Abstract
Observation of others in pain induces positive elevation (pain effect) in late event-related potentials (ERP). This effect is associated with top-down attention regulating processes. It has previously been shown that stimulus exposure duration can affect top-down attentional modulation of response to threat-related stimuli. We investigated the effect of exposure duration on ERP response to others in pain. Two late ERP components, P3 and late positive potentials (LPP), from 18 healthy people were measured while they viewed pictures of hands in painful or neutral situations for either 200 or 500 ms, during two task conditions (pain judgment and counting hands). P3 and LPP pain effects during the pain judgment condition were significantly greater with 500 ms than 200 ms stimulus presentation. Ours is the first study to suggest that engagement of empathy-related self-regulatory processes reflected in late potentials requires longer exposure to the pain-related stimulus. Although this is important information about the relationship between early sensory and subsequent brain processing, and about engagement of self-regulatory processes, the neural basis of this time-dependence remains unclear. It might be important to investigate the relationship between stimulus duration and empathic response in clinical populations where issues of self-regulation, empathic response and speed of information processing exist.
Keywords: empathy for pain, emotional regulation, P3, late positive potential (LPP), stimulus exposure duration
INTRODUCTION
‘Empathy’ consists of phenomena ranging from feelings of concern for other people that create a desire to help them, emotions that match another person’s emotions, knowing what another person is thinking or feeling, to blurring the line between self and other (Hodges and Klein, 2001). The experience of empathy can lead to sympathy (which includes an other-oriented motivation) or personal distress leading to withdrawal from the stressor, decreased likelihood of prosocial behavior (Decety, 2010). Some aspects of empathy can be observed in human beings as early as infancy, and appear to be present in other species as well (Singer, 2012). The word ‘Empathy’ was coined in 1903 by Edward Titchener, but the phenomenon of Empathy was described as early as the fourth century B.C. by the Taoist, Zhuangzi.
In the field of social neuroscience, the majority of empathy studies has used the perception of ‘pain in others’ since the first study using functional magnetic resonance imaging (fMRI) in 2004 (Singer, 2012). According to a meta-analysis on 32 studies that investigated empathy for pain using fMRI, bilateral anterior insula cortex (AI) and the border of anterior medial cingulate cortex (aMCC) and posterior anterior cingulate cortex were the most consistently activated regions (Lamm et al., 2011). This activation pattern overlaps with the neural network involved in the direct experience of pain. Moreover, another meta-analysis of fMRI studies of empathy not restricted to painful stimuli also identified dorsal anterior cingulate cortex–aMCC–supplementary motor area and bilateral AI as being consistently activated (Fan et al., 2011).
Recent studies also suggest that emotional and cognitive processes mediate the empathic response (Shamay-Tsoory et al., 2007, 2009; Fan and Han, 2008). While an early component is automatically elicited by perception of another’s emotional state (emotional contagion) (Shamay-Tsoory et al., 2007, 2009; Fan and Han, 2008), a late component involves cognitive perspective taking and mentalizing (Shamay-Tsoory et al., 2009). Previous work using event-related potentials (ERPs) demonstrated more positivity to painful than neutral stimuli, with this ERP positivity to painful stimuli modulated by various types of top-down attention demands particularly in late ERP waves such as ‘P300/P3’ (Fan and Han, 2008; Li and Han, 2010; Ibanez et al., 2011; Cheng et al., 2012; Meng et al., 2012). A long-lasting and later ERP positivity [late positive potential (LPP)] is also associated with affective stimuli, especially aversive/unpleasant pictures and images of people in pain, and associated with emotional regulation (Olofsson et al., 2008; Hajcak et al., 2010). P300s and LPPs are thought by many to represent different brain sources and to reflect different aspects and stages of cognitive–affective processing (Foti and Hajcak, 2008; Olofsson et al., 2008; Dunning and Hajcak, 2009; Macnamara et al., 2009; Weinberg and Hajcak, 2011; Cheng et al., 2012).
It has been repeatedly reported that stimulus exposure duration can affect top-down attentional process with threat-related stimuli. Onnis et al. (2011), summarizing behavioral studies with the attentional bias paradigm, noted enhanced vigilance to threat-related stimuli or difficulties disengaging attention from threat-related stimuli at 100–200 ms stimulus presentation durations, and attentional avoidance to threat-related stimuli usually at display times of 500 ms and longer. A recent behavioral study links these findings to empathy studies by noting that visual processing of painful stimuli as used in empathy studies can be associated with potential threat and activates a threat-detection system and possibly a general aversive response in the observer (Yamada and Decety, 2009).
It has been previously reported that stimulus exposure duration can affect ERP responses. For example, P300s to non-affective pictures are greater with short stimuli than long (Berti and Schroger, 2001; Brisson and Jolicoeur, 2007). Whereas, with affective picture, late positivity components were clearer with long than short duration stimuli, and increased significantly across presentation times (Pegna et al., 2008; Genetti et al., 2009). However, to the best of our knowledge, there is no study comparing long and short stimulus exposure using pictures of others in pain.
In this study, we evaluated the effect of exposure duration of pictures of people in pain on pain-related positivity in P3 and LPP. As these late responses are associated with top-down modulation, we hypothesized that they would differ between long vs short stimulus duration.
METHODS
Participants
Eighteen healthy people (12 male and six female; seven African American and 11 Caucasian), as screened for psychiatric disorders with the Structured Clinical Interview Non-Patient Edition (First et al., 1998) by a licensed clinical psychologist, participated in this study (mean age; 39.8 years). They were recruited through advertisements (flyers, internet posts), and were without history of psychiatric illness, neurological disease, brain injury, or developmental disability. All participants were right handed, native English speakers with normal or corrected to normal vision. This study was approved by Yale Human Investigation Committee. After complete description of the study, subjects provided written informed consent.
Measures
Stimuli and procedure
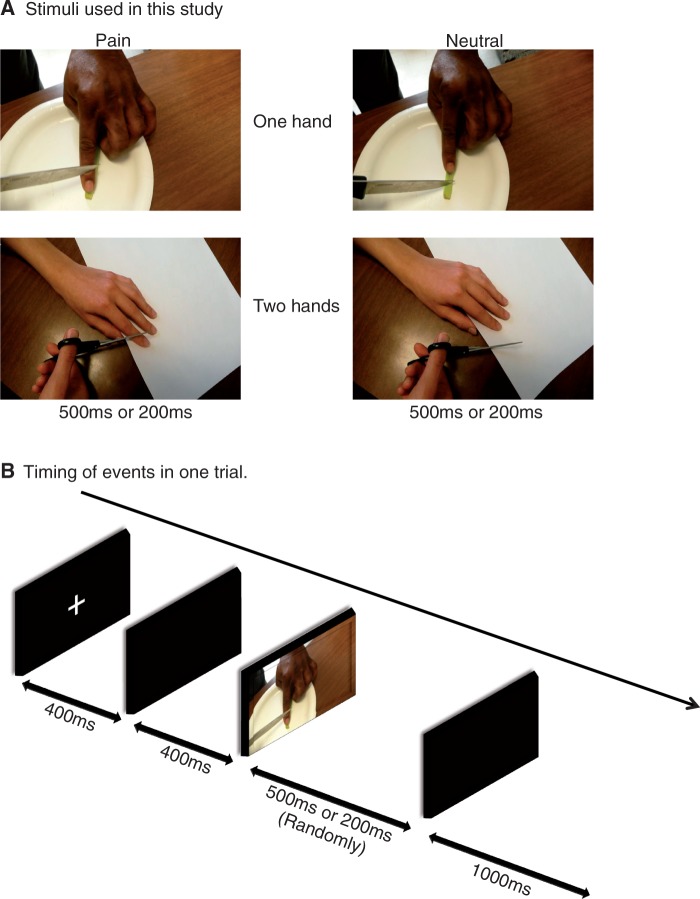
Stimuli were pictures of one or two hands in painful or non-painful situations as used in previous fMRI and ERP studies of healthy subjects (Fan and Han, 2008) (Figure 1). The pain-related pictures illustrate accidents that may happen in everyday life such as a hand trapped in a drawer or cut by scissors. Each painful picture was matched with a neutral picture which showed the same situation without accident or injury. Prior to the study, 90 candidate visual stimuli were rated by eight judges using the Wong-Baker faces pain rating scale (6-point scale, from 0 ‘no hurt’ to 5 ‘hurts worst’) (Hockenberry et al., 2005). Pictures that received an average score below 1.25 were considered neutral and ones above 2.50 were considered painful. Thirty-four neutral and 34 pain-related pictures were selected for the electroencephalographic (EEG) session such that half depicted one hand and the other half two hands and half were African-American hands and half Caucasian hands. The pictures were presented in the center of a black background of a 15 inch color monitor. Each stimulus was 22.5 × 13.5 cm (width × height), subtending a visual angle of 12.7 × 7.7° at a viewing distance of 100 cm. In the ERP session, there were two main conditions: (i) participants judged pain vs neutral in painful and neutral pictures, labeled pain judgment condition (PC) and (ii) participants counted the number of hands in painful or neutral pictures, labeled counting hands condition (CC). Participants responded to each stimulus pressing buttons using their right index and middle fingers. Each condition consisted of three blocks of 136 randomized trials in which each of the 68 pictures was presented twice. Each block started with the presentation of instructions for 5 s, which defined the task (i.e. PC or CC). Each trial consisted of a fixation cross presented at the center of the screen for 400 ms, a black screen for 400 ms, and a picture duration varying randomly between 200 and 500 ms, followed by a 1000 ms response period. The button press responses were collected simultaneously with the EEG recordings.
Fig. 1.
Illustration of the experimental procedure.
EEG data recording
EEG was continuously recorded using a Biosemi Activetwo system (Biosemi B.V, Amsterdam, The Netherlands) from 32 pin-type active scalp electrodes using the 10–20 system, with the addition of two flat-type active external mastoid electrodes, and one flat-type active external electrode on the nose. Additionally, four electrodes were used to measure electrooculogram (EOG) (for horizontal EOG, two electrodes were placed on the outer canthus of the left and right eye; for vertical EOG, two electrodes were placed above and below the left eye). As the Biosemi system does not need a reference electrode while recording, all electrodes were referenced off-line to the algebraically computed average of the left and right mastoids.
Data were sampled at 1024 Hz and filtered at band-pass 0.1–40 Hz, and then epoched to 200 ms pre-stimulus and 900 ms post-stimulus. Baseline correction was applied to the 200 ms prior to stimulus presentation. The behavioral response time and the ERP latency were measured relative to the stimulus onset. The ERPs in each condition were averaged separately off-line. Artifact rejection was performed for any trial that determined by visual inspection to include artifacts from eye blinks, eye movements, or muscle potentials exceeding ±100 µV at electrode sites F3, Fz, F4, FC1, FC2, FC5, FC6, C3, Cz, C4, Cp1, Cp2, Cp5, Cp6, P3, Pz, P4, PO3, PO4, O1, Oz and O2. The mean number of accepted responses for each condition over the channels was more than 50 for all subjects and did not differ significantly between genders and ethnicity. Rejection rates were 11.5% in the PC and 10.7% in the CC. The rejection rate was higher with pain stimuli [pain stimuli = 12.3%, neutral stimuli = 10.0%, F(1, 17) = 9.944, P = 0.006]. Neither the main effect of duration nor any interactions between duration and stimulus type or condition were significant.
ERP data analysis
In order to limit false positives from multiple comparisons, and consistent with the published literature, statistical analyses were conducted on recordings at midline frontal (Fz), central (Cz), parietal (Pz) and occipital (Oz) electrodes. ERP waveforms were created by averaging all trials separately by condition (PC or CC), stimulus type (painful or neutral) and stimulus duration (200 or 500 ms) for each individual. As the previous research suggested that late positive ERP components approximately before 600 ms (P3 or ‘early’ LPP) may reflect different processes in the allocation of attention to emotion than later components (i.e. >600 ms) (Azizian and Polich, 2007; Olofsson et al., 2008; Foti et al., 2009; Weinberg and Hajcak, 2010, 2011), we examined two late positive components, P3 and LPP, separately. The mean amplitude was calculated from each time window as follows: 380–480 ms as P3 and 700–900 ms as LPP. As we applied stimuli that terminate before the whole epoch ends, we examined the effect of stimulus offset. According to visual inspection, the offset-related positive–negative complex was evident in Oz. The latencies for positive and negative offset peaks were around 110 and 145 ms, respectively. Therefore, the offset responses for 200 ms stimuli, to the degree present, would be expected to occur around 310–345 ms, and for 500 ms stimuli around 610–645 ms. In addition, we added −20 ∼ +20 ms around the average stimuli offset to account for variability in the color monitor refresh rate. Thus, we made the P3 window (380–480 ms) short enough to avoid possible visual offset-related ERP components of 200 ms stimuli and possible confounds of the 500 ms stimuli offset variability related to the monitor refresh rate. We also delayed the start of the LPP window (700–900 ms) to ensure that the possible visual offset-related ERP components of 500 ms stimuli were not contaminating the LPP window. According to the visual inspection of ERP waveform for each participant, there was no evidence of these ‘positive–negative peak’ offsets during the P3 and LPP epochs as defined, in any of the study electrodes. In order to provide assurance that the strict limitations to avoid contamination by offset waves did not itself create artifacts, we also examined the entire response period (350–900 ms), an extended P3 epoch (350–550 ms) and an intermediate epoch (550–700 ms).
Additionally, individual difference waves (DW) were calculated by subtracting neutral stimuli ERP from painful stimuli ERP in each condition and stimulus duration, separately. Individual DW mean amplitudes were calculated in each component following the same time intervals as above.
Empathy assessment
The Interpersonal Reactivity Index (IRI) is a self-assessment questionnaire consisting of four 7-item sub-scales, each covering a separate facet of empathy (Davis, 1983). Participants score each item from a 5-point scale selecting the descriptor that best suits him/her. Two subscales measure cognitive elements of empathy: Perspective Taking scale measures the reported tendency to adopt the psychological point of view of others in everyday life and the Fantasy scale (FS) measures the tendency to imaginatively transpose oneself into fictional situations. The second pair of subscales measures emotional aspects of empathy: Empathic Concern (EC) assesses the tendency to experience feelings of sympathy and compassion for unfortunate others and Personal Distress (PD) taps the tendency to experience discomfort in response to distress in others. Higher scores on the PD subscale indicate more distress. The total scores for each element of empathy range from 0 to 28.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences (SPSS 19). Measures were checked for their distributional properties using the box-plot function. There were no extreme outliers; therefore, all data were retained. ERP amplitudes, behavioral reaction times (RTs) and performance (response accuracies; the arcsine transformation was applied) were subjected to analysis of variance with stimuli (painful stimuli vs neutral), condition (PC vs CC) and duration (500 vs 200 ms) as within subject factors. Channel location (Fz, Cz, Pz and Oz) was also included as a within subject factor in ERP amplitudes analysis. We also analyzed Pearson’s correlation between DW mean amplitudes and four IRI subscales. Significant main effects and interactions were clarified with post hoc tests. The significance level for all analyses was set at 0.05 two-tailed.
RESULTS
Behavioral data
Response accuracy was higher with 500 ms stimuli than 200 ms stimuli [F(1, 17) = 21.644, P < 0.001] (Table 1). The main ‘condition’ effect [F(1, 17) = 5.025, P = 0.039] and ‘condition*stimuli’ interaction [F(1, 17) = 7.002, P = 0.017] were also significant. Regardless of duration or stimulus type, participants responded more accurately during the counting condition. In addition, accuracy was relatively higher for the neutral stimuli than painful during the counting condition, whereas this trend was reversed during the PC.
Table 1.
Behavioral response rate and RT
| Pain stimuli |
Neutral stimuli |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 500 ms |
200 ms |
500 ms |
200 ms |
||||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Pain condition | |||||||||
| Response rate | Correct | 85.87% | 11.30% | 81.13% | 11.25% | 83.33% | 11.15% | 79.93% | 13.02% |
| Incorrect | 9.72% | 9.80% | 11.52% | 8.44% | 12.61% | 8.63% | 12.80% | 8.52% | |
| Miss | 4.41% | 6.55% | 7.35% | 7.85% | 4.06% | 6.59% | 7.27% | 10.26% | |
| Response rate; Arcsine transformed | Correct | 69.40 | 9.30 | 64.96 | 7.79 | 66.78 | 8.02 | 64.30 | 9.01 |
| Incorrect | 27.28 | 8.35 | 18.57 | 7.45 | 16.67 | 8.33 | 15.42 | 6.99 | |
| RT (ms) | Correct | 718.32 | 109.11 | 692.39 | 87.81 | 767.33 | 104.21 | 728.66 | 92.56 |
| Incorrect | 889.90 | 244.34 | 694.90 | 154.81 | 699.91 | 179.03 | 662.32 | 150.35 | |
| Counting condition | |||||||||
| Response rate | Correct | 88.56% | 9.57% | 86.66% | 9.33% | 90.52% | 8.42% | 88.54% | 11.80% |
| Incorrect | 8.20% | 7.40% | 9.91% | 6.58% | 5.61% | 5.76% | 7.65% | 8.20% | |
| Miss | 3.24% | 6.26% | 3.43% | 6.52% | 3.87% | 7.43% | 3.81% | 8.40% | |
| Response rate; Arcsine transformed | Correct | 71.75 | 8.60 | 69.57 | 7.55 | 73.48 | 7.81 | 72.43 | 10.43 |
| Incorrect | 29.91 | 14.65 | 15.98 | 9.93 | 20.01 | 6.75 | 12.66 | 5.78 | |
| RT (ms) | Correct | 596.25 | 119.97 | 566.87 | 71.89 | 596.19 | 111.48 | 561.27 | 68.64 |
| Incorrect | 864.92 | 244.69 | 705.61 | 174.14 | 782.00 | 210.31 | 662.26 | 204.88 | |
RT on correct response was longer with 500 ms than 200 ms stimuli [F(1, 17) = 7.588, P = 0.014]. The main effects of ‘condition’ [F(1, 17) = 311.646, P < 0.001], ‘stimuli’ [F(1, 17) = 13.812, P = 0.002] and the ‘condition*stimuli’ interaction [F(1, 17) = 14.300, P = 0.001] were also significant. Participants needed significantly longer time to respond in the PC than in the counting condition when responding correctly. In the PC, the neutral stimuli required longer RT than the painful stimuli, whereas the two did not differ in the counting condition. RT on incorrect trials was similar to that on correct trials except for the absence of slower responses with 500 ms stimuli and the presence of a significant ‘duration*stimuli’ interaction [F(1, 16) = 12.082, P = 0.003] with slower response with pain stimuli than with neutral stimuli when making mistakes with 200 ms duration stimuli the reverse with 500 ms duration.
Behavioral responses did not show any significant correlation with IRI subscales or DW amplitude (pain–neutral) in pain judging condition. Additionally, mean RT for both correct and incorrect responses during pain judging condition was essentially same (correct RT = 726.68 ± 98.42 ms; incorrect RT = 736.76 ± 182.13 ms).
ERP data
P3 components
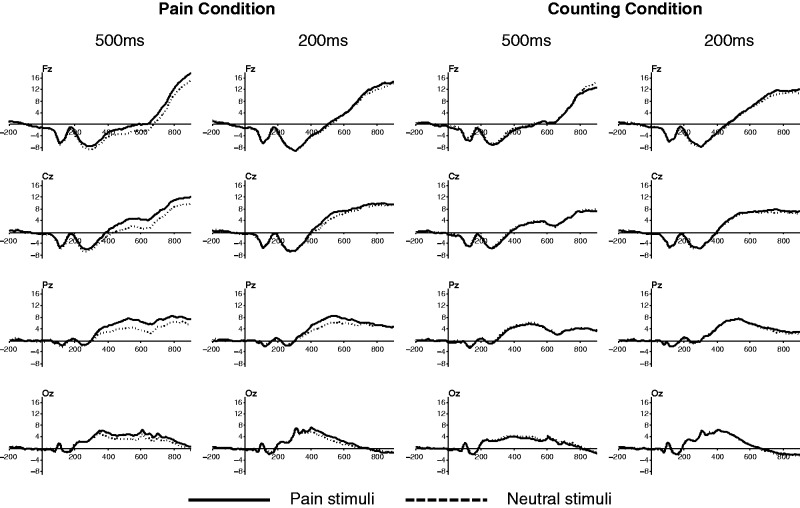
There was a significant main effect of ‘channel’ [F(3, 51) = 31.935, P < 0.001] (Figures 2 and 3). Post hoc analysis revealed the highest P3 amplitude in Pz [as typically seen, e.g. Polich (2007)] and the lowest in Fz [Fz = −2.317 µV, Cz = 1.287 µV, Pz = 4.728 µV, Oz = 4.643 µV; all pairwise comparisons were P < 0.05 (Bonferroni corrected), except for the comparison between Pz and Oz]. The interaction between ‘channel*duration’ [F(3, 51) = 8.255, P < 0.001] was significant. The effect of ‘duration’ was significant only in Oz [F(1, 17) = 10.82, P = 0.004] with higher P3 amplitude with 200 ms than 500 ms stimuli.
Fig. 2.
ERP waves for each condition.
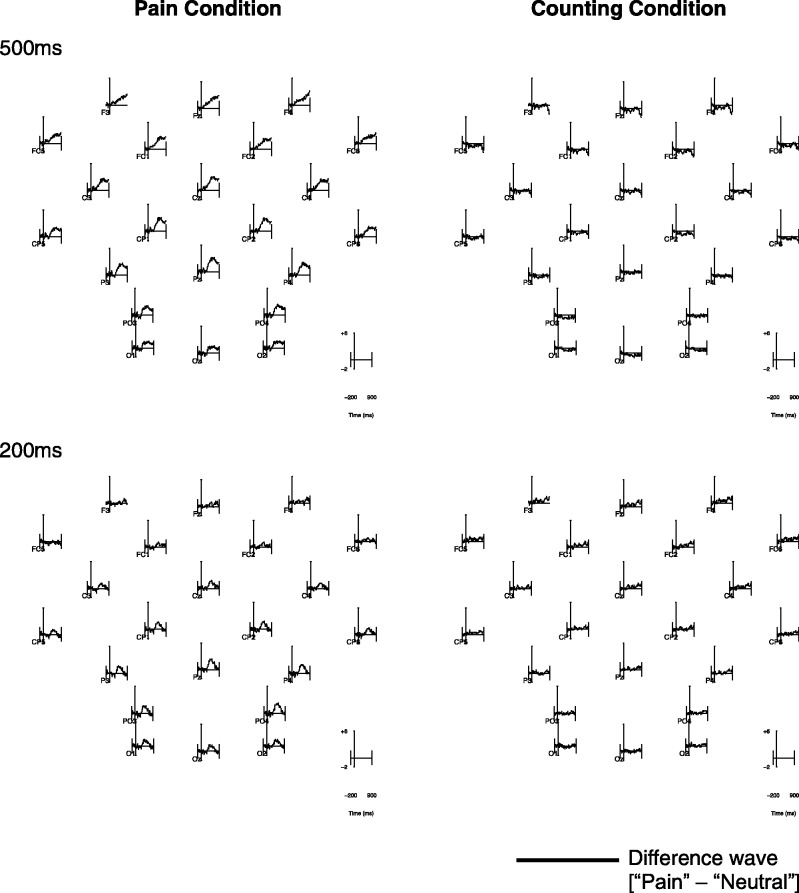
Fig. 3.
ERP scalp map of DWs (pain minus neutral) for each condition, duration separately.
The main effect of ‘stimuli’ [F(1, 17) = 10.930, P = 0.004] and ‘channel*stimuli’ interaction [F(3, 51) = 4.25, P = 0.009] was significant. Further analysis revealed more positive waves with painful than neutral stimuli (pain effect) significantly at Cz [F(1, 17) = 11.134, P = 0.004], Pz [F(1, 17) = 18.227, P = 0.001] and Oz [F(1, 17) = 5.605, P = 0.03], and a trend level at Fz [F(1, 17) = 3.783, P = 0.069].
‘Condition*stimuli’ [F(1, 17) = 35.652, P < 0.001] and ‘channel*condition*stimuli’ [F(3, 51) = 6.686, P = 0.001] were also significant. All electrodes showed significant ‘condition*stimuli’ interactions [F(1, 17) = 5.706–53.856, P < 0.05]. According to the post hoc analysis, the pain effect was significant in the PC [F(1, 17) = 6.75–52.91, P < 0.05], but not the hand counting condition [F(1, 17) = 0.01–2.50, P > 0.1], and was most robust in Pz [F(1, 17) = 52.91, P < 0.001].
The ‘condition*duration*stimuli’ [F(1, 17) = 5.736, P = 0.028] and ‘channel*condition*duration*stimuli’ [F(1, 17) = 6.145, P = 0.001] interactions were also significant. Post hoc analysis revealed that the pain effect during PC was more evident with 500 ms than 200 ms stimuli, especially in Cz and Fz [‘condition*stimuli’ interaction; 500 ms stimuli: F(1, 17) = 25.434–52.009, P < 0.001; 200 ms stimuli: F(1, 17) = 0.018–3.37, P > 0.05].
LPP components
There was a significant main effect of ‘channel’ [F(3, 51) = 31.928, P < 0.001], with LPP amplitude highest in Fz and decreased toward Oz [Fz = 11.19 µV, Cz = 8.095 µV, Pz = 4.903 µV, Oz = 0.124 µV; all pairwise comparisons were P < 0.05 (Bonferroni corrected), except for the comparison between Fz and Cz]. The ‘channel*duration’ interaction [F(3, 51) = 8.255, P < 0.001] was significant. Post hoc analysis revealed that the effect of ‘duration’ was significant in Pz and Oz [Pz: F(1, 17) = 7.635, P = 0.013; Oz: F(1, 17) = 20.393, P < 0.001] with higher LPP amplitude with 500 ms stimuli than 200 ms in these electrodes.
The main effect of ‘stimuli’ [F(1, 17) = 5.946, P = 0.026] and ‘condition*stimuli’ [F(1, 17) = 8.624, P = 0.009] interaction was significant. As in P3, painful stimuli showed more positive LPP than that of neutral stimuli (pain effect) during the PC [F(1, 17) = 9.893, P = 0.006], but not the hand counting condition [F(1, 17) = 0.208, P > 0.1].
As in P3, there were significant ‘channel*condition*duration*stimuli’ [F(1, 17) = 2.950, P = 0.041] and ‘condition*duration*stimuli’ [F(1, 17) = 11.463, P = 0.004] interactions. Post hoc analysis revealed that pain effect during PC was more evident with 500 ms stimuli in all channel locations [‘condition*stimuli’ interaction; 500 ms stimuli: F(1, 17) = 9.829–21.001, P < 0.006; 200 ms stimuli: F(1, 17) = 0.169–0.791, P > 0.1], but was most robust in Cz [F(1, 17) = 21.001, P < 0.001].
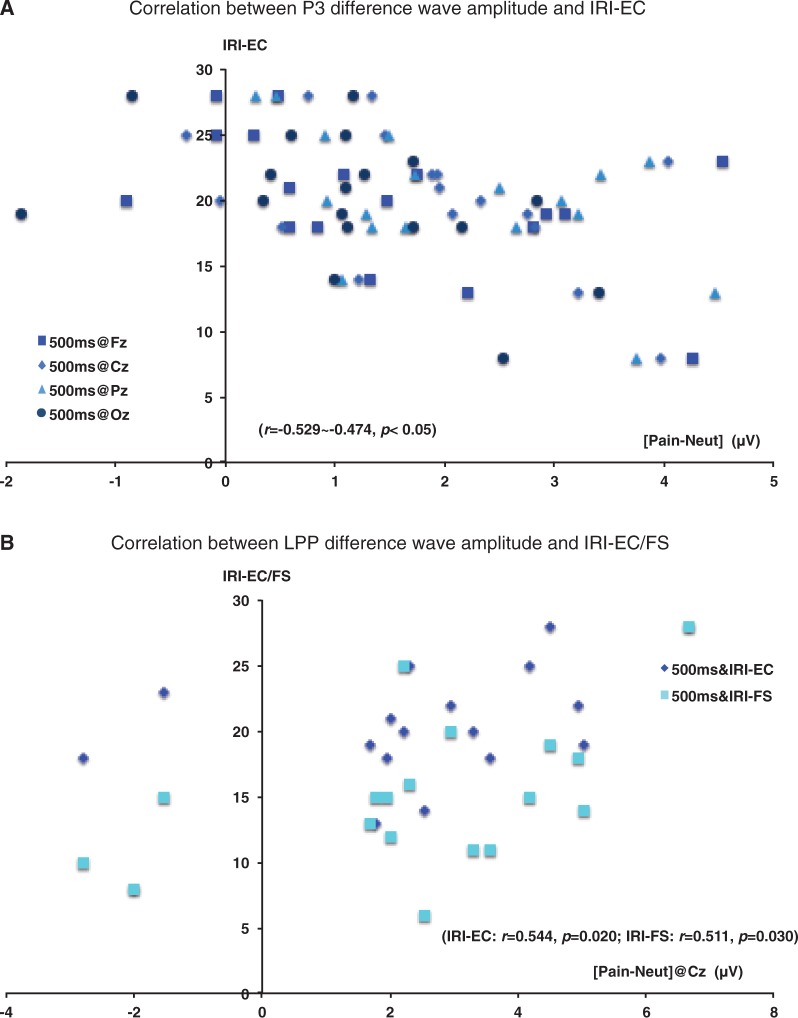
Correlation between ‘pain effect’ and self-reported empathy
As we found significant ‘pain effect’ on both P3 and LPP only during the PC, we focused here on the correlation between the DW amplitude (pain–neutral) during the PC and the IRI subscales (Figure 4). No correlations were significant with 200 ms duration. With 500 ms duration stimuli, there was a negative correlation between IRI-EC subscale and P3 DW amplitude from all electrodes (500 ms stimuli: Fz: r = −0.508, Cz: r = −0.474, Pz: r = −0.529, Oz: r = −0.466, all P < 0.05). Correlations between IRI-EC subscales and P3 DW 200 ms did not approach significance (r = −0.15 to 0.06) and the difference of the correlation coefficients between IRI-EC with P3 DW 500 ms and that with 200 ms approached significance in Pz (P = 0.075). In addition, LPP DW amplitude in Cz with 500 ms duration showed positive correlation with IRI-EC subscale and IRI-FS subscale (500 ms stimuli: IRI-EC r = 0.544, IRI-FS r = 0.511, both P < 0.05). Correlations between both subscales and LPP DW 200 ms did not approach significance (r = −0.142 to 0.184) and the difference of the correlation coefficients between IRI-FS with LPP DW 500 ms and that with 200 ms was significant (P < 0.05).
Fig. 4.
Correlation between DW (pain minus neutral) amplitude during pain judging task and self-reported empathy.
Additional analysis: late positivity as a whole, longer P3 window and early LPP
These analyses show essentially the same effects of ‘duration’ as do the analyses primary analyses protected from contamination by stimulus offset waves. For the whole epoch, the pain DW during pain judging condition was greater following 500 ms stimuli than following 200 ms stimuli [F(1, 17) = 9.743, P = 0.006]. With a longer P3 window, the effects are identical to the results in the original manuscript, although the significance levels are somewhat less robust [F(1, 17) = 4.878, P = 0.041]. The intermediate period (early LPP) also showed a greater pain DW with 500 ms stimuli [F(1, 17) = 5.523, P = 0.031].
DISCUSSION
In this study, P3 and LPP responses were greater to pain than non-pain stimuli during the PC, consistent with recent literature (Fan and Han, 2008). The important new finding is that the P3 and LPP pain effects during the PC were significantly greater with 500 ms than 200 ms stimulus presentation. It has been shown that ERP indices of empathic response to others in pain can be modulated by attention manipulations and other top-down processes (Fan and Han, 2008; Li and Han, 2010; Ibanez et al., 2011; Cheng et al., 2012; Meng et al., 2012). Threatening stimuli, such as negative picture/words (Meng et al., 2012) and strangers’ faces (Ibanez et al., 2011) shown prior to the pain-related stimuli, elevate the P3 pain effect. On the other hand, when subjects are asked to take the perspective of others (Li and Han, 2010) or they themselves are physicians, the P3 pain effect is smaller (Decety et al., 2010). In our results, the ‘pain effect’ during the P3 time window was greater in subjects who reported that in general they were less likely to experience feelings of sympathy and compassion for unfortunate others (EC). The opposite association was seen with the LPP ‘pain effect’, subjects with greater LPP ‘pain effect’ said that they were more likely to experience empathic concern and to imaginatively transpose themselves into fictional social situations.
With 500 ms but not 200 ms stimuli, empathic responsiveness showed a positive correlation with LPP amplitude and a negative correlation with P3 amplitude. It might be possible to think that the larger LPPs in high trait empathic people reflect more active self-regulation of emotional response, and the smaller P3 responses are the result of that self-regulation. We propose that the ability to regulate ones own emotional response facilitates empathic response to others in pain. Other researchers have suggested that the later LPP (i.e. >600 ms) is indicative of increasing influence of elaborative top-down processes (Azizian and Polich, 2007; Olofsson et al., 2008; Foti et al., 2009; Weinberg and Hajcak, 2010, 2011). Li and Han (2010) found that the P3 (370–420 ms) response to others in pain was attenuated when the participants were told to take the other’s perspective while performing a pain judgment task, a top-down regulation of their own experience (Li and Han, 2010). Decety et al. (2010) also found lower P3 amplitudes (360–400 ms) in physicians who generally have a necessity to regulate their emotional responses while they work with others in pain or even inflict pain in the course of their treatments. Leutgeb et al. suggested that participants with successful cognitive behavioral therapy have learned to maintain their attention to phobic stimuli in order to regulate their emotional responses, and show increased LPP amplitude but not P300 amplitude, after successful treatment (Leutgeb et al., 2009, 2012).
It is particularly interesting that these self-regulatory responses seem to be engaged when stimuli are presented for 500 ms but not when they are presented for 200 ms. Understanding of this primary finding is further informed by behavioral data showing slower and more accurate responses with 500 ms compared with 200 ms stimuli, and P3 amplitude to pain and neutral stimuli that were undifferentiated and greater with 200 ms than 500 ms stimuli. This constellation of behavioral and ERP findings suggests that the longer processing associated with 500 ms stimuli is associated with general attenuation of ‘automatic’ attention processing reflected in P3 amplitude, greater differentiation of stimuli (i.e. pain is different from neutral) and further regulation of behavioral response related to the nature of the stimuli (i.e. empathic concern). This view is consistent with the previous studies cited above showing that maintaining focus on the evocative stimuli and regulating emotional response to them go together and are associated with greater LPP amplitudes. For reasons not currently understood, it may require longer processing of stimuli themselves in order to engage higher brain processes.
This study has several limitations. Our sample size was relatively small, limiting power to avoid possible false negative results in our findings. Second, some of the results, particularly the results from correlation analysis were not in the significant level after Bonferroni correction. However, we see the correlations as exploratory and hypothesis generating. Finally, comparison of stimuli of two distinct durations might be confounded by stimulus offset responses which begin at different time points for the two stimuli. This problem is unlikely to be the basis of the differences we found in responses to the 200 and 500 ms stimuli, however, for several reasons. First, the offset-related positive–negative complex was clearly evident in Oz but there was no evidence of it at other leads; the clear demarcation at Oz provides confidence in the absence of the response at other locations. Second, findings related to stimulus duration were present when comparing DWs between pain and non-pain stimuli. Offset effects would be expected to be largely similar with the two types of stimuli and thus if present would ‘subtract’ out of the analyses. Secondary differences in the offset waves between pain and non-pain stimuli are possible, and if present could themselves contribute to our findings, but these differences would most likely be very small in the leads of interest given that the primary offset waves are not even visible. Additionally, it is possible that the aspects of the ERP could be related to initiation of response which comes sooner for the 200 ms stimuli, to contribute to differences of interest in late ERP responses following the 200 and 500 ms stimuli; however, the ERPs related to response generation per se would themselves have to be different and substantial. Two things mitigate against this factor being of significance. First, in a seminal paper, McCarthy and Donchin (1981) demonstrated that P3 latency is relatively independent of response execution. Second, the peaks and epochs we are looking at are well established in the literature in relation to stimulus onset (Fan and Han, 2008), and are clearly visible in our response waves for both stimulus durations. Indeed, consistent with the McCarthy and Donchin finding, the same time windows and peaks are used in studies which each use only one stimulus duration but among which use stimuli of different durations (Leutgeb et al., 2009, 2012; Cheng et al., 2012; De Sanctis et al., 2013; Weinberg et al., 2012; Horan et al., 2013; Moran et al., 2013).
In conclusion, the duration of exposure to pictures of others in pain affects late ERP responses associated with empathy. Ours and previous findings are consistent with a model where pain response as reflected in P3 amplitude is subject to modulation by regulatory processes reflected in LPP amplitude. Ours is the first study to suggest that engagement of self-regulatory processes depends on longer exposure to the pain-related stimulus. It might be important to investigate the relationship between stimulus duration and empathic response in clinical populations where issues of self-regulation, empathic response and speed of information processing exist.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Authors would like to thank Dr Jason Johannesen for his informative comments on the previous version of this article, and would also like to thank the State of Connecticut, Department of Mental Health and Addiction Services through its support for the Connecticut Mental Health Center. Dr Wexler designed the study and wrote the protocol. Dr Ikezawa performed the statistical analyses, and wrote the first draft of the paper. All authors contributed to and have approved the final manuscript.
This study was supported by funding from the National Institute of Mental Health (R01 MH084079) to B.E.W. The sponsor played no role in this study or publication.
REFERENCES
- Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from event-related potentials. Journal of Cognitive Neuroscience. 2007;19:2071–81. doi: 10.1162/jocn.2007.19.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti S, Schroger E. A comparison of auditory and visual distraction effects: behavioral and event-related indices. Brain Research. Cognitive Brain Research. 2001;10:265–73. doi: 10.1016/s0926-6410(00)00044-6. [DOI] [PubMed] [Google Scholar]
- Brisson B, Jolicoeur P. The N2pc component and stimulus duration. Neuroreport. 2007;18:1163–6. doi: 10.1097/WNR.0b013e3281e72d1b. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Development and Psychopathology. 2012;24:623–36. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual-differences in empathy—evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–26. [Google Scholar]
- De Sanctis P, Foxe JJ, Czobor P, et al. Early sensory-perceptual processing deficits for affectively valenced inputs are more pronounced in schizophrenia patients with a history of violence than in their non-violent peers. Social Cognitive and Affective Neuroscience. 2013;8:678–87. doi: 10.1093/scan/nss052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Developmental Neuroscience. 2010;32:257–67. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Yang CY, Cheng Y. Physicians down-regulate their pain empathy response: an event-related brain potential study. Neuroimage. 2010;50:1676–82. doi: 10.1016/j.neuroimage.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Hajcak G. See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46:28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46:160–73. doi: 10.1016/j.neuropsychologia.2007.07.023. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 1998. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–88. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–30. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Genetti M, Khateb A, Heinzer S, Michel CM, Pegna AJ. Temporal dynamics of awareness for facial identity revealed with ERP. Brain and Cognition. 2009;69:296–305. doi: 10.1016/j.bandc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35:129–55. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Wilson D, Winkelstein ML. Wong’s Essentials of Pediatric Nursing. 7th edn. St. Louis: MO: Mosby; 2005. [Google Scholar]
- Hodges SD, Klein JKK. Regulating the costs of empathy: the price of being human. The Journal of Socio-Economics. 2001;30:437. [Google Scholar]
- Horan WP, Hajcak G, Wynn JK, Green MF. Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychological Medicine. 2013:1–15. doi: 10.1017/S0033291713000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Hurtado E, Lobos A, et al. Subliminal presentation of other faces (but not own face) primes behavioral and evoked cortical processing of empathy for pain. Brain Research. 2011;1398:72–85. doi: 10.1016/j.brainres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schafer A, Kochel A, Schienle A. Exposure therapy leads to enhanced late frontal positivity in 8- to 13-year-old spider phobic girls. Biological Psychology. 2012;90:97–104. doi: 10.1016/j.biopsycho.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb V, Schafer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Li W, Han S. Perspective taking modulates event-related potentials to perceived pain. Neuroscience Letters. 2010;469:328–32. doi: 10.1016/j.neulet.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Macnamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–43. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Meng J, Hu L, Shen L, et al. Emotional primes modulate the responses to others' pain: an ERP study. Experimental Brain Research. 2012;220:277–86. doi: 10.1007/s00221-012-3136-2. [DOI] [PubMed] [Google Scholar]
- Moran TP, Jendrusina AA, Moser JS. The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research. 2013;1516:66–75. doi: 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnis R, Dadds MR, Bryant RA. Is there a mutual relationship between opposite attentional biases underlying anxiety? Emotion. 2011;11:582–94. doi: 10.1037/a0022019. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Landis T, Khateb A. Electrophysiological evidence for early non-conscious processing of fearful facial expressions. International Journal of Psychophysiology. 2008;70:127–36. doi: 10.1016/j.ijpsycho.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Shur S, Barcai-Goodman L, Medlovich S, Harari H, Levkovitz Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Research. 2007;149:11–23. doi: 10.1016/j.psychres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Singer T. The past, present and future of social neuroscience: a European perspective. NeuroImage. 2012;61:437–49. doi: 10.1016/j.neuroimage.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23:2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hilgard J, Bartholow BD, Hajcak G. Emotional targets: evaluative categorization as a function of context and content. International Journal of Psychophysiology. 2012;84:149–54. doi: 10.1016/j.ijpsycho.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Yamada M, Decety J. Unconscious affective processing and empathy: an investigation of subliminal priming on the detection of painful facial expressions. Pain. 2009;143:71–5. doi: 10.1016/j.pain.2009.01.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.