Abstract
In the beginning, that is from the 1960's, when a link between menopause and osteoporosis was first identified; estrogen treatment was the standard for preventing bone loss, however there was no fracture data, even though it was thought to be effective. This continued until the Women's Health Initiative (WHI) study in 2001 that published data on 6 years of treatment with hormone therapy that showed an increase in heart attacks and breast cancer. Even though the risks were small, 1 per 1500 users annually, patients were worried and there was a large drop off in estrogen use. In later analyses the WHI study showed that estrogen reduced fractures and actually prevented heart attacks in the 50-60 year age group. Estrogen alone appeared to be safer to use than estrogen + the progestin medroxyprogesterone acetate and actually reduced breast cancer.
At the same time other drugs were being developed for bone that belong to the bisphosphonate group and the first generation of compounds showed moderate potency on bone resorption. The second and third generation compounds were much more potent and in a series of large trials were shown to reduce fractures. For the last 15 years the treatment of osteoporosis belonged to the bisphosphonate compounds, most of which reduce fracture rates by 50 percent. With the exception of gastrointestinal irritation the drugs are well tolerated and highly effective. The sophistication of the delivery systems now allow treatment that can be given daily, weekly, monthly and annually either orally or intravenously
Bone remodeling is a dynamic process that repairs microfractures and replaces old bone with new bone. In the last 10 years there has been a remarkable understanding of bone biology so that new therapies can be specifically designed on a biological basis. The realization that RANKL was the final cytokine involved in the resorption process and that marrow cells produced a natural antagonist called Osteoprotegerin (OPG) quickly led to two lines of therapy. First OPG was used as a therapy to block RANKL was initially successful but later antibodies against OPG developed and this line of treatment had to be discontinued. The next step was to develop a monoclonal antibody against RANKL and this proved to be highly effective in blocking bone resorption. It led to development of a drug Denosumab that successful reduces fractures and is now one of the therapeutic options for osteoporosis treatment.
On the anabolic side bone biology research showed that osteocytes produces sclerostin an inhibitor of the anabolic WNT signaling pathway. Recent development of a monoclonal antibody against sclerostin has shown remarkable anabolic activity in bone showing large increases in bone density and fracture trials are now underway. The newer treatments for osteoporosis are likely to be based on our understanding of bone biology and the design of new highly specific compounds with fewer side effects.
This review summarizes the diagnosis of postmenopausal osteoporosis and various available nonpharmacological and pharmacological therapies available for its management.
Pathophysiology of bone loss
Bone remodeling is the process by which old bone is replaced by new bone. The normal bone remodeling process consists of five phases: the resting phase activation, resorption, reversal, and formation.
➢ In the activation phase of remodeling, osteoclasts are recruited to the surface of the bone.
➢ In the resorption phase, osteoclasts generate an acidic microenvironment between the cell and the surface of the bone, dissolving or resorbing the mineral content of the bone.
➢ In the reversal phase osteoclasts undergo apoptosis and osteoblasts are recruited to the bone surface.
➢ In the formation phase, osteoblasts then deposit collagen; this is mineralized to form new bone.
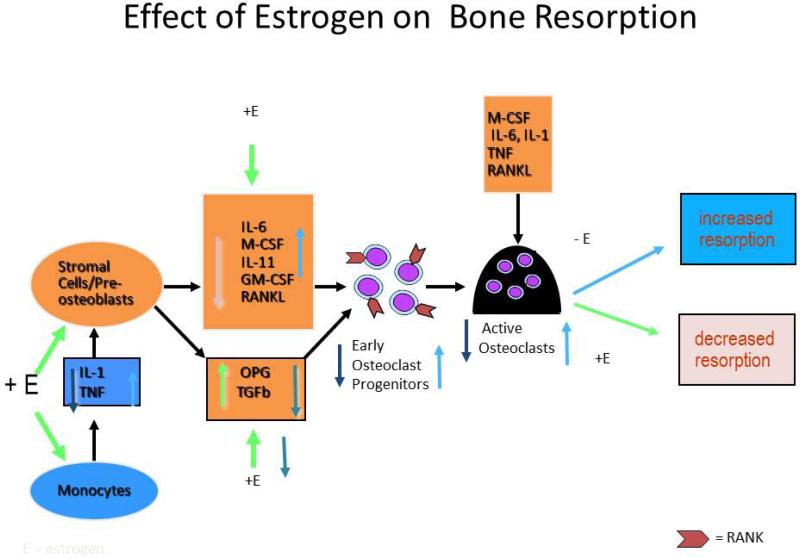
At menopause estrogen deficiency impairs the normal cycle by increasing osteoclastic resorption activity without a corresponding increase in osteoblastic activity and the amount of bone resorbed therefore is greater than the amount deposited leading to a net loss of bone. This process was originally described as ‘uncoupling’. The cellular changes that occur in estrogen deficiency are now quite well understood. (FIGURE 1). There is an increased production of Tumor necrosis factor (TNFα) and cells of the stromal / osteoblastic lineage become more sensitive to IL-1. IL-1 and TNF stimulate stromal cells / preosteoblasts to release several cytokines- IL-6, macrophage colony stimulating factor (M-CSF), IL-11, granulocyte macrophage colony-stimulating factor (GM-CSF), transforming growth factor (TGF). The final cytokine in the osteoclastogenesis cascade is RANK ligand (receptor activator of nuclear factor B ligand) which is produced from osteoblasts and binds to its receptor RANK on osteoclasts (1, 2). RANKL has a natural antagonist osteoprotegerin (OPG) that is a soluble receptor that is secreted by the stromal osteoblast lineage cells (3). OPG is stimulated by estrogen (3). In retrospect we now realize that the uncoupling factor secreted by the osteoblasts is RANKL. These factors increase bone resorption by increasing the pool size of pre-osteoclasts in bone marrow (1) and are down regulated by estrogen. The important action of estrogen is to increase OPG secretion (3) and decrease M-CSF (1) and RANK (4).
Figure 1.
Cellular changes that occur with estrogen changes. + E depicts effects in presence of estrogen; -E depicts effects in absence of estrogen. IL-1 is Interleukin 1, TNF-Tumor Necrosis Factor, OPG-Osteoprotegerin. Estrogen decreases osteoclastogenesis and increases osteoclast apoptosis. Estrogen reduces osteoclastogenesis by suppressing IL-1 and TNF and increasing the sensitivity of stromal cells/preosteoblasts to IL-1, thus suppressing MCSF, RANKL, and perhaps most notably, IL-6. In addition, estrogen stimulates the production of OPG, the potent inhibitor of osteoclastogenesis. Estrogen also reduces the responsiveness of osteoclast precursors to RANKL. Estrogen also promotes osteoclastic apoptosis, thereby reducing osteoclast lifespan. This effect appears to be mediated by TGFβ.
Age related changes in calcium and vitamin D metabolism
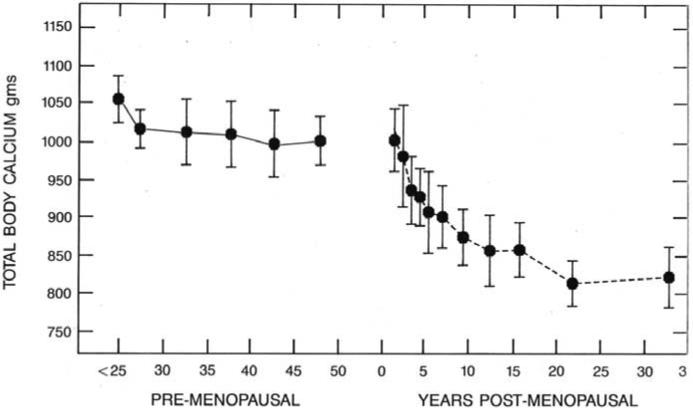
During the first phase of menopausal bone loss women are in marked negative calcium balance. Using total body calcium measurements one can estimate that the average loss of calcium daily in the first 3-4 years is - 200mg daily, this gradually decreases to -45mg daily in years 5—10 post menopause. This is shown in Figure 2.
Figure 2.
Figure depicting the decline of total body Calcium with years since menopause.
Other age related changes develop in the sixties. There is an age related decrease in calcium absorption that contributes to further negative calcium balance (5, 6). Malabsorption of calcium causes secondary hyperparathyroidism that increases bone resorption causing further bone loss. In much older people, age 80 + years the calcium absorption response relative to serum 1,25 dihydroxyvitamin D is lower than in young people suggesting that part of the aging problem is intestinal resistance to endogenous circulating 1, 25 dihydroxyvitamin D. In addition there is a decline in the formation of 1, 25-dihydroxyvitamin D production in the aging kidney and at a GFR of 50-60 50mls/min the production of 1,25dihydroxyvitamin D decrease about 50 percent. Many old people over age 80 years have a GFR< 50mls/min. Thus, there seems to be two separate steps involved in malabsorption of calcium, a primary as yet unspecified defect involving the gut and the other is due to decreased production of 1, 25dihydroxyvitamin D by the aging kidney. Vitamin D deficiency will aggravate the situation by causing secondary hyperparathyroidism and increasing bone resorption. Estrogen however still plays a role in aging. BMD is higher if serum estradiol is more than 15 pg / ml and lower if serum estradiol is < 5 pg/ml (7) and fractures are increased when serum estradiol is less than 5pg/ml (8).
Change in bone mineral density with age
Peak bone mass is reached in the mid-twenties for spine and hip but other bones such as the radius reach a peak at age 40 years. At the time of menopause there is a rapid acceleration in bone loss that starts the year before menopause and continues for another 3 years (9) before de-accelerating, even so the rate of bone loss in the years 4-8 years past menopause is still high. The average decrease in BMD during the menopausal transition is about 10 percent so this means that half the women are losing even rapidly, perhaps as much as 10-20 percent in those 5-6 years around menopause. 25 percent of postmenopausal women can be classified as fast bone losers measured by rates bone loss and bone resorption markers (10). If the average age of the menopause is 51 years then an early menopause at age 41 would ‘age’ the bone 10 years earlier than normal- unless treated. This explains why 15-20 percent women in the early sixties have vertebral fractures and the effect of early menopause on bone.
Osteoporosis
Osteoporosis is characterized by decreased bone strength that predisposes to an increased risk of fracture (5). Bone strength = Bone mineral density + bone quality.
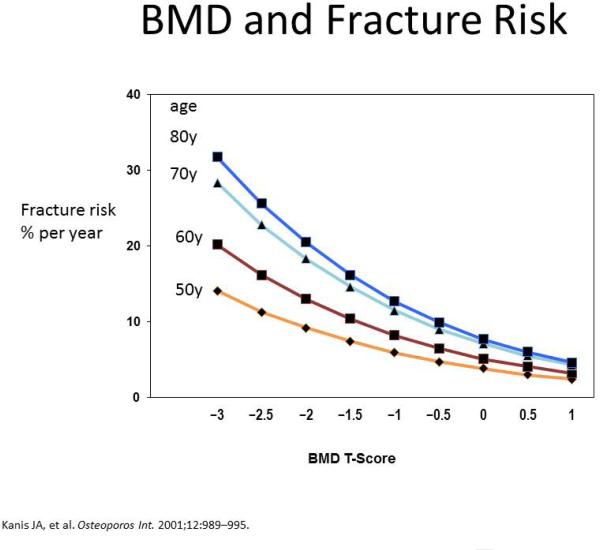
Bone density has been measured in many ways in the past, densitometry of the radius, calcaneus, ultrasound of the heel, radius and phalanges, X-ray of the metacarpal and phalanges, computerized tomography of the spine and now dual energy absorptiometry (DXA) has become the standard. Bone density declines with age and by age seventy years bone mass has decreased by 30-40 percent. The term bone quality implies that there is an increased tendency to fracture. It is well exemplified in the clinic where patients, usually elderly, present with vertebral fractures but have completely normal bone density measured on DXA. This decrease in bone quality is due to several factors, age is associated with low quality bone and the same bone density at age 75 and 55 is associated with the fracture risk that is 3-4 fold higher in older people. Figure 3 summarizes fracture risk as a function of age and BMD –T score. The factors that play a causative role in the aging process of bone are
➢ Increased bone resorption - increases fracture risk by twofold.
➢ Accumulation of micro damage – can be visible on bone biopsy.
➢ Numerous areas of demineralization throughout bone–visible on histology.
➢ Loss of horizontal struts in trabecular bone architecture - seen in specimens.
Figure 3.
Figure depicting the decrease of T score with age and simultaneous increase in fracture risk.
Prevalence of osteoporotic fractures
The prevalence of osteoporotic fractures rises from 4% in women at age 50 - 59 to 52% of women age >80 years (11). There is a temporal sequence in osteoporotic fractures, the first sign being fractures of the lower end of radius starting at age 50 years, followed by vertebral fractures at age 60-75 years and hip fractures starting in the late 70‘s. For a white American woman at age 50, the risk of suffering an osteoporotic fracture in her remaining lifetime has been estimated at 40%, with two thirds of the fractures occurring after age 75.
Risk Factors of osteoporosis
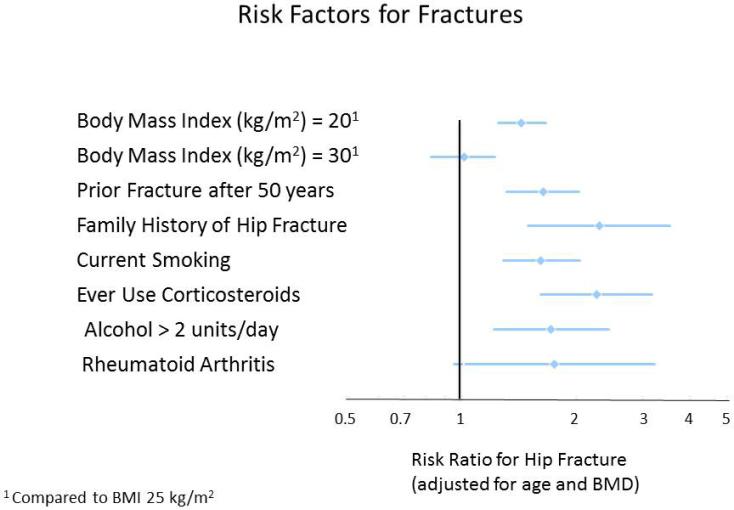
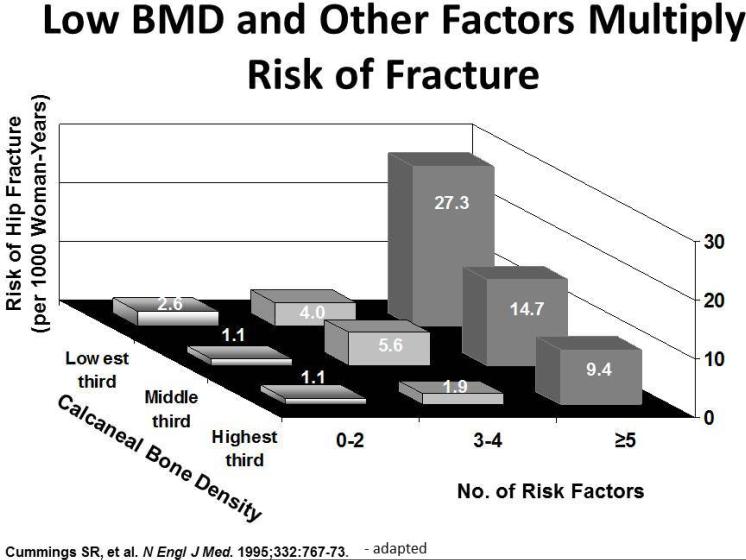
Many factors, including age, gender, race (Caucasians especially), genetics, reproductive status, low calcium intake, and exercise, affect bone mass. In addition, osteoporosis is a recognized complication of specific diseases and disorders. Table 1, figures 4 & 5 summarizes the risk factors for osteoporosis.
Table 1.
Risk factors for Osteoporosis
| Family history | Parental history of hip facture |
|---|---|
| Medical history | • Advanced age • Hyperthyroidism (including iatrogenic) or hyperparathyroidism • Celiac and other malabsorption syndromes • BMI < 20 kg/m2 or weight loss • Medication history, particularly chronic glucocorticoid use and medications that decrease Calcium, Vit D absorption (Anti-convulsants) • Rheumatoid arthritis • Chronic Liver disease • Low femoral neck BMD • Chronic Obstructive Pulmonary Disease (COPD) • Organ Transplantation • Type 1 Diabetes Mellitus |
| For women | • Estrogen deficiency (primary or secondary) • Early menopause (< 45 years), including surgical |
| Lifestyle | • Prolonged immobility and lack of weight-bearing exercise • Current Smoking • Daily alcohol consumption > 3 units (1 unit = 5 oz wine, 1.5 oz spirits, 12 oz beer) • Inadequate calcium and vitamin D intake (Nutritional deficiency) • Lack of sunlight exposure (may cause vitamin D deficiency) |
Figure 4.
The International Osteoporosis Foundation and the National Osteoporosis Foundation have recommended that risk of fracture be expressed as a fixed-term absolute risk, for example the probability in percent over a 10-year period. This figure shows other BMD-independent risk factors for fracture that the IOF and NOF have integrated to establish absolute risk of fracture in men and women.
Figure 5.
This figure depicts how risk factors multiply the fracture risk.
Assessment of risk of osteoporosis or fracture: FRAX approach
The fracture risk (FRAX) of a patient over 10 years can be estimated as low (< 10% in next 10 years), moderate (10 - 20% in next 10 years), or high (> 20% in next 10 years) using known risk factors and femoral neck bone mineral density (12). The models were developed from large population-based cohorts from Europe, North America, Asia and Australia. FRAX is a better risk tool for older women after age 60 years who are at risk for hip fractures, Spine DEXA cannot be used in the FRAX analysis because elderly people with osteoarthritis show an artefactual increase in spine BMD by ~10 percent and this limits the use of FRAX in predicting which women in the age group 50-60 years will develop spine osteoporosis in the next 10-15 years.
Screening recommendations for practice
In a review by Nelson HD et al (9) it was stated that, for women 55 to 59 years of age, the number needed to screen (NNS) over five years was more than 4,000 to prevent one hip fracture and 1,300 to prevent one vertebral fracture. The NNS to prevent one hip fracture over five years declines with age, to 1,856 for women 60 to 64 years of age, 731 for women 65 to 69 years of age, and 143 for women 75 to 79 years of age. The U.S. Preventive Services Task Force (USPSTF) guideline recommends screening DXA in all women 65 years and older, as well as in women <65 years of age with one or more risk factors and history of fracture. If the BMD is not in the osteoporosis range the
USPSTF recommends a 15 year hiatus before repeating another BMD
BMD may not be indicated for people with chronic back pain, screening women aged < 65 years unless significant clinical risk factors have been identified. Some believe that there is no point confirming osteoporosis if there is a fragility fracture; however without DEXA one is unsure of the severity of the osteoporosis. It has also been suggested that women on hormonal therapy for menopausal symptom relief do not need DEXA screening, however because of rapid bone loss after discontinuing HT /ET it would be helpful to identify those with low BMD.
Diagnosis of Osteoporosis
Osteoporosis is diagnosed clinically when there is a presence of fragility fracture or BMD measured by bone densitometry that is less than or equal to 2.5 standard deviations below that of a young adult reference population. By convention the value is described as a T-score. Z score refers to a BMD value that is -2.5 below the mean for that age. It does not apply to measurements using quantitative computerized scans (QCT) that usually measure about 1 T score lower than DXA because QCT measures bone in the spine which is about 90-95 percent trabecular and is less dense or compact. In contrast DXA measures spine and hip which are approximately 50 percent cortical and trabecular bone. The World Health Organization has established the following diagnostic guidelines:
➢ T-score + 2.5 to -1.0 is normal.
➢ T score between -1.0 and -2.5 is osteopenia
➢ T-score -2.5 or below is osteoporosis.
➢ Presence of fragility fracture independent of T score
In the Study of Osteoporotic Fractures by Stone KL et al., (13), 28% of hip fractures, 25% of vertebral fractures, and 13% of all fractures occurred in women with total hip BMD of -2.5 or less that is not in the osteoporotic range. A hip T score between -1.5 to -2.5 was present in 51% of hip fracture subjects. In a 2-year follow up of women older than age 65, 49% of hip fractures occurred in women with total hip BMD T-scores worse than -2.5; 28% occurred in women with T-scores -2.0 to -2.5. This explains that in addition to diagnosis through DXA, osteoporosis can be diagnosed clinically when there is any type of osteoporotic fracture regardless of the T-score.
Sometimes it would be difficult to define osteopenia, which is a bone density T score between -1.0 and -2.5. For example in premenopausal women at age 40 years half of the population has osteopenia but this is part of the normal (statistical) range and can be described as low bone mass. Thus the diagnostic criteria should not be applied to healthy pre-menopausal women because we expect half the population to have a negative T score. In post-menopausal women when DXA is measured for the first time the clinician may be unsure whether the low bone density measurement is part of the low normal range or represents bone loss. In the NORA study, a T score of > - 1.8 seemed to separate women with fractures or not (14).
Investigations at the time of diagnosis
The range of tests will depend on the severity of the disease, age at presentation, and the presence or absence of fractures. The aims of the clinical history, physical examination, and clinical tests are to exclude diseases that mimic osteoporosis (e.g. osteomalacia, myeloma), identify the cause of osteoporosis and contributory factors, assess the risk of subsequent fractures, select the most appropriate form of treatment. The number of postmenopausal women with osteoporosis from a secondary cause is unknown, but thought to be low. A careful history and physical examination may identify common causes of secondary osteoporosis. If clinical evaluation does not raise suspicion of a secondary cause, there is currently little evidence to warrant additional testing in postmenopausal women. Approximately 50 percent of pre- and peri-menopausal women with osteoporosis have an associated underlying cause. There are no evidence-based guidelines to direct the evaluation of a suspected secondary cause of osteoporosis. In pre- and peri-menopausal women, a basic laboratory evaluation should be considered if there is no clear etiology evident by history and physical examination. Procedures in the investigation of osteoporosis are summarized in Table 2.
Table 2.
Procedures in the investigation of osteoporosis
| Routine: |
| ○ History and physical examination |
| ○ Blood cell count, sedimentation rate or C-reactive protein, Serum Calcium, Albumin, Creatinine, phosphate, alkaline phosphatase, Liver transaminases |
| ○ Thyroid function tests |
| ○ Bone densitometry (DXA) |
| ○ 25 OH Vitamin D |
| • If suspicion for secondary osteoporosis following tests are included in initial work up: |
| ■ Lateral radiographs of lumbar and thoracic spine/DXA-based vertebral imaging |
| ■ Protein immunoelectrophoresis and urinary Bence-Jones proteins (Myeloma) |
| ■ Serum prolactin |
| ■ 24 hour urinary cortisol/dexamethasone suppression test (Cushing's) |
| ■ Endomysial and/or tissue transglutaminase antibodies (Celiac disease) |
| ■ Isotope bone scan |
| ■ Markers of bone turnover. |
| ■ Urinary calcium excretion, Intact parathyroid hormone (Hyperparathyroidism) |
Management of Osteoporosis
Non pharmacological and pharmacological interventions are recommended, depending on fracture risk assessment. Risk can be stratified according to the FRAX system
Low risk: Provide lifestyle advice and adequate daily intake of total calcium of 1200mg and vitamin D 800 IU
Moderate risk: Medication is usually not necessary but can be considered in addition to lifestyle advice and adequate daily intake of calcium and vitamin D. When considering therapy it is important to discuss options with the patient and look for additional clinical risk factors such as loss of vertebral height, fractures, women receiving aromatase inhibitor therapy for breast malignancy, long-term or repeated systemic corticosteroid use (oral or parenteral) that does not meet the conventional criteria for recent prolonged systemic corticosteroid use (i.e., ≥ 3 months consecutive) therapy at a dose of prednisone ≥ 7.5 mg per day or equivalent) and recurrent falls.
High risk: Consider pharmacological treatment in addition to lifestyle advice and adequate intake of calcium and vitamin D. Patients with hip and other fragility fractures are considered to be high risk. Individuals can be considered as candidates for medication after implementing fall prevention strategies and providing lifestyle advice. Therapy available for management of osteoporosis is summarized in Table 3.
Table 3.
Therapies for osteoporosis
| Name of drug | Points to consider | Dosing regimen |
|---|---|---|
| Alendronate oral (Fosamax) & Risedronate oral (Actonel) |
Post-menopausal women: Prevents vertebral, non-vertebral, and hip fractures. Glucocorticoid induced osteoporosis (GIO) : Some evidence of decreased vertebral fracture risk |
Alendronate 5 & 10 mg daily 35 & 70 mg weekly Risedronate 5 mg daily 35 mg weekly 150 mg monthly |
| Ibandronate oral & IV (Boniva) | Prevents spinal fractures No proven benefit in hip and non-vertebral fractures |
Ibandronate 2.5 mg po daily 150 mg po monthly,3 mg IV/3 months |
| Zolendronic acid IV (Reclast) |
Post-menopausal women: prevents vertebral, non-vertebral, and hip fractures GIO: maintains BMD Cost effectiveness may limit use Consider for high-risk patients who are unable to tolerate oral therapy or have poor adherence Caution: When used in high doses, increases the risk of osteonecrosis of jaw. |
Zolendronic acid 5 mg IV yearly Approved for the prevention of osteoporosis in postmenopausal women given as a single intravenous dose every 2 years |
| Raloxifene oral (Evista) |
Post-menopausal women: reduces the incidence of vertebral fractures May be considered in post-menopausal women who are unable to tolerate/take bisphosphonates and have no history of thromboembolic disease Caution: May increase the risk of venous thromboembolic disease and stroke |
|
| Denosumab SC (Prolia) |
Postmenopausal women: prevents vertebral, non-vertebral, and hip fractures Cost and lack of long term safety data may limit use |
Denosumab 60 mg SC q6 monthly |
| Teriparatide SC (Forteo) | Post-menopausal women: prevents vertebral and non-vertebral fractures in postmenopausal women with severe OP Some evidence of benefit in the treatment of GIO Cost and need for daily subcutaneous injection may limit use Consider for patients at increased risk of fracture or lack of response to other therapies Maximum lifetime exposure is 24 months Bisphosphonates must be discontinued prior to treatment Gains in BMD decline once treatment with teriparatide is discontinued; consider anti-resorptive therapy after completing treatment course |
|
| Calcitonin |
Post-menopausal women: Reduces incidence of vertebral fractures however evidence for benefit is limited Consider as an alternative when other more effective drugs cannot be used Effective in decreasing acute pain associated with vertebral osteoporotic fractures Calcitonin injection is currently not approved for the treatment of OP; it is sometimes prescribed for patients who have pain due to acute vertebral fractures The intranasal spray formulation is used for OP. Caution: Concerns about increase in cancer risk with use of intranasal spray. |
|
| HRT |
Post-menopausal women: Shown to prevent vertebral, hip and non-vertebral fractures Is not recommended for the sole indication of OP prevention and for long term use for this indication; consider benefits versus risks May be appropriate for OP prevention when it is already being used for the management of menopausal symptoms |
High dose: 2 mg Estradiol 100 mcg transdermal estradiol Medium dose:0.625 mg conjugated estrogens 1 mg oral estradiol 50 mcg transdermal estradiol Low dose: 0.3 mg conjugated estrogens 0.5 mg oral estradiol 25 mcg transdermal estradiol |
The major goals of treatment for osteoporosis are prevention of fractures, maintenance or increase in BMD, improvement in physical function.
Non pharmacological management (Indicated for low, medium and high risk levels)
Nutrition: Institute measures that reduce modifiable risk factors through dietary and lifestyle changes.
Calcium: Most postmenopausal women consume inadequate amounts of dietary calcium; therefore, supplementation is needed. In a double blinded trial (15), it was shown that there was decrease in fracture rates in older women with 80 percent or greater adherence to calcium supplementation. For optimal absorption, a single dose of calcium supplement should contain about 500 mg of elemental calcium usually given as citrate or carbonate. All calcium supplements may cause constipation and gastrointestinal upset and these side effects are to be discussed with the patients before initiating the treatment for better compliance. The absorption of numerous medications, most notably levothyroxine, quinolones, tetracycline, phenytoin (Dilantin), ACE inhibitors, iron and bisphosphonates can be significantly decreased when given with calcium and these medications should be given several hours before or after calcium supplements.
A daily intake of 1,200 mg of calcium is recommended for all women with osteoporosis. We prefer to use natural sources of calcium instead of supplements if possible. Recent studies have suggested that higher doses of calcium supplementation may cause increase kidney stone formation and myocardial infarction (16).
Vitamin D: Recommend 800IU per day of vitamin D3, including supplements if necessary, to adults over the age of 50. There is no urgent need to use 50,000IU weekly. The goal of treatment is to maintain a serum 25-hydroxyvitamin D level greater than 20 ng per mL (50 nmol per L) (17).
Protein: Recommend an adequate intake of dietary protein (1g/kg/day) (18).
Exercise: Regular weight-bearing and muscle-strengthening reduce the risk of falls and fractures by improving agility, strength, posture, and balance, as well as general health benefit.
Smoking: Tobacco products are detrimental to the skeleton as well as to overall health.
Alcohol: Intake of 3 or more units (5oz wine, 1.5oz spirits, 12oz beer) per day is detrimental to bone health and increases the risk of falling.
Falls prevention strategies: Falls prevention is the first line of treatment for those at high risk for falling. Consider referral to geriatric medicine, a falls prevention program, and physical therapy.
Pharmacological interventions
Pharmacological therapy in treatment of osteoporosis is suggested in
➢ All postmenopausal women who have had an osteoporotic vertebral or hip fracture
➢ All postmenopausal women who have BMD values consistent with osteoporosis (i.e., T-scores equal to or worse than -2.5) at the lumbar spine, femoral neck, or total hip region
➢ All postmenopausal women who have T-scores from -1.0 to -2.5 and a 10-year risk, based on the FRAX calculator of a 20 % risk of a major osteoporotic fracture (spine, hip, shoulder and wrist) or 3% risk of hip fracture.
Pharmacological interventions can be classified into anti resorptive agents that prevent the bone resorption and anabolic agents that help in the new bone formation. Approved anti-resorptive agents in USA include hormone replacement therapy (HRT), bisphosphonates, SERMS, Denosumab. Anabolic agents include Strontium (only available in Europe) and Teriparatide.
Anti-resorptive agents
Hormone Replacement therapy
ET/HT can still be considered a first line choice for prevention of bone loss and fracture in the early postmenopausal period for a period of 5 years. In women with a low risk of adverse events classically associated with HT/ET newer analyses show that treatment can be continued with an acceptable risk benefit ratio.
For many years the mainstay of prevention of postmenopausal bone loss was hormone therapy estrogen + progestin (HT) or estrogen therapy (ET). Years from menopause has one of the largest effects on the rate of bone loss because bone resorption is highest in the first 3-4 years after menopause. Response to treatment is always highest during this time because stopping resorption causes rapid filling in of the resorption or remodeling space and the higher the bone resorption, the greater the increase in BMD. The most effective way to treat bone loss in postmenopausal women is by using a high dose of HT/ET for 6 months to rapidly reduce bone resorption and then reduce the dose one level lower for subsequent years. HT and ET were commonly used in the last 30 years but their use declined following publication of the Women's Health Initiative (WHI) study in 2001 (19). However, the average age of the women in WHI study was 63 and few of them only have had menopausal symptoms. This WHI results might not apply for early post-menopausal group. In a recent analysis of hysterectomized women it was shown that avoiding estrogen has led to ~50,000 extra deaths from 2002-2011 (20). In a review article (21) of oral estradiol and didrogesterone combination, it was concluded that the combination is very effective in relieving menopausal symptoms and prevention of osteoporosis while maintaining a good safety profile. In a meta-analysis of 57 trials (about 10,000 women), on an average the increase in BMD after 2 years was 6.8 % in the spine and 4.1 % in the hip in estradiol/conjugated estrogens treated group and no difference was noted in results between prevention and treatment trial (22). Since 2002 there have been more than 10 double blind trials lasting 2-3 years and were mainly using HT therapy. The results from these trials are very similar to those reported in a meta-analysis (23).
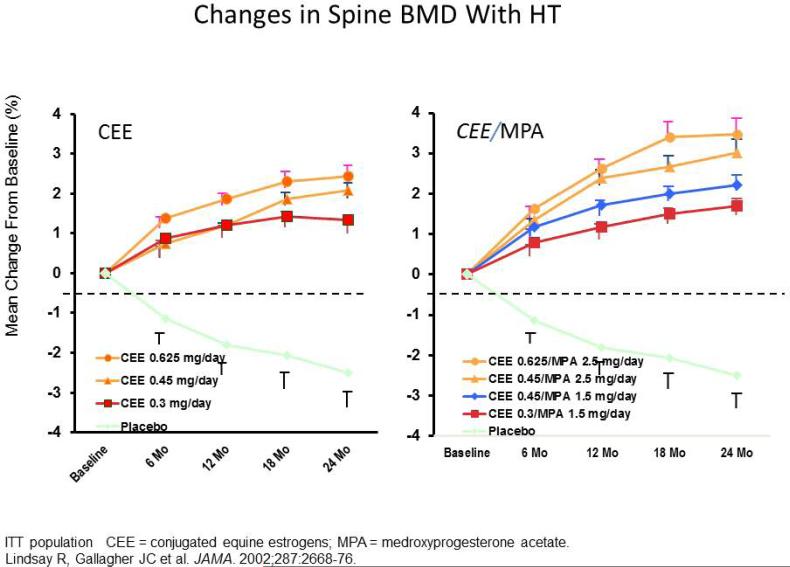
In HOPE trial, 822 women were randomized to either daily conjugated equine estrogens 0.3, 0.45 or 0.625mg or to the same doses with continuous daily medroxyprogesterone acetate 1.5, 2.5mg as well as calcium 600mg. After 2 years treatment there were significant increases in BMD on all hormone doses compared to placebo, the difference being about 3-5 percent for spine BMD and 1-3 percent for total hip. There was a 1 percent higher BMD in the HT compared to the ET group probably due to the progestin effect on bone (figure 6) (24). In the safety evaluation of HT/ET on the uterus endometrial biopsies showed that increase of endometrial hyperplasia and was dose related; 25 percent on 0.625mg, 15 percent on 0.45mg and 2 percent on 0.3mg. In the HT groups it was zero (25). Thus over at least 2 years there is a favorable benefit –risk profile. The dose response effect on BMD is similar to oral HT/ET, the average increase in spine BMD compared to placebo was 7.7 percent on 100mcg, 7.3percent on 75mcg, 6.3 percent on 50mcg and 3.9 percent on 25mcg (23). Based on the increase in BMD is the measure of potency of HT/ET and the dose response results, hormone treatments can be classified into high, medium and low doses (Table 3).
Figure 6.
The Women's Health, Osteoporosis, Progestin, Estrogen (Women's HOPE) trial was a prospective, randomized, double-blind, placebo-controlled, multicenter trial that investigated the efficacy and safety of lower doses of conjugated equine estrogens (CEE) alone and with continuous medroxyprogesterone acetate (MPA) in early postmenopausal women (who were within 4 years of their last menstrual period).
Effect of estrogen on Fractures
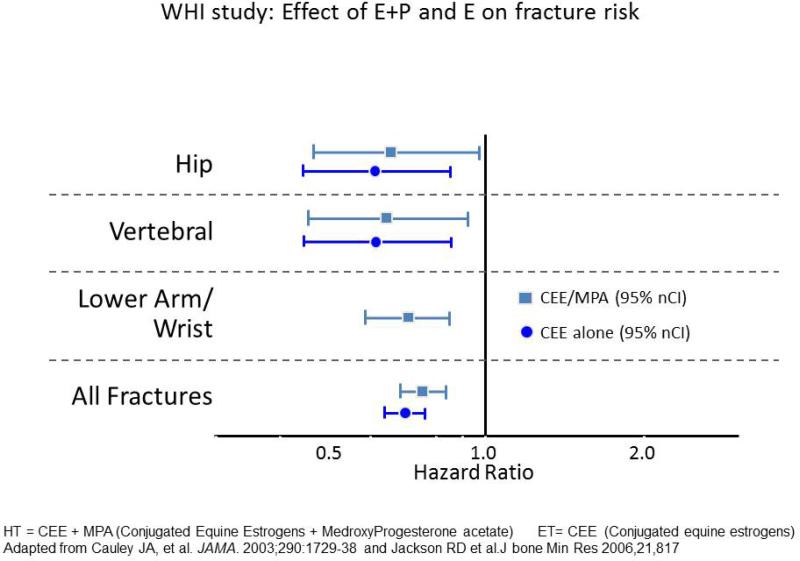
Retrospective analysis of different trials or case cohort studies show a significant decrease of about 30-50 percent in hip fractures and other fractures in estrogen treated groups (23). In a meta-analysis of 22 trials, estrogen has shown to reduce non-vertebral fractures in the under 60 age group by 33 percent compared to a non-significant reduction of 12 percent in the over 60's (26). In WHI study 16608 women were randomized to HT or placebo and 10,600 randomized to ET or placebo. After one year about half the women were randomized also to vitamin D 400 IU and calcium 1000 mg. The fracture risk started to decrease after 3 years on ET and after 2 years on HT. In the HT arm after 5.6 years there was a 33 percent reduction in hip fractures and 24 percent reduction in all fractures (27). In the ET trial of 10,739 women the follow up extended to 7.1 years and there was a similar reduction in hip fractures of 35 percent and 29 percent in all fractures. To summarize, HT/ET reduce hip fractures by 35 percent, but because a substantial number of these women were on calcium and vitamin D it is not clear yet how much of the fracture reduction is due to HT/ET, calcium and vitamin D or both agents (28). Figure 8 summarizes WHI fracture trial.
Figure 8.
Although it is FDA-approved for osteoporosis prevention, but not treatment, results from the Women's Health Initiative (WHI) HT trials provide randomized clinical trial evidence for the efficacy of estrogen used alone (E only) or with a progestin (E+P) in reducing the incidence of hip, vertebral, nonvertebral, and total fractures as shown in this slide.
Studies of HT/ET in older women
Use of estrogen in older women showed an increase of 6% in spine BMD, 5% in total hip BMD and 4% in femoral neck BMD compared to placebo (29). Another study demonstrated increased spine BMD by 6.6%, femoral neck BMD 3.2%, and total hip BMD 3.1% compared to placebo and the effect was greater when combined with alendronate (30). Two lower dose studies also showed promising evidences in increasing spine BMD (31).
Combination Therapy with Estrogen Therapy/Hormone Therapy
There have been several studies where estrogen was combined with other agents. Combination therapy (estrogen plus bisphosphonate) has been more effective in increasing BMD than either agent alone in some but not in all studies. One study showed that adding calcium supplementation to ET increased spine BMD by 2% and femoral neck BMD by 1.5% compared to ET/HT alone (32). Alendronate combined with estradiol/norethisterone acetate showed no increase in total hip BMD over those taking either medication separately (33). Alendronate (30), risedronate (34), and calcitriol (29) are other agents that have been combined with 0.625 mg CEE. In all studies the combination of two agents produced spine BMD that was 2% higher than the single agent and a 1–2% higher total hip BMD. The biochemical markers of bone resorption continued to suppress on combination therapy indicating that single therapy does not fully suppress resorption. However, not enough data is available on further fracture reduction.
New Hormone preparations
Since the decline in the use of HT and ET and concerns about the adverse effects of the progestin there has been development of other HT based preparations. The development of Tissue Selective Estrogen Complex (TSEC) that pairs conjugated estrogens with Bazodoxifene which is a selective estrogen receptor modulator (SERM).
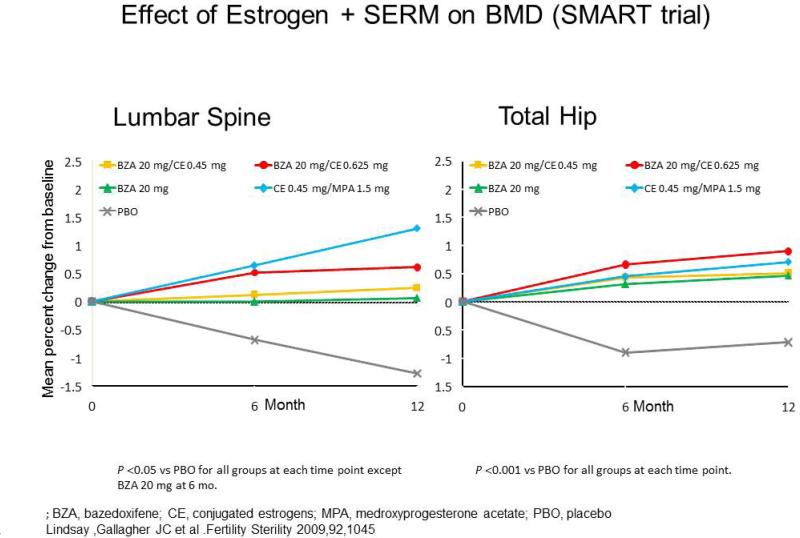
In a study of 2315 postmenopausal women enrolled into a 2-year study of Bazodoxifene doses 10, 20, 40mg were combined with conjugated estrogens 0.45 and 0.625mg and compared to raloxifene and placebo. The mean increase in spine BMD was always significantly greater with the TSEC than raloxifene or placebo (figure 7) (35). With regard to safety 20mg bazodoxifene prevented endometrial hyperplasia in more than 99 percent of women and these results suggest a favorable benefit/risk profile for this type TSEC. The application of Bazedoxifene/CEE is under review by FDA.
Figure 7.
Effect of tissue selective estrogen complex (TSEC) (CEE + Bazedoxifene) on lumbar spine and total hip BMD.
Discontinuation of estrogen
Three studies, two in elderly (36, 37) and one in younger women (30) has shown that withdrawal of estrogen produces rapid bone loss BMD and within one year most of the previous increase in BMD accumulated over 3-4 years had disappeared. In a study of early post-menopausal women, age 55 years, women were randomized to HT, alendronate 5mg and placebo. Both the HT and alendronate increased BMD by 5-6 percent. After 4 years, treatment was stopped and there was a rapid decrease in spine BMD of 7 percent in the HT group whereas the alendronate group showed a smaller decline of 2.4 percent presumably because of accumulation of alendronate in bone (38). Another similar analysis showed a significant increase in hip fractures over 6.5 years. Within 2 years hip fractures increased 50 percent and by 77 percent after 5 years (39). It is recommended therefore that women who discontinue ET/HT should undergo a BMD assessment and if the T score is below -2.0, then another therapy such as a bisphosphonate can be used to prevent further bone loss.
Risk-benefit ratio in young and elderly (> 60 years) postmenopausal women
The WHI trial has shown that compared with the placebo, HT resulted in a small but measurable risk of heart attack, stroke, venous thromboembolism and breast cancer. There was a reduced risk of colorectal cancer, fractures. ET did not increase the risk for heart attack or breast cancer and reduced fractures. There was a slight increased risk of stroke. A secondary reanalysis (40) by age group or years since menopause showed that these cardiovascular events occurred primarily in women older than 70 years and not in the younger women. In women within 10 years of menopause there was a reduced risk of heart attacks and reduced mortality suggesting that the timing of initiation of HT is important for the prevention of heart disease. These findings are in accordance with those from observational studies, such as the Nurses’ Health Study In these studies (41), HT was associated with a reduced risk of major coronary events in women 10 years or less post-menopause (ET: -34%, RR, 0.66; 95% CI, 0.54-0.80; EPT: -28%, RR, 0.72; 95% CI, 0.56-0.92) (42) and in postmenopausal women with no previous heart disease.
The North American Menopause Society guidelines suggest that as long as the lowest effective dose of HT is used, extending treatment for an individual woman's treatment goals is acceptable when the benefits of menopause symptom relief outweigh potential risks and for further prevention of osteoporotic fracture or preservation of bone mass in women with an established reduction in bone mass when other therapies are not suitable. Determination of the lowest effective dose of HT should be based on vasomotor symptom relief.
BISPHOSPHONATES
Bisphosphonates suppress bone turnover, prevent bone loss and preserve bone architecture by tightly adhering to bone surfaces and by inhibiting the enzyme farnesyl pyrophosphate synthase which is required for formation of the cytoskeleton in osteoclasts, thereby inhibiting bone resorption. Alendronate, ibandronate, risedronate and zoledronic acid are approved for the prevention (Table 4) and treatment of postmenopausal osteoporosis in the United States.
Table 4.
Osteoporosis prevention studies of bisphosphonates:
| Bisphosphonate | Study duration | Sample size | Outcome of the study |
|---|---|---|---|
| Alendronate 2.5/5 mg (41) | 6 years | 585 women 6 years post-menopausal | Increase in lumbar spine BMD by 1.5% (2.5 mg)-2.4 % (5 mg) compared to −3.2% on placebo. |
| Alendronate 5 mg (30) | 5 years | 447 women 3 years post-menopausal | Increase in spine BMD by 2.5% and after treatment is discontinued BMD declined by 1.8 to 5.7% at various sites. |
| Risedronate 2.5 mg and 5 mg (42,43) | 2 years | 383 Post-menopausal women within 3-4 years menopause | Increase in spine BMD by 4.5 % and hip BMD by 3.3% compared to placebo |
| Ibandronate 0.5 mg, 1 mg, 2.5 mg (44) | 2 years | 653 women 8-9 years post-menopausal | Dose related increase in spine BMD was noted. 1.9 % increase in spine BMD (2.5 mg) compared to baseline. |
| Ibandronate 20 mg/week (45) | 2 years | 630 post-menopausal women | Increase in spine BMD by 4.0 % and hip BMD by 2.7 % compared to that of placebo |
| Zolendronate 5 mg (46) | 1,2 years | 581 osteopenic women >45 years | Increase in spine BMD by 5.2 % at end of first year and 4.4 % at second compared to decline in BMD by 1.3% with placebo |
Bisphosphonates in treatment of osteoporosis
Alendronate was first approved in 1995 for treatment of osteoporosis after completion of the first large osteoporosis trial of 1996 patients with osteoporosis. In the first year they were given 5mg and this was increased to 10mg in the next 2 years. Spine fractures were reduced by 50 percent and hip fractures were significantly reduced although the numbers were small (49). The effects of discontinuing alendronate treatment (5 or 10 mg/d) after 5 years were compared with continuing treatment for 10 years in the Fracture Intervention Trial Long-term Extension (FLEX trial). Results from this trial indicated that 5 years after discontinuation of alendronate, BMD at the total hip decreased by -2.4% and spine by -3.7 % and was significantly lower compared to those who continued on alendronate although BMD levels were still higher than those recorded ten years earlier at baseline. Although continuing alendronate treatment significantly lowered the risk of clinically recognized vertebral fractures compared with placebo (2.4% Vs 5.3%, respectively), non-vertebral fracture risk was not significantly different between women who continued alendronate treatment (19%) and those who discontinued (18.9%) suggesting some loss of efficacy (50).
Risedronate, a third-generation bisphosphonate, has been shown in large well designed studies to increase or maintain bone density and reduce the risk of spine and non-spine fractures. In a study of 2458 women with osteoporosis were randomized to 2.5mg, 5mg or placebo. The 2.5mg arm was discontinued and after 3 years spine fractures were reduced by 41 percent and non-vertebral fractures by 39 percent compared to placebo spine BMD increased 5.4 percent and hip BMD by 2.8 percent (49). Similar findings were reported in a trial from Europe (51).
Zoledronic acid is the first and currently the only once-a-year osteoporosis medication. Studies have shown that zoledronic acid increases bone density and reduce fractures in the hip, spine and total body. In a study after 3 years of infusion of 5 mg of zolendronic acid/year, spine fractures were reduced by 70 percent, non-vertebral fractures by 25 percent and hip fractures by 41 percent (53). In a long term 6 year follow up 1233 patients were randomized to continue Zolendronate or change to placebo. Femoral neck BMD declined and spine fractures were 50 percent lower in those who were treated for 6 years suggesting continued benefit (54). There were transient increases in serum creatinine > 0.5mg/dl. It is possible to give it once every 2 years for prevention
Ibandronate reduces the incidence of spine fractures by about 50% over three years. Data do not yet confirm that ibandronate can reduce the risk of hip and other non-spine fractures. However, ibandronate increases bone density substantially throughout the skeleton. In Ibandronate studies there were difficulties in determining the effective dose but eventually a treatment dose of daily 2.5mg daily and an intermittent dose of 20mg every other day for 12 doses repeated every 3 months was evaluated. On both regimens spine fractures were reduced by 62 percent and there was no effect on non-vertebral fractures. However, in a post hoc analysis of the more osteoporotic patients whose femoral neck BMD was below -3.0, non-vertebral fractures were reduced 69 percent (55).
In summary all bisphosphonates are powerful anti resorptive agents and reduce fractures by 40-70 percent. It is not clear whether there are real differences in potency or whether the results reflect the different degrees of severity of osteoporotics entered into study. Only one study prospectively planned a study of hip fractures and that showed a reduction in hip fractures only in women under age 80 year and not over age 80 years (56). However, it is likely that all of these bisphosphonates will reduce fractures given the results. These regimens offer patients many choices and this can improve compliance. Tables 3 and 5 summarizes the available bisphosphonates and their doses (Calcitonin, Estrogen and Teriperatide are included in the table).
Table 5.
Therapies for osteoporosis and their effects
| Fracture prevention | Spine fractures | Hip fractures | Non-Vertebral fractures | |
|---|---|---|---|---|
| Anti Resorptive agents | ||||
| Alendronate | x | x | x | x |
| Ibandronate | x | x | - | - |
| Risedronate | x | x | x | x |
| Zolendronate | x | x | x | x |
| Calcitonin | - | x | - | - |
| Estrogen + Progesterone | x | x | x | x |
| Denosumab | x | x | x | x |
| Raloxiefene | x | x | - | - |
| Anabolic agents | ||||
| Teriparatide | - | x | - | x |
Limitations of bisphosphonates
Some concerns associated with bisphosphonates limit their utility for certain women. Oral absorption is very low-less than 1 percent of the dose and must be taken on an empty stomach for maximal absorption, post-dose patients should sit upright. A new formulation of risedronate allows it to be taken after food (Atelvia). Gastrointestinal intolerability, ranging from mild acid reflux to serious esophageal ulcers, is a significant side effect. Intravenous administration of bisphosphonates may be accompanied by acute phase reaction symptoms, although they are generally mild to moderate in severity and become less frequent with repeated intravenous infusions and can usually be managed with acetominophen. Musculoskeletal pain is one of the rare side effects of bisphosphonates. No identifiable cause and incidence of this potential side effect is known. Some patients feel better after discontinuation the drug while others appear to have slow or incomplete resolution. Transient hypocalcaemia with secondary hyperparathyroidism is a recognized but underappreciated consequence of bisphosphonate administration. This hypocalcaemia occurs most frequently after IV infusion. All patients who are to begin receiving either oral or IV bisphosphonate therapy should have adequate calcium and vitamin D intake. If there are any concerns about nutritional status or absorptive capacity, serum levels of 25-hydroxyvitamin D, calcium, phosphorus, and urinary calcium excretion should be assessed and abnormalities are to be addressed before initiating bisphosphonate therapy. Both oral and IV bisphosphonate therapy have been rarely associated with ocular inflammation, ocular pain and photophobia. Onset is idiosyncratic and can occur in weeks, months, or even years after bisphosphonate initiation. Given the complexity of diagnosis and treatment, ophthalmologic referral is recommended. Bisphosphonates have also been associated with reports of acute renal failure and it may be advisable to only treat patients in whom GFR is > 30ml/min. Atrial fibrillation has been a concern with bisphosphonate use. However, no clear association between atrial fibrillation and bisphosphonate use has been established. To date, neither a convincing mechanism nor a dose dependent effect and duration of bisphosphonate therapy has been proposed for this potential risk. One possible explanation is, patients receiving bisphosphonate therapy are primarily older and represent the population most likely to have atrial fibrillation independent of bisphosphonate use.
Recently there have been long-term safety concerns about bisphosphonates that include a possible increased risk of atypical femoral fractures and osteonecrosis of the jaw. It is likely that both are very rare adverse events and are time related. It has been estimated that these risks in osteoporotics are 1 in 50,000 to 1 in 150,000. They are more common in patients with cancer but these patients are given much higher doses of bisphosphonates for metastatic bone disease as well as taking chemotherapy that is toxic to bone cells (57). Atypical femoral fractures typically occur in the proximal or mid femoral diaphysis, either spontaneously or result from low-energy trauma. These are transverse or oblique (≤30°) fractures and have delayed healing. Case reports have shown that these fractures have occurred in patients who have received prolonged bisphosphonate therapy, and are often preceded by a prodrome of thigh pain, vague discomfort, or subjective weakness. This has raised the concern about long-term bisphosphonate therapy that might lead to over-suppression of bone remodeling, an impaired ability to repair skeletal microfractures, and increased skeletal fragility. These safety concerns have raised question about how long to treat osteoporosis with bisphosphonates which has led to concept of ‘drug holiday’.
What is the optimum duration of bisphosphonate therapy and when to consider a ‘drug holiday’?
Bisphosphonates can accumulate in the body because of the drug's long elimination half-lives and accumulation of the drug may increase risk of adverse effects due to over suppression of bone turnover. Theoretically, a ‘drug holiday’ may be a viable option to decrease risks of bisphosphonate accumulation with the possibility of continued protection from fractures. Based on the fracture risk the duration of treatment and length of holiday should be individualized (58). It has been suggested that bisphosphonates are not indicated in the first place and can be safely discontinued if there are no risk factors for fractures, early postmenopausal and if T score not in osteoporotic range. For those with mild fracture risk that includes elderly postmenopausal (>10 years menopausal), T score in the osteopenic range, parental history of fractures it is recommended to treat with bisphosphonates for 3-5 years and if BMD is stable over time a drug holiday might be considered after 5 years of treatment. In > 10 year postmenopausal women with moderate risk of fracture, initial T score in osteoporotic range, no risk factors it is better to treat with bisphosphonate for 5-10 years, then offer a drug holiday for 3-5 years if BMD is stabilized or increased over time. In high risk fracture individuals with risk fractures of fracture and/or history of osteoporotic fracture treat with bisphosphonate for 10 years then offer a drug holiday for 1-2 years. The effect of Risedronate on bone is less prolonged than for alendronate. In this high risk fracture individuals it is always prudent to add either teriperatide or raloxifene during the ‘drug holiday’.
After initiation of drug holiday, risk re-assessment should be done after 1 year for risedronate as it has lowest skeletal affinity, 1-2 years for alendronate and 2-3 years for zoledronic acid by measuring BMD and/or bone turn over markers. Checking BMD at two year intervals is generally practiced. If the BMD shows a consistent decrease greater than 6%, then consider restarting bisphosphonates or another medication. Some institutions follow urinary N-telopeptide cross linked type 1 collagen (NTx) – a bone resorption marker urinary test annually to follow the patient on drug holiday (59). If the urine Ntx value is less than 40 nmol bone collagen equivalents per millimole of creatinine (nmol BCE/mmol Cr) it is suggested to continue drug holiday and monitor urinary Ntx yearly. If urinary Ntx increases to more than 40 nmol BCE/mmol Cr, consider starting patient on other anti-resorptive agent. Thus, the duration of therapy and the length of drug holiday should be individualized based on risk/benefit ration and should be monitored closely by follow up BMD or by measuring BTMs.
SERMS
The past decade has witnessed the design and development of compounds that act as estrogens on some tissues but anti-estrogens on others. The only approved second generation SERM for osteoporosis, raloxifene, is an estrogen agonist at bone and liver, maintains BMD and lowers LDL cholesterol concentrations. Moreover, it does not stimulate the endometrium and is a potent anti -estrogen at the breast. SERMs are an appropriate alternative for women who cannot take bisphosphonates due to side effects such as gastrointestinal intolerance, acid reflux and those who cannot adhere to the dosing requirements for bisphosphonates.
Many trials have demonstrated the beneficial effect of raloxifene on bone mineral density. In a meta-analysis of seven trials examining the effects of raloxifene versus placebo on bone mineral density, raloxifene increased bone mineral density of the lumbar spine of 1.8 % and hip 2.1% after two years of treatment (60). In a three-year analysis of the two largest prevention trials, 1,145 postmenopausal women 60 years or younger and an average of 5 years postmenopausal without osteoporosis were randomly assigned to raloxifene versus placebo (61). All groups received 400 to 600 mg of elemental calcium. The change in lumbar spine BMD from baseline to 3 years was -1.32% with placebo, 0.71% with raloxifene 30 mg, 1.28% with raloxifene 60 mg, and 1.20% with raloxifene 150 mg. Moreover, biochemical markers of bone turnover were suppressed to the normal premenopausal range in raloxifene-treated women. In another analysis (62) of two studies involving 328 women who were on average age of 55 years and who were 5 years post-menopause at baseline, 5 years of treatment with raloxifene 60 mg was associated with maintenance of BMD and reduced risk of developing fractures compared with placebo. Relative to placebo, the mean percentage of change in BMD was increased by 2.8% at the lumbar spine and 2.6% at the hip (both P < 0.001) with raloxifene treatment.
Raloxifene is also effective for the treatment of established osteoporosis. In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, a total of 7705 women who had a mean age of 65 years (low bone mass group) and 68 years (osteoporosis group) and were followed up for a total of 8 years, were randomly assigned to raloxifene (60 or 120 mg) or placebo daily). Lumbar spine and femoral neck BMD increased from 2.0 to 2.7 percent in the raloxifene groups compared with placebo. The reduction in fractures was 30 percent on 60mg and 50 percent on 120mg dose. There was no effect on non-vertebral fractures. But importantly treatment with raloxifene 60 mg reduced the incidence of invasive and invasive estrogen receptor-positive breast cancers by 65-78 percent (both P < 0.05) (63).
Third-generation SERMS in the management of postmenopausal osteoporosis
Third generation SERMS, Bazedoxifene and Lasofoxifene are approved in the European Union for the treatment of osteoporosis in postmenopausal women at increased risk of fracture.
Bazodoxifene is a novel third generation SERM approved in European Union and is currently under review in United States. Unlike Raloxifene, in clinical trials, it prevented both vertebral and non-vertebral fractures in high risk women and had neutral effects on reproductive tracts with positive effects on lipid profile. In a 2-year prevention study conducted in healthy postmenopausal women (N=1583; mean age, 57.6±6.5 years) who were at risk for osteoporosis, women were randomly assigned to daily oral doses of Bazodoxifene 10, 20, or 40mg, raloxifene 60mg, or placebo for 2 years. The increase in spine BMD compared with placebo was 1.08±0.28% for Bazodoxifene 10mg, 1.41±0.28% for 20mg, 1.49±0.28% for 40mg, and 1.49±0.28% for Raloxifene 60mg (p<0.001 versus placebo for all). Similar improvements in BMD were observed with all doses of Bazodoxifene at the total hip and with Bazodoxifene 20 and 40mg at the femoral neck (64).
In a pivotal phase III treatment study of Bazodoxifene conducted in 7492 healthy postmenopausal women age, 66 years women were randomly assigned to daily oral doses of Bazodoxifene 20 or 40mg, Raloxifene 60mg, or placebo. There was a reduction in spine fractures on all doses at 3 years of 42 percent for Bazodoxifene 20mg, 37% for 40mg dose, and 42% for Raloxifene 60mg (65). In another trial of486 women age 57 years) BZA 20mg was associated with significantly greater lumbar spine BMD (0.41%) compared with placebo (−0.32%; p<0.01). Similar small increases in BMD were observed at the hip sites (66).
Lasofoxifene is another third generation SERM with excellent oral bioavailability. It has a high affinity for both ERα and ERβ, approximately the same as estradiol, and about 10-fold higher than the other SERMs- raloxifene, tamoxifen and droloxifene. Two doses of lasofoxifene 0.25 and 0.5mg were evaluated in a randomized trial (PEARL study). In the three-year analysis lasofoxifene 0.25mg and 0.5mg daily increased lumbar spine BMD by 3.0%and 3.1% respectively, increased femoral neck BMD by 2.9%-3.0% respectively relative to placebo. Lasofoxifene 0.25 mg and 0.5 mg/d reduced the risk of vertebral fractures by 31% and 42%, respectively (P < 0.002), while nonvertebral fractures were significantly reduced by 22% with the 0.5 mg/d dose. The higher dose of lasofoxifene reduced breast cancer by 81 percent and the lower dose by 49 percent (67).
DENOSUMAB
Denosumab, a human monoclonal antibody that specifically binds RANKL, blocks the binding of RANKL to RANK thereby reducing bone resorption and increasing bone density. It is administered as a subcutaneous injection every 6 months. The FDA has approved denosumab 60mg for treatment of osteoporosis. However, it is not yet approved for prevention (pending long-term safety data). Denosumab is shown to improve bone mineral density (BMD) in postmenopausal women with low BMD.
In the FREEDOM trial, 7868 postmenopausal women (60 to 90 years of age) with osteoporosis were randomly assigned to subcutaneous denosumab (60 mg every six months) or placebo for three years, denosumab increased BMD of the lumbar spine and total hip compared with placebo (9.2 versus 0 percent and 4.0 versus -2.0 percent, respectively) and significantly reduced the risk of new vertebral fractures by 68% (P < 0.001), hip fractures by 40% (P = 0.04), and non-vertebral fractures by 20% (68). In FREEDOM extension trial (69) of 4550 postmenopausal women, the FREEDOM denosumab group is continued on 3 more years of denosumab for a total of 6 years and women from the FREEDOM placebo group are given 3 years of denosumab. Lumbar spine and hip BMD increased significantly at the end of 6 years (15.2% and 7.2% respectively). In the cross over group lumbar spine and total hip BMD increased by 9.4% and 4.8% respectively. In the sixth year of the extension trial, six cases of osteonecrosis of jaw were observed in the long-term group. Atypical fracture was reported in one participant. The most common side effects of denosumab include urinary and respiratory tracts infections, cataracts, constipation, rashes and joint pain.
Calcitonin
Salmon Calcitonin, most widely used, is highly potent in humans because its affinity is 40 times higher than human calcitonin for the human calcitonin receptor. Calcitonin is approved in the United States for administration by the subcutaneous, intramuscular, and intranasal routes. Calcitonin has a beneficial short-term effect on acute pain relief in patients who have sustained a vertebral fracture. The effect of calcitonin in reducing fractures is less clear than for other drugs because the 200IU dose of nasal spray calcitonin reduced spine fractures by 33 percent but the 100 IU and 400 IU doses were ineffective (70). Recently two FDA advisory committees have concluded that use of a nasal spray formulation of the peptide hormone salmon calcitonin is associated with an increased risk of cancer. The FDA now has to decide whether to approve their recommendations. This new cancer concern arose from the results of an unpublished meta-analysis that included 18 studies of nasal Calcitonin in which the risk of any cancer was 1.54 times greater (95% CI: 1.06, 2.23) in patients who used the drug compared to controls (71). An earlier 5-year trial in about 1200 postmenopausal women with osteoporosis also found that there is a small, but statistically significant, increase in the risk of any malignancy (OR 1.62, 95% CI: 1.00, 2.61) in nasal calcitonin treated group compared to that of placebo (71). With these concerns, the drug will no longer be available in Canada from October 1, 2013. However, the injectable products will still be available with a new warning about the cancer risk and it is advised to limit their use to the shortest time possible.
Teriparatide
Teriparatide (rDNA origin) injection contains recombinant human parathyroid hormone hPTH(1-34)] that has an identical sequence to the 34 N-terminal amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone. Teriparatide stimulates bone formation and remodeling thereby increasing BMD as measured by DXA and improves micro architecture. Treatment with teriparatide is the only proven and approved method of increasing bone mass and improving bone architecture.
Multiple cellular pathways in the osteoblast are activated by PTH. Target genes induced by intermittent PTH include IGF-I, amphiregulin, 1, alpha hydroxylase, Runx2, TGF-beta, RANKL, and M-CSF, while sclerostin, an inhibitor of the Wnt signaling pathway, is suppressed by treatment with PTH. Activation of these pathways results in osteoblastic recruitment to the remodeling unit. Bone formation begins within the first month of PTH treatment and peaks six to nine months after initiation of daily PTH. Since the remodeling unit is always coupled (i.e., bone formation equals bone resorption), once preosteoblasts are stimulated, they release cytokines that can activate osteoclasts resulting in bone resorption. Bone resorption in patients on PTH begins at about six months and peaks after 12 months of treatment. Increased bone formation in the first three months precedes bone resorption and places the remodeling unit in a positive balance. Though most of the gains in BMD occur in the first few months, anti-fracture efficacy is evident only after six months or more of treatment (72).
In the Fracture Prevention Trial, 1637 women with prior vertebral fractures, average age 69 years, were given PTH 1-34 ( teriparatide) 20 or 40 mcg subcutaneously daily. Spine BMD increased 9.7 percent on the 20mcg dose and 13.7 percent on 40 mcg. The relative risks for vertebral fractures for the 20 and 40 mcg doses compared with placebo were 0.35 (95% CI 0.22-0.55) and 0.31 (95% CI 0.19-0.50) respectively and for non-vertebral fractures the relative risks were 0.47 and 0.46, respectively (95% CI, 0.25-0.88 and 0.25-0.861) (72). Ongoing toxicology studies in rats showed that longer duration and larger dose and of therapy was correlated with the appearance of osteosarcomas. For this reason, the FDA has limited the use of Teriparatide to 2 years and should not be used in patients with history of Paget's disease or any cancer. Since it was approved there have been no reported cases of osteosarcoma in human subjects.
In the Treatment of Osteoporosis with Parathyroid Hormone 1-84 study, 2532 postmenopausal women with or without prior vertebral fractures were randomly assigned to 100 mcg of PTH 1-84 or placebo daily by subcutaneous injection (73) for 18 months. Women receiving PTH1-84 had greater increases in lumbar spine and femoral neck BMD compared with placebo (6.9 and 2.5 percent, respectively). Volumetric trabecular BMD measured by quantitative computed tomography [QCT] at L3 and at the hip increased by 37 and 4.7 percent more respectively in the PTH group than in the placebo group. Hypercalcemia was more of a problem than with 1-34.
In the European Study of Forsteo (EUROFORS), women who had received one year of teriparatide were randomly assigned for a second year to continue teriparatide or to switch to either raloxifene or placebo (74). BMD in the women who received teriparatide for a second year continued to increase (10.7% increase), whereas BMD was maintained in the women who received raloxifene and decreased in women who received placebo. Thus, after PTH treatment is discontinued, an antiresorptive preferably a bisphosphonate, should be used to preserve or increase gains in BMD acquired with PTH alone. The main adverse effects of PTH 1-84 are hypercalcemia, hypercalciuria, nausea, and headache.
Combinations of anabolic agent (Teriparatide) and anti-resorptives
Combined estrogen plus PTH therapy appears to be more effective than hormone therapy alone (75). In one trial of 34 postmenopausal women randomly assigned to receive hormone therapy (estrogen-progestin) alone, or combined with PTH 1-34 (25 mcg/day) for 36 months, those receiving combination therapy had a greater increase in spine and hip BMD (13 and 2.7 percent respectively) than those receiving hormone therapy alone (75). Clinical trials have shown negative effects with teriparatide and bisphosphonate combination when they are given together, although sequential therapy has proved more promising (76-79). In a 2-year double-blind, placebo-controlled trial (76), 238 women with osteoporosis received Teriparatide 100 μg/d for year 1 followed by alendronate 10 mg daily for year 2 (sequential therapy), Teriparatide plus alendronate for year 1 followed by alendronate for year 2 (combination therapy), alendronate for 2 years, or Teriparatide for year 1 followed by placebo for year 2. After 1 year, BMD at the spine increased in all women. The increases in the Teriparatide-alone group were twice as large as those observed in the other 2 groups. Thus, no synergy was found in this trial between teriparatide and alendronate. Indeed, observed changes in bone density and BTMs suggested that the combination of these agents may reduce the anabolic effects of teriparatide. Two other studies (78, 79) has shown that after discontinuation of PTH, alendronate treatment resulted in further significant increases in BMD at both the spine and hip, which suggests again that sequential treatment with PTH followed by a bisphosphonate appears to be an effective strategy to increase BMD. Tsai JN et al (80) evaluated the effect of combination of teriparatide and denosumab on BMD. At 12 months, lumbar spine BMD increased more in the combination group (9·1%) than in the teriparatide (6·2%), (p=0·0139) or denosumab (5·5%), p=0·0005) groups. Femoral-neck BMD also increased more in the combination group (4·2%) than in the teriparatide (0·8%), p=0·0007) and denosumab (2·1%), (p=0·0238) groups, as did total-hip BMD (combination, 4·9%; teriparatide, 0·7%, (p<0·0001); denosumab 2·5%, (p=0·0011). Combined teriparatide and denosumab increased BMD more than either agent alone and might, therefore, be useful to treat patients at high risk of fracture.
New therapies for osteoporosis
Risideronate DR
This is a newer formulation of bisphosphonate with pH sensitive enteric coating that helps the drug to bypass the esophagus and stomach and the drug is absorbed in small intestine. This drug can be taken after breakfast. The EDTA (chelating agent) is intended to reduce the binding of risedronate to dietary calcium. In a 2 year randomized controlled study, Risedronate DR 35 mg (Delayed Release) given weekly compared with Risedronate IR 5 mg (Instant Release) showed that Risedronate 35 mg DR weekly is as effective as risedronate 5 mg IR daily in increasing spine and hip BMD, so it is approved for use although there is no fracture data (81).
Oral Calcitonin
Oral calcitonin may provide an additional treatment alternative for women with postmenopausal osteoporosis. In phase III Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) study (n=565, mean age: 66.5), women randomized to oral recombinant salmon Calcitonin 0.2 mg/day had a mean increase in lumbar spine bone mineral density (BMD) (1.5%±3.2%) that was greater than those randomized to synthetic salmon calcitonin nasal spray 200 IU/day (0.78%±2.9%) or placebo (0.5%±3.2%). Lumbar spine BMD change in those receiving nasal calcitonin did not differ from placebo. Oral calcitonin treatment also resulted in greater improvements in trochanteric and total proximal femur BMD than the calcitonin nasal spray. Reductions in bone resorption markers with oral were greater than those observed in nasal spray or placebo recipients. However there was no significant effect in reducing spine fractures (82).
Sclerostin Inhibitors
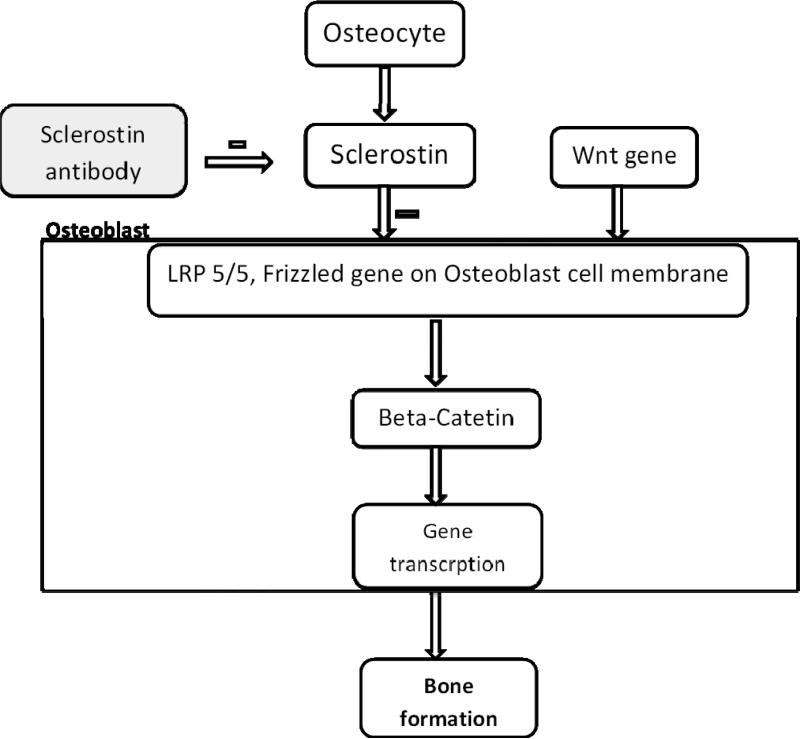
Sclerostin, a protein that is produced by osteocyte acts by antagonizing Wnt signaling system on cell membranes of osteoblasts thus having anti-anabolic effects on bone. The binding of Wnt proteins to the LRP5/6-Frizzled co-receptor on the cell membrane of osteoblasts leads to intracellular changes, in turn regulating gene transcription that promotes osteoblastic bone formation. Sclerostin inhibitors are monoclonal antibodies that enhance Wnt signaling pathway and thus increasing osteoblastic bone formation. In animal models and in a phase I trial in healthy adults, administration of a sclerostin monoclonal antibody increased bone mass (83). Sclerostin and Wnt2 pathway is summarized in figure 11.
Figure 11.
Figure summarizing Sclerostin and Wnt 2 pathway. Sclerostin antibody binds and inhibits the anti-anabolic effect of inhibits sclerostin protein.
Integrin antagonists
The alphaVbeta3 integrin (vitronectin) receptor plays a pivotal role in bone resorption. Integrins mediate the adhesion of osteoclasts to the bone surface, inhibiting bone resorption and thus increasing BMD (84).
Cathepsin-K inhibitors
Cathepsin K is a protease expressed in osteoclasts that plays a role in osteoclast-mediated bone resorption. Cathepsin K degrades type 1 collagen organic bone. Cathepsin-K inhibitors (eg, Odanacatib) inhibit matrix dissolution, decrease bone resorption, and thus improve BMD in postmenopausal women (85). There is a significant reduction in fractures (Binkley). There were no clinically relevant safety concerns yet(86).
MONITORING THE RESPONSE TO THERAPY
Monitoring the response to therapy is important for identifying patients who may require a change in therapy. This includes clinical reassessment, laboratory and/or radiological evaluation (DXA scan)
Clinical re-assessment
Re-assess patients as clinically indicated to monitor side effects, compliance, height loss, incident fractures, and risk of falls, which may alter patient management.
Bone turnover markers and DXA scan are used for treatment monitoring
Bone turnover markers (BTMs)
Bone turnover markers (BTMs) indicate either osteoclastic bone resorption (breakdown products of type I collagen in bone: N-telopeptides, C-telopeptides, deoxypyridinoline) or osteoblast function (bone matrix synthesis: bone-specific alkaline phosphatase, procollagen type I N-terminal propeptide, osteocalcin). BTMs can be measured in serum or urine. These tests may show an individual patient's response to therapy earlier than BMD changes, sometimes within 24 hours and can be used as a measure of the potency of a drug in suppressing bone resorption. However, the biological variability in day to day excretion of bone markers is large and makes useful clinical interpretation in an individual is problematic but these tests have been very useful in treatment monitoring and also to decide on ‘drug holiday’ for bisphosphonates (described above). Check spot urine NTX and serum CTX at baseline and repeat in six months. If > 50% decrease in urine NTX and > 30% decrease for serum CTX drug is having desired effect.
Follow-up BMD measurements
For patients on OP medication, repeat BMD examinations may not be justified based on current evidence. If a BMD is to be done, any changes would be difficult to detect prior to 2 years. For these reasons insurance will only cover the cost of a scan every 2 years but there are times when annual monitoring is clinically helpful. Drugs may decrease a patient's risk of fracture even when there is no apparent decrease in BMD. Changes of less than 2 – 3% in spine BMD and 5–6% at the hip may be due to precision error. Consider more frequent testing in specific high-risk situations (e.g., multiple risk factors, or receiving ≥ 7.5 mg prednisone daily or its equivalent for 3 months consecutively who require a baseline examination and repeat scans at 6-month intervals while on treatment).
Women > 65 years will usually lose bone. A stable BMD value on treatment may reflect successful treatment and appreciable decreases in fracture risk may accompany minor increases in BMD. Minor increases in BMD may also be due to testing variance. Ideally, any follow-up BMD testing is recommended to be done on the same DXA machine and at the same time of year.
Summary
In women within 10 years of the menopause ET and HT may be considered as first-line therapy in preventing bone loss and fractures in women who has menopausal symptoms (hot flashes, vasomotor symptoms), whereas SERMs and bisphosphonates represent secondary options. Bisphosphonates should be the first choice for older patients over age 60 years with osteoporosis or marked osteopenia (T score -1.8) and risk factors. Intravenous bisphosphonates given once a year are available for individuals with gastrointestinal intolerance to oral bisphosphonates. There is some lingering anti-fracture effect after treatment is stopped. So, it is reasonable to consider a ‘drug holiday’ from bisphosphonate therapy. However, the duration of treatment and the length of ‘drug holiday’ should be individualized based on the risk/benefit ratio. SERMs are an appropriate alternative for women who cannot take bisphosphonates due to their complex dosing, who have renal insufficiency, who experience gastrointestinal intolerability. Denosumab could be used as initial therapy in certain patients at high risk for fracture, such as older patients who have difficulty with the dosing requirements of oral bisphosphonates and in patients who are intolerant of or unresponsive to other therapies and in those with impaired renal function. Teriparatide is used to treat people who have a high risk of getting fractures and we generally use it when the T score is -3.0 or lower (Figures 9 and 10).
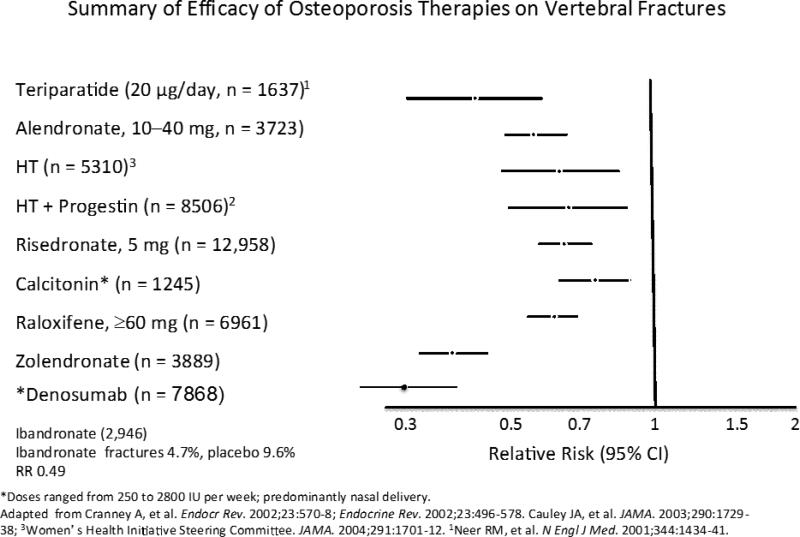
Figure 9.
The relative risk (95% CIs) for vertebral fractures with each therapy is shown in this slide. Significant treatment effects were not seen for calcium and the P value for effect of calcitonin was 0.05. All other therapies significantly reduced the incidence of vertebral fractures. *Denosumab: * Cummings et al N Engl J Med. 2009
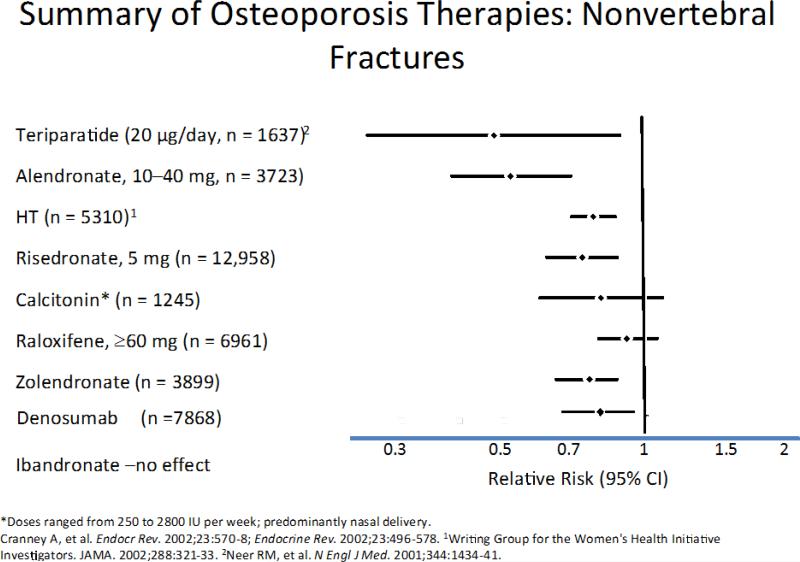
Figure 10.
The relative risk (95% CIs) for nonvertebral fractures with each therapy is shown in this slide. Significant treatment effects were seen only with HT (including the WHI trial), alendronate (10–40 mg), risedronate and Tereperatide. *Denosumab: * Cummings et al N Engl J Med. 2009
In addition to considering pharmacological intervention, all postmenopausal women should be advised on lifestyle modifications that reduce the risk of bone loss and fracture such as exercise, adequate protein intake of 1gm per kg of body weight and avoiding risk factors such as smoking. There are recommendations that women should have a total calcium intake of 1200mg/daily and if possible the calcium should come from the dietary intake. Vitamin D 800 IU daily is recommended by the institute of Medicine (87), certainly in the winter months but may not be needed in summer in those who get sun exposure. High risk groups such as nursing home patients should take it daily.
Highlights.
➢ ET/HT is first choice for osteoporosis prevention in women < 10 years of menopause
➢ Bisphosphonates is first choice for osteoporosis In older women ≥ 60 years
➢ SERMs are an alternative for women who cannot take bisphosphonates or HT/ET
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 3.Hofbauer LC, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 4.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligandinduced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullamore JR, Wilkinson R, Gallagher JC, Nordin BE. Marshall DHEffect of age on calcium absorption. Lancet. 1970 Sep 12;2(7672):535–7. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- 6.Albright F, Smith PH, Richardson AM. Postmenopausal osteoporosis. JAMA. 1941;116:2465–2474. [Google Scholar]
- 7.Rapuri PB, Gallagher JC, Haynatzki G. Rapuri PB, Gallagher JC, Haynatzki G, editors. Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J Clin Endocrinol Metab. 2004 Oct;89(10):4954–62. doi: 10.1210/jc.2004-0434. [DOI] [PubMed] [Google Scholar]
- 8.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 9.Gail A Greendale MaryFran Sowers, Weijuan Han Mei-Hua Huang, Finkelstein Joel S, Crandall Carolyn J, Lee Jennifer S, Karlamangla Arun S. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women's Health Across the Nation (SWAN) J bone Min Res. 2012;27(1):111. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res. 1999 Sep;14(9):1614–21. doi: 10.1359/jbmr.1999.14.9.1614. [DOI] [PubMed] [Google Scholar]
- 11.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Collaborating Centre for Metabolic Bone Diseases [October 28, 2009];FRAXA WHO Fracture Risk Assessment Tool. Available at: http://www.shef.ac.uk/FRAX/.
- 13.Stone KL, Seeley DG, Lui LY, et al. for the Study of Osteoporotic Fractures Research Group BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 14.Siris ES, et al. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 15.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women.Arch Intern Med. 2006 Apr 24;166(8):869–75. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 16.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13(6):501–505. doi: 10.1007/s001980200061. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Writing Group for the Women's Health Initiative Investigators JAMA. 2002;288:321–33. [Google Scholar]
- 20.Sarrel PM, Njike VY, Vinante V, Katz DL. The Mortality Toll of Estrogen Avoidance: An Analysis of Excess Deaths Among Hysterectomized Women Aged 50 to 59 Years. Am J Public Health. 2013 Sep;103(9):1583–1588. doi: 10.2105/AJPH.2013.301295. Epub 2013 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson JC, Panay N, Pexman-Fieth C. Oral estradiol and dydrogesterone combination therapy in postmenopausal women: Review of efficacy and safety. Maturitas. 2013 Sep;76(1):10–21. doi: 10.1016/j.maturitas.2013.05.018. doi: 10.1016/j.maturitas.2013.05.018. Epub 2013 Jul 6. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D, Cranney A. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev. 2002 Aug;23(4):529–39. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher JC. Effect of estrogen on bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. American Society for Bone and Mineral Research; Washington, DC: 2006. pp. 327–330. [Google Scholar]
- 24.Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668–2676. doi: 10.1001/jama.287.20.2668. [DOI] [PubMed] [Google Scholar]
- 25.Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy.Fertil Steril. 2009 Sep;92(3):1018–24. doi: 10.1016/j.fertnstert.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 26.Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. AMA. 2001 Jun 13;285(22):2891–7. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 27.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women's Health Initiative randomized trial. JAMA. 2003;290(13):1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 28.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T. Women's Health Initiative Investigators. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: Results from the Women's Health Initiative randomized trial. J Bone Miner Res. 2006;21(6):817–28. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher JC, Fowler SE, Detter JR, et al. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001 Aug;86(8):3618–28. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 30.Greenspan SL, Resnick NM, Parker RA. Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA. 2003 May 21;289(19):2525–33. doi: 10.1001/jama.289.19.2525. [DOI] [PubMed] [Google Scholar]
- 31.Huang AJ, Ettinger B, Vittinghoff E, et al. Endogenous estrogen levels and the effects of ultra-low-dose transdermal estradiol therapy on bone turnover and BMD in postmenopausal women. J Bone Miner Res. 2007 Nov;22(11):1791–7. doi: 10.1359/jbmr.070707. [DOI] [PubMed] [Google Scholar]
- 32.Nieves JW, Komar L. Cosman F Calcium potentiates the effect of estrogen and calcitonin on bone mass: review and analysis. J Clin Endocrinol Metab. 2001 May;86(5):1890–7. doi: 10.1093/ajcn/67.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Eviö S, Tiitinen A, Laitinen K, et al. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. Am J Clin Nutr. 1998 Jan;67(1):18–24. doi: 10.1210/jc.2003-030198. [DOI] [PubMed] [Google Scholar]
- 34.Harris ST, Eriksen EF, Davidson M. Effect of combined risedronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2004 Feb;89(2):626–31. doi: 10.1210/jcem.86.5.7505. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. doi: 10.1016/j.fertnstert.2009.02.093. [DOI] [PubMed] [Google Scholar]
- 36.Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004 Nov-Dec;11(6 Pt 1):622–30. doi: 10.1097/01.gme.0000123641.76105.b5. [DOI] [PubMed] [Google Scholar]
- 37.Karim R, Dell RM, Greene DF, Mack WJ, et al. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. doi: 10.1097/gme.0b013e31821b01c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004 Nov-Dec;11(6 Pt 1):622–30. doi: 10.1097/01.gme.0000123641.76105.b5. [DOI] [PubMed] [Google Scholar]
- 39.Karim R, Dell RM, Greene DF, Mack WJ, et al. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. doi: 10.1097/gme.0b013e31821b01c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 41.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 42.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006;15:35, 44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- 43.McClung MR, Wasnich RD, Hosking DJ, et al. Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. J Clin Endocrinol Metab. 2004;89:4879–4885. doi: 10.1210/jc.2003-031672. [DOI] [PubMed] [Google Scholar]
- 44.Hooper MJ, Ebeling PR, Roberts AP, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebocontrolled trial. Climacteric. 2005;8:251–262. doi: 10.1080/13697130500118126. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen L, Charles P, Bekker PJ, et al. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998;83:396–402. doi: 10.1210/jcem.83.2.4586. [DOI] [PubMed] [Google Scholar]
- 46.McClung MR, Wasnich RD, Recker R, et al. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res. 2004;19:11–18. doi: 10.1359/JBMR.0301202. [DOI] [PubMed] [Google Scholar]
- 47.Tanko LB, Felsenberg D, Czerwinski E, et al. Oral weekly ibandronate prevents bone loss in postmenopausal women. J Intern Med. 2003;254:159–167. doi: 10.1046/j.1365-2796.2003.01174.x. [DOI] [PubMed] [Google Scholar]
- 48.McClung M, Miller P, Recknor C, et al. Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet Gynecol. 2009;114:999–1007. doi: 10.1097/AOG.0b013e3181bdce0a. [DOI] [PubMed] [Google Scholar]
- 49.Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 50.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 51.Harris ST, Watts NB, Genant HK, et al. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group Effects of risedronate treatment on vertebral and non-vertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 52.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 53.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 54.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27(2):243. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or inter- mittently on fracture risk in postmenopausal osteoporosis. 2004;J. Bone Miner. Res.;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 56.McClung MR, Geusens P, Miller PD, et al. Hip Intervention Program Study Group Effect of risedronate on the risk of hip fracture in elderly women. N. Engl. J. Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 57.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 58.Dima L, Diab, Watts Nelson B. Use of drug holidays in women taking bisphosphonates. NAMS practice pearl. doi: 10.1097/GME.0b013e31829ef343. [DOI] [PubMed] [Google Scholar]
- 59.Ott Susan M. What is the optimal duration of bisphosphonate therapy?. Cleaveland clinic journal of Medicine. doi: 10.3949/ccjm.78a.11022. [DOI] [PubMed] [Google Scholar]
- 60.Cranney A, Tugwell P, Zytaruk N, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IV. Meta-analysis of raloxifene for the prevention and treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:524. doi: 10.1210/er.2001-4002. [DOI] [PubMed] [Google Scholar]
- 61.Johnston CC, Jr, Bjarnason NH, Cohen FJ, et al. Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three-year data from 2 double-blind, randomized, placebo-controlled trials. Arch Intern Med;2000;160:3444. doi: 10.1001/archinte.160.22.3444. [DOI] [PubMed] [Google Scholar]
- 62.Jolly EE, Bjarnason NH, Neven P, et al. Prevention of osteoporosis and uterine effects in postmenopausal women taking raloxifene for 5 years. Menopause. 2003;10:337–344. doi: 10.1097/01.GME.0000058772.59606.2A. [DOI] [PubMed] [Google Scholar]
- 63.Ettinger B, Black DM, Cummings SR, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 64.Miller PD, Chines AA, Christiansen C. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008 Apr;23(4):525–35. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 65.Silverman SL, Chines AA, Kendler DL, et al. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int. 2012 Jan;23(1):351–63. doi: 10.1007/s00198-011-1691-1. [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Tsai KS, Kim GS. Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int. 2011 Feb;22(2):559–65. doi: 10.1007/s00198-010-1259-5. Epub 2010 Jun 10. [DOI] [PubMed] [Google Scholar]
- 67.Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010 Feb 25;362(8):686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 68.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 69.Bone HG, Chapurlat R, Brandi ML, et al. The Effect of 3 or 6 Years of Denosumab Exposure in Women With Postmenopausal Osteoporosis: Results From the FREEDOM Extension. J Clin Endocrinol Metab. 2013 Aug 26; doi: 10.1210/jc.2013-1597. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chesnut CH, 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000 Sep;109(4):267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 71. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Rep roductiveHealthDrugsAdvisoryCommittee/UCM341781.pdf.
- 72.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 73.Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 74.Eastell R, Nickelsen T, Marin F, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009;24:726. doi: 10.1359/jbmr.081215. [DOI] [PubMed] [Google Scholar]
- 75.Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 76.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 77.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 78.Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 79.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 80.Tsai JN, Uihlein AV, Lee H. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013 Jul 6;382(9886):50–6. doi: 10.1016/S0140-6736(13)60856-9. doi: 10.1016/S0140-6736(13)60856-9. Epub 2013 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McClung MR, Balske A, Burgio DE, et al. Treatment of postmenopausal osteoporosis with delayed-release risedronate 35 mg weekly for 2 years. doi: 10.1007/s00198-012-2175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Binkley N, Bolognese M, Sidorowicz-Bialynicka A. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. doi: 10.1002/jbmr.1602. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 84.Murphy MG, Cerchio K, Stoch SA, et al. Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2005;90:2022. doi: 10.1210/jc.2004-2126. [DOI] [PubMed] [Google Scholar]
- 85.Bone HG, McClung MR, Roux C, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 86.Eastell R, Nagase S, Ohyama M, et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J Bone Miner Res. 2011;26:1303. doi: 10.1002/jbmr.341. [DOI] [PubMed] [Google Scholar]
- 87.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]