Key Points
Physiological shear stress promotes megakaryocytic maturation, DNA synthesis, phosphatidylserine exposure and caspase-3 activation.
Shear enhances the production and function of PLPs and Mk-derived microparticles possessing a novel function.
Abstract
In vivo visualization of thrombopoiesis suggests an important role for shear flow in platelet biogenesis. In vitro, shear stress was shown to accelerate proplatelet formation from mature megakaryocytes (Mks). Yet, the role of biomechanical forces on Mk biology and platelet biogenesis remains largely unexplored. In this study, we investigated the impact of shear stress on Mk maturation and formation of platelet-like particles (PLPs), pro/preplatelets (PPTs), and Mk microparticles (MkMPs), and furthermore, we explored a physiological role for MkMPs. We found that shear accelerated DNA synthesis of immature Mks in an exposure time– and shear stress level–dependent manner. Both phosphatidylserine exposure and caspase-3 activation were enhanced by shear stress. Exposure to physiological shear dramatically increased generation of PLPs/PPTs and MkMPs by up to 10.8 and 47-fold, respectively. Caspase-3 inhibition reduced shear-induced PLP/PPT and MkMP formation. PLPs generated under shear flow displayed improved functionality as assessed by CD62P exposure and fibrinogen binding. Significantly, coculture of MkMPs with hematopoietic stem and progenitor cells promoted hematopoietic stem and progenitor cell differentiation to mature Mks synthesizing α- and dense-granules, and forming PPTs without exogenous thrombopoietin, thus identifying a novel and unexplored potential physiological role for MkMPs.
Introduction
Megakaryocytes (Mks) are derived from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM), and as they mature they migrate to the endothelial lining of BM sinusoids where they extend proplatelets (PPTs) through gaps of the endothelium into circulation.1,2 Mks encounter biomechanical stresses as they deform to penetrate gaps of the sinusoid wall and shear stresses upon exposure to blood flow (see supplemental Figure 1 on the Blood Web site).3 Upon entering circulation, Mk fragments or whole Mks are exposed to shear stresses of a broad range and duration in different parts of circulation (supplemental Figure 1 and supplemental Table 1). Released Mk fragments mature into platelets in circulation, while released whole Mks are eventually captured in the pulmonary vasculature where they give rise to platelets (supplemental Figure 1).4-7
Following a pioneering visualization study3 identifying a physiological shear-stress range of 1.3 to 4.1 dyn/cm2 for platelet biogenesis in the BM, a role for shear stress was supported by an in vitro study demonstrating that a high shear rate (1800 s−1; corresponding to ∼16 dyn/cm2, almost fourfold higher than the upper physiological limit in the BM) accelerates PPT formation and platelet biogenesis from cultured, mature Mks.4 However, the cellular events underlying the effects of mechanical stresses on Mk maturation and platelet biogenesis remain largely unexplored.4,8 Shear and other biomechanical stresses affect different cell types in biologically multifaceted and complex ways. For example, shear stress is an important differentiation signal for embryonic stem cells,9,10 endothelial progenitor cells circulating in peripheral blood,11-13 and mesenchymal stem cells.14,15 Many cellular processes are affected by shear forces, including the cell cycle,11,16,17 migration,17,18 apoptosis,19-22 and differentiation.11,23
In this study, we probed the role of shear stress on Mk maturation by examining its impact on DNA synthesis, polyploidization, phosphatidylserine (PS) exposure, caspase-3 activation and the generation of PPTs, platelet-like particles (PLPs), and Mk-derived microparticles (MkMPs). And, for the first time, we identify a potential biological role for MkMPs.
Material and methods
Part of our methods describing protein, cytokine, and antibody sources, Mk culture, flow cytometry analysis of DNA synthesis and Annexin V binding, immunofluorescent staining, isolation of PLPs, platelet-stimulation assays, the thrombopoietin (TPO) enzyme-linked immunosorbent assay, the preparation of human platelets and platelet-derived microparticles (PMPs), and the transmission electron microscopy analysis are detailed in the supplemental Methods. Blood for isolation of human platelets was collected by venipuncture from adult human volunteers after providing written informed consent as approved by the Institutional Review Board at University of Delaware (protocol #190471-3). The study was conducted in accordance with the Declaration of Helsinki.
Shear-stress experiments: exposure of Mk cells to shear flow
Rectangular flow slides (µ-Slide I0.6 Luer, ibidi) were coated with 50 μg/mL von Willebrand factor (VWF) and ∼300 000 cultured Mks were seeded onto each slide. Mks on slides were cultured overnight (21 hours) before being exposed to shear flow. Iscove modified Dulbecco medium (IMDM) supplemented with 10% BIT9500, 50 ng/mL recombinant human TPO (rhTPO), 50 ng/mL rhSCF, 0.5 μg/mL human low density lipoprotein, and 6.25 mM nicotinamide was perfused over Mks on slides by 2 syringe pumps (Dual NE-4000 pump; New Era Pump Systems) to achieve the desirable shear-stress level (supplemental Figure 3). For 5-bromo-2′-deoxyuridine (BrdU) incorporation assays, the medium was supplemented with 10 µM BrdU (BD Biosciences). During shear flow, some Mks were detached from the slide surface and released into the circulating medium. These cells were considered as nonadherent Mks. Adherent Mks were harvested for analysis using a nonenzymatic cell dissociation buffer (Sigma-Aldrich). For immunofluorescent experiments, adherent Mks were fixed with 2% paraformaldehyde directly on slides. For caspase inhibition experiments, Mks were treated with caspase inhibitors, 10 μM z-VAD.fmk (Bachem) or 10 μM z-DEVD.fmk (Bachem) starting on day 9. Inhibitor-treated Mks were seeded into flow slides at day 9 or day 11, were exposed to shear flow (2.5 dyn/cm2 for 0.5 hour) in medium supplemented with the same inhibitor, and were harvested for PPT, PLP, and CD41+ MP counting.
Isolation and characterization of MkMPs
For both static cultures and cultures exposed to shear flow, Mk cells were removed from the culture medium by centrifugation (150 × g for 10 minutes). Following that, PLPs were removed by centrifugation at 1000 × g for 10 minutes. MkMPs were enriched by ultracentrifugation24 (25 000 rpm for 1 hour at 4°C; Beckman Coulter Optima Max Ultracentrifuge) and then washed 3 times in IMDM medium. CD41, CD42b, and CD62P expression of MkMPs was examined by flow cytometry. Concentrations of MkMPs (and of PMPs and Mks) were measured by flow cytometry using 1.34 μm microbeads (Spherotech) as a standard.
Coculture of CD34+ cells or hematopoietic progenitor cells with MkMPs
A total of 60 000 CD34+ cells (or cultured hematopoietic progenitor cells (HPCs) from day 3 culture of CD34+ cells with or without TPO) were incubated with 10 MkMPs or PMPs per CD34+ cell or HPC in 50 µL IMDM medium for 1 hour at 37°C to enhance the contact between MPs and cells. After that, the cells with the MPs were diluted in 600 µL IMDM medium supplemented with 5% BIT9500 and 50 ng/mL rhSCF, and cultured at 37°C and 20% O2 until they were harvested at day 8 for CD41 and ploidy flow cytometry analysis.25 At day 9, cells in coculture were examined using multiphoton confocal microscope (Zeiss 510 NLO) and Differential Interference Contrast images were collected. At day 10, cells from coculture were seeded onto human fibrinogen-coated coverslips and cultured overnight for staining for β1 tubulin (TUBB1), VWF, and serotonin (5-HT) at day 11. Cells from vehicle control were fixed first and cytospun onto the coverslip (Shandon Cytospin 4; Thermo Scientific) before immunofluorescent staining. At day 11, some cells were harvested for transmission electron microcopy (TEM) imaging.
Results
Shear enhances DNA synthesis and Mk polyploidization in a largely dose and maturation stage dependent way
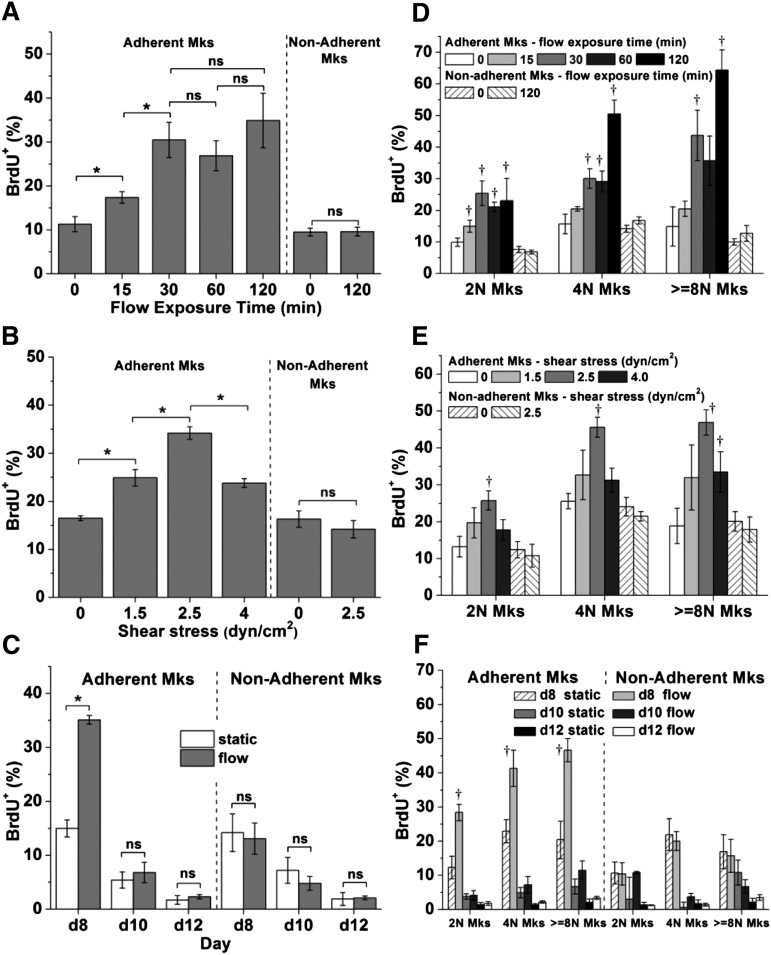
Mk cells engage in endomitosis as they mature and become polyploid.1 We hypothesized that biomechanical forces, such as physiological shear forces,3 would impact DNA synthesis. To investigate this hypothesis, Mk cells at days 8, 10, or 12 were exposed to shear flow using a validated perfusion system (supplemental Figure 3). For our experiments, we used shear in the physiological range of 1.3 to 4.1 dyn/cm2.3 First, we exposed day 8 Mk cells to 2.5 dyn/cm2 for 0 (static control), 15, 30, 60, or 120 minutes. Exposure to shear flow increased DNA synthesis of Mk cells by up to threefold, and the increase was exposure time–dependent (Figure 1A). Mks responded to shear stress quickly, certainly within 15 minutes, and after 30 minutes no further increase in DNA synthesis was observed. This is physiologically relevant. It has been reported that, in vivo, the time needed for trans-sinusoidal migration of murine Mk fragments into the blood stream is about 30 minutes.3 In subsequent experiments, an exposure time of 30 minutes was used to investigate the impact of shear-stress level on Mks. Low levels of shear stress (1.5 dyn/cm2) enhanced DNA synthesis of day 8 Mks by 51% compared with the static conditions (Figure 1B). Exposure to 2.5 dyn/cm2 increased DNA synthesis further by 37% over that of 1.5 dyn/cm2, or 107% over static control (Figure 1B). However, at 4.0 dyn/cm2 (near the upper limit of the physiological range), DNA synthesis was similar to that at 1.5 dyn/cm2. To investigate if shear flow differentially affects DNA synthesis at different differentiation stages, Mks at days 8, 10, and 12 were exposed to 2.5 dyn/cm2 for 30 minutes. Our data (Figure 1C) show that exposure to shear flow results in increased DNA synthesis only of day 8 Mk cells, which were at an immature stage. Mks at days 10 or 12 were more mature, thus displaying much lower DNA synthesis compared with day 8 cells. We also examined if shear affects Mks of different ploidy classes (2N, 4N, ≥ 8N Mks) differently. DNA synthesis of each ploidy class showed trends similar to those of the total Mk population (Figure 1D-F). However, DNA synthesis of Mks with 4N and higher ploidy classes increased further when 2.5 dyn/cm2 was applied for 120 minutes (Figure 1D). These data suggest that even short exposure to circulatory shear promotes the maturation of less mature day 8 Mk cells by enhanced polyploidization.
Figure 1.
Shear stress enhances DNA synthesis of immature Mks. (A-C) The BrdU+ percentage of all adherent and nonadherent Mks or (D-F) Mks with different ploidy classes (2N, 4N, and ≥ 8N) upon exposure to various shear-stress conditions. After shear-flow application, Mks were cultured in the presence of BrdU for a total of 4 hours. Cells were then harvested for CD41 and propidium iodide (DNA) staining and analyzed by flow cytometry. (A,D) At day 8, Mks were exposed to shear stress at level of 2.5 dyn/cm2 for 0 (static control), 15, 30, 60, and 120 minutes. (B,E) At day 8, Mks were exposed to shear stress at 0 (static control), 1.5, 2.5, and 4.0 dyn/cm2 for 30 minutes. (C,F) At days 8, 10, and 12, Mks were exposed to 2.5 dyn/cm2 for 30 minutes. Error bars indicate standard error of mean (SEM) of 3 biological replicates. *P < .05; †P < .05 compared with corresponding static control. ns, not significant.
We also examined the impact of biomechanical forces on nonadherent cells. In contrast to adherent Mk cells, DNA synthesis of nonadherent Mk cells in these experiments was not affected compared with static conditions (Figure 1A-C).
Could the effect of shear stress on DNA synthesis be due to differential retention of adherent Mk cells because adherent Mks cells were more active in synthesizing DNA? Our data suggest that this is not the case. Indeed, the percentage of CD41+ cells and ploidy distribution among adherent Mks under various (stress level and duration) flow conditions and static conditions were similar (supplemental Figure 4). In addition, we observed decreased DNA synthesis after the shear stress level was increased from 2.5 dyn/cm2 to 4 dyn/cm2 (Figure 1B); and finally, DNA synthesis of Mks at days 10 and 12 were not accelerated by shear stress.
Shear stress promotes PS surface exposure on maturing Mk cells
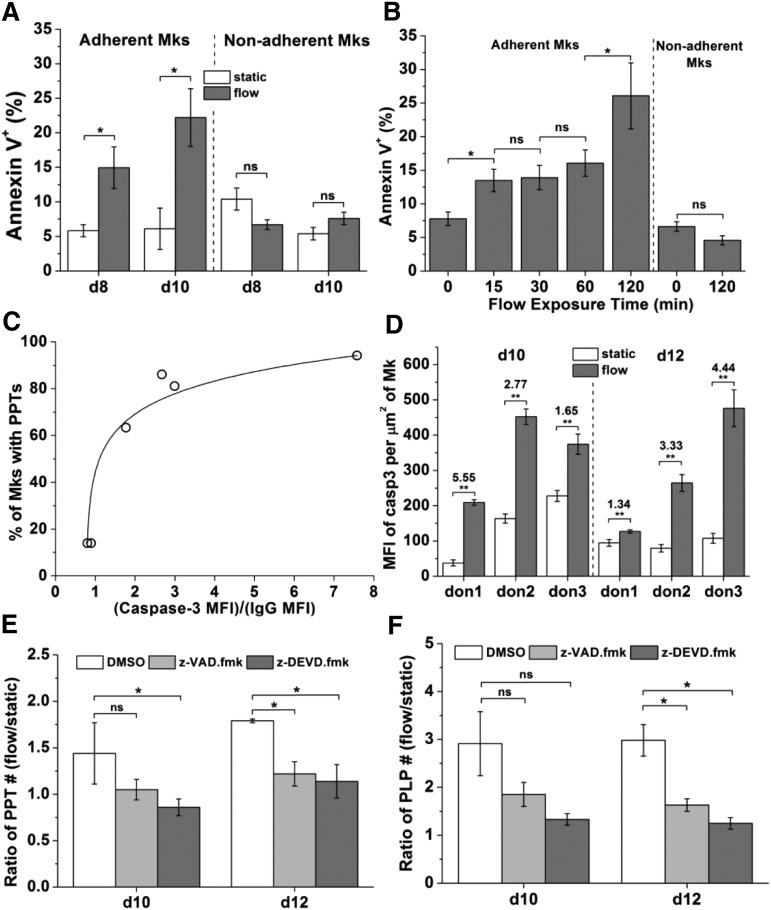
Previous studies in our laboratory and other laboratories have shown that PS becomes exposed on the extracellular side of the Mk membrane when HPSCs (both human and murine) were differentiated into Mks.26-30 PS exposure is also important in platelet activation.31 Low level of PS exposure has been also implicated in microparticle formation from different cell types.32 Thus, we wanted to examine if PS exposure is part of the mechanism through which Mks respond to shear to form various size particles. In this study, to differentiate human CD34+ cells into Mks, we used a new protocol that gives rise to functional PLPs in vitro.33 Using flow cytometric and microscopic analyses, we confirmed that PS was also exposed on the surface of maturing (>day 8) Mks generated by this protocol at low level (data not shown). To investigate if shear promotes PS externalization, fluid flow at 1 dyn/cm2 was applied to day 8 and day 10 Mks for 2 hours. Cells were harvested immediately after the shear-flow application for analysis. Shear resulted in a significantly increased Annexin V+ fraction (by ∼160% and 260% at days 8 and 10, respectively) of adherent Mks, but not so for nonadherent Mks (Figure 2A). We also examined the impact of shear exposure time on PS externalization by exposing day 10 Mks to 2.5 dyn/cm2 for 0 (static condition), 15, 30, 60, and 120 minutes. PS externalization responded quickly to shear stress: the Annexin V+ fraction of adherent Mk cells increased by more than 70% after 15 minutes (Figure 2B). PS externalization plateaued for exposure times between 15 and 60 minutes, but increased further at 120 minutes (Figure 2B). Shear flow did not affect PS externalization of nonadherent Mks (P > .10) (data for 0 and 120 minutes are shown; Figure 2B).
Figure 2.
Shear stress promotes PS externalization and caspase-3 activation. Caspase-3 is involved in shear-stress enhanced PPTs and PLPs formation. (A) Percent of Annexin V+ Mks at days 8 and 10 after shear-flow application at 1 dyn/cm2 for 2 hours vs static control. Adherent and nonadherent Mks were analyzed separately. (B) Percent of Annexin V+ day 10 Mks exposed to 2.5 dyn/cm2 for 0 (static control), 15, 30, 60, or 120 minutes. (C) Correlation between caspase-3 activation and PPT formation of Mks under static culture conditions. X-axis: caspase-3 activation level defined as the ratio of MFI of active caspase-3 over IgG control. Y-axis: percent of Mks bearing PPTs. (D) MFI of active caspase-3 per µm2 of adherent day 10 and 12 Mks from different donors (don) after shear-flow exposure at 1.0 dyn/cm2 for 2 hours vs static control. The ratio of MFI of caspase-3 of Mks under shear-flow conditions over static control is indicated above the bars. Under shear flow, MFI values for caspase-3 activation were well above the MFI for isotype control, so there was no need to correct for isotype control. (E-F) At days 10 and 12, DMSO (vehicle control) or z-VAD.fmk (pan-caspase inhibitor) or z-DEVD.fmk (caspase-3 inhibitor) treated Mks were exposed to shear flow at 2.5 dyn/cm2 for 0.5 hour. After shear-flow exposure, PPTs and PLPs were harvested and counted. The number of PPTs (E) or PLPs (F) from 1 slide of Mks exposed to shear flow was normalized by number of PPTs or PLPs on a slide under static conditions, and the resulting ratios are plotted. Error bars indicate SEM of 3∼4 biological replicates in panel (A-B,E-F) and 6∼10 different images in panel (D). *P < .05; **P < .01. ns, not significant.
Caspase-3 activation in maturing Mk cells is accelerated by exposure to shear flow
Caspase-3 activation has been shown to be involved in microparticle formation from various cell types34 such as from platelets35 and endothelial cells.36 Since shear accelerates the formation of active particles from Mk cells,4 we wanted to investigate if shear stress affects caspase-3 activation in Mks. First, we confirmed that caspase-3 indeed was activated during in vitro Mk maturation. Caspase-3 was activated at days 10 and 12 when Mks projected PPTs under our culture protocol; active caspase-3 accumulated largely around the nucleus, but PPTs did not stain for active caspase-3 (supplemental Figure 5). We also observed a correlation between the activation level of caspase-3 in Mks (represented as the ratio of mean fluorescence intensity [MFI] of active caspase-3 over isotype control) and PPT formation (Figure 2C), which is consistent with previous studies.29 Next, we investigated if shear stress enhances caspase-3 activation in maturing Mks, notably at days 10 and 12. Exposure of Mks for 2 hours to 1 dyn/cm2 enhanced caspase-3 activation at both days 10 and 12 by 1.34- to 5.6-fold, depending on the donor and day (Figure 2D). One mechanism by which shear stress may promote PPT/PLP formation is by enhancing caspase-3 activation. To investigate this hypothesis, Mks were treated with z-VAD.fmk (pan-caspase inhibitor) or z-DEVD.fmk (caspase-3 inhibitor), and were then exposed to shear flow. After flow application, PLPs (d = 1 to 3 μm37) and PPTs (d = 3 to 10 μm37) were harvested and counted. The effect of shear stress on particle generation was assessed using the ratio of PLP or PPT number from 1 slide of Mks under flow conditions over that under static conditions. At day 10, z-VAD.fmk had no statistically significant effect on the PLP and PPT ratios, and z-DEVD.fmk decreased only the PPT ratio (Figure 2E-F). However, at day 12, both z-VAD.fmk and z-DEVD.fmk decreased both the PPT and PLP ratios (Figure 2E-F). These data suggest that at the day 10 early maturation stage, at which time Mks were starting to project PPTs and very few PLPs were formed, the caspase-3 inhibitor inhibited the effect of shear stress on PPT formation but not on PLP formation. At day 12, when Mks produced more PPTs and started to form a significant number of PLPs, caspase inhibitors attenuated the effect of shear on PPT and PLP generation. These data suggest that caspase-3 activation plays a role in the mechanism by which shear stress enhances PPT and PLP formation.
Shear stress enhances the generation of functional PLPs, as well as PLP activity
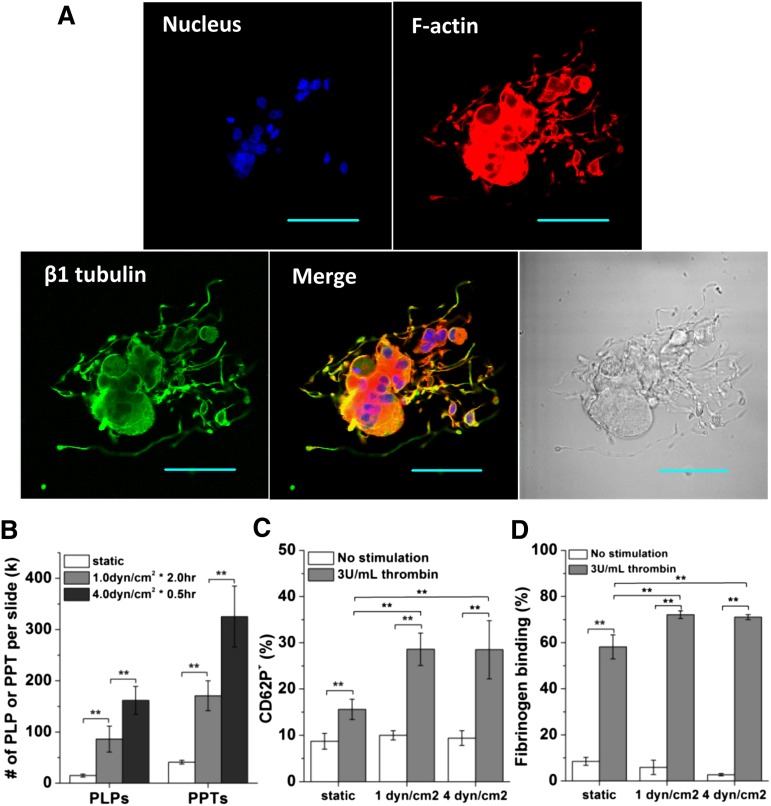
It was previously shown that shear accelerates the formation of functional platelets.4 Here, we aimed to investigate and quantify the effect of shear stress on the generation of Mk fragments with platelet-like properties at day 12 when Mks had extensive PPTs (Figure 3A). After a 2-hour exposure of adherent Mks to low shear flow at 1 dyn/cm2, 5.8 times more PLPs were formed compared with static conditions, while exposure to high shear flow at 4 dyn/cm2 for 0.5 hour yielded even more PLPs (∼10.8; Figure 3B). Moreover, the number of PPTs increased by 4.1- and 7.9-fold after exposure to low and high shear flow, respectively (Figure 3B). These data show that, in vitro at least, exposure to physiological levels of shear results in a dramatic increase in both PLP and PPT formation from mature Mks. The enhanced formation of PLPs and PPTs by shear stress is supported by enhanced fragmentation of Mks by shear stress, as detailed in the supplemental Results.
Figure 3.
Shear stress enhances the production of PPTs and PLPs. PLPs generated under shear flow display enhanced in vitro functional activity. (A) A mature and polyploid Mk displays extensive PPTs in static culture at day 12. Scale bar: 50 µm. (B-D) Mks at day 12 were exposed to shear flow at 1 dyn/cm2 for 2 hours or 4.0 dyn/cm2 for 0.5 hour. Both adherent and nonadherent cell fragments were analyzed post shear exposure. Numbers (B) of PLPs and PPTs per slide post shear exposure vs static control. Two functionality assays, CD62P exposure (C) and fibrinogen binding (D), were performed on the harvested PLPs and demonstrate enhanced activity for PLPs generated under shear flow. Error bars indicate SEM of 3∼4 biological replicates. **P < .01.
Next, we examined the impact of shear flow on the functionality of the generated PLPs. Is it possible that the fast generation of PLPs under shear flow results in lesser quality of PLPs, or perhaps the opposite? To do so, we employed 2 platelet-function assays: CD62P exposure and fibrinogen-binding, both using the physiological activator, human thrombin. For PLPs generated from Mks under low shear conditions, the fraction of PLPs expressing CD62P increased by almost threefold (from 10% to 29%) upon thrombin activation, while for PLPs generated from Mk cells under static conditions this fraction increased by 1.8-fold (from 9% to 16%; Figure 3C). After activation with thrombin, the percentage of PLPs generated from Mks under low shear conditions that bind fibrinogen increased by 12-fold (from 6% to 72%), while that of PLPs from static culture increased by ∼6.5-fold (from 9% to 58%; Figure 3D). The quality of PLPs generated under high shear conditions was similar to that of PLPs generated under low shear conditions (Figure 3C-D). Taken together, these data suggest that PLPs produced from Mks upon exposure to shear flow have better functionality than PLPs generated under static conditions. To conclude, exposure to shear, even briefly, results in dramatic increases in both the number and quality of PLPs when compared with static controls.
Shear stress dramatically enhances the generation of MkMPs
When we examined the size distribution of cell fragments released from Mks under both static and shear-flow conditions, in addition to PLPs and PPTs, we found a distinct population of very small particles (Figure 4A). We ran a microbead (d = 1.34 µm) control to confirm that these particles were on average considerably smaller than 1.34 µm (Figure 4B). Mature Mks and activated platelets can give rise to MPs that are smaller than platelets.38,39 Surface staining demonstrated that most of these particles were CD41+ and CD42b+, but many were CD41+ and CD42b− (Figure 4C). In order to identify the origin of these CD41+ particles, we examined them for CD62P expression. CD62P is expressed on PMPs but not on MkMPs.38 We found that, on average, 16% of the CD41+ MPs were CD62P+, thus suggesting that most of these MPs were MkMPs deriving from Mks rather than from activated PLPs (Figure 4D), which presumably can generate MPs similar to PMPs (ie, CD62P+ MPs).
Figure 4.
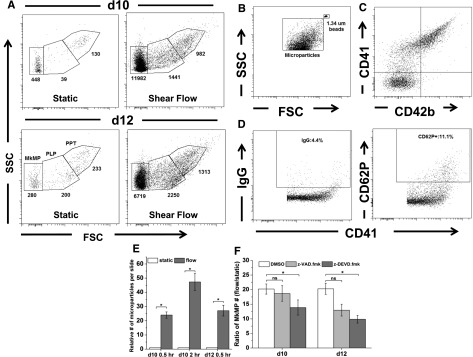
Shear stress exposure results in dramatically enhanced generation of MkMPs through activation of caspase-3. (A) Day 10 and 12 Mks were exposed to medium flow at a shear stress of 2.5 dyn/cm2 for 0.5 hour. Whole cells were removed and the same amount of samples, as assessed by internal microbeads control, from slides exposed to shear flow and static control were analyzed by flow cytometry. PPTs were located on the high gate, PLPs in the middle gate, and MkMPs in the low gate. The number of particles in each gate is displayed below each gate. (B) MkMPs were smaller than microbeads of 1.34 µm diameter. (C) Expression of CD41 and CD42b on MkMPs from day 12 Mk cells. (D) CD62P expression and the corresponding IgG control of CD41+ MPs from day 12 Mk cells. (E) The relative number of CD41+ microparticles generated from day 10 or day 12 Mk cells either under static conditions or upon application of shear flow at 2.5 dyn/cm2 for 0.5 hour (days 10 and 12) or 2 hours (day 10). (F) At days 10 and 12, DMSO (vehicle control) or z-VAD.fmk (pan-caspase inhibitor) or z-DEVD.fmk (caspase-3 inhibitor) treated Mks were exposed to shear flow at 2.5 dyn/cm2 for 0.5 hour. After shear flow exposure, the number of isolated MkMPs was measured by flow cytometry. The number of MkMPs from 1 slide of Mks under shear flow was normalized by the number of MkMPs on a slide maintained under static culture conditions, and the resulting ratios were plotted. Error bars indicate SEM of 3 biological replicates. *P < .01 in panel (E) and *P < .05 in panel (F).
Cultures of Mk cells post-shear exposure contained a dramatically larger number of MkMPs compared with those from Mks grown under static conditions (Figure 4A). Thus, Mk exposure to shear results in increased MkMP formation in addition to enhanced PLP generation. Exposure to 2.5 dyn/cm2 for 0.5 hour resulted in increased MkMP generation by 24- and 27-fold at days 10 and 12, respectively (Figure 4E). For day 10 Mks, exposure to 2.5 dyn/cm2 for 2 hours resulted in a 47-fold increase in MkMP generation (Figure 4E). Next, we investigated if caspases mediate the shear stress-enhanced generation of MkMPs. As described earlier, Mks were treated with z-VAD.fmk or z-DEVD.fmk before exposure to shear stress at days 10 and 12. The ratio of the number of MkMPs from 1 slide of Mks under shear flow to the number of MkMPs under static conditions was used to assess the effect of shear stress on MkMP generation. The results (Figure 4F) show that only treatment with caspase-3 inhibitor (z-DEVD.fmk) attenuated the effect of shear stress, suggesting that caspase-3 is involved in shear-enhanced MkMP generation.
Novel biological activity of MkMPs: promoting Mk differentiation of HSPCs
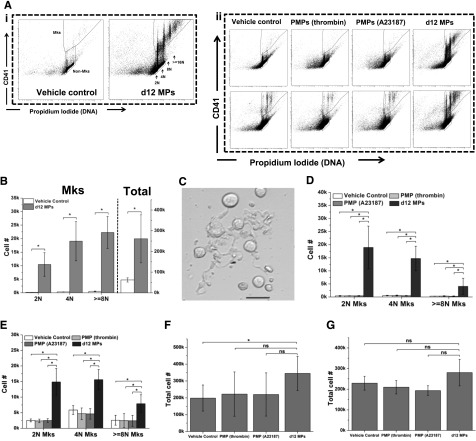
To our knowledge, a physiological function for MkMPs has not yet been reported. We hypothesized that a role of MkMPs might be to accelerate hematopoietic progenitor differentiation into Mks. In early experiments, we found that MkMPs cocultured with HPCs from day 5 of Mk culture from CD34+ cells promoted HPC survival and Mk differentiation under serum- and TPO-free conditions (data not shown). Therefore, we examined this effect in more detail using MPs generated from day 12 Mks. We will refer to these MPs as MkMPs although they may contain a small fraction (∼16%) of CD62P+ MPs. MkMPs were cocultured with CD34+ cells in a medium without TPO but with 50 ng/mL rhSCF (for enhancing cell survival), and the outcomes were examined after 8 days of culture. In the vehicle control culture, barely any CD34+ cells could differentiate into Mks by day 8 (Figure 5Ai,B). However, we observed a dramatic induction of Mk differentiation (as assessed by CD41 expression and polyploidization) in the day 8 culture of CD34+ cells cocultured since day 0 with MkMPs (Figure 5Ai,B-C). In addition, MkMPs promoted cell proliferation; the total cell number was increased 4.2-fold compared with vehicle control (Figure 5B). We also examined if MkMPs could stimulate partially differentiated HPCs. CD34+ cells were cultured in medium with or without TPO for 3 days and then cocultured with MkMPs without TPO for 5 more days to day 8. Day 3 HPCs from culture without TPO gave rise to very few Mks in the vehicle control culture, but MkMPs induced dramatically higher (by >10 000-fold) differentiation into Mks (Figure 5Aii,D) with a concomitant 1.7-fold increased cell expansion (Figure 5F) compared with the vehicle control. In the vehicle control cultures, day 3 HPCs from culture with TPO developed into Mks by day 8 even without further TPO stimulation (Figure 5Aii,E). However, coculture with MkMPs resulted in 5.9-, 2.7-, and 3.0- higher numbers of Mks with 2N, 4N, and >8N ploidy (Figure 5Aii,E), although the total cell number was not increased (Figure 5G). Taken together, these data show that MkMPs promote Mk differentiation of HSPCs at different differentiation stages and that the effect is more pronounced on more primitive, uncultured CD34+ cells.
Figure 5.
MkMPs promote Mk differentiation of CD34+ cells and HPCs. (A) Representative graph of flow cytometry ploidy analysis of cells from various coculture conditions at day 8. (i) CD34+ cells were cocultured with or without MkMPs starting at day 0. (ii) HPCs from day 3 culture without (top panel) or with (bottom panel) TPO were cocultured without or with MkMPs, and PMPs from day 3 to day 8. The same fraction of cells from each sample was analyzed. (B-C) At day 0, 60 000 CD34+ cells were cocultured with or without (vehicle control) MkMPs in a medium without TPO. The numbers (B) of total cells and Mks with 2N, 4N, and ≥ 8N ploidy were counted at day 8. Some Mks started to form PPTs at day 9 of coculture (C). Scale bar: 20 μm. (D-G) At day 3, 60 000 HPCs from culture with (D,F) or without (E,G) TPO were cultured in TPO-free medium with or without the addition of MkMPs or PMPs. Total cells (F-G) and Mks (D-E) with 2N, 4N, and ≥ 8N ploidy were counted at day 8. Error bars indicate SEM of 3∼4 biological replicates. *P < .01. ns, not significant.
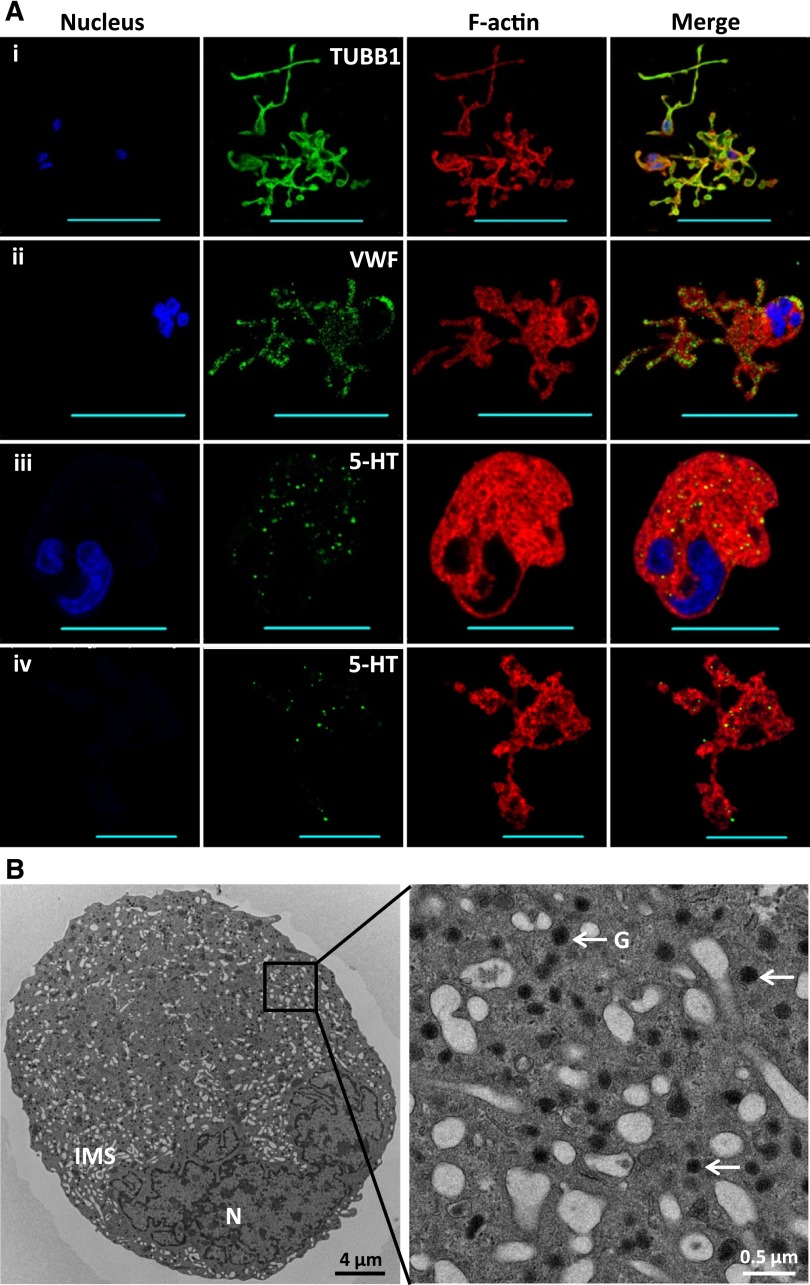
In order to further characterize the Mks generated from CD34+ cells cocultured with MkMPs, the coculture was prolonged to day 11. At day 9, we found that some Mks started to form PPTs (Figure 5C). At day 11, cells were stained for β1 tubulin, VWF, and serotonin to examine PPT structures and the formation of α- and dense-granules, respectively. Fluorescent imaging demonstrated that Mks generated from the cocultures displayed normal microtubule PPT structures and synthesized both types of platelet granules (Figure 6A). TEM imaging (Figure 6B) of Mks from day 11 of coculture confirmed that numerous platelet granules were packed in Mk cells, which also displayed the characteristic invaginated membrane system. These data demonstrate that Mks generated from coculture of CD34+ cells with MkMPs display normal developmental characteristics.
Figure 6.
Mks generated from MkMP coculture display characteristic PPT structures and synthesize both α- and dense-granules. CD34+ cells were cocultured with MkMPs starting at day 0. (A) At day 11, cells were stained for β1 tubulin (TUBB1 [i]), VWF [ii], and serotonin (5-HT [iii-iv]) to visualize PPT structures, α-granules, and dense-granules, respectively. Panels (iii) and (iv) displaying serotonin staining of both cells with a nucleus (panel iii) and anuclear cellular fragments (PPTs; panel iv) to demonstrate the early development of dense-granules in cells prior to fragmentation. Scale bar: 50 µm (panels i-ii) and 20 µm (panels iii-iv). (B) TEM thin section of an Mk from day 11 of the coculture. G, granules; IMS, invaginated membrane system; N, nucleus.
Since day 12 MPs contained ∼16% CD62P+ MPs with PMP characteristics, we examined if PMPs generated from activated human platelets by thrombin or the calcium ionophore A23187 could have an effect similar to that of MkMPs. Compared with vehicle control, coculture of day 3 HPCs (from cultures with or without TPO) with either type of PMPs did not affect the Mk differentiation of HPCs compared with control (Figure 5Aii,D-G). These data demonstrate that PMPs cannot promote Mk differentiation of HPCs, thus suggesting that the day 12 MP effect derives from the ∼84% of CD62P− MkMPs.
Although the protocol for harvesting MkMPs employs rigorous centrifugal enrichment and triple washing in IMDM medium, we carried out experiments to show that the impact of MkMPs in promoting the Mk differentiation of HSPCs was not due to TPO attached to MkMPs (see supplemental Results).
Discussion
The pioneering study of Dunois-Lardé et al4 demonstrated the impact of high shear rate (1800 s−1) on cell remodeling and cytoskeleton reorganization of mature Mks leading to accelerated platelet generation. Here, we examined the impact of shear stress in the physiological range3 on DNA synthesis and the formation of various Mk particles (PLPs, PPTs, and MkMPs). DNA synthesis and PS externalization of immature Mks were enhanced by shear stress in an exposure time– and shear shear level–dependent manner. PS externalization, caspase-3 activation, and fragmentation of mature Mks were enhanced by shear stress. Enhanced PLP and PPT generation accompanied this phenomenon. This suggests that elevated hemodynamic stress would enhance platelet generation. In healthy adults, both acute and prolonged exercise leads to elevated platelet counts by ∼25% and at least 57%, respectively.40,41 Significantly, we demonstrated that not only mature Mks but also immature Mks can sense and then respond to shear stress. The latter may pertain to Mk progenitor cells circulating in blood under physiological or pathological conditions (supplemental Figure 1). For example, as stated above, whole Mks released into systemic circulation are eventually captured in the pulmonary vasculature where they are exposed to a broad range of biomechanical forces as they give rise to platelets (supplemental Figure 1 and supplemental Table 1).4-7
Furthermore, shear stress dramatically increased MkMP generation by 30- to 40-fold. Flaumenhaft et al38 demonstrated that the CD41+ MPs in human plasma are mainly derived from Mks rather than activated platelets. When mature Mks enter BM sinusoids and are exposed to shear circulatory forces, numerous MkMPs are likely formed in addition to platelets (supplemental Figure 2). PMP generation from platelets on immobilized VWF surfaces is also promoted by high shear.42 While the cellular mechanisms leading to membrane vesiculation and MP release remain an active research field, studies from PMP biogenesis suggest that PS externalization and caspase-3 activation play an important role in MP generation.34 In our study, we found that caspase-3 activation and PS externalization were enhanced by shear stress, thus suggesting that shear–stress–enhanced MkMP generation may be mediated by PS externalization and caspase-3 activation. The latter is supported by the data from the caspase-3 inhibition assays.
A potential physiological function for MkMPs was also investigated. We demonstrated that MkMPs promote the survival and Mk differentiation of HSPCs in the absence of added TPO. Thus, one possible role for MkMPs in circulation may be to promote differentiation of circulatory HSPCs or perhaps re-enter the hematopoietic BM compartment aiming to target HSPCs for accelerated megakaryopoiesis under stress (supplemental Figure 2). This would be somewhat analogous to a recently proposed model of stem-cell plasticity.43 A similar role has been suggested from experiments employing MPs from other cell types. MPs generated during macrophage differentiation of THP-1 cells induced differentiation of resting THP-1 cells into macrophages through microRNA-223 transfer.44 Deregibus et al45 found that endothelial-progenitor cell-derived MPs carried messenger RNA to and triggered angiogenesis of endothelial cells. Thus, it is possible that MkMPs act in the similar way to deliver RNAs carrying Mk differentiation signals to HSPCs. Using ribonuclease digestion studies, we found that ribonuclease treatment did not totally abolish the effect of MkMPs (data not shown), thus suggesting that MkMPs promote HPC differentiation only partially through RNA. Martínez et al46 found that T-cell microvesicles induced differentiation of K562 and CD34+ cells toward Mk lineage, and hedgehog morphogens harbored by microvesicles were responsible for this effect. Thus, it is possible that MkMPs carry Mk differentiation signals in the form of proteins or lipids. These signals and the mechanisms by which MkMPs target and enter HSPCs are currently under investigation in our laboratory.
Although it was recently reported that caspase-3 and the classical apoptotic pathways are dispensable for steady-state platelet biogenesis in mice,47-49 other studies have shown that activation of caspase-3 and 9 is required for PPT formation.29,50,51 It is not clear how these reports can be reconciled. What is certain is that Mk cells are destructed by some death mechanism, and that there may exist several such death mechanisms including canonical apoptosis, as well as others, that may be engaged in restoring platelet biogenesis in mice, whereby core components of canonical apoptosis have been deleted.47-49 While canonical apoptosis may be indeed dispensable in steady-state murine platelet biogenesis, logically, this does not prove that canonical apoptosis of Mks is not involved in normal Mk fragmentation that leads to platelet biogenesis. This is supported by in vivo murine data, whereby deletion of the key antiapoptotic component, Bcl-xL, of canonical apoptosis affects transient but not steady-state platelet biogenesis.52 Significantly, in humans, there is strong evidence that some components, at least of canonical apoptosis, are necessary for platelet biogenesis.53 Furthermore, caspase-3 and other caspases are expressed in platelets and are important in platelet activation, aggregation, and other events necessary for thrombopoiesis.35,54-56 In the context of MkMP formation and the engagement of caspase-3, the involvement and importance of proteolytic caspase activity independent of cell death in the generation at endothelial microparticles is worth noting.57 A more recent report shows that mechanical forces stimulate the generation of endothelial microparticles independent of canonical apoptosis, but requires the action of caspases.36 Another study by Abid Hussein et al58 shows that various types of circulating MPs from healthy human plasma contain active caspase-3, including CD61+ MPs, most of which are likely derived from Mks.38 It could well be then that the generation of MkMPs does not require canonical apoptosis but rather caspase activity, and as we show in this study, PS externalization as well.
Acknowledgments
The authors thank Jeffrey Caplan (Bioimaging Center, University of Delaware) for assistance with immunofluorescence microscopy and Dongjun Li (former member in Wolfe Laboratory) for assistance with platelet collection.
This work was supported by a grant from the National Institutes of Health, National Heart, Lung and Blood Institute (R21HL106397).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.T.P. and J.J. designed the study; J.J. carried out experiments and analyzed the data; E.T.P. and J.J. wrote the manuscript; D.S.W. assisted with the preparation of PMPs and functional assays of PLPs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eleftherios T. Papoutsakis, Delaware Biotechnology Institute, 15 Innovation Way, Newark, DE 19711; e-mail: epaps@udel.edu.
References
- 1.Schulze H, Shivdasani RA. Mechanisms of thrombopoiesis. J Thromb Haemost. 2005;3(8):1717–1724. doi: 10.1111/j.1538-7836.2005.01426.x. [DOI] [PubMed] [Google Scholar]
- 2.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44(4):575–582. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- 3.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 4.Dunois-Lardé C, Capron C, Fichelson S, Bauer T, Cramer-Bordé E, Baruch D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009;114(9):1875–1883. doi: 10.1182/blood-2009-03-209205. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes R, Wang Y, Hirsch J, et al. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120(11):3917–3922. doi: 10.1172/JCI43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157(1):69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geddis AE, Kaushansky K. Immunology: the root of platelet production. Science. 2007;317(5845):1689–1691. doi: 10.1126/science.1148946. [DOI] [PubMed] [Google Scholar]
- 9.Illi B, Scopece A, Nanni S, et al. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96(5):501–508. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288(4):H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Takahashi T, Asahara T, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol (1985) 2003;95(5):2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 12.Obi S, Masuda H, Shizuno T, et al. Fluid shear stress induces differentiation of circulating phenotype endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303(6):C595–C606. doi: 10.1152/ajpcell.00133.2012. [DOI] [PubMed] [Google Scholar]
- 13.Ye C, Bai L, Yan ZQ, Wang YH, Jiang ZL. Shear stress and vascular smooth muscle cells promote endothelial differentiation of endothelial progenitor cells via activation of Akt. Clin Biomech (Bristol, Avon) 2008;23(Suppl 1):S118–S124. doi: 10.1016/j.clinbiomech.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Knippenberg M, Helder MN, Doulabi BZ, Semeins CM, Wuisman PIJM, Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11(11-12):1780–1788. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 15.Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol Bioeng. 2007;96(3):584–595. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- 16.Zeng L, Xiao Q, Margariti A, et al. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174(7):1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardy Y, Resnick N, Nagel T, Gimbrone MA, Jr, Dewey CF., Jr Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol. 1997;17(11):3102–3106. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo R, Gaetano C, Melillo G, Toschi E, Remuzzi A, Capogrossi MC. Shear stress downregulation of platelet-derived growth factor receptor-beta and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation. 2000;102(2):225–230. doi: 10.1161/01.cir.102.2.225. [DOI] [PubMed] [Google Scholar]
- 19.Leytin V, Allen DJ, Mykhaylov S, et al. Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun. 2004;320(2):303–310. doi: 10.1016/j.bbrc.2004.05.166. [DOI] [PubMed] [Google Scholar]
- 20.Shive MS, Salloum ML, Anderson JM. Shear stress-induced apoptosis of adherent neutrophils: a mechanism for persistence of cardiovascular device infections. Proc Natl Acad Sci USA. 2000;97(12):6710–6715. doi: 10.1073/pnas.110463197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shive MS, Brodbeck WG, Colton E, Anderson JM. Shear stress and material surface effects on adherent human monocyte apoptosis. J Biomed Mater Res. 2002;60(1):148–158. doi: 10.1002/jbm.10035. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald TN, Shepherd BR, Asada H, et al. Laminar shear stress stimulates vascular smooth muscle cell apoptosis via the Akt pathway. J Cell Physiol. 2008;216(2):389–395. doi: 10.1002/jcp.21404. [DOI] [PubMed] [Google Scholar]
- 23.Metallo CM, Vodyanik MA, de Pablo JJ, Slukvin II, Palecek SP. The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol Bioeng. 2008;100(4):830–837. doi: 10.1002/bit.21809. [DOI] [PubMed] [Google Scholar]
- 24.Baj-Krzyworzeka M, Majka M, Pratico D, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30(5):450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrken PG, Apostolidis PA, Lindsey S, Miller WM, Papoutsakis ET. Tumor suppressor protein p53 regulates megakaryocytic polyploidization and apoptosis. J Biol Chem. 2008;283(23):15589–15600. doi: 10.1074/jbc.M801923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mostafa SS, Miller WM, Papoutsakis ET. Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. Br J Haematol. 2000;111(3):879–889. [PubMed] [Google Scholar]
- 27.Chen C, Fuhrken PG, Huang LT, et al. A systems-biology analysis of isogenic megakaryocytic and granulocytic cultures identifies new molecular components of megakaryocytic apoptosis. BMC Genomics. 2007;22(8):384. doi: 10.1186/1471-2164-8-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apostolidis PA, Woulfe DS, Chavez M, Miller WM, Papoutsakis ET. Role of tumor suppressor p53 in megakaryopoiesis and platelet function. Exp Hematol. 2012;40(2):131–142, e4. doi: 10.1016/j.exphem.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100(4):1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 30.Huang HL, Chen YC, Huang YC, et al. Lapatinib induces autophagy, apoptosis and megakaryocytic differentiation in chronic myelogenous leukemia K562 cells. PLoS ONE. 2011;6(12):e29014. doi: 10.1371/journal.pone.0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenwaelder SM, Yuan Y, Josefsson EC, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114(3):663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 32.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 33.Panuganti S, Schlinker AC, Lindholm PF, Papoutsakis ET, Miller WM. Three-stage ex vivo expansion of high-ploidy megakaryocytic cells: toward large-scale platelet production. Tissue Eng Part A. 2013;19(7-8):998–1014. doi: 10.1089/ten.tea.2011.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335(1):143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 35.Shcherbina A, Remold-O’Donnell E. Role of caspase in a subset of human platelet activation responses. Blood. 1999;93(12):4222–4231. [PubMed] [Google Scholar]
- 36.Vion AC, Birukova AA, Boulanger CM, Birukov KG. Mechanical forces stimulate endothelial microparticle generation via caspase-dependent apoptosis-independent mechanism. Pulm Circ. 2013;3(1):95–99. doi: 10.4103/2045-8932.109921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thon JN, Macleod H, Begonja AJ, et al. Microtubule and cortical forces determine platelet size during vascular platelet production. Nat Commun. 2012;3:852. doi: 10.1038/ncomms1838. [DOI] [PubMed] [Google Scholar]
- 38.Flaumenhaft R, Dilks JR, Richardson J, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113(5):1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 40.Davis RB, Boyd DG, McKinney ME, Jones CC. Effects of exercise and exercise conditioning on blood platelet function. Med Sci Sports Exerc. 1990;22(1):49–53. [PubMed] [Google Scholar]
- 41.Wang JS, Jen CJ, Kung HC, Lin LJ, Hsiue TR, Chen HI. Different effects of strenuous exercise and moderate exercise on platelet function in men. Circulation. 1994;90(6):2877–2885. doi: 10.1161/01.cir.90.6.2877. [DOI] [PubMed] [Google Scholar]
- 42.Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood. 2006;107(9):3537–3545. doi: 10.1182/blood-2005-02-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quesenberry PJ, Dooner MS, Aliotta JM. Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol. 2010;38(7):581–592. doi: 10.1016/j.exphem.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–995. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 46.Martínez MC, Larbret F, Zobairi F, et al. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood. 2006;108(9):3012–3020. doi: 10.1182/blood-2006-04-019109. [DOI] [PubMed] [Google Scholar]
- 47.Josefsson EC, James C, Henley KJ, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208(10):2017–2031. doi: 10.1084/jem.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Debrincat MA, Josefsson EC, James C, et al. Mcl-1 and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood. 2012;119(24):5850–5858. doi: 10.1182/blood-2011-12-398834. [DOI] [PubMed] [Google Scholar]
- 49.Josefsson EC, Burnett DL, Lebois M, et al. Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat Commun. 2014;5:3455. doi: 10.1038/ncomms4455. [DOI] [PubMed] [Google Scholar]
- 50.Clarke MCH, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003;160(4):577–587. doi: 10.1083/jcb.200210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozuma Y, Yuki S, Ninomiya H, Nagasawa R, Kojima H. Caspase activation is involved in early megakaryocyte differentiation but not in platelet production from megakaryoyctes. Leukemia. 2009;23(6):1080–1086. doi: 10.1038/leu.2009.7. [DOI] [PubMed] [Google Scholar]
- 52.Kaluzhny Y, Yu G, Sun S, et al. BclxL overexpression in megakaryocytes leads to impaired platelet fragmentation. Blood. 2002;100(5):1670–1678. doi: 10.1182/blood-2001-12-0263. [DOI] [PubMed] [Google Scholar]
- 53.Morison IM, Cramer Bordé EM, Cheesman EJ, et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40(4):387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 54.Leytin V. Apoptosis in the anucleate platelet. Blood Rev. 2012;26(2):51–63. doi: 10.1016/j.blre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Rosado JA, Lopez JJ, Gomez-Arteta E, Redondo PC, Salido GM, Pariente JA. Early caspase-3 activation independent of apoptosis is required for cellular function. J Cell Physiol. 2006;209(1):142–152. doi: 10.1002/jcp.20715. [DOI] [PubMed] [Google Scholar]
- 56.Cohen Z, Wilson J, Ritter L, McDonagh P. Caspase inhibition decreases both platelet phosphatidylserine exposure and aggregation: caspase inhibition of platelets. Thromb Res. 2004;113(6):387–393. doi: 10.1016/j.thromres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Sapet C, Simoncini S, Loriod B, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108(6):1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 58.Abid Hussein MN, Nieuwland R, Hau CM, Evers LM, Meesters EW, Sturk A. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J Thromb Haemost. 2005;3(5):888–896. doi: 10.1111/j.1538-7836.2005.01240.x. [DOI] [PubMed] [Google Scholar]