential induction of MSC aggregates in chondrogenic and osteogenic media resulted in the formation of a chondro-osseous “organoid” with bony collar forming around a core of mineralizing cartilage.77
The increased ECM secretion and retention in the aggregates also promote the autocrine signaling, presumably due to the local growth factor enrichment via ECM binding. For example, the organization of MSC into 3D aggregates results in a 25-fold increase in bone morphogenetic protein (BMP)-2 expression compared to monolayer culture.89 Similarly, the aggregation of human synovial MSCs led to the increased BMP-2 expression and the enhanced chondrogenesis in vitro and in vivo.22 These results suggest that endogenous growth factors such as BMP-2 may have a more pronounced impact on MSC fate in an ECM-rich microenvironment of the 3D aggregates. Given the prominent role of endogenous growth factors, understanding their impact on 3D aggregates is an important area meriting future investigation.
Effects of cell–cell contacts, cadherins, and gap junctions
It has been demonstrated that cell–cell contacts provide additional signaling to improve adipogenic and osteogenic differentiation,67 through the increased gap junction or cadherin signaling.59,90 In chondrogenic differentiation, the widely used micromass culture induces an artificial condensation event and creates a cellular microenvironment that promotes the formation of cartilage tissue in response to a chondrogenic cytokine milieu.75 Additionally, cell–cell contact in the aggregates is required to activate Notch signaling to initiate chondrogenesis.91
Cadherins are a class of intracellular adhesion proteins that provide recognition signals in cell sorting and aggregation during tissue development. N-cadherin is expressed in the condensed mesenchyme; and blocking N-cadherin reduced chondrogenesis and perturbed limb development in vivo.92 In vitro, increased expressions of N-cadherin and P-selectin were observed in A-MSC and G-MSC aggregates generated on chitosan membrane, affecting the differentiation of MSCs into osteogenic, myogenic, and adipogenic cells.93,94 Studies have shown that the inhibition of N-cadherin impaired osteoblast differentiation, which can be compensated by cadherin-11.95 N-cadherin is shown to regulate MSC fate through sequestration of β-catenin and modulation of its cellular abundance.95,96 Upon destabilization of N-cadherin/β-catenin complexes, β-catenin is liberated and the canonical Wnt signaling is activated. Consequently, β-catenin translocated to the nucleus and acted as a transcription factor of osteogenic genes to regulate osteogenic signaling.96,97 Conversely, suppression of N-cadherin and cadherin-11 expressions attenuated osteogenesis and promoted adipogenic differentiation through the modulation of β-catenin signaling.93,98 N-cadherin is also required to initiate chondrogenesis of MSCs mediated through transforming growth factor β but needs to be cleaved at the later stage of chondrogenic differentiation.99 A switch from N-cadherin to OB-cadherin was observed to reduce chondrogenic differentiation but increase osteogenic differentiation.100 Besides regulating MSC differentiation, N-cadherin also modulates the secretion of proangiogenic factors. For instance, N-cadherin increased VEGF expression in CB-MSCs by the activation of extracellular signal-regulated kinases (ERKs) and enhancing cell–cell contacts.101 It is apparent that cadherin-mediated cell–cell adhesion plays a fundamental role in regulating MSC properties in the aggregates.
Connexin-43, a major gap junction protein, is upregulated upon cell–cell contact in 3D MSC aggregates and is required for osteogenic differentiation.102,103 Connexin-43 is also differentially regulated during adipogenic differentiation with increased expression during the early stage of adipogenesis followed by downregulation in maturation of adipocytes.104 The inhibition of connexin-43 during osteogenic differentiation of BM-MSCs leads to the enhanced adipogenesis, highlighting the competition between osteogenesis and adipogenesis.105 The enhanced connexin-43 expression was also observed during chondrogenic differentiation.106 These reports support the notation that cell–cell contacts and expression of adhesion molecules are important to regulate MSC differentiation potential in the 3D aggregates.
Effects of oxygen diffusion and biomechanical forces
The multicellular aggregate structure creates a heterogeneous microenvironment with size-dependent gradients of nutrients, oxygen, and cytokines.107 Among these factors, oxygen availability has been frequently suggested as a primary factor that influences MSC fate. G-MSC and A-MSC aggregate formation has been reported to induce a mild hypoxic environment, increasing the expression of hypoxia-inducible factor (HIF)-1α and HIF-2α.12,38 The mild hypoxia in the aggregates has been suggested as the primary mechanism for the upregulated secretion of proangiogenic factors, such as VEGF, FGF-2, HGF, and CXCR4.12,38 Secretion of PGE2, an anti-inflammatory cytokine, is also related to hypoxia, although the mechanism is yet to be determined.18 The hypoxic environment in the interior of aggregates is also a contributing factor for the increased ECM expression, including fibronectin, collagen I, vitronectin, and collagen IV.82 An increased protection against reactive oxygen species through the increased superoxide dismutase 2 expression was also observed in G-MSCs and A-MSC aggregates.12 Similarly, the protection against apoptosis was observed in MSC aggregates through the increased expression of Bcl-XL possibly due to the hypoxic environment.38 However, whether oxygen gradient in the aggregates is solely responsible or even sufficient to induce the observed alternation in MSC behaviors and secretome requires in-depth experimental and modeling analysis.107 Although oxygen tension is a primary factor that activates HIF transcription, glycolytic metabolites such as pyruvate and lactate can also activate and stabilize HIF under normoxic condition.108,109 Therefore, the molecular profiles of the 3D MSC aggregates remain to be fully characterized.
It is important to note that the aggregate fabrication methods may involve vastly different biomechanical forces, which influence the aggregate properties. For example, MSC self-assembly on nonadherent surface and in hanging drop culture rely on spontaneous cell–cell interactions, which may select a subpopulation of cells while shedding other cells and debris from the aggregates.5 In contrast, forced aggregation by centrifugation may subject cells to considerable mechanical forces, generating dissimilar microenvironment in the aggregates that significantly influence MSC fate. Although cell aggregation typically occurs during a short duration, the initial impact of mechanical forces may result in significant changes in cell properties and the trajectory of their developmental patterns.110 Thus, the role of mechanical forces in aggregate fabrication and their subsequent influence on cell biological and biomechanical properties need further investigation.
Possible signaling pathways
Analyses at molecular level have been attempted to elucidate the possible pathways activated in 3D MSC aggregates.5,6,79,100 For example, genes related to MSC adhesion, including ECM proteins, antioxidant activities, and molecular signaling, were upregulated.5 Especially, several Wnt genes and proteins, such as Wnt3a and Wnt5a, were found to be differentially upregulated in biomaterial-derived A-MSC aggregates.100 The Wnt3a-mediated canonical signaling and Wnt5a-mediated noncanonical signaling cross-talked with ERK1/2 or Smad2/3 pathways to regulate osteogenic or chondrogenic differentiations, respectively.100 Consistent with the reduced proliferation, a downregulation of cell cycle genes, actin binding proteins, and cytoskeleton organization was also observed.5,79 While these studies provide mechanistic insight, it is important to note that the regulatory mechanisms are multifactorial and likely involve multiple signaling pathways and their convergence.
Expansion of MSC Aggregates in Bioreactors
It is estimated that myocyte deficit in infarction-induced heart failure requires the order of 109 cells, but only 104–105 cells are typically generated from 2D cultures. In addition, the biomolecules released from MSCs are in the range of ∼10 ng based on clinical scale of MSC mass (∼108 cells), suggesting that high MSC doses are likely required for clinical applications.111,112 Production of MSC aggregates for clinical application requires bioreactor systems that provide homogenous physicochemical environment with a tight control of various parameters, such as oxygen tension, mechanical stresses, pH, and nutrient feeding.113–115
Long-term culture of MSC aggregates
The MSC aggregates are able to maintain high cell viability with enhanced functional properties during long-term culture, although their proliferation potential is rather limited.5 Aggregates containing 0.5–2×105 cells and a diameter of 100–300 μm had comparable viability to 2D monolayer for 17–30 days.34,35,50 Independent of the aggregation method, increased compactness has been reported in aggregates of A-MSC or BM-MSC over the prolonged culture.5,35,50,61,72 In long-term culture, the proliferation capacity of placental and A-MSC aggregates generated on chitosan membranes was reduced.71,72 The incorporation of hyaluronan in chitosan membrane increased the survival and proliferation ability presumably due to increased cell migration and matrix synthesis.72
It has been suggested that cell proliferation is inversely related to aggregate size due to diffusion limitations, and large aggregates have reduced proliferation potential compared to small ones.107,116 However, the MSC aggregates are heterogeneous in both molecular profiles and biomechanical microenvironments. Apart from biomechanical analysis at individual cell and aggregate levels, quantitative measurement and analysis of the spatial distribution of oxygen, nutrient, and regulatory macromolecules in the 3D aggregates are required to definitively determine the role of diffusion in the aggregates. This analysis is complicated by the adaptive cellular responses and accumulation of endogenous growth factors, which may significantly influence cellular metabolism and macromolecule distribution in the aggregates.5,117 While depletion of oxygen due to molecular diffusion is thought to induce the formation of a hypoxic zone inside the aggregate, the extent of oxygen depletion and its influence on cellular properties remain to be determined. MSCs are known to survive under near-anoxic condition and may reduce oxygen consumption in the aggregates, altering oxygen distribution.118 Additionally, MSCs are significant source of endogenous growth factors and the accumulation of these factors in the aggregates is a critical property but remains to be investigated. Thus, quantitative understanding of the temporal and spatial characteristics of MSC aggregates is required for in-depth study of their biological and functional properties.
Bioreactor systems for cultivating MSC aggregates
Several types of bioreactor systems have been shown to support MSC aggregates in suspension for long-term culture, including stirred tank bioreactors, rotating wall bioreactors, and perfusion bioreactors (Table 5). Although MSC aggregates have limited proliferation capacity, the dynamic bioreactor culture appears to enhance their biological function. The proliferation of MSC aggregates in both spinner flask and rotating wall bioreactor has been demonstrated to be comparable to 2D cultures, whereas cell size and surface antigen expression were altered and adipo-osteogenic differentiation of bioreactor-expanded MSC aggregates was enhanced.6 The secreted IL-24 was upregulated in bioreactors, indicating that the secretion profiles of MSCs were different from static cultures.6 Similarly, the secretion of VEGF, HGF, and FGF-2 was promoted in spinner flasks for A-MSCs.38 Applied microgravity into rotating wall vessel bioreactor enhanced the adipogenesis of BM-MSC aggregates.6 Rotating wall vessel and bioreactors delivering cyclic compression enhanced the chondrogenic differentiation of A-MSCs and BM-MSCs.119–121 The regulation of osteo-adipogenic differentiation of MSC aggregates in bioreactors may involve cytoskeletal remodeling and the modulation of RhoA/ROCK signaling.122,123 These preliminary studies suggest the potentials of bioreactor systems for MSC aggregate cultures.
Table 5.
Bioreactor Expansion of MSC Aggregates
| Type of bioreactor | MSC source | Culture parameters | Effect on proliferation | Effect on differentiation | Ref. |
|---|---|---|---|---|---|
| Spinner flask | Human bone marrow | 30 rpm Seeded at 2×104 cells/mL |
No effect compared to 2D | Improved osteogenesis after 14–21 days | 6 |
| Human adipose tissue | 70 rpm Seeded at 6×105 cells/mL |
ND | Enhance the secretion of VEGF, HGF, and FGF-2 | 38 | |
| Rotating wall vessel | Human bone marrow | 15 rpm Seeded at 2×104 cells/mL |
No effect compared to 2D | Improved adipogenesis after 14–21 days | 6 |
| Rabbit bone marrow | 11–25 rpm Seeded at 1–1.5×106 cells/mL |
ND | Improved chondrogenesis: threefold increase in aggregans; increase collagen type II, GAG secretion | 121 | |
| Human adipose tissue | 11–25 rpm Seeded at 106 cells/mL |
ND | Improved chondrogenesis after 21 days; 20- to 30-fold increase of SOX9 and aggregan compared to normal gravity | 120 | |
| Cyclic compression | Rabbit bone Marrow | 10% magnitude at a frequency of 1 Hz for 4 h; seeded at 3×107 cells/mL | No effect compared to unloaded controls | Improved chondrogenesis after 14 days; twofold increase in collagen type II compared to unloaded pellets | 119 |
GAG, glycosaminoglycan.
Physicochemical environment in the bioreactors
Different from static cultures, the cells in bioreactors are exposed to shear stress with the enhanced mass transfer, which were shown to modulate MSC proliferation and differentiation as well as the secretion of proangiogenic factors (i.e., VEGF).122,124,125 Shear stress in bioreactors has been shown to transduce mechanical signals into biochemical signals to modulate MSC differentiation. For example, mitogen-activated protein kinase signaling, including ERK1/2, p38, and c-Jun N-terminal kinase pathways, are influenced by shear stress through the cell surface signaling to activate the GTPase.113
Novel design of bioreactors that provide homogenous shear stress may be required in the formation and expansion of MSC aggregates. Spinner flasks displayed heterogeneous shear stress (0–2.83 dyn/cm2), fluid velocity, and Kolmogorov eddy size, which may limit their biological effects on MSCs.126 In perfusion chambers, oscillating fluid flow provided better mechanical signaling compared to the unidirectional counterpart.127,128 Nevertheless, such bioreactor requires cell adhesion to exogenous surface, whereas cell surface adhesion may change the properties of aggregates. Alternatively, bi-axial wavy-walled bioreactors provided modular and more homogeneous shear stress compared to the uniaxial counterpart, which could be a flexible and scalable system for MSC aggregates.129,130
Evidences were provided that the bioreactors enhanced oxygen and nutrient diffusion inside aggregates (100–300 μm) compared to static cultures.131,132 The dynamic culture conditions create different oxygen profiles in MSC aggregates, which may affect the cell metabolism and phenotype. Control of oxygen tension inside bioreactors has been shown to induce biological signaling to modulate MSC fate decision.133,134 Hypoxia (1% O2) was shown to reduce MSC senescence and reduce the expression of osteogenic genes via HIF-TWIST signaling.135,136 Similarly, hypoxia (2% O2) stabilized HIF-α, which acted as the transcription factor for SOX9 and promoted chondrogenic gene expression.137 Adipogenic differentiation was reduced under hypoxia through the activation of an endoplasmic reticulum stress protein.138 As observed in perfusion cultures, hypoxia also enhanced the secretion of proangiogenic factors.139 Thus, the control of oxygen diffusion is essential for mass scale production of functional MSC aggregates in bioreactors.
Application of MSC Aggregates in Preclinical Studies
Single-cell transplantation of MSCs has low retention and engraftment. Compared to single cells, transplantation of MSC aggregates offers the following advantages. (i) The upregulated secretion of trophic, antiapoptotic, and anti-inflammatory factors enhances MSC therapeutic efficacy5,80; (ii) the local enrichment of growth factors and ECM proteins provides a protective environment against the cytotoxicity at the injury site, improving cell survival and long-term engraftment; (iii) the cell–cell contacts and ECM proteins associated with aggregates improve cell adhesion and retention.140 Owing to these properties, MSC aggregates have demonstrated positive outcomes in bone and cartilage regeneration, wound healing, neoangiogenesis, and cardiac transplantation in various animal models (Table 6).
Table 6.
In Vivo Application of MSC Aggregates
| MSC source | Methods of aggregation | Transplanted tissues | In vivo effects | Ref. |
|---|---|---|---|---|
| Bone and cartilage regeneration | ||||
| Rabbit bone marrow | Fragment of 3D cell sheet | Subcutaneous site; Mandibule (fractured) | Twofold increase in bone formation compared to single-cell injection; | 141 |
| Rabbit bone marrow | 3D cell sheet | Subcutaneous dorsal pocket | New bone formation; enhanced compressive strength | 142 |
| Human bone marrow | Pellet culture | Skin | 24% increase in bone and 74% in marrow formation compared to controls | 143 |
| Human and rabbit synovium | Hanging drop | Left knee joint | Successful cartilage regeneration, the defect was fully filled with cartilage matrix | 22 |
| Rabbit articular cartilage and synovium | Low attachment dish with rotary shaker | Osteochondral defects in the legs | Effective restoration of articular cartilage; in vivo ECM production | 147 |
| Wound healing and neoangiogenesis | ||||
| Human gengiva | Low attachment dish | Tongue (oral mucositis) | Increased regeneration of damaged crypts, basal and epithelia layer, increased proliferation of mucosal epithelial cells compared to single cells | 12 |
| Human adipose tissue | Spinner flask | Ischemic limbs | Three to fivefold increase in local HGF, VEGF, and FGF-2; two to fivefold host cells expressing α-actin, ICAM-1, VCAM-1, and PECAM-1 compared to dissociated cells | 38 |
| Human umbilical cord | Hanging drop | Ischemic limbs | Increased number of microvessels and smooth muscle α-actin compared to dissociated cells | 148 |
| Human adipose tissue | Hanging drop | Diabetic wounds | A significant increase in the rate of wound closure compared to single cells due to ECM proteins and secreted soluble factors (e.g., HGF, MMP-2) | 79 |
| Cardiac tissue repair | ||||
| Human umbilical cord | Low attachment plate | Myocardium (infarcted) | Fourfold decrease in LV end-diastolic and systolic diameters, or LV fractional shortening compared to single cells | 59 |
| Rat bone marrows | Thermal responsive hydrogel | Intramyocardial injection | 23% greater LV fractional shortening when compared with the dissociated MSCs | 48 |
| Mice bone marrow | Thermal responsive hydrogel | Skeletal muscle | Cells entrapped into the muscular interstices; better retention of the transplanted cells | 33 |
| Human adipose tissue | Modified hanging drop | Intramyocardial injection | Improved cell retention, survival, and integration in a porcine model | 21 |
LV, left ventricular; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; PECAM-1, platelet endothelial cell adhesion molecule 1; MMP-2, matrix metalloproteinase 2.
MSC aggregates in bone and cartilage regeneration
Multicellular MSC aggregates have been used to generate bone and cartilage tissue. Implantation of intact BM-MSC aggregates in both heterotopic and orthotopic sites of the delayed healing model has shown better cell survival and enhanced bone healing via endochondral and intramembranous ossification compared to the dissociated BM-MSCs.141,142 Hypertrophic chondrocytes derived from BM-MSC aggregates implanted subcutaneously in mice provided a template for in situ endochondral ossification.143 Compared to dissociated cells, the preserved endogenous ECM proteins and cell–cell contacts promoted cell retention and survival and provided the microenvironment inductive to osteogenic differentiation. In addition, studies indicated that while in vitro spontaneous differentiation started from the center of MSC aggregates, the mineralization in the osteogenic medium began from the periphery to the interior of the aggregates in vivo.144 Moreover, the time for osteogenic priming was inversely related to the cell outgrowth from the aggregate.145,146 In vivo studies in rabbits showed that aggregates of MSCs could adhere promptly on the osteochondral defects by surface tension and stay without any loss, improving cartilage regeneration.22 Aggregate composites of chondrocytes and synovium-derived MSCs were injected to the osteochondral defects in a rabbit model, and effective restoration of articular cartilage and in vivo ECM production were observed.147 Thus, compared to dissociated cells, the MSC aggregates provide multiple cellular components that benefit tissue repair and regeneration.
MSC aggregates enhanced wound healing and neoangiogenesis
MSC aggregates enhanced wound healing and neoangiogenesis due to the secretion of angiogenesis factors and endogenous ECMs. G-MSC aggregates were shown to reverse chemotherapy-induced oral mucositis and epithelial layer damage of mucositic tongues compare to adherent MSCs.12 Following intravenously injection, G-MSCs displayed higher homing ability to the injury sites, due to the increased expression of CXCR4.12 In addition, improvement of ischemic limb salvage was shown following CB-MSC and A-MSC aggregate transplantation due to the enhanced vascularization compared to dissociated MSCs.38,148 A-MSC aggregates showed a significant increase in the rate of wound closure compared to single cells due to the significantly elevated ECM secretions (e.g., tenascin C, collagen VI, and fibronectin) and the secreted soluble factors (e.g., HGF, matrix metalloproteinase-2).79 Prevention of anoikis through the preservation of ECM proteins was proposed as a potential mechanism of the increased survival of A-MSC aggregates. Moreover, the enhanced expression of proangiogenic factors of MSC aggregates promoted von Willebrand factor expression and capillary density.38 Hence, methods to enhance MSC secretion of angiogenesis factors and ECMs should improve the therapeutic potential in wound healing and neoangiogenesis.
MSC aggregates enhanced cardiac transplantation
Cardiac functional improvement after myocardial infarction in rats was observed following UC-MSC aggregate transplantation.59 The reduced cardiac fibrosis and the increased capillary density were associated with the enhanced engraftment of UC-MSC aggregates compared to single cells, owing to the increased VEGF secretion by UC-MSCs in the aggregates.2,59 Additionally, direct transplantation of MSC aggregates in ischemic cardiac injury site may improve cell retention, survival, and engraftment due to the presence of endogenous ECM. Indeed, transplantation of MSC aggregates in skeletal muscle provided adequate physical size to entrap the cells into the muscular interstices and offered a favorable ECM environment to enhance the retention of the transplanted cells.33 Transplantation of MSC aggregates with endogenous ECMs in a rat model also increased vascular density and improved cardiac function.48 The echocardiography and catheterization measurements showed a superior heart function for MSC aggregates compared to the dissociated single cells.48 A transcatheter-based electromechanical mapping-guided intramyocardial transplantation of A-MSC aggregates in the porcine heart showed the improved survival and tissue integration, whereas single-cell injection displayed low level and random distribution.20,21 Thus, in vitro preconditioning MSCs in aggregates may be an effective strategy to enhance cell survival and the secretion of trophic factors in vivo for myocardial disease therapy.
Conclusions and Perspectives
MSC 3D aggregates have many attractive properties, but understanding the mechanism of MSC aggregate formation and the structure–function relation is crucial to further improve their prospects in clinical applications. The cellular and physicochemical characteristics of the 3D aggregate microenvironment need to be elucidated using both modeling and experimental approach to better define MSC aggregates' structure–function relations and to guide their clinical applications. In addition to cell adhesion molecules, the effects of cortical tension of individual cells and mechanical forces employed during aggregate fabrication on MSC biological properties need to be delineated. Scalable aggregate fabrication and expansion methods that allow a tight control of aggregate size in long-term culture are important to sustain the viability and functionality of MSC aggregates. Advances in these areas are expected to significantly accelerate the translation of MSC therapy to clinical applications.
Acknowledgments
This work is supported by the James King Biomedical Research Program (3KF05 and 4KB09) and the American Heart Association Grant-in-Aid (10GRNT3860040) to T.M.
Disclosure Statement
The authors have no conflicts of interest.
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Ranganath S.H., Levy O., Inamdar M.S., and Karp J.M.Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10,244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira F.G., Carvalho M.M., Sousa N., and Salgado A.J.Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci 70,3871, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uccelli A., Benvenuto F., Laroni A., and Giunti D.Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol 24,59, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Bartosh T.J., Ylostalo J.H., Mohammadipoor A., Bazhanov N., Coble K., Claypool K., Lee R.H., Choi H., and Prockop D.J.Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 107,13724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frith J.E., Thomson B., and Genever P.G.Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods 16,735, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., and Frolova G.P.Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6,230, 1968 [PubMed] [Google Scholar]
- 8.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., and Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 9.da Silva Meirelles L., Chagastelles P.C., and Nardi N.B.Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119(),2204, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Pozzobon M., Piccoli M., and De Coppi P.Sources of mesenchymal stem cells: current and future clinical use. Adv Biochem Eng Biotechnol 2012. DOI: 10.1007/10_2012_161 [DOI] [PubMed] [Google Scholar]
- 11.Sart S., Schneider Y.J., and Agathos S.N.Ear mesenchymal stem cells: an efficient adult multipotent cell population fit for rapid and scalable expansion. J Biotechnol 139,291, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q., Nguyen A.L., Shi S., Hill C., Wilder-Smith P., Krasieva T.B., and Le A.D.Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev 21,937, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J., and Wang C.Y.The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19,35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco P., Robey P.G., and Simmons P.J.Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2,313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caplan A.I.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213,341, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Galipeau J.The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15,2, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd Z.E., Kloster A., Di Halvorsen Y., Storms R.W., Goh B., Kilroy G., Wu X., and Gimble J.M.Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24,376, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Ylostalo J.H., Bartosh T.J., Coble K., and Prockop D.J.Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30,2283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng N.C., Chen S.Y., Li J.R., and Young T.H.Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med 2,584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmert M.Y., Wolint P., Wickboldt N., Gemayel G., Weber B., Brokopp C.E., Boni A., Falk V., Bosman A., Jaconi M.E., and Hoerstrup S.P.Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 34,6339, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Emmert M.Y., Wolint P., Winklhofer S., Stolzmann P., Cesarovic N., Fleischmann T., Nguyen T.D., Frauenfelder T., Boni R., Scherman J., Bettex D., Grunenfelder J., Schwartlander R., Vogel V., Gyongyosi M., Alkadhi H., Falk V., and Hoerstrup S.P.Transcatheter based electromechanical mapping guided intramyocardial transplantation and in vivo tracking of human stem cell based three dimensional microtissues in the porcine heart. Biomaterials 34,2428, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S., Muneta T., Tsuji K., Ichinose S., Makino H., Umezawa A., and Sekiya I.Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res Ther 14,R136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katagiri H., Muneta T., Tsuji K., Horie M., Koga H., Ozeki N., Kobayashi E., and Sekiya I.Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem Biophys Res Commun 435,603, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Lin R.Z., and Chang H.Y.Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 3,1172, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Moscona A., and Moscona H.The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat 86,287, 1952 [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosawa H.Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng 103,389, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Rodriguez D., Guevorkian K., Douezan S., and Brochard-Wyart F.Soft matter models of developing tissues and tumors. Science 338,910, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Huh D., Hamilton G.A., and Ingber D.E.From 3D cell culture to organs-on-chips. Trends Cell Biol 21,745, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastrana E., Silva-Vargas V., and Doetsch F.Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8,486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Su P., Xu C., Yang J., Yu W., and Huang D.Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett 32,1339, 2010 [DOI] [PubMed] [Google Scholar]
- 31.DeLise A.M., Fischer L., and Tuan R.S.Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8,309, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Ichinose S., Tagami M., Muneta T., and Sekiya I.Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res 322,217, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Lee W.Y., Chang Y.H., Yeh Y.C., Chen C.H., Lin K.M., Huang C.C., Chang Y., and Sung H.W.The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials 30,5505, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Itaka K., Ohba S., Nishiyama N., Chung U.I., Yamasaki Y., and Kataoka K.3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 30,2705, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Baraniak P.R., and McDevitt T.C.Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res 347,701, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langenbach F., Berr K., Naujoks C., Hassel A., Hentschel M., Depprich R., Kubler N.R., Meyer U., Wiesmann H.P., Kogler G., and Handschel J.Generation and differentiation of microtissues from multipotent precursor cells for use in tissue engineering. Nat Protoc 6,1726, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Potapova I.A., Brink P.R., Cohen I.S., and Doronin S.V.Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem 283,13100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhang S.H., Cho S.W., La W.G., Lee T.J., Yang H.S., Sun A.Y., Baek S.H., Rhie J.W., and Kim B.S.Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32,2734, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., and de Boer J.Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31,108, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Elbert D.L.Bottom-up tissue engineering. Curr Opin Biotechnol 22,674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean D.M., Napolitano A.P., Youssef J., and Morgan J.R.Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J 21,4005, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Achilli T.M., Meyer J., and Morgan J.R.Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 12,1347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foty R.A., and Steinberg M.S.The differential adhesion hypothesis: a direct evaluation. Dev Biol 278,255, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Steinberg M.S.On the mechanism of tissue reconstruction by dissociated cells, Iii. free energy relations and the reorganization of fused, heteronomic tissue fragments. Proc Natl Acad Sci USA 48,1769, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kronenberg H.M.Developmental regulation of the growth plate. Nature 423,332, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Hall B.K., and Miyake T.All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22,138, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Ghone N.V., and Grayson W.L.Recapitulation of mesenchymal condensation enhances in vitro chondrogenesis of human mesenchymal stem cells. J Cell Physiol 227,3701, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Wang C.C., Chen C.H., Hwang S.M., Lin W.W., Huang C.H., Lee W.Y., Chang Y., and Sung H.W.Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells 27,724, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kim J., and Ma T.Endogenous extracellular matrices enhance human mesenchymal stem cell aggregate formation and survival. Biotechnol Prog 29,441, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Hildebrandt C., Buth H., and Thielecke H.A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell 43,91, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Steinberg M.S.On the mechanism of tissue reconstruction by dissociated cells. I. Population kinetics, differential adhesiveness, and the absence of directed migration. Proc Natl Acad Sci USA 48,1577, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brodland G.W.The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng 124,188, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Amack J.D., and Manning M.L.Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338,212, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Krieg M., Arboleda-Estudillo Y., Puech P.H., Kafer J., Graner F., Muller D.J., and Heisenberg C.P.Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol 10,429, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Nesti L.J., Caterson E.J., Wang M., Chang R., Chapovsky F., Hoek J.B., and Tuan R.S.TGF-beta1-stimulated osteoblasts require intracellular calcium signaling for enhanced alpha5 integrin expression. Ann N Y Acad Sci 961,178, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Lin R.Z., Chou L.F., Chien C.C., and Chang H.Y.Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res 324,411, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ivascu A., and Kubbies M.Diversity of cell-mediated adhesions in breast cancer spheroids. Int J Oncol 31,1403, 2007 [PubMed] [Google Scholar]
- 58.Wuchter P., Boda-Heggemann J., Straub B.K., Grund C., Kuhn C., Krause U., Seckinger A., Peitsch W.K., Spring H., Ho A.D., and Franke W.W.Processus and recessus adhaerentes: giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res 328,499, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Lee E.J., Park S.J., Kang S.K., Kim G.H., Kang H.J., Lee S.W., Jeon H.B., and Kim H.S.Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther 20,1424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dromard C., Bourin P., Andre M., De Barros S., Casteilla L., and Planat-Benard V.Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp Cell Res 317,770, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Kapur S.K., Wang X., Shang H., Yun S., Li X., Feng G., Khurgel M., and Katz A.J.Human adipose stem cells maintain proliferative, synthetic and multipotential properties when suspension cultured as self-assembling spheroids. Biofabrication 4,025004, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baraniak P.R., Cooke M.T., Saeed R., Kinney M.A., Fridley K.M., and McDevitt T.C.Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J Mech Behav Biomed Mater 11,63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caicedo-Carvajal C.E., Shinbrot T., and Foty R.A.Alpha5beta1 integrin-fibronectin interactions specify liquid to solid phase transition of 3D cellular aggregates. PLoS One 5,e11830, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Nemir S., and West J.L.Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng 38,2, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Sun Y., Chen C.S., and Fu J.Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys 41,519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng R., Yao X., Cao B., Tang J., and Ding J.The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials 33,6008, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Kilian K.A., Bugarija B., Lahn B.T., and Mrksich M.Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA 107,4872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leight J.L., Liu W.F., Chaturvedi R.R., Chen S., Yang M.T., Raghavan S., and Chen C.S.Manipulation of 3D cluster size and geometry by release from 2D micropatterns. Cell Mol Bioeng 5,299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ungrin M.D., Joshi C., Nica A., Bauwens C., and Zandstra P.W.Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One 3,e1565, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng N.C., Wang S., and Young T.H.The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 33,1748, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Huang G.S., Dai L.G., Yen B.L., and Hsu S.H.Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 32,6929, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Miyagawa Y., Okita H., Hiroyama M., Sakamoto R., Kobayashi M., Nakajima H., Katagiri Y.U., Fujimoto J., Hata J., Umezawa A., and Kiyokawa N.A microfabricated scaffold induces the spheroid formation of human bone marrow-derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation. Tissue Eng Part A 17,513, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Gigout A., Buschmann M.D., and Jolicoeur M.Chondrocytes cultured in stirred suspension with serum-free medium containing pluronic-68 aggregate and proliferate while maintaining their differentiated phenotype. Tissue Eng Part A 15,2237, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U.In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238,265, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Winter A., Breit S., Parsch D., Benz K., Steck E., Hauner H., Weber R.M., Ewerbeck V., and Richter W.Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 48,418, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Muraglia A., Corsi A., Riminucci M., Mastrogiacomo M., Cancedda R., Bianco P., and Quarto R.Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci 116(),2949, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Jing Y., and Jian-Xiong Y.3-D spheroid culture of bone marrow mesenchymal stem cell of rhesus monkey with improved multi-differentiation potential to epithelial progenitors and neuron in vitro. Clin Exp Ophthalmol 39,808, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Amos P.J., Kapur S.K., Stapor P.C., Shang H., Bekiranov S., Khurgel M., Rodeheaver G.T., Peirce S.M., and Katz A.J.Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 16,1595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Francesco F., Tirino V., Desiderio V., Ferraro G., D'Andrea F., Giuliano M., Libondi G., Pirozzi G., De Rosa A., and Papaccio G.Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One 4,e6537, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J., and Ma T.Regulation of autocrine fibroblast growth factor-2 signaling by perfusion flow in 3D human mesenchymal stem cell constructs. Biotechnol Prog 28,1384, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Kim J., and Ma T.Autocrine fibroblast growth factor 2-mediated interactions between human mesenchymal stem cells and the extracellular matrix under varying oxygen tension. J Cell Biochem 114,716, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Mathews S., Bhonde R., Gupta P.K., and Totey S.Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation 84,185, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Chiellini C., Cochet O., Negroni L., Samson M., Poggi M., Ailhaud G., Alessi M.C., Dani C., and Amri E.Z.Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol 9,26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S.Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6,483, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Gao L., McBeath R., and Chen C.S.Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28,564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y.K., Yu X., Cohen D.M., Wozniak M.A., Yang M.T., Gao L., Eyckmans J., and Chen C.S.Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev 21,1176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruiz S.A., and Chen C.S.Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26,2921, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kabiri M., Kul B., Lott W.B., Futrega K., Ghanavi P., Upton Z., and Doran M.R.3D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochem Biophys Res Commun 419,142, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Tang J., Peng R., and Ding J.The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials 31,2470, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Oldershaw R.A., and Hardingham T.E.Notch signaling during chondrogenesis of human bone marrow stem cells. Bone 46,286, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Oberlender S.A., and Tuan R.S.Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120,177, 1994 [DOI] [PubMed] [Google Scholar]
- 93.Shin C.S., Lecanda F., Sheikh S., Weitzmann L., Cheng S.L., and Civitelli R.Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem 78,566, 2000 [PubMed] [Google Scholar]
- 94.Yeh H.Y., Liu B.H., and Hsu S.H.The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials 33,8943, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Mbalaviele G., Shin C.S., and Civitelli R.Cell-cell adhesion and signaling through cadherins: connecting bone cells in their microenvironment. J Bone Miner Res 21,1821, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Zhong Z., and Williams B.O.Integration of cellular adhesion and Wnt signaling: Interactions between N-cadherin and LRP5 and their role in regulating bone mass. J Bone Miner Res 27,1849, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hay E., Buczkowski T., Marty C., Da Nascimento S., Sonnet P., and Marie P.J.Peptide-based mediated disruption of N-cadherin-LRP5/6 interaction promotes Wnt signaling and bone formation. J Bone Miner Res 27,1852, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Di Benedetto A., Watkins M., Grimston S., Salazar V., Donsante C., Mbalaviele G., Radice G.L., and Civitelli R.N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. J Cell Sci 123(),2640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tuli R., Tuli S., Nandi S., Huang X., Manner P.A., Hozack W.J., Danielson K.G., Hall D.J., and Tuan R.S.Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278,41227, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Hsu S.H., and Huang G.S.Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials 34,4725, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Lee E.J., Choi E.K., Kang S.K., Kim G.H., Park J.Y., Kang H.J., Lee S.W., Kim K.H., Kwon J.S., Lee K.H., Ahn Y., Lee H.J., Cho H.J., Choi S.J., Oh W.I., Park Y.B., and Kim H.S.N-cadherin determines individual variations in the therapeutic efficacy of human umbilical cord blood-derived mesenchymal stem cells in a rat model of myocardial infarction. Mol Ther 20,155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang J.C., Hsu S.H., and Su H.L.The regulation of the gap junction of human mesenchymal stem cells through the internalization of quantum dots. Biomaterials 30,1937, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Kamijo M., Haraguchi T., Tonogi M., and Yamane G.Y.The function of connexin 43 on the differentiation of rat bone marrow cells in culture. Biomed Res 27,289, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Yeganeh A., Stelmack G.L., Fandrich R.R., Halayko A.J., Kardami E., and Zahradka P.Connexin 43 phosphorylation and degradation are required for adipogenesis. Biochim Biophys Acta 1823,1731, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Schiller P.C., D'Ippolito G., Brambilla R., Roos B.A., and Howard G.A.Inhibition of gap-junctional communication induces the trans-differentiation of osteoblasts to an adipocytic phenotype in vitro. J Biol Chem 276,14133, 2001 [DOI] [PubMed] [Google Scholar]
- 106.Loty S., Foll C., Forest N., and Sautier J.M.Association of enhanced expression of gap junctions with in vitro chondrogenic differentiation of rat nasal septal cartilage-released cells following their dedifferentiation and redifferentiation. Arch Oral Biol 45,843, 2000 [DOI] [PubMed] [Google Scholar]
- 107.Van Winkle A.P., Gates I.D., and Kallos M.S.Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs 196,34, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Semenza G.L.HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20,51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palomaki S., Pietila M., Laitinen S., Pesala J., Sormunen R., Lehenkari P., and Koivunen P.HIF-1alpha is upregulated in human mesenchymal stem cells. Stem Cells 31,1902, 2013 [DOI] [PubMed] [Google Scholar]
- 110.Bartosh T.J., Ylostalo J.H., Bazhanov N., Kuhlman J., and Prockop D.J.Dynamic compaction of human mesenchymal stem/precursor cells (MSC) into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6 and STC1). Stem Cells 2013. DOI: 10.1002/stem.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sensebe L., Bourin P., and Tarte K.Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther 22,19, 2011 [DOI] [PubMed] [Google Scholar]
- 112.Wagner J., Kean T., Young R., Dennis J.E., and Caplan A.I.Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol 20,531, 2009 [DOI] [PubMed] [Google Scholar]
- 113.Yeatts A.B., Choquette D.T., and Fisher J.P.Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 1830,2470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dos Santos F.F., Andrade P.Z., da Silva C.L., and Cabral J.M.Bioreactor design for clinical-grade expansion of stem cells. Biotechnol J 8,644, 2013 [DOI] [PubMed] [Google Scholar]
- 115.Rodrigues C.A., Fernandes T.G., Diogo M.M., da Silva C.L., and Cabral J.M.Stem cell cultivation in bioreactors. Biotechnol Adv 29,815, 2011 [DOI] [PubMed] [Google Scholar]
- 116.Sart S., Ma T., and Li Y.Cryopreservation of pluripotent stem cell aggregates in defined protein-free formulation. Biotechnol Prog 29,143, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Mylotte L.A., Duffy A.M., Murphy M., O'Brien T., Samali A., Barry F., and Szegezdi E.Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells 26,1325, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Deschepper M., Manassero M., Oudina K., Paquet J., Monfoulet L.E., Bensidhoum M., Logeart-Avramoglou D., and Petite H.Proangiogenic and prosurvival functions of glucose in human mesenchymal stem cells upon transplantation. Stem Cells 31,526, 2013 [DOI] [PubMed] [Google Scholar]
- 119.Huang C.Y., Hagar K.L., Frost L.E., Sun Y., and Cheung H.S.Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22,313, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Yu B., Yu D., Cao L., Zhao X., Long T., Liu G., Tang T., and Zhu Z.Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway. Biochem Biophys Res Commun 414,412, 2011 [DOI] [PubMed] [Google Scholar]
- 121.Ohyabu Y., Kida N., Kojima H., Taguchi T., Tanaka J., and Uemura T.Cartilaginous tissue formation from bone marrow cells using rotating wall vessel (RWV) bioreactor. Biotechnol Bioeng 95,1003, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Meyers V.E., Zayzafoon M., Douglas J.T., and McDonald J.M.RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 20,1858, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arnsdorf E.J., Tummala P., Kwon R.Y., and Jacobs C.R.Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 122(),546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li D., Tang T., Lu J., and Dai K.Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng Part A 15,2773, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Kim J., and Ma T.Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol Bioeng 109,252, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Sucosky P., Osorio D.F., Brown J.B., and Neitzel G.P.Fluid mechanics of a spinner-flask bioreactor. Biotechnol Bioeng 85,34, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Liu L., Yu B., Chen J., Tang Z., Zong C., Shen D., Zheng Q., Tong X., Gao C., and Wang J.Different effects of intermittent and continuous fluid shear stresses on osteogenic differentiation of human mesenchymal stem cells. Biomech Model Mechanobiol 11,391, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Salvi J.D., Lim J.Y., and Donahue H.J.Finite element analyses of fluid flow conditions in cell culture. Tissue Eng Part C Methods 16,661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hutmacher D.W., and Singh H.Computational fluid dynamics for improved bioreactor design and 3D culture. Trends Biotechnol 26,166, 2008 [DOI] [PubMed] [Google Scholar]
- 130.Zhang Z.Y., Teoh S.H., Teo E.Y., Khoon Chong M.S., Shin C.W., Tien F.T., Choolani M.A., and Chan J.K.A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 31,8684, 2010 [DOI] [PubMed] [Google Scholar]
- 131.Mueller-Klieser W.F., and Sutherland R.M.Influence of convection in the growth medium on oxygen tensions in multicellular tumor spheroids. Cancer Res 42,237, 1982 [PubMed] [Google Scholar]
- 132.Kinney M.A., Sargent C.Y., and McDevitt T.C.The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng Part B Rev 17,249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lovett M., Rockwood D., Baryshyan A., and Kaplan D.L.Simple modular bioreactors for tissue engineering: a system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng Part C Methods 16,1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grayson W.L., Zhao F., Izadpanah R., Bunnell B., and Ma T.Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 207,331, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Tsai C.C., Chen Y.J., Yew T.L., Chen L.L., Wang J.Y., Chiu C.H., and Hung S.C.Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 117,459, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Yang D.C., Yang M.H., Tsai C.C., Huang T.F., Chen Y.H., and Hung S.C.Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One 6,e23965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robins J.C., Akeno N., Mukherjee A., Dalal R.R., Aronow B.J., Koopman P., and Clemens T.L.Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 37,313, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Tamama K., Kawasaki H., Kerpedjieva S.S., Guan J., Ganju R.K., and Sen C.K.Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem 112,804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Potier E., Ferreira E., Andriamanalijaona R., Pujol J.P., Oudina K., Logeart-Avramoglou D., and Petite H.Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 40,1078, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Kelm J.M., and Fussenegger M.Scaffold-free cell delivery for use in regenerative medicine. Adv Drug Deliv Rev 62,753, 2010 [DOI] [PubMed] [Google Scholar]
- 141.Ma D., Zhong C., Yao H., Liu Y., Chen F., Li J., Zhao J., Mao T., and Ren L.Engineering injectable bone using bone marrow stromal cell aggregates. Stem Cells Dev 20,989, 2011 [DOI] [PubMed] [Google Scholar]
- 142.Ma D., Ren L., Liu Y., Chen F., Zhang J., Xue Z., and Mao T.Engineering scaffold-free bone tissue using bone marrow stromal cell sheets. J Orthop Res 28,697, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Farrell E., Both S.K., Odorfer K.I., Koevoet W., Kops N., O'Brien F.J., Baatenburg de Jong R.J., Verhaar J.A., Cuijpers V., Jansen J., Erben R.G., and van Osch G.J.In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 12,31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Langenbach F., Naujoks C., Laser A., Kelz M., Kersten-Thiele P., Berr K., Depprich R., Kubler N., Kogler G., and Handschel J.Improvement of the cell-loading efficiency of biomaterials by inoculation with stem cell-based microspheres, in osteogenesis. J Biomater Appl 26,549, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Langenbach F., Naujoks C., Kersten-Thiele P.V., Berr K., Depprich R.A., Kubler N.R., Kogler G., and Handschel J.Osteogenic differentiation influences stem cell migration out of scaffold-free microspheres. Tissue Eng Part A 16,759, 2010 [DOI] [PubMed] [Google Scholar]
- 146.Ferrera D., Poggi S., Biassoni C., Dickson G.R., Astigiano S., Barbieri O., Favre A., Franzi A.T., Strangio A., Federici A., and Manduca P.Three-dimensional cultures of normal human osteoblasts: proliferation and differentiation potential in vitro and upon ectopic implantation in nude mice. Bone 30,718, 2002 [DOI] [PubMed] [Google Scholar]
- 147.Lee J.I., Sato M., Kim H.W., and Mochida J.Transplantatation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. Eur Cell Mater 22,275, 2011; discussion 290 [DOI] [PubMed] [Google Scholar]
- 148.Bhang S.H., Lee S., Shin J.Y., Lee T.J., and Kim B.S.Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A 18,2138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]