Abstract
Milk protein allergy–induced reactions from lactose-containing dry powder inhalers (DPIs) have not been widely described in the literature. Lactose is a common inactive ingredient in many pharmaceutical products that is used to enhance the stability of active substances in medicinal products, including asthma medications. Contamination of lactose with milk proteins has been identified in reports of inhaled corticosteroid product lot testing. Serious respiratory sequelae may follow after the inhalation of a DPI corticosteroid in a patient with milk protein allergy because DPIs that contain lactose may be contaminated with milk proteins. Lactose-containing DPIs are contraindicated in patients with milk protein allergy. Although manufacturers identify this contraindication in product package inserts, some drug references may not include this information and health care professionals may lack awareness. Clinicians should consider reviewing multiple medication resources for warnings and contraindications of medications to prevent complications. We describe a refractory asthma exacerbation secondary to a hypersensitivity reaction following administration of a lactose-containing DPI corticosteroid and long-acting β2 agonist combination in a child with a milk protein allergy.
INDEX TERMS: asthma, dry powder inhalers, lactose, milk protein, pediatrics
INTRODUCTION
Inhaled corticosteroids (ICSs) are first-line asthma maintenance therapy in children and adults as recommended by the National Heart, Lung, and Blood Institute asthma guidelines.1 ICSs are available as a dry powder inhaler (DPI) and as a hydrofluoroalkane (HFA) formulation for single agents or for combined therapy with long-acting β2 agonists. DPI formulations may include a stabilizing lactose excipient. Lactose, found in milk, is a common inactive ingredient in some asthma medications and is used to enhance the stability of active substances in medicinal products. Literature and manufacturer labeling of lactose-containing DPIs indicate purification processes have resulted in lactose contamination with milk proteins2 that may result in further complications if inhaled by patients with milk protein allergy. DPI manufacturer package inserts include reports of anaphylactic reactions in patients with milk protein allergies and list milk protein allergy as a contraindication, but this reaction is not discussed in all medication resources. Only one case report exists in the literature that describes a pediatric patient with persistent asthma who developed anaphylaxis after treatment with a lactose-containing DPI.3 This child with severe milk protein allergy previously received the same lactose-containing DPI without complications, but a new lot of the product resulted in a reaction. To our knowledge, we present the first refractory asthma exacerbation in a child with milk protein allergy resulting from a hypersensitivity reaction following the administration of a combination fluticasone and salmeterol DPI (Advair Diskus, GlaxoSmithKline, Research Triangle Park, NC).
CASE
A 9-year-old African American boy with a history of moderate persistent asthma presented to the emergency department with a complaint of shortness of breath. He required admission for an asthma exacerbation. Patient triggers included a sick contact with an upper respiratory infection and a recent weather change, causing windy and dusty conditions in West Texas. Past medical history was significant for moderate to severe persistent asthma, diagnosed at the age of 1 year by his primary care physician based on clinical symptoms, and a strong family history of asthma in both parents and two siblings. His home asthma regimen included albuterol sulfate via a handheld nebulizer as needed. He was on nebulized budesonide as a younger child but had not been on maintenance therapy for the last few years. His parents reported his asthma had not been a problem for the period he was off his controller medication and only used albuterol a couple of times per week. However, assessment of his symptoms would later reveal that his asthma was more severe than the family recognized. The family listed no known drug allergies, but they did identify a food allergy to milk and indicated that it caused problems with his asthma when he was a toddler.
Following the review of his clinic chart, we found the patient presented as a young infant with irritability and blood in his stool. He was diagnosed with a milk protein allergy and was treated by changing his formula to a hydrolyzed formula (Similac Expert Care Alimentum, Abbott Nutrition, Columbus, Ohio). He also had eczema, and it was noted that his asthma and eczema would flare for several days following ingestion of products containing milk. He had no history of seeing an allergist, and no formal testing of a milk allergy protein had been pursued as is typical with milk protein allergy. No previous adverse reactions to inhaled asthma medications were documented.
Prior to admission for his asthma exacerbation, the patient received albuterol sulfate with an ipratropium bromide treatment via nebulizer in the emergency department. Intravenous methylprednisolone was also administered using a dose of 2 mg/kg per dose. Oxygen saturation was 94% on room air; however, his lung exam revealed poor air movement and tachypnea with increased work of breathing. On the pediatric ward, we initiated nebulized albuterol sulfate every 3 hours, and prednisolone liquid at a dosage of 1 mg/kg/day. His condition improved; physical examination revealed increased air movement, and his albuterol treatments were to be spaced to every 4 hours. After further evaluation of the patient’s severe chronic asthma symptoms, we initiated a maintenance regimen to include an ICS and a long-acting β2 agonist combination device in preparation for discharge planning. We chose to start this maintenance therapy in the hospital to teach the family about asthma and demonstrate the use of the device to be included in his routine at home. He continued to receive prednisolone to treat his exacerbation. At the time of his hospitalization, the combination product readily available was Advair Diskus DPI, with the recent introduction of Advair HFA (Glaxo-SmithKline) with the same active ingredients. Initially, Advair Diskus DPI was ordered for that evening, and upon clarification of the patient’s milk protein allergy, the order was changed to the Advair HFA product. We notified the family of the required change in medication and the rationale. However, the pharmacy had already delivered the Advair Diskus to the pediatric ward and the patient received one dose in the early afternoon prior to the change of the order. He developed subsequent chest tightness over the next few hours without requiring oxygen therapy, and required an increased frequency of albuterol treatments to every 1 to 2 hours for the next 12 hours. That evening he correctly received the HFA formulation.
On the second day, we initiated azithromycin suspension for possible Mycoplasma pneumoniae when his condition did not improve. Albuterol treatments were spaced to every 3 hours. On the third day, his prednisolone dosage was increased to 2 mg/kg/day and his albuterol remained at every 3 hours. However, this did not improve his pulmonary status. He continued to have problems with poor air movement, and 1 day later we consulted pulmonology, and oral steroids were increased to 4 mg/kg/day. Albuterol sulfate treatments remained at every 3 hours. An outpatient allergy workup was recommended. He remained in the hospital for 2 additional days and then met criteria for discharge home with nebulized albuterol treatments spacing to every 4 hours. At discharge, the patient had no further episodes of hypersensitivity reaction following the administration of the HFA formulation. During the 6-day hospital stay he remained clinically stable but became refractory to treatment following the DPI dose. He required an increased frequency of albuterol treatments, with an inability to extend the treatment interval. This case prompted us to investigate why an asthma exacerbation that was improving as expected turned into a prolonged hospitalization for this child. At the same time, it provided awareness of this problem for all health care professionals in our hospital and led to the close following of patients with milk allergies.
DISCUSSION
Food allergies affect about 6% of pediatric patients,4 with some allergies persisting into adulthood, and eggs, peanuts, and milk result in the majority of allergic reactions in young children.5 Milk allergy rates account for 3% of food allergies in people of all ages as self-reported symptoms and are described as the highest in comparison with peanut, egg, fish, and crustacean shellfish allergies.5 Milk protein allergies may be classified into type I or type IV reactions. The severity of type I or immunoglobulin E–mediated allergic reactions is attributed to the quantity of the allergen, the route (inhalation, ingestion, or injection) of the exposure, and the duration of treatment, as seen with drug allergies,6 with larger volumes of allergens increasing the severity of the reaction.7 Clinical manifestations generally start within minutes and may include skin rashes, vomiting, or airway swelling with difficulty breathing requiring removal of the offending agent, and possible pharmacological intervention.5,8 Milk protein allergy is often caused by a delayed hypersensitivity reaction, which is a type IV or cell-mediated allergic reaction.
The cell-mediated response results in damage to intestinal cells. Damage to stomach lining results in allergies to antigens, as is seen with milk protein allergy. Milk protein allergy often causes abdominal pain, blood in the stool, irritability, and skin rash, and may require more steroid use in asthma.5 The diagnosis is made clinically and treatment involves removing milk protein from the diet. Milk protein allergy is often confused with lactose intolerance. Lactose intolerance is not an allergic reaction.5 Individuals with lactose intolerance have problems digesting foods containing lactose because of a deficiency in lactase, an enzyme found throughout the small intestine. After consuming foods containing lactose, patients present with diarrhea, bloating, and abdominal pain. Treatment involves either avoiding these offending foods or replacing the enzyme using an oral supplement. In this case, the patient developed complications following a dose of a lactose-containing DPI because of a type IV hypersensitivity reaction. After a review of the literature, no other similar cases were found in the literature.
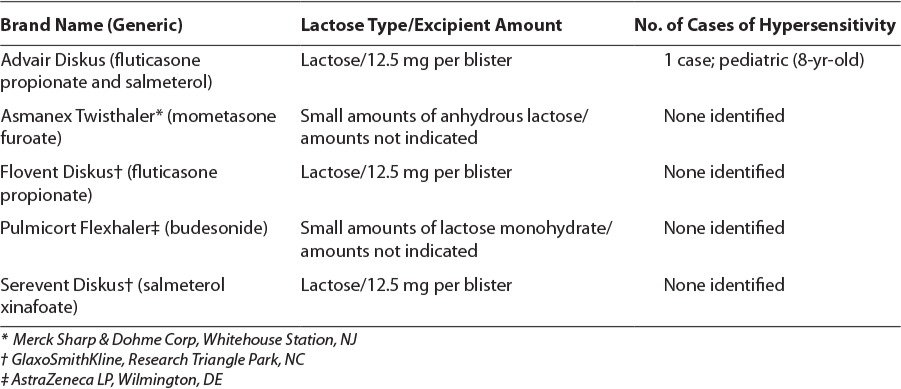
A search of PubMed, the Database of Abstracts of Reviews of Effects (DARE), and Google Scholar through November 2013 using the search terms “asthma,” “lactose,” “anaphylaxis,” “hyper-sensitivity reaction,” “milk protein,” and “dry powder inhaler” revealed one letter to the editor case report3 involving shortness of breath after consecutive doses of inhaled Advair Diskus. A comparable search on http://www.google.com provided reports, journal search results, child health alert, blogs, and concerns of lactose-containing DPIs in asthma. Reports of anaphylaxis or hypersensitivity reactions associated with lactose in food additives, occupational environments, lactulose, and intravenous methylprednisolone are found in the literaure.3,9–12 In the Table, we present a review of asthma medications in the United States that contain lactose.13–17 A total of 5 of the 17 inhaled asthma medications contain lactose, all of which are DPIs. Lactose is also found in Spiriva HandiHaler (Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT), Foradil Aerolizer (Merck & Co Inc, Whitehouse Station, NJ), and some Canadian product formulations.
Table.
Nowak-Wegrzyn et al18 provided a report of milk protein contamination in DPIs and brought awareness to the lack of broad contraindications or warnings in package inserts for individuals with milk protein allergies. Their abstract details the evaluation of 4 lactose-containing DPIs (two lots of each product) for milk proteins, with the detection of milk proteins in all product lots. The authors recommended future clinical challenge studies to determine the significance of lactose-containing DPI milk protein contamination and suggest that food allergens may induce acute bronchospasms. Lactose purity may vary between medication lots among manufacturers, requiring caution in susceptible patients.
Nowak-Wegrzyn and colleagues3 provide a case report of milk protein–contaminated DPIs in an 8-year-old patient with severe milk protein allergy and persistent asthma. The patient was treated with Advair Diskus for several months without any complications. Following 3 consecutive doses from a new product lot, the patient developed chest tightness and distress, requiring treatment with diphenhydramine and an inhaled bronchodilator. A product challenge resulted in chest tightness and hypotension, requiring treatment with epinephrine, diphenhydramine, and prednisone. The patient was subsequently initiated on the single-agent formulations without complications. This letter to the editor suggests lactose purity may vary between medication lots among manufacturers, requiring caution in susceptible patients.
Despite the reports of reactions following the use of lactose-containing DPIs in milk protein allergy, there is evidence that suggests patients with a milk allergy had no reported reaction following the use of a lactose-containing DPI.19 Spiegel and Anolik19 performed a chart review of more than 8000 asthma patients and identified 278 who were allergic to milk. Of these milk-allergic patients, 21 patients of undefined age received treatment with a lactose-containing DPI. Evaluation of more than 700 months of treatment with a DPI indicated no reaction following the use of a lactose-containing DPI. The authors indicated the amount of milk protein contamination might have been minimal, thereby avoiding a reaction in their small population. Spiegel and Anolik19 suggest that despite a rare occurrence of milk protein reactions, the avoidance of DPIs should not occur, but rather close observation of the patient is indicated.
Maiello et al9 reported a severe allergic reaction with a lactose-containing oral lactulose solution in a 4-year-old patient with milk protein allergy. Dry cough, similar to that experienced during an asthma exacerbation, occurred without other symptoms. Subsequently, a lactulose challenge of the patient produced a cough with wheezing, oral itching, diffuse erythema with conjunctival hyperemia, and sneezing. The patient received cetirizine and albuterol, with a resolution of symptoms 3 hours later. Oral asthma medications containing lactose include montelukast, zafirlukast, prednisone, and prednisolone tablet formulations, with the exception of Orapred Orally Disintegrating Tablet (Shionogi Inc, Florham Park, NJ), that may cause an allergic reaction and may warrant caution in patients with milk protein allergy.
To our knowledge, our case report is the first to present a refractory asthma exacerbation in a child with a milk protein allergy resulting from hypersensitivity following the administration of a combination fluticasone and salmeterol DPI. In our patient’s case, the onset of hypersensitivity occurred after the DPI dose, with subsequent symptoms of shortness of breath. Prior to that dose, he had demonstrated improvement. After the DPI dose, his asthma became refractory to treatment. Following a review of his medication documentation, we concluded a probable adverse drug reaction using the Naranjo20 probability scale (score 6) following the administration of the DPI formulation in place of the HFA, with a resultant hypersensitivity reaction, and the refractory asthma exacerbation with a prolonged hospital course. No other possible etiologies were identified for the refractory asthma exacerbation. We avoided rechallenge with the DPI formulation because it was considered potentially dangerous and unnecessary given the availability of the HFA formulation. Following the event we informed the patient’s parents and hospital staff of the administration of the DPI and educated everyone to avoid DPIs in the future. Five years later the family members have continued their diligence and avoided the use of lactose-containing DPI inhalers, preventing similar complications. He still cannot tolerate milk protein, and his family relocated prior to allergy workup in our city.
Anaphylactic reactions from lactose-containing DPIs are described in manufacturer package inserts, but currently the incidence of anaphylaxis or hypersensitivity reactions is unknown. Package inserts provide warnings, contraindications, patient education, and lactose dosage contents for health care professionals. Despite this information being present in manufacturer materials, quick access summary drug resources commonly used by medical professionals do not contain this information, or it requires the review of several areas within the reference to identify the information. This omission can create distress and confusion among parents and physicians.
CONCLUSION
This case highlights that patients with milk protein allergy and asthma require careful assessment of medication options. Clinicians should consider multiple medication resources for reviewing warnings and contraindications of medications to prevent complications. Greater awareness of the contraindication of DPIs in milk protein allergy is important for health care professionals, including physicians, practitioners, pharmacists, nurses, and respiratory therapists. Those regularly prescribing DPIs in asthma patients should be aware of milk protein allergies that may result in hypersensitivity reactions. Lactose-containing inhaled medications should not be administered to patients with milk protein allergies.
ACKNOWLEDGMENT
We would like to thank Lara W. Johnson, MD, MHS, for the medical editorial assistance with this manuscript.
ABBREVIATIONS
- DPI
dry powder inhaler
- HFA
hydrofluoroalkane
- ICS
inhaled corticosteroids
Footnotes
Disclosure The authors declare no conflicts or financial interests in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.National Heart, Lung, and Blood Institute. Expert panel report 3 (EPR3): guidelines for the diagnosis and management of asthma. https://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. Accessed September 21, 2013.
- 2.Edge S, Mueller S, Price R et al. Factors affecting defining the quality and functionality of excipients used in the manufacture of dry powder inhaler products. Drug Dev Ind Pharm. 2008;34(9):966–973. doi: 10.1080/03639040802192814. [DOI] [PubMed] [Google Scholar]
- 3.Nowak-Wegrzyn A, Shapiro GG, Beyer K et al. Contamination of dry powder inhalers for asthma with milk proteins containing lactose. J Allergy Clin Immunol. 2004;113(3):558–560. doi: 10.1016/j.jaci.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Rona RJ, Keil T, Summers C et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Boyce JA, Assa'ad A, Burks AW et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(suppl 6):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology: Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez DA, Bahna SL. Food hypersensitivity by inhalation. Clin Mol Allergy. 2009;7:4. doi: 10.1186/1476-7961-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spergel JM, Pawlowski NA. Food allergy: mechanisms, diagnosis, and management in children. Pediatr Clin North Am. 2002;49(1):73–96. vi. doi: 10.1016/s0031-3955(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 9.Maiello N, Del Giudice MM, Capristo C et al. Severe allergic reaction to lactulose in a child with milk allergy. Ann Allergy Asthma Immunol. 2011;107(1):85. doi: 10.1016/j.anai.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Savvatianos S, Giavi S, Stefanaki E et al. Cow's milk allergy as a cause of anaphylaxis to systemic corticosteroids. Allergy. 2011;66(7):983–985. doi: 10.1111/j.1398-9995.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 11.Eda A, Sugai K, Shioya H et al. Acute allergic reaction due to milk proteins contaminating lactose added to corticosteroid for injection. Allergol Int. 2009;58(1):137–139. doi: 10.2332/allergolint.C-07-59. [DOI] [PubMed] [Google Scholar]
- 12.Morisset M, Moneret-Vautrin D, Commun N et al. Allergy to cow milk proteins contaminating lactose, common excipient of dry powder inhalers for asthma. J Allergy Clin Immunol. 2006;117(2):S95. [Google Scholar]
- 13.Advair Diskus (fluticasone and salmeterol) [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2011. [Google Scholar]
- 14.Asmanex Twisthaler (mometasone) [package insert] Whitehouse Station, NJ: Merck Sharp & Dohme Corp; 2013. [Google Scholar]
- 15.Flovent Diskus (fluticasone) [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2011. [Google Scholar]
- 16.Pulmicort Flexhaler (budesonide) [package insert] Wilmington, DE: AstraZeneca LP; 2010. [Google Scholar]
- 17.Serevent Diskus (salmeterol) [package insert] Research Triangle Park, NC: Glaxo-SmithKline; 2012. [Google Scholar]
- 18.Nowak-Wegrzyn AH, Bardina L, Beyer K et al. Detection of milk proteins in dry powder inhalers containing lactose. J Allergy Clin Immunol. 2002;109(1):S258–S259. doi: 10.1016/j.jaci.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel WA, Anolik R. Lack of milk protein allergic reactions in patients using lactose containing dry powder inhalers (DPIs) J Allergy Clin Immunol. 2010;125(2):AB69. [Google Scholar]
- 20.Naranjo CA, Busto U, Sellers EM et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


