Abstract
Objective
Palliative radiotherapy represents an important treatment option among patients with advanced cancer, though research shows decreased use among older patients. This study evaluates age-related patterns of palliative radiation use among an elderly Medicare population.
Methods
We identified 63,221 patients with metastatic lung, breast, prostate, or colorectal cancer diagnosed between 2000-2007 from the Surveillance, Epidemiology, and End Results (SEER)– Medicare Linked Database. Receipt of palliative radiotherapy was extracted from Medicare claims. Multivariate Poisson regression determined residual age-related disparity in the receipt of palliative radiotherapy after controlling for confounding covariates including age-related differences in patient and demographic covariates, length of life, and patient preference towards aggressive cancer therapy.
Results
The use of radiation decreased steadily with increasing patient age. Forty-two percent of patients aged 66-69 received palliative radiotherapy. The rate of palliative radiation decreased to 38%, 32%, 24%, and 14% among patients aged 70-74, 75-79, 80-84, and over 85, respectively. Multivariate analysis found that confounding covariates attenuated these findings, though the decreased relative rate of palliative radiotherapy among the elderly remained clinically and statistically significant. On multivariate analysis compared to patients 66-69 years old, those aged 70-74, 75-79, 80-84, and over 85 had a 7%, 15%, 25%, and 44% decreased rate of receiving palliative radiation, respectively (all p<0.0001).
Conclusion
Age disparity with palliative radiotherapy exists among older cancer patients. Further research should strive to identify barriers to palliative radiation among the elderly, and extra effort should be made to give older patients the opportunity to receive this quality of life enhancing treatment at the end-of-life.
INTRODUCTION
Palliative radiotherapy represents a standard treatment option in advanced cancer to improve patient quality of life. Among its many indications in the palliative setting, radiotherapy can improve pain from tumor metastases, improve neurologic function from brain or spine metastases, or help relieve compressive or obstructive symptoms caused by tumor bulk. Numerous prospective clinical trials spanning decades have shown the utility of this important treatment modality [4,7,15].
Despite the proven benefit of palliative radiation, multiple studies have found decreased use among the elderly [10-13]. The underlying causes remain unclear, though many potential confounding factors could influence this age-related disparity in palliative radiotherapy. Older patients often have more comorbidity, shorter survival, and less proclivity to receive aggressive cancer care, all which could impact a patient’s decision or ability to receive palliative radiotherapy. To date, research has not assessed the use of palliative radiotherapy adjusting for these confounding factors. The purpose of this study is to define the age-related patterns of palliative radiotherapy in a population-based cohort with a specific focus on identifying and adjusting for confounding factors.
MATERIALS AND METHODS
Dataset
To evaluate the patterns of palliative radiotherapy among the elderly we used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. SEER consists of US cancer registries that track cancer diagnoses for approximately 28% of the US population. The Medicare program provides health insurance for US residents over the age of 65. The SEER-Medicare linkage contains healthcare claims data for Medicare patients within SEER. This dataset allows for population-based longitudinal tracking of healthcare delivery across the US. This research was found exempt from institutional review board approval.
Study cohort
This study included patients with lung, breast, prostate, or colorectal cancer, which represent the four most common types of cancer diagnosed within the United States [14]. Our initial query identified 115,834 patients between 2000 and 2007 with stage IV cancer at diagnosis. We sequentially excluded those with multiple primary cancer diagnoses (13%), as well as diagnosed on death certificate or autopsy only (0.2%). This study accounted for differences in survival time as well as aggressiveness of care at the end-of-life, and to allow for complete patient follow-up we excluded the small fraction of patients alive at the end of the study period (7%). Finally, we excluded patients with incomplete data including non-continuous Medicare part A or part B (6%), or enrollment in part C (26%) from 12 months prior to diagnosis through death. The final study cohort included 63,221 patients. The median age of excluded patients was slightly higher than those in the final study cohort (76 years vs. 75 years).
Study Endpoints
This study's primary endpoint was the receipt of palliative radiotherapy in patients with metastatic cancer. This primary endpoint was ascertained from Medicare claims data using the following daily radiation treatment and weekly management Healthcare Common Procedure Coding System (HCPCS) codes: 77371 - 77373, 77417, 77419 - 77420, 77425, 77427, 77430 - 77432, 77435, 77401 - 77416, 77418, 77422 - 77423, 77470, 77499, 77520, 77522 - 77523, 77525, G0173 - G0174, G0243, G0251, and G0338 - G03340. These radiation codes encompass external beam treatments including 2-dimensional (2D), 3D conformal, intensity modulated radiotherapy, and stereotactic radiotherapy. A course of radiation was defined as a cluster of radiation claims without a break of more than 14 days. Cancer patients often receive multiple courses of radiation, and we assumed that a break in radiation codes of 14 days or more indicated multiple separate courses. Length of an individual course of radiation was defined as the time difference between the first and last radiation claims.
Bone and brain metastases represent two of the most common indications for palliative radiation, and we sought to evaluate these sites further. ICD-9 diagnosis codes from radiation billing claims were used to identify patients treated with bone or brain metastases. ICD-9 diagnosis codes 196 through 198 refer to secondary or metastatic neoplasms, with specific codes for bone (198.5), and brain metastases (198.3) [1]. We assumed that patients were treated for bone or brain metastases when the corresponding ICD-9 code was present, in absence of another metastatic neoplasm IDC-9 diagnosis codes.
Study Covariates
Patient and demographic related variables obtained from SEER include age, race, gender, marital status, year of diagnosis, geographic location, and median household income. Geographic region was grouped into East (Connecticut and New Jersey), Midwest (Detroit and Iowa), South (Atlanta, rural Georgia, Kentucky, and Louisiana), and West (San Francisco, Hawaii, New Mexico, Seattle, Utah, San Jose, Los Angeles, and greater California). Median household income was determined from the 2000 census using census track data preferentially over zip code data, and using race- and age-adjusted data preferentially over unadjusted data. The proximity of patient to radiation facility was estimated from the number of radiation oncologists per 1,000 square miles in the county where the patient resided, as determined from the Area Resource File [2].
This study focused on potential confounding factors that could impact the relationship between age and palliative radiotherapy. Specifically, we focused on patient comorbidity, length of life, and patient tendency towards aggressiveness of care, as we hypothesized that these covariates would vary by patient age, and could potentially independently impact the use of palliative radiotherapy. Comorbidity during the 12 months prior to diagnosis was determined from Medicare data using the Deyo adaptation [5] of the Charlson comorbidity index [3]. Length of life, or survival time, was defined as the time from diagnosis through death. Patient tendency towards aggressiveness of care is not explicitly captured in Medicare data; therefore we used proxy covariates to estimate how receptive a patient would be towards receiving active treatment. Specific measures of aggressiveness included either the receipt of chemotherapy at any point after diagnosis, or the use of aggressive end-of-life measures. The use of chemotherapy was defined as described elsewhere [16]. The use of aggressive care at the endof-life [6] was defined as a composite covariate that included any of the following: receiving chemotherapy within 14 days of death, starting a new chemotherapy agent in last month of life, more than one emergency room visit or hospitalization in last month of life, admission to an ICU or spending more than 2 weeks in a hospital in the last month of life, or death in an acute care hospital.
Statistical Analysis
Continuous covariates such as age were re-classified into categorical covariates. Age was categorized into 5-year increments to allow demonstration of age-specific trends in palliative radiation. Patients over 85 were included in a single category due to small patient numbers. The association of age and palliative radiotherapy was determined with a Poisson regression with robust error estimation [19]. We constructed sequential multivariate Poisson regression models to examine potential confounding covariates which impact the age disparity associated with palliative radiation. We selected clinically relevant covariates a priori to enter into the multivariate model. We used a Poisson regression over a conventional logistic regression because Poisson regressions have the advantage of producing relative risks, which can be more interpretable than odds ratios. Reported p-values are all two sided, and were determined to be significant if less than 0.05. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics for the 63,221 patients stratified by age are presented in Table 1. We found significant age-related differences in patient characteristics (Chi-squared p<0.05 for comparison between age and each characteristic in Table 1). Compared to the younger cohort, older patients were less likely to have lung cancer, and more likely to have breast, prostate, or colorectal cancer. Older patients were more likely to be female, unmarried, live in metropolitan areas, and have higher comorbidity scores. Older patients were less likely to be treated with chemotherapy, and were less likely to have aggressive cancer care at the end-of-life. The median survival was shorter for older patients compared to younger patients. The median survival for patients 66-69 was 6 months compared to 3 months for those 85 and over.
Table 1.
Patient Characteristics according to age.
| Age (years) |
|||||
|---|---|---|---|---|---|
| Characteristic | 66-69 (n=11,749) | 70-74 (n=15,688) | 75-79 (n=15,707) | 80-84 | >85 (n=8,431) |
| Year of diagnosis | |||||
| 2000 | 11% | 12% | 12% | 11% | 11% |
| 2001 | 11% | 13% | 12% | 11% | 11% |
| 2002 | 12% | 13% | 12% | 13% | 11% |
| 2003 | 13% | 13% | 13% | 12% | 12% |
| 2004 | 14% | 13% | 14% | 14% | 14% |
| 2005 | 14% | 13% | 13% | 14% | 14% |
| 2006 | 13% | 12% | 13% | 13% | 14% |
| 2007 | 12% | 11% | 11% | 12% | 14% |
| Primary tumor origin | |||||
| Lung | 73% | 72% | 69% | 61% | 47% |
| Breast | 5% | 5% | 5% | 6% | 8% |
| Prostate | 6% | 7% | 8% | 11% | 16% |
| Colorectal | 16% | 17% | 18% | 22% | 29% |
| Gender | |||||
| Male | 57% | 55% | 53% | 50% | 46% |
| Female | 43% | 45% | 47% | 50% | 54% |
| Race | |||||
| White | 83% | 84% | 84% | 85% | 85% |
| Black | 12% | 10% | 9% | 8% | 9% |
| Other | 5% | 6% | 7% | 7% | 6% |
| Marital status | |||||
| Married | 54% | 53% | 50% | 44% | 31% |
| Other | 46% | 47% | 50% | 56% | 69% |
| Charlson comorbidity score | |||||
| 0 | 55% | 50% | 48% | 47% | 48% |
| 1 | 26% | 28% | 27% | 28% | 27% |
| 2 | 10% | 12% | 14% | 14% | 13% |
| ≥3 | 9% | 10% | 11% | 12% | 12% |
| Median income | |||||
| Bottom quintile | 22% | 21% | 20% | 19% | 18% |
| 2nd quintile | 21% | 20% | 20% | 20% | 19% |
| 3rd quintile | 20% | 20% | 20% | 20% | 20% |
| 4th quintile | 19% | 19% | 21% | 20% | 21% |
| Top quintile | 18% | 19% | 20% | 21% | 22% |
| Geographic region | |||||
| East | 19% | 23% | 24% | 25% | 26% |
| Midwest | 16% | 16% | 16% | 16% | 16% |
| South | 25% | 22% | 20% | 18% | 17% |
| West | 40% | 39% | 40% | 41% | 42% |
| Metropolitan area | |||||
| Yes | 81% | 82% | 84% | 85% | 85% |
| No | 19% | 18% | 16% | 15% | 15% |
| Density of radiation oncologists (per 1,000 square miles) | |||||
| <1 | 26% | 24% | 22% | 20% | 19% |
| 1-10 | 29% | 29% | 28% | 28% | 27% |
| 11-20 | 10% | 10% | 11% | 12% | 12% |
| 21-30 | 8% | 8% | 9% | 9% | 10% |
| 31-40 | 9% | 9% | 10% | 10% | 12% |
| ≥40 | 18% | 19% | 20% | 21% | 21% |
| Aggressiveness of care composite score | |||||
| 0 | 52% | 53% | 55% | 59% | 63% |
| 1 | 18% | 19% | 18% | 18% | 18% |
| ≥2 | 30% | 28% | 26% | 23% | 19% |
| Treated with chemotherapy | |||||
| Yes | 58% | 53% | 46% | 35% | 21% |
| No | 42% | 47% | 54% | 65% | 79% |
Among the whole study cohort, 19,836 patients (31%) received palliative radiotherapy. The rates of palliative radiotherapy decreased steadily with age (Table 2). Among those 66-69 years of age 42% received palliative radiotherapy, which decreased to 14% among patients 85 years and over. The declining trend in the use of palliative radiotherapy was relatively consistent and statistically significant across a wide array of patient covariates (Table 2, p-value for declining trend p<0.05 for each patient subgroup). Among those treated with chemotherapy 46% received palliative radiation, compared to those not treated with chemotherapy where 19% received palliative radiation. We found a decreasing rate of palliative radiation with increasing age in both patients receiving and not receiving chemotherapy (Table 2).
Table 2.
Fraction of patients receiving palliative RT stratified by age.
| Characteristic | Age (years) |
||||
|---|---|---|---|---|---|
| 66-69 | 70-74 | 75-79 | 80-84 | ≥85 | |
| All patients | 42% | 38% | 32% | 24% | 14% |
| Year of diagnosis | |||||
| 2000 | 42% | 36% | 31% | 22% | 13% |
| 2001 | 43% | 38% | 35% | 24% | 14% |
| 2002 | 46% | 36% | 31% | 23% | 16% |
| 2003 | 41% | 40% | 32% | 25% | 15% |
| 2004 | 42% | 39% | 32% | 24% | 13% |
| 2005 | 42% | 39% | 31% | 25% | 14% |
| 2006 | 41% | 36% | 29% | 26% | 16% |
| 2007 | 43% | 35% | 31% | 25% | 14% |
| Primary tumor origin | |||||
| Lung | 48% | 43% | 36% | 30% | 19% |
| Breast | 44% | 38% | 36% | 25% | 18% |
| Prostate | 46% | 41% | 36% | 26% | 18% |
| Colorectal | 16% | 13% | 10% | 7% | 4% |
| Gender | |||||
| Male | 41% | 37% | 32% | 25% | 16% |
| Female | 44% | 38% | 30% | 23% | 13% |
| Race | |||||
| White | 43% | 38% | 32% | 25% | 15% |
| Black | 36% | 30% | 26% | 18% | 10% |
| Other | 41% | 38% | 31% | 26% | 14% |
| Marital status | |||||
| Married | 45% | 41% | 35% | 27% | 18% |
| Other | 38% | 34% | 28% | 22% | 13% |
| Charlson comorbidity | score | ||||
| 0 | 44% | 40% | 34% | 26% | 16% |
| 1 | 43% | 39% | 33% | 25% | 14% |
| 2 | 37% | 33% | 28% | 21% | 12% |
| ≥3 | 31% | 27% | 23% | 21% | 12% |
| Median income | |||||
| Bottom quintile | 37% | 32% | 27% | 20% | 12% |
| 2nd quintile | 41% | 36% | 31% | 25% | 14% |
| 3rd quintile | 42% | 38% | 31% | 23% | 14% |
| 4th quintile | 45% | 41% | 32% | 27% | 16% |
| Top quintile | 47% | 41% | 35% | 26% | 16% |
| Geographic region | |||||
| East | 45% | 39% | 33% | 24% | 16% |
| Midwest | 44% | 37% | 31% | 22% | 12% |
| South | 43% | 37% | 31% | 25% | 13% |
| West | 40% | 37% | 31% | 25% | 15% |
| Metropolitan area | |||||
| Yes | 42% | 38% | 32% | 24% | 15% |
| No | 42% | 37% | 31% | 24% | 12% |
| Density of radiation oncologists (per 1,000 square miles) | |||||
| <1 | 42% | 37% | 31% | 22% | 12% |
| 1-10 | 43% | 39% | 32% | 25% | 15% |
| 11-20 | 41% | 40% | 31% | 26% | 16% |
| 21-30 | 43% | 39% | 31% | 24% | 14% |
| 31-40 | 40% | 35% | 33% | 26% | 17% |
| ≥40 | 42% | 37% | 31% | 23% | 14% |
| Aggressiveness of care composite score | |||||
| 0 | 45% | 41% | 34% | 26% | 16% |
| 1 | 44% | 35% | 29% | 22% | 12% |
| ≥2 | 37% | 32% | 27% | 21% | 13% |
| Treated with chemotherapy | |||||
| yes | 55% | 50% | 44% | 36% | 27% |
| no | 25% | 23% | 21% | 18% | 11% |
We next evaluated age-related differences in specific attributes of palliative radiation. Among those who received palliative radiation, younger patients were more likely to receive longer courses of radiation. The median length of a course of radiation was 18 days (interquartile range 12-30 days) for those aged 66-69, which decreased to 16 days (interquartile range 11-28 days) for those 85 and over. The average patient received 1.25 courses of radiation per year of life after diagnosis, though younger patients were more likely to receive more than one course of radiotherapy. Of patients 66-69 years old, 27% received more than one course of palliative radiation, which steadily decreased to 15% among those 85 or older.
Further evaluation into the specific indication for radiation revealed specific age-related trends. Among those who received palliative radiotherapy, 6,123 received radiation for bone metastases, and 5,730 received radiation for brain metastases. While the use of bone and brain radiation decreased with increasing age, the declining use of brain radiation was more pronounced (Figure 1). With radiation for bone metastases, the use among patients aged 66-69 was 9%, which decreased to 4% among those over 85. With radiation for brain metastases, the use among patients aged 66-69 was 13%, which decreased to 2% among those over 85.
Figure 1.
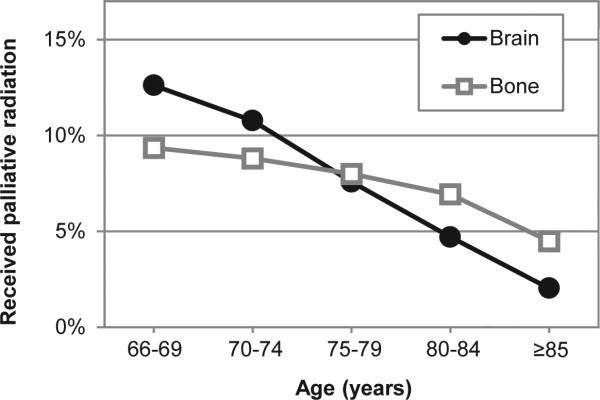
Palliative radiation indication stratified by patient age. This figure demonstrates the use of palliative radiation according to patient age and indication for radiation. The indications for radiation were defined from ICD-9 diagnosis codes from radiation billing claims within Medicare, and include bone metastases (gray squares), and brain metastases (black circles).
Next we sought to determine if potentially confounding covariates impacted the age-related differences in the receipt of palliative radiation. We addressed this question with a multivariate analysis (Table 3). The unadjusted relative rate of palliative radiation decreased steadily with advancing age. Compared to patients 66-69 years old, those aged 70-74, 75-79, 80-84, and over 85 had an 11%, 25%, 42%, and 66% decreased rate of receiving palliative radiation, respectively (all p<0.0001). Adjusting for patient comorbidity, patient characteristics, survival time, and aggressiveness of care all attenuated the relative rate of palliative radiotherapy, though patient demographics including the density of radiation oncologist per square mile did not appear to impact the age-related disparity in palliative radiotherapy. After controlling for all known confounders the age-related discrepancy in palliative radiation among the elderly remained substantial. Compared to patients 66-69 years old, those aged 70-74, 75-79, 80-84, and over 85 had a 7%, 15%, 25%, and 44% decreased rate of receiving palliative radiation, respectively (all p<0.0001).
Table 3.
Multivariate analysis of palliative radiotherapy.
| Relative rate of receiving palliative radiation (95% CI) |
|||||
|---|---|---|---|---|---|
| Model | Age 66-69 | Age 70-74 | Age 75-79 | Age 80-84 | Age ≥85 |
| Unadjusted | 1.00 | 0.89 (0.86-0.92) | 0.75 (0.72-0.77) | 0.58 (0.55-0.60) | 0.34 (0.32-0.36) |
| Prior model adjusted for primary tumor site | 1.00 | 0.89 (0.87-0.92) | 0.76 (0.74-0.78) | 0.61 (0.58-0.63) | 0.39 (0.36-0.41) |
| Prior model adjusted for geography † | 1.00 | 0.89 (0.87-0.92) | 0.76 (0.73-0.78) | 0.60 (0.58-0.63) | 0.38 (0.36-0.41) |
| Prior model adjusted for patient demographics ‡ | 1.00 | 0.89 (0.86-0.91) | 0.76 (0.73-0.78) | 0.61 (0.59-0.63) | 0.40 (0.37-0.42) |
| Prior model adjusted for comorbidity § | 1.00 | 0.90 (0.87-0.92) | 0.77 (0.75-0.80) | 0.62 (0.60-0.65) | 0.41 (0.38-0.43) |
| Prior model adjusted for survival time ∥ | 1.00 | 0.91 (0.89-0.94) | 0.80 (0.78-0.83) | 0.67 (0.64-0.69) | 0.45 (0.43-0.48) |
| Prior model adjusted for aggressiveness of care ¥ | 1.00 | 0.93 (0.91-0.96) | 0.85 (0.82-0.87) | 0.75 (0.73-0.78) | 0.56 (0.53-0.59) |
This table represents sequentially constructed multivariate Poisson regression models.
Geography refers to geographic region, metropolitan area, and density of radiation oncologists.
Patient demographics refer to gender, race, median income level, and year of diagnosis.
Comorbidity refers to the Charlson comorbidity score.
Survival time refers to the time from diagnosis through death.
Aggressiveness of care covariates include the aggressiveness at end-of-life score (defined in the methods), and the receipt of chemotherapy. All p-values<0.0001.
Finally, we conducted a subset analysis on healthy patients without comorbidity, who received chemotherapy, and lived at least 6 months (n=10,834). Multivariate analysis on this subset controlling for all know confounders found similar results. Among this favorable subset of patients on multivariate analysis compared to patients aged 66-69 years old, those aged 70-74, 75-79, 80-84, and over 85 had an 8%, 14%, 27%, and 39% decreased rate of receiving palliative radiation, respectively (all p<0.0001).
DISCUSSION
The principal finding of this study relates to the declining rates of palliative radiotherapy among elderly patients with metastatic cancer. This analysis found that confounding covariates accounted for a portion of this age-related difference, though the final multivariate analysis shows a clinically relevant decrease in palliative radiotherapy among older patients. Furthermore, our findings of decreased radiation use among older patients held steady among a healthy subset of patients who lived at least 6 months.
Many potential age-related barriers could impact a patient's decision or ability to receive palliative radiation. Typical courses of radiation include outpatient daily treatments extending over several days or a few weeks. Older people may lack the ability or support system to get to treatment, and lack of transportation has shown to be a potential barrier to cancer care among the elderly in general [9]. Of note, this study found that the median course of radiation stretched over 16-18 days depending on patient age. The longer length of a course of radiation could impact a patient's decision to pursue radiation, or could impact a providers willingness to refer patients to a radiation oncologist. Increased use of shorter courses of treatment for certain indications such as bone metastases [4] could reduce barriers for older patients wishing to receive radiation. Another factor to consider relates to physician bias. Physicians may consciously or subconsciously feel that older patients would not tolerate or be interested in palliative radiotherapy, which means that an informed discussion with patients may never take place. Increased physician education among referring physicians as well as radiation oncologists, or more involvement of radiation oncology in the palliative care team could help increase utilization of palliative radiotherapy. Another important factor to consider is patient preference. Older patients may simply not want to receive palliative radiotherapy, which could mean that the age-differences we see in this study simply reflect age-specific differences in patient choice. While research has not evaluated patient preferences with respect to palliative radiation, studies surveying patient preference for chemotherapy do not find age-related differences [18]. Ultimately, the underlying cause of the age-related differences in palliative radiotherapy remains unknown, and future research should focus on identifying potentially surmountable barriers.
An important issue relevant to this study relates to the question of overall effectiveness of palliative radiotherapy among the elderly. Few reported studies compare the efficacy of palliative radiation across different age groups. With bone metastases, a secondary analysis of the Dutch bone metastases study found that patients over 75 had a 67% response rate to palliative bone metastases radiation, slightly lower than patients under 65 (78% response rate) [17]. While age is a well-documented adverse prognostic factor in patients with brain metastases [8], research has not thoroughly addressed whether the effectiveness or toxicity of brain radiation varies with patient age. If the efficacy or side effects of radiation depends on the age of the patient, then the declining use of radiation could be appropriate. Further study focusing on age-specific quality of life metrics with palliative radiation is warranted.
This observational population-based study has limitations worth mentioning. Our covariates of patient comorbidity, income level, proximity to radiation oncologist, all reflect estimates. Any age-related bias in these estimates could introduce residual confounding and impact our multivariate analysis. Additionally, our estimate of patient tendency towards aggressiveness of cancer care partly depends on measures of aggressive care at the end-of-life, which may not accurately reflect a patient's inclination towards receiving aggressive cancer care in general. Also, the administrative data in this project lacks patient-level detail; therefore, we cannot directly assess a medical chart to determine the clinical scenario, indication, or value of palliative radiation. Plus, we cannot account for possible age-specific differences in disease biology, disease presentation, and patterns of progression, any of which could explain the differences in the need for or willingness of a patient to receive palliative radiotherapy. Additionally, we cannot determine patient performance status, which could vary with age, and could potentially account for decreased rates of palliative radiation among the elderly. Another limitation relates to our inability to analyze patients with Medicare Part C, which represents patients enrolled in Health Maintenance Organizations (HMOs) which are not required to submit detailed Medicare claims. This lack of HMO patients leads to an inability to generalize our study findings to this cohort. Finally, as noted above, this study does not address patient preference, which limits our ability to determine whether these age-related differences in palliative radiation represent disparity. While studies evaluating chemotherapy preference do not find age-specific differences [18], this question should be reassessed in the palliative radiotherapy setting.
Despite these limitations, this study shows a substantial decrease in the use of palliative radiotherapy among older patients with metastatic cancer. Given the increasingly aging population, as well as the potential quality of life benefit with palliative radiation, future research should strive to define and overcome potential barriers to treatment.
ACKNOWLEDGEMENTS
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
Support: This work was supported by the National Institute on Aging T35 grant AG26757 (JW), a Young Investigator Award from the American Society of Clinical Oncology (JDM), NIH KL2 RR031978 (JDM), and a research collaboration grant from Varian Medical Systems (to JDM and QTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
REFERENCES
- 1.American medical association Ama phyician icd-9-cm. 2009.
- 2.U.S. Department of health and human services; 2009-2010 area resource file (arf) Http://datawarehouse.Hrsa.Gov/datadownload/arf/arf2009-2010.Zip. Downloaded on april 15, 2012.
- 3.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 4.Chow E, et al. Palliative radiotherapy trials for bone metastases: A systematic review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Cherkin DC Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 6.Earle CC, et al. Trends in the aggressiveness of cancer care near the end of life. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 7.Fairchild A, et al. Palliative thoracic radiotherapy for lung cancer: A systematic review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 8.Gaspar L, et al. Recursive partitioning analysis (rpa) of prognostic factors in three radiation therapy oncology group (rtog) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin JS, Hunt WC Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Guadagnolo BA, et al. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the united states. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:80–87. doi: 10.1200/JCO.2012.45.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayman JA, et al. Use of palliative radiotherapy among patients with metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:1001–1007. doi: 10.1016/j.ijrobp.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, et al. Factors affecting the use of palliative radiotherapy in ontario. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:137–144. doi: 10.1200/JCO.2001.19.1.137. [DOI] [PubMed] [Google Scholar]
- 13.Murphy JD, et al. Patterns of care in palliative radiotherapy: A population-based study. Journal of oncology practice / American Society of Clinical Oncology. 2013;9:e220–227. doi: 10.1200/JOP.2012.000835. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Naishadham D Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 15.Tsao MN, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. The Cochrane database of systematic reviews. 2012;4:CD003869. doi: 10.1002/14651858.CD003869.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren JL, et al. Utility of the seer-medicare data to identify chemotherapy use. Medical care. 2002;40:IV–55-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 17.Westhoff PG, de Graeff A, Reyners AK, Rodenhuis CC, van Vulpen M, Leer JWH, Marijnen CAM, van der Linden YM. Response and quality of life in elderly with painful bone metastases: Results from a randomized radiotherapy study icon. 2nd ESTRO Forum; Geneva, Switzerland: 2013. [Google Scholar]
- 18.Yellen SB, Cella DF Leslie WT. Age and clinical decision making in oncology patients. Journal of the National Cancer Institute. 1994;86:1766–1770. doi: 10.1093/jnci/86.23.1766. [DOI] [PubMed] [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]


