Summary
T-cell activation is mediated by antigen-specific signals from the TCRζ/CD3 and CD4–CD8–p56lck complexes in combination with additional co-signals provided by coreceptors such as CD28, inducible costimulator (ICOS), cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death (PD-1), and others. CD28 and ICOS provide positive signals that promote and sustain T-cell responses, while CTLA-4 and PD-1 limit responses. The balance between stimulatory and inhibitory co-signals determines the ultimate nature of T-cell responses where response to foreign pathogen is achieved without excess inflammation and autoimmunity. In this review, we outline the current knowledge of the CD28 and CTLA-4 signaling mechanisms [involving phosphatidylinositol 3 kinase (PI3K), growth factor receptor-bound protein 2 (Grb2), Filamin A, protein kinase C θ (PKCθ), and phosphatases] that control T-cell immunity. We also present recent findings on T-cell receptor-interacting molecule (TRIM) regulation of CTLA-4 surface expression, and a signaling pathway involving CTLA-4 activation of PI3K and protein kinase B (PKB)/AKT by which cell survival is ensured under conditions of anergy induction.
Keywords: CD28, CTLA-4, TRIM, PKB/AKT, apoptosis
Introduction
CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) are the most characterized of the immunoglobulin (Ig) family of coreceptors. Their genes are located next to each other on human chromosome 2q33 (1) and mouse chromosome 1 (2). Both form homodimers and bind the ligands CD80 (B7-1) and CD86 (B7-2), using a signature MYPPPY binding motif (3). CD80 binds CTLA-4 and CD28 with different affinities (Kd values of approximately 12 and 200 nM, respectively) (4). The higher affinity binding of CTLA-4 is due to a periodic arrangement in which bivalent homodimers bridge bivalent CD80 molecules (5, 6). Although T-cell activation can occur with a potent T-cell receptor (TCR) signal alone (i.e. high avidity peptide), CD28 coligation is required in most responses to peptide antigen (7–9). Without CD28 coligation, TCR often induces a non-responsive, anergic state or cell death (10). T cells from CD28-deficient mice show reduced proliferation in response to peptide antigens (11), although repeated antigen stimulation or long-term viral infection can bypass the requirement for CD28 (12). CD28 cosignals can stabilize messenger RNA (mRNA) of cytokines and amplify the activation of nuclear factor of activated T cells (NFAT) and nuclear factor κB (NF-κB) (13–15).
By contrast, CTLA-4 dampens T-cell responses in a manner that can protect against the development of autoproliferative or autoimmune disease (16–18). CTLA-4-deficient (Ctla-4−/−) mice show a profound post-thymic, hyperproliferative phenotype of death within 3 weeks of age, which is due to massive tissue infiltration and organ destruction (19, 20). Antibody cross-linking is also inhibitory (8, 21, 22), although it is unclear whether there is a straightforward connection between these two sets of observations. Antibody cross-linking effects operate in trans and not efficiently in cis (23).
Several highlighted features relate to CTLA-4. First, it is primarily located in intracellular compartments such as the trans-Golgi network (TGN), endosomes, and lysosomes (24, 25). Secondly, the coreceptor can increase T-cell adhesion and motility, and in the process, can reverse the classic TCR-induced ‘stop signal’ needed for firm contact between T cells and antigen-presenting cells (APCs) (26–29). This ‘reverse-stop signaling model’ provides a mechanism by which CTLA-4 could raise the threshold of TCR signaling by limiting contact between T cells and APCs and the aggregation of TCR molecules in the immunological synapse (IS) (27). It could also account for increased tissue infiltration by Ctla-4−/− T cells, and the fact that the coreceptor is not expressed on naive and resting T cells which require longer dwell times with APCs for activation (26, 27). Thirdly, coligation of CTLA-4 with TCRs blocks the formation of ZAP-70-containing microclusters in T cells (29). Micro-clustering of kinases and adapters is needed for effective transmission of signals from the TCR complex (30). Disruption of these clusters coincided with a loss of calcium mobilization that is needed for T-cell proliferation (29). Lastly, recent evidence indicates that CTLA-4 is needed for the optimal function of regulatory T cells (Tregs) (31). The deletion of CTLA-4 in forkhead box P3 (FoxP3)-positive cells reduced Treg suppressor function, and delayed the onset of autoimmune disease. Similarly, FoxP3 transgene expression in the Ctla-4−/− mouse had previously been shown to delay disease onset (32). These observations support a role for CTLA-4 in influencing Treg function and for Tregs in reducing the severity of the Ctla-4−/− disease phenotype. Tregs could account for ‘cell extrinsic’ regulation (i.e. factors that operate outside of the CTLA-4-expressing cell) of the proliferation of Ctla-4−/− T cells; however, genetic manipulation did not fully reverse disease and, hence, this is unlikely to be the causal element of the autoproliferative disorder. In this context, ‘cell intrinsic’ (i.e. mediated by CTLA-4 within a given cell) mechanisms that induce intracellular signals also negatively modulate the behavior of T cells.
CD28 and CTLA-4 surface expression
Despite their structural similarity, CD28 and CTLA-4 differ markedly in their localization in T cells. CD28 is expressed on resting and activated cells, while CTLA-4 expression is induced in response to TCR ligation, and in particular to TCR/CD28 costimulation. The exception is the Treg cell that constitutively expresses the coreceptor (33, 34). CD28 is expressed as the result of biosynthesis, and subsequent translocation to the cell surface. Several factors that control its internalization have been identified. We initially showed that the binding of phosphatidylinositol 3-kinase (PI3K) to a YMNM consensus motif in the cytoplasmic domain is needed (35) (Fig. 1). Through phosphorylation, PI3K produces the lipids phosphatidylinositol (3,4)-biphosphate (PIP2) and phosphatidylinositol (3–5)-triphosphate (PIP3) (collectively known as D-3 lipids) that bind to the inner leaflet of the plasma membrane. The enzyme is comprised of various p85 subunits complexed to different p110 catalytic chains. The src homology 2 (SH2) domain of the p85 chain binds the phosphorylated YMNM motif of CD28 (36–39). CD28–PI3K complexes are preferentially internalized relative to PI3K-free receptor, while mutations in the YMNM motif that differentially reduce PI3K binding closely correlated with impaired coreceptor endocytosis (35). This internalization occurred in a clathrin-dependent manner (35). Mutations at Y-FMNM and Y-FRS disrupted PI3K binding and endocytosis, while YMNM-C had a partial effect. PI3K associates with clathrin-coated vesicles and the microtubule cytoskeleton (40–42). Recent evidence has also implicated Wiskott–Aldrich syndrome protein (WASP) and its binding to sorting nexin 9 (SNX9) in the process (43). WASP had previously been shown to be needed for TCRζ/CD3 complex clustering where mutations are responsible for the immunodeficiency disorder Wiskott–Aldrich syndrome (WAS) (44). Sorting nexins are membrane proteins involved in endocytosis, membrane trafficking, and protein sorting (45, 46). In the context of CD28, SNX9 was found to bind WASP via its SH3 domain, while using its phagocyte oxidase homology (PX) domain to interact with the p85 subunit of PI3K and its product PIP3 (43). CD28 internalization is markedly enhanced by SNX9 overexpression, and severely impaired by the expression of an SNX9 mutant (SNX9ΔPX) lacking p85-binding capacity. These findings provide a mechanism by which PI3K, WASP, and SNX9 can become coordinated in the control of CD28 internalization (Fig. 1).
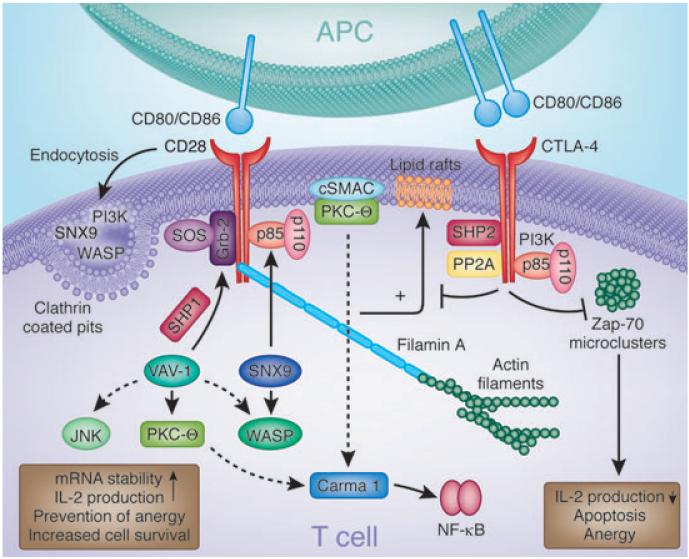
Fig. 1. Signaling molecules involved in CD28 and CTLA-4 function.
CD28 and CTLA-4 associate with various intracellular signaling proteins. CD28 associates with PI3K and Grb2 via SH2 domain binding to the Tyr–Val–Asn–Met (YMNM) motif. PI3K generates PI3,4P2 and PI3,4,5P3 lipids, while Grb2 binds to the exchange factor Sos1, an activator of the GTPase p21ras. CD28–Grb2 binding is also needed for the phosphorylation and activation of Vav1. In turn, Vav1 can activate Rac1 that activates the serine/threonine kinase JNK, may activate Cdc42 in the activation of WASP, and is needed for the membrane association of PKCθ. Protein tyrosine phosphatase 1 (SHP-1) can also associate with Vav1, Grb2, and Sos1, dampening signals from CD28. The CD28–PYAPP motif (located further to the C-terminus) has been reported to bind to FLNA and colocalizes with PKCθ and CD28. Mutations in the PYAPP motif block PKCθ formation in a cSMAC. CD28 endocytosis is regulated by the combination of mediators, PI3K, WASP, and SNX9. Mutations that disrupt PI3K binding impair internalization via a clathrin-dependent mechanism. SNX9 binds WASP via its SH3 domain and uses its PX domain to interact with the p85 subunit of PI3K and its product PIP3. WASP, SNX9, PI3K, and CD28 colocalize within clathrin-containing endocytic vesicles after TCR/CD28 costimulation. On the other hand, CTLA-4 binds to PI3K using its YVKM motif as well as phosphatases SHP-2 and PP2A. Phosphatases have been postulated to generate negative signals. CTLA-4 can also block the expression of lipid rafts (GEMs) and the induction by the TCR of ZAP-70 microcluster formation. CTLA-4, cytotoxic T-lymphocyte antigen-4; PI3K, phosphatidylinositol 3-kinase; SH2, src homology 2; WASP, Wiskott–Aldrich syndrome protein; PKCθ, protein kinase C h; cSMAC, central supramolecular activation cluster; SNX, sorting nexin; TCR, T-cell receptor; YVKM, Val–Tyr–Val–Lys–Met; SHP-1, SH2-domain-containing protein tyrosine phosphatase 1; GEM, glycolipid-enriched microdomain.
By contrast, the majority of CTLA-4 is localized in several intracellular compartments: the TGN, endosomes, and lysosomes (24, 47–50). Only small amounts of CTLA-4 can be detected on the surface of cells, even when optimally expressed following T-cell activation. The mechanism by which CTLA-4 is transported to the cell surface is of key importance, given that minor differences in expression can have potentially major effects on T-cell function and in the development of autoimmunity. Single-nucleotide polymorphisms (SNPs) linked to autoimmune diseases such as type 1 diabetes and rheumatoid arthritis generally occur in regions involved in gene expression (51, 52). Furthermore, dysregulation of intracellular expression of CTLA-4 has been proposed to account for the lymphoproliferative disease in Chediak–Higashi syndrome (53). Despite these findings, the full range of components that control surface transport remains unclear (Fig. 2). Calcium influx is important as calcium ionophores induce the release of CTLA-4 from the TGN to the cell surface (48, 49). TCR ligation causes the directional release of this coreceptor to the site of ligation (48, 54). We recently showed that the 30-kDa type III transmembrane protein termed TCR-interacting molecule (TRIM) controls this event (25) (Fig. 2). TRIM is a 30-kDa type III transmembrane protein expressed in T cells and natural killer (NK) cells (55, 56). Its cytoplasmic tail contains several tyrosine motifs with the potential to bind to SH2 domains of signaling proteins such as the p85 subunit of PI3K (55). An association with the TCRζ/CD3 complex has been reported, but with unclear consequences (55).
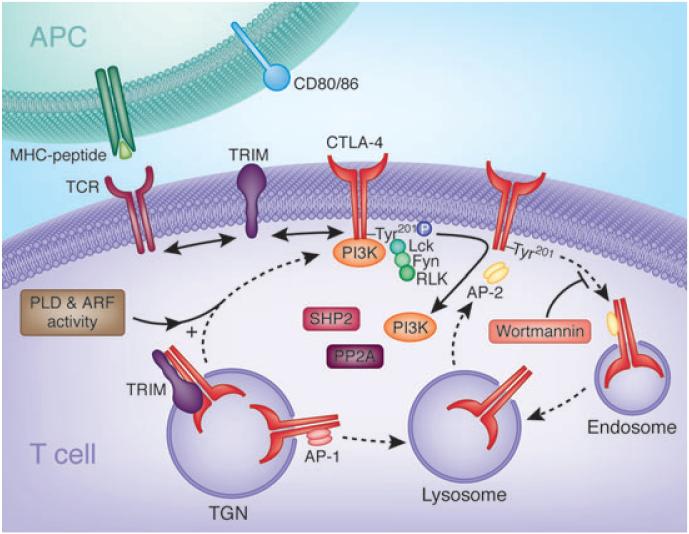
Fig. 2. Regulation of CTLA-4 surface expression and internalization.
Newly synthesized CTLA-4 binds to the transmembrane adapter TRIM in the TGN promoting the formation of CTLA-4-containing vesicles and their transport to the cell surface. On the cell surface, CTLA-4 and TRIM no longer associate allowing TRIM to interact with other receptors, possibly the TCR complex. CTLA-4 externalization is also dependent on general factors such as PLD and GTPase ARF-1. Shuttling to the lysosomal compartment from the TGN occurs due to adapter AP-1 binding to GVY201VKM motif in CTLA-4. On the surface, CTLA-4 becomes phosphorylated on the Y201VKM by kinases Lck, Fyn, and Rlk leading to the association of PI3K and possibly other proteins. Phosphorylation retards internationalization. Dephosphorylation allows binding to the clathrin adapter AP-2 to the GVY201VKM motif and rapid internalization to endosomes and lysosomes. Upon T-cell activation, CTLA-4 enriched lysosomes (and endosomes) are recycled to the cell surface. This process involves Wortmannin-sensitive lipid kinases but not PI3K (174). CTLA-4, cytotoxic T-lymphocyte antigen-4; TRIM, T-cell receptor-interacting molecule; TGN, trans-Golgi network; TCR, T-cell receptor; PLD, phospholipase D; ARF, adenosine diphosphate ribosylation factor; AP, adapter protein; PI3K, phosphatidylinositol 3-kinase.
Valk et al. (25) found that TRIM binds to CTLA-4 and co-localizes with the coreceptor in the TGN. Anti-CTLA-4 coprecipitated TRIM (but not CD28), while immunofluoresence staining showed CTLA-4 and TRIM colocalization in the TGN. By contrast, TRIM was not found with CTLA-4 on the cell surface or in endosomes. Overexpression of TRIM with pulse-chase analysis showed an increased rate of CTLA-4 surface expression. Downregulation of TRIM expression by short-hairpin RNAs (shRNAs) inhibited CTLA-4 surface expression, while the expression of TCR and CD28 was unaffected. Furthermore, downregulation of TRIM markedly reduced the presence of CTLA-4-carrying vesicles proximal to the TGN (25) (Fig. 3). As seen in Fig. 3A, numerous CTLA-4 labeled vesicles can be detected in wildtype T cells (see arrow). These include TGN-proximal vesicles, lysosomal vesicles, and endosomal vesicles. They exist distinct from the TGN stained with anti-CTLA-4 and anti-Syntaxin, a TGN marker (upper right panels). As seen in Fig. 3B, reduction in TRIM expression using shRNA resulted in a marked loss of CTLA-4 stained vesicles. Instead, CTLA-4 remained restricted to the TGN (also right panels). This loss of CTLA-4 vesicular formation was observed in cell lines and primary T cells, and was accompanied by increased TCR-mediated interleukin-2 (IL-2) production (25). Taken together, these observations are consistent with a chaperone role for TRIM in the transport of CTLA-4 to the cell surface (25). While the interaction between TRIM and CTLA-4 strongly suggests specificity in the formation of TGN vesicles, the nature of these TGN-proximal vesicles and a role for TRIM on general aspects of TGN transport also will require further investigation.
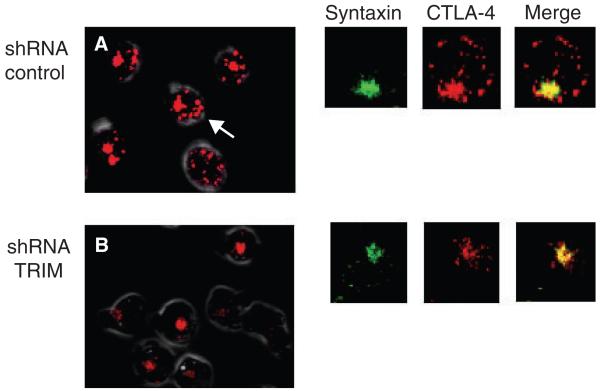
Fig. 3. TRIM controls the generation of TGN proximal vesicles with CTLA-4.
(A) Pattern of intracellular CTLA-4 staining in wildtype T cells (shRNA control) (left panel). Arrows point to CTLA-4-labeled vesicles. Right panels: cells labeled with anti-Syntaxin (TGN marker), anti-CTLA-4, and merged images. Anti-CTLA-4 labels both TGN and intracellular vesicles (TGN proximal and endosomal/lysosomal). (B) Pattern of intracellular CTLA-4 staining in TRIM knockdown cells (left panel). There is a general loss of CTLA-4 labeled vesicles. Instead, CTLA-4 is restricted to the Golgi area. Right panels: This loss of CTLA-4 vesicles was confirmed by analysis of a magnified single-cell using anti-Syntaxin and anti-CTLA-4 followed by merging of the images. TRIM, T-cell receptor-interacting molecule; TGN, trans-Golgi network; CTLA-4, cytotoxic T-lymphocyte antigen-4; shRNA, short-hairpin RNA.
The mild immune-deficient phenotype of TRIM knockout mice suggests that other non-raft transmembrane adapter proteins such as SHP2-interacting transmembrane adapter protein (SIT) or linker for activation of X cells (LAX) may compensate for the absence of TRIM (57). LAX-deficient T cells are hyper-responsive to anti-CD3- and anti-IgM-mediated stimulation (58), and SIT-deficient mice show an increased susceptibility to develop experimental autoimmune encephalomyelitis (59).
CTLA-4 surface expression is also dependent on guanosine triphosphatases (GTPases), adenosine diphosphate ribosylation factor-1 (ARF-1) and phospholipase D (PLD). ARF family GTPases and PLD are needed for the budding of vesicles at the Golgi apparatus (60–62). PLD inhibitors or dominant-negative mutants of ARF-1 or PLD inhibit the release of CTLA-4 to the cell surface (63), while other regulators of trafficking [i.e. soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs)] are also needed for CTLA-4 expression. Unlike TRIM, these mediators play general roles in the transport of multiple receptors to the surface of mammalian cells.
Following cell surface expression, other mechanisms affect CTLA-4 surface expression and internalization (Fig. 2). The duration of expression is determined by the opposing effect of phosphorylation and adapter protein-2 (AP-2) binding (49, 64–68). Clathrin adapter heterotetrameric complexes AP-1 and AP-2 are implicated in the selective recognition and recruitment of proteins into coated pits. As expressed at the plasma membrane, AP-2 is well known to mediate the endocytosis of multiple receptors (69). Effective targeting to coated pits requires that receptors contain specific internalization signals such as tyrosine-based motifs (i.e. YXXØ where X stands for any amino acid and Ø for a bulky hydrophobic residue) or dileucine motifs (70). While CTLA-4 lacks dileucine motifs, it has a Gly–Val–Tyr–Val–Lys–Met (GVYVKM) motif for AP-2 binding (49). Interestingly, a smaller region within the same motif (i.e. YVKM) binds to PI3K when phosphorylated (71). Protein tyrosine kinases p56lck, p59fyn, and resting lymphocyte kinase (Rlk) can phosphorylate CTLA-4, promote PI3K binding, and inhibit endocytosis (65–68). Once dephosphorylated, by an as of yet unidentified phosphatase, CTLA-4 binds to the AP-2 complex (49, 65, 66, 72, 73). Increased surface expression of mutant CTLA-4 with an FVKM motif (24, 48) may also be related to defective AP-2 binding. Following internalization into endosomes, CTLA-4 can either be re-expressed on the cell surface, or shuttled to the lysosomal compartment for degradation (see model; Fig. 2). Whether AP-2 binding is solely responsible for this rapidity of CTLA-4 internalization remains unclear. The level of surface expression of CTLA-4 may also be enhanced by activation-induced secretion of CTLA-4 enriched lysosomes (50).
In contrast to the plasma membrane localized coreceptor, Golgi-associated CTLA-4 binds to AP-1 via the same non-phosphorylated GVYVKM motif as used by AP-2 (49) (Fig. 2). The AP-1 complex is structurally similar to the AP-2 complex, but is localized in the TGN and lysosomes. Association of CTLA-4 with AP-1 mediates the shuttling from the TGN to endosomal and lysosomal compartments for degradation (49). By controlling the overall abundance of CTLA-4 in the TGN, AP-1 can determine overall levels of CTLA-4 expression.
CD28-mediated cosignaling
CD28 is a 44-kDa homo-dimeric protein involved in T-cell costimulatory signaling pathways. The cytoplasmic domain of CD28 lacks intrinsic catalytic activity, and instead, the coreceptor signals by binding to intracellular signaling proteins. Key to this recruitment is the tyrosine and proline-based motifs that bind to SH2 and SH3 domains, respectively. As mentioned, one prominent motif is the YMNM sequence, which when tyrosine phosphorylated is bound by the SH2 domain of PI3K and Grb2 (36–39). Cross-linking of CD28 increases tyrosine phosphorylation as mediated by the src-family kinases p56lck and p59fyn (74). PI3K production of PIP2 and PIP3 lipids bind the pleckstrin homology (PH) domains within proteins such as phosphoinositide-dependent protein kinase 1 (PDK1), which in turn activates protein kinase B (PKB/AKT). PDK1 and PKB can themselves then phosphorylate and regulate multiple pathways linked to protein synthesis, cellular metabolism, and cell survival (16, 75). In this way, CD28–PI3K and PKB provide signals for increased cell metabolism (76).
Although the CD28–PI3K connection has been extensively studied, there is still controversy over its relevance to cytokine production and T-cell proliferation. PI3 kinases are divided into four classes (IA, IB, II, and III) based on their structural characteristics and substrate specificity. Class IB PI3K deficiency affects T-cell development and function (77). Anti-CD3-mediated proliferation is reduced in p110γ−/− T cells, an effect that can be partially restored by CD28 coligation (77). Despite this, there are mixed results in the literature where mutation of the Y (i.e. loss of Grb2 and PI3K binding) and M residues (i.e. loss of PI3K binding alone) interferes with the induction of IL-2 in T-cell hybridomas (37, 78–80), while in vivo responses showed either no (81) or partial dependency on CD28–PI3K (82, 83). Part of this confusion may be explained by a recent report that CD28 can enhance PIP3 production at the T-cell synapse independently of its YMNM–PI3K-recruitment motif (84), perhaps by increasing the efficiency of conjugate formation and TCR signaling. In this context, the requirement for CD28–YMNM–PI3K may be particularly important in response to low-affinity peptide, an effect that is yet to be measured. The activation of PI3K by the TCR and CD28 differ in that the former varies with ligand affinity, while PI3K-binding affinity to the phosphorylated CD28–YMNM motif remains constant, determined by p85 SH2 domain binding (16). The efficacy of CD28 engagement with PI3K will depend on receptors and signals that phosphorylate the YMNM motif. Low-affinity TCR ligation produces low PIP3 levels and weak T-cell/APC conjugation via suboptimal activation of the ‘inside-out’ pathway needed to activate lymphocyte function-associated antigen 1 (LFA-1) adhesion (85). This suboptimal level of D-3 lipid production may require CD28 activation of PI3K to provide sufficient levels of activation signals for proliferation.
By contrast, it is clear that CD28–pYMNM (and CTLA-4–pYVKM) binding to PI3K generates prosurvival signals that prevent T-cell apoptosis (16, 81, 83, 86). CD28 has long been known to rescue cells from TCR-driven antigen-induced cell death (AICD) (81, 83, 87–89). Apoptosis can be induced by two pathways, either via death receptors [Fas ligand (FasL/CD95L)/Fas (CD95)] or mitochondria-associated proteins (i.e. the Bcl family). In the context of Fas/FasL, CD28 utilizes at least three different mechanisms: decreasing FasL expression, increasing expression of c-FLICE inhibitory protein (c-FLIP) (87), or interfering with the formation of the death-inducing signaling complex (DISC) (90). In the mitochondria-associated pathway, CD28 can activate PKB/ATK (91, 92), and increase the expression of the prosurvival factors Bcl-2 and Bcl-XL (93). CD28-deficient mice reconstituted with a disrupted YMNM motif for PI3K binding were unable to upregulate Bcl-XL (81, 83). This incapacity is most probably due to the inability to recruit and activate PI3K and subsequently activate PKB. Transgenic expression of an activated form of PKB leads to constitutively elevated Bcl-XL expression (90).
The YMNM motif can also accommodate the binding of the adapter protein Grb2, by virtue of the YXNX sequence (80, 94, 95). Grb2 is comprised of one SH2 domain flanked by two SH3 domains, which bind to the exchange factor Son of Sevenless, an activator of the GTPase p21ras. The loss of Grb2 binding by mutation of the asparagine (N) residue led to a loss of CD28-mediated phosphorylation of the guanine nucleotide exchange factor Vav1 and the activation of the serine/threonine kinase c-Jun kinase (JNK) (80). CD28 and Grb2 further cooperated with Vav1 in the activation of NFAT/AP-1 transcription (96). Grb2-like adapters such as Grb2-related adapter protein 2 (Gads) and Grb2-related adapter protein (Grap) failed to substitute efficiently for Grb2. Vav1 also coprecipitated with CD28 (96). Vav1 has a Dbl-homology (DH) domain with guanine diphosphate (GDP)–GTP exchange factor (GEF) activity for the activation of the GTP-binding proteins Ras-related C3 botulinum toxin substrate 1 (Rac1) [and possibly cell division cycle 42 (Cdc42)] and acts upstream to activate JNK (97). CD28–Grb2 may therefore act in a pathway where Vav1 activates Rac1, which in turn activates JNK. Confirmation of the importance of Grb2 binding came with the report showing that insertion of the N in the inducible costimulator (ICOS) YXXM motif conferred an ability of this coreceptor to bind Grb2 and produce IL-2 (98). Wildtype ICOS is otherwise unable to induce the production of this cytokine. Furthermore, Vav1 mutants that lack GEF activity can block CD28-mediated activation of JNK (91, 99, 100). Vav1 also facilitates clustering of the TCRζ/CD3 complex (101–103), while Rac1 can remodel the actin cytoskeleton, an event needed for TCR clustering. In this context, CD28 ligation can remodel the actin cytoskeleton (104, 105). The effect on TCR clustering via Vav1 could therefore potentially explain the previously reported cooperative effects of CD28 on TCR signaling (106, 107).
Further downstream of the YMNM motif, CD28 has two proline-rich regions P175RRPGP and P187YAPA (numbering in mouse CD28; PYAPP in human). P175RRPGP has been reported to bind to the SH3 domains of Tec family kinases IL2-inducible T-cell kinase (Itk), Rlk and Tec, while the P187YAPP motif has been found to bind to the src kinase p56lck, Grb2 and Filamin A (FLNA) (80, 95, 108, 109). The function of P175RRPGP binding to Tec kinases is unclear, although this kinase can upregulate cytokine production. On the other hand, T cells that are deficient in the ITK kinase are hyper-responsive to CD28 coligation, and hence the kinase may also negatively affect costimulation (110). By contrast, the P187YAPP motif recruits several signaling proteins including PKCθ to the IS (111). Lymphocyte-specific protein tyrosine kinase (Lck) was originally reported to bind this P187YAPP motif (109), and may help sustain kinase signaling, although its relevance is still somewhat unclear. Similarly, the Grb2 SH3 domain can bind this motif and reinforce Grb2 SH2 binding to the YMNM motif (80). However, this interaction may be supplementary rather than obligatory (80). By contrast, FLNA, an actin-binding scaffold protein has recently been reported to bind to the motif (108) where it colocalizes with PKCθ (108, 111, 112) (Fig. 1). Hayashi and Altman (112) showed that FLNA is required for PKCθ activity, while Tavano et al. (108) showed that FLNA binds to the P187YAPP motif and this site is needed for the potentiating effects of CD28 on lipid raft expression. Knockdown of FLNA by shRNA inhibited CD28-mediated raft accumulation at the IS (108) and Sanchez-Lockhart et al. (113) showed that the motif is needed for CD28 localization to the central supramolecular activation cluster (cSMAC). Yokosuka et al. (111) then showed that the P187YAPP motif was needed for PKCθ movement to the same region in the IS. Mutation of the site disrupted the ability of CD28 to preferentially accumulate at the cSMAC, and the distribution of PKCθ at the IS mirrored CD28 localization. Together, these observations fit a potential scenario where the recruitment of PKCθ to cSMAC requires the binding of FLNA to the P187YAPP motif in CD28 (108, 113). FLNA may act by inducing signals (i.e. sphingosine kinase 1 and p21-activated kinase), or providing a platform of PKCθ movement to clusters or the cSMAC via actin remodeling. The association of Vav1 with Grb2 may also have a part to play as its GEF activity promotes PKCθ translocation to the membrane (106, 114). Further, dominant-negative PKCθ can block the activation of JNK in certain T cells (100, 115, 116). Lastly, mutation of the same site can increase PI3K binding to CD28 and its rate of endocytosis by twofold (35). There is now little doubt that the CD28–PYAPP motif is one key regulator of CD28 cosignaling, although the exact mechanism by which it operates remains to be resolved.
Connected to this finding is the additional observation that CD28 can activate the transcription factor, NF-κB (Fig.1). NF-κB activation is regulated by upstream serine/threonine kinases, the IκB kinases (IKKs), which promote the phosphorylation of IκB and degradation by ubiquitination, allowing NF-κB to enter the nucleus (117, 118). A complex of three proteins, caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA1), Bcl10, and mucosa-associated lymphoid tissue (MALT) lymphoma translocation gene 1 (MALT1) lie downstream of PKCθ, and are involved in antigen receptor-mediated activation of NF-κB (119–124). Stimulation of T cells with APCs or cross-linking of TCR/CD3 plus CD28 by antibodies can activate the NF-κB pathway, whereas the TCR signal alone may be unable to do so, suggesting CD28 plays a key role in NF-κB activation (125–127). Mutation of Y-188 diminishes CD28-dependent activation of NF-κB (113). Takeda et al. (127) reported that CD28-mediated NF-κB activation by Bcl10 was dependent on PKCθ and CARMA1 and required Gads/Grb2 binding to CD28, but not PI3K binding. Taken together, these findings could connect CD28, Grb2 with FLNA and PKCθ in the activation of CARMA1–Bcl10 pathway leading to NF-κB function.
Finally, Kon-Kozlowski et al. (128) have shown that the SH2-domain-containing protein tyrosine phosphatase 1 (SHP-1) associates with Vav1, Grb2, and Sos1. Given the interaction of Grb2 with CD28, it raises the possibility that binding of SHP-1 to Grb2 and Vav1 potentially modulates signaling events mediated by CD28 (Fig. 1). This modulation of signaling was seen further in SHP-1-deficient CD4+ thymocytes, which have a reduced requirement for CD28 costimulation in order to produce IL-2. Thus, indicating SHP-1 raises the threshold for TCR activation by influencing costimulatory pathways (129, 130). Furthermore, IL-10, a suppressor cytokine of T-cell proliferation and cytokine production, has also been found to affect CD28 cosignaling by means of suppressing PI3K binding via SHP-1 (130–132). Other phosphatases, such as SHIP (132) and SHP-2 (133), have also been speculated to have some effect on aspects of CD28 signaling, although the molecular basis behind these effects remains to be elucidated.
Whether CD28 induces distinct signals or simply amplifies TCR signaling has been the subject of controversy (16, 106, 107). The pathways outlined above are probably engaged by both the CD28 and TCR receptors. Despite this, CD28 ligation itself fails to activate T cells, except for unusual superagonist antibodies (134). Amplification of the TCR signal could occur due to the ability of CD28 to increase lipid rafts or glycolipid-enriched microdomains (GEMs) (135). However, questions have been raised regarding the relevance of rafts in T-cell signaling, and, in either case, the claim that CD28 simply augments TCR signaling is probably an oversimplification. The ultimate nature of signaling networks depends critically on the stoichiometry of different components in the activation networks. CD28 preferential engagement of mediators, such as PI3K and FLNA, will undoubtedly alter the qualitative nature of the downstream pathways engaged.
CTLA-4-mediated cosignaling
Despite its structural similarity to CD28, CTLA-4 has an opposing effect on T-cell immunity by dampening or actively inhibiting T-cell activation. The mechanism(s) by which this inhibition is achieved has been a matter for great debate with the postulation of multiple models. In general, CTLA-4 is thought to raise the threshold for TCR signaling, thereby preventing responses to self-antigen (18, 136). As mentioned, both cell extrinsic or intrinsic mechanisms influence CTLA-4 function. Cell extrinsic models include the secretion of soluble CTLA-4 (sCTLA-4), the production of indoleamine 2,3-dioxygenase (IDO) and the involvement of Tregs (as reviewed in 27). Cell intrinsic mechanisms have included CTLA-4 ectodomain competition for CD28 binding to CD80/86 (137), the presence of associated phosphatases (138–142), blockade of lipid-raft expression (143–146) and microcluster formation (29). It is likely that most of these factors contribute to the overall function of CTLA-4 in different contexts.
Three isoforms of CTLA-4 exist: a full-length isoform (flCTLA-4), a form lacking the ectodomain (CTLA-4i), and a soluble form (sCTLA-4) that lacks exon 3 encoding the transmembrane region (52). Unlike the flCTLA-4 that is expressed only on activated T cells, CTLA-4i is restricted to resting cells. Transgenic overexpression of CTLA-4i or flCTLA-4 in Ctla4−/−) mice can prevent the development of autoimmune disease (147). On the other hand, elevated levels of sCTLA-4 have been described in patients with autoimmunity (148, 149). The mechanism by which elevated sCTLA-4 could protect against autoimmunity is not clear, and depends on whether this form competes or mimics the effects of flCTLA-4. It could operate to inhibit CD28-driven activation or, conversely, increase IDO activity, or simply reflect an overall increase in CTLA-4 expression (i.e. both sCTLA-4 and CTLA-4fl). IDO production and Tregs are unlikely to fully account for the disease, as the IDO-knockout mouse does not present a Ctla4−/− phenotype (34). Similarly, the overexpression of FoxP3 on a Ctla4−/− mouse delays but does not resolve disease (32).
The importance of cell intrinsic regulation has come from studies showing that coligation of the coreceptor with TCR can induce anergy (21, 22) and, secondly, that transgenic expression of CTLA-4 lacking the cytoplasmic domain on a Ctla4−/− background does not restore a normal phenotype as observed with flCTLA-4 (107, 137). Intrinsic receptor signaling could operate in normal T cells or Tregs. In naive non-Tregs, CD28 engagement would be followed by CTLA-4 expression on activated cells. CTLA-4 mRNA expression can be detected in a few hours following activation and anti-CTLA-4 inhibition can be detected within 4 h of TCR/CD28-induced activation (150, 151). It is still unresolved as to whether CTLA-4 acts directly on TCR and/or CD28 signaling. The disease phenotype of the Ctla4−/− mouse can be reversed by the blockade of CD28 signaling (152), an observation that may reflect a direct effect on CD28 signaling, or the fact that response to low-affinity self-antigen is dependent on CD28 co-stimulation. In this context, anti-CTLA-4 coligation can inhibit both TCR and TCR/CD28 activation of cells (153).
CTLA-4 lacks a classic immunoreceptor tyrosine-based inhibitory motif (ITIM) that is found in other inhibitory receptors. Despite this, two phosphatases, SHP-2 and the serine–threonine phosphatase protein phosphase 2A (PP2A), have been reported to associate (Fig. 1). SHP-2 targets phosphotyrosine residues, whereas PP2A dephosphorylates serine and threonine residues. Binding of SHP-2 to CTLA-4 depends on the YVKM motif (141), although it occurs indirectly (154). PP2A has been reported to require this YVKM motif, and/or the membrane-proximal Lys residues of CTLA-4 (142, 155). The limitation of the SHP-2 model is that it also associates with CD28, and is generally needed for positive signaling (156, 157). In certain cells, CTLA-4 inhibits proliferation without detectable association with the phosphatase (158), while conversely in naive CD8+ T cells, CTLA-4 is not inhibitory, even when associated with SHP-2 (159). The limitation of the PP2A model is that it also associates with CD28 and the stoichiometry of binding is low. While PP2A could act to inhibit PKB activity and metabolism, it is unclear whether PP2A actively inhibits or whether it suppresses the inhibitory signals generated by CTLA-4 (142, 155).
In another model proposed by our group, CTLA-4 potently inhibits TCR- and TCR–CD28-mediated raft expression and the induction of signaling microclusters (29, 143, 146) (Fig. 1). TCR ligation and CD28 cosignals induce the appearance of lipid rafts or GEMs on the surface of cells, platforms that carry signaling proteins (160, 161). Martin et al. (143) showed that CTLA-4 potently inhibits TCR- and TCR–CD28-mediated raft expression. This inhibition was accompanied by a reduction in the presence of phospho-LAT in the domains. The elegance of the raft model lies in the simplicity of CD28 and CTLA-4 targeting the same event in opposing ways. In this model, costimulatory receptors would function by simply regulating the availability of crucial signal mediators that are required for effective TCR signaling. It would also explain previous results showing that CTLA-4 reduces the phosphorylation of proximal signaling proteins such as TCRζ (141) and general reduction in TCR gene induction from gene profiling (162). The one limitation of this model is that while the induction of raft expression on TCR-activated naive cells is completely blocked by CTLA-4, this inhibition is less effective on pre-activated cells (i.e. <30 reduction). Further downstream, CTLA-4 also generically inhibits cell cycle progression and activation of transcription factors NF-κB, NFAT, and AP1 (23, 163).
Similarly, coligation of the TCR and CTLA-4 effectively blocks the formation of ζ-chain-associated protein kinase 70 (ZAP-70)-containing microclusters in T cells (29) (Fig. 1). Microclusters form shortly after TCR ligation and involve the TCR complex as well as signaling mediators such as ZAP-70, SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) and Gads. Some of these mediators exist in overlapping clusters (i.e. Gads and SLP-76), while other complexes carrying ZAP-70 can transiently interact with the TCR or SLP-76 clusters (164, 165). Peripheral microclusters can sustain signaling that is terminated in the cSMAC (166). CTLA-4 coligation blocked ZAP-70 microcluster formation in the majority of cells from the earliest time point and throughout the incubation period (i.e. 5–10 min). This inhibition may be related to an intrinsic ability to interfere with clustering or the interference due to increased cell motility. The block in microcluster formation occurred concurrently with a loss of calcium mobilization that is needed for T-cell proliferation (29).
It is also now clear that CTLA-4 can induce positive signaling events in T cells. CTLA-4 binds to PI3K with the same avidity as to CD28 and other growth factor receptors (71). Like CD28, it also activates JNK in CD4+ T cells, but inhibits extracellular signal-regulated kinases (ERKs) (167). CTLA-4 therefore differentially regulates members of the mitogen-activated protein kinase (MAPK) family. Inhibition of ERKs could account for negative signaling, while the activation of JNK could contribute to the effects of CTLA-4 on T-helper cell type 1 (Th1) versus Th2 cell differentiation, in which CTLA-4 and JNK1 have both been found to be required for Th1 cell differentiation (168). It can also enhance LFA-1 adhesion, and polarization dependent on PI3K, Vav1, Cdc42, and myosin light chain kinase (26, 27, 169).
Recently, we have shown that CTLA-4 binding to PI3K leads to the activation of PDK1 that in turn activates PKB/AKT by phosphorylation of Thr-308 (86) (Figs 4 and 5). Anti-CTLA-4 ligation, either alone or with anti-CD3, induced and/or increased in PKB/AKT phosphorylation at the activation site Thr-308 (Fig. 4, lanes 4, 6). This phosphorylation occurred at a level similar to anti-CD28 (lane 3). Surprisingly, the same conditions of coligation induce classic CTLA-4-dependent non-responsiveness or anergy (22, 86). Further, under these same conditions, CTLA-4 coligation acted to reverse anti-CD3-induced AICD as measured by propidium iodide/annexin V staining (i.e. 54% cell death relative to 24% in anti-CD3 treated cells alone) (Fig. 4B, upper panels). To connect this event with CTLA-4-induced PKB/AKT phosphorylation, inhibitors of AKT/PKB were then used to assess their effects on the CTLA-4 reversal of AICD. The addition of Akt inhibitor II completely reversed the pro-survival effect induced by CTLA-4 coligation (i.e. 71% cell death relative to 77% in anti-CD3 treated cells alone) (Fig. 4B, lower panels). A similar reversal was observed with PI3K inhibitors (86). These observations showed that CTLA-4 is endowed with an ability to reverse AICD under conditions of anergy induction.
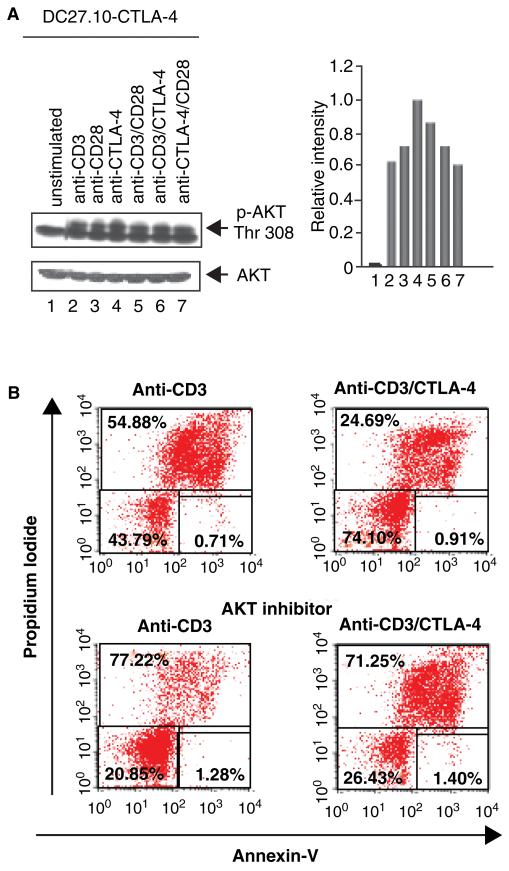
Fig. 4. CTLA-4 coligation can rescue T cells from AICD under conditions of anergy induction.
(A) CTLA-4 coligation induces AKT–Thr–308 phosphorylation. DC27.10–CTLA-4 cells were left untreated (lane 1) or stimulated for 30 min with anti-CD3 (lane 2), anti-CD28 (lane 3), anti-CTLA-4 (lane 4), anti-CD3/CD28 (lane 5), anti-CD3/CTLA-4 (lane 6), and anti-CD28/CTLA-4 (lane 7) antibodies. The DC27.10 cell is a mouse hybridoma that has been stably transfected with CTLA-4 (i.e. DC27.10–CTLA-4). Cell lysates were subjected to immunoblotting with anti-phospho-PKB/AKT (Thr-308) antibody (lanes 1–7). Lower panel: equal amounts of cell lysates were detected by anti-PKB/AKT blotting (lanes 1–7). Histogram depiction of phosphorylated PKB/AKT as detected by densitometric reading. Similar observations were made in peripheral T cells (86). (B) CTLA-4 coligation can rescue T cells from AICD in an AKT/PKB-dependent manner. Pre-activated PBLs were stimulated with anti-CD3 (left panel) and anti-CD3/CTLA-4 (right panel) in the absence (upper panel) or presence of AKT inhibitor (AKT inhibitor II) (lower panel). CTLA-4 coligation reversed cell death induced by anti-CD3 (i.e. 54% to 24% cell death), an event reversed by the inhibition of AKT/PKB (i.e. 77% and 71% cell death) and PI3K (data not shown). Cell viability was assessed by staining with Annexin V-Cy5 and propidium iodide and analyzed using FACS. CTLA-4, cytotoxic T-lymphocyte antigen-4; AICD, antigen-induced cell death; PKB, protein kinase B; PBL, peripheral blood lymphocyte; PI3K, phosphatidylinositol 3-kinase.
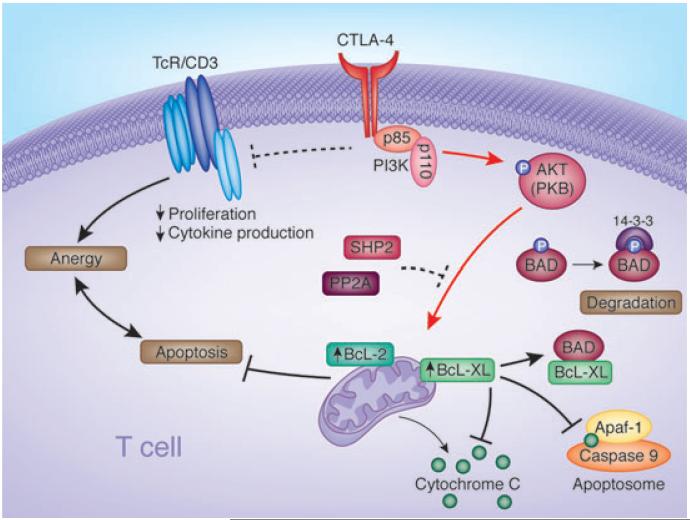
Fig. 5. Diagram outlining the mechanism of CTLA-4 induced prosurvival signaling pathways.
CTLA-4 coligation can reverse AICD induced by ligation of the TCR complex. In the signaling pathway, CTLA-4–PI3K activates PKB/Akt by phosphorylation at Thr-308. CTLA-4 also inactivates proapoptotic BAD by phosphorylation at Ser-136. BAD phosphorylation at Ser-136 leads to binding to 14–3–3 proteins and frees Bcl-XL and Bcl-2 to mediate mitochondrial-dependent cell survival. BAD otherwise promotes cell death by sequestering Bcl-XL/Bcl-2 leading to the release of cytochrome c and apoptosis. Inactivated BAD induced by CTLA-4 ligation is also accompanied by increased Bcl-XL/Bcl-2 expression. CTLA-4–PI3K-PKB/Akt maintenance of cell survival under conditions of anergy induction provides a potential mechanism to ensure long-term tolerance in immunity. CTLA, cytotoxic T-lymphocyte antigen; AICD, antigen-induced cell death; TCR, T-cell receptor; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; BAD, Bcl-2 antagonist of cell death.
The mechanism by which this occurs downstream is not fully resolved, but is accompanied by CTLA-4-induced phosphorylation of the pro-apoptotic protein Bcl-2 antagonist of cell death (BAD) (Fig. 4) (86). BAD, a pro-apoptotic member of the Bcl-2 protein family, promotes apoptosis through heterodimerization with anti-apoptotic proteins such as Bcl-2 and Bcl-XL (170). BAD is phosphorylated by PKB/AKT, an event that can prevent binding to the factors Bcl-XL and Bcl-2 in their mediation of mitochondrial-dependent cell survival (171) (Fig. 4). BAD phosphorylation leads to binding and degradation by 14–3–3 proteins (171). In the process, CTLA-4 ligation alone or coligation was also observed to increase the expression of Bcl-XL and Bcl-2 (86). The ability of CTLA-4–PI3K–PKB/AKT to maintain cell survival under conditions of anergy induction would provide a mechanism to ensure long-term tolerance in immunity, as has been observed using CTLA-4 and anti-CD45RB reagents in transplant rejection (172, 173).
Concluding remarks
CD28 and CTLA-4 play dominant roles in determining the outcome of the T-cell responses by determining the balance of stimulatory and inhibitory cosignals in the regulation of inflammation versus autoimmunity. Understanding the mechanisms that control their expression as well as intracellular signaling will be essential. Distinct and overlapping processes control their localization and cell surface expression. These include PI3K, WASP, and SNX9 in the case of CD28, and TRIM and AP-2 in the case of CTLA-4. Targeting unique aspects of these pathways should allow for the modulation of co-receptor function and immunity. On the other hand, despite years of investigation, new findings on CD28 and CTLA-4 cytoplasmic signaling sites and signaling mechanisms continue to be uncovered that include PI3K, Grb2, FLNA, PKCθ, phosphatases, and others. Increasing evidence indicates that CTLA-4 can no longer be regarded as a simple ‘negative signaling coreceptor’, but rather it has the capacity to generate positive signals that affect adhesion, motility, and prosurvival pathways. Although the mediators engaged by coreceptors are also induced by the TCR complex, they are not equally engaged by each of these receptors. The differential engagement of pathways by the TCR versus coreceptors in the presence of weak versus potent peptide agonists will ultimately determine the balance of cytokines and effector functions in T-cell biology.
References
- 1.Naluai A, et al. The CTLA4/CD28 gene region on chromosome 2q33 confers susceptibility to celiac disease in a way possibly distinct from that of type 1 diabetes and other chronic inflammatory disorders. Tissue Antigens. 2001;56:350–355. doi: 10.1034/j.1399-0039.2000.560407.x. [DOI] [PubMed] [Google Scholar]
- 2.Howard T, Rochelle J, Seldin M. Cd28 and Ctla-4, two related members of the Ig supergene family, are tightly linked on proximal mouse chromosome 1. Immunogenetics. 1991;33:74–76. doi: 10.1007/BF00211698. [DOI] [PubMed] [Google Scholar]
- 3.Balzano C, Buonavista N, Rouvier E, Golstein P. CTLA-4 and CD28: similar proteins, neighbouring genes. Int J Cancer Suppl. 1992;7:28–32. [PubMed] [Google Scholar]
- 4.van der Merwe P, Bodian D, Daenke S, Linsley PS, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikemizu S, et al. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12:51–60. doi: 10.1016/s1074-7613(00)80158-2. [DOI] [PubMed] [Google Scholar]
- 6.Stamper CC, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 7.Bluestone J. New perspectives of CD28-B7 mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 8.Linsley PS. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule 4 receptor during T-cell activation. J Exp Med. 1995;182:289–292. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noel PJ, Boise LH, Thompson CB. Regulation of T cell activation by CD28 and CTLA4. Adv Exp Med Biol. 1996;406:209–217. doi: 10.1007/978-1-4899-0274-0_22. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 11.Shahinian A, et al. Differential T-cell costimulatory requirement in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 12.Kündig TM, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 13.Rincon M, Flavell RA. AP-1 transcriptional activity requires both T cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalli K, Huntoon C, Bell M, McKean DJ. Mechanism responsible for T-cell antigen receptor- and CD28- or interleukin 1 (IL-1) receptor-initiated regulation of IL-2 gene expression by NF-kappaB. Mol Cell Biol. 1998;18:3140–3148. doi: 10.1128/mcb.18.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-kappaB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 16.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 17.Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res. 2003;28:241–253. doi: 10.1385/IR:28:3:241. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 19.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 20.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 21.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 23.Fraser JH, Rincon M, McCoy KD, Le Gros G. CTLA-4 ligation attenuates AP-1, NFAT and NF-kappaB activity in activated T cells. Eur J Immunol. 1999;29:838–844. doi: 10.1002/(SICI)1521-4141(199903)29:03<838::AID-IMMU838>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Leung HT, Bradshaw J, Cleaveland JS, Linsley PS. Cytotoxic T lymphocyte-associated molecule-4, a high avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplamic tail. J Biol Chem. 1995;270:25107–25114. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- 25.Valk E, Leung R, Kang H, Kaneko K, Rudd CE, Schneider H. T cell receptor-interacting molecule acts as a chaperone to modulate surface expression of the CTLA-4 coreceptor. Immunity. 2006;25:807–821. doi: 10.1016/j.immuni.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 27.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 28.Downey J, Smith A, Schneider H, Hogg N, Rudd CE. TCR/CD3 mediated stop-signal is decoupled in T-cells from Ctla4 deficient mice. Immunol Lett. 2008;115:70–72. doi: 10.1016/j.imlet.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider H, Smith X, Liu H, Bismuth G, Rudd C. CTLA-4 expression disrupts ZAP-70 microcluster formation, T-cell/APC conjugation and calcium mobilization. Eur J Immunol. 2007;38:40–47. doi: 10.1002/eji.200737423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 31.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 32.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 33.Perkins D, et al. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156:4154–4159. [PubMed] [Google Scholar]
- 34.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cefai D, Schneider H, Matangkasombut O, Kang H, Brody J, Rudd CE. CD28 endoytosis is targeted by mutations that disrupt phosphatidylinositol 3-kinase binding and costimulation. J Immunol. 1998;160:2223–2230. [PubMed] [Google Scholar]
- 36.Prasad KVS, et al. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)–Met–Xaa–Met motif. Proc Natl Acad Sci USA. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pages F, et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signaling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 38.Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol-3-kinase in Jurkat cells. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.August A, Dupont B. CD28 of T lymphocytes associates with phosphatidylinositol 3-kinase. Int Immunol. 1994;6:769–774. doi: 10.1093/intimm/6.5.769. [DOI] [PubMed] [Google Scholar]
- 40.Kapeller R, Chakrabarti R, Cantley L, Fay F, Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphatidylinositol-3′ kinase complexes: potential interactions with the microtubule cytoskeleton. Mol Cell Biol. 1993;13:6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherr CJ, Borzillo GV, Kato J, Shurtleff SA, Downing JR, Roussel MF. Regulation of cell growth and differentiation by the colony-stimulating factor 1 receptor. Semin Hematol. 1991;28:143–151. [PubMed] [Google Scholar]
- 42.Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- 43.Badour K, et al. Interaction of the Wiskott–Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells. Proc Natl Acad Sci USA. 2007;104:1593–1598. doi: 10.1073/pnas.0610543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snapper SB, Rosen FS. The Wiskott–Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 45.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins – unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 46.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 47.Alegre M-L, et al. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 48.Linsley PS, Bradshaw J, Greene J, Peach R, Bennet KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 49.Schneider H, et al. Cytolytic T lymphocyte-associated antigen-4 and the TCRζCD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- 50.Iida T, et al. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165:5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- 51.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases – a general susceptibility gene to autoimmunity? Genes Immun. 2000;1:170–184. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 52.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 53.Barrat FJ, et al. Defective CTLA-4 cycling pathway in Chediak–Higashi syndrome: a possible mechanism for deregulation of T lymphocyte activation. Proc Natl Acad Sci USA. 1999;96:8645–8650. doi: 10.1073/pnas.96.15.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 55.Bruyns E, et al. T cell receptor (TCR) interacting molecule (TRIM), a novel disulfide-linked dimer associated with the TCR–CD3-zeta complex, recruits intracellular signaling proteins to the plasma membrane. J Exp Med. 1998;188:561–575. doi: 10.1084/jem.188.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirchgessner H, et al. The transmembrane adaptor protein TRIM regulates T cell receptor (TCR) expression and TCR-mediated signaling via an association with the TCR zeta chain. J Exp Med. 2001;193:1269–1284. doi: 10.1084/jem.193.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolsch U, et al. Normal T-cell development and immune functions in TRIM-deficient mice. Mol Cell Biol. 2006;26:3639–3648. doi: 10.1128/MCB.26.9.3639-3648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu M, et al. Negative regulation of lymphocyte activation by the adaptor protein LAX. J Immunol. 2005;174:5612–5619. doi: 10.4049/jimmunol.174.9.5612. [DOI] [PubMed] [Google Scholar]
- 59.Simeoni L, et al. The transmembrane adapter protein SIT regulates thymic development and peripheral T-cell functions. Mol Cell Biol. 2005;25:7557–7568. doi: 10.1128/MCB.25.17.7557-7568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beraud-Dufour S, Balch W. A journey through the exocytic pathway. J Cell Sci. 2002;115:1779–1780. doi: 10.1242/jcs.115.9.1779. [DOI] [PubMed] [Google Scholar]
- 61.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 62.Freyberg Z, Siddhanta A, Shields D. ‘Slip, sliding away’: phospholipase D and the Golgi apparatus. Trends Cell Biol. 2003;13:540–546. doi: 10.1016/j.tcb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Mead KI, et al. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- 64.Yi LA, Hajialiasgar S, Chuang E. Tyrosine-mediated inhibitory signals contribute to CTLA-4 function in vivo. Int Immunol. 2004;16:539–547. doi: 10.1093/intimm/dxh055. [DOI] [PubMed] [Google Scholar]
- 65.Bradshaw JD, et al. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–15982. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- 66.Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- 67.Schneider H, Schwartzberg PL, Rudd CE. Resting lymphocyte kinase (Rlk/Txk) phosphorylates the YVKM motif and regulates PI 3-kinase binding to T-cell antigen CTLA-4. Biochem Biophys Res Commun. 1998;252:14–19. doi: 10.1006/bbrc.1998.9559. [DOI] [PubMed] [Google Scholar]
- 68.Miyatake S, Nakaseko C, Umemori H, Yamamoto T, Saito T. Src family tyrosine kinases associate with and phosphorylate CTLA-4 (CD 152) Biochem Biophys Res Commun. 1998;249:444–448. doi: 10.1006/bbrc.1998.9191. [DOI] [PubMed] [Google Scholar]
- 69.Bonifacino JS, Dell’Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 71.Schneider H, Prasad KVS, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Allison JP. Interaction of CTLA-4 with AP-50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci USA. 1997;94:9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiratori T, et al. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 74.Raab M, Cai Y-C, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56lck and p59fyn regulate CD28 recruitment of phosphatidylinositol 3-kinase, growth factor receptor-bound GRB-2 and T cell-specific protein-tyrosine kinase ITK: implications for costimulation. Proc Natl Acad Sci USA. 1995;92:8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alessi D, et al. 3-Phosphoinositide-dependent proteinkinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 76.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 77.Sasaki T, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 78.Cai Y-C, Cefai D, Schneider H, Raab M, Nabavi N, Rudd CE. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 79.Cefai D, Cai Y-C, Hu H, Rudd CE. CD28 co-stimulatory regimes differ in their dependence on PI-3 kinase: common cosignals induced by CD80 and CD86. Int Immunol. 1996;8:1609–1616. doi: 10.1093/intimm/8.10.1609. [DOI] [PubMed] [Google Scholar]
- 80.Kim H-H, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in cosignaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- 81.Okkenhaug K, et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;325:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 82.Harada Y, et al. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 83.Burr JS, et al. Cutting edge: distinct motifs within CD28 regulate cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 84.Garcon F, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Rudd CE. SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion. Trends Cell Biol. 2008;18:486–493. doi: 10.1016/j.tcb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider H, Valk E, Leung R, Rudd CE. CTLA-4 activation of phosphatidylinositol 3-kinase (PI 3-K) and protein kinase B (PKB/Akt) sustains T-cell anergy without cell death. PLoS ONE. 2008;3:e3842. doi: 10.1371/journal.pone.0003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirchhoff S, Muller WW, Li-Weber M, Krammer PH. Up-regulation of c-FLIPshort and reduction of activation-induced cell death in CD28-costimulated human T cells. Eur J Immunol. 2000;30:2765–2774. doi: 10.1002/1521-4141(200010)30:10<2765::AID-IMMU2765>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 88.Sperling A, Auger J, Ehst B, Rulifson I, Thompson C, Bluestone J. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 89.Radvanyi L, et al. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 90.Jones RG, et al. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J Exp Med. 2002;196:335–348. doi: 10.1084/jem.20020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood JE, Schneider H, Rudd CE. TcR and TcR-CD28 engagement of protein kinase B (PKB/AKT) and glycogen synthase kinase-3 (GSK-3) operates independently of guanine nucleotide exchange factor VAV-1. J Biol Chem. 2006;281:32385–32394. doi: 10.1074/jbc.M604878200. [DOI] [PubMed] [Google Scholar]
- 92.Appleman LJ, van Puijenbroek AAFL, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 93.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. [PubMed] [Google Scholar]
- 94.Schneider H, Cai Y-C, Prasad KVS, Shoelson SE, Rudd CE. T cell antigen CD28 binds to the GRB-2/SOS complex, regulators of p21ras. Eur J Immunol. 1995;25:1044–1050. doi: 10.1002/eji.1830250428. [DOI] [PubMed] [Google Scholar]
- 95.Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J Biol Chem. 1998;273:21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- 96.Schneider H, Rudd C. CD28 and Grb2, relative to Gads or Grap, preferentially co-operate with Vav1 in the activation of NFAT/AP-1 transcription. Biochem Biophys Res Commun. 2008;369:616–621. doi: 10.1016/j.bbrc.2008.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harada Y, et al. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marinari B, et al. Vav cooperates with CD28 to induce NF-kB activation via a pathway involving Rac-1 and mitogen-activated kinase kinase 1. Eur J Immunol. 2002;32:447–456. doi: 10.1002/1521-4141(200202)32:2<447::AID-IMMU447>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 100.Moller A, Dienz O, Hehner SP, Droge W, Schmitz ML. Protein kinase C theta cooperates with Vav1 to induce JNK activity in T-cells. J Biol Chem. 2001;276:20022–20028. doi: 10.1074/jbc.M011139200. [DOI] [PubMed] [Google Scholar]
- 101.Fischer KD, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 102.Holsinger LJ, et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 103.Krawczyk C, et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 104.Kaga S, Ragg S, Rogers KA, Ochi A. Stimulation of CD28 with B7-2 promotes focal adhesion-like cell contacts where Rho family small G proteins accumulate in T cells. J Immunol. 1998;160:24–27. [PubMed] [Google Scholar]
- 105.Raab M, Pfister S, Rudd CE. CD28 signaling via Vav/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity. 2001;15:921–933. doi: 10.1016/s1074-7613(01)00248-5. [DOI] [PubMed] [Google Scholar]
- 106.Michel F, et al. CD28 utilizes Vav-1 to enhance TCR-proximal signaling and NF-AT activation. J Immunol. 2000;165:3820–3829. doi: 10.4049/jimmunol.165.7.3820. [DOI] [PubMed] [Google Scholar]
- 107.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 108.Tavano R, et al. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 109.Holdorf AD, et al. Proline residues in CD28 and Src homolgy (SH)3 domain of LCK are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking ltk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 111.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayashi K, Altman A. Filamin A is required for T cell activation mediated by protein kinase C-theta. J Immunol. 2006;177:1721–1728. doi: 10.4049/jimmunol.177.3.1721. [DOI] [PubMed] [Google Scholar]
- 113.Sanchez-Lockhart M, Graf B, Miller J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J Immunol. 2008;181:7639–7648. doi: 10.4049/jimmunol.181.11.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 115.Ghaffari-Tabrizi N, et al. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur J Immunol. 1999;29:132–142. doi: 10.1002/(SICI)1521-4141(199901)29:01<132::AID-IMMU132>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 116.Sun Z, et al. PKC-theta is required for TCR-induced NF-kB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 117.Lai JH, Tan TH. CD28 signaling causes a sustained down-regulation of I kappa B alpha which can be prevented by the immunosuppressant rapamycin. J Biol Chem. 1994;269:30077–30080. [PubMed] [Google Scholar]
- 118.Harhaj EW, Maggirwar SB, Good L, Sun SC. CD28 mediates a potent costimulatory signal for rapid degradation of IkappaBbeta which is associated with accelerated activation of various NF-kappaB/Rel heterodimers. Mol Cell Biol. 1996;16:6736–6743. doi: 10.1128/mcb.16.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruland J, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 120.Wang D, et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 121.Hara H, et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity. 2003;18:763–775. doi: 10.1016/s1074-7613(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 122.Wang D, et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanner MJ, Hanel W, Gaffen SL, Lin X. CARMA1 coiled-coil domain is involved in the oligomerization and subcellular localization of CARMA1 and is required for T cell receptor-induced NF-kappaB activation. J Biol Chem. 2007;282:17141–17147. doi: 10.1074/jbc.M700169200. [DOI] [PubMed] [Google Scholar]
- 124.Jun JE, et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity. 2003;18:751–762. doi: 10.1016/s1074-7613(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 125.Annibaldi A, et al. CD28 ligation in the absence of TCR promotes RelA/NF-kappaB recruitment and trans-activation of the HIV-1 LTR. Eur J Immunol. 2008;38:1446–1451. doi: 10.1002/eji.200737854. [DOI] [PubMed] [Google Scholar]
- 126.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takeda K, et al. CD28 stimulation triggers NF-kappaB activation through the CARMA1–PKCtheta–Grb2/Gads axis. Int Immunol. 2008;20:1507–1515. doi: 10.1093/intimm/dxn108. [DOI] [PubMed] [Google Scholar]
- 128.Kon-Kozlowski M, Pani G, Pawson T, Siminovitch KA. The tyrosine phosphatase PTP1C associates with Vav, Grb2, and mSos1 in hematopoietic cells. J Biol Chem. 1996;271:3856–3862. doi: 10.1074/jbc.271.7.3856. [DOI] [PubMed] [Google Scholar]
- 129.Sathish JG, et al. Requirement for CD28 costimulation is lower in SHP-1-deficient T cells. Eur J Immunol. 2001;31:3649–3658. doi: 10.1002/1521-4141(200112)31:12<3649::aid-immu3649>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 130.Taylor A, et al. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 132.Freeburn RW, Wright KL, Burgess SJ, Astoul E, Cantrell DA, Ward SG. Evidence that SHIP-1 contributes to phosphatidylinositol 3,4,5-trisphosphate metabolism in T lymphocytes and can regulate novel phosphoinositide 3-kinase effectors. J Immunol. 2002;169:5441–5450. doi: 10.4049/jimmunol.169.10.5441. [DOI] [PubMed] [Google Scholar]
- 133.Tarazona R, et al. Inhibition of CD28-mediated natural cytotoxicity by KIR2DL2 does not require p56(lck) in the NK cell line YT-Indy. Mol Immunol. 2002;38:495–503. doi: 10.1016/s0161-5890(01)00092-x. [DOI] [PubMed] [Google Scholar]
- 134.Beyersdorf N, Hanke T, Kerkau T, Hünig T. Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases. Ann Rheum Dis. 2005;64(Suppl. 4):iv 91–iv 95. doi: 10.1136/ard.2005.042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 136.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 137.Masteller EM, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 138.Chuang E, et al. Regulation of cytotoxic T lymphocyte-associated molecule-4 by Src kinases. J Immunol. 1999;162:1270–1277. [PubMed] [Google Scholar]
- 139.Lee KM, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 140.Cilio CM, Daws MR, Malashicheva A, Sentman CL, Holmberg D. Cytotoxic T lymphocyte antigen 4 is induced in the thymus upon in vivo activation and its blockade prevents anti-CD3-mediated depletion of thymocytes. J Exp Med. 1998;188:1239–1246. doi: 10.1084/jem.188.7.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marengère LEM, Waterhouse P, Duncan GS, Mittrücker H-W, Feng G-S, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase Syp association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 142.Chuang E, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 143.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chikuma S, Imboden JB, Bluestone JA. Negative regulation of T cell receptor-lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2003;197:129–135. doi: 10.1084/jem.20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Darlington PJ, et al. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rudd CE, Martin M, Schneider H. CTLA-4 negative signaling via lipid rafts: a new perspective. Sci STKE. 2002;2002:PE18. doi: 10.1126/stke.2002.128.pe18. [DOI] [PubMed] [Google Scholar]
- 147.Vijayakrishnan L, et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20:563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 148.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164:5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 149.Liu MF, Wang CR, Chen PC, Fung LL. Increased expression of soluble cytotoxic T-lymphocyte-associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol. 2003;57:568–572. doi: 10.1046/j.1365-3083.2003.01232.x. [DOI] [PubMed] [Google Scholar]
- 150.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 151.Lindsten T, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 152.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Egen J, Kuhns M, Allison J. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 154.Schneider H, Rudd CE. Tyrosine phosphatase SHP-2 binding to CTLA-4: absence of direct YVKM/YFIP motif recognition. Biochem Biophsy Res Commun. 2000;269:279–283. doi: 10.1006/bbrc.2000.2234. [DOI] [PubMed] [Google Scholar]
- 155.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 156.Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gadina M, Stancato LM, Bacon CM, Larner AC, O’Shea JJ. Involvement of SHP-2 in multiple aspects of IL-2 signaling: evidence for a positive regulatory role. J Immunol. 1998;160:4657–4661. [PubMed] [Google Scholar]
- 158.Schneider H, da Rocha Dias S, Hu H, Rudd CE. A regulatory role for cytoplasmic YVKM motif in CTLA-4 inhibition of TCR signaling. Eur J Immunol. 2001;31:2042–2050. doi: 10.1002/1521-4141(200107)31:7<2042::aid-immu2042>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 159.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 160.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 161.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Riley JL, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Olsson C, Riesbeck K, Dohlsten M, Michaelsson E. CTLA-4 ligation suppresses CD28-induced NF-kappaB and AP-1 activity in mouse T cell blasts. J Biol Chem. 1999;274:14400–14405. doi: 10.1074/jbc.274.20.14400. [DOI] [PubMed] [Google Scholar]
- 164.Bunnell SC, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 166.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schneider H, et al. Cutting Edge: CTLA-4 (CD152) differentially regulates mitogen-activated protein kinases (extracellular signal-regulated kinase and c-Jun N-terminal kinase) in CD4+ T cells from receptor/ligand-deficient mice. J Immunol. 2002;169:3475–3479. doi: 10.4049/jimmunol.169.7.3475. [DOI] [PubMed] [Google Scholar]
- 168.Oosterwegel MA, et al. The role of CTLA-4 in regulating Th2 differentiation. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 169.Wei B, da Rocha Dias S, Wang H, Rudd CE. CTL-associated antigen-4 ligation induces rapid T-cell polarization that depends on phosphatidylinositol 3-kinase, Vav-1, Cdc42 and myosin light chain kinase. J Immunol. 2007;179:400–408. doi: 10.4049/jimmunol.179.1.400. [DOI] [PubMed] [Google Scholar]
- 170.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 171.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 172.Zheng XG, Turka LA. Blocking T-cell costimulation to prevent transplant rejection. Transplant Proc. 1998;30:2146–2149. doi: 10.1016/s0041-1345(98)00568-5. [DOI] [PubMed] [Google Scholar]
- 173.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2:58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 174.Oki S, Kohsaka T, Azuma M. Augmentation of CTLA-4 expression by Wortmannin: involvement of lysosomal sorting properties of CTLA-4. Int Immunol. 1999;11:1563–1571. doi: 10.1093/intimm/11.9.1563. [DOI] [PubMed] [Google Scholar]