Abstract
Background
Overgrowth of calcium oxalate on Randall's plaque is a mechanism of stone formation among idiopathic calcium oxalate stone-formers (ICSFs). It is less clear how stones form when there is little or no plaque.
Design, setting, participants, & measurements
Participants were a consecutive cohort of ICSFs who underwent percutaneous nephroscopic papillary mapping in the kidney or kidneys containing symptomatic stones and a papillary tip biopsy from a representative calyx during a stone removal procedure between 2009 and 2013. The distribution of Randall's plaque coverage was analyzed and used to divide ICSFs into those with a high (≥5%; mean, 10.5%; n=10) versus low (<5%; mean, 1.5%; n=32) amount of plaque coverage per papilla. Demographic and laboratory features were compared between these two groups.
Results
Low-plaque stone formers tended to be obese (50% versus 10%; P=0.03) and have a history of urinary tract infection (34% versus 0%; P=0.04). They were less likely to have multiple prior stone events (22% versus 80%; P=0.002) and had a lower mean 24-hour urine calcium excretion (187±86 mg versus 291±99 mg; P<0.01). Morphologically, stones from patients with low amounts of plaque lacked a calcium phosphate core by microcomputed tomography. Papillary biopsies from low plaque stone-formers revealed less interstitial and basement membrane punctate crystallization.
Conclusions
These findings suggest that other pathways independent of Randall's plaque may contribute to stone pathogenesis among a subgroup of ICSFs who harbor low amounts of plaque.
Keywords: kidney stones, hypercalciuria, obesity
Introduction
The incidence of kidney stones appears to have increased over recent decades so that 10% or more of Americans now experience this painful condition (1). Nevertheless, how stones form and grow within the kidney remains unclear. Idiopathic calcium oxalate (CaOx) stone-formers (ICSFs) constitute a majority of affected individuals, and overgrowth of CaOx upon renal papillary Randall's plaque has been proposed as a potential mechanism of stone formation in this group (2–5). Randall's plaque is composed of calcium phosphate (CaP) and appears to originate within basement membranes of the thin loops of Henle (6). Previous studies have suggested that plaque is common in the kidneys of ICSFs, being found in 91% of individual papillae (7).
Plaque, however, is not unique to stone-formers and is often present in the kidneys of non–stone formers (8). Furthermore, some ICSFs with small amounts of plaque can have sufficient stone burdens to require percutaneous nephrolithotomy (PCNL) (9). These observations led us to hypothesize that mechanisms independent of Randall's plaque can contribute to stone formation and growth, particularly among ICSFs who harbor low amounts of plaque.
Materials and Methods
Study Population
The Mayo Institutional Review Board approved this study. After informed consent, nonpregnant stoneformers >18 years of age undergoing PCNL for urolithiasis were prospectively enrolled. The patients completed a structured questionnaire, and all medical records were reviewed to obtain risk factors for kidney stone formation. A consecutive cohort of 137 stone-formers was enrolled before the procedure between 2009 and 2013. In the current study, analysis was limited to 42 ICSFs who had at least one stone composed of >50% calcium oxalate without any uric acid, struvite, or cystine and were without the systemic disorders of primary hyperparathyroidism, sarcoidosis, vitamin D excess, hyperthyroidism, or renal tubular acidosis (10–13). The other 95 stone formers were excluded because their major stone components were uric acid, struvite, or CaP. Twenty-four-hour urine studies were obtained with patients receiving a random diet. The non–stone-forming controls consisted of 10 patients with a diagnosis of upper tract urothelial carcinoma and no history of nephrolithiasis who underwent papillary plaque mapping at the time of surgery but did not complete 24-hour urine studies.
Percutaneous access was placed in an upper- or lower-pole posterior renal calyx to maximize stone removal. One fragment of the removed stone was sent for culture using a sterile no-touch technique, another for composition by infrared spectroscopy, and the remainder for composition and morphology by microcomputed tomography (mCT). All mCT images were reviewed by an experienced observer (J.C.W.) blinded to the endoscopic and clinical findings (14). After all stone material was removed, the internal papillary structures of the kidney were systematically evaluated and videotaped using a flexible digital nephroscope. The location of each papilla was determined using fluoroscopy with instillation of contrast material through the nephroscope. Once the entire kidney was mapped, a papillary-tip biopsy specimen was taken endoscopically from a representative calyx containing plaque or plug (when present). Only the kidney containing the symptomatic stone was mapped and biopsied, not the asymptomatic contralateral kidney. The site of biopsy was chosen to be a representative sample and to confirm endoscopic findings but did not necessarily correlate exactly with stone location. While controls underwent nephroscopic papillary mapping, most did not have biopsy.
Image Analysis
Representative still images of each papilla were captured from the video footage. A single urologist who performed the surgery (A.E.K.) assisted by an image-processing technician reviewed all videos and images to determine the location and distribution of the papillae, plaque, and plugs. As described previously (11), the area of the papillae, individual plaques, and plugs, were outlined using video-editing software to calculate the percentage surface area of plaque and plug.
Urine Chemistries
Twenty-four-hour urine collections obtained within 6 months of the PCNL date were analyzed. All were obtained before surgery or a minimum of 8 weeks afterward. A minority of patients were receiving medications at the time of urine studies, including thiazides (n=3) and citrate (n=3). All urine measurements were performed in the Mayo Clinic Renal Testing Laboratory (Rochester, MN). EQUIL2 was used to calculate urinary supersaturations (15). Data from inappropriate collections (>20% discrepancy of measured versus expected creatinine excretion) were not used.
CaOx Crystal Growth Inhibition Assay
As described previously (11), an aliquot from the 24-hour urine collection was spun at 3600 rpm, 4°C, for 10 minutes to prepare a total urinary protein isolate. The supernatant was concentrated with a Vivaspin 6 concentrator (5000 MW cutoff; Sartorius, Goettingen, Germany), then rinsed three times with PBS (6 ml) containing Roche Complete protease inhibitor (1 tablet per 1000 ml) in the same Vivaspin concentrator. The protein concentration in 200 µl of the resulting filtrate was measured using a bicinchoninic acid assay kit (Pierce, Rockford, IL), after which PBS was added to achieve a final concentration of 1 mg/ml.
CaOx monohydrate (COM) crystal–growth inhibition was measured using the seeded crystal-growth assay of Nakagawa and colleagues (16). This assay quantitates the ability of total urinary protein to inhibit deposition of soluble [14C]oxalate upon a COM crystal slurry, with results expressed as a percentage of a blank sample (no protein added).
Kidney Stone Culture
Stone specimens submitted for culture and antimicrobial susceptibilities in the Mayo Microbiology Laboratory were crushed using a disposable sterile tissue grinder (Covidien Precision, REF 3500SA) with 0.5 ml of sterile saline solution. The homogenized specimen was inoculated into thioglycolate broth and onto trypticase soy agar with 5% sheep blood and eosin methylene blue agar plates.
Histology
Papillary-tip biopsy specimens were fixed in 10% formalin overnight, dehydrated through graded ethanol, and paraffin embedded. Serial sections were cut at 5 µm and placed on charged slides. One slide was prepared using hematoxylin and eosin–staining and the adjacent one was stained for calcifications using the method of Yasue (17). Slides were reviewed by a board-certified renal pathologist (L.H.H.) who was blinded to all patients’ clinical and laboratory characteristics. The number of tubules with Yasue-stained deposits within lumens or adjacent basement membrane and/or interstitium was quantitated as a percentage of the total number of distal tubules present on adjacent hematoxylin and eosin–stained sections. Each parameter was scored semiquantitatively as the percentage of tubules that were Yasue-positive using the scale of mild (≤25%), moderate (26%–50%), and severe (>50%).
Statistical Analyses
The distribution of papillary plaque coverage in ICSFs compared with controls was used to determine a threshold for defining the high- and low-plaque groups. All urinary biochemistries were expressed as mean±SD or median (25th, 75th percentiles) if not normally distributed. The mean values were compared by two-sample t test. The Fisher exact test was used in the analysis of categorical data. The Kruskal–Wallis test was used to analyze non–normally distributed values. All reported P values were two-sided. To limit false-positive results in the presence of multiple testing, P values from 0.01 to 0.05 are considered marginal and those ≤0.01 are considered to represent statistically significant findings.
All statistical analyses were performed using JMP, version 9.0 (SAS Institute Inc., Cary, NC).
Results
Study Sample
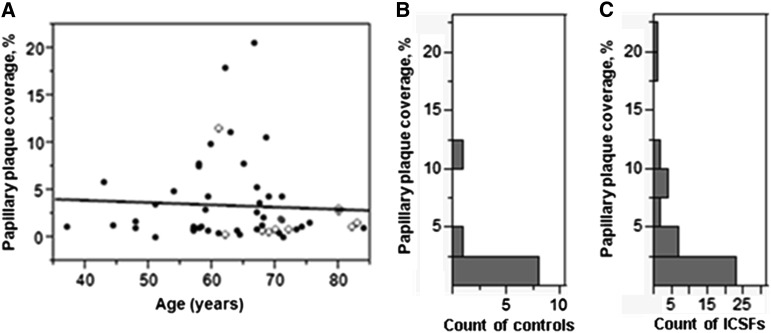
We investigated a consecutive cohort of 42 ICSFs undergoing PCNL for symptomatic urolithiasis. Non–stone-forming controls (n=10) were undergoing ureteroscopic or percutaneous renal surgery for nonstone indications (urothelial cancers). As previously described (11), all underwent nephroscopic papillary mapping between 2009 and 2013 at the Mayo Clinic. Thirty-six ICSFs completed a 24-hour urine collection to measure a supersaturation profile, macromolecular CaOx crystal–growth inhibition, and serum electrolyte analysis, including creatinine (11). The ICSF group contained 15 men and 27 women age 62±10 years; controls consisted of eight men and two women age 73±8 years. Percentage area of papillary plaque coverage did not vary with age among either ICSFs (P=0.82) or controls (P=0.37) (Figure 1A).
Figure 1.
Distribution of papillary surface Randall's plaque coverage among controls and idiopathic calcium oxalate stone-formers (ICSFs). (A) Randall's plaque coverage was not related to the age of ICSFs (closed circles) or controls (open circles). (B) Nine of 10 controls had <5% plaque coverage (median, 0.9%). (C) The ICSF group appears to contain a group of persons with higher amounts of plaque (≥5%).
The median percentage of papillary plaque coverage in controls was 0.9%, with 90% having plaque coverage <5% (Figure 1B). The median percentage of papillary plaque coverage in ICSFs was 1.5%, while 75% of ICSFs had plaque coverage similar to that in controls (<5%) (Figure 1C). Thus, the distribution of papillary surface plaque coverage reveals two distinct groups of ICSFs: those who are similar to controls, with <5% papillary surface plaque (low-plaque group, lower three quartiles), and those with ≥5% papillary surface plaque (high-plaque group, upper quartile) (Figure 1, B and C).
Clinical Characteristics and Urine Chemistries
We examined differentiating features of the clinical and laboratory profiles of low- versus high-plaque ICSFs. As shown in Table 1, low-plaque ICSFs tended to be obese (50% versus 10%; P=0.03) and have a history of urinary tract infection (UTI; 34% versus 0%; P=0.04). Low-plaque ICSFs were less likely to have multiple prior stone events (22% versus 80%; P=0.002). The negative association of multiple prior stone events and low plaque ICSFs was not substantially changed after adjustments for age, sex, obesity, and history of UTI (P=0.004). High- versus low-plaque ICSFs did not differ significantly by age or prevalence of diabetes or hypertension. None of the ICSFs had CKD (eGFR<60 ml/min per 1.73 m2).
Table 1.
Clinical characteristics
| Characteristic | ICSFs with High Plaque (n=10) | ICSFs with Low Plaque (n=32) | P Value |
|---|---|---|---|
| Age (yr) | 61±7 | 62±10 | 0.78 |
| Men, n (%) | 6 (60) | 8 (25) | 0.06 |
| BMI (kg/m2) | 27±2.5 | 31±7.6 | 0.11 |
| BMI≥30 kg/m2, n (%) | 1 (10) | 16 (50) | 0.03 |
| History of prior stone events, n (%) | 10 (100) | 21 (66) | 0.04 |
| Prior stone events ≥4, n (%) | 8 (80) | 7 (22) | 0.002 |
| History of UTI, n (%) | 0 (0) | 11 (34) | 0.04 |
| Hypertension, n (%) | 4 (40) | 13 (42) | 0.92 |
| Diabetes mellitus, n (%) | 1 (10) | 10 (31) | 0.25 |
Values expressed with a plus/minus sign are the mean±SD. P value was from two-sample ttest or two-tail Fisher exact test. BMI, body mass index; UTI, urinary tract infection; ICSF, idiopathic calcium oxalate stone-former.
Low-plaque ICSFs had a significantly lower 24-hour calcium excretion (187±86 mg versus 291±99 mg) and marginally lower 24-hour sulfate excretions (Table 2). Multivariate modeling was not attempted because of the relatively low number of high-plaque patients. High- versus low-plaque ICSFs did not differ by calculated CaOx or CaP urinary supersaturation. Low-plaque ICSFs had a lower 24-hour urine volume than did high-plaque ICSFs. CaOx crystal growth inhibition activity did not differ between low- and high-plaque groups.
Table 2.
Urine chemistry values
| Variable | ICSF with High Plaque (n=9) | ICSF with Low Plaque (n=27) | P Value |
|---|---|---|---|
| pH | 6.3±0.4 | 6.2±0.6 | 0.71 |
| Osmolality | 444±128 | 473±167 | 0.73 |
| Urine volume (L/24 hr) | 2.6 (1.9, 3.0) | 1.6 (1.3, 2.3) | 0.01 |
| Uric acid (mg/24 hr) | 555±244 | 502±184 | 0.49 |
| Sodium (mmol/24 hr) | 170±97 | 134±45 | 0.14 |
| Potassium (mmol/24 hr) | 59 (48, 84) | 44 (40, 67) | 0.16 |
| Calcium (mg/24 hr) | 291±99 | 187±86 | <0.01 |
| Phosphate (mg/24 hr) | 980±620 | 679±257 | 0.05 |
| Magnesium (mg/24 hr) | 112±32 | 91±38 | 0.17 |
| Chloride (mmol/24 hr) | 148±88 | 126±46 | 0.32 |
| Creatinine (mg/24 hr) | 1369±593 | 1134±365 | 0.16 |
| Sulfate (mmol/24 hr) | 21±11 | 15±5 | 0.04 |
| Citrate (mg/24 hr) | 660±329 | 625±286 | 0.72 |
| Oxalate (mmol/24 hr) | 0.3±0.1 | 0.3±0.2 | 0.86 |
| Calcium phosphate brushite DG | −0.1 (−0.7, 0.8) | −0.7 (−1.4, 0.8) | 0.36 |
| Calcium oxalate DG | 1.7 (0.9, 2.0) | 1.9 (0.8, 2.0) | 0.93 |
| Uric acid DG | −3.1 (−3.8, −0.2) | −1.0 (−2.9, 0.6) | 0.24 |
| Apatite DG | 3.8 (3.4, 6.1) | 4.1 (2.6, 6.0) | 0.40 |
| Na urate DG | 0.5 (−0.8, 1.0) | 0.8 (−0.02,1.5) | 0.18 |
| CGI | 0.34±0.05 | 0.37±0.03 | 0.64 |
Values expressed with a plus/minus sign are the mean±SD. All other values are the median (25th, 75th percentile). P value was from two-sample t-test or Kruskal–Wallis test. DG, delta Gibbs free energy term; CGI, crystal growth inhibition activity.
Kidney Stone Culture
At the time of the surgical procedure, a portion of the stone was sterilely sampled for culture. Six of 32 low-plaque ICSFs had a positive stone culture, while none of the high-plaque group did. Diverse organisms were cultured, including Corynebacterium species (n=2), Escherichia coli (n=1), Enterococci (n=1), Gardnerella vaginalis (n=1), and Pseudomonas species (n=1). During a PCNL procedure performed on 10 independent patients (not part of the current cohort), the surgical forceps were placed through the scope, dipped into the sterile tube with irrigant flow running, and then sent for culture. All of these controls were negative for infection, indicating that the positive stone cultures do not appear to represent environmental contaminants. The plaque percentage was lower among the low-plaque ICSFs with positive stone cultures than among those with negative cultures, but this did not reach statistical significance (1.4% versus 4.0%; P=0.19). Consistent with the stone culture results, 11 of 32 low-plaque ICSFs had a history of UTI, while zero of 10 high-plaque ICSFs had a history of UTI (P=0.04). The plaque percentage trended lower among low-plaque ICSFs with a history of UTI compared with those without a history of UTI (1.2% versus 4.3%; P=0.04).
Stone Morphology by mCT
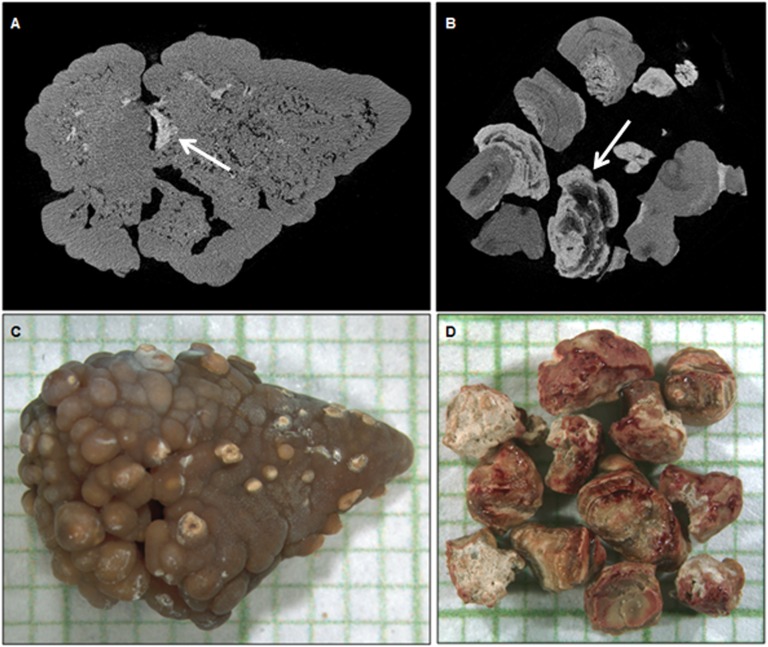
To assess stone morphology, a single observer (J.C.W.) blinded to other clinical features examined the mCT images and determined whether there was evidence that the stone had originated on a Randall's plaque. The identification of the stone as being consistent with formation on Randall's plaque is based on the presence of a significant region of interior CaP (white region in Figure 2A) surrounded by CaOx, as previously described by Miller and colleagues (18). Stones that were composed mostly of calcium oxalate and that showed a small apatite region consistent with formation of Randall's plaque was classified as a “likely Randall's plaque stone” (Figure 2, A and C). “Unlikely Randall's plaque stones” were those that lacked features to suggest they had grown from a Randall's plaque (Figure 2, B and D). If the fragments examined by mCT were inconclusive, the stone was classified as indeterminate. Most of the stones in both high-plaque (nine of 10) and low-plaque (18 of 30) ICSFs were indeterminate. Among the remainder, the single high-plaque ICSF had a likely Randall's plaque stone compared with six of 12 low-plaque ICSFs. None of the high-plaque ICSFs had an unlikely Randall's plaque stone compared with six of 12 (50%) low-plaque ICSFs. Thus, we found no evidence that any stones from high-plaque ICSFs did not grow anchored to a plaque, while up to one half of the stones from low-plaque ICSFs may have developed via a nonplaque pathway.
Figure 2.
Calcium oxalate (CaOx) stones likely or unlikely to have arisen upon a Randall's plaque. Microcomputed tomographic sections (A and B) through the stones shown in the micrographs on a millimeter background grid (C and D), respectively. The CaOx monohydrate (COM) stone in A and C represents a likely Randall's plaque stone containing internal apatite (arrow). Conversely, in the unlikely Randall's plaque stone in B and D, it appears as if CaOx formed around a lower-attenuation unspecified material, and apatite (arrow) subsequently deposited upon the COM surface.
Histology of Papillary Biopsy Specimens
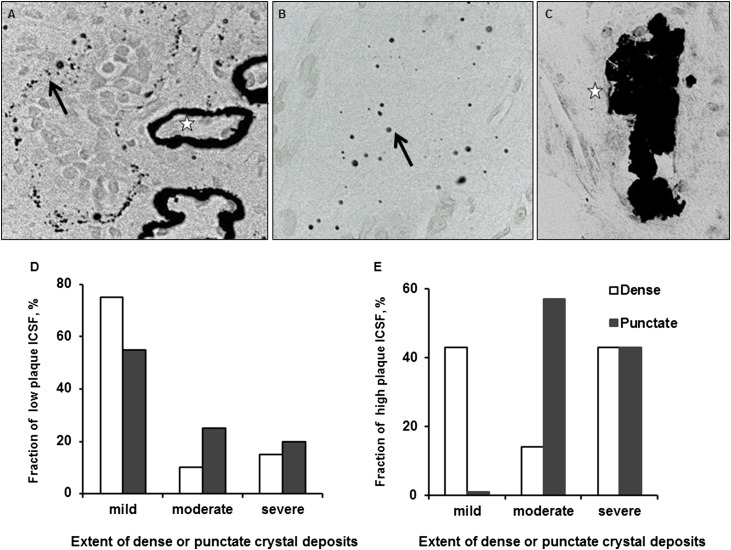
We also assessed histologic plaque patterns among the seven high-plaque and 20 low-plaque ICSFs with papillary biopsy tissues available. Morphologically, Yasue-positive interstitial plaque was dense (stars in Figure 3A) or punctate (arrows in Figure 3, A and B). Yasue-positive tubular plugs were also observed (Figure 3C). Almost all dense plaque was observed around basement membranes (stars in Figure 3A), while punctate plaques were seen surrounding the basement membrane or scattered in the interstitium (arrows in Figure 3, A and B). The quantity of dense plaque was positively associated with the quantity of punctate plaque staining (P=0.002), suggesting that the two abnormalities were related, perhaps along a spectrum. The amount of plaque was semiquantitatively scored as mild (≤25%), moderate (26%–50%) or severe (>50%) in relation to the number of adjacent tubules involved. Among low-plaque ICSFs, 20% had severe punctuate plaque staining compared with 43% of high-plaque ICSFs (P=0.04) (Figure 3D). Similarly, only 15% of low-plaque ICSFs had severe dense plaque staining compared with 43% of high-plaque ICSFs (P=0.25) (Figure 3E). Thus, the histologic samples are consistent with the video-mapping data that were used to classify patients into the low- and high-plaque groups and also verifies that the video-mapping cutoff of 5% plaque coverage separated patients into two clinical groups with different underlying histologic features. Histologic Yasue-positive–collecting duct plugs were observed in a large percentage of ICSFs (Figure 3C) and did not appear to differentiate high- versus low-plaque groups (43% versus 60%, respectively; P=0.43). The mean papillary surface area covered by plugs quantitated by endoscopic mapping also did not differ between the low-plaque versus high-plaque groups (0.35% versus 0.26%; P=0.68).
Figure 3.
Calcium deposits by Yasue staining. (A) Light-microscopic image of a papillary biopsy specimen from an ICSF. Calcium deposits are stained black and appear as punctate (arrow) or dense (star) regions surrounding tubular basement membranes. (B) Light-microscopic image of punctate calcium deposits within interstitial tissue (arrow). (C) Light micrograph of a collecting duct plug (star). (D) Fraction of low-plaque ICSFs (n=22) by the extent of dense and punctate crystal deposits. (E) Fraction of high-plaque ICSFs (n=7) by the extent of dense and punctate crystal deposits. For all images, original magnification×400.
Discussion
Previous studies performed in a group of highly selected ICSFs with rather marked hypercalciuria suggested that higher papillary plaque coverage was associated with more frequent stone events (9), higher urine calcium, lower urine volume, and lower urine pH (8). The current study, performed in a consecutive sample of ICSFs who were not selected by urine chemistry levels, suggests that high-plaque ICSFs are characterized by greater urine calcium excretion and more frequent stone events. These features are consistent with the prior report that implicated hypercalciuria as a key feature in plaque pathogenesis (8).
Unlike the previous cohort, our high-plaque ICSFs did not manifest a lower urine pH (8), although they did have a higher urine sulfate. This might suggest higher protein intake and a tendency toward greater acid loads. In addition, in our study the high-plaque group had higher urine volume, rather than the lower urine volume that had been previously observed (8). However, the current high-plaque ICSFs had more prior stone events and thus may have already increased their fluid intake. In part because of their high urine volume, the high-plaque group also did not display an increased CaOx supersaturation compared with the low-plaque group (19).
Certain kidney stones (those made of struvite) cannot arise without underlying urinary tract infection. Nonstruvite stones, and especially those of ICSFs, have not typically been associated with infection. However, our data suggest that the role of bacterial infection in stone formation merits further study. In particular, the current study suggests that bacterial infection could contribute to stone formation in at least a subset of the low-plaque group. Although the mechanisms that link the two remain to be elucidated, it is possible that micro-organisms in the collecting system alter the local microenvironment or provide a nidus for stone formation via a mechanism totally independent of plaque. Indeed, recent animal studies demonstrate that the inflammasome, a part of the innate immune system, appears to play an important role in CaOx crystal–mediated renal damage (19,20). It is also conceivable that alterations in the urinary tract associated with microbial growth lead to inflammasome activation and provide a nidus for CaOx stone formation.
Our study also suggests obesity could predispose to stones via a nonplaque mechanism. Several studies have reported that obese patients have a higher risk of nephrolithiasis (21). The prevalence of uric acid and CaOx stones is higher in obese than in nonobese patients (22), possibly because of the inverse correlation between urine pH and body mass index (23). Previous studies reported that the association of obesity with nephrolithiasis is stronger in women than in men (24), and in the current cohort the percentage of women was higher in low-plaque group.
Our study has certain limitations, including the relatively small number of high-plaque stone-formers, the cross-sectional nature of the data, the incomplete availability of data (histology) in some patients, and the fact that a few patients had been previously treated with medications. The small group size could also limit power for correlations with stone morphology, in particular given the high number of indeterminant stones. It is also possible that a stone could form on Randall's plaque, yet subsequent addition of apatite layers from the urinary space make identification of the underlying plaque difficult. However, the interobserver specificity of a typical Randall's plaque stone by mCT, when present, is high (14). Furthermore, in this study assignment of the Randall's plaque class for each specimen was done in a blinded fashion such that the observer had no knowledge of patient data; this yielded an unbiased and independent classification. Finally, although many studies have used a cutoff of >50% of CaOx for ICSFs (10–13), others have used a more rigorous number of >70%. However, when we repeated all analyses using this more stringent cutoff, results were not substantially changed.
In conclusion, our study provides new insight regarding the clinical, urinary chemistry, and stone morphologic characteristics of ICSF with low amounts of Randall's plaque. The findings indicate that a subgroup of ICSFs may have a different mechanism of stone formation and growth independent of plaque. Further studies need to be performed to determine how these factors translate into the pathogenesis of stones.
Disclosures
None.
Acknowledgments
The abstract of this work was selected for an oral presentation at the American Society of Nephrology Kidney Week 2013 in Atlanta, Georgia.
The authors thank Mark Korinek for technical assistance and Brittni Barnett for study coordinator support.
This work was supported by the Mayo Clinic O’Brien Urology Research Center P50-DK083007 from the National Institute of Diabetes and Digestive and Kidney Diseases and grant UL1-TR000135 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Scales CD, Jr, Smith AC,Hanley JM, Saigal CS, Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller NL, Gillen DL, Williams JC, Jr, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Matlaga BR, Munch LC, Lingeman JE: A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int 103: 966–971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evan AP, Unwin RJ, Williams JC, Jr: Renal stone disease: A commentary on the nature and significance of Randall’s plaque. Nephron, Physiol 119: 49–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller NL: The origin and significance of Randall’s plaque in nephrolithiasis. J Urol 186: 783–784, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Coe FL, Evan AP, Lingeman JE, Worcester EM: Plaque and deposits in nine human stone diseases. Urol Res 38: 239–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M: Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matlaga BR, Williams JC, Jr, Kim SC, Kuo RL, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Munch LC, Lingeman JE: Endoscopic evidence of calculus attachment to Randall's plaque. J Urol 175: 1720–1724, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, Munch LC, Coe FL: Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int 64: 2150–2154, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kim SC, Coe FL, Tinmouth WW, Kuo RL, Paterson RF, Parks JH, Munch LC, Evan AP, Lingeman JE: Stone formation is proportional to papillary surface coverage by Randall’s plaque. J Urol 173: 117–119, discussion 119, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Evan AP, Weinman EJ, Wu XR, Lingeman JE, Worcester EM, Coe FL: Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res 38: 439–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linnes MP, Krambeck AE, Cornell L, Williams JC, Jr, Korinek M, Bergstralh EJ, Li X, Rule AD, McCollough CM, Vrtiska TJ, Lieske JC: Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int 84: 818–825, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evan AP, Lingeman JE, Coe FL, Worcester EM: Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin Nephrol 28: 111–119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight J, Jiang J, Wood KD, Holmes RP, Assimos DG: Oxalate and sucralose absorption in idiopathic calcium oxalate stone formers. Urology 78: e9–, e13., 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarse CA, Hameed TA, Jackson ME, Pishchalnikov YA, Lingeman JE, McAteer JA, Williams JC, Jr: CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urol Res 35: 201–206, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa Y, Abram V, Parks JH, Lau HS, Kawooya JK, Coe FL: Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. J Clin Invest 76: 1455–1462, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasue T: Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2: 83–95, 1969 [Google Scholar]
- 18.Miller NL, Williams JC, Jr, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Munch LC, Handa SE, Lingeman JE: In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randall’s plaque. BJU Int 105: 242–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ: Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123: 236–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS: NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hayek S, Schwen ZR, Jackman SV, Averch TD: The impact of obesity on urine composition and nephrolithiasis management. J Endourol 27: 379–383, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Chou YH, Su CM, Li CC, Liu CC, Liu ME, Wu WJ, Juan YS: Difference in urinary stone components between obese and non-obese patients. Urol Res 39: 283–287, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Najeeb Q, Masood I, Bhaskar N, Kaur H, Singh J, Pandey R, Sodhi KS, Prasad S, Ishaq S, Mahajan R: Effect of BMI and urinary pH on urolithiasis and its composition. Saudi J Kidney Dis Transplant 24: 60–66, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Nowfar S, Palazzi-Churas K, Chang DC, Sur RL: The relationship of obesity and gender prevalence changes in United States inpatient nephrolithiasis. Urology 78: 1029–1033, 2011 [DOI] [PubMed] [Google Scholar]