Abstract
BACKGROUND AND OBJECTIVE:
Environmental or lifestyle exposures in utero may influence the development of childhood asthma. In this meta-analysis, we aimed to assess whether maternal obesity in pregnancy (MOP) or increased maternal gestational weight gain (GWG) increased the risk of asthma in offspring.
METHODS:
We included all observational studies published until October 2013 in PubMed, Embase, CINAHL, Scopus, The Cochrane Database, and Ovid. Random effects models with inverse variance weights were used to calculate pooled risk estimates.
RESULTS:
Fourteen studies were included (N = 108 321 mother–child pairs). Twelve studies reported maternal obesity, and 5 reported GWG. Age of children was 14 months to 16 years. MOP was associated with higher odds of asthma or wheeze ever (OR = 1.31; 95% confidence interval [CI], 1.16–1.49) or current (OR = 1.21; 95% CI, 1.07–1.37); each 1-kg/m2 increase in maternal BMI was associated with a 2% to 3% increase in the odds of childhood asthma. High GWG was associated with higher odds of asthma or wheeze ever (OR = 1.16; 95% CI, 1.001–1.34). Maternal underweight and low GWG were not associated with childhood asthma or wheeze. Meta-regression showed a negative association of borderline significance for maternal asthma history (P = .07). The significant heterogeneity among existing studies indicates a need for standardized approaches to future studies on the topic.
CONCLUSIONS:
MOP and high GWG are associated with an elevated risk of childhood asthma; this finding may be particularly significant for mothers without asthma history. Prospective randomized trials of maternal weight management are needed.
Keywords: childhood asthma, asthma risk factors, maternal obesity, gestational weight gain, meta-analysis
Obesity is a major public health problem, affecting >35% of the adult population in the United States1 and complicating up to 20% of pregnancies in this country.1 Maternal obesity is defined as a BMI ≥30 kg/m2, and maternal overweight is defined as a BMI between 25 and 29.9 kg/m2.2 Maternal obesity is further stratified into classes: class I (BMI 30–34.9), class II (BMI 35–39.9), and class III (BMI ≥40).3 Maternal obesity in pregnancy (MOP) has been associated with adverse pregnancy outcomes, including hypertensive disorders of pregnancy, gestational diabetes, and need for operative delivery.4,5 Maternal obesity also has a significant impact on fetal development, the neonatal period, and overall childhood development. It has been associated with an elevated incidence of fetal neural tube defects such as spina bifida and anencephaly.6,7 Moreover, higher gestational weight gain (GWG) increases the risk of small size for gestational age and of iatrogenic preterm birth.8
In recent years, there has been growing interest on how in utero exposures predispose infants to diseases throughout the life span. For example, British epidemiologist David Barker9 reported that people with a history of low birth weight were at elevated risk of coronary artery disease later in life and, in what came to be known as the Barker hypothesis,10 theorized that the origins of complex diseases may stem from intrauterine exposures. Thus, MOP may significantly affect life ex utero for years to come. Given the substantial impact of maternal obesity on not only maternal but also fetal and neonatal outcomes, in 2009 the Institute of Medicine3 recommended that obese women limit their GWG to between 11 and 20 pounds.
Asthma, a complex disease that affects ∼7 million children in the United States,11 can result from interactions between hereditary and environmental factors12,13 beginning in utero. For example, maternal smoking during pregnancy increases the risk of asthma in offspring14 and may hinder age-dependent improvement in airway hyperreactivity among children with asthma.15
Along these lines, there is growing evidence that MOP or high GWG is associated with elevated risk of asthma or wheeze (a symptom of asthma) in offspring.16–18 It is thought that in utero exposure to different dietary patterns or to the proinflammatory milieu of obesity may affect fetal immune or pulmonary development, thus leading to asthma.17 Therefore, the aims of this meta-analysis were to quantitatively estimate the effect of MOP or GWG on childhood asthma or wheeze, to generate a pooled analysis of studies available in the literature, and to explore factors that may modify the effect of MOP on childhood asthma.
Methods
We prospectively registered the protocol for this meta-analysis in PROSPERO on September 5, 2013: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005490.
Sources and Study Selection
We searched PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature, Scopus, the Cochrane Database, and Ovid for observational studies evaluating the association between MOP or GWG and asthma or wheeze in childhood, published up to October 2013. Initial inclusion criteria were as follows:
Study design: Observational studies published in English (or in languages other than English when able to translate into English or when enough information was available in an English abstract for use in the data analysis).
Population: Children in whom outcomes were measured between birth and <18 years of age. Mothers in whom BMI at the beginning of pregnancy was ascertained directly or by report at some point during pregnancy or whose GWG was ascertained directly or by report in the third trimester of pregnancy.
Outcomes: Wheeze or asthma during childhood, determined by report, doctor’s diagnosis, medical record review, or evaluation by the study team. When >1 time point for such assessment existed, only the latest point available was included.
Two authors (E.F. and O.M.Y.) independently retrieved and screened all studies according to these predetermined selection criteria. In addition, we manually screened references in the selected articles for additional relevant studies. Initial selection was based on title and abstract screening, and final selection was performed using full texts. Exclusion criteria were ineligible study design (eg, interventional study for management of MOP or GWG or for prevention of childhood asthma or wheeze), ineligible population (eg, adults), ineligible outcomes (eg, other than childhood asthma or wheeze), and insufficient information in English to determine inclusion criteria and abstract risk estimates. Differences of opinion for inclusion were resolved by discussion.
Data Extraction and Quality Assessment
Using a uniform data extraction form, 2 of the authors (E.F. and O.M.Y.) independently extracted from full-text articles all data on references (first author, year of publication), number of participants, timing of MOP or GWG determination (eg, gestational age at which “final” maternal weight was ascertained), BMI or GWG (means or categories) as reported by the original studies, outcome definitions, ages at which outcomes were measured, total number of participants, number of participants with and without the outcomes when available, odds ratios (ORs) or risk ratios with their corresponding confidence intervals (CIs), and relevant covariates as reported in the original studies (eg, mean maternal age, race, smoking exposure during pregnancy or childhood). Disagreements on data extraction between the 2 authors were resolved through mutual discussion and, if needed, consultation with a third author (J.C.C.). Agreement between the reviewers on study selection was determined by using the Cohen k statistic (κ). The quality of reporting in the included studies was assessed by using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist.19 Studies were graded as A (>85% of STROBE criteria fulfilled), B (50–85%), or C (<50%).
Analysis
Data collected were pooled to generate summary estimates; each study was weighed by its inverse effect size variant. To evaluate the association of MOP or GWG, we calculated ORs for the development of childhood asthma or wheeze. Many studies provided different categories for MOP and GWG; therefore, when possible we contacted the corresponding authors of the original studies to provide us with estimates for BMI and GWG (in kilograms) as continuous variables. We tested for heterogeneity in results across studies by using a Cochran Q statistic; the I2 statistic was used to quantify the extent of true heterogeneity. ORs and their CIs were calculated by using random effects models. Egger tests were used to assess for potential publication bias. Subgroup analyses by outcome definition and age group and meta-regression analyses were conducted to explore potential sources of heterogeneity and assess effect modification by different covariates such as maternal age, race, and smoking exposure. All analyses were performed in Stata version 12 (Stata Corp, College Station, TX), and a P of .05 was considered statistically significant.
Results
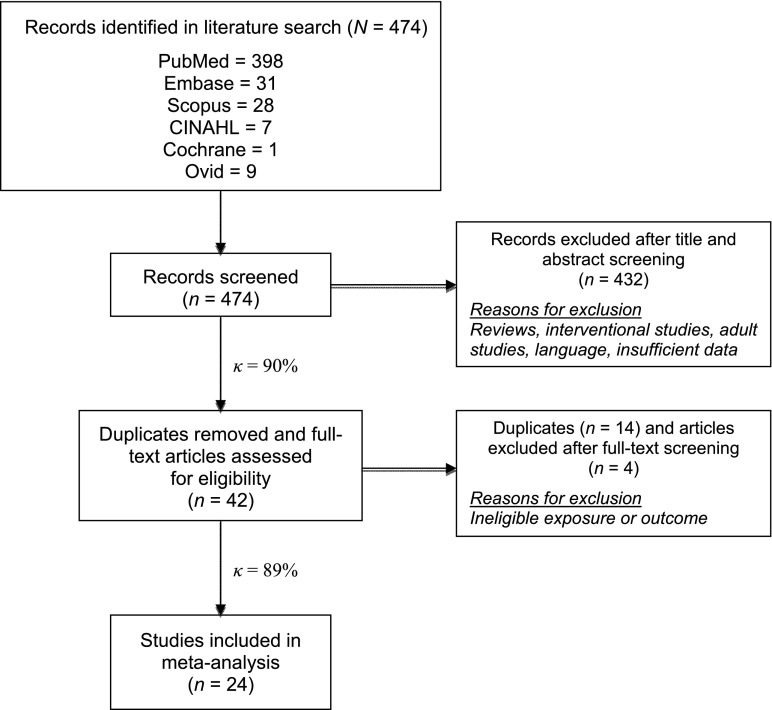
A total of 474 studies were identified (Fig 1): 398 from PubMed, 31 from Embase, 28 from Scopus, 7 from Cumulative Index to Nursing and Allied Health Literature, 1 from the Cochrane Database, and 9 from Ovid; no additional references were identified from reviewing references in relevant articles. Of these, 14 studies with a total of 108 321 mother–child pairs were included in the meta-analysis16–18,20–30 (Table 1). There was complete agreement on 469 of 474 articles after title and abstract screening (interreader agreement κ = .90) and on 40 of 42 articles after full-text screening (κ = .89).
FIGURE 1.
Flowchart of study selection. κ indicates Cohen’s κ agreement coefficient (1.0 = perfect agreement). (Flowchart adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement for systematic reviews and meta-analyses.50)
TABLE 1.
Characteristics of Studies Included in the Meta-analysis
| Reference | N | Age of Children at Outcome | City or Country | Ascertainment of Exposure; Outcome | Risk Estimates as Originally Reported | Comments | Quality Scorea |
|---|---|---|---|---|---|---|---|
| Caudri et al 201316 | 3963 | 8 y | Netherlands | Self-report; self-report | Persistent wheeze: Continuous prepregnancy BMI, OR = 1.26 (CI, 1.01–1.57). | Adjusted for gender, parental allergy, smoke exposure, breastfeeding, day care, pregnancy duration, older siblings, birth wt, maternal age, study region, parental education, and maternal asthma. | A |
| Guerra et al 201317 | 1107 | 14 mo | Spain | Self-report | Frequent wheeze: Continuous BMI, OR = 1.08 (CI, 1.01–1.15). BMI categories: BMI <18.5, OR = 1.7 (CI, 0.3–8.3); BMI 25–30, OR = 1.0 (CI, 0.4–2.6); BMI >30, OR = 4.2 (CI, 1.5–11.3). | Adjusted for maternal socioeconomic status, child’s gender and wt for length, type of delivery, preterm birth, birth wt, day care, breastfeeding, maternal age, parity, and smoking, and parental asthma. | A |
| Håberg et al 200918 | 32 281 | 18 mo | Norway | Self-report; self-report | Wheeze: BMI categories: BMI 25–30, RD = 0.4 (CI, –1.1, 1.8); BMI >30, RD = 3.3 (CI, 1.2, 5.3). | Adjusted for maternal age, parity, education, income, asthma, and smoking in pregnancy, parental smoking after birth, breastfeeding, day care, child’s gender, low birth wt, preterm birth, and cesarean section. Continuous data obtained directly from the author. | A |
| Halonen et al 201320 | 261 | 9 y | United States (Tucson, AZ) | Medical records; measured | Asthma: Prepregnancy BMI tertiles: Middle tertile OR = 0.5 (CI, 0.2–1.3); high OR = 0.4 (CI, 0.2–1.1). GWG categories: High GWG, OR = 3.4 (CI, 1.6–7.2). | Adjusted for maternal age, ethnicity, and parity, parental smoking, and child’s gender. Continuous data obtained directly from the author. | A |
| Harpsøe et al 201321 | 38 874 | 7 y | Denmark | Self-report; self-report | Asthma ever: BMI categories: BMI <18.5, OR = 1.03 (CI, 0.88–1.22); BMI 25–30, OR = 1.22 (CI, 1.12–1.33); BMI 30–35, OR = 1.56 (CI, 1.36–1.79); BMI >35, OR = 1.55 (CI, 1.23–1.95). | Adjusted for maternal age, race, socioeconomic status, smoking during pregnancy, atopy, day care use, atopy, cesarean delivery, child gender, and number of siblings. Continuous data obtained directly from the author. | A |
| GWG categories: <5 kg, OR = 1.17 (CI, 0.94–1.45); 5–9 kg, OR = 1.02 (CI, 0.91–1.15); 16–19 kg, OR = 0.97 (CI, 0.89–1.06); 20–24 kg, OR = 1.15 (CI, 1.05–1.27); >25 kg, OR = 1.19 (CI, 1.04–1.35). | |||||||
| Current asthma: BMI categories: BMI <18.5, OR = 1.07 (CI, 0.85–1.34); BMI 25–30, OR = 1.24 (CI, 1.10–1.38); BMI 30–35, OR = 1.58 (CI, 1.32–1.90); BMI >35, OR = 1.48 (CI, 1.08–2.04). | |||||||
| GWG categories: <5 kg, OR = 0.84 (CI, 0.60–1.17); 5–9 kg, OR = 1.11 (CI, 0.96–1.29); 16–19 kg, OR = 0.90 (CI, 0.80–1.02); 20–24 kg, OR = 1.15 (CI, 1.01–1.31); >25 kg, OR = 1.23 (CI, 1.03–1.46). | |||||||
| Kumar et al 201022 | 1191 | 3 y | United States (Boston, MA) | Self-report | Recurrent wheezing: BMI categories: BMI 25–30, OR = 1.58 (CI, 0.75–3.30); BMI >30, OR = 3.51 (CI, 1.68–7.32). | Adjusted for maternal age, race, education, atopy, child’s gender, premature delivery, smoking during pregnancy and at home, fetal growth retardation, and large size for gestational age. Continuous data obtained directly from the author. | A |
| Leermakers et al 201323 | 4656 | 4 y | Netherlands | Self-report; measured | Current wheeze: | Study included 2 subgroups. Adjusted for maternal age, parity, ethnicity, education, smoking during pregnancy, pets, gestational complications, gestational age at measurement, child’s gender, gestational age at birth, birth wt, breastfeeding, day care, and child’s height and wt at follow-up. Continuous data obtained directly from the author. | A |
| No family history: Continuous BMI, OR = 1.02 (CI, 0.95–1.08). Categories: BMI <20, OR = 0.93 (CI, 0.79–1.09); BMI 25–30, OR = 1.09 (CI, 0.95–1.26); BMI >30, OR = 0.93 (CI, 0.73–1.19). Continuous GWG OR = 1.10 (CI, 1.03–1.16). | |||||||
| Positive family history: Continuous BMI, OR = 1.06 (CI, 0.98–1.43). Categories: BMI <20, OR = 1.19 (CI, 0.98–1.43), BMI 25–30, OR = 0.95 (CI, 0.80–1.14), BMI >30, OR = 1.41 (CI, 1.06–1.87). Continuous GWG, OR = 1.09 (CI, 1.01–1.17). | |||||||
| Oliveti et al 199624 | 262 | 4–9 y | United States (Cleveland, OH) | Medical records | Asthma: GWG category (<9 kg): OR = 3.42 (CI, 1.72–6.79). | Adjusted for maternal asthma history, bronchiolitis, maternal smoking, lack of prenatal care, use of oxygen at birth, prematurity, low birth wt <2500 g, and 5-min Apgar score. | B |
| Patel et al 201225 | 6945 | 15–16 y | Northern Finland | Medical records | Wheeze ever: BMI categories for wheeze (ever) or asthma (ever): BMI <18.5, OR = 0.80 (CI, 0.58–1.09); BMI 25–30, OR = 1.18 (CI, 0.95–1.47); BMI <30, OR = 0.99 (CI, 0.66–1.48). Continuous BMI, OR = 1.028 (CI, 1.005–1.051). | Adjusted for socioeconomic status, marital status, maternal education, maternal asthma, birth wt, parental smoking during gestation, and adolescent BMI at age 15 y. | A |
| Current wheeze: BMI categories for wheeze (current) or asthma (current): BMI <18.5, OR = 0.87 (CI, 0.57–1.04); BMI 25–30, OR = 1.13 (CI, 0.85–1.30); BMI <30, OR = 1.54 (CI, 0.97–2.44). Continuous BMI, OR = 1.047 (CI, 1.019–1.077). | |||||||
| Pike et al 201326 | 940 | 6 y | United Kingdom | Measured | Continuous BMI: | Adjusted for maternal education, asthma, smoking in pregnancy, parity, child’s gender, birth wt, breastfeeding, and other outcome-specific covariates. | A |
| Asthma ever: OR = 1.06 (CI, 0.91–1.24). | |||||||
| Current asthma: OR = 1.10 (CI, 0.92–1.32). | |||||||
| Wheeze ever: OR = 1.08 (CI, 1.02–1.13). | |||||||
| Current wheeze: OR = 1.02 (CI, 0.87–1.20). | |||||||
| Reichman and Nepomnyaschy 200827 | 1971 | 3 y | United States | Medical records | Asthma ever: BMI >30, OR = 1.34 (CI, 1.03–1.76). | Adjusted for maternal age, education, income, race, smoking during pregnancy, parental smoking, preterm delivery, child age, gender, and BMI. Continuous data obtained directly from the author. | A |
| Rusconi et al 200728 | 15 609 | 6–7 y | Italy | Self-report; self-report | Persistent wheezing: GWG <9 kg, OR = 1.08 (CI, 0.84–1.39). GWG >15 kg, OR = 1.20 (CI, 0.98–1.48). | Adjusted for maternal age, education, smoking, study center, parental asthma or atopy, child gender, low birth wt, siblings, socioeconomic status, season, and person who completed the questionnaire. Author responded but continuous data not available. | B |
| Scholtens et al 201029 | 3963 | 8 y | Netherlands | Self-report | Continuous BMI, no family history: | Adjusted for maternal education, smoking, asthma, breastfeeding, mode of delivery, birth wt, and child’s BMI at 8 y of age. | A |
| Asthma: OR = 0.98 (CI, 0.94–1.02). | |||||||
| Wheeze: OR = 1.01 (CI, 0.69–1.07). | |||||||
| Continuous BMI, positive family history: | |||||||
| Asthma: OR = 1.05 (CI, 1.01–1.10). | |||||||
| Wheeze: OR = 1.06 (CI, 1.01–1.12). | |||||||
| Wright et al 201330 | 261 | 2 y | United States (Boston, MA) | Measured | Repeated wheeze: BMI >30, OR = 2.65 (CI, 1.01–6.95). | Adjusted for maternal age, race, education, prenatal smoking, atopy, child’s gender, birth wt adjusted for gestational age, and serum cortisol levels (afternoon slope). Continuous data unavailable. | A |
Table shows characteristics of individual studies, including outcome, obesity categories, and ORs as defined and reported in the original manuscript, as well as reported covariates used in their adjusted models.
Quality of reporting assessment based on STROBE statement criteria.19
Characteristics of Included Studies
Included studies were published between March 1996 and October 2013. Twelve studies reported maternal BMI (continuous or categorical) closely before or at the beginning of pregnancy16–18,20–23,25–27,29,30 and were included in the analysis of MOP; 5 studies were included in the analysis of GWG.20,21,23,24,28 Although many studies categorized MOP and GWG in different ways, response from the authors of the original studies was very positive, and the majority of them provided us with estimates for continuous BMI18,20–23,27 and GWG (in kilograms)20,21 (or kindly responded that continuous data were not available28). For prepregnancy BMI, 7 studies used maternal self-report of height or weight,16–18,21–23,29 and 5 used medical records or directly measured both.20,25–27,30 For GWG, 4 studies used the difference between weight in the third trimester and that in the first trimester,20,23,24,28 and 1 used maternal report of weight gain.21 Outcomes reported varied and included wheezing (ever, current, transient, or persistent) and asthma (ever or current); 12 studies used self-report (of which 3 studies specified “report of physician-diagnosed asthma”20,21,27), and 2 studies reported using physician diagnosis or medical record review.22,24 For comparability purposes, we grouped (when possible) the outcomes into 2 broader categories: “asthma/wheeze ever”17,18,20–22,25–30 and “current asthma/wheeze.”21,23–26,29
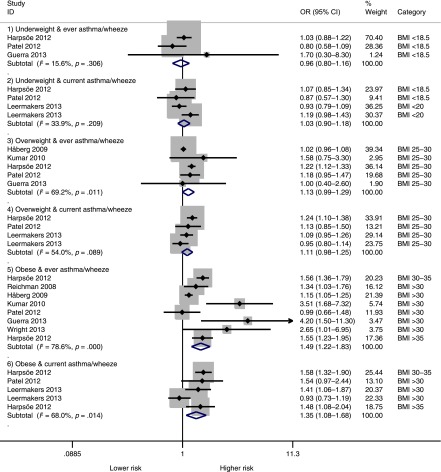
Maternal Obesity During Pregnancy
Table 1 shows the original studies with the MOP as reported (categorical or continuous) and the reported outcomes. Given heterogeneity of outcomes, we grouped them into “asthma/wheeze ever” and “current asthma/wheeze.” Figure 2 shows the pooled analysis by BMI categories. Compared with children from mothers of normal weight, those whose mothers were obese had higher odds of asthma or wheeze ever (OR = 1.49; 95% CI, 1.22–1.83; P < .001) and current asthma or wheeze (OR = 1.36; 95% CI, 1.08–1.68; P = .008). Maternal overweight showed nonsignificant trends for asthma or wheeze ever (OR = 1.13; 95% CI, 0.99–1.29; P = .06) and current asthma or wheeze (OR = 1.11; 95% CI, 0.98–1.25; P = .09). When analyzed together, maternal overweight or obese (BMI >25) led to higher odds of asthma or wheeze ever (OR = 1.31; 95% CI, 1.16–1.49; P < .001) and current asthma or wheeze (OR = 1.21; 95% CI, 1.07–1.37; P = .003) (Supplemental Figure 6). Maternal underweight was not associated with asthma or wheeze (P = .71).
FIGURE 2.
Pooled analysis by BMI categories and childhood asthma or wheeze. Maternal obesity by BMI categories was significantly associated with asthma or wheeze ever and current asthma or wheeze during childhood. Maternal overweight categories showed nonsignificant trends toward increased asthma or wheeze. Note that ORs are for each category as compared with the “normal weight” category (BMI ∼18.5–24.9). Some studies had 2 subgroups (with and without family history of asthma or atopy) and may have 2 entries for the same weight category. Maternal underweight was not significantly associated with childhood asthma or wheeze.
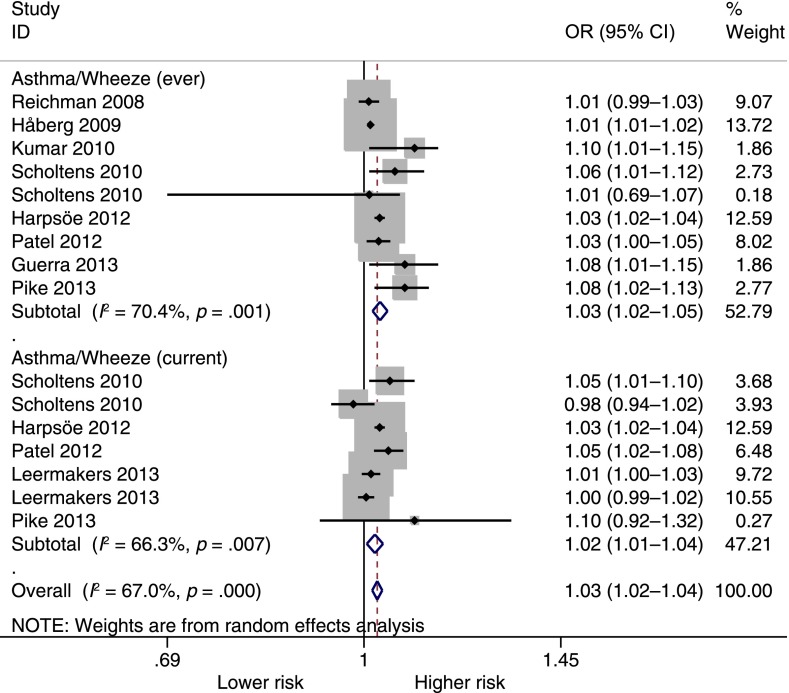
Figure 3 shows the pooled analysis of maternal BMI and asthma or wheeze. In this analysis, maternal BMI was significantly associated with higher odds of asthma or wheeze ever (OR = 1.03 per each 1 kg/m2 increase; 95% CI, 1.02–1.05; P < .001) and current asthma or wheeze (OR = 1.02 per kg/m2; 95% CI, 1.01–1.04; P = .009). There was significant heterogeneity for both outcomes (asthma or wheeze ever, I2 = 70.4%; current asthma or wheeze, I2 = 66.3%). The Egger test showed there could be significant publication bias for asthma or wheeze ever (P = .04) but not for current asthma or wheeze (P = .99).
FIGURE 3.
Pooled analysis by continuous BMI and asthma or wheeze. Increasing maternal BMI was significantly associated with both asthma or wheeze ever and current asthma or wheeze during childhood. Note that ORs are for each unit increase in BMI. Some studies23,29 had 2 subgroups (with and without family history of asthma or atopy) and may have 2 entries.
Gestational Weight Gain
Table 1 shows the original studies with GWG as reported (categories or continuous). Figure 4 shows the pooled analysis by reported GWG categories. Children whose mothers were in the higher GWG categories had significantly higher odds of asthma or wheeze ever (OR = 1.16; 95% CI, 1.001–1.34; P = .049) but not of current asthma or wheeze (OR = 1.08; 95% CI, 0.89–1.31; P = .45). Figure 5 shows the pooled analysis of maternal (continuous) GWG and asthma or wheeze. In this analysis, maternal GWG was significantly associated with higher odds of current asthma or wheeze (OR = 1.015 per 1-kg increase in GWG; 95% CI, 1.01–1.02; P < .001) but not with asthma or wheeze ever (OR = 1.04 per kg; 95% CI, 0.97–1.11; P = .27). Both for GWG categories and for continuous GWG, the number of studies was small (n = 2–3). The Egger test showed no significant evidence of publication bias for current asthma or wheeze (P = .52), but it could not be calculated for asthma or wheeze ever because of the small number of studies. Subnormal GWG was not associated with asthma or wheeze (P = .19; Supplemental Figure 7), but the number of studies reporting was small (n = 3).
FIGURE 4.
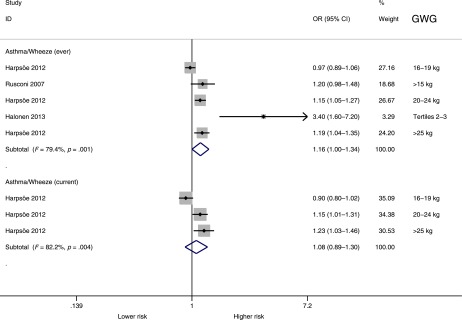
Pooled analysis by GWG categories and asthma or wheeze. High maternal GWG was significantly associated with asthma or wheeze ever but not with current asthma or wheeze (P = .47) during childhood. Note that categories of GWG vary between studies. Subnormal GWG was not significantly associated with childhood asthma or wheeze (see Supplemental Fig 7).
FIGURE 5.
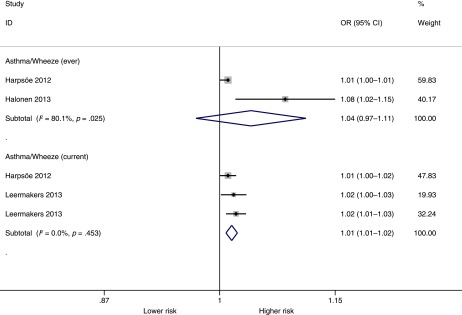
Pooled analysis by continuous GWG and asthma or wheeze. Increasing GWG (in kg) was significantly associated with current asthma or wheeze but not with asthma or wheeze ever (P = .27) during childhood. Note that ORs are for each 1-kg increase in GWG. Note the small number of studies in each outcome (n = 2).
Subgroup Analysis and Meta-regression
Meta-regression was performed for BMI with covariates available for extraction from the original studies (at the study level [eg, mean or median maternal age in the study] or at the category level when available [eg, mean or median maternal age within each BMI category in the study]). For continuous BMI, meta-regression showed a negative association of borderline significance between maternal history of asthma and the study effect size: The risk of childhood asthma or wheeze with increasing maternal BMI was higher when the prevalence of maternal asthma was lower (β = 0.90; 95% CI, 0.80–1.01; P = .07); this effect modification explained part of the study heterogeneity (I2 decreased to 33.6%; R2 = 81.5%). We were unable to perform subgroup analysis or meta-regression for GWG because of the small number of studies.
Discussion
In this meta-analysis, we report that children whose mothers were obese during pregnancy (defined by BMI categories or by higher continuous BMI) are at higher risk of asthma or wheeze. Maternal underweight did not appear to increase the risk of asthma or wheeze in childhood, although this analysis included only a few studies with small sample sizes and may have been underpowered.
Maternal obesity could influence the risk of asthma in the offspring through several mechanisms. Obesity is associated with a chronic, low-grade inflammatory state.31 For example, it has been associated with elevated levels of inflammatory cytokines implicated in asthma, such as tumor necrosis factor α (TNF-α), interleukin 6, and transforming growth factor β-1. Leptin, a proinflammatory adipokine, not only is elevated in the maternal circulation of pregnant obese women but also is higher in the cord blood of their children than in children whose mothers are not obese.32 Conversely, adiponectin, which has antiinflammatory properties and has shown inverse associations with asthma symptoms33 and airway inflammation,34 is decreased in obesity and is also decreased in newborns of obese mothers.35 Alternatively, maternal obesity may influence the pathogenesis of asthma through exposure of the developing fetus to different dietary patterns.36 For example, obese women are more likely to have low serum levels of vitamin D,37 and maternal vitamin D insufficiency or deficiency has been associated with elevated risk of childhood asthma.38 Similarly, maternal consumption of other foods during pregnancy (eg, meat, dairy, or fats) has been associated with childhood asthma.39,40 Finally, shared genetic polymorphisms or epigenetic changes as a result of maternal obesity could be causing, mediating, or modifying the elevated risk of asthma in their offspring.
Individual studies assessing whether maternal history of asthma modifies the effect of MOP on childhood asthma have shown conflicting results.23,25,29 In our meta-regression analysis, there was significant modification of the effect of MOP on asthma by maternal asthma: There was a more pronounced increase in the risk of childhood asthma when the prevalence of maternal asthma was lower. Although such analysis is only exploratory and should be interpreted with caution, it could indicate that the risk conferred by the mother’s asthma supersedes that conferred by maternal obesity. The heritability of asthma has been estimated to be ∼0.82–0.91,12,41 and studies with higher prevalence of maternal asthma could be underpowered to detect an effect of maternal obesity. Alternatively, this finding may suggest that the risk from maternal obesity is stronger for nonatopic asthma. Many42–44 but not all45 studies of obesity and asthma have reported this association to be stronger among nonatopic children or adults.
GWG was reported in fewer studies. Although there seems to be a higher risk of asthma or wheeze with higher GWG, the observed associations varied depending on the way the exposure was reported (categorical or continuous GWG) and the definition of asthma or wheeze. GWG has also been associated with elevated maternal46,47 and cord blood47 leptin levels. Halonen et al20 reported that persistently elevated TNF-α from birth to 3 months of age was associated with asthma and decreased lung function at age 9 years and that maternal GWG was the strongest predictor of an elevated TNF-α level. These findings suggest that GWG and maternal obesity may contribute to the onset of childhood asthma via similar proinflammatory mechanisms. Additional studies are needed to reach more definitive conclusions in regard to GWG and childhood asthma or wheeze.
Studies addressing maternal obesity and childhood asthma that did not meet inclusion criteria for our meta-analysis should be mentioned. In a Swedish cohort of ∼431 000 first-born children, Lowe et al48 reported that MOP was associated with greater use of inhaled corticosteroid use in boys and girls up to age 12 years and in girls up to age 16 years; these results suggest that MOP may also be a risk factor for more severe or persistent childhood asthma. Furthermore, in a study by Watson and McDonald,49 both maternal baseline skinfold thickness (as a surrogate of percentage body fat) and increase in skinfold thickness during pregnancy were independently associated with greater risk of asthma in childhood, whereas BMI was not significant. The authors proposed that skinfold measurement may be more sensitive than BMI; this extends to maternal obesity in our recent report that for studies of asthma, the child’s BMI may not be the best or sole indicator of adiposity.45
The current study has several limitations. We included only articles published in English or with sufficient information in English to abstract data for analysis, which may not fully represent all studies conducted on the subject. There was high variability among studies. We dealt with this variability in 3 ways: To account for effect size variability, we used random effects models; to account for the variability in the way BMI and GWG were categorized in the original studies, we obtained effect sizes using continuous BMI or GWG from many of the authors of the original reports; and we performed meta-regression analyses to detect significant effect modifiers. However, as with any meta-analysis, we were limited to the covariates available to us from the original articles. Finally, maternal obesity is also a risk factor for childhood obesity, and the elevated risk of asthma could result partly from the child’s own obesity; however, such confounding is unlikely given that most of the included studies adjusted for birth weight16,17,22,23,25,26,29,30 or for the child’s weight or BMI at the time of assessment of the outcome.17,23,25,27,29
Conclusions
We report that MOP is a significant risk factor for the development of childhood asthma or wheeze. GWG may also increase the risk of childhood asthma or wheeze, but we were limited by the small number of studies. The effect of maternal obesity on childhood asthma may be more pronounced when the prevalence of maternal asthma is lower.
Supplementary Material
Glossary
- CI
confidence interval
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- GWG
gestational weight gain
- MOP
maternal obesity in pregnancy
- OR
odds ratio
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TNF-α
tumor necrosis factor α
Footnotes
Dr Forno formulated the idea for the study and participated in the study design, literature searches, study selection, data abstraction, analysis, and interpretation and the writing of the manuscript; Dr Young participated in the study design, literature searches, study selection, data abstraction, analysis, and interpretation and the writing of the manuscript; Dr Kumar participated in the data interpretation and the writing of the manuscript; Dr Simhan contributed to the data analysis and interpretation and participated in the writing of the manuscript; Dr Celedón contributed to the data analysis and interpretation and participated in the writing of the manuscript; and all authors approved the final manuscript as submitted. Dr Forno and Dr Young contributed equally to this manuscript.
This trial has been registered at PROSPERO (www.crd.york.ac.uk/prospero) (identifier CRD42013005490).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Forno was supported by NIH grant K12-HD052892. Dr Celedón was supported by NIH grants HL079966 and HL117191 and an endowment from the Heinz Foundation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Celedón served as a one-time consultant for Genentech in 2011 on an issue unrelated to this manuscript; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;131:1–8. PubMed PMID: 24152742 [PubMed]
- 2.World Health Organization (WHO). Obesity and overweight: WHO. Fact sheet no. 311. 2013. Available at: www.who.int/mediacentre/factsheets/fs311/en/. Accessed April 17, 2014
- 3.Institute of Medicine (IOM) Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academy of Sciences, Institute of Medicine; 2009 [Google Scholar]
- 4.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–224 [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists . ACOG committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213–217 [DOI] [PubMed] [Google Scholar]
- 6.Waller DK, Mills JL, Simpson JL, et al. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol. 1994;170(2):541–548 [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198(6):611–619 [DOI] [PubMed] [Google Scholar]
- 8.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007;110(4):752–758 [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580 [DOI] [PubMed] [Google Scholar]
- 10.Paneth N, Susser M. Early origin of coronary heart disease (the “Barker hypothesis”). BMJ. 1995;310(6977):411–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. PubMed PMID: 22617340 [PubMed]
- 12.Thomsen SF, van der Sluis S, Kyvik KO, Skytthe A, Skadhauge LR, Backer V. Increase in the heritability of asthma from 1994 to 2003 among adolescent twins. Respir Med. 2011;105(8):1147–1152 [DOI] [PubMed] [Google Scholar]
- 13.Scirica CV, Celedón JC. Genetics of asthma: potential implications for reducing asthma disparities. Chest. 2007;132(5 suppl):770S–781S [DOI] [PubMed] [Google Scholar]
- 14.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163(2):429–436 [DOI] [PubMed] [Google Scholar]
- 15.Cohen RT, Raby BA, Van Steen K, et al. Childhood Asthma Management Program Research Group . In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol. 2010;126(3):491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caudri D, Savenije OEM, Smit HA, et al. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clin Exp Allergy. 2013;43(12):1395–1405 [DOI] [PubMed] [Google Scholar]
- 17.Guerra S, Sartini C, Mendez M, et al. Maternal prepregnancy obesity is an independent risk factor for frequent wheezing in infants by age 14 months. Paediatr Perinat Epidemiol. 2013;27(1):100–108 [DOI] [PubMed] [Google Scholar]
- 18.Håberg SE, Stigum H, London SJ, Nystad W, Nafstad P. Maternal obesity in pregnancy and respiratory health in early childhood. Paediatr Perinat Epidemiol. 2009;23(4):352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349 [DOI] [PubMed] [Google Scholar]
- 20.Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-α production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013;188(1):35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harpsøe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131(4):1033–1040 [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Story RE, Pongracic JA, et al. Maternal pre-pregnancy obesity and recurrent wheezing in early childhood. Pediatr Allergy Immunol Pulmonol. 2010;23(3):183–190. PubMed PMID: 22375278. PubMed Central PMCID: 3281288 [DOI] [PMC free article] [PubMed]
- 23.Leermakers ET, Sonnenschein-van der Voort AM, Gaillard R, et al. Maternal weight, gestational weight gain and preschool wheezing. The Generation R Study. Eur Respir J. 2013;42(5):1234–1243. PubMed PMID: 23471348 [DOI] [PubMed]
- 24.Oliveti JF, Kercsmar CM, Redline S. Pre- and perinatal risk factors for asthma in inner city African-American children. Am J Epidemiol. 1996;143(6):570–577 [DOI] [PubMed] [Google Scholar]
- 25.Patel SP, Rodriguez A, Little MP, et al. Associations between pre-pregnancy obesity and asthma symptoms in adolescents. J Epidemiol Community Health. 2012;66(9):809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike KC, Inskip HM, Robinson SM, et al. Southampton Women’s Survey Study Group . The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax. 2013;68(4):372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichman NE, Nepomnyaschy L. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Matern Child Health J. 2008;12(6):725–733 [DOI] [PubMed] [Google Scholar]
- 28.Rusconi F, Galassi C, Forastiere F, et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am J Respir Crit Care Med. 2007;175(1):16–21 [DOI] [PubMed] [Google Scholar]
- 29.Scholtens S, Wijga AH, Brunekreef B, et al. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond). 2010;34(4):606–613 [DOI] [PubMed] [Google Scholar]
- 30.Wright RJ, Fisher K, Chiu YH, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187(11):1186–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919, quiz 920 [DOI] [PubMed] [Google Scholar]
- 32.Ferretti G, Cester A, Bacchetti T, et al. Leptin and paraoxonase activity in cord blood from obese mothers [published online ahead of print October 23, 2013]. J Matern Fetal Neonatal Med. 2013. PubMed PMID: 24147648 [DOI] [PubMed]
- 33.Kattan M, Kumar R, Bloomberg GR, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125(3):584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118(2):389–395 [DOI] [PubMed] [Google Scholar]
- 35.Vega-Sanchez R, Barajas-Vega HA, Rozada G, Espejel-Nuñez A, Beltran-Montoya J, Vadillo-Ortega F. Association between adiposity and inflammatory markers in maternal and fetal blood in a group of Mexican pregnant women. Br J Nutr. 2010;104(12):1735–1739 [DOI] [PubMed] [Google Scholar]
- 36.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20(6):682–687 [DOI] [PubMed] [Google Scholar]
- 37.Scholl TO, Chen X. Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev. 2009;85(4):231–234 [DOI] [PubMed] [Google Scholar]
- 38.Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strøm M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease: a prospective study in 44,825 Danish mother–child pairs. BMC Pregnancy Childbirth. 2013;13(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatzi L, Garcia R, Roumeliotaki T, et al. INMA Study Group. RHEA Study Group . Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother–child cohort studies. Br J Nutr. 2013;110(11):2058–2068 [DOI] [PubMed] [Google Scholar]
- 40.Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal fat intake during pregnancy and wheeze and eczema in Japanese infants: the Kyushu Okinawa Maternal and Child Health Study. Ann Epidemiol. 2013;23(11):674–680 [DOI] [PubMed] [Google Scholar]
- 41.van Beijsterveldt CE, Boomsma DI. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. Eur Respir J. 2007;29(3):516–521 [DOI] [PubMed] [Google Scholar]
- 42.Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma. 2010;47(7):822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenger RV, Gonzalez-Quintela A, Vidal C, et al. Exploring the obesity–asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy. 2012;42(8):1237–1245 [DOI] [PubMed] [Google Scholar]
- 44.Telenga ED, Tideman SW, Kerstjens HA, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67(8):1060–1068 [DOI] [PubMed] [Google Scholar]
- 45.Forno E, Acosta-Perez E, Brehm J, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133(5):1308–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferraro ZM, Qiu Q, Gruslin A, Adamo KB. Excessive gestational weight gain and obesity contribute to altered expression of maternal insulin-like growth factor binding protein-3. Int J Womens Health. 2013;5:657–665. PubMed PMID: 24124394. PubMed Central PMCID: 3794982 [DOI] [PMC free article] [PubMed]
- 47.Karakosta P, Georgiou V, Fthenou E, et al. Maternal weight status, cord blood leptin and fetal growth: a prospective mother–child cohort study (Rhea study). Paediatr Perinat Epidemiol. 2013;27(5):461–471 [DOI] [PubMed] [Google Scholar]
- 48.Lowe AJ, Ekeus C, Bråbäck L, Rajaleid K, Forsberg B, Hjern A. Impact of maternal obesity on inhaled corticosteroid use in childhood: a registry based analysis of first born children and a sibling pair analysis. PLoS ONE. 2013;8(6):e67368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson PE, McDonald BW. Subcutaneous body fat in pregnant New Zealand women: association with wheeze in their infants at 18 months. Matern Child Health J. 2013;17(5):959–967 [DOI] [PubMed] [Google Scholar]
- 50.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.