Abstract
Bone tissue engineering promises to restore bone defects that are caused by severe trauma, congenital malformations, tumors, and nonunion fractures. How to effectively promote the proliferation and osteogenic differentiation of mesenchymal stem cells (MSCs) or seed cells has become a hot topic in this field. Many researchers are studying the ways of conferring a pro-osteodifferentiation or osteoinductive capability on implants or scaffold materials, where osteogenesis of seed cells is promoted. Graphene (G) provides a new kind of coating material that may confer the pro-osteodifferentiation capability on implants and scaffold materials by surface modification. Here, we review recent studies on the effects of graphene on surface modifications of implants or scaffold materials. The ability of graphene to improve the mechanical and biological properties of implants or scaffold materials, such as nitinol and carbon nanotubes, and its ability to promote the adhesion, proliferation, and osteogenic differentiation of MSCs or osteoblasts have been demonstrated in several studies. Most previous studies were performed in vitro, but further studies will explore the mechanisms of graphene's effects on bone regeneration, its in vivo biocompatibility, its ability to promote osteodifferentiation, and its potential applications in bone tissue engineering.
Introduction
Bone is a dynamic organ that has the ability to repair minor injuries by remodeling. However, for large bone defects caused by severe trauma, congenital malformations, tumors, and nonunion fractures, remodeling is limited. Bone tissue engineering, which aims at regenerating bone, is a promising solution for this problem. In essence, it requires a scaffold that enables cell attachment and maintenance of cell function, along with a rich source of seed cells combined with selected osteoinductive growth factors.1 Multiple materials have been explored as scaffolds, but some challenges remain, such as plastic deformation, thrombosis and, especially, and lack of osteoinductive or pro-osteodifferentiative potential. As seed cells, mesenchymal stem cells (MSCs) have multilineage potential and can differentiate into osteoblasts, adipocytes, and chondrocytes.2 The effective promotion of the osteogenic differentiation of MSCs is one of the core issues in this field. In the past, multiple growth factors and inducers were administered to promote differentiation. However, most of them are complicated and expensive to produce and are easily degraded in vivo.3 Therefore, pursuing active osteoinductive or pro-osteodifferentiative properties that confer stable and long-lasting promotive or inductive effects in vivo on implants or scaffold materials has become a hot topic. Graphene (G) may have the potential to confer the pro-osteodifferentiation capability on implants or scaffold materials and to even promote the osteogenesis of MSCs based on current investigations.4–12 Graphene comprises excellent physicochemical and structural properties, such as high elasticity and mechanical strength and large surface area13,14; therefore, it is very interesting to further examine whether graphene can be a promising nano-material for promoting surface modifications of implants or scaffold materials in bone tissue engineering.
Graphene and Its Derivatives
The Sp2-carbon nanomaterial family typically includes zero-dimensional fullerene, one-dimensional (1D) carbon nanotubes (CNTs), two-dimensional (2D) graphene, and three-dimensional (3D) diamond and graphite. Graphene has been discussed in theory for about 60 years. In 2004, graphene was first isolated by Novoselov and Geim.13 Since then, graphene has attracted much interest from various scientific fields, and its discovery won the 2010 Nobel Prize in physics.
Graphene is a one-atom-thick sheet of carbon atoms arranged in a 2D honeycomb structure. The strong carbon-carbon bonding in the plane, the aromatic structure, the presence of free π electrons, and reactive sites for surface reactions make graphene a unique material with excellent properties: the thinnest material ever measured; a large specific surface area (2600 m2 g−1)15; a Young's modulus of 1 TPa and intrinsic strength of 130 GPa, which is close to that predicted by theory16,17; a high room temperature electron mobility of 2.5×105 cm2 V−1 s−1 (theoretical limit ∼2×105 cm2 V−1 s−1)18,19; very high thermal conductivity, reaching 5000 W m−1 K−1; optical adsorption of exactly π≈2.3%20,21; and complete impermeability to any gases.22 Its room temperature quantum Hall Effect and room temperature ferromagnetism are also reported.23,24 In addition, graphene can be synthesized in a relatively pure form, meaning that biological cell testing would be less affected by impurities.25–29 However, some of these characteristics have been achieved only for the highest-quality samples (mechanically exfoliated graphene13) and for graphene deposited on special substrates such as hexagonal boron nitride.18,30 Equivalent characteristics have not been observed on graphene prepared using other techniques.
The market for graphene applications is essentially driven by progress in the production of graphene with properties that are appropriate for its specific application. Currently, graphene can be prepared using mechanical exfoliation,13 chemical vapor deposition (CVD),31 epitaxial growth,32 and chemical exfoliation.33 Among them, CVD has emerged as an important and the most successful method for the production of large-scale and high-quality graphene sheets for various applications, including bone tissue engineering, as the method was first reported in 2009.34,35 After growth of large-scale graphene by CVD on copper foils or nickel film, the copper or nickel are etched and the graphene can be transferred to distinct substrates using polymethylmethacrylate, according to the experimental design.36 By contrast, mechanical exfoliation suffers from low yields, making it difficult to apply to industrial-scale production.15
Graphene oxide (GO) is an oxidized derivative of graphene.37 The most widely used approach to GO synthesis is based on the principle first introduced by Hummers and Offeman (commonly referred to as Hummers method), which involves the oxidation of graphite by treatment with potassium permanganate and sulfuric acid.38 Many techniques have been used in the fabrication of GO thin films, such as spin coating,39,40 Langmuir–Blodgett techniques,41,42 flow-directed assembly,43 evaporation-induced self-assemblies on water,44 and the bubble-deposition method.45 GO contains a range of reactive oxygen functional groups, including epoxide, carboxyl, and hydroxyl groups. They are presented on the basal plane and edges of GO and enable greater interactions with proteins through covalent, electrostatic, and hydrogen bonding. GO sheets, because of their derivatization, are more hydrophilic, and the hydrogen bonds between their polar functional groups and water molecules offer reasonable colloidal dispersibility under appropriate pH conditions.46
Reduced graphene oxide (rGO) can be obtained by thermal, chemical, and UV treatment of GO under reducing conditions with hydrazine or other reducing agents.47 It is mainly produced to restore the electrical conductivity and optical absorbance of GO while reducing the oxygen content, surface charge, and hydrophilicity.48
For better utilization of graphene in biology and medicine, appropriate functionalization of pristine graphene and the immobilization of biomaterials on it are necessary. This is important in biocompatibility, because the functional groups can create defects on the graphene surface and reduce the strong hydrophobic interaction of graphene with cells and tissues.49 Biomolecules such as proteins, DNA, and small molecules have been reported to functionalize graphene. The biological activity of graphene-based materials can be associated with their capacities to affect molecular processes and functions.50
Based on current experiments with regard to cell behaviors in these materials (Table 1), the applications of graphene and its derivatives can be categorized into three types: G sheet,4,5,51 GO flakes,6,8,9,11,12,52,53 and rGO flakes, which are directly reduced from GO.7,10,54 Although GO or rGO films and sheets have been used in different experiments, the main form of a single layer of GO or rGO film or sheet is as a flake or microflake with a diameter less than several micrometers.5–7,10,51–53 However, CVD-grown graphene can be formed as a large-scale sheet as long as a sufficiently large substrate is used for growing the product, Currently, it is easy to coat scaffolds or substrates with a planar or regular surface. However, GO and rGO can be easily coated onto scaffolds with irregular or 3D structures, and they can be easily grafted with other chemicals and form new hybrid surfaces or materials.8–10,12,52,53
Table 1.
The Cell Behaviors on Graphene and Its Derivatives
| Graphene types | Preparation methods | Application modes | Cell types | Outcomes | Possible mechanisms | Reference |
|---|---|---|---|---|---|---|
| G | CVD | Sheet | Human OB, hMSCs | The cells on G adhered and proliferated better than on an SiO2 substrate | The electrical conductivity of G is of particular importance, because electricity as well as cocktails of growth factors and substrate properties is able to stimulate cell growth and differentiation. | Kalbacova et al.51 |
| G | CVD | Sheet | hMSCs | G provides a promising biocompatible scaffold that does not curb the proliferation of hMSCs. The osteogenic differentiation rate induced by G is comparable to BMP-2. | Surface morphology; lateral stress | Nayak et al.4 |
| G, GO | CVD | Sheet | hMSCs | The strong noncovalent binding abilities of G enable it to act as a preconcentration platform for osteogenic inducers, which accelerate the osteogenesis of MSCs. However, G suppresses adipogenesis of MSCs. GO does not interfere with adipogenesis of MSCs. | G has the ability to bind dexamethasone and β-glycerolphosphate, which is good for osteogenesis. Insulin is denatured on adsorption of G through a strong π-π interaction. GO does not interfere with adipogenesis due to electrostatic binding with insulin. | Lee et al.5 |
| GO | Modified Hummers method | Nanosheets | hASCs | The GO film proved to be a suitable environment for the time-dependent viability of hASCs. The enhanced differentiation of hASCs included osteogenesis, adipogenesis, and epithelial genesis; while the chondrogenic differentiation of hASCs was decreased, compared tissue culture polystyrene as a control substrate. | It is hypothesized that a combination of factors, including nanoscale structure, strong stiffness, roughness, reactive oxygen functional groups, and adsorption of biomolecules on GO films, affects the hASCs behaviors. | Kim et al.6 |
| rGO | Modified Hummers method; Spin coating | Film | hFOB | rGO is biocompatible with hFOB, whereas single-walled carbon nanotube network is inhibitory to the proliferation of hFOB. | The distinct nanotopographic features of these two kinds of nanocarbon substrates cause the difference. | Agarwal et al.54 |
| GO-CaCO3 G-CaCO3 GO-HA G-HA |
Modified Hummers method | Film | Mouse OB | Compared with bare GO and G films, GO/G-CaCO3 composites exhibited remarkably enhanced HA formation when incubated in a simulated body fluid solution. The GO/G-HA composites supported high viability of OB with an elongated morphology. | GO/G-HA hybrid materials induce a 3D matrix adhesion of OB with high cell viability and provide a similar microenvironment to that found in vivo | Kim et al.52 |
| FRGO | Hummer's method; low-temperature thermal reduction method | Film | OB | The surface oxygen content of FRGO significantly influences the cellular behaviors, with the best performance for cell attachment, proliferation, calcium deposition, and collagen secretion being obtained in moderately reduced FRGO. Cell performance significantly decreased as the FRGO was highly reduced. Moderate performance was found in nonreduced pure GO and control glass slides. | Enhancement of cell adhesion and proliferation may be induced by enhanced extracellular matrix protein adsorption in moderately reduced FRGO by noncovalent interactions. | Shi et al.7 |
| Nylon 6, 6-GO hybrid | Hybrid Nylon 6,6 with 0.25 wt% graphene oxide. | Film | Mouse pre-OB | The cell attachment and proliferation on Nylon 6, 6-GO hybrid polymer greatly exceed those of Nylon 6,6. | The incorporation of GO in Nylon 6,6 matrix modifies the physiochemical properties of the surface, including chemistry and wettability, favoring attachment, cell-substrate interactions, and biological response required for cell attachment and proliferation. Furthermore, the negative polarity of G is instrumental in favorably influencing biological function. | Misra and Chaudhari53 |
| Chitosan-GO scaffolds | Covalent linking of the carboxyl groups of GO with the amino groups of CS | Nanosheets | Mouse pre-OB | The covalent incorporation of GO into a chitosan network favorably modulated the biological response of osteoblasts, such that cell attachment, proliferation, and formation of extracellular matrix are significantly enhanced. | The significant enhancement of biological function is related to a combination of a number of physico-chemical factors, including a large surface area, nanoscale roughness, the presence of pendant groups, a hydrophilic nature, and a high water retention ability. | Depan et al.8 |
| GO-modified CS scaffold | The carboxyl groups of GO are covalently attached with the amine group of CS. | Nanosheets | Mouse pre-OB | Biological functions of the pre-OB including cell attachment, proliferation, growth and mineralization, are significantly enhanced in the presence of GO, compared with unmodified chitosan. | The difference of cell behavior is related to the degree and topography of protein adsorption on the scaffolds. BSA is widely spread on CS-GO as small globules, whereas the globules are large and randomly distributed on pure CS. This subtle but important difference in the adsorption of protein is responsible for the observed differences in cellular interactions. | Depan and Misra9 |
| rGO-CS substrata | Hummers method; hydrazine treatment; spin-coating | Nanosheets; film | hMSCs | G-incorporated CS substrate promotes adhesion and differentiation of hMSCs. | rGO-CS substrata with asymmetrical nanotopology and its secondary effects such as stiffness and roughness provided a suitable environment for adhesion and proliferation of hMSCs as well as enhanced cell-substrata interactions and cell-cell contacts. | Kim et al.10 |
| 3D GFs | Growing graphene on 3D Nickel scaffolds | Film | hMSCs | 3D GFs can maintain hMSCs viability and promote osteogenic differentiation without the need for extrinsic biochemical manipulation. | The mechanism driving spontaneous differentiation remains unclear, but is suspected to be an intrinsic cellular response to the stress derived from the interaction with a high stiffness material. | Crowder et al.11 |
| GO-ac | Hummer's method; self-assemblies on water | Film | hMSCs | hMSCs show spontaneous osteogenic differentiation on rough GO-ac film without the use of any chemical inducers. The extent of mineralization increases with increasing roughness of the GO-ac film. | The rough topology of the cross-linked film creates increased cytoskeletal tension to grow and differentiate. The rough surface topology provides more anchoring points for the adhesion and proliferation of hMSCs. | Tang et al.12 |
G, graphene; GO, graphene oxide; rGO, reduced graphene oxide; OB,osteoblasts; BSA, bovine serum albumin; HA, hydroxyapatite; CS, chitosan; BMP-2, bone morphogenetic protein-2; CVD, chemical vapor deposition; FRGO, few-layer rGO films; GO-ac, acrylated GO; hASCs, human adipose-derived stem cells; hMSCs, human mesenchymal stem cells; hFOB, human fetal OB; MSC, mesenchymal stem cells; 3D, three-dimensional; 3D GFs, 3D graphene foams.
Graphene and its derivatives, owing to their unique optical, mechanical, chemical, and electrical properties, have been extensively studied by materials scientists, physicists, and chemists.31 Their uses are expanding beyond electronic and chemical applications toward biomedical areas, such as drug delivery, cancer therapies, biosensing, and tissue engineering.55,56
Biocompatibility or Toxicity of Graphene and Its Derivatives
So far, only a limited number of publications have attempted to address the interactions of graphene and its derivatives with living systems.57,58 However, existing studies with regard to the biocompatible effects of G and its derivatives often show contradictory or inconclusive results.
Most of the in vitro studies have reported that cell viability decreases after exposure to graphene and its derivatives at concentrations around 10 μg mL−1.59–62 Chng and Pumera investigated the cytotoxic effects of GOs prepared by different oxidative treatments (the Staudenmaier, Hoffmann, Hummers, and Tour methods). The results showed differing cytotoxicities of GO. The extent of oxidation may play a critical role in toxicity imparted by GOs, which may stem from the oxygen content (C/O ratio) and the amount of carbonyl groups present.63 Furthermore, Liao et al. reported that the particle size, particulate state, oxygen content, and the surface charge of graphene have a large impact on the biological and toxicological response to red blood cells. At the smallest size, GO showed the greatest hemolytic activity, whereas aggregated graphene sheets exhibited the lowest hemolytic activity. Their study also demonstrated that compacted graphene sheets are more damaging to human skin fibroblasts than the less densely packed GO.60 As for the mechanism, Zhang et al. reported that CVD-grown graphene increased the activation of caspase 3 (apoptosis marker), release of lactate dehydrogenase, and generation of reactive oxygen species (ROS) in neural pheochromocytoma-derived PC12 cells.64 Liao et al. also reported that aggregated graphene sheets induced a high level of ROS in human skin fibroblasts.60 However, when cancer cell lines were used, decreases in cell viability were lower in most cases.59,65,66
There are a few in vivo studies with regard to the biocompatibility of graphene and its derivatives.57,58 Sahu et al. have developed a simple method to prepare a thermosensitive hydrogel system based on nano-sized GO by adding a small amount of Pluronic block copolymer as a physical crosslinker, without any chemical modification of GO. It was reported that the gel formation was long lasting and stable. When injected subcutaneously into mice, the histological investigation revealed that these GO-Pluronic hydrogels did not show any severe chronic inflammatory response and might be biocompatible for future in vivo applications.67 Duch et al. directly administered solutions of aggregated graphene, Pluronic dispersed graphene and GO into the lungs of mice and observed that GO caused severe persistent lung inflammation. In contrast, this toxicity was significantly reduced in the case of pristine graphene after liquid phase exfoliation, and was further minimized when the unoxidized graphene was well dispersed with the block copolymer Pluronic. It also demonstrated that the covalent oxidation of graphene is a major contributor to its pulmonary toxicity and suggested that the dispersion of pristine graphene in the Pluronic provides a pathway for the safe handling and potential biomedical application of 2D carbon nanomaterials.68 As for in vivo studies on the biocompatibility in other living organisms, Gollavelli and Ling showed that multi-function graphene (coated with polyacrylic acid and fluorescein o-methacrylate) was biocompatible with zebrafish and did not induce any significant abnormalities.69 Yan et al. found that an intravitreal injection of GO into rabbits' eyes did not lead to gross changes in the eyeballs' appearance, intraocular pressure, electroretinogram, or on histological examination.70 Among the in vivo studies, only one study has reported possible long-term toxicology of graphene in living systems. It is reported that PEGylated nanographene sheets did not cause appreciable toxicity to treated mice over a period of 3 months, as evidenced by blood biochemistry, hematological analysis, and histological examinations.71 The in vivo effect of graphene and its derivatives might be related to their method of synthesis, physicochemical properties, concentration, time of exposure, administration route, and choice of animals used. Most studies report no occurrence of adult animal death.67,68,70,71 Most of the available literature considers graphene and its derivatives to be hemocompatible.71,72 However, several reports showed an inflammatory reaction.67,68 In summary, the literature is still limited and not sufficient to reach any conclusions. Further investigations are needed for confirmation.
It should be mentioned that most of the available data with regard to the toxicity and biocompatbility of graphene and its derivatives were obtained from studies on its potential use as a vehicle in drug delivery.57,58,67,71,73 In this case, graphene and its derivatives are normally systemically administered,67,71 or directly administered to organs of animals.68,70 Thus, their biological reactions may be more obvious and direct. By contrast, when they are applied as scaffold materials in bone tissue engineering, G and its derivatives will be more locally sequestered in situ, and the inflammation or toxic reaction may be quite different from systemic administration. However, little is known about the inflammation or toxic reaction of graphene and its derivatives when they are used as scaffolds in constructing tissue-engineered bones, because in vivo bone regenerative models of graphene or its derivatives have not been available.
The Effects of Graphene on the Surface Modification of Implant or Scaffold Materials
Graphene, as a 2D, single-atom-thick nanomaterial, can be coated on implants or scaffold materials. The graphene coat will enhance biocompatibility, mechanical, and other properties. Graphene has a great potential as a surface modification material for implants and scaffolds.
Graphene coatings for enhanced hemo- and biocompatibility of nitinol stents
The novel material nitinol (NiTi) is often used in biomedical implant manufacturing due to its superior properties.74,75 However, its metal nature results in metal leaching, lack of cell adhesion, and proliferation and thrombosis when in contact with flowing blood, which limits its application. An ideal surface coating is expected to have high adhesion strength, be chemically inert, and show hemo- and biocompatibility. Previously, numerous materials, including diamond-like carbon (DLC), SiC, TiN, and many polymer materials, have been tested, but found wanting.76–78 For example, DLC coatings show reduced wear, corrosion, and debris formation. DLC coatings also reduced thrombogenicity by minimizing platelet adhesion and activation. However, some contradictory results were reported, suggesting that no significant improvement could be observed. In addition, instability of the DLC coating caused by its high level of residual stress and poor adhesion in aqueous environments should be carefully considered.76 Podila et al. used graphene-coated NiTi (Gr-NiTi) as a scaffold and found that graphene met all the earlier criteria. Gr-NiTi supported excellent smooth muscle and endothelial cell growth, leading to better cell proliferation. They also showed that the serum albumin adsorption on Gr-NiTi was higher than fibrinogen, an important and well-known criterion for promoting a lower thrombosis rate. In addition, spectroscopic measurements confirmed the lack of charge transfer between graphene and fibrinogen, suggesting that graphene coatings inhibited platelet activation on the surface of the implants.79 These hemo- and biocompatible properties, along with their high strength, chemical inertness, and durability, indicate that graphene is an ideal coating for biomedical implants and devices.
Graphene coatings for improving CNTs' resistance to fatigue
CNTs are another kind of carbon nanomaterial that combine elasticity, mechanical resilience, and low density, and they have been exploited as scaffold materials.80 However, all CNT-based materials explored to date have undergone structural collapse or significant plastic deformation, with a reduction in compressive strength, which limits their applications.81–83 Kim et al. fabricated graphene-coated CNTs and found that they exhibited no change in mechanical properties after more than 1×106 compressive cycles, and their original shape recovered quickly after compression release. Moreover, the coating did not affect the structural integrity of the nanotubes or the compressibility and porosity of the nanotube network. The coating also increased Young's modulus and energy storage.84 The application of graphene coating to improve CNTs' properties will make these materials attractive for artificial muscles and scaffolds for bone tissue engineering.
Reinforcement effects of graphene materials on polymer polyprophlenefumarate
Polyprophlenefumarate (PPF), an injectable, cross-linkable, and biodegradable polymer, has been widely investigated for applications in bone tissue engineering as the polymer matrix. A major motivation behind these studies is to enhance the mechanical properties of the biodegradable polymer for improved structural integrity when implanted under load-bearing conditions.85 Lalwani et al. reinforced PPF using various 2D nanostructures: single- and multi-walled GO nanoribbons, and GO nanoplatelets. Compression and flexural testing showed significant enhancement in the mechanical properties of the 2D-reinforced PPF nanocomposites, which were better when compared with 1D nanostructures (single- or multi-walled CNTs). The result also indicated that the extent of mechanical reinforcement was closely dependent on the nanostructure morphology and followed the trend of nanoplatelets>nanoribbons>nanotubes. The study demonstrated that harnessing the reinforcing potential of 2D nanostructures could lead to a whole new class of ultrastrong, lightweight biomaterials for tissue engineering applications.86
The Effects of Graphene on the Adhesion, Proliferation, and Differentiation of MSCs
As a possible surface modification material in bone substitutes, the effects of graphene on MSCs or osteoblasts (osteogenically differentiated MSCs) have been investigated (Table 1).
The adhesion of MSCs on graphene
Adhesion is a crucial prerequisite to many cell functions, such as proliferation, synthesis of proteins, and formation of mineral deposits. Kalbacova et al. reported that bone marrow-derived MSCs (BMMSCs) cultured on SiO2 substrates formed focal adhesions (FAs) that were large and homogenously distributed on the cell periphery, resulting in a state of quiescence. By contrast, the FAs in contact with CVD grown graphene films are smaller, weaker, and concentrated on the protruding ends of the cells, which corresponds to an active state of the cells.51 However, Kim et al. found that adipose-derived stem cells (ASCs), with similar characteristics to BMMSCs, showed increased adhesion when grown on GO films, indicated by a large number of FAs and a higher correlation between the orientations of actin filaments and vinculin bands compared with ASCs grown on glass substrates.6 In addition, Some et al. discovered that ASCs attached at higher levels to graphene derivative-poly (L-lysine) (PLL) composite-coated coverslips compared with coverslips coated with only GO or PLL.87 In another study by Kim and his colleagues, rGO-chitosan substrata were fabricated after spin coating of rGO-chitosan composites on bare glass. They found that rGO-chitosan substrata, regardless of the incorporated graphene concentration, provided a suitable environment for hMSC adhesion: hMSCs on the rGO-chitosan substrata showed a large number of FAs and enhanced expression of the integrin β1.10 Other studies have reported that osteoblasts or preosteoblasts, when cultured on chitosan-GO scaffolds, Nylon 6,6-GO hybrid, and few-layer reduced graphene oxide films (FRGO), showed better cell adhesion.7–9,53
Therefore, there is still some controversy in terms of cell adhesion morphology when MSCs are in contact with G or GO. The inconsistency may be accounted for by the difference in cell types, the graphene derivatives, the substrates, and the manufacturing methods of G or GO.
The proliferation of MSCs on graphene
After incubation for 48 h, Kalbacova et al. found that BMMSCs homogenously covered the CVD grown graphene film in a confluent layer, but formed separate islands on the SiO2 substrate. Cell counting confirmed that more cells were present on the CVD-grown graphene film than on the SiO2 substrate.51 Likewise, Lee et al. reported a higher density of cells on CVD-grown graphene and GO substrates than that on a polydimethylsilxane (PDMS) substrate.5 Meanwhile, Nayak et al. studied the influence of graphene on cell growth using four substrates with variable stiffness and surface roughness, and discovered that, independent of the substrate (PDMS, polyethylene [PET], glass slide, or silicon wafer), there was no significant difference in cell viability between graphene-coated and uncoated substrates.4
Tang et al. explored cross-linked GO films as a biocompatible scaffold for MSCs and found that GO films also facilitated the growth of BMMSCs.12 For ASCs, Some et al. reported that a graphene derivative-PLL composite had more than double the ability to proliferate ASCs compared with PLL alone, indicating that these materials are not cytotoxic for the cells.87 In addition, the GO film and rGO-chitosan substrata (5%) proved to be a suitable environment for the time-dependent viability of ASCs and BMMSCs.6,10
In addition, the proliferation of osteoblasts or preosteoblasts on graphene-related materials, including rGO, GO/graphene-hydroxyapatite, chitosan-graphene oxide (CS-GO), and Nylon 6,6-GO, was enhanced.7–9,53,54
However, Kim et al. evaluated the cytotoxicity of GO in ASCs and found that a moderate concentration of GO (<0.1 mg/mL) resulted in good ASCs viability, but a high concentration of GO showed some cytotoxicity toward ASCs.6 Similarly, Wang et al. demonstrated that GO could induce significant cytotoxicity of human fibroblast cells at a concentration above 50 mg/L.88 In another study by Kim et al., the proliferation rate of hMSCs decreased with the incorporation of higher amounts of rGO into the rGO-chitosan substrata.10 On the other hand, G and GO exhibited negligible in vitro toxicity to BMMSCs, ASCs, or osteoblasts when they were used as coating materials, as mentioned earlier. Thus, further systemic studies are required to wholly understand the potential biological effects and to address concerns over health hazards before any practical application.
The differentiation of MSCs into osteogenic lineages on graphene
A study by Kim et al. showed that GO/graphene-CaCO3, hybrid materials exhibited remarkably enhanced hydroxyapatite formation when incubated in a simulated body fluid solution compared with bare GO and graphene films.52 Later, other studies indicated that the formation of extracellular matrix of osteoblasts or preosteoblasts on CS-GO and FRGO was significantly enhanced.7–9
In addition, positive results for the osteogenic differentiation of MSCs on graphene have been obtained.4–6,12 Nayak et al. cultured BMMSCs in osteogenic medium without bone morphogenetic protein-2 (BMP-2), which did not lead to osteogenic differentiation over the whole duration of the experiment (15 days). However, once the substrates (PDMS, PET, glass slide, and silicon wafer) were coated with CVD-grown graphene, BMMSCs successfully differentiated into osteoblasts, which was confirmed by quantitative alizarin red staining. Interestingly, both BMP-2-treated and CVD-grown graphene-coated substrates induced cell differentiation at the same rate, suggesting that graphene might be a driving force of bone cell formation.4 In another study, after 12 days of osteogenic induction, there was a sevenfold increase in the extent of mineralization in BMMSCs cultured on CVD-grown graphene compared with those on PDMS.5 Tang et al. reported spontaneous osteogenic differentiation of MSCs on a cross-linked GO film, without the use of any chemical inducers. Furthermore, the extent of mineralization increased with an increase in the roughness of the GO film. Thus, the GO film may represent a chemical-free method of inducing osteoblastic differentiation.12 The enhanced differentiation of ASCs, including osteogenesis and adipogenesis, on GO film has also been reported.6 In addition, Alizarin Red S staining and western blot analysis by Kim et al. showed up-regulation of the osteogenic differentiation of hMSCs in the rGO-chitosan substrata compared with the chitosan substrata and tissue culture polystyrene, in both the proliferative and osteogenic induction media.10 These recent investigations indicate an active or potential pro-osteodifferentiation capability of G or GO.
Cell behavior of MSCs on 3D graphene
All the reports mentioned earlier investigated stem cell behavior on 2D graphene sheets. Crowder et al. employed 3D graphene foams (3D GFs) as culture substrates for BMMSCs for the first time. GFs were fabricated by growing graphene on 3D nickel scaffolds, and the nickel was subsequently removed by FeCl3 etching. GFs were shown to maintain BMMSCs viability and to stimulate changes in morphology. When cultured on GFs for 7 days, BMMSCs exhibited spindle-shaped, elongated morphology with thin, aligned nuclei and strongly expressed both osteocalcin and osteopontin, indicating spontaneous osteogenic differentiation of BMMSCs without the need for extrinsic biochemical manipulation.11
Mechanism of Graphene's Positive Effects on the Proliferation and Osteogenic Differentiation of MSCs
The ability of graphene to promote the proliferation and osteogenic differentiation of BMMSCs and ASCs has been demonstrated (Fig. 1). It has been reported that topography, chemistry, and physical properties of biomaterials are critical parameters for directing cell fates.81 In recent years, the mechanism has been explored from different aspects (Table 1).
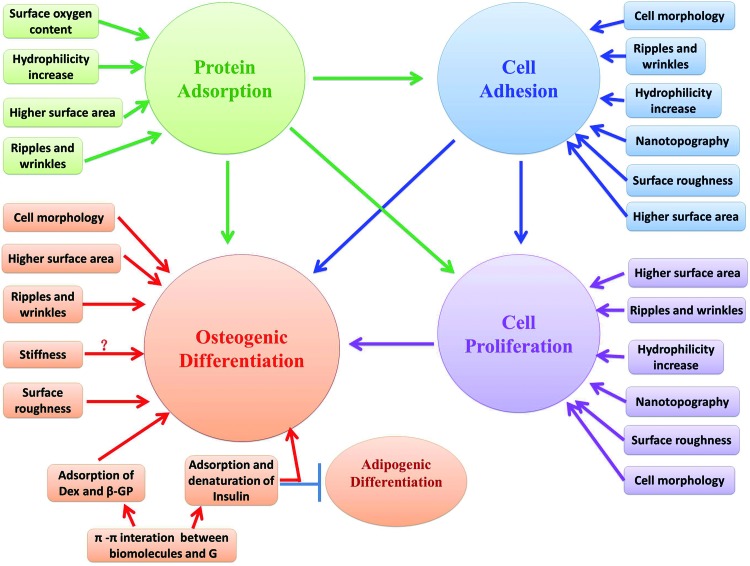
FIG. 1.
Schematic diagram depicting the possible mechanisms of the biological effects of graphenes on mesenchymal stem cells or osteoblastic cells in vitro. ? Means inconclusive results. Arrow, promotion; bar, inhibition. G, graphene; Dex, dexamethasone; β-GP, β-glycerophosphate. Color images available online at www.liebertpub.com/teb
Adhesion mechanism
FAs are large protein complexes that indicate the connections between cells and the extracellular matrix. FAs play a key role in the mediation of adhesion and migration of cells.89 Several studies have suggested that nanoscale structures of substrates may regulate FAs. Kim et al. hypothesized that the unique nanotopography of the GO film would influence the formation of FAs.6
Cell morphology mechanism
Micromorphology regulates multiple biological processes, including adhesion,90 proliferation,91 and differentiation.92 Moreover, studies suggest that cell shape is a key regulator in MSCs' commitment to osteoblasts.93 BMMSCs cultured on graphene exhibited a spindle shape with noticeable filopodia extensions and cellular protrusions.4,5,51 Thus, the elongated MSC morphology on graphene may permit higher rates of proliferation.
Mechanical mechanism
CVD graphene consists of many ripples and wrinkles on the micrometer scale, caused by the difference between the thermal expansion coefficients of Copper and graphene. rGO also showed wrinkles in the atomic force microscopy image because of the spin-coating process.54 Some et al. and Misra and Chaudhari assumed that the higher surface area might be the cause of higher adhesion and proliferation.53,87 Nayak et al. thought that the ripples and wrinkles might play a role in protein adsorption, cell adhesion, proliferation, and differentiation.4
Recent studies suggest that nanostructured and micro-rough topographies might also mediate osteogenic differentiation.94,95 The study by Tang a et al. proposed that the rough topology of the cross-linked GO film created increased cytoskeletal tension for hMSCs to grow, which had been shown to guide differentiation into osteoblasts.12 Kim et al. hypothesized that the unique characteristics of the nanoscale topographical (asymmetrical nanotopology) aspect of graphene and its secondary effects, such as stiffness and roughness within substrata, might play a crucial role in the enhancement of hMSC differentiation.10
Misra and Chaudhari reported that the addition of GO to Nylon 6,6 led to a small decrease in the contact angle, making the hybrid polymer relatively more hydrophilic. A hydrophilic surface is expected to influence cell assembly and conformation of cytoskeletal protein, such that hydrophilicity favors cell attachment and proliferation.53
Engler et al. found that stiff substrates promoted osteogenic differentiation.96 However, the elasticity of graphene and PDMS are in the same order of magnitude (∼3–7 MPa); therefore, the differences in cell differentiation were unlikely to be caused by differences in substrate stiffness. In addition, graphene is very thin; therefore, the substrate supporting graphene has a dominant influence on overall stiffness. Hence, Nayak et al. and Lee et al. speculated that stiffness is not a differentiating factor.4,5
Biochemical and molecular mechanisms
Lee et al. tested the adsorption capacity of the substrate and found that graphene adsorbed approximately 8% of serum proteins, compared with <1% adsorption on PDMS on day 1. Serum contains many extracellular matrix globular proteins and glycoproteins, such as fibronectin, which plays a major role in facilitating cell adhesion. Lee et al. also found that graphene had a higher adsorption capacity for dexamethasone and β-glycerophosphate compared with PDMS after 1 day of incubation. This may be attributed to π-π stacking between the aromatic rings in the biomolecules and the graphene basal plane.5 Dexamethasone is a synthetic glucocorticoid that alters the expression levels of many proteins and enzymes which are required during osteogenic differentiation. However, dexamethasone should act synergistically with β-glycerophosphate to synthesize new mineralized bone matrix. Insulin is the main mediator for fatty acid synthesis. Graphene also shows high adsorption of insulin, which is a key regulator for the synthesis of fatty acids; however, insulin on graphene was denatured through graphene's strong π-π interaction. Thus, graphene suppressed adipogenic differentiation of BMMSCs.5 In addition, it has been reported that there is an inverse relationship between osteogenesis and adipogenesis in MSC differentiation.97
Depan et al. also tested the adsorption capacity of substrates and found that, in the case of pure CS, the adsorption of bovine serum albumin was random and large globular aggregates were observed. By contrast, in the CS-GO scaffolds, the globules were small and uniformly spread over the surface of the scaffold. GO possess carboxylic, hydroxyl, and epoxide groups, which render the scaffolds hydrophilic. These hydrophilic functional groups interact with the functional groups of protein molecules via electrostatic and van der Waals forces to induce the adsorption of protein molecules. Thus, the significant differences in protein adsorption can be attributed to an increase in the hydrophilicity of the hybrid scaffolds induced by the introduction of hydrophilic GO.9
As for FRGO, Shi et al. reported that the surface oxygen content of FRGO has a strong influence on protein adsorption. Quantitative measurements indicated that the amounts of adsorbed fetal bovine serum on moderately reduced FRGO was significantly higher than that on nonreduced, highly reduced FRGO and the control glass slides.7
The mechanism driving the spontaneous differentiation of MSCs toward the osteoblastic lineage on 3D GFs remains unclear. It is suspected that the intrinsic properties of 3D GFs resulted in increased cytoskeletal tension and, thus, guided the cell behavior.11 However, detailed studies are required to fully understand the mechanism.
Conclusions and Prospects
The unique properties of graphene make it a promising material for various fields. Here, we reviewed recent studies with regard to graphene's effects on the adhesion, proliferation, and differentiation of MSCs, and its potential to promote osteodifferentiation, which point to its future use in surface modification of implants or scaffold materials. To date, studies on graphene's contribution to bone tissue engineering are still quite new, and knowledge on the effects of graphene is limited. Within the limitation of the present investigation, we believe that graphene may have a promising future, and further research will realize its potential. In spite of the significant progress mentioned earlier, there are still some important challenges. First, the potential long-term toxicity and the nonbiodegradable nature of graphene should be further investigated. Second, our understanding of graphene-cell interactions and its internal mechanisms are incomplete, and many hypotheses remain to be tested. Third, a comparison of the osteogenic effects of graphene with those of current successful implants or scaffold materials should be performed before we can conclude whether graphene is a promising nano-material for promoting surface modification of implants or scaffold materials. Lastly, the exact effects of graphene on cells, tissues, or organs, and their metabolic pathway in vivo remain unclear and require further studies.
Acknowledgments
This study was supported by grants from the Program for New Century Excellent Talents in University from Ministry of Education of China (NCET-11-0026) and Peking University 985 Program.
Disclosure Statement
The authors indicated no potential conflicts of interest.
References
- 1.Rose F.R., and Oreffo R.O.Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun 292,1, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., and Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Treiser M.D., Yang E.H., Gordonov S., Cohen D.M., Androulakis I.P., Kohn J., Chen C.S., and Moghe P.V.Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci U S A 107,610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayak T.R., Andersen H., Makam V.S., Khaw C., Bae S., Xu X., Ee P.L., Ahn J.H., Hong B.H., Pastorin G., and Ozyilmaz B.Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 5,4670, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Lee W.C., Lim C.H., Shi H., Tang L.A., Wang Y., Lim C.T., and Loh K.P.Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5,7334, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Kim J., Choi K.S., Kim Y., Lim K.T., Seonwoo H., Park Y., Kim D.H., Choung P.H., Cho C.S., Kim S.Y., Choung Y.H., and Chung J.H.Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J Biomed Mater Res A 101,3520, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Shi X.T., Chang H.X., Chen S., Lai C., Khademhosseini A., and Wu H.K.Regulating cellular behavior on few-layer reduced graphene oxide films with well-controlled reduction states. Adv Funct Mater 22,751, 2012 [Google Scholar]
- 8.Depan D., Girase B., Shah J.S., and Misra R.D.Structure-process-property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater 7,3432, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Depan D., and Misra R.D.The interplay between nanostructured carbon-grafted chitosan scaffolds and protein adsorption on the cellular response of osteoblasts: structure-function property relationship. Acta Biomater 9,6084, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Kim Y.R., Kim Y., Lim K.T., Seonwoo H., Park S., et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J Mater Chem B 1,933, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Crowder S.W., Prasai D., Rath R., Balikov D.A., Bae H., Bolotin K.I., and Sung H.J.Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 5,4171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L.A., Lee W.C., Shi H., Wong E.Y., Sadovoy A., Gorelik S., Hobley J., Lim C.T., and Loh K.P.Highly wrinkled cross-linked graphene oxide membranes for biological and charge-storage applications. Small 8,423, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Novoselov K.S., Geim A.K., Morozov S.V., Jiang D., Zhang Y., Dubonos S.V., Grigorieva I.V., and Firsov A.A.Electric field effect in atomically thin carbon films. Science 306,666, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Wei W., and Qu X.Extraordinary physical properties of functionalized graphene. Small 8,2138, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Yang M., Yao J., and Duan Y.Graphene and its derivatives for cell biotechnology. Analyst 138,72, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Lee C., Wei X., Kysar J.W., and Hone J.Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321,385, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Novoselov K.S., Fal'ko V.I., Colombo L., Gellert P.R., Schwab M.G., and Kim K.A roadmap for graphene. Nature 490,192, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Mayorov A.S., Gorbachev R.V., Morozov S.V., Britnell L., Jalil R., and Ponomarenko L.A.Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett 11,2396, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Morozov S.V., Novoselov K.S., Katsnelson M.I., Schedin F., Elias D.C., and Jaszczak J.A.Giant intrinsic carrier mobilities in graphene and its bilayer. Phys Rev Lett 100,016602, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Balandin A.A.Thermal properties of graphene and nanostructured carbon materials. Nat Mater 10,569, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Nair R.R., Blake P., Grigorenko A.N., Novoselov K.S., Booth T.J., and Stauber T.Fine structure constant defines visual transparency of graphene. Science 320,1308, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Bunch J.S., Verbridge S.S., Alden J.S., van der Zande A.M., Parpia J.M., and Craighead H.G.Impermeable atomic membranes from graphene sheets. Nano Lett 8,2458, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Novoselov K.S., Jiang Z., Zhang Y., Morozov S.V., Stormer H.L., Zeitler U., Maan J.C., Boebinger G.S., Kim P., and Geim A.K.Room-temperature quantum Hall effect in graphene. Science 315,1379, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Huang Y., Song Y., Zhang X., Ma Y., Liang J., and Chen Y.Room-temperature ferromagnetism of graphene. Nano Lett 9,220, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Yan L., Zheng Y.B., Zhao F., Li S., Gao X., Xu B., Weiss P.S., and Zhao Y.Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chem Soc Rev 41,97, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Salas E.C., Sun Z., Luttge A., and Tour J.M.Reduction of graphene oxide via bacterial respiration. ACS Nano 4,4852, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Park S., Mohanty N., Suk J.W., Nagaraja A., An J., Piner R.D., Cai W., Dreyer D.R., Berry V., and Ruoff R.S.Biocompatible, robust free-standing paper composed of a TWEEN/graphene composite. Adv Mater 22,1736, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Liu S., Zeng T.H., Hofmann M., Burcombe E., Wei J., Jiang R., Kong J., and Chen Y.Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5,6971, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Chang Y., Yang S.T., Liu J.H., Dong E., Wang Y., Cao A., Liu Y., and Wang H.In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett 200,201, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Dean C.R., Young A.F., Meric I., Lee C., Wang L., and Sorgenfrei S.Boron nitride substrates for high-quality graphene electronics. Nat Nanotechnol 5,722, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Zhang L., and Zhou C.Review of chemical vapor deposition of graphene and related applications. Acc Chem Res 46,2329, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Berger C., Song Z., Li X., Wu X., Brown N., Naud C., Mayou D., Li T., Hass J., Marchenkov A.N., Conrad E.H., First P.N., and de Heer W.A.Electronic confinement and coherence in patterned epitaxial graphene. Science 312,1191, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Stankovich S., Dikin D.A., Dommett G.H., Kohlhaas K.M., Zimney E.J., Stach E.A., Piner R.D., Nguyen S.T., and Ruoff R.S.Graphene-based composite materials. Nature 442,282, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Li X., Cai W., An J., Kim S., Nah J., Yang D., Piner R., Velamakanni A., Jung I., Tutuc E., Banerjee S.K., Colombo L., and Ruoff R.S.Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324,1312, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Bae S., Kim H., Lee Y., Xu X., Park J.S., Zheng Y., Balakrishnan J., Lei T., Kim H.R., Song Y.I., Kim Y.J., Kim K.S., Ozyilmaz B., Ahn J.H., Hong B.H., and Iijima S.Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat Nanotechnol 5,574, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Reina A., Jia X., Ho J., Nezich D., Son H., Bulovic V., Dresselhaus M.S., and Kong J.Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9,30, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Singh V., Joung D., Zhai L., Das S., Khondaker S.I., and Seal S.Graphene based materials: past, present and future. Prog Mater Sci 56,1178, 2011 [Google Scholar]
- 38.Hummers W.S., Jr., and Offeman R.E.Preparation of graphitic oxide. J Am Chem Soc 80,1339, 1958 [Google Scholar]
- 39.Yamaguchi H., Eda G., Mattevi C., Kim H., and Chhowalla M.Highly uniform 300 mm wafer-scale deposition of single and multilayered chemically derived graphene thin films. ACS Nano 4,524, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Becerril H.A., Mao J., Liu Z., Stoltenberg R.M., Bao Z., and Chen Y.Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2,463, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Kim J., Cote L.J., Kim F., Yuan W., Shull K.R., and Huang J.Graphene oxide sheets at interfaces. J Am Chem Soc 132,8180, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Cote L.J., Kim F., and Huang J.Langmuir-Blodgett assembly of graphite oxide single layers. J Am Chem Soc 131,1043, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Dikin D.A., Stankovich S., Zimney E.J., Piner R.D., Dommett G.H., Evmenenko G., Nguyen S.T., and Ruoff R.S.Preparation and characterization of graphene oxide paper. Nature 448,457, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Chen C.M., Yang Q.H., Yang Y.G., Lv W., Wen Y.F., Hou P.X., Wang M.Z., and Cheng H.M.Self-assembled free-standing graphite oxide membrane. Adv Mater 21,3007, 2009 [Google Scholar]
- 45.Azevedo J., Costa-Coquelard C., Jegou P., Yu T., and Benattar J.J.Highly ordered monolayer, multilayer, and hybrid films of graphene oxide obtained by the bubble deposition method. J Phys Chem C 115,14678, 2011 [Google Scholar]
- 46.Shih C.J., Lin S., Sharma R., Strano M.S., and Blankschtein D.Understanding the pH-dependent behavior of graphene oxide aqueous solutions: a comparative experimental and molecular dynamics simulation study. Langmuir 28,235, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Park S., An J., Jung I., Piner R.D., An S.J., and Li X.Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett 9,1593, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Bagri A., Mattevi C., Acik M., Chabal Y.J., Chhowalla M., and Shenoy V.B.Structural evolution during the reduction of chemically derived graphene oxide. Nat Chem 2,581, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Salavagione H.J., Martinez G., and Ellis G.Recent advances in the covalent modification of graphene with polymers. Macromol Rapid Commun 32,1771, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Menard-Moyon C., Kostarelos K., Prato M., and Bianco A.Functionalized carbon nanotubes for probing and modulating molecular functions. Chem Biol 17,107, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Kalbacova M., Broz A., Kong J., and Kalbac M.Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 48,4323, 2010 [Google Scholar]
- 52.Kim S., Ku S.H., Lim S.Y., Kim J.H., and Park C.B.Graphene-biomineral hybrid materials. Adv Mater 23,2009, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Misra R.D., and Chaudhari P.M.Cellular interactions and stimulated biological functions mediated by nanostructured carbon for tissue reconstruction and tracheal tubes and sutures. J Biomed Mater Res A 101,528, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Agarwal S., Zhou X., Ye F., He Q., Chen G.C., Soo J., Boey F., Zhang H., and Chen P.Interfacing live cells with nanocarbon substrates. Langmuir 26,2244, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Feng L., and Liu Z.Graphene in biomedicine: opportunities and challenges. Nanomedicine (Lond) 6,317, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Chung C., Kim Y.K., Shin D., Ryoo S.R., Hong B.H., and Min D.H.Biomedical applications of graphene and graphene oxide. Acc Chem Res 46,2211, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Pinto A.M., Goncalves I.C., and Magalhaes F.D.Graphene-based materials biocompatibility: a review. Colloids surf B Biointerfaces 111C,188, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Jastrzebska A.M., Kurtycz P., and Olszyna A.R.Recent advances in graphene family materials toxicity investigations. J Nanopart Res 14,1320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai X., Tan S., Yu A., Zhang J., Liu J., and Mai W.Sodium 1-naphthalen esulfonate-functionalized reduced graphene oxide stabilizes silver nanoparticles with lower cytotoxicity and long-term antibacterial activity. Chem Asian J 7,1664, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Liao K.H., Lin Y.S., Macosko C.W., and Haynes C.L.Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces 3,2607, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Sasidharan A., Panchakarla L.S., Chandran P., Menon D., Nair S., and Rao C.N.Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 3,2461, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Hong B.J., Compton O.C., An Z., Eryazici I., and Nguyen S.T.Successful stabilization of graphene oxide in electrolyte solutions: enhancement of biofunctionalization and cellular uptake. ACS Nano 6,63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chng E.L., and Pumera M.The toxicity of graphene oxides: dependence on the oxidative methods used. Chemistry 19,8227, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Ali S.F., Dervishi E., Xu Y., Li Z., Casciano D., and Biris A.S.Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 4,3181, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Peng C., Hu W., Zhou Y., Fan C., and Huang Q.Intracellular imaging with a graphene-based fluorescent probe. Small 6,1686, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Wen H., Dong C., Dong H., Shen A., Xia W., and Cai X.Engineered redox-responsive PEG detachment mechanism in PEGylated nano-graphene oxide for intracellular drug delivery. Small 8,760, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Sahu A., Choi W.I., and Tae G.A stimuli-sensitive injectable graphene oxide composite hydrogel. Chem Commun 48,5820, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Duch M.C., Budinger G.R., Liang Y.T., Soberanes S., Urich D., and Chiarella S.E.Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett 11,5201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gollavelli G., and Ling Y.C.Multi-functional graphene as an in vitro and in vivo imaging probe. Biomaterials 33,2532, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Yan L., Wang Y., Xu X., Zeng C., Hou J., and Lin M.Can graphene oxide cause damage to eyesight? Chem Res Toxicol 25,1265, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Yang K., Wan J., Zhang S., Zhang Y., Lee S.T., and Liu Z.In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 5,516, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Sasidharan A., Panchakarla L.S., Sadanandan A.R., Ashokan A., Chandran P., and Girish C.M.Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 8,1251, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Nahain A.A., Lee J.E., Jeong J.H., and Park S.Y.Photoresponsive fluorescent reduced graphene oxide by spiropyran conjugated hyaluronic Acid for in vivo imaging and target delivery. Biomacromolecules 14,4082, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Shah A.K., Sinha R.K., Hickok N.J., and Tuan R.S.High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone 24,499, 1999 [DOI] [PubMed] [Google Scholar]
- 75.Wu S., Liu X., Wu G., Yeung K.W., Zheng D., Chung C.Y., Xu Z.S., and Chu P.K.Wear mechanism and tribological characteristics of porous NiTi shape memory alloy for bone scaffold. J Biomed Mater Res A 101,2586, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Roy R.K., and Lee K.R.Biomedical applications of diamond-like carbon coatings: a review. J Biomed Mater Res B Appl Biomater 83,72, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Huang N., Yang P., Leng Y.X., Chen J.Y., Sun H., Wang J., Wang G.J., Ding P.D., Xi T.F., and Leng Y.Hemocompatibility of titanium oxide films. Biomaterials 24,2177, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Gutensohn K., Beythien C., Bau J., Fenner T., Grewe P., Koester R., Padmanaban K., and Kuehnl P.In vitro analyses of diamond-like carbon coated stents. Reduction of metal ion release, platelet activation, and thrombogenicity. Thromb Res 99,577, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Podila R., Moore T., Alexis F., and Rao A.Graphene coatings for biomedical implants. J Vis Exp e50276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edwards S.L., Werkmeister J.A., and Ramshaw J.A.Carbon nanotubes in scaffolds for tissue engineering. Expert Rev Med Devices 6,499, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Zanello L.P., Zhao B., Hu H., and Haddon R.C.Bone cell proliferation on carbon nanotubes. Nano Lett 6,562, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Abarrategi A., Gutierrez M.C., Moreno-Vicente C., Hortiguela M.J., Ramos V., Lopez-Lacomba J.L., Ferrer M.L., and Del M.F.Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 29,94, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Firkowska I., Olek M., Pazos-Perez N., Rojas-Chapana J., and Giersig M.Highly ordered MWNT-based matrixes: topography at the nanoscale conceived for tissue engineering. Langmuir 22,5427, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Kim K.H., Oh Y., and Islam M.F.Graphene coating makes carbon nanotube aerogels superelastic and resistant to fatigue. Nat Nanotechnol 7,562, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Yaszemski M.J., Payne R.G., Hayes W.C., Langer R.S., Aufdemorte T.B., and Mikos A.G.The ingrowth of new bone tissue and initial mechanical properties of a degrading polymeric composite scaffold. Tissue Eng 1,41, 1995 [DOI] [PubMed] [Google Scholar]
- 86.Lalwani G., Henslee A.M., Farshid B., Lin L., Kasper F.K., Qin Y.X., Mikos A.G., and Sitharaman B.Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 14,900, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Some S., Ho S.M., Dua P., Hwang E., Shin Y.H., Yoo H., Kang J.S., Lee D.K., and Lee H.Dual functions of highly potent graphene derivative-poly-L-lysine composites to inhibit bacteria and support human cells. ACS Nano 6,7151, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Wang K., Ruan J., Song H., Zhang J., Wo Y., Guo S., and Cui D.Bioccompatibility of graphene oxide. Nanoscale Res Lett 6,8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Humphries J.D., Wang P., Streuli C., Geiger B., Humphries M.J., and Ballestrem C.Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179,1043, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamilton D.W., and Brunette D.M.The effect of substratum topography on osteoblast adhesion mediated signal transduction and phosphorylation. Biomaterials 28,1806, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Chen C.S., Mrksich M., Huang S., Whitesides G.M., and Ingber D.E.Geometric control of cell life and death. Science 276,1425, 1997 [DOI] [PubMed] [Google Scholar]
- 92.Roskelley C.D., Desprez P.Y., and Bissell M.J.Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci U S A 91,12378, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S.Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6,483, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., Wilkinson C.D., and Oreffo R.O.The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6,997, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Oh S., Brammer K.S., Li Y.S., Teng D., Engler A.J., Chien S., and Jin S.Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A 106,2130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Kawai M., and Rosen C.J.PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6,629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]