Abstract
SUMMARY
The pathogenicity and clinical pertinence of diffusely adhering Escherichia coli expressing the Afa/Dr adhesins (Afa/Dr DAEC) in urinary tract infections (UTIs) and pregnancy complications are well established. In contrast, the implication of intestinal Afa/Dr DAEC in diarrhea is still under debate. These strains are age dependently involved in diarrhea in children, are apparently not involved in diarrhea in adults, and can also be asymptomatic intestinal microbiota strains in children and adult. This comprehensive review analyzes the epidemiology and diagnosis and highlights recent progress which has improved the understanding of Afa/Dr DAEC pathogenesis. Here, I summarize the roles of Afa/Dr DAEC virulence factors, including Afa/Dr adhesins, flagella, Sat toxin, and pks island products, in the development of specific mechanisms of pathogenicity. In intestinal epithelial polarized cells, the Afa/Dr adhesins trigger cell membrane receptor clustering and activation of the linked cell signaling pathways, promote structural and functional cell lesions and injuries in intestinal barrier, induce proinflammatory responses, create angiogenesis, instigate epithelial-mesenchymal transition-like events, and lead to pks-dependent DNA damage. UTI-associated Afa/Dr DAEC strains, following adhesin-membrane receptor cell interactions and activation of associated lipid raft-dependent cell signaling pathways, internalize in a microtubule-dependent manner within urinary tract epithelial cells, develop a particular intracellular lifestyle, and trigger a toxin-dependent cell detachment. In response to Afa/Dr DAEC infection, the host epithelial cells generate antibacterial defense responses. Finally, I discuss a hypothetical role of intestinal Afa/Dr DAEC strains that can act as “silent pathogens” with the capacity to emerge as “pathobionts” for the development of inflammatory bowel disease and intestinal carcinogenesis.
INTRODUCTION
Human Escherichia coli strains are classified as commensal microbiota E. coli, enterovirulent E. coli, and extraintestinal pathogenic E. coli (ExPEC) on the basis of their genetic features and clinical outcomes (1). Their serotypes are based on virulence factors present in small or large virulence-associated plasmids or chromosomal pathogenicity islands (PAIs) (2) and the molecular and cellular mechanisms by which the intestinal disease is thought to be provoked. For the pathogenic enteric E. coli strains, six pathotypes, i.e., enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and diffusely adhering E. coli (DAEC), were first defined by James P. Nataro and James B. Kaper (3). Recently (4, 5), a seventh group of enteric E. coli strains has been defined, the Crohn's disease-associated adherent-invasive E. coli pathotype (AIEC) (6), which have particular mechanisms of pathogenesis (7). It is noticeable that, distinct from enterovirulent E. coli in expressing particular virulence determinants and developing pathogenesis in extraintestinal tissues, ExPEC strains include uropathogenic E. coli (UPEC) (8), sepsis-associated E. coli (SEPEC) (9), and neonatal meningitis-associated E. coli (NEMEC) (10).
The diffusely adherent E. coli (DAEC) class of pathogenic E. coli (1, 3) was previously subdivided into two subclasses: DAEC expressing Afa/Dr adhesins (Afa/Dr DAEC) and DAEC not expressing Afa/Dr adhesins (11). The subclass of DAEC that does not express Afa/Dr adhesins has recently evolved. Indeed, the main member of this subclass, i.e., the diarrhea-associated DAEC expressing the aidA gene, encoding an adhesin involved in diffuse adherence (AIDA-I) (12–15), belongs to the newly defined second class of EPEC designated “atypical EPEC” (aEPEC) since it is eae positive. The EPEC class of enterovirulent E. coli has been recently subdivided into two subclasses: typical EPEC (tEPEC) and atypical EPEC (aEPEC) (4). The aEPEC subclass (16) comprises eae-positive strains that express a wide range of genes, such as aida-1, fimA, ecpA, csgA, elfA, hcpA, and lda, which code for known adhesive factors triggering localized adherence-like (LAL), DA, or aggregative (AA) patterns of adhesion, and that do not express-bundle forming pili (BFP), a type IV pilus encoded by the EPEC adherence factor (EAF) plasmid (pEAF), which allows interconnection between bacteria within the dense microcolonies that form the localized adhesion (LA) pattern of tEPEC.
Afa/Dr DAEC strains are associated with urinary tract infections (UTIs), pregnancy complications, and diarrhea in children of ages 18 months to 5 years, but they can also be asymptomatic intestinal microbiota strains in children and adults (11, 17). Five phylogenetic groups, including the main phylogenetic groups A, B1, B2, and D, have been identified in Gram-negative species using multilocus enzyme electrophoresis and sequence typing methods. Afa/Dr DAEC strains belong to the phylogenetic B2 group (18, 19). In commensal E. coli from humans (in Europe, the United States, Australia, and Japan), B2 group E. coli strains are predominant (20), and it is noteworthy that these E. coli strains displayed a high capacity to colonize epithelia (21–23). The name “Afa/Dr DAEC” was proposed in 2005 to define a family of human UTI- or diarrhea-associated clinical E. coli isolates harboring adhesins encoded by the afa (24–28), dra (29, 30), and daa (31, 32) operons, having a similar genetic organization and displaying a similar receptor specificity for human decay-accelerating factor (hDAF) and members of the family of human carcinoembryonic antigen cell adhesion molecules (hCEACAMs) (11). It is important to note that the name “Dr family” has been used by Bogdan Nowicki and coworkers as dictated by the receptor specificity of Afa, Dr, and F1845 adhesins for the Dr blood group antigen (33, 34). In this review, I summarize recent advances in our understanding of Afa/Dr DAEC pathogenesis in the urinary and intestinal tracts by analyzing how the Afa/Dr DAEC virulence factors contribute to cause disease in humans.
EPIDEMIOLOGY
Detection
In order to detect E. coli bearing Afa/Dr adhesins, phenotype and genotype methods have been developed. Scaletsky et al. (35) and Nataro et al. (36), investigating the adhesion of diarrheagenic E. coli onto cultured, nonintestinal, undifferentiated epithelial Hep-2 and HeLa cells, were the first to observe three specific patterns of adhesion: diffuse adherence (DA), resulting in adherent bacteria being randomly distributed on all the whole cell surface; localized adherence (LA), where adherent bacteria form organized microcolonies randomly distributed on the cell surface; and aggregative adherence (AA), in which adherent bacteria form typical “stacked-brick” microcolonies randomly distributed on the cell surface. However, this cell adhesion assay is not suitable for the detection of enteric Afa/Dr DAEC, since several aEPEC strains also developed a DA pattern of adhesion (16). Moreover, DA adhesion onto Hep-2 or HeLa cells has been also observed for UPEC strains expressing Afa-I (37), Afa-III (28), and Dr (38).
Goluszko et al. (39) have proposed a HeLa cell receptor assay designated the diffuse clustering assay (DCA), which associates the cell diffuse adhesion of Afa/Dr DAEC with the property of Afa/Dr adhesins to promote hDAF receptor clustering around adhering bacteria (40–42). However, the DCA did not detect all the E. coli strains bearing Afa/Dr adhesins, considering that AfaE-VII and AfaE-VIII adhesins did not recognize hDAF (26). This is a particular drawback for the detection of human AfaE-VIII adhesin-positive ExPEC strains (43–45). Moreover, the DCA could give overestimated results since several aEPEC strains have been found to be daaC positive (13, 46–53).
To detect daaC, daaE, afaB, and afaC sequences, probes and PCR primers have been developed (32, 45, 54, 55). DNA probes included the following: drb (56), a 260-bp PstI fragment of the pIL14 plasmid (afa-1 operon) coding for the AfaE-I adhesin of uropathogenic Afa/Dr DAEC KS52 strain (25); daaC, a 300-bp PstI fragment of the plasmid pSSS1 daa operon (57); a 390-bp I fragment of the pSLM852 daa operon (58); a DNA fragment homologous to daaE (59); and a probe designed from the M030 sequence found to be specifically present in wild-type, diarrhea-associated Afa/Dr DAEC strain C1845 (60, 61).
PCR approaches have been developed, including primers designed to amplify a 750-bp fragment of the afaB gene (62) and the 390-bp PstI fragment of the pSLM852 daa operon (63). Others PCR assays have been developed to detect all the Afa/Dr adhesins, including the afa1 and afa2 primers designed on the partial sequence of the afa-1 gene operon overlapping the afaB and afaC genes (54), primers for afaE-I, afaE-II, afaE-III/draE, and afaEV (64); and primers afa-f and afa-r, which flanked a 672-bp DNA segment internal to the afaC gene of the afa-3, afa-7, and afa-8 operons. Yamamoto et al. (65) established a multiplex PCR to detect UPEC-associated genes, including afa genes. Multiplex PCRs to detect UPEC-associated genes have been described, including afa1 (65) or genes expressed by diarrheagenic E. coli, including daaD (66–68).
The specificity of the daaC probe for the characterization of diarrheagenic Afa/Dr DAEC has recently been questioned. Smith et al. (69) found that 80 of the 86 EAEC strains positive for the AEAC probe (1-kb EcoRI-PstI fragment from pCVD432) (70) also hybridized with the probe derived from the daaC gene. Gomes et al. (71) reported that 5 of the 197 daaC-positive E. coli isolates hybridized with the AAEC probe (eaeA). Recently, Snelling et al. (72) have revealed that the daaC probe cross-reacts with strains belonging to EAEC. This is due to 84% identity between the daaC locus and the EAEC fimbria II cluster gene aafC at the nucleotide level (73). Moreover, Montiero et al. (74), in a study investigating the presence of dispersin in pathogenic and nonpathogenic intestinal E. coli isolates, observed the presence of agg3C-positive E. coli strains despite the absence of expression of the pilin-encoding gene agg3A and have suggested that the agg3C primers may have cross-reacted with an Afa/Dr usher-encoding gene(s). Indeed, the biogenesis of the well-characterized EAEC adhesins AAF-I, -II, and -III and Hda (75–78) involved a periplasmic chaperone, an outer membrane usher protein, a major adhesin subunit, and a capping subunit (79–81). Afa/Dr DAEC and EAEC strains are cause of diarrheal illness in young children. The cross-reaction makes it difficult to establish a clear identification of Afa/Dr DAEC in relation to diarrhea, notably in regions of the world in which EAEC and Afa/Dr DAEC strains are known to be responsible for acute diarrhea in children. It is obvious that new PCR probes that are more specific for diarrhea-associated Afa/Dr DAEC are called for future epidemiological studies. Blanc-Potard et al. (60) have identified M030, S109, and S111 sequences in the diarrhea-associated, wild-type strain C1845. These sequences are highly widespread (77 to 80%) among Afa/Dr strains, but have low prevalence (12 to 23%) in non-Afa/Dr strains. Additionally, analysis shows that only the M030, S109, S111, and S164 sequences are present in diarrhea-associated Afa/Dr DAEC strains and absent from non-Afa/Dr ECOR strains and diarrhea-associated clinical isolates (60). Moreover, M030 positivity has been found in human enteric DAEC isolates belonging to phylogroups A, B2, and D (61). In contrast, M030 positivity has been found to be absent in ETEC, EAEC, EPEC, and EHEC isolates (61). Epidemiological studies associating probes designed from these sequences and associating probes specific for EAEC remain to be conducted in areas such as Latin America, where Afa/Dr DAEC and EAEC have been found to be prevalent in children with acute and persistent diarrhea illness (see below).
Urinary Tract Infections
The role of UPEC expressing Afa/Dr adhesins in recurrent UTIs has been clearly established (82, 83). afaBC/daaC positivity has been found in UPEC strains belonging to the B2 phylogroups (61). Epidemiological studies show that E. coli isolates expressing Afa/Dr adhesins are involved in cystitis in children (25 to 50%) and pyelonephritis in pregnant women (30%) (34, 53, 55–57, 84–93). In addition, these pathogenic E. coli strains cause UTIs in pregnant women (30, 90, 91, 94–97). In patients with a first UTI, the presence of E. coli isolates expressing Afa/Dr adhesins leads to an elevated occurrence of a second UTI (56, 57, 64, 98, 99). In patients with pyelonephritis, there was a variable distribution of afaE subtypes in afa-positive strains (100). Zhang et al. (64) have found that UTI-associated and fecal E. coli isolates were afaE1 positive (18%), afaE2 positive (1.3%), afaE3 positive (1.3%), draE positive (12%), daaE positive (1.3%), and draE-afaE3 hybrid positive (12%). Some human pyelonephritis E. coli isolates have been found to be positive for afa1-afa2 and afa-f-afa-r PCR probes and in some cases to express the afaE1 (5%), afaE8 (39%), and afaEX (20%) operons (45). A UTI-associated E. coli strain generally expresses a multiplicity of adhesive factors. Foxman et al. (92), analyzing E. coli strains isolated from women with first-time UTIs, observed that drb-probe positive E. coli isolates displayed positivity with the type 1 pilus probe (80 to 100% positivity) and the P fimbria probe (50% positivity) and no positivity for the S fimbria probe. Szemiako et al. (101) have observed that combinations of genes encoding two adherence factors (P and Dr fimbriae or S and Dr fimbriae) in UTI-associated E. coli isolates result in an increased risk of translocation to the vascular system, leading to bacteremia. Moreover, daaC-positive, UTI-associated clinical isolates have been found to express aerobactin (89), hemolysin (56, 86, 89, 90, 92, 102, 103), and cytotoxic necrotizing factor (CNF) (19, 56, 89, 92). The same numbers of strains expressing the drb probe have been found in isolates from the urinary tract or rectum of women with UTIs (104).
Afa/Dr adhesins are frequently found in E. coli associated with pyelonephritis in pregnant women with gestational complications (30, 62, 90, 96, 97). In addition, Afa/Dr DAEC strains are associated with preterm labor/birth (82, 95, 105). Sledzinska et al. (97) have reported the presence of an E. coli strain harboring the combination of P and Dr fimbriae in a case of fatal sepsis in a pregnant woman who developed pyelonephritis.
Diarrhea
It is well established that pathogenic ETEC, tEPEC, aEPEC, and AIEC colonize the small intestine, EAEC colonizes small intestine and/or colon, EHEC colonizes the distal ileum and colon, and EIEC colonizes the colon (4, 5). In contrast, the intestinal site(s) colonized by diarrhea-associated Afa/Dr DAEC currently remains to be determined. There is an absence of a role of Afa/Dr DAEC in diarrhea in adults. Indeed, when the wild-type strain C1845 was inoculated in adult volunteers, none of the patients developed diarrhea, despite the strain being detected in duodenal cultures and stools (106). Moreover, examination of a large number of human diarrhea-associated DAEC strains has shown that only two carried the daaE gene, suggesting that the F1845 fimbria is rare among diarrheagenic DAEC strains (59). In addition, epidemiological studies in various areas of the world are inconclusive with regard to a role of daaC-positive E. coli strains in diarrhea in children or adults (13, 48, 50, 99, 107–115). However, the relationship between Afa/Dr DAEC and diarrhea in children as a function of age has been more convincingly demonstrated in age cross-sectional studies showing an increased incidence in children <1 to 5 years of age. These studies were conducted in the United States (116), Mexico (117), and different South American countries, including Chile (118), Brazil (58, 71, 119–121), Colombia (122), Peru (123–127), and Argentina (128), as well as in Thailand (50), Bangladesh (129), Japan (119), New Caledonia (45, 63, 130), various places in Africa (131–133), and European countries, including the United Kingdom (134) and France (135, 136). In Afa/Dr DAEC strains isolated from stools of children, the sat gene has been found in almost half of the diarrhea-associated Afa/DrDAEC strains and was not present in all the non-diarrhea-associated Afa/DrDAEC strains (137). Why and how Afa/Dr DAEC isolates are potential pathogens in children with an age-dependent occurrence remain to be determined. A possible explanation, but one that does not exclude other possible causes, is that in children in this age range, the intestinal epithelial barrier is not structurally and functionally mature, and therefore the strong host defense responses against infection by Afa/Dr DAEC, which are described below, are not yet functional.
The observation of daaC positivity of some aEPEC strains is indicative that Afa/Dr adhesins are expressed by enterovirulent E. coli strains other than Afa/Dr DAEC (13, 46–53). Moreover, E. coli isolates displaying daaC and afaBC positivities have been found among AIEC, inflammatory bowel disease (IBD)-associated, and intestinal cancer-associated (6, 138–140) strains.
Intestinal Asymptomatic Portage
Most of the epidemiological studies conducted in various areas of the world that were intended to identify Afa/Dr DAEC as cause of diarrhea in children over 5 years of age and in adults were inconclusive, as the same numbers of daaC-positive strains were found in cases and controls (13, 48, 50, 71, 99, 107–115, 141). This observation highlights the existence of asymptomatic carriers of intestinal Afa/Dr DAEC strains and suggests that these pathogenic E. coli strains can be tolerated or controlled if the mature intestinal epithelial barrier is in a healthy condition.
VIRULENCE FACTORS
Afa/Dr Adhesins
The processes by which epithelia are infected by pathogenic E. coli start by the attachment of bacteria to specific host cells. To do this, pathogenic bacteria express a wide variety of surface-exposed adhesins responsible for specific binding to structural or functional cell membrane-associated molecules (142). The attachments onto the target host cells allow enteric and urinary tract bacterial pathogens to resist clearance by peristalsis and micturition, respectively. The bacterial adhesion to target host cells can be more than a simple attachment due to pathogen-specific recognition of host cell membrane-associated molecules, since several of these molecules functioned intrinsically as signaling molecules or after recognition/activation recruited cytosolic signaling molecules (143). Attachment by fimbrial or afimbrial structures allows bacterial pathogens to interact with the host cell membrane to ensure the optimal delivery of their cytotonic or cytotoxic toxins in the vicinity of their membrane-associated receptors, triggering signaling events that affect transport/secretion functions or the cell structural organization. For other pathogenic bacteria, adhesive factors allow the intimate association of bacteria with the cell membrane that is necessary for the initiation and completion of signaling-controlled structural lesions, which in turn dramatically impair host cell functions. For invasive bacterial pathogens, attachment initiates an orderly series of signaling-controlled events that lead to host cell membrane rearrangements that are necessary for the achievement of bacterial cell entry followed by the development of sophisticated bacterial intracellular lifestyles.
Two major classes of adhesins are present on the bacterial surface of Gram-negative pathogens: the fimbrial adhesins, consisting of linear homopolymers or heteropolymers, and the afimbrial adhesins, formed of single proteins or homotrimers (142). For the completion of fimbrial and afimbrial adhesins in Gram-negative pathogens, different secretion systems have been identified, including Sec-independent and Sec-dependent pathways (144). The major families of adhesive proteins (145) include the classical chaperone/usher pathway-dependent fimbrial adhesins (146, 147), the alternate chaperone/usher pathway-dependent E. coli surface pili (148), the extracellular nucleation precipitation-dependent curly or thin aggregative fimbrial adhesins (149), the type I secretion system-dependent afimbrial adhesins (150), the type III secretion system-dependent integral outer membrane proteins (151), the polymerization-assembled type IV pili (152), and the type V secretion system-dependent nonfimbrial trimeric autotransported adhesins (153).
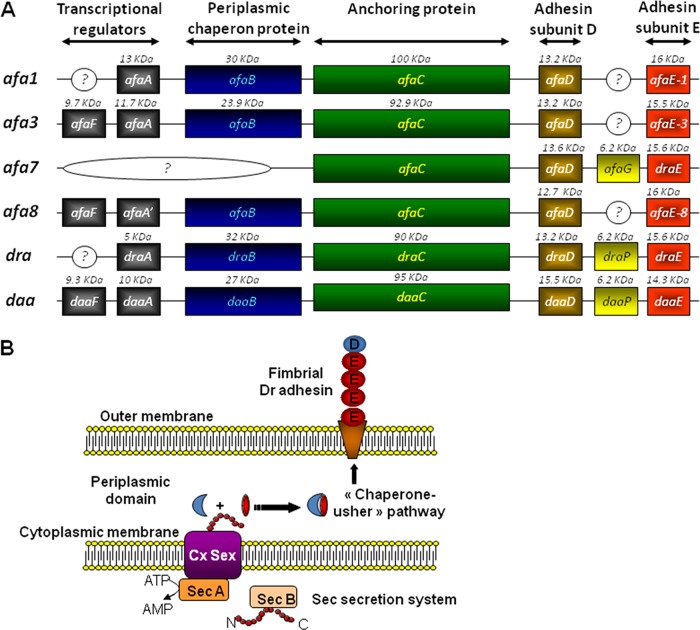
The Afa/Dr family of adhesins contains fimbrial (29, 32, 75, 76, 154, 155) or afimbrial (25–27, 30, 37, 44, 64, 84, 156) adhesins (Table 1). These adhesins are encoded by genes present in operons containing five major genes, including highly conserved genes A to D, encoding accessory proteins, and more divergent genes E, encoding the adhesin subunits (Fig. 1A). Assembly via the FGS (with a short F1-G1 loop) and FGL (with a long F1-G1 loop) classes of periplasmic chaperones has been described, and the FGL chaperone/usher protein secretion system assembles Afa/Dr adhesins (146, 147) (Fig. 1B). The structural organization of long and short Afa/Dr adhesins develops by the assembly of the bacterial membrane usher, successive E adhesin subunits, and one D subunit capping the structure. It is interesting to note that the chaperone usher functions in UPEC to form adhesive structures such as the P pili, resulting in the orderly assembly of PapA, PapK, PapE, PapF, and adhesive PapG subunits, and type 1 pili, formed by assembly of FimA, FimF, FimG, and adhesive FimH subunits (146, 147). The PapG subunit, localizing at the tip of the fimbria, triggers recognition of host cell membrane-associated globoseries glycolipids, and the FimH subunit triggers the mannose-dependent recognition. Crystallographic and nuclear magnetic resonance (NMR) studies coupled or not coupled with mutagenesis have been used to define the functional domains of the DraE and AfaE-III-Dsc adhesins, which are required for binding to host receptors such as hDAF (157–160), hCEACAMs (160), and collagen type IV (161, 162), and to explain the differential sensitivity to chloramphenicol (161, 162).
TABLE 1.
Characteristics of Afa/Dr adhesins and Afa/Dr-related adhesins
| Adhesin | Type | Host | Receptors |
||
|---|---|---|---|---|---|
| Type IV collagen | hDAF | hCEACAMs | |||
| AfaE-I | Afimbrial | Human | Negative | Positive | Positive |
| AfaE-II | Afimbrial | Human | Unknown | Positive | Unknown |
| AfaE-III | Afimbrial | Human | Negative | Positive | Positive |
| AfaE-V | Afimbrial | Human | Unknown | Positive | Positive |
| AfaE-VII | Afimbrial | Bovine | Unknown | Negative | Unknown |
| AfaE-VIII | Afimbrial | Human/animal | Unknown | Negative | Negative |
| Dr | Fimbrial | Human | Positive | Positive | Positive |
| Dr-II | Afimbrial | Human | Negative | Positive | Negative |
| F1845 | Fimbrial | Human | Negative | Positive | Positive |
| Distant membersa | |||||
| NFA-I | Afimbrial | Human | Unknown | Positive | Unknown |
| AAF-I | Fimbrial | Human | Unknown | Unknown | Unknown |
| AAF-II | Fimbrial | Human | Unknown | Unknown | Unknown |
| AAF-III | Fimbrial | Human | Unknown | Unknown | Unknown |
| HdaA | Fimbrial | Human | Unknown | Unknown | Unknown |
Like Afa/Dr adhesins, AAF-I, -II, and -III and HdaA promote an MRHA phenotype in human erythrocytes (78).
FIG 1.
Genetic organization of Afa/Dr operons (A) and assembly of Dr adhesin via the chaperone-usher pathway (B).
Afa adhesins.
Agnès Labigne and Chantal Le Bouguénec have extensively described the pathogenicity mechanisms of E. coli strains bearing the afimbrial adhesins (Afa) encoded by the afa operons (Table 1). The first Afa adhesin was isolated from the wild-type prototype UPEC strain KS52 by Labigne et al. (25, 37). The 6.7-kb chromosomal DNA fragment essential for a mannose-resistant hemagglutination (MRHA) phenotype in human erythrocytes and for adhesion onto uroepithelial cells contains five genes: afaA, afaE, afaD, afaB, and afaC (24). Adhesins AfaE-II and AfaE-III were then isolated from two other UPEC strains, A22 and A30, by Labigne et al. (84). Le Bouguénec et al. (27) isolated from the UPEC strain A30 a 9-kb plasmid region containing the afa-3 gene cluster. The afa-3 gene cluster contains six genes designated afaA to afaF (163). The AfaE-III and DraE adhesin subunits displayed 98% identity (of 160 amino acids, 157 are similar) (27, 30), and the afa-3 gene cluster and daa operon are closely similar (31, 32, 164, 165). The atomic resolution structure for the AfaE-III subunit has been determined (158, 159, 166, 167). Nuclear magnetic resolution and biophysical studies have revealed that the structural organization of Afa-III adhesin develops by assembly onto the bacterial membrane usher of AfaE-III adhesins subunits (158, 159) capped by the AfaD-III subunit (166–168). The AfaD-III subunit also has the ability to separate from the Dr fimbriae (42). A diffuse and not well-ordered cell surface localization of the AfaD-III subunit has been observed by immunoelectron microscopy (42, 168, 169). Using chimeras constructed from the afa-3 and daa operons, a study has revealed that the afimbrial or fimbrial morphologies of the adhesins were influenced by the order in the genes coding for the afimbrial or fimbrial adhesin subunits (28). The AfaE-III adhesin subunit is involved in recognizing host cell receptors (158, 159) such as the DraE and DaaE adhesin subunits (170). As shown in the analysis of epidemiological studies below, Afa-possessing E. coli strains have been found to be expressed by UPEC, diarrhea-associated E. coli, and E. coli isolated from the feces of asymptomatic patients.
The afa-7 and afa-8 operons encode the AfaE-VII and AfaE-VIII adhesins (26, 44) (Table 1). Like the Afa-III adhesin, the Afa-VII and Afa-VIII adhesins can aggregate to form amorphous masses (26, 42). Among the four sRNA genes (171) present in the PAIAL862 strain and expressed by afa-8-positive E. coli (44), the AfaR small RNA, the transcription of which is temperature controlled, regulates the expression of the AfaD-VIII subunit (172). It is noticeable that despite the presence of the Afa-VIII adhesin in human intestinal E. coli isolates (43), these E. coli isolates have never been found to be responsible for diarrhea in humans (45). In contrast, in calves, pigs, and poultry with diarrhea there was the presence of E. coli isolates expressing sequences of the afa-8 operon (173).
Dr adhesins.
Bogdan Nowicki and coworkers have magnificently demonstrated the role of E. coli expressing Dr adhesins in pyelonephritis, recurrent bladder cystitis, and pregnancy complications and have also dissected their molecular and cellular mechanisms of infection. The human pyelonephritis-associated wild-type prototype UPEC strain E. coli IH11128 (O75) has a K5 capsule, lacks the flagellar antigen, expresses a type 1 pilus, develops MRHA, does not exert hemolytic activity, and does not produce colicin (155) (Table 1). Strain IH11128 exhibits mannose-resistant Dr fimbriae (29). Five Dr operon-associated proteins with molecular masses of 15.5, 5, 18, 32, and 90 kDa are necessary for the development of the complete MRHA (55).
The role of the FGL chaperone/usher biogenesis pathway of Dr fimbriae has been investigated in detail (174–178). To allow proper folding of the DraE adhesin subunits to occur, the DraC usher creates an assembly and secretion platform, and premature DraE subunit-subunit association is prevented by the DraB chaperone. When the DraC is lacking, there are no protein subunit secretion and fimbria assembly, since the protein complexes amass in the periplasm. Mutagenesis of the DraC N terminus shows that DraC-F4A, DraC-C64, DraC-C100A, and DraC-W142A play a pivotal role in the bioassembly of fimbriae. In the case of the E subunit, two conserved cysteine residues forming a disulfide bond are important for stabilizing elements of the immunoglobulin fold of the Dr fimbriae. With regard to the DraD subunit (179), it has been reported that when the DraE subunit assembles, the DraD subunit localizes at the tip of the fiber (158, 159, 166, 167, 180). Jedrzejczak et al. (181) have shown that the DraD subunit localizes at the tip, because it lacks a donor strand and as a consequence functions only as an acceptor. However, expression of the DraD subunit has been found to be independent of the DraC usher, and DraD appears not to be necessary for the polymerization of DraE subunits (182). Recently, Zalewska-Piatek et al. (183) showed that the DraD subunit can be produced by a chaperone/usher-independent, type II secretion-dependent process that allows the translocation of the DraD subunit onto the cell surface of Dr-positive E. coli.
The major structural subunit DraE is involved in host cell receptor recognition (170), as are the AfaE-III (158, 159) and DaaE adhesin subunits (170). Calculation of the electrostatic potentials of the DraE structure shows an electronegative area around the cluster of amino acids involved in binding onto hDAF (Asp61, Asp63, and Asp75) (157). In the genes encoding Dr fimbriae, single-nucleotide polymorphisms conferring an adaptive advantage have been identified (184). Dr fimbriae are unique among Afa/Dr adhesins in expressing chloramphenicol sensitivity for binding onto host cell receptors, whereas binding of Afa-I to -III and F1845 is not affected (27, 185, 186). Korotkova et al. (187) have interestingly shown that genes encoding Dr fimbriae form eight structural groups displaying a high level of amino acid sequence diversity among them. It is noticeable that a functional analysis has revealed the presence of distinctly different binding phenotypes controlling affinity to hDAF, capability to bind collagen type IV and hCEACAMs, and sensitivity of adhesiveness capacity to chloramphenicol. Since the AfaD/DraD/DaaD subunits localize at the tip of the Afa/Dr fimbriae, their possible involvement in adhesion onto epithelial cells and, in addition, that of the AfaE/DraE/DaaE subunits has been envisaged. Conflicting results have been obtained. Recombinant DraE−/DraD+ or AfaE−/D+-III E. coli failed to adhere to differentiated primary bladder cells (188) and CHO-hDAF-α5β1 cells (189), respectively. In contrast, Zalewska-Piatek et al. (190) have reported that in HeLa cells, DraE−/DraD+ E. coli displays a low level of adhesion, ∼3-fold lower that of DraE+/DraD+ E. coli. In contrast to the chloramphenicol-sensitive adhesion of DraE, the DraD-induced binding is chloramphenicol insensitive (190).
The Dr-II adhesin has been isolated from the human pyelonephritis-associated strain EC7372 (Table 1). Compared to the members of the Afa/Dr adhesin family, the Dr-II adhesin displays poor sequence identity (17 to 20%) (30). Dr-II has 96% identity with the nonfimbrial adhesin I (NFA-I) expressed by UTI-associated E. coli (191). Interestingly, NFAs and Afa/Dr adhesins have a very similar genetic organization, and the nfa gene cluster encodes NfaA subunits assembled via the chaperone-usher pathway (191).
F1845 adhesin.
Steve L. Moseley and coworkers discovered the diarrhea-associated E. coli expressing F1845 adhesin and beautifully described the structural aspects of the interaction between Afa/Dr adhesins and their epithelial cell hDAF and hCEACAM receptors. The human wild-type prototype diarrheagenic strain C1845 expresses a fimbrial adhesin, designated F1845 (Table 1). The order and regulation of the genes necessary for F1845 adhesin assembly have been identified (31, 32, 164, 165, 192–194). The F1845 and Dr adhesins display 57% identity (91 amino acids of 160 are identical) (30). Five polypeptides (10, 95, 27, 15.5, and 14.3 kDa) are encoded by daaA, daaB, daaC, daaD, and daaE genes, respectively. The major structural subunit, DaaE, is involved in host cell receptor recognition like the AfaE-III (158, 159) and DraE adhesin subunits (170). Bilge et al. (31) have demonstrated that the fimbrial gene expression in the daa operon was regulated by both phase variation and environmental regulatory mechanisms. White-Ziegler et al. (193) have reported that in response to multiple environmental signals, the histone-like H-NS acts as an overall regulator by controlling transcription of the daa operon.
Flagella
The biogenesis of flagella involves the coordinated structural assembly of flagellar proteins (195). A variety of flagellar structural proteins and capping proteins compose the flagellar propeller (195), and cytoplasmic membrane proteins compose the force-generating unit of the flagellar motor (196). In an aqueous environment, many bacterial species move by rotating their flagella, allowing individual bacteria to swim in three dimensions (197). Moreover, flagellar swarming coordinates the movement of bacteria across the host cell surface (198). Flagella expressed by UPEC contribute to colonization of the epithelium, dissemination to the kidney by ascending progression from the bladder, and biofilm formation (199). It has been observed that UPEC strains expressing type 1 pili or P fimbriae are less flagellated and display repressed motility, suggesting that when fimbrial expression is switched off, UPEC strains are motile (200, 201). Afa/Dr DAEC strains express or do not express flagella. The prototype pyelonephritis-associated, wild-type Afa/Dr DAEC strain IH11128, expressing a type 1 pilus, does not possess flagellar antigens (155). In contrast, the UPEC wild-type strain A30, which does express AfaE-III adhesin, is positive for flagellar antigen (unpublished data), and the animal wild-type Afa-VIII-positive strain AL511 is H8 positive (202). It is worth mentioning that that the prototype diarrheagenic wild-type Afa/Dr DAEC strain C1845 (32) does not express flagellar antigens (unpublished data). According to Arikawa et al. (203), only seven of the 19 afaE1-, afaE2, or afaEX-positive, diarrhea-associated E. coli isolates they examined are motile. In contrast, Meraz et al. (107), who examined 18 DAEC isolates, found that all nine diarrhea-associated, afaE1- or afaEX-positive E. coli isolates are motile. These findings indicate that UPEC and diarrhea-associated Afa/Dr DAEC display heterogeneous flagellum expression.
Secreted Autotransporter Toxin
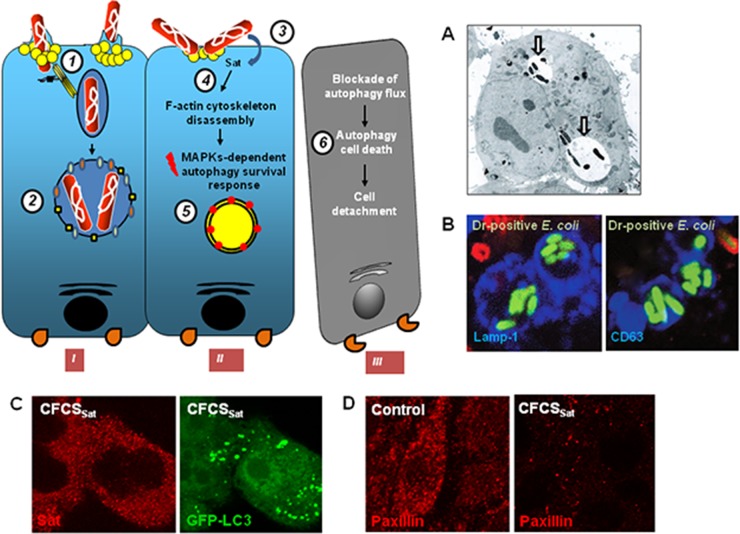
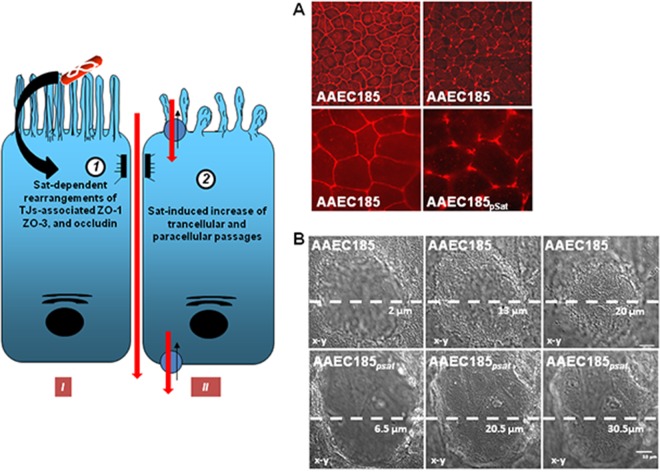
Secreted autotransporter toxin (Sat) belongs to the type V secretion pathway-dependent subfamily of serine protease autotransporters of Enterobacteriaceae (SPATE) toxins (81, 153, 204). As the result of differences in the toxins structures and activities, there are two classes of SPATE toxins. Class I includes plasmid-encoded toxin (Pet) of EAEC, extracellular serine protease, plasmid encoded (EspP) of EHEC, EspC of EPEC, SigA of Shigella flexneri and EAEC, Sat of intestinal E. coli and ExPEC, and the hypothetical EspC-like SPATE toxins with EcPCN033-C1sp (NCBI accession number EGP21815.1) of ExPEC, EcNA114-C1sp (NCBI accession number AEG39156.1) of UPEC, and EcM605-C1sp (NCBI accession number ZP_08351236.1) of AIEC (204). Class II includes protein involved in intestinal colonization (Pic) of Shigella, EAEC, and UPEC, SepA of Shigella, EatA of ETEC, vacuolating autotransporter toxin (Vat)-like toxins of UPEC, SEPEC, and NEMEC, EcRN587-C2sp (NCBI accession number EFZ76879.1) of EAEC and EPEC, and EpeA of Shiga toxin-producing E. coli (204). Class I SPATE toxins are generally cytotoxic, whereas class II display diverse activities, including the cleavage of mucus, which provides a competitive advantage for host epithelium colonization (81, 204). The sat gene has been characterized in the UPEC prototype strain CFT073 (205), where it resides within PAI-IICFT073 (206–209). The sat gene is prevalent in UPEC strains, including those bearing Afa/Dr adhesins (8, 56, 137, 206, 208, 210–214), resident intestinal microbiota E. coli stains, and pathogenic strains of E. coli, including EAEC (137, 215–218), and Shigella isolates (219, 220). The sat gene has been found present in daaC-positive E. coli strains isolated from stools of children with diarrhea in Brazil and France (137, 218, 221). In Afa/Dr DAEC isolates, the sat gene has been found to be expressed equivalently by diarrheic and asymptomatic adults (222). Interestingly, the sat gene is prevalently expressed in Afa/Dr DAEC isolated from children in a context of diarrhea (222).
Hemolysin
The pyelonephritogenic strain EC7372, which expresses Dr-II adhesin (30), is the only Afa/Dr DAEC strain that produces a functional hemolysin. Indeed, unlike other Afa/Dr DAEC strains, strain EC7372 promotes a strong cellular lysis in epithelial cells preceded by apoptosis (102). On the basis of results reported by Blanc-Potard et al. (60), the hemolysin-positive strain EC7372 carries both the hly and pap operons and seems to have acquired a larger part of the PAIsCFT073 (207–209) than Afa/Dr DAEC. The recombinant E. coli strain EC901, which carries plasmid pBJN406 and contains the draA to -E genes involved in expression of Dr fimbriae (223), has been observed to display a curious hemolytic activity. Insertion mutations in draD and draE, but not in draA, draB, and draC, abolish hemolytic activity, indicating that this activity is supported by the extracellular domain of Dr fimbriae. This observation is intriguing, since strain IH11128, gestational pyelonephritis Dr-positive E. coli isolates (94, 155), and clinical Dr-positive E. coli isolates (60) all lack either hemolytic activity or hly gene expression. In contrast, the wild-type O75X strain IH11032 does display hemolytic activity (155). Moreover, four afaE1-positive and one afaEX-positive diarrheagenic E. coli isolates have been found to trigger hemolysis, while 14 other afaE1-positive and one afaEX-positive isolates do not (203). Collectively, these findings show that Afa/Dr DAEC strains are heterogeneous in terms of α-hemolysin expression, suggesting a variable distribution of the part of PAICFT073 containing the hly gene among the Afa/Dr DAEC strains.
Other Factors
Blanc-Potard et al. (60) identified several short sequences (73 to 495 bp) that are prevalent in Afa/Dr adhesin-positive E. coli clinical isolates in comparison with E. coli clinical isolates not expressing Afa/Dr adhesins (GenBank accession numbers AZ935556 to AZ935604). Several sequences are homologous to virulence genes expressed in other pathotypes of E. coli, including genes for two siderophores (irp2 and iuc), a catechol siderophore receptor (iroN), and two transport systems (shu and modD) (60). Interestingly, several C1845-specific sequences display no likeness with known sequences (60). Importantly, the diarrhea-associated wild-type C1845 strain does not express the genes encoding ETEC and EAEC virulence factors and is devoid of genes encoding EPEC and EHEC virulence factors, including the genes of the locus of enterocyte effacement (LEE) island involved in the type III secretion system (T3SS) or T3SS-associated effector proteins and not hybridized with eae probes (60). The wild-type C1845 and IH11128 strains expressed a part of PAICFT073 (207–209) not including the hlyA, hlyD, hp1-hp4, papG, or papF sequences (60). A remnant of the pap operon which has the F10 papA allele but lacks most of the central region of the pap operon has been detected. It is noteworthy that regions of the PAICFT073 complete genome sequence (207, 209, 224) have been found in E. coli strains of the B2 phylogenic group (208) and are prevalently expressed in ExPEC strains of group B2 involved in UTIs (8). Moreover, parts of PAICFT073 have recently been found in intestinal commensal E. coli strains, particularly those of phylogenic group B2 (225–227), and in an AIEC strain (228).
The PAIAL862 expressed by afa-8-positive E. coli strains (44) includes the deoK gene, which confers metabolic adaptability and increases the competitive advantage with regard to host infectivity (229). The locus designated vpe (virulence-associated phosphotransferase) contains the vpeA, vpeB, and vpeC genes, which encode, respectively, the EIIA, EIIB, and EIIC constituents of a putative carbohydrate-specific permease of the SgaTBA family (230). This locus is present in the pyelonephritis-associated strain AL511, which expresses the afa-8 operon (43), which confers an ability to adapt for kidney and intestinal colonization (231). The presence of the vpe locus in other UTI- and diarrhea-associated Afa/Dr DAEC strains has not been documented.
The capacity to form filamentous forms results from a plasticity capacity developed by a bacterial pathogen in order to escape host defenses when in an intracellular location or to assemble to form a biofilm-like structure that leads to resistance to anti-infective treatments, such as antibiotics (199). Some excellent experiments have demonstrated that type 1 pilus-positive UPEC strains, after internalization into superficial epithelial cells known as “umbrella cells” lining the luminal surface of the bladder, form biofilm-like bacterial assemblages designated “intracellular bacterial communities” (IBCs) that function as transient protective structures for UPEC intracellular growth (199, 232). UPEC cells in IBCs constitute reservoirs of UPEC, which, after switching to filamentous forms, become detached from the bacterial community and may be flushed out of the host cells. Zalewska-Piatek et al. (233) were the first to observe that that Dr-positive E. coli formed biofilms. This phenomenon means that Dr-positive E. coli strains form live filamentous bacteria, depending on their nutritional environment (190, 233). It has been observed that adhering Dr-positive E. coli forms filamentous forms at the cell surface of CHO-hDAF-α5β1 (189) or CHO-hDAF (234) cells. Filamentous bacteria residing within the phagosome escaped phagosomal killing as the bacteria manipulated the phagosome compartment by blocking the acquisition of hydrolytic components (235, 236). Even though the intracellular vacuole-containing Dr-positive E. coli in HeLa cells lack the characteristics of a degradative compartment (189), no filamentous forms of Afa- or Dr-positive E. coli residing intracellularly have ever been observed. This aspect of Afa/Dr pathogenesis remains to be explored in the appropriate model of bladder epithelial cells. Bacterial biofilm formed by UPEC after aggregation of three-dimensional structured cells connected by self-produced exopolysaccharide matrix plays a major role in persistent and chronic UTIs (199). Exopolysaccharide production, which plays a pivotal role in biofilm completion, has been found in UTI-associated E. coli strains expressing Dr (190, 233) or Afa-VIII (231) adhesins. Interestingly, exopolysaccharide production is controlled by the vpeBC gene (231), which is present in the vpe locus of afa-8-positive E. coli (43). DraE+/DraD+ E. coli strains form dense biofilms, and DraD, whether associated with fimbriae or not, plays a role in biofilm formation (190, 233).
A large variety of bacteria have been found to produce toxins, named cyclomodulins, that dramatically interfere with the cell cycle (237). Cyclomodulins produced by pathogenic E. coli included colibactin, cycle-inhibiting factor (Cif), cytotoxic necrotizing factor (CNF), and cytolethal distending toxin (CDT) (238). Currently, the two known genotoxins are colibactin and CDT (238). The cluster of genes known as the “pks island” (239) encodes a multienzymatic machinery for synthesizing the hybrid, nonribosomal, peptide-polyketide genotoxin colibactin (240). It has been suggested that the pks island may affect the host immune response and could be involved in chronic inflammation, in the accumulation of genomic instability, and in tumor progression (241). Whether the pks island contains other genes encoding additional bacterial factors and whether the pks-related colibactin is a prototype of a family of molecules or not remain to be investigated. The pks genomic island is present in the prototype Afa/Dr DAEC wild-type IH11128 and C1845 strains (J. P. Nougayrede and E. Oswald, unpublished result) and in colonic afa-I-positive E coli strains isolated from patients with IBD and colorectal cancer (140). The pks island has been also found in ExPEC strains of phylogenetic group B2 (242), in fecal E. coli strains isolated from healthy patients but not in pathogenic EPEC and EHEC isolates (243), in group B2 E. coli strains that are long-term colonizers of the intestine (22), in E. coli isolated from the mucosa of patients with IBD (244), in mucosa-associated or internalized E. coli of tumors and mucosa of colorectal cancer patients (244–246), and in urosepsis E. coli strains (247). It was noted that the intestinal microbiota E. coli strain Nissle 1917 expresses the pks genomic island and displays similarities with the prototype Afa/Dr DAEC wild-type C1845 and IH11128 strains, since it harbors parts of PAICFT073 that lack the expression of α-hemolysin and P fimbriae but includes iron uptake systems (225, 227). This probiotic E. coli strain with diverse activities (248) is intriguing since its promotion of gut homeostasis activity in response to mucosal injury cannot be dissociated from the presence of the pks island (249). Whether the presence of the pks island in intestinal E. coli and ExPEC strains is deleterious for the host or without pathological consequences remains to be investigated.
MECHANISMS OF PATHOGENICITY
Host Cell Receptors for Afa/Dr Adhesins
On the basis of the differential recognition of human epithelial cell membrane-associated receptors by Afa/Dr adhesins (Table 1), Afa/Dr DAEC strains have been subdivided into two subclasses (11). The first subclass includes E. coli strains harboring the Afa-I (25, 37), Afa-II (27), Afa-III (27), Afa-V (64), Dr (29, 155), Dr-II (30), and F1845 (32) adhesins recognizing hDAF, which also may or may not recognize members of the hCEACAM family. The second subclass includes strains that express Afa-VII (26, 44) and Afa-VIII (26, 44) adhesins that do not recognize hDAF. In addition, the NFA-I adhesin of UPEC (191) belongs to the Afa/Dr family of adhesins (Table 1). Moreover, despite a similar genetic organization with the gene clusters triggering the biogenesis of Afa/Dr adhesins, the EAEC adhesins AAF-I (77), AAF-II (76), AAF-III (75), and Hda (78) are distant pathogenic factors of the Afa/Dr family of adhesins (Table 1). The four major characteristics of EAEC pathogenesis (79–81) are as follows: (i) adherence to the intestinal mucosa via adhesins (18, 75–78), (ii) the formation of typical “stacked-brick” microcolonies as each bacterium interacts with others, (iii) production of enterotoxins and cytotoxins, and (iv) the development of a severe mucosal inflammation. Boisen et al. (78), analyzing this superfamily of adhesins, have proposed a pertinent phylogram composed of three distinct clusters. The first cluster comprises Afa-I, Afa-II, Afa-III, Afa-V, Dr, Dr-II, and F1845, the second comprises AAF-I, AAF-II, and AAF-III, and the third comprises Afa-VII, Afa-VIII, and Hda. It is worth mentioning that cluster 3 (78) also includes the nonfimbrial M-agglutinin encoded by the bma gene cluster of UPEC (250).
hDAF.
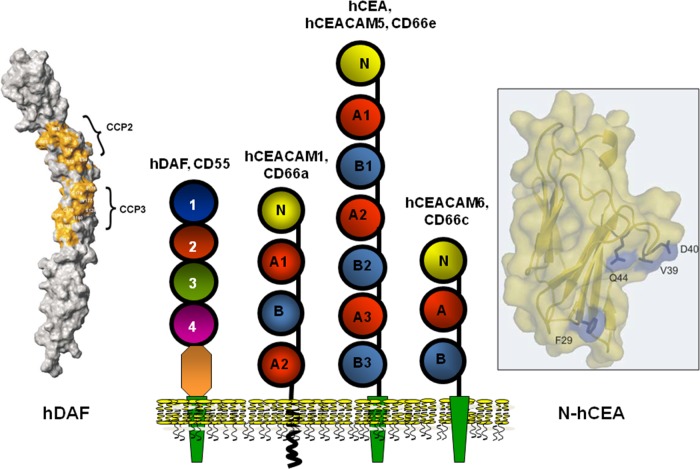
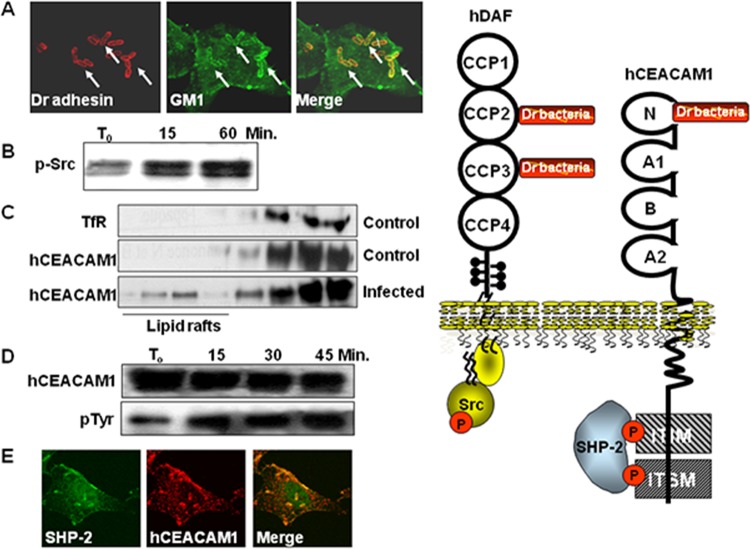
Nowicki et al. (33) were the first to report that human decay-accelerating factor (hDAF) (CD55) expressing the antigens of the Cromer blood group system (251) acts as an epithelial cell receptor for E. coli expressing Afa/Dr adhesins (Fig. 2) (Table 1).
FIG 2.
Membrane-associated proteins expressed by human epithelial cells that function as receptors for Afa/Dr adhesins. Center, representations of the structures of hDAF and hCEACAMs. Left, surface representation of hDAF. Right, homology model of human N-CEA. (Representations of hDAF and N-hCEA reprinted from reference 158 with permission of Elsevier and from reference 160 with permission of the publisher, respectively.)
(i) Structure and functions.
DAF is a complement-regulating protein with an Mr of 55,000 to 70,000 (251). The physiological function of DAF is to control the amplification of the complement cascade by a direct interaction with membrane-bound C3b or C4b, which in turn impedes the ulterior uptake of C2 and factor B. Membrane-bound DAF is formed by a membrane glycosylphosphatidylinositol (GPI) anchor followed by a serine/threonine/proline (STP)-rich region and by four complement control protein repeat (CCP) domains, previously named short consensus repeats (SCRs) (Fig. 2). Modeling of the extracellular domain of DAF reveals that CCPs are organized in a helical manner. While CCP-1 had no effect on hDAF regulatory activity, deletion of CCP-2, CCP-3, or CCP-4 entirely abolished the regulatory activity. Interaction of DAF with the convertases is mediated predominantly by two patches approximately 13 Å apart, one centered around Arg69 and Arg96 on CCP-2 and the other around Phe148 and Leu171 on CCP-3 (252). Phe123 and Phe148, localizing at the interface between CCP-2 and CCP-3, and also Phe154, which is present in the CCP-3 cavity, are pivotal for the regulatory activity (253). The GPI anchor increases the lateral mobility of DAF within the cell membrane in relation to its localization into membrane-associated lipid rafts, and the O-glycosylated STP serves as a spacer for the projection of the hDAF functional domains at the cell membrane (253).
(ii) Receptor for Afa/Dr adhesins.
hDAF is one of the receptors recognized by Afa/Dr adhesins in epithelial cells (Fig. 2) (Table 1). It is noteworthy that Afa/Dr adhesins bind specifically to hDAF but not to rodent or pig DAF (254). Dr fimbria binding develops in the digestive, urinary, genital, and respiratory epithelia and in skin (255), consistent with the hDAF expression (251). Only uropathogenic and diarrhea-associated E. coli strains bearing the F1845, AfaE-I, AfaE-III, AfaE-V, Dr, and Dr-II adhesins recognized hDAF as a receptor (62, 160, 256). In contrast, the Afa-VIII adhesin expressed by human ExPEC does not recognize hDAF (26, 43, 44). It has been established from functional studies and atomic resolution models that Afa/Dr adhesins recognize the CCP-2 and CCP-3 on hDAF (41, 158, 159, 257–259) (Fig. 2). In contrast, gestational pyelonephritis-associated E. coli expressing dra-related X adhesins recognized the CCP-3 and CCP-4 domains of hDAF (62). In the CCP-3, a single point substitution (Ser155-Ala and Ser165-Leu, mimicking the Dra-to-Drb allelic polymorphisms) results in a complete loss of Dr fimbria binding to hDAF (257, 260). The amino acids (148 to 171), in particular Ser155, present at the surface of CCP-3 controlled the Dr adhesin binding (260). A surface plasmon resonance study of Afa-III adhesin binding onto CCP domains of hDAF has revealed that a construct formed of CCP-1 and -2 did not show any measurable binding to the AfaE-III adhesin subunit, while constructs formed of CCP-2, -3, and -4, CCP-2 and -3, or CCP-3 and -4 allowed AfaE-III binding with affinities comparable to that for the entire hDAF, confirming the previously observed importance of the combined CCP-2 and CCP-3 domains for the recognition of hDAF by Afa/Dr adhesins (158, 159). The Dr adhesin-binding and complement-regulating epitopes of hDAF have been found to be distinguishable and are approximately 20 Å apart (260). However, Anderson et al. (158) observed that the binding of AfaE-III to hDAF antagonized the hDAF regulatory activity.
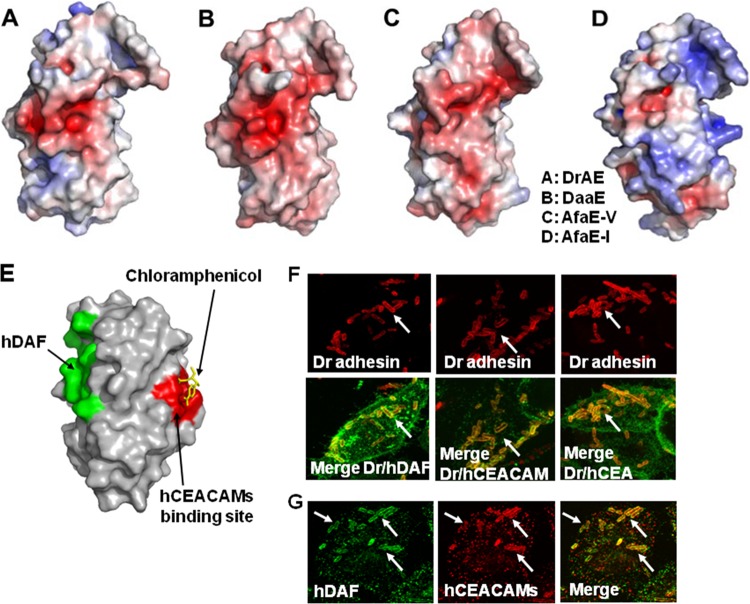
The AfaE-I, AfaE-III, AfaE-V, DraE, and DaaE subunits function as receptor ligands for hDAF (27, 160, 170) (Fig. 3A to D). In DraE/AfaE-III subunits, the hDAF-binding site forms a large convex surface involving seven β strands (158, 159). The residues Asp61, Ile73, and Asn77 have been found to be important for binding to hDAF (158, 159). Mutagenesis and crystallographic studies of DaaE have been conducted in order to define the detailed molecular interactions between Afa/Dr adhesins and hDAF (157). Five daaE mutants (T8N, A60V, D61A, D63V, and T133S) showed a 30 to 50% reduced ability to bind onto CHO cells transfected for hDAF expression (157). Mapping the sites of DaaE reveals that positions Asp61 and Asp63 are necessary for binding to hDAF, and calculation of the electrostatic potentials of the DaaE structure has revealed an electronegative region around the cluster of amino acids involved in hDAF recognition (Asp61, Asp63, and Glu126) (157). Moreover, the ability of the DraE adhesin to bind hDAF has been found influenced by individual amino acid changes at positions 10, 63, 65, 75, 77, 79, and 131 of the mature DraE sequence (261).
FIG 3.
Receptor clustering by Afa/Dr DAEC. (A to D) Representations of the DraE, DaaE, AfaE-V, and AfaE-I adhesin subunits, respectively. Surface electrostatic potentials of the DraE, DaaE, AfaE-V, and AfaE-I adhesins (red indicates the negative charges and blue the positive charges) are shown. (Reprinted from reference 157 with permission of the publisher.) (E) Representation of DraE adhesin subunit-associated surfaces allowing the specific recognition of hDAF or N-hCEA. Green, surface recognition of hDAF. Red, surface recognition of N-hCEA. Yellow, chloramphenicol bound onto the domain of AfaE-III that recognizes N-hCEA. (Reprinted from reference 160 with permission of the publisher.) (F) Micrographs showing the observation by confocal laser scanning microscopy (CLSM) of hDAF, hCEACAM1, and hCEA receptor clustering around Dr adhesin-positive E. coli adhering to untransfected HeLa cells constitutively expressing hDAF and to transfected HeLa cells expressing hCEACAM1 and hCEA. Yellow shows colocalization of immunolabeling of Dr adhesin (red) and hDAF, hCEACAM1, or hCEA (green). (Reprinted from reference 274 with permission of the publisher. Copyright 2004 Blackwell Publishing Ltd.) (G) Receptor clustering of hDAF (green) and hCEACAM1 (red) around Dr adhesin-positive E. coli adhering onto transfected HeLa cells expressing hCEACAM1. Yellow, colocalization of immunolabelings of hDAF and hCEACAM1. Arrows show immunolabelings of interest around adhering bacteria. (Reprinted from reference 274 with permission of the publisher. Copyright 2004 Blackwell Publishing Ltd.)
Binding of the DraE adhesin subunit onto hDAF is sensitive to chloramphenicol, which also inhibits the hDAF-dependent MRHA of human erythrocytes (chloramphenicol-sensitive hemagglutination [CSHA]) (33, 256). In HeLa cells, the presence of chloramphenicol diminished the adhesion of DraE+/DraD+ E. coli by ∼3-fold and totally abolished the adhesion of DraE+/DraD− E. coli but did not change the adhesiveness capacity of DraE−/DraD+ E. coli (190). According to Swanson et al. (262), the domains involved in the CSHA are present within the N-terminal domain of the DraE subunit. According to Pettigrew et al. (161, 162), a hydrophobic pocket including Gly113, Gly42, Pro40, Pro43, Ile111, Tyr115, and Ile114 plays a pivotal role in the chloramphenicol-binding site in the DraE subunit. The inhibition of the binding of the DraE subunit onto hDAF by chloramphenicol has received a structural explanation, since by covering the functional portion of the adhesin subunit, chloramphenicol disrupts the recognition of hDAF (161, 162). In contrast to the case for the Dr adhesin, chloramphenicol does not affect the hDAF-dependent MRHA exerted by the AfaE-I, AfaE-III, and F1845 adhesins (27, 256). This is a result of a difference in expression of amino acids between the adhesin subunits (161, 162). Moreover, it has been established that binding of chloramphenicol onto the DraE subunit develops via the interaction of its chlorine “tail” rather than its benzene ring (161, 162). Analyzing structural chloramphenicol modifications, Pettigrew et al. (162) have demonstrated that acylating the 3-hydroxyl group has no effect on the binding onto hDAF.
(iii) Receptor for microbial pathogens and viruses.
The cell membrane-bound hDAF is also hijacked by viruses, including coxsackievirus serotypes B1, B3, and B5 (263, 264) and coxsackievirus A21 (265), enteroviruses (266), and echoviruses (267, 268). Various different hDAF sites are recognized by echoviruses (269). It is worth underlining that like Afa/Dr DAEC (254), echoviruses and coxsackieviruses (270) express high specificity for hDAF. In addition, hDAF acts as a receptor for hantavirus (271). Moreover, epithelial hDAF has been identified as a gastric epithelial receptor for Helicobacter pylori and has been found to be upregulated by the pathogen in relation to inflammatory responses (272, 273).
hCEACAMs.
Guignot et al. (41) were the first to show that hCEA (CEACAM5, CD66e) is recruited around the prototype Dr adhesin- or F1845 adhesin-positive wild-type Afa/Dr DAEC strains IH11128 and C1845, respectively, adhering to cultured human enterocyte-like Caco-2 cells and that an anti-CD66 antibody inhibits this bacterial adhesion (Fig. 2) (Table 1). Berger et al. (274), using Chinese hamster ovary (CHO) cells and human cervical cancer HeLa cell lines transfected for the expression of each of the human carcinoembryonic antigen-related cellular adhesion molecules (hCEACAMs) (CEACAM1 to 8), found that the Dr, F1845, and AfaE-III adhesins bound only to cells expressing epithelial hCEACAM1, hCEA, or hCEACAM6, whereas the AfaE-I and Dr-II adhesins did not (Fig. 2) (Table 1). Korotkava et al. (160) demonstrated Afa-V adhesin binding to hCEA (Table 1). In addition, the Dr, F1845, and AfaE-III adhesins recognize the nonepithelial CEACAM3 as a receptor (188, 189). In contrast, the murine CEACAM1 is not recognized by Afa/Dr adhesins (274).
Twelve members, i.e., CEACAM1 (biliary glycoprotein [BGP], CD66a), CEACAM3 (CEA gene family member 1 [CGM1], CD66d), CEACAM4 (CGM7), CEA (carcinoembryonic antigen, CD66e), CEACAM6 (nonspecific cross-reacting antigen [NCA], CD66c), CEACAM7 (CGM2), CEACAM8 (CGM6, CD66b), CEACAM16, and CEACAM18 to -21, compose the family of CEACAMs (275). CEACAM proteins generally have one variable (V)-like Ig domain, identified as the N domain (except CEACAM16, which has two N domains), but they differ in the number of constant C2-like Ig domains as well as in their membrane anchorage (Fig. 2). CEACAM5, CEACAM6, CEACAM7, and CEACAM8 are anchored within the cell membrane through a GPI linkage, whereas six other CEACAM family members (CEACAM1, CEACAM3, CEACAM4, CEACAM19, CEACAM20, and CEACAM21) are anchored via bona fide transmembrane domains (275) (Fig. 2). CEACAM16 is devoid of any membrane anchorage and is the only known secreted CEACAM. The CEACAM1 cytoplasmic domain has immunoreceptor tyrosine-based inhibitory motifs (ITIMs), whereas CEACAM3, CEACAM4, CEACAM19, and CEACAM20 carry immunoreceptor tyrosine-based activation motifs (ITAMs) (Fig. 2). All family members are highly glycosylated on their extracellular domains, and as a function of the cell type and differentiation state of the cells, the level of glycosylation of each CEACAM may vary, since multiple glycoforms of the same protein have been characterized. Epithelial, endothelial, and hematopoietic cells variously expressed CEACAMs (275, 276). CEACAMs function mainly as adhesion molecules engaged in homotypic and/or heterotypic intercellular adhesion, and several CEACAMs exert regulating cell signaling activities (275, 276). CEACAMs are engaged during complex biological processes such as cancer progression, inflammation, immune responses, angiogenesis, and apoptosis (275, 276).
(i) hCEACAM1 structure and functions.
CEACAM1 was present in leukocytes, including granulocytes, activated T cells, B cells, and CD16−/CD56+ natural killer cells (275). It was present in endothelial cells (275). CEACAM1 was also expressed in epithelial of the stomach, intestine, bile ducts, kidney, prostate, endometrium, and mammary ducts (275). The gene encoding CEACAM1 contains 9 exons that, after alternatively splicing, generate 11 different isoforms with long or short cytoplasmic tails and long or short cytoplasmic domains (275). The long cytoplasmic domain contains two ITIMs (Fig. 2). ITIMs after tyrosine phosphorylation associate with diverse cytoplasmic signaling molecules, including the tyrosine kinases of the Src family, the tyrosine phosphatase Src homology 2 (SH2) domain-containing protein tyrosine phosphatase 1 (SHP-1) or 2 (SHP-2), and Shc (275). In contrast, the cytoplasmic S domain lacks the presence of tyrosine residues (275). The two major isoforms CEACAM1-L and CEACAM-1S can be cell coexpressed, but CEACAM1-L isoforms predominate in most cell types (275). However, the CEACAM1-L/CEACAM-1S ratio can vary as a function of the cell types and the cell differentiation states. In polarized epithelial cells, the CEACAM1-L and CEACAM-1S isoforms are expressed at both the apical domain and cell-cell contact areas (277, 278). CEACAM1 acts as a cell-cell adhesion molecule by hemophilic interaction (275). Down-expression of CEACAM1 occurred in several tumor types, such as breast, prostate, and colorectal cancer, and high levels of CEACAM1 expression are related to poor prognosis and tumor metastasis (275).
(ii) hCEA structure and functions.
hCEA is a GPI-anchored protein (275) (Fig. 2). hCEA was initially defined as a tumor-associated marker, since it is overproduced in an elevated number of carcinomas. Overexpression is often associated with enhanced metastatic potential and thus with poor prognosis (279, 280). However, it is important to remember that despite its name, hCEA is normally expressed in tissues, including intestinal M cells, enterocytes, and colonic cells, in which it is abundantly expressed at the brush border (281). hCEA is localized within cell membrane-associated lipid rafts via its GPI anchor and can act as a cell membrane-bound cell signaling receptor (275). In addition, hCEA is present in the intestinal apical glycocalyx. The physiological role played by hCEA remains unknown, but it has been shown to mediate cell-to-cell Ca2+-independent, homotypic interactions.
(iii) hCEACAM6 structure and functions.
Like CEA, CEACAM6 is a GPI-anchored protein (275) (Fig. 2). In colorectal cancers, the deregulation of CEACAM6 expression suggests a role in tumor onset (275). CEACAM6 is brush border expressed in polarized epithelial intestinal cells. CEACAM6 has the capacity to signal in cells. For example, following CEACAM6 cross-linking, there was subsequent activation of Src that led to the tyrosine phosphorylation of focal adhesion kinase (FAK), in turn triggering cross talk with αvβ3 integrin, and cell interaction with extracellular matrix molecules (ECMs) (282, 283).
(iv) Receptors for Afa/Dr adhesins.
Human UPEC and diarrhea-associated Afa/Dr DAEC strains expressing the Dr, Afa-III, Afa-V, and F1845 adhesins recognize the epithelial hCEACAM1, hCEA, and hCEACAM6 and the nonepithelial hCEACAM3 as host cell receptors (41, 160, 187–189, 274, 284) (Fig. 2) (Table 1). The Afa-I adhesin does not recognize hCEACAMs well, and the Dr-II adhesin fails to recognize hCEACAMs (274). It is not known whether hCEACAMs are recognized by the Afa-VIII adhesin. By surface plasmon resonance (SPR) binding analysis, the N-terminal domain of hCEA has been identified as being recognized by the DraE, AfaE-I, AfaE-III, AfaE-V, and DaaE adhesin subunits (160) (Fig. 2). The recognition of the N-terminal domain of nonepithelial hCEACAM3 observed by SPR analysis (187) has been confirmed in transfected cells expressing hCEACAM3 (188). The AfaE-I subunit displays lower-affinity binding to hCEA than the DraE, AfaE-III, AfaE-V, and DaaE adhesin subunits (160), which is consistent with a previous observation in transfected hCEACAM-expressing epithelial cells (274). For the hCEA/DraE interaction, the N-terminal 58 amino acids of hCEA are necessary, since the N-terminal F29I, I91A, and L95A hCEA mutants showed a decreased affinity for the DraE adhesin subunit (160, 284). Coupled mutagenesis analysis has identified residues F29, Q44, and D40, localizing in the exposed loops of the GFCC′C″ face of the N-terminal domain of hCEA, as being involved in DraE adhesin subunit binding. In contrast, the hCEACAM8 N-terminal domain is not recognized by Afa/Dr adhesins (160), which is consistent with previous results in transfected CHO cells expressing hCEACAM8 (274). A nuclear magnetic resonance (NMR) analysis of the hCEA binding site of DraE and AfaE-III-dsc adhesin subunits has revealed a site that overlaps a surface area of approximately 1,446 Å, localizing primarily in the A, B, E, and D strands (160) at the opposite end of the β sheet including the binding site for hDAF (158) (Fig. 3E). Korotkova et al. (188) analyzed the receptor clustering induced by E. coli expressing DraE, the DraE D61A adhesin subunit mutant deficient in hDAF binding, or the NfaE adhesin subunit, which binds only to hDAF, and showed that the DraE-expressing E. coli recruited both hDAF and hCEACAMs, that the DraE-D61A adhesin subunit-expressing E. coli recruited only hCEACAMs, and that the NfaE adhesin subunit-expressing E. coli recruited only hDAF when adhering to primary epithelial bladder cells expressing hDAF and hCEACAMs.
The P40S, P43V, R86G, G113A, and Y115A mutations in the DraE adhesin subunit severely affect binding to hCEA (160). As for hDAF (161, 162, 170, 185, 187, 261), the binding of DraE adhesin subunit-expressing E. coli to hCEA was inhibited by chloramphenicol, whereas the binding of the AfaE adhesin subunit III-expressing E. coli to hCEA was resistant to chloramphenicol (160).
Some hCEACAMs can form homophilic (hCEACAM1/hCEACAM1, hCEA/hCEA, and hCEACAM6/hCEACAM6) and heterophilic (hCEA/hCEACAM1, hCEA/hCEACAM6, and hCEA/hCEACAM8) complexes which form strong intercellular adhesion bonds that are involved in cell-to-cell interactions (275). Although no relationship has been established with microbial pathogenesis, the recognition of the N-terminal domain of hCEA by the DraE adhesin subunit leads to an unexpected structural consequence (284). The binding of the DraE adhesin subunit to the N-terminal domains of the hCEA/hCEA dimer is followed by the dimerization of the complex. It is noteworthy that in the physiological situation of the epithelia, the homophilic and heterophilic dimers of hCEACAM are not accessible to Afa/Dr DAEC, since they are located at the junctional domain of polarized epithelial cells.
(v) Receptors for microbial pathogens.
hCEACAM1, hCEA, and hCEACAM6 have been shown to be recognized by some strains of E. coli and some Salmonella species, probably at extramembrane glycosylated domains (285–287). Moreover, hCEACAM6 functions as a cell receptor for the AIEC strain LF82 (288). hCEACAMs are important for the pathogenicity of Neisseria, since after they are recognized by opacity proteins (Opa), these membrane-bound molecules triggered cell signaling, allowing the bacteria to penetrate into human tissues (289). As described above for Afa/Dr adhesins, Opa interactions with hCEACAM1, hCEACAM3, hCEA, and hCEACAM6 have been identified, whereas Opa do not interact with hCEACAM4, hCEACAM7, and hCEACAM8. It is worth underlining that Opa52 binds hCEACAM1, hCEACAM3, hCEA, and hCEACAM6, Opa53 specifically recognizes hCEACAM1, Opa54 binds to hCEACAM1 and hCEA, and Opa55 is hCEA specific. It is important to note that CEACAM recognition by Neisseria Opa (290) and Afa/Dr adhesins (274) is highly human specific. hCEACAM1 is also recognized as a receptor by the outer membrane protein P5 of typeable and nontypeable Haemophilus influenzae which can cause diseases including otitis media, conjunctivitis, sinusitis, pneumonia, and chronic bronchitis and the progression of chronic obstructive pulmonary disease (COPD) (291). Moreover, a major outer membrane protein of Moraxella catarrhalis strains associated with sinusitis, exacerbations of asthmatic conditions, and otitis and a cause of lower respiratory tract infections in adults, especially in patients with COPD, also interacts with CEACAM1 (292). It is interesting to note that like Afa/Dr DAEC (160, 284), the N-terminal domains of CEACAMs are targeted by the adhesive factors of Neisseria (293), H. influenzae (291), M. catarrhalis (292), and mouse hepatitis virus strain A59 virus (294, 295). In contrast, the FimH variant of the AIEC prototype wild-type strain LF82 probably recognizes glycosylated epitopes at the IgAs domain of CEACAM6 (288). In addition, CEACAM1-4L acts as a receptor for lipopolysaccharide (LPS) and lipooligosaccharide (296), in turn promoting Toll-like receptor 4 (TLR4)-dependent cell signaling responses (296, 297).
Basement membrane type IV collagen.
Basement membrane-associated proteins include fibronectin, laminin, tenascin, and heparin sulfate proteoglycans and type IV collagen (298). Type IV collagen interacts with integrins expressed at the membrane basal domain of polarized intestinal cells to structurally form the epithelium (299). Dr adhesin is unique in the Afa/Dr family of adhesins as recognizing the 7s domain of the type IV collagen (186, 300) (Table 1). By SPR analysis, the resonance signal indicates that the DraE adhesin subunit and type IV collagen form a stable complex (187). Carnoy and Moseley (185) have shown that mutations at positions 32, 40, 54, 88, 90, and 113 of the DraE adhesin subunit affect the type IV collagen binding and chloramphenicol sensitivity of binding, without affecting the hDAF-binding capability. The amino acids Pro40, Pro43, Ile114, and Tyr115 are also important for DraE adhesin subunit/type IV collagen interactions, since mutations P40A, P43V, I114A, and Y115A lead to a complete loss of recognition (187). Moreover, mutations in the two conserved cysteine residues forming a disulfide bond, which is necessary for stabilizing elements of the immunoglobulin fold of the Dr adhesin (174), abolish both MRHA and binding to type IV collagen (185). The role of type IV collagen in the pathogenesis of Afa/Dr DAEC remains largely elusive. It has been reported that the type IV collagen-binding phenotype is necessary for Dr adhesin-positive E. coli to induce pyelonephritis in a mouse model (301). However, the basolateral localization of type IV collagen in the epithelium prevents it from functioning as a receptor for Dr adhesin during Afa/Dr DAEC intestinal and urinary tract infections, since epithelial colonization strikingly develops at the cell domain facing the luminal compartments, which are devoid of type IV collagen expression. In the context of diseases in which the epithelia are structurally deregulated, the basement membrane domain becomes available for pathogenic bacteria that recognize basement membrane-associated molecules as receptors.
β1 Integrin.
AfaED-dsc, but not AfaE-dsc, interacts with two integrins: α5β1 and αvβ3 (167). However, there was a low-affinity interaction between DraD subunit and β1 integrin as observed by SPR analysis (167, 188). Considering the presence of DGR tripeptides and an RDG sequence in the AfaD-III subunit, Cota et al. (167) have proposed that the recognition of β1 integrin at a low level of affinity results in binding by these two nonsequential motifs. Intriguingly, when Korotkova et al. (188) used SPR analysis to test the binding to β1 integrin of the whole Dr adhesin, they found no detectable association, suggesting that the association detected with the DraD adhesin subunit cannot reflect the normal bacterial situation in which the Dr adhesin is well formed and expressed at the E. coli cell surface.
Receptor Clustering and Cell Signaling
As recently reviewed by Schmick and Bastiaens (302), the signaling activity at cellular membranes depends on constant membrane reshaping plus interactions with the dynamic cytoskeleton, thereby regulating the potency of molecular reactions between membrane-associated structural components and signaling molecules. The epithelial membrane-bound proteins that function as receptors for Afa/Dr adhesins are known to trigger cell signaling after antibody ligation (303, 304) or activation by chemical molecules (305–307). It is noteworthy that the physiological ligands that produce cell signaling by hDAF and hCEACAMs are not known. Adhesive factors of bacterial pathogens and viruses have been reported to trigger cell signaling in epithelial cells expressing hDAF and/or hCEACAM1 and CEACAM6 (308, 309). Whether cell signaling is induced following the recognition of hCEA by adhesive factors of bacterial pathogens remains in debate. Afa/Dr adhesins have been found to trigger various different cell signaling pathways after recognition of hDAF and hCEACAM1 in epithelial cells, some of which are involved in a wide variety of cellular injuries or cell responses.
Mobilization of adhesin receptors and constituents of cell membrane-associated lipid rafts.
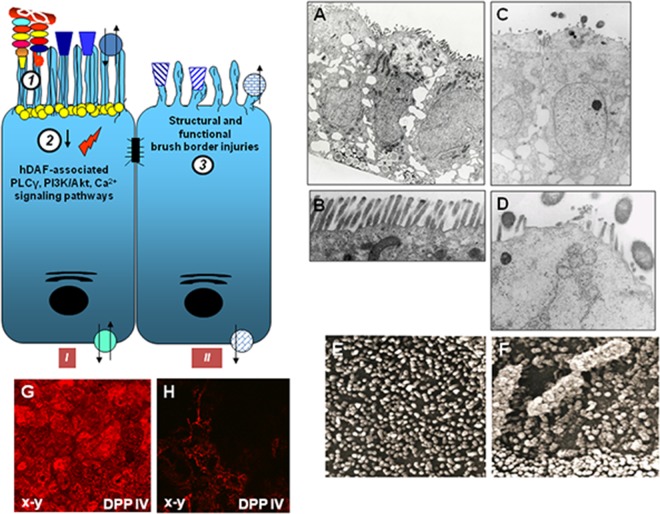
Membrane lipid rafts are heterogeneous sterol-sphingolipid-enriched domains that can dynamically associate and dissociate (310, 311). How lipids and proteins assemble for the structural and functional organization of the membrane lipid rafts remains not entirely understood. In the cell membrane, these dynamic entities assemble molecules expressing the GPI anchor, acylation, or certain transmembrane domains which, following the recruitment and connection with cytoplasmic effectors, function as platforms of signal transduction. Different models of lipid raft organization have been proposed, including the model for the apical membrane of epithelial cells proposed by Kay Simons and coworkers (312) consisting of a continuous lipid raft phase within which isolated non-lipid raft-phase domains are randomly distributed. It has been proposed that the family of integral membrane flotillin/reggie proteins facilitates the physical organization of lipid raft macrodomains (313–315). Moreover, a particular lattice network of filaments named the cortical actin cytoskeleton underlies the plasma membrane and allows a connection with lipid rafts (316). In addition, other proteins, including supervillin, myosin-IIA, myosin IG, and ezrin-radixin-moesin (ERM), associate with lipid rafts to establish a connection with the cortical actin cytoskeleton (316). By their adhesins that recognize signaling molecules associated with lipid membrane rafts, Afa/Dr DAEC activates diverse signaling pathways that produce deleterious effects on the host cells but also various cellular defense responses against infection (Fig. 4). Wild-type Afa/Dr DAEC and recombinant strains of E. coli that express the AfaE-I, AfaE-II, and AfaE-III Dr adhesin subunits, the Dr-II adhesin, or the F1845 adhesin promote the hDAF receptor clustering around bacteria adhering to the epithelial cell surface (39–41, 45, 102, 188, 189, 258, 317, 318) (Fig. 3F and G). Using draE mutants with impaired type IV collagen and chloramphenicol binding sensitivity but retaining hDAF-binding capability (185), it has been observed that D54V, D54Y, T90M, and I113T DraE adhesin subunit mutants conserved the property of inducing hDAF receptor clustering around adhering bacteria, while the D54G mutant (Asp54 was replaced with glycine) and the D54C mutant (Asp54 was replaced with cysteine) lose the receptor-clustering activity (41). Das et al. (319) observed that hDAF receptor clustering around adhering bacteria was lower in HeLa and CHO-hDAF cells infected with E. coli mutants in which mutations at the T31A and Q34A amino acids of the DraE adhesin subunit hydrophobic II domain had been induced. Guignot et al. (41) determined the roles of hDAF epitopes in hDAF receptor mobilization around adhering Dr-positive bacteria. Using hDAF mutants expressed by stably transfected CHO cells, it has been found that the absence of CCP2 or CCP3 entirely abolished the receptor-clustering activity. Absence of the CCP-4 domain did not affect receptor clustering, whereas the role of the CCP-1 domain remains uncertain (41, 258). Moreover, the lack of the heavily O-glycosylated STP region abolished the receptor-clustering activity (41).
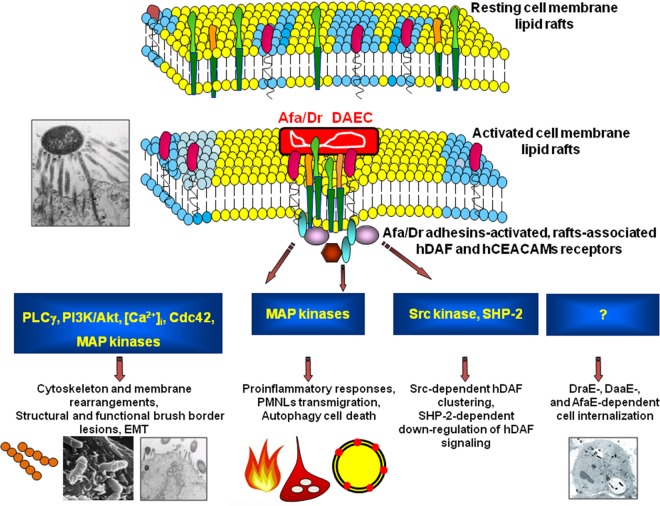
FIG 4.
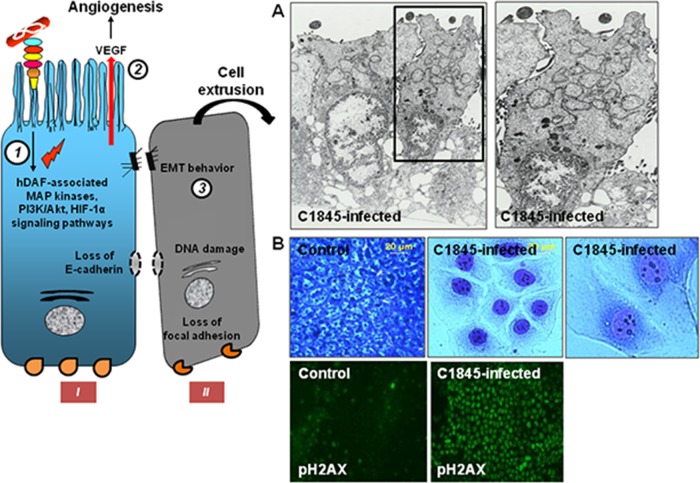
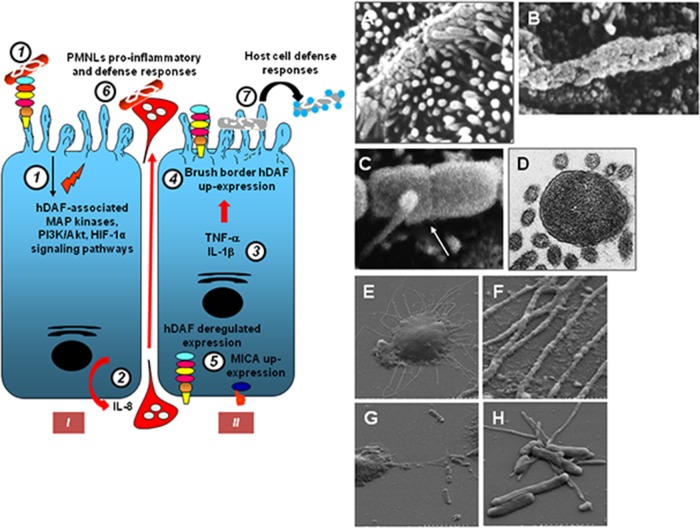
Summary of lipid raft-associated signaling pathways involved in Afa/Dr DAEC pathogenesis. A high-magnification micrograph shows a bacterium interacting with a large number of microvilli at an early time postinfection. Afa/Dr adhesins recognize as receptors the GPI-anchored hDAF, hCEA, and hCEACAM6 and the transmembrane hCEACAM1 proteins. hDAF, hCEA, and hCEACAM6 are endogenously associated with lipid rafts, and a part of hCEACAM1 is translocated within membrane lipid rafts after Afa/Dr DAEC infection. hDAF-dependent signaling involving protein tyrosine kinase(s), phospholipase Cγ, phosphatidylinositol 3-kinase (PI3K), protein kinase C, and an increase in [Ca2+]i leads to structural and functional lesions at the brush border of enterocyte-like cells. hDAF-, hCEA-, and hCEACAM6-dependent signaling involving the Rho GTPase Cdc42 and ERM proteins leads to membrane elongation. hDAF-dependent signaling involving MAPKs and PI3K/Akt lead to HIF-α-dependent VEGF production and epithelial-mesenchymal transition (EMT). hDAF-dependent signaling involving MAPKs leads to proinflammatory cytokines responses, PMNL transmigration, and autophagy followed by cell detachment. Src kinase is necessary for hDAF clustering around adhering bacteria. Phosphorylation of hCEACAM1-4L at ITIMs and recruitment of SHP-2 lead to a negative regulation of phosphorylation of Src associated with hDAF signaling. The DraE-, DaaE-, and AfaE-triggered dynamic microtubule-dependent internalization of bacteria is a lipid raft-dependent phenomenon involving hDAF, hCEACAM1, hCEA, and hCEACAM6.
Guignot et al. (41), when they first identified hCEA as a receptor for Dr and F1845 adhesins in stably transfected HeLa cells expressing hCEA, observed the hCEA receptor clustering around Dr adhesin-positive E. coli infecting HeLa cells (Fig. 3F and G). Berger et al. (274), when identifying the epithelial hCEACAM1 and hCEACAM6 as additional receptors for the Dr adhesin, F1845 adhesin, and AfaE-III adhesion subunit, observed hCEACAM receptor clustering around adhering Dr adhesin-positive E. coli (Fig. 3F and G). Like hDAF receptor clustering, the hCEA receptor clustering is not promoted by the DraE adhesion subunit mutant D54C (41). Consistent with the presence of distinct hDAF and hCEA binding domains in the DraE adhesin subunit (160) (Fig. 3E), colocalization of hDAF and hCEACAM immunolabeling develops around Dr adhesin-positive E. coli adhering to HeLa cells constitutively expressing hDAF and transfected for the expression of hCEA (274) (Fig. 3G).
Membrane-associated lipid rafts are currently defined as dynamic sterol-sphingolipid-enriched nanoscale domains of different sizes containing GPI-anchored proteins (310–312). Interestingly, quite large and highly stably organized “super lipid rafts” are present at the membrane of the brush border of enterocytes (320, 321). Adherence of Dr adhesin-positive bacteria to epithelial HeLa cells constitutively expressing hDAF, to stably transfected CHO cells expressing hDAF, hCEA, and/or hCEACAM6, or to HeLa cells transfected for stable expression of hCEA or hCEACAM6 results in recruitment of raft markers GM1 and VIP21/caveolin (188, 317) (Fig. 5A). A similar recruitment of VPI21/caveolin has been observed around Dr adhesin-positive E. coli infecting primary bladder epithelial cells (188). The mobilization of lipid rafts has also been observed for viruses recognizing hDAF (322, 323) and Opa-expressing Neisseria recognizing hCEACAMs (324, 325). The cortical actin cytoskeleton provides a structural organization of lipid rafts, and a well-organized actin cytoskeleton is required for the completion of regulating raft-associated signaling events (316). In stably transfected cells expressing hDAF, hCEA, or hCEACAM6, the receptor clustering around adhering Dr adhesin-positive E. coli is associated with the clustering of fine rings of cytoskeleton-associated proteins, such as F-actin, α-actinin, and phosphorylated ezrin (40, 188, 317). In contrast and surprisingly, there was an absence of recruitment of F-actin around Dr adhesin-positive E. coli infecting primary bladder epithelial cells constitutively expressing hDAF (188). The Afa/Dr adhesin-induced F-actin mobilization differed markedly from the dramatic F-actin mobilization induced by EPEC (326) and Salmonella (327). Fine rings of F-actin have also been observed to be associated with Opa-expressing Neisseria infecting hCEACAM1-, hCEA-, and hCEACAM6-expressing cells, whereas dense rings of F-actin ringed the Opa-expressing Neisseria adhering to hCEACAM3-expressing cells (328). The fine rings accompanied the low level of F-actin-independent cell entry into hCEACAM1-, hCEA-, and hCEACAM6-expressing cells, and the dense rings accompanied the high level of F-actin-dependent, small Rac1- and Cdc42 GTPase-triggered cell entry into hCEACAM3-expressing cells (329, 330). It is noticeable that the low level of Afa/Dr DAEC internalization into hCEA- and hCEACAM6-expressing HeLa and CHO cells is not affected by cytoskeleton blockers (189, 284, 317, 331). Moreover, the recruitment of F-actin around adhering Dr adhesin-positive bacteria plays no role in bacterial internalization, since although the DraE adhesion subunit mutant D54G has impaired F-actin mobilization, it displays an unchanged level of cell entry (317). Collectively, the results obtained with Afa/Dr DAEC indicated that the recruitment of F-actin forming a fine ring around adhering bacteria probably reflects the physical mobilization of F-actin-containing lipid rafts by the adhering bacteria rather than the recruitment of F-actin for subsequent bacterium-triggered cellular events.
FIG 5.
hDAF- and hCEACAM1-4L-associated lipid raft signaling pathways involved in Afa/Dr DAEC pathogenesis. (A) Micrographs show the observation by CLSM of the mobilization of the lipid raft marker ganglioside GM1 around Dr adhesin-positive E. coli adhering to HeLa cells. Yellow, colocalization of Dr adhesin and GM1 immunolabelings. (Reprinted from reference 317.) (B) Dr adhesin-induced phosphorylation of Src in infected HeLa cells. (C) Passage of hCEACAM1 into lipid rafts in transfected hCEACAM1-HeLa cells infected with Dr adhesin-positive E. coli. TfR, non-lipid raft transferrin receptor. (D) Recruitment of SHP-2 around Dr adhesin-positive E. coli adhering to transfected HeLa cells expressing hCEACAM1. (E) Time-dependent association of SHP-2 with hCEACAM1 in transfected HeLa cells expressing hCEACAM1 and infected with Dr adhesin-positive E. coli. (In panels B to E, data and micrographs reprinted from reference 318 with permission.) The drawing on the right indicates the sites of phosphorylation observed in hDAF and hCEACAM1-4L after Afa/Dr DAEC infection.
During the adhesion step preceding cell entry, the α5β1 integrin has been observed to be mobilized so as to form fine rings around adhering Dr adhesin- or AfaE-III adhesion subunit-positive bacteria (188, 189, 317, 332). In addition, AfaE/D adhesin subunit-coated beads adhering onto HeLa cells are decorated by rings of positive immunofluorescence for α5β1 integrin (167). Moreover, it was noted that the clustering of β1 integrin around E. coli adhering to CHO-hDAF-α5β1 cells occurred for bacteria expressing DraE or AfaE-III adhesin subunits alone, regardless of the presence or absence of DraD or AfaD-III adhesin subunits (189). The mobilization of β1 integrin by Afa/Dr DAEC adhering to the cell surface of undifferentiated epithelial cells could result from the mobilization of lipid rafts containing integrins. Indeed, integrins have been found in lipid rafts engaged in cell adhesion (333–335), cell migration (336, 337), and contractile forces for cell invasion (337–339). As discussed above for F-actin mobilization, the recruitment of β1 integrin by adhering Afa/Dr DAEC can reflect the physical mobilization of β1 integrin-containing lipid rafts by the adhering bacteria.
hDAF-dependent signaling.
hDAF has a signal transduction capacity associating Src tyrosine kinases p56lck and p59fyn (251). Phosphorylation of Src develops in epithelial HeLa cells infected with Dr-positive E. coli (318) (Fig. 5B). When examining the involvement of Src kinases in Dr adhesin-induced hDAF signaling, Queval et al. (258) observed the recruitment of phosphorylated Src kinases together with hDAF around adhering recombinant Dr adhesin-positive E. coli in infected hDAF-transfected CHO cells and in constitutively hDAF-expressing HeLa and human embryonic kidney HEK293 cells. CCP-4 of hDAF plays a crucial role in the recruitment of phosphorylated Src kinases and Src kinase activation, while deletion of CCP-1 had no effect. Moreover, small interfering RNA (siRNA) silencing of c-Src in HeLa cells abolishes hDAF clustering around adhering Dr-positive bacteria, while siRNA silencing of the Src kinases Yes, Fyn, and Lyn does not (258). Finally, the observation that the D54C DraE adhesion subunit mutant fails to induce hDAF clustering and Src recruitment confirms the predominant role of this domain of the DraE adhesin subunit in triggering cell responses.
A variety of cell signaling pathways are activated after the recognition of hDAF by Afa/Dr adhesins. Phosphatidylinositol 3-kinase (PI3K) is activated in undifferentiated intestinal INT407 cells (340), and phosphorylated PI3K is recruited around Dr adhesin-positive E. coli adhering to human differentiated primary bladder cells expressing hDAF and hCEACAMs (188). Triggered by the Dr and F1845 adhesins, an hDAF-dependent activation of mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase 1/2 (Erk1/2), p38 mitogen-activated protein kinase (p38), and stress-activated protein kinase/c-Jun N-terminal kinase SAPK/JNK (JNK), occurred in enterocyte-like Caco-2 cells and colonic T84 cells (341–345). Src kinase and NF-κB and AKT signaling pathways are hDAF-dependently activated in C1845-infected T84 cells (345). Moreover, hDAF-dependent activation of tyrosine kinases and protein kinase C (PKC) develops in Afa/Dr DAEC-infected polymorphonuclear leukocyte (PMNL)-like cells (346). In addition, MAPK and NF-κB activation occurred in PMNL-like cells triggered by type 1 pili and independently of hDAF recognition (347).
hCEACAM-dependent signaling.
Like the Opa52 protein of Neisseria, which recognizes the N-terminal domain of CEACAM1-4L and promotes its association with the tyrosine phosphatases SHP-1 and SHP-2 in the cytoplasmic ITIM domains (348), Dr adhesin-expressing E. coli binding to HeLa cells transfected for the expression of hCEACAM1-4L leads to the translocation of a part of CEACAM1-4L into the membrane lipid rafts and phosphorylation of its ITIM and SHP-2 recruitment (318) (Fig. 5C to E). In turn, hCEACAM1-4L has been found to play a key role in downregulating the activity of the protein tyrosine Src kinase associated with hDAF signaling (318).
Urinary Tract Infections and Pregnancy Complications
Recurrent cystitis in the bladder and acute pyelonephritis in the kidney, corresponding to 80% of all UTIs, result from infection by UPEC (199, 349). Bacteriuria is the clinical sign of UTI. Recurrent cystitis is a major health problem. Indeed, a recurrence of cystitis within 3 to 4 months develops in 20 to 30% of women who had developed a first acute infection. Lower UTIs affect the urethra and bladder, whereas upper UTIs affect the ureters and kidneys, and both can be either uncomplicated or complicated. Cell insults together with an intense mucosal inflammatory response, including the recruitment of neutrophils, lead to cystitis in bladder and pyelonephritis in kidney. It should be noted that asymptomatic bacteriuria (ABU) has been observed in 2 to 20% of the population, depending on age and gender (199). The infecting E. coli strain involved in ABU colonizes the urothelium and may remain present for months or years, resulting in a low-level deleterious tissue attack effect and an innate immune response that is too weak to cause symptoms. The observation that UPEC virulence gene sequences remained present in ABU strains has led to the suggestion that the lower virulence of ABU results from a shift from UPEC as a result of genome reduction caused by inactivation of virulence genes as well as by deletions or by the accumulation of point mutations (350, 351), although other causes can also be involved (352).
UPEC strains have an elevated organ tropism and ascend the urinary tract from the urethra to the bladder and kidneys (1, 199, 232). To do this, UPEC expresses a wide variety of adhesive factors, including type 1 pili, type IV pili, and P, S, F1C, Auf, Yad, Ygi, and F9 fimbriae (199, 353). Moreover, UPEC has developed a reciprocal regulation of adhesive factors and motility (199). UPEC strains have developed sophisticated strategies to avoid clearance by micturition involving colonization of the urothelium and cell internalization, which thus allow them to survive and evade host innate immune defenses. For pathogenesis, UPEC expresses PAIs of different sizes containing assemblages of genes encoding virulence factors such as adhesins, invasins, capsule, proteases, and multiple siderophore systems, including aerobactin, IroN, and IreA (199). Moreover, UPEC secretes cytotoxic toxins, including the repeat-in-toxin α-hemolysin (HlyA), cytotoxic necrotizing factor (CNF-1), and diverse SPATE toxins, such as Sat, Pic, PicU, Tsh, and Vat. In addition, UPEC adapts to survive in the urine by expressing factors involved in scavenging of nutrients.
Internalization.
Cell entry of E. coli expressing the Dr, F1845, or Afa-III adhesin has been examined in cervical epithelial HeLa cells and endometrial cells constitutively expressing hDAF but not hCEACAMs, in undifferentiated intestinal cells and differentiated primary epithelial bladder cells constitutively expressing hDAF and hCEACAMs, and in CHO or HeLa cells transfected for stable expression of hDAF, hCEACAM1, hCEA, or hCEACAM6 (38, 42, 188, 189, 234, 259, 317, 319, 331, 332, 354). Afa/Dr DAEC displays a rate of cell entry similar to that observed with UPEC expressing type 1 pili (199, 232). Dr and Afa-III adhesin-positive E. coli used a membrane zipper-like mechanism to enter the HeLa cells (42, 317), and Afa-III adhesin-positive E. coli entered the cells via a single internalization vacuole (42). The cell membrane lipid rafts play a pivotal role in the internalization of Dr- and Afa-III adhesin-positive E. coli into cultured cervical HeLa cells, primary cultured human bladder cells, and epithelial cells transfected to express hDAF, hCEACAM1, hCEA, or hCEACAM6 by a mechanism involving cell microtubules but not microfilaments (38, 188, 189, 259, 317, 331). A particular network of microtubules, i.e., the dynamically unstable microtubule network, has been found to be involved in the cell entry of Dr adhesin-positive E. coli (331). Rana et al. (234), using a PCR-based proximity ligation assay to detect protein-protein interactions, observed a strong fluorescent signal of hDAF/tubulin in Dr-positive E. coli-infected HeLa cells resulting from the proximity between the two molecules, suggesting that hDAF and microtubules can be physically associated after infection. The Afa/Dr DAEC cell entry resembles the lipid raft- and microtubule-dependent zipper-like uptake of Opa-expressing Neisseria into transfected CHO and HeLa cells expressing hCEA or hCEACAM6 (330). It was noted that a few invasive bacteria, including, for example, Haemophilus influenzae, Klebsiella pneumoniae, and pilus type 1-expressing UPEC, also require a functional host microtubule network for the invasion of host epithelial cells (355). Consistent with the F-actin microfilament-independent cell entry of Dr-positive E. coli, the DraE adhesin subunit mutant strains D54G and D54C, showing absence and presence of changes in F-actin mobilization around adhering bacteria, respectively, displayed unchanged levels of cell internalization (317). Microtubule-dependent internalization of Dr adhesin-positive E. coli within primary bladder epithelial cells has been found to result from the engagement of the actin-binding proteins ezrin/radixin/moesin (ERM) (188). Observations that phosphorylation of ERM accompanied the Dr-positive E. coli cell entry (188) agree well with the know role of ERM, which together with Rho GTPases act in the remodeling of host cell cytoskeleton (356). It has been noticed that the efficiency of bacterial cell entry resulting from the action of T3SS-dependent effectors seems to be higher than that resulting from microtubule-dependent processes triggered by bacteria that do not express the T3SS.
Selvaragan et al. (259) have determined the roles of hDAF epitopes in the cell entry of Dr adhesin-positive bacteria using hDAF deletion mutants expressed by stably transfected CHO cells. The absence of CCP-2 or CCP-3 entirely abolished the cell entry of Dr-positive bacteria, whereas the lack of CCP-1 or CCP-4 had no effect. Deletion of the heavily O-glycosylated STP region abolished the cell entry. In contrast, replacing the GPI anchor with the transmembrane anchor of HLA-B44 or membrane cofactor protein did not modify the cell entry. Rana et al. (234) have examined for adhesion/invasion five hDAF mutants previously used to map the extracellular CCP-2/CCP-3 domains of hDAF involved in Dr adhesin-positive E. coli adhesion (260). Compared to hDAF, the Phe123-Ala mutant conserves both normal binding activity and invasion rate, the Ser165-Ala mutant conserves normal binding activity but displays a lower invasion rate, and the Gly159-Ala mutant shows both reduced binding capacity and a lower invasion rate. In contrast, the Phe148-Ala and Phe154-Ala mutants display normal binding capacity combined with an increased rate of invasion.
In terms of cell signaling, protein tyrosine kinases (PTKs), phospholipase Cγ (PLC-γ), and protein kinase C (PKC) blockers have no effect on the cell internalization of Dr adhesin-positive E. coli (317). In contrast, the PI3K-dependent signaling pathway is engaged in the internalization of Dr adhesin-positive E. coli into hCEACAM1-, hCEA-, and hCEACAM6-expressing epithelial cells but not hDAF-expressing cells (188). While Src-dependent signaling does not play any role in internalization (188), Src and phosphorylated Src have been observed to be recruited around adhering Dr-positive E. coli (258).
The β1 integrin is the host cell receptor triggering the zipper-like internalization of type 1 pilus-expressing UPEC (199, 232). A polyclonal antibody directed against anti-α5β1 integrin abolishes the basolateral entry of Dr adhesin-positive E. coli within undifferentiated intestinal Caco-2 cells (331). Comparison of the cell entry of beads coated with rAfaD-III adhesin subunit into mouse endodermal carcinoma F9-TKO cells stably transfected or not to express β1 integrin shows that the presence of the integrin increased the bead internalization level 2.3-fold (332). In addition, in HeLa cells subjected to incubation with AfaD-III or AfaD-VIII adhesin subunits, membrane immunoprecipitation showed that AfaD-III and -VIII adhesin subunits coprecipitated with β1 integrin (332). The role of α5β1 integrin as a receptor for internalization of E. coli expressing Dr- or Afa-III adhesin subunits into epithelial cells has been recently revisited (189). The overexpression of α5β1 integrin as a result of transfection in the CHO B2 clone, which does not express α5β1 integrin, does not increase the levels of adhering and internalized Dr- or Afa-III adhesin-positive E. coli compared to that in untransfected cells. To test whether the coexpression of α5β1 integrin together with hDAF, hCEACAM1, hCEA, or hCEACAM6 influences the adhesion and/or cell entry of Dr adhesin-positive E. coli, CHO cells transfected for the stable expression of hDAF, hCEAM1, hCEA, or hCEACAM6 were transfected again with the gene coding for the α5 or β1 integrin subunit (189). In all cases, the presence of α5β1 integrin together with hDAF or CEACAMs did not increase the levels of adhering and internalized Dr adhesin-positive E. coli. In addition, the knockdown of the gene coding for β1 integrin by siRNAs in HeLa cells constitutively expressing α5β1 integrin did not affect the level of adhering and internalized Dr- or Afa-III adhesin-expressing E. coli compared to that in untreated cells (189). It is important to note that the fact that integrins are located exclusively at the basolateral domain and never at the brush border of the polarized intestinal epithelial cells renders the integrins inaccessible for intestinal epithelium infection by enterovirulent Afa/Dr DAEC residing within the luminal compartment.
The identity of the virulence factor(s) of Afa/Dr DAEC involved in the invasion of epithelial cells has long been debated. It has been proposed by Le Bouguénec and coworkers (357) that the D adhesin subunits encoded by the dra, daa, and afa-1 to -3 operons function as an invasin for the entry of Afa/Dr DAEC into urothelial and bladder cells. DraD and AfaD adhesin subunits have been identified at the tips of Afa/Dr fibrils (167, 180). AfaD protein, unlike the AfaE protein, was able to detach from the bacterium (28, 168, 169). Using recombinant E. coli producing the AfaD or AfaE-III adhesin subunit, it has been observed that the AfaE-III adhesin subunit triggers the binding of the recombinant E. coli to cells and that the AfaD adhesin subunit mediates the cell entry within epithelial HeLa cells (42, 168). When HeLa cells are infected with an Afa-III adhesin-positive E. coli strain, the AfaE-III protein remains localized at the membranes of infected cells, and only the AfaD-III protein coats the internalized bacteria residing within an internalization vacuole (169). In addition, coating polycarbonate beads with AfaD protein allows the beads to penetrate the epithelial cells (28). Moreover, using colloidal gold-tagged AfaE-III and AfaD proteins, it has been shown that AfaE-III–gold complexes associate with the cell surface, whereas AfaD-gold complexes are internalized within the cells (42). In human undifferentiated Caco-2, T84, and INT407 cells, urothelial T24 cells, and cervical HeLa cells, rAfaD-III- and rAFaD-VIII adhesin subunit-coated beads invaded the cells, whereas rAfaE-III adhesin subunit-coated beads were not internalized (332). In contrast, according to Nowicki and coworkers, the DraE adhesin subunit alone is sufficient to allow the entry of Dr adhesin-positive bacteria into epithelial cells. Indeed, both the purified Dr fimbriae and latex beads coated with Dr adhesin are internalized into HeLa cells (259, 319). Moreover, the insertional draE, draC, and draB and adherent draD mutants were unable to enter epithelial cells, and complementation of the draE mutation restored the invasion property (38). Mapping of the DraE adhesin subunit shows that amino acids localized on hydrophilic domain II, and in particular the V28, T31, G33, Q34, L35, T36, and P40 amino acids, reduced or abolished bacterial cell entry into transfected CHO-hDAF cells and HeLa cells constitutively expressing hDAF but did not affect attachment (319). The role of E and/or D adhesin subunits in the internalization of Afa/Dr DAEC has been recently reexamined using appropriate E. coli mutant strains and cultured unpolarized epithelial cells and bladder cells (188, 189). Korotkova et al. (188) found that expressing combinations of the DraE+/DraD+ and DraE+/DraD− adhesin subunits allowed E. coli to enter differentiated primary bladder cells, while the combination of DraE−/DraD+ adhesin subunits did not. Guignot et al. (189) have found that in CHO cells transfected to express hDAF and α5β1 integrin, recombinant E. coli expressing combinations of DraE+/DraD+ or AfaE+/AfaD+-III adhesin subunits adhered and were internalized and that deletion of the DraD adhesin subunit did not modify the level of internalized bacteria. Collectively, these last findings fit in well with the previous results reported by Nowicki and coworkers (38, 319), and taken together they clearly establish that the DraE and AfaE-III adhesin subunits are necessary and sufficient to promote the cell entry of Dr and Afa-III adhesin-expressing E. coli into epithelial cells. However, one question remains unresolved concerning the functions of the DraD, AfaE, and DaaD adhesin subunits in the pathogenesis of Afa/Dr DAEC when the proteins are detached from the fimbriae (42) or when each of the proteins are present alone at the bacterial cell surface (42, 168, 169). It is noteworthy that the DraD adhesin subunit, whether fimbria associated or not, seems to function in the formation of biofilm by Dr adhesin-expressing E. coli (190, 233).
Intracellular lifestyle.
When internalized within nonphagocytic epithelial cells, enteric bacterial pathogens reside and survive in the cell cytoplasm in small or large vacuoles, where they may or may not replicate (358); only a few invasive enteric bacterial pathogens have developed strategies to escape from the vacuole to gain access to and proliferate within the host cell cytosol (359). After entering HeLa cells inside a single internalization vacuole, Afa/Dr DAEC survived within large late vacuoles (38, 40, 42, 94, 189, 234, 259, 319, 331, 332) (Fig. 6A). As revealed by transmission electron microscopy examination, the late large vacuole containing internalized Dr or Afa-III adhesin-expressing bacteria appears to result from the fusion of early vacuoles formed during the initial step of the internalization process, each containing a single bacterium (42, 234, 259, 319). Recent data have shown that the internalization vacuoles containing Dr adhesin-positive bacteria interacted with the cell endocytic pathway. This pathway includes a complex and multifunctional set of vesicular compartments essentially derived from internalization of the plasma membrane, resulting in the formation and maturation of well-formed endocytic compartments (360). These compartments are sequentially modified by acquiring diverse vesicular elements to form the early endosomes and subsequently the late endosomes. In the last step, the intraluminal vesicles are taken for delivery of their contents to the lysosome, which is a stable organelle that avoids self-degradation (361). The intracellular vacuoles containing Dr adhesin-positive E. coli display some of the characteristics of late endosomes, such as the membrane expression of the Lamp-1, Lamp-2, and CD63 proteins but not of cathepsin D, and are acidic (189) (Fig. 6B). This resembles the characteristics of the UPEC UTI89-containing vacuoles, which are positive for Lamp-1 and CD63, cathepsin D negative, and acidic (362). Surprisingly, despite the facts that Dr adhesin-positive bacteria bind to membrane-associated hDAF during the first step of cell association (41, 257–260) and that hDAF has been observed associated with the membrane invagination during the first step of adhesion/internalization (45), the membranes of the large late vacuoles containing the internalized Dr-positive bacteria do not contain hDAF (189).
FIG 6.
Cellular events observed in cervical epithelial HeLa cells infected with uropathogenic Afa/Dr DAEC or subjected to Sat toxin treatment. The drawing on the left summarizes the observed cellular events. Internalization of bacteria occurs in nonpolarized epithelial cells via the recognition of membrane-associated hDAF, or hCEACAM1, hCEA, and hCEACAM6 by a mechanism involving lipid rafts and dynamic microtubules (1). Internalized Afa/Dr DAEC cells have survived within large vacuoles, which seems to result from the fusion of early vacuoles, each containing one bacterium formed during the initial step of internalization, that tested positive for early and late endosome markers but not for the autophagy LC3 marker (2). The secreted and cell internalized Sat toxin (3) promotes a dramatic loss of F-actin stress fibers (4), and in turn the intoxicated cell engage an autophagy cell survival response (5). The massive appearance of autophagosomes is followed by the blockade of autophagy flux, leading to the lack of maturation of autophagosomes into autophagolysosomes (5). As the result of autophagic cell death, the cells lose the focal adhesion contacts and become detached from the substratum (6). (A) Transmission electron micrograph showing vacuoles containing internalized Afa-III adhesin-positive E. coli in HeLa cells. Arrows indicate the vacuole-containing internalized bacteria. (Reprinted from reference 332 with the permission of the publisher. Copyright 2003 Blackwell Publishing Ltd.) (B) High-magnification micrographs show the observation by CLSM of Lamp1 or CD63 immunolabeling (blue) in membranes of vacuoles containing the internalized Dr adhesin-positive E. coli in infected HeLa cells (green). Red, extracellular adhering bacteria. (Reprinted from reference 189.) (C) High-magnification micrographs show the observation by CLSM of Sat immunolabeling present in the cytoplasm of cells treated with cell-free culture supernatant of AAEC185pSatIH11128 containing the secreted Sat toxin (CFCSSat) (red) and the appearance of green fluorescent protein (GFP)-LC3 autophagic vacuoles in CFCSSat-treated cells (green). (Reprinted from reference 374 with the permission of the publisher. Copyright 2011 Blackwell Publishing Ltd.) (D) High-magnification micrographs show the observation by CLSM of paxillin immunolabeling (red). Note the disappearance of paxillin in in CFCSSat-treated cells. (Reprinted from reference 374 with the permission of the publisher. Copyright 2011 Blackwell Publishing Ltd.)
Cellular autophagy (363) is an evolutionarily conserved process by which cellular cytosolic structures and cell constituents are degraded and recycled (364). Some intracellular bacteria are detected and eliminated via the autophagic pathway, and several intracellular bacteria have developed sophisticated avoidance strategies, but this pathway may also serve as a protected niche providing a source of nutrients for intracellular bacteria (365). Infecting UPEC was rapidly cleared in the presence of a deficiency of the key autophagy protein Atg16L1, suggesting that UPEC may subvert autophagy proteins to establish latency (366). However, the autophagy pathway can favor the pathogen, since internalized bacteria present within intracellular bacterial communities in superficial bladder cells colocalized with Atg16L1 and LC3 puncta (366). No interaction has been observed between the Dr adhesin-positive E. coli-containing vacuoles and the autophagic pathway (189, 234). Moreover, induction of autophagy in Dr adhesin-positive E. coli-infected cells has no effect on the survival of internalized bacteria (137). It is noteworthy that autophagy clears intracellular AIEC and that induction of autophagy accelerates this phenomenon (367–369). These results indicate that by blocking the association of vacuole-containing bacteria with the autophagosome, Dr adhesin-positive E. coli has developed a strategy to escape the host defense autophagy. The same phenomenon has been observed for several invading pathogens (370).
Internalized Dr adhesin-positive E. coli survived up to 72 h postinfection in HeLa cells transfected to express hDAF and to a lesser extent in cells expressing hCEACAM1, hCEA, or hCEACAM6 (189). Dr adhesin-positive bacteria intracellularly present within a vesicular compartment in the host cell have no impact on the cell survival (189, 331) and do not affect functional epithelial intestinal cell differentiation (331). It has been recently observed that a Ser165-Ala mutation in the CCP-3 extracellular domain of hDAF promotes the dormant vacuolar persistence of internalized Dr adhesin-positive E. coli, whereas a Phe154-Ala mutation promotes the multiplication of vacuolar bacteria (234). Whether these mutations in the extracellular domain of cell membrane-associated hDAF can impact the lifestyle of internalized Dr adhesin-positive E. coli remains unclear and needs to be explained, considering that hDAF is not present at the membrane of intracellular vesicles containing the internalized bacteria (189). On the basis of these results, it looks as if internalized Dr adhesin-positive E. coli cells enter the endocytic pathway (364, 371) in order to reach a protective niche with a low-pH environment where they can live and remain metabolically active in order to replicate or enter dormancy. The observation that the Dr adhesin-positive E. coli-containing large vacuoles are phenotypically quite similar to lysosomes indicates that internalized bacteria have developed a capacity to actively modify this compartment so as to create a distinct compartment. The mechanism(s) by which internalized uropathogenic Afa/Dr DAEC cells modify the vacuolar compartment and lead it to engage in dormancy or intracellular replication remains to be elucidated. A process designated “nutritional virulence” (372) has recently been defined as used by several invasive bacteria which, to allow themselves to grow, subvert the host cell machinery so as to create a vesicular developmental niche where they receive components from the secretory pathway as a source of nutrients. It will be interesting to investigate whether internalized Afa/Dr DAEC cells do or do not develop a “nutritional virulence” mechanism in order to permit their intracellular residence and survival. The observation of an intracellular lifestyle of Dr adhesin-positive E. coli with intracellular survival is interesting in term of persistence and recurrence of infection. Indeed, it is worth mentioning that type 1 pilus-positive UPEC after cell entry resided in endocytic vesicles in which its replication is restricted and which form an intracellular niche that is protected from host immunity responses and killing of intracellular bacteria by antibiotics (232). This intracellular lifestyle permits the long-term maintenance of quiescent UPEC in the bladder cells (232).
Cell detachment.
To protect the epithelia against bacterial colonization and invasion and to maintain tissue homeostasis, the host has developed defense systems including exfoliation and cell death of bacterially infected cells (370). The eviction of cells from the epithelium can occur by a process known as extrusion following detachment from the extracellular matrix and loss of cell-to-cell contact or toward the basal surface by delamination after disruption of cell-to-cell contacts (373). For example, exfoliation of fully differentiated umbrella epithelial cells infected by FimH-positive E. coli is an innate host defense mechanism that has the effect of removing adhering and invaded bacteria from the bladder (199, 232). However, some invasive bacterial pathogens have developed countermeasures intended to antagonize the host epithelial turnover to exploit infected epithelial cells as a survival and replicative niche (370). As an adverse effect for the host, the detachment of cells containing intracellular UPEC promotes the reinitiation of the UPEC infectious cycle (199, 232).
A cell detachment effect triggered by the Sat toxin expressed by Afa/Dr DAEC has been recently described (137, 206, 210–212). Sat promotes a profound disorganization of the F-actin cytoskeleton in HeLa cells that is followed by a host cell survival response involving the noncanonical autophagy pathway (374). The autophagy pathway induced is not intended to destroy the intracellular toxin, since the toxin is never present in autophagic vesicles. The Sat-intoxicated cells displayed a massive intracellular appearance of autophagic vacuoles which, at the autophagosome step, fail to mature into autophagolysomes. In turn, the Sat-intoxicated cells overexpressing autophagosomes die as a result of autophagy cell death. A similar effect has been reported for α-hemolysin of Streptococcus aureus (375). The Sat-induced autophagy cell death is accompanied by a dramatic disassembly of the focal adhesion-associated vinculin and paxillin, which finally promotes cell detachment (374). It is noteworthy that Capello et al. (376) have observed that the class I SPATE toxin Pet of EAEC induces the cleavage of FAK and a deterioration of focal adhesion complexes resulting from the redistribution of paxillin and vinculin and the depletion of phosphotyrosines. Together with a loss of F-actin stress fibers and α-actinin and spectrin network disassembly, these dramatic rearrangements of structural and functional proteins result in cell rounding and detachment of Pet-intoxicated cells from the substratum (376–378). Interestingly, the UPEC pore-forming HylA triggering cell exfoliation of bladder epithelial cells induces cell rounding and a complete loss of the microtubule network and F-actin stress fibers, accompanied by the degradation of paxillin (379).
Inflammatory responses.
New information on immune responses accompanying UPEC infection have recently been obtained in mice models (380, 381). UPEC infection is accompanied by a strong inflammatory response, including the production of cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-8, IL-17a, and granulocyte colony-stimulating factor (199, 382). Moreover, neutrophils and macrophages play a pivotal role in the defense against UTI (383). Although not observed in urinary cell models, it is important to note that the UTI-associated wild-type Afa/Dr DAEC strain IH11128 promotes an hDAF- and MAPK-dependent production of IL-8 (341) followed by transepithelial migration of PMNLs (341), triggering the production of TNF-α (342).
Animal models of UTIs.
Dr adhesin-positive E. coli leads to chronic pyelonephritis in experimental mice (301, 384). In contrast, wild-type strain IH11128, an isogenic mutant that is devoid of Dr adhesin, does not cause kidney infection or cellular lesions and is gradually eliminated compared to the wild-type strain. In the kidney tissue of Dr adhesin-positive E. coli-infected mice, Dr antigen was present in the injured parenchymal regions characterized by histological changes indicating tubulointerstitial nephritis (384). It is notable that mouse DAF (mDAF) probably plays no role in this Dr adhesin-induced chronic ascending pyelonephritis in mice, since Hudault et al. (254) have demonstrated that Dr adhesin binds specifically to hDAF but not to rodent DAF. Importantly, Selvarangan et al. (301) have demonstrated the crucial role of the type IV collagen-binding capability of Dr adhesin for pathogenesis in mouse kidney. Indeed, an isogenic DraE adhesin subunit mutant lacking binding to type IV collagen fails to persist within the mouse renal tissues, and the transcomplementation of the mutant strain, restoring the type IV collagen-binding activity, allows the reestablishment of a long-term renal infection. It was noticed that immunization of LPS-nonresponder C3H/HeJ mice with purified Dr protein resulted in delayed mortality when the immunized mice were infected by instilling wild-type Dr adhesin-positive strain IH11128 into the bladder, and incubation of IH11128 with sera of immunized mice resulted in a marked decrease in bacterial adhesion to ex vivo specimens of mouse bladders and kidneys (385). However, additional bacterial factors may also be involved, since Meitinen et al. (386) observed that after injection of O75X fimbriae in mice, there was an absence of histological damage despite persistent O75X deposits in glomeruli. Interestingly, the Sat toxin of the prototype UPEC wild-type strain CFT073 has been found to promote renal histological lesions in mice (387). It remains to be determined whether Sat expressed by strain IH11128 plays a role in the IH11128-induced mice kidney lesions.
Pregnancy Complications
Bogdan Nowicki and coworkers have illuminated the risk generated by UTIs caused by UPEC harboring the Dr adhesin in women who have undergone pregnancy termination or preterm labor (105), and this is related to the levels of tissue expression of hDAF (82, 388–390) and TLR4 (390, 391). Dr adhesin-positive E. coli strains have been found to be often present in pyelonephritis-associated E. coli strains isolated from pregnant females (30, 62, 90, 91, 96, 97). Nowicki and coworkers (82, 105) have convincingly demonstrated the role of Dr-positive UPEC in pregnancy complications. In the uterus, hDAF is present in the endometrial glands, spiral arterioles, and myometrial arteries. In these tissues, the density of hDAF expression correlates with a high level of regulation of the complement-induced cell damage (392, 393). Moreover, it has been demonstrated that interindividual differences in hDAF density in the endometrium influence the tissue colonization by Dr adhesin-expressing E. coli (393, 394).
A set of elegant studies have provided a clear demonstration of the role of UPEC expressing the Dr and Afa adhesins in the lethal outcome of gestational infection. Nowicki et al. (395) have shown that the level of uterine Dr adhesin-positive E. coli infection in pregnant rats was higher than that in nonpregnant rats. In an experimental model of nonpregnant lipopolysaccharide-hyporesponder C3H/HeJ mice, Kaul et al. (396) observed that Dr adhesin-positive E. coli-induced chronic pyelonephritis resulted in a high level of preterm deliveries compared to that in mice infected with an E. coli isogenic Dr mutant. A high rate of maternal mortality has been observed in rats infected with an AfaE+/AfaD+ strain that causes high rates of maternal mortality (397). Mice infected with the AfaE+/AfaD− strain show a 2-fold-decreased level of maternal mortality and infection with AfaE−/AfaD+ strain results in a 5-fold decrease in death, while in contrast, the AfaE−/AfaD− double mutant fails to promote death, even though all the mutants showed an equal infection level in uteri. The observation that the decrease in the mortality rate paralleled the decrease in the invasiveness capacity of a Dr adhesin-positive E. coli strain (397) correlates well with the in vitro observation by Goluszko et al. of a high rate of invasiveness capability in Dr adhesin-positive E. coli isolated from pregnant women with UTIs (94).
It has been proposed that infectious complications of pregnancy such as host gestation-dependent sensitivity to UPEC are related to the host nitric oxide (NO) status. When Dr-positive E. coli invades human endometrial adenocarcinoma Ishikawa cells, there is a decrease of cell entry after an induction of NO production and an increase of cell entry after NO inhibition (354). Interestingly, elevation of NO production is accompanied by a significantly reduced expression of hDAF protein and mRNA (354). Mechanistically, NO triggers a displacement of hDAF from the membrane lipid rafts coupled to its internalization within human endometrial cells (398). In rodents, an increase in rat uterine NO synthase (NOS) activity has been observed during pregnancy, and one function of NO generated in the uterus, which declines at term (399), is to inhibit uterine contractility (400). It has been observed, interestingly, that urogenital tract colonization by Dr adhesin-positive E. coli is followed by a defense mechanism involving the production of NO (401). Moreover, there is a localized increase in type II NOS expression and NO production after intrauterine Dr-positive E. coli infection in pregnant versus nonpregnant rats (402). Comparing LPS responder (C3H/HeN) and nonresponder (C3H/HeJ) mice and Dr adhesin-positive E. coli and P fimbria-positive E. coli infections, Nowicki et al. (403) observed that the infection level in the Dr-positive E. coli-infected C3H/HeN group treated with an inhibitor of NO, nitro-l-arginine methyl ester (l-NAME), was about 100-fold higher than that in the P adhesin-positive E. coli-infected, l-NAME-treated group. Dr adhesin-positive E. coli infection in mice (403) and AfaE/AfaD adhesin subunit-positive E. coli infection in rats (404) are followed by complications in pregnancy and death. Moreover, the death rate was increased by treatment with the NO blocker l-NAME in both mice and rat (403, 404). As indicated above for Dr adhesin-induced pyelonephritis in the mouse model, the role of the mouse DAF in Dr adhesin-induced complications during pregnancy in the rat model is intriguing, considering that the rodent DAF is clearly not recognized by Afa/Dr adhesins (254). Banadakoppa et al. (405) recently demonstrated an NO-independent regulation of hDAF expression in endometrial Ishikawa and cervical HeLa cells involving the PI3K/Akt pathway engaging the PI3K/Akt regulatory protein PTEN. Interestingly, the PI3K/Akt pathway negatively regulated the membrane expression of hDAF and consequently downregulated the adhesion of Dr adhesin-positive E. coli. As underlined by Nowicki and coworkers, two independent host cell systems, NO and PI3K/Akt, by the downregulation of hDAF expression at cell membrane level represent a functional host pathogen strategy to achieve a well-controlled limited level of infection by Dr adhesin-positive E. coli.
Intestinal Tract Infection
Facing the luminal compartment, the intestinal epithelium is lined by a monolayer of highly polarized epithelial cells, including four extremely specialized cell phenotypes, each with specialized functions: enterocytes, neuroendocrine cells, goblet cells, and Paneth cells. Enterocytes, neuroendocrine cells, and goblet cells are constantly renewed by detachment from the villus tip (373) via a specific type of apoptosis known as “anoikis” (406) and are replaced by a cell cycle renewal characterized by a differentiation/migration process occurring along the crypt-villus axis and starting from stem cells localizing at the crypt of the villus (407). Polarized intestinal epithelial cells express an apical domain facing the luminal compartment. In the cell lateral domain, the cell-to-cell junctional domain, including the tight junction (TJ), the adherens junctions (AJs), and the desmosome, establish tight contacts with neighboring cells and seal the intestinal cell barrier (299, 408). In addition, the basal domain establishes a connection with the basement membrane. Structural or functional breaches of the intestinal epithelial barrier by enterovirulent bacteria lead to diseases (409).
Structural and functional injuries at the intestinal epithelial barrier.
Afa/Dr DAEC strains infecting cultured enterocyte-like Caco-2 (410–412) and colonic T84 (342) cells attach at the brush border. Adhesion of F1845 adhesin-positive E. coli strain C1845 parallels the cell differentiation-dependent appearance of the brush border (413). As the result of the recognition of brush border-associated hDAF, recombinant Dr adhesin- and Afa-I adhesin-positive E. coli strains adhere to cultured human enterocyte-like HT-29 and Caco-2 cells (414). Similarly, Adlerberth et al. (415) have found that Dr adhesin-positive E. coli cells adhere at the brush border of freshly isolated ileal enterocytes or colonic cells. The wild-type strain C1845 displays a low level of cell entry when infecting the permissive epithelial Hep-2 cell line, 20-fold lower than that of the prototype invasive AIEC wild-type strain LF82 but equaling the rate of internalization of noninvasive EHEC and EAEC strains (416). Moreover, Afa/Dr DAEC strains are noninvasive when they infect cultured enterocyte-like cells via the natural apical infection route. Indeed, Guignot et al. (331) have shown that after apical infection of cultured human enterocyte-like Caco-2/TC7 cells, the level of internalized Afa/Dr DAEC was very low, indicating that these bacteria are noninvasive when in contact with the brush border of human enterocytes lining the intestinal epithelial barrier. In contrast, when Afa/Dr DAEC infected the Caco-2/TC7 cells basolaterally, it was internalized by a mechanism involving the basolaterally expressed β1 integrin. It should be noted, however, that Afa/Dr DAEC never uses the basolateral domain of intestinal epithelial barrier as its natural way of infection. However, treating Caco-2/TC7 cells with Ca2+-free medium containing EGTA, disrupting intercellular junctions and exposing the junctional domain of the cells, results in an increased level of internalized Afa/Dr DAEC infecting the brush border. This indicates that when Afa/Dr DAEC infects a diseased intestinal epithelial barrier in which the closure at the junctional domain is impaired, it can enter the cells.
(i) Structural lesions at the brush border.
The highly differentiated apical pole structure of enterocytes is composed of a dense array of microvilli, considerably increasing the intestinal surface area (299). The formation of the brush border in enterocytes during the establishment of the apical-basal polarity results from a complex and highly regulated epithelial polarity program (408). Microvilli are formed by assembly of parallel arrays of actin filaments that create the actin bundle, and myosin-1a links the actin network to the plasma membrane (417, 418). In addition, zonula occludens (ZO) proteins required for TJ assembly also regulate the organization of the apical cytoskeleton, particularly the perijunctional antimyosin ring, and in turn function in the polarized organization of the cells (419).
The molecular and cellular mechanisms of the intestinal pathogenesis of Afa/Dr DAEC have been investigated using cultured fully differentiated human intestinal Caco-2 cells and T84 cells, which structurally and functionally mimic enterocytes and colonic cells of the intestinal barrier (420, 421). Unlike that of other enteric pathogenic E. coli strains (4, 5, 422), the intestinal pathogenesis of Afa/Dr DAEC is triggered predominantly via interactions between Afa/Dr adhesin and brush border membrane-associated proteins functioning as signaling receptors (410–412). Adhering diarrhea-associated wild-type strain C1845 and recombinant F1845 adhesin-positive E. coli induced hDAF-dependent injuries in microvilli in fully differentiated Caco-2 and T84 cells (341, 410) (Fig. 7A to F). Bacteria at the sites of attachment were trapped by an elevated number of microvilli as a result of contact with the tips of microvilli (Fig. 4). Elongation of microvilli occurs, and finally, the lesion results in a loss of microvilli (Fig. 7C to F). This brush border injury results in rearrangements of structural brush border proteins, including the disappearance of the F-actin cytoskeleton and clumping of villin, fimbrin, and α-actinin within cytoplasmic aggregates (410, 412). Loss of the brush border has been also observed after apical infection of human enterocyte and cultured enterocyte-like cells by EPEC and EHEC (326, 423). The EPEC- and EHEC-triggered attaching and effacing (A/E) lesions at the microvilli of the intestinal brush border follow a common mechanism of virulence resulting from the expression of the PAI locus of enterocyte effacement (LEE) controlling via a type III secretion system (T3SS) the completion of a bacterial syringe allowing the translocation of virulence factors into target intestinal host cells (4, 5, 422). Even though the disappearance of brush border microvilli induced by Afa/Dr DAEC, EPEC, and EHEC infection is morphologically similar, the mechanism controlling the deleterious effect of Afa/Dr DAEC at the brush border is very different from those for EPEC and EHEC, since Afa/Dr DAEC does not express any T3SS and is devoid of the EPEC and EHEC virulence factors involved in A/E lesions (60).
FIG 7.
Structural and functional brush border lesions caused by diarrhea-associated Afa/Dr DAEC in enterocyte-like cells. The drawing on the left summarizes the observed cellular events. Afa/Dr DAEC interact with the brush border-associated hDAF and hCEACAM1, hCEA, and hCEACAM6 receptors (1). In turn, hDAF-associated signaling pathways, including protein tyrosine kinase(s), phospholipase Cγ, phosphatidylinositol 3-kinase, protein kinase C, and Ca2+, are activated (2). Loss of the brush border results in the disassembly of the microvillus cytoskeleton and induces defective expression of functional proteins, such as SI, DPP IV, SGLT1, and GLUT5 (3). (A and B) Low and high magnifications of transmission electron micrographs showing the well-ordered brush border microvilli of uninfected cells. (C to F) Low and high magnifications of transmission electron and scanning electron micrographs show the disappearance of the brush border at a late time postinfection. (Micrographs in panels A to F reprinted from reference 410.) (G and H) Micrographs show the observation by CLSM of immunofluorescence labeling of brush border-associated DPP IV (x-y section). (Reprinted from reference 412.)
Mechanistically, the lesions induced by diarrhea-associated wild-type strain C1845 in the F-actin cytoskeleton result from F1845 adhesin-triggered, hDAF-dependent signaling events. In permanently undifferentiated human embryonic intestinal INT407 and undifferentiated intestinal Caco-2 cells, both expressing hDAF, infection by the wild-type strain C1845 and by an F1845 adhesin-expressing recombinant E. coli strain triggers F-actin cytoskeleton disassembly accompanied by the appearance of cytoplasmic phosphotyrosylated proteins and the activation of signaling molecules, including PTKs, phospholipase Cγ, PI3K, PKC, and Ca2+ (340). In enterocyte-like Caco-2 cells, the F1845 adhesin-triggered disassembly of brush border cytoskeleton-associated proteins is Ca2+ signaling dependent (412).
Elongation of microvilli precedes the disappearance of brush border in diarrhea-associated wild-type strain C1845-infected enterocyte-like Caco-2 cells (410). Cell membrane extensions attached or entrapping adhering Afa/Dr DAEC have been observed in infected epithelial cells. Yamamoto et al. (51) observed that cell membrane extensions are connected to the daaC-positive strain D2 adhering to Hep-2 and HeLa cells. Cookson and Nataro (424) observed cell membrane extensions in close contact with adhering bacteria in C1845-infected Hep-2 cells. Berger et al. (274) have dissected the mechanism by which Afa/Dr DAEC promotes cell membrane extensions. In epithelial cells infected with a Dr adhesin-expressing E. coli strain, the phenomenon results from the recognition by Dr adhesin of membrane-associated GPI-anchored receptor hDAF, hCEA, or hCEACAM6. In contrast, the phenomenon does not occur after recognition of the transmembrane receptor hCEACAM1. Mechanistically, the Dr adhesin-induced cell membrane extension is microfilament dependent and follows the activation of the small GTPase Cdc42 and the phosphorylation of ERM (274). The observation that phosphorylation of ERM accompanied the Dr adhesin-induced cell membrane extensions (274) is consistent with the known roles of Rho GTPases and ERM in the completion of the actin cytoskeleton (356). Indeed, the low-molecular-weight GTPases RhoA, Rac, and Cdc42 are enzymes that in the host cells control a wide range of physiological processes, including membrane trafficking, cytoskeletal dynamics, and nuclear importation and signal transduction pathways (425). The endogenous activators of the Rho family GTPases are guanine nucleotide exchange factors and GTPase-activating proteins (426). Bacterial pathogens hijacking the low-molecular-weight GTPases are critical targets of bacterial effector proteins (427).
After infection of cultured human enterocyte-like Caco-2 cells with the diarrhea-associated wild-type strain C1845, there was an original cell lesion characterized by the release of the tips of microvilli that were in close contact with the infecting bacteria (410). Indeed, disrupted tips of microvilli in contact with adhering bacteria vesiculated and remained attached to the bacteria. The released C1845 bacteria are newly positive for the brush border-associated functional dipeptidylpeptidase IV (DPP IV), indicating a decoration by detached membrane microvilli (410). Under physiological conditions, vesicles expressing membrane-bound sucrase-isomaltase (SI) and maltase-glucoamylase aminopeptidase N (APN) are spontaneously formed at the microvillar tips by a mechanism involving the membrane-binding actin-based motor Myo1a, but not Myo2, and are subsequently shed into the luminal compartment (428, 429). Interestingly, the released vesicles contain particularly high levels of proteins that are preferentially partitioned into lipid rafts (429). Several vesicle cargoes containing brush border hydrolases and several proteins that have immunological function or are involved in inflammatory responses have been identified (429). This Afa/Dr DAEC-induced sacrificial cellular effect, which has as a direct consequence the extrusion of brush border-attached bacteria, probably has a limited and/or temporary impact on intestinal function, since the intestinal epithelial cells are entirely renewed at between 3 and 5 days (299, 373, 406).
(ii) Functional lesions at the brush border.
In enterocyte-like Caco-2 cells (430–434), the asymmetric presence of membrane-associated functional proteins is monitored during the epithelial polarity program by biosynthetic and recycling routes mediating the apical or basolateral delivery of proteins possessing these specific apical or basolateral sorting signals (408). Functional proteins, including hydrolases, transporters, exchangers, some members of the aquaporin (AQP) family, and glycophosphatidylinositol (GPI)-anchored proteins, are expressed at the enterocyte brush border (299). It is interesting to note that large and highly well-organized “super lipid rafts” have been detected at the membrane of the brush border of enterocytes (320, 321, 435) containing some of the major brush border-associated functional proteins (436), including maltase-glucoamylase aminopeptidase N (APN) and sucrase-isomaltase (SI) (437), Na+/H+ exchanger (NHE) isoforms 1 to 3 (438), downregulated in adenoma Cl−/base exchanger (DRA) (439), Cl−/HCO3−/OH− exchanger (440), and peptide transporter 1 (PEPT1), which is responsible for the uptake of di- and tripeptides (441). Sophisticated strategies including the hijacking of the cellular machinery have been developed by enteric pathogenic E. coli, which result in the alteration of activities of membrane-associated transporters, ion channels, and/or exchangers and water channels, in turn modifying the normal transports of nutrients and the water balance (4, 5, 409, 422, 442). Accompanying the C1845-induced brush border structural injuries in enterocyte-like Caco-2 cells (410, 412), the distribution at the brush border of functional intestinal proteins such as SI and DPP IV hydrolases, sodium/glucose cotransporter 1 (SGLT1), and the fructose GLUT5 transporter was profoundly impaired (412) (Fig. 7H and I). The default of SI and DPP IV expression at the brush border induced by strain C1845 relates directly to the promoted disassembly of the brush border cytoskeleton, since stabilizing the F-actin cytoskeleton by jaspakinoline treatment abolishes the disappearance of SI and DPP IV (411). In C1845-infected cells, there is a strong decrease of enzyme activity of SI and DPP IV (411, 412). It was logical to assume that this decrease in enzyme activity was related to the disorganization of the microvillus cytoskeleton and the disappearance of microvilli. However, it was not. Indeed, unlike C1845-induced, signaling-dependent cytoskeleton disassembly (340, 412), the loss of SI and DPP IV enzyme activity is independent of PTK, phospholipase Cγ, PI3K, PKC, and Ca2+ signaling pathways and also of C1845-induced cytoskeleton disassembly (411). This indicates that when SI and DPP IV are delocalized from the brush border in infected enterocyte-like cells, the hydrolases relocated into the cytoplasm lose their enzyme activity. In addition, the biosynthesis of the two hydrolases is severely affected without any change in mRNA levels and protein stability (411).
(iii) Structural and functional lesions at the junctional domain.
The junctional domain in epithelia plays a critical role in health, and barrier dysfunction at the junctional domain can lead to disease (409). The adherence junctions (AJs) and desmosomes act as adhesive domains between intestinal epithelial cells. Indeed, cadherin-based cell-cell junctions are mechanical connections creating contractile force which, after interaction with the contractile antimyosin cortex, actively couples neighboring cells in the intestinal epithelial barrier (443, 444). The most apical junctional complex is TJs, which are highly regulated and formed by the assembly of specialized proteins such as the cytoplasmic ZO-1, ZO-2, and ZO-3 proteins connected with the F-actin cytoskeleton, the transmembrane occludin connected both with ZO proteins, cytoskeleton, and junctional adhesion molecules, and claudins connected with ZO proteins (445, 446). Functionally, TJs act as a “fence” separating the apical and basolateral membrane domains of polarized intestinal cells. This function results in the segregation in each membrane domain of cell proteins and lipids. Moreover, TJs, by the sealing of intercellular space, act as a “gate” regulating the paracellular passage of small particles and solutes. On the basis of functional studies (447), it has been recently evidenced that the gate activity includes two functional paracellular pathways: the first one, named the leaky pathway, engaging occludin that controls the paracellular passage of larger molecules, and the second, named the high-capacity pore pathway, engaging the claudin family of proteins functioning as cation-selective and anion-selective protein-forming channels and as protein-forming channels without a clear established selectivity. Enterovirulent bacteria have developed sophisticated strategies to breach the intestinal barrier by means of effectors that by signaling-dependent mechanisms target the structural molecules that compose the TJs (448). Pathogen-induced intestinal barrier deficiencies have been linked to the onset of inflammation and diarrhea (409).
The infection of fully differentiated Caco-2/TC7 cell monolayers by strain C1845, which expresses F1845 adhesin, is followed by an elevated level of paracellular permeability without affecting the transepithelial resistance (449). At an early stage of infection, the distributions of TJ-associated occludin and ZO-1 proteins are profoundly modified, showing the disappearance of the proteins at the TJs, whereas there was no change in expression of the AJ-associated E-cadherin. The TJ lesions develop from an F1845 adhesin-induced, hDAF-dependent mechanism. Guignot et al. (137) identified the toxin Sat of wild-type strain IH11128 as the virulence factor that triggers the TJ lesions (Fig. 8A). Sat induces rearrangements of the TJ-associated proteins ZO-1, ZO-3, and occludin without modifying significantly the claudin-1 distribution. In Sat-treated cells, there was a dramatic decrease of the membrane expression of ZO-1 and ZO-3 and a slight decrease in the membrane expression of phosphorylated and nonphosphorylated forms of occludin. In turn, Sat increases the paracellular permeability, since there is elevation the paracellular passage of mannitol without affecting the passage of nonionic molecules with higher molecular masses (137, 449). In addition, Sat induces the formation of fluid domes by increasing the transcellular passage (137) (Fig. 8B). These fluid-filled, blister-like structures known as fluid domes are randomly distributed areas that evolve permanently into the cell monolayers as the result of transcellular passage of fluids accumulating basolaterally (450, 451). This result is interesting since Taddei et al. (221) have reported that Sat can induce secretory activity, resembling the enterotoxic activity of ETEC, in rabbit ileum tissues mounted in an Ussing chamber, with a dramatic fluid accumulation in rabbit ileum loops.
FIG 8.
Structural and functional injuries at the tight junction caused by Afa/Dr DAEC in enterocyte-like cells. The drawing on the left summarizes the observed cellular events. The secreted and internalized Sat toxin induces the reorganization of the TJ-associated proteins ZO-1 and occludin without affecting E-cadherin expression (1). Sat induces an increase in transcellular and paracellular passages (2). (A) Low- and high-magnification micrographs show the immunofluorescence labeling of structural TJ-associated ZO-1 protein observed by CLMS in control and AAEC185psat-infected cells. Note the Sat-induced disappearance of the protein from the cell-to-cell contacts. (Reprinted from reference 137 with permission.) (B) Fluid domes observed by phase-contrast CLSM in control and AAEC185psat-infected cells. Note the Sat-induced increase of the fluid dome height and surface.
Inflammatory responses.
Diverse host cell proinflammatory responses followed the infection of enterocyte-like and colonic cell lines by Afa/Dr DAEC. Flagella expressed by EPEC, EAEC, and EHEC contribute to favor epithelium colonization and proinflammatory responses, such as the induction of proinflammatory IL-8 production (4, 5). Motile, AfaE1-, AfaE2-, or afaEX-positive diarrhea-associated strains induced the production of high levels of IL-8 in T84 and Caco-2 cells (203, 452). Meraz et al. (107) have reported that nine motile diarrhea-associated AfaE1- or AfaEX-positive E. coli strains induced the production of IL-8 in T84 cells. Paul Hofman and coworkers (341, 342, 344, 453) have nicely dissected the Afa/Dr adhesin-triggered, hDAF-dependent signaling pathways controlling the production of proinflammatory cytokines and related cellular events in colonic T84 cells. The nonflagellated, wild-type Afa/Dr DAEC C1845 and IH11128 strains were able to promote the basolateral secretion of IL-8 in monolayers of polarized intestinal T84 cells (341). The production is daa or dra operon dependent, involves binding onto brush border-associated hDAF, and develops by activation of the extracellular signal-regulated protein kinase (Erk1/2), p38, and c-Jun NH2-terminal kinase signaling pathways. Arikawa et al. (203) observed that 12 nonmotile, AfaE1-, AfaE2-. or AfaEX-positive, diarrhea-associated strains also induced the production of IL-8, but at a level nine times lower than that found in the motile strain. Flagella isolated from motile Afa/Dr DAEC to produce IL-8 recognize the basolateral Toll-like receptor 5 (TLR5), and in addition, TJ function is observed to be reduced (454). To explain this phenomenon, it has been proposed that an unknown additional virulence factor causing the structural opening of TJs allows flagellin to reach basolaterally expressed TLR5 (454).
PMNLs play a pivotal role in maintaining intestinal homeostasis and are critical actors in the innate immune response that protects the host against microbial pathogens by generating a diversified arsenal of antimicrobial molecules, including reactive oxygen species, antimicrobial peptides, myeloperoxidase, hydrolytic enzymes, proteases, cationic phospholipase, and metal chelators, and by forming cell extensions known as neutrophil extracellular traps (NETs) (455). PMNL infiltration resulting from recruitment by chemokines produced by macrophages or epithelial cells at the site of insult is a hallmark of the host inflammatory response to infection with various different enteric bacterial pathogens. The effects of Afa/Dr DAEC strains on the transepithelial migration of PMNLs have been demonstrated in polarized monolayers of human colonic T84 cells cocultured with freshly isolated PMNLs (341). The transepithelial migration of PMNLs induced by Afa/Dr DAEC follows F1845 and Dr adhesin-induced hDAF-dependent basolateral production of IL-8 after activation of Erk1/2, p38, and JNK MAPKs. Afa/Dr DAEC-induced PMNL transmigration triggers cell synthesis of TNF-α, and IL-1β, in turn inducing the up-expression of hDAF at the brush border of the cells and increasing the adhesion of Afa/Dr DAEC bacteria (342). In addition, there was an abnormal appearance of hDAF at the basolateral domain of cells (342). The Afa/Dr DAEC-induced transmigration of PMNLs leading to a cytokine-triggered up-expression of hDAF was consistent with previously observed IL-1β-induced up-expression of hDAF (456–459). The up-expression of hDAF at the brush border is probably a cell defense response, since it has been demonstrated that hDAF functions as an antiadhesive molecule accelerating the release from the luminal surface of PMNLs that have undergone transepithelial migration (460, 461). It remains to be determined whether the abnormal basolateral expression of hDAF in infected colonic T84 cells is also a cell defense response that in turn can block the basolateral recruitment and transmigration of PMNLs.
The major histocompatibility complex (MHC) class I-related molecules A and B (MICA and MICB) are distant homologues of MHC class I molecules (462). Below the epithelium, resident lymphocytes can be activated by these nonclassical MHC class I molecules to display a diverse array of immune responses (463). Together with UL-16-binding proteins, MICA and MICB are ligands of human NKG2D (464), an activating natural killer (NK) receptor expressed on both tumor-infiltrating lymphocytes and tumors cells. NKG2D exerts cytolytic destruction of cells through recognition of its cognate ligands. NK cells circulate through the blood, lymphatics, and tissues, patrolling for the presence of pathogen-infected cells (465). MICA and MICB are more highly expressed at the surface of epithelial cells in colonic biopsy specimens from Crohn's disease (CD)-affected patients than in those from controls (466). Tieng et al. (467) have observed that the upregulation of MICA in enterocyte-like Caco-2 cells infected by AfaE-III adhesin subunit-positive E. coli is mediated by the specific interaction between the adhesin subunit and hDAF.
Pathogenic bacteria use quorum sensing (QS) to regulate several traits that allow them to establish and maintain infection in their host; these include motility, biofilm formation, and virulence-specific genes (468). In E. coli, QS involves autoinducers AI-2 and AI-3, depending on the function encoded by the luxS gene. It has been observed that the Dr adhesin-positive IH11128 strain produces high levels of AI-2 at the end of the exponential phase of growth (unpublished result). Sperandio et al. (469), observing that an EHEC luxS mutant responds to a eukaryotic cell signal activating the expression of its virulence genes, have identified epinephrine as promoting a “language” by which bacteria and host cells communicate. Diard et al. (343, 470) have demonstrated that norepinephrine is involved in Afa/Dr DAEC pathogenesis. In the wild-type strain IH11128, the thermoregulated production of Dr adhesin, which is optimal during the logarithmic phase of anaerobic growth, has been found to be accompanied by a release of Dr fimbriae without cell lysis (470). Norepinephrine increases the Dr adhesin release by affecting the production of the fimbrial subunits. Indeed, norepinephrine promotes the differential induction of genes draC and draE by a regulatory mechanism; i.e., the level of the draE transcript is highly increased, while expression of draC transcript is increased to a lesser extent, relative to basal expression (343). Like with the wild-type IH11128 (341), the released Dr adhesin induces the phosphorylation of Erk1/2, in turn promoting the production of IL-8 in enterocyte-like Caco-2/TC7 cells (343).
Proinflammatory effects of Afa/Dr DAEC after direct contact with PMNL-like PLB-985 cells have been described by Sylvie Chollet-Martin and coworkers (346, 347, 471). A rapid and massive release of reactive oxygen species and preformed intragranular mediators (myeloperoxidase and IL-8) develops in PMNL-like PLB-985 cells infected with the wild-type IH11128 and C1845 strains (347). The phenomenon is triggered not by Afa/Dr adhesins but by the type 1 pili (347) expressed by wild-type Afa/Dr DAEC strains (155).
Adherence to hDAF expressed at the membrane of freshly isolated PMNLs mediated by Dr adhesin did not lead to a significant increase of bacterial killing, indicating that the Afa/Dr DAEC could overcome the microbicidal activity of PMNLs (472). In contrast with the fact that PMNLs that have transmigrated display greater phagocytic activity against E. coli (473), PMNLs that have not transmigrated or transmigrated across T84 cell monolayers after Afa/Dr DAEC infection display low phagocytic activity, suggesting that Afa/Dr DAEC can block this activity (453).
Interaction of the wild-type Afa/Dr DAEC C1845 and IH11128 strains or recombinant Dr adhesin-positive E. coli with freshly isolated human PMNLs results in an increased rate of cell apoptosis evidenced by morphological nuclear changes, DNA fragmentation, cleavage of pro-caspase 3 and stimulation of the caspase activity, and up-expression of annexin V (453, 472). Since this phenomenon is not blocked by anti-hDAF and -hCEA antibodies, it seems to be linked to a leukocyte agglutination process rather than to the recognition of these signaling receptors. Intriguingly, phosphatidylserine appears at the cell membrane of PMNL-like PLB-985 cells, resulting from an Afa/Dr adhesin-triggered, hDAF-dependent activation of tyrosine kinase, and protein kinase C signaling occurs, but without apoptosis (346). Low levels of phagocytosis of Afa/Dr DAEC bacteria were observed in both nontransmigrated and transmigrated PMNLs, suggesting a bacterium-triggered diminished leukocyte phagocytic capacity to escape host defenses (453).
Angiogenesis.
Angiogenesis is a new component of IBD pathogenesis. Cane et al. (345) have reported that infection of cultured human colonic T84 cells by the diarrhea-associated E. coli strain C1845 is followed by an increase in vascular endothelial growth factor (VEGF) mRNA expression and production of VEGF protein. Members of the VEGF family are secreted, dimeric glycoproteins of ∼40 kDa consisting of five members, VEGF-A, -B, -C, and -D and a placenta growth factor, which function as regulators of vasculogenesis, i.e., the vascular development that occurs during embryogenesis, and the angiogenesis process that forms blood vessels (474). Importantly, the VEGF secreted during C1845-infection of T84 cells is bioactive, since the cell-free spent culture medium is able to induce tubulogenesis. This phenomenon results from the recognition of hDAF by F1845 adhesin followed by activation of a Src protein kinase upstream of the activation of the Ras/Raf/MAPK and PI3K/Akt signaling pathways (345). Upregulation of VEGF has also been observed in epithelial cells infected with an Afa-I adhesin-positive E. coli strain (140). Moreover, the F1845 adhesin-triggered, DAF-dependent up-production of VEGF and also IL-8 in T84 cells has been found to be controlled by an MAPK- and PI3K-dependent induction of the hypoxia-induced factor 1-α (HIF-1α), revealing a connection between hDAF-associated signaling and a translational mechanism(s) (344). It is noteworthy that HIF-1 consists of two subunits, HIF-1α and HIF-1β, and that HIF-1α, which is degraded via an oxygen-dependent process involving prolyl hydroxylases, functions as an oxygen sensor (475). It is interesting to note that the CD-associated AIEC strain LF82 also induces, by an unknown factor, the production of HIF-1 α protein and the activation of VEGF/VEGR receptor (VEGFR) signaling in T84 cells (476). All the above-reported cell responses to Afa/Dr DAEC infection are related mainly to recognition of intestinal epithelial cell-expressed hDAF by Afa/Dr adhesins. Considering that hCEACAM1, hCEA, and hCEACAM6 are known to be involved in immune responses (477), it remains to be shown whether these hCEACAMs also trigger host cell immune responses after recognition by Afa/Dr adhesins.
EMT events.
Under physiological conditions, the intestinal epithelium undergoes controlled constant cell shedding. In epithelia, a programmed cell death process known as “anoikis,” a Greek word meaning “homelessness,” occurs when polarized epithelial cells forming an epithelial barrier are detached from the appropriate lateral domain and extracellular matrix (406). The control of anoikis in differentiated epithelial intestinal cells is a differentiation-dependent process involving the engagement of β1 and β4 integrins and activation of FAK, Src kinase, and the Erk1/2 and Pi3K/Akt signaling pathways (406). The diarrhea-associated C1845 affects the AJs of intestinal epithelial polarized colonic T84 cells. Indeed, an F1845 adhesin-triggered, hDAF-dependent loss of AJ-associated E-cadherin protein and cytokeratin 18 (CK18) has been observed, resulting from activation of HIF-1α (344). Moreover, CK18 plays a cytoskeletal function as a component of the intermediate filaments and acts as a target for caspase-mediated cleavage during cell apoptosis (478). In parallel, C1845 infection promotes a rise in fibronectin expression, accompanied by the up-expression of Twist1 mRNA (344), a helix-loop-helix transcriptional factor belonging to a small group of core transcription factors that also includes Snail, Slug, and Sip1 and which are involved in controlling the epithelial-mesenchymal transition (EMT) (479). The EMT is a transcriptional and morphological program observed during the progression of diseases, including cancers (480, 481). Three distinct subtypes of epithelial cells that transition into mesenchymal cells have been defined, depending on the physiological tissue context (481). Type 1 occurs in embryogenesis and organ development, type 2 develops in tissue regeneration and organ fibrosis, and type 3 is associated with cancer progression. However, partial EMT can develop as a function of the tissue and signaling contexts, resulting in a loss of only some of the polarized characteristics such as cell contact dissolution and actin cytoskeleton reorganization. The two major hallmarks of EMT are the dissolution of epithelial cell-cell adhesion contacts and the massive actin cytoskeleton reorganization followed by the loss of cell polarization. Transcription factors drive EMT by downregulating genes, including those encoding proteins maintaining epithelial cell-cell adhesion domains. After a long time of infection, C1845 infection promotes depolarization of the enterocyte-like Caco-2 cells, which is characterized by the complete disorganization of the apical domain and the loss of cell-to-cell contacts accompanied by the appearance of undifferentiated cells that become detached from the cell monolayers (Fig. 9A) (unpublished results). The C1845-induced cell shedding resembles the cascapase-1-dependent cell shedding observed in T84 cells (482) and the TNF-α-induced cell shedding observed in mouse intestinal and Madin-Darby canine kidney cells (482, 483). It has been mentioned above that in C1845-infected T84 cells, the induced transepithelial migration of PMNLs is followed by the production of TNF-α (342). Collectively, these results show that diarrhea-associated Afa/Dr DAEC promotes cell depolarization and injuries at the two major sites of the junctional domain of polarized epithelial cells forming the intestinal epithelial cell barrier, i.e., TJs and AJs, and in turn induces EMT-like behavior.
FIG 9.
Diarrheagenic Afa/Dr DAEC strain C1845 promotes epithelial-mesenchymal transition (EMT)-like behavior, cell extrusion, and pks-dependent damage in human intestinal cells. The drawing on the left summarizes the observed EMT-related cellular events. An F1845-triggered hDAF-, MAPKs-, PI3K-, and HIF-1α-dependent production of VEGF develops in colonic-like T84 cells (1 and 2). An F1845-triggered hDAF-, MAPKs-, PI3K-, and HIF-1α-dependent EMT-like behavior develops, characterized by the typical changes in expression of mesenchymal markers, such as the upregulation of fibronectin, the downregulation of CK18, and the disappearance of AJ-associated E-cadherin (1 and 3). Finally, depolarized intestinal cells lose the lateral cell-to-cell and basal contacts and become detached from the cell monolayer. (A) Low-magnification transmission electron micrographs show the dedifferentiation of Caco-2/TC7 cells infected with the diarrhea-associated strain C1845, characterized by a disorganization of the apical domain and by the appearance of nucleus fragmentation and loss of nucleus dense electron material, indicating cell death (left micrograph). The boxed area indicates a cell with a funnel form engaging in shedding from the cell monolayer as the result of the wide opening of the lateral cell-to-cell junctional domain and detachment at the basal domain. The micrograph on the right shows a high magnification of the cell engaging in detachment and containing a high number of vesicles in the cytoplasm. (B) Low- and high-magnification micrographs show the morphological changes in Giemsa-stained C1845-infected undifferentiated Caco-2/TC7, cells characterized by an enlargement of the cell body and nucleus. Low-magnification micrographs show the increase of the phosphorylation of nuclear H2AX, the most sensitive marker of DNA damage. (Courtesy of J. P. Nougayrede and E. Oswald, reproduced with permission.)
pks-related cell injuries.
The presence of the pks genomic island coding for the genotoxin colibactin in the prototype wild-type Afa/Dr DAEC strains leads to the appearance of known pks-related cell changes (240). Indeed, in C1845-infected undifferentiated Caco-2/TC7 cells there was an enlargement of the cell body and nucleus and an increase of the nuclear phosphorylation of histone H2A variant H2AX (Fig. 9B) (Nougayrede and Oswald, unpublished result). These cell injuries were abrogated when the cells were infected with a mutant colibactin strain (clbA::frt) deleted for the clbA gene, encoding the phosphopantetheinyl transferase ClbA within the pks island (484), and were restored by the complementation of the mutant strain with p-clbA (Nougayrede and Oswald, unpublished result). It is noteworthy that the pks-triggered enlarged cells cell have been recently characterized as senescent cells bearing massive and irreparable damage and secreting protumorigenic factors that damage adjacent cells (485, 486).
Host cell defense responses.
Infection of enterocyte-like Caco-2 cells by wild-type strain C1845 or recombinant E. coli expressing F1845 adhesin is followed by a strong antibacterial cell response at the brush border, since at a late time of infection the adhering bacteria are dramatically damaged (487) (Fig. 10A and B). This defense response seems to be correlated with the fact that Caco-2 cells express intestinal antimicrobial peptides in a differentiation-dependent manner (487, 488).
FIG 10.
Proinflammatory and defense responses in enterocyte- and colonic-like cells and PMNLs after infection with the diarrhea-associated strain C1845. The drawing on the left summarizes the observed cellular events. An hDAF-dependent MAPK-, PI3K/Akt-, and HIF-1α-triggered signaling (1) leads to the production of the proinflammatory cytokine IL-8, which promotes the transepithelial migration of PMNLs (2), which in turn promotes the production of proinflammatory cytokines TNF-α and IL-1β (3) and leads to the up-expression of brush border-associated hDAF (4) and basolateral MICA (5) and the abnormal expression of hDAF at the basolateral domain (5). Afa/Dr DAEC interacting with transmigrated PMNLs induces cytotoxic proinflammatory responses and a defense response involving NET-triggered bacterial killing (6). As the result of a host intestinal cell defense response, bacteria attached at the brush border are entrapped by microvilli and killed (7). In addition, bacteria attached at the brush border are extruded from the intestinal cells when the tips of microvilli are shed (7). (A) A high-magnification scanning electron micrograph shows an altered bacterium entrapped by numerous microvilli in Caco-2 cells. (Reprinted from reference 487 with permission of Elsevier.) (B) A high-magnification scanning electron micrograph shows a highly damaged adhering bacterium at a late time of infection. (Reprinted from reference 487 with permission of Elsevier.) (C) A high-magnification scanning electron micrograph shows a bulbous membrane protrusion at the tip of elongated microvilli in close contact with an adhering bacterium. (Reprinted from reference 410.) (D) A high-magnification transmission electron micrograph shows a high number of cell membrane vesicles in close contact with a bacterium that is detached from the brush border. (Reprinted from reference 410.) (E and F) Low- and high-magnification field emission scanning electron micrographs show the NETs expressed by phorbol myristate acetate (PMA)-stimulated PMNL-like PLB-985 cells. (Reprinted from reference 471.) (G and H) Low- and high-magnification field emission scanning electron micrographs show NETs expressed by infected PMNL-like PLB-985 cells and in contact with diarrhea-associated C1845 bacteria. (Reprinted from reference 471.)
As described above, the intestinal cells after infection by Afa/Dr DAEC seem to react so as to resolve brush border colonization by releasing the tips of microvilli that were in close contact with the infecting bacteria (410) (Fig. 10C and D). Bacteria detached from the brush border express vesicles formed of microvillus membranes, indicating extrusion of brush border-colonizing bacteria. An increase in the microvillar vesicles shed after tEPEC infection of Caco-2BBe clone cells has been reported (489). Interestingly, the interaction of these vesicles with the luminal pathogen blocks the tEPEC intimate attachment onto the microvilli of enterocyte-like HT-29 cells (489). Collectively these results indicate that the accelerated formation and shedding of microvillar vesicles constitute an efficient host intestinal cell defense response. Hammarstrom and Baranov (281) have interestingly postulated that the vesiculation of the microvillus membrane to form vesicles is an effective host defense mechanism intended to extrude adhering enterovirulent bacteria from the luminal surface of the intestinal epithelium and/or to limit the deleterious impact of enterovirulent bacteria.
NETs are formed by a nuclear DNA skeleton and transport antibacterial molecules, including antimicrobial peptides, histones, and proteases (490, 491). In the human myeloid cell line PLB-985, which has the capability to differentiate into fully mature neutrophil-like cells, wild-type strain C1845 infection is followed by the projection of NETs, which by entrapment of bacterial cells trigger the killing of bacteria (471) (Fig. 10E to H). However, this defense response has an unexpected cell cytotoxic consequence, since the induced NETs promote a profound disorganization of the F-actin cytoskeleton by contact with the Caco-2/TC7 cells (471). It is worth mentioning that NETs induce cell death in endothelial cells (492, 493).
CLINICAL CONSIDERATIONS
Reservoir and Transmission
There has been no report of the isolation of E. coli expressing the major human AfaE-I to -III, Dr, or F1845 adhesins in a large variety of animal species. In contrast, the afimbrial Afa-VII and Afa-VIII adhesins are present in diarrhea- or septicemia-associated E. coli strains in calves (26, 44). Moreover, the AfaE-VIII adhesin is expressed in ExPEC infecting calves (43, 54, 494). The gene afa-8 and afa/dr-related genes have been also been found in porcine, poultry, and cattle E. coli isolates (45, 173, 494–496). Examination of E. coli isolates from dogs with UTIs has revealed the presence of P- and S-positive isolates and the absence of Afa-positive isolates (497). There is an absence of evidence of direct animal-to-human transmission.
Treatments
Historically, antibiotics have provided a very effective way of resolving UTIs. The current routine treatment for UTIs is antibiotic therapy, most commonly trimethoprim-sulfamethoxazole (TMP-SMX) or ciprofloxacin. However, patients with chronic or recurrent cystitis require long-term treatment with antibiotics, and as UPEC strains have an unusual intracellular lifestyle, conventional antibiotics are not very effective when the bacteria reside within intracellular vacuoles or are present in biofilm-like structures (498–505).
Afa/Dr DAEC-associated diarrhea in children has been described as being generally limited in duration. Diarrhea, as defined by the World Health Organization (WHO), is characterized by the presence of three or more stools or watery stools within a 1-day period. Diarrhea is defined as being acute when it lasts less than 14 days and as persistent when the episode has lasted 14 days or more. In general, to prevent dehydration and nutritional default in children with infectious acute diarrhea, it has been recommended to provide an oral rehydration solution (ORS) and to continue feeding (506). Since ORS administration has no effect on the duration, severity, or frequency of infectious diarrheal episodes, ORS administration has been associated with adjuvant therapies such as luminally acting antisecretory drugs and antibiotics (507). However, these treatments can cause adverse effects outside or inside the gastrointestinal tract; one such effect that occurs if broad-spectrum antibiotics are used is the emergence of Clostridium difficile-associated diarrhea resulting from an antibiotic-induced imbalance of the intestinal microbiota (508, 509). It should be noted that cost and availability are limiting factors for the use of antisecretory drugs and antibiotics, particularly in developing countries. Another therapeutic strategy used to accompany ORS (which is low cost but for which availability may be a problem, particularly in developing countries) is the use of human intestinal microbiota probiotic Lactobacillus strains secreting antibiotic-like molecules that have demonstrated experimental in vitro and in vivo effects against the major diarrhea-associated bacterial pathogens (510).
Antibiotic Resistance
The increasing prevalence of antibiotic resistance of bacterial pathogens is a major health problem (511, 512). There has been a steady and rapid increase of UPEC resistance to antibiotics accompanied by the problematic occurrence of multidrug-resistant UPEC strains over the last decade (199, 349, 498). For examples, a recent international study revealed that more than 10% of E. coli cystitis isolates are resistant to at least three classes of antibiotics (513). Moreover, a recent survey of more than 12 million clinical isolates across the United States from 2000 to 2011 revealed an increase of resistance to 9 antibiotics, including TMP-SMX, ciprofloxacin, nitrofurantoin, and ceftriaxone (514). Not surprisingly, resistance to antibiotics has been reported for Afa/Dr DAEC, including resistance to ampicillin (90, 124, 515, 516), TMP-SMX (515–518), fosfomycin (517), piperacillin (515), tetracycline (124, 515), ciprofloxacin (518), co-trimoxazole (124), nitrofurantoin (518), fosfomycin (517), tetracycline (519), penicillin (516), oxacillin (516), bactericin (516), cloxacillin (516), chloramphenicol (519), and nalidixic acid (124, 516). In addition, the fact that Dr adhesin-positive E. coli has the capability to form biofilm (190, 233) suggested that like FimH-positive UPEC (199, 232), uropathogenic Afa/Dr DAEC strains have developed strategies to escape antimicrobial host defense systems and to reduce their sensitivity to antibiotics.
Vaccines and Pilicides
A therapeutic strategy has been developed to disarm pathogens in the host by the use of substances that mimic bacterial virulence factors such as adhesive factors, bacterial effectors, and toxins or that display antagonistic activities against the bacterial production of these factors (520, 521). Touchon et al. (522) underlined that this could make it difficult to develop a vaccine against ExPEC infections. It is noteworthy that an emerging therapeutic strategy consists of developing antagonists of QS systems in order to inhibit the bacterium-bacterium and bacterium-host cell communications involved in virulence (523). Brumbaugh and Mobley (520) have recently summarized the advances in vaccine strategy against UPEC and enteric virulent E. coli. These include compounds targeting surface polysaccharides and formulations composed of several virulence factors such as hemolysin, type 1, P, Dr, and S fimbrial adhesins, CNF-1, siderophores, and markers of PAIsCFT073 and which display serotypes O1, -4, -6, -17, -75, and -77, K1, -3, -5, -13, and -95, and flagellar H:1, H:5, H:7, and H:33. Other strategies are the targeting of membrane proteins from UPEC strains or the use of mixtures of inactivated UPEC strains or genetically engineered vaccines. Although promising, the use of antagonists of adhesive factors to combat bacterial colonization of epithelia is complicated by the fact that a single pathogenic E. coli strain expresses a multiplicity of adhesive factors, each of which displays specific host cell receptor recognition. Consequently, it appears evident that it may be necessary to target more than one virulence factor for successful vaccine or pilicide therapies. Another subunit vaccine strategy against ExPEC has been designed to target immunodominant epitopes of the virulence-associated ExPEC proteins FyuA, IroN, ChuA, IreA, Iha, and Usp (524). For Afa/Dr DAEC infection, a vaccine strategy has been experimentally tested using the Dr adhesin in the mouse pyelonephritis model (385). High titers of serum anti-Dr antibodies develop after vaccinating mice with purified Dr adhesin, and this is accompanied by a significant reduction in mortality but, surprisingly, without affecting the levels of bladder or kidney colonization by Dr adhesin-positive E. coli. Ex vivo, preincubating Dr adhesin-positive E. coli with Dr adhesin-immunized mouse serum reduces the bacterial adherence to mouse bladders and kidneys, whereas preincubating urine from Dr adhesin-immunized mice fails to produce this inhibitory activity.
Therapeutic-strategy-based pilicides have been developed in order to inhibit the completion of fimbrial and nonfimbrial adhesins at the cell surface of bacterial pathogens, including UPEC and enteric pathogenic E. coli (525, 526). Pilicides are a class of low-molecular-weight compounds which, by blocking chaperone and usher functions, inhibit pilus/fimbria completion without killing or affecting the growth of bacteria. Consequently, pilicides can prevent the first critical adhesion step required for the successful urinary tract colonization by UPEC strains involved in cystitis and pyelonephritis. On the bases that AfaC/DraC/DaaC ushers are pivotal for Afa/Dr adhesins biogenesis to occur and that the draC and afaC-III genes encoding the Afa/Dr ushers display 100% identity, it has been postulated that 2-pyridone pilicide compounds could inhibit the biogenesis of Afa/Dr adhesins (83, 176, 177). This pilicide strategy has been found to be effective against Dr adhesin biogenesis, where it produces a marked reduction of the expression of Dr adhesin and the disappearance of its properties of adherence to hDAF (175, 527).
CONCLUDING REMARKS AND FUTURE DIRECTIONS
The importance of human Afa/Dr DAEC in UTIs and pregnancy complications has been convincingly demonstrated both experimentally and clinically. Moreover, increasing epidemiological evidences has been reported during the last decade on the role of human intestinal Afa/Dr DAEC in triggering established acute diarrhea in infants <5 years. In contrast, epidemiological studies indicate that human intestinal Afa/Dr DAEC strains are probably not responsible for established acute diarrhea in adults. A robust PCR method for detecting and identifying diarrhea-associated Afa/Dr DAEC strains remains to be developed, as the PCR methods currently used in a clinical setting are problematic with regard to the recently observed cross-reaction between the most commonly used Afa/Dr DAEC daaC primer and EAEC primers.
In spite of the remarkable results obtained over the past decade, the cellular and molecular mechanisms of Afa/Dr DAEC pathogenesis remain incompletely elucidated. Several aspects deserve particular attention in the future. Whole-genome sequencing is called for to identify more potential virulence factors in the wild-type Afa/Dr DAEC prototype strains in order to complete our understanding of the pathogenesis of these pathogenic E. coli strains. Much of our current knowledge about Afa/Dr DAEC intestinal pathogenesis has been obtained from in vitro observations using cultured human epithelial colon cancer cells, which, despite mimicking the situation in vivo, do not entirely reflect the healthy human situation and have several drawbacks (421). The high specificity of Afa/Dr adhesins for human epithelium-associated molecules acting as receptors and triggering cell signalization and cellular effects complicates the experimental examination of the cellular and molecular mechanisms of Afa/Dr DAEC pathogenesis. In comparison with the in vitro situation, the use of transgenic mouse models constructed for the epithelial expression of hDAF and hCEACAMs has not given convincing results in terms of cellular lesions even in the presence of Afa/Dr DAEC intestinal colonization (unpublished results). This is probably due to the endogenous presence of mouse DAF and CEACAMs and to the observation of a less-than-optimal expression of the membrane-bound human receptors (unpublished results) in the mouse epithelial tissue and/or defective connection to the mouse endogenous signaling pathways that have been observed in vitro to be involved in Afa/Dr DAEC pathogenesis. To overcome these problems, human intestinal in vitro organ culture (IVOC) models (528) can offer valuable tools for the experimental investigation of the mechanisms of virulence of intestinal Afa/Dr DAEC. Various IVOC systems corresponding to an ex vivo human situation have been described, including those constituting polarized IVOCs established from pediatric duodenum biopsy specimens or specimens of human colon taken at the vicinity of tumors. These IVOC systems have been used mainly to study the mechanisms of virulence of enterovirulent E. coli, including ETEC, EPEC, EHEC, EAEC, and AIEC (528).
Repeated observations in epidemiological studies of the intestinal presence of daaC-positive DAEC strains in adults in the absence of acute diarrhea are intriguing. This asymptomatic carriage deserves further exploration. Indeed, the Afa/Dr DAEC strains are clearly pathogenic with regard to the deleterious effects observed in vitro in human intestinal cell models, some of which resemble the pathogenic effects of EPEC and EHEC even though they result from different cellular and molecular pathogenic mechanisms. This reinforces our previous hypothesis that intestinal Afa/Dr DAEC strains act as “silent pathogens” that are well controlled under healthy conditions by the intestinal antimicrobial molecules generated by dedicated cells of the host intestinal barrier and by the barrier effect exerted by the intestinal microbiota (11). The above-described deleterious cellular effects of intestinal Afa/Dr DAEC make us think that these microbiota-resident E. coli in healthy humans could be good candidates for belonging to the recently defined class of “pathobionts” (529). Indeed, recent evidence suggests that the observed deleterious cellular effects may be caused by specific bacterial species with pathogenic potential that were present as symbionts in the healthy host intestinal microbiota. Although the pathophysiological mechanisms of pathobionts remain largely unknown, it appears that genetic factors, environmental factors, and/or changes in host defense systems may expose the host to developing diseases triggered by these potentially pathogenic microbiotal bacteria. Observations that some strong cell defense responses, including the extrusion of infecting bacteria from the brush border, cell detachment of intoxicated cells, and killing by NETs of activated PMNLs developed after Afa/Dr DAEC infection, all support the existence of efficient control by the host of these pathogenic E. coli strains in healthy situations. This leads to the question of how a resident intestinal microbiota Afa/Dr DAEC strain can emerge as an enterovirulent pathogen. Several circumstances allow the occurrence of diseases triggered by pathobionts. For example, dysbiosis, a shift in the makeup of the intestinal microbiota community and/or its abundance, leads to a change in the equilibrium between putative protective species and harmful pathogens (530). Another E. coli pathobiont candidate is the E. coli strain C25, isolated from the feces of a healthy human (531, 532). This strain expresses the SPATE toxin Pic (533), translocates across colonic T84 cell monolayers, promotes alterations in localization of claudin-1, activates NF-κB signaling, and induces the production of IL-6, IL-8, and TNF-α (533–535), all effects that are exacerbated in a proinflammatory situation (536).
The causes of IBD have not been entirely identified but are known to be related to a susceptible human genome (537), a dysfunction of the immune system (537), a massive and/or selective modification in the composition of the intestinal microbiota (538), and the intervention of opportunistic enterovirulent bacteria (529, 539). With regard to the observed asymptomatic carriage of intestinal daaC-positive E. coli strains, some of the above-reported deleterious cellular effects of intestinal Afa/DrDAEC, including marked proinflammatory responses, indicated that these strains could play a role in intestinal inflammation. Afa/Dr DAEC belongs to phylogenic group B2, which has been observed to be prevalently associated with IBD (138, 217). It is difficult to address this question experimentally because of the lack of experimental animal models mimicking the human situation. Furthermore, epidemiological clinical studies are difficult to conduct because this would require isolating the mucosa-associated intestinal Afa/Dr DAEC in patients with chronic inflammatory diseases. Indeed, the in vitro results show that these strains are very effectively excluded from the brush border by the host defenses. Moreover, the sampling techniques used to isolate the mucosa-associated bacteria, which are localized exclusively on the luminal surface of the intestinal barrier, are also problematic, since these bacteria can be easily lost during the technical process. It has been found to be easier to isolate AIEC from patients with chronic inflammatory diseases simply because it is intracellularly localized in cells lining the epithelial barrier. Future progress can be expected to provide answers to these questions.
There are emerging links between microbial and parasite infections and oncogenesis (540). Moreover, the relationships and mechanisms through which disturbance of intestinal microbiota, infection, and inflammation increase cancer risks and promote tumor development were recently reviewed (541, 542). A small number of microbial pathogens have been identified as critical causes of specific chronic inflammatory conditions or malignancies. The best-characterized example of a bacterial contribution to cancer is H. pylori infection, which is well established as a major risk factor for gastritis leading to gastric cancer as the result of the deleterious activity of its cytotoxin-associated gene A protein (543). However, the simple observation that some bacteria are present at the site of cancerous intestinal tumors does not constitute a proof of causality. A link of causality is difficult to demonstrate here because there is a long time lag between initiation of a cellular process of carcinogenesis and the onset of overt disease. Indeed, the bacterium-triggered event can occur long before any sign of a cancer tumor manifests itself, and indeed the pathogen may no longer be present. It is becoming clear that some proinflammatory bacterial pathogens can contribute to initiating, promoting, and/or progressing cancer by their virulence factors, including toxins, which directly disrupt cellular signaling pathways that control inflammatory processes and damage DNA. Some demonstrated aspects of the mechanisms of pathogenicity of intestinal and uropathogenic Afa/Dr DAEC strains suggest a possible connection with the initiation or development of intestinal and urinary tract cancers. Some of the deleterious cellular responses triggered by Afa/Dr DAEC described above suggest that they can be connected to the initiation of cellular events in relation to intestinal cancer. The pks island (238, 540) is present in Afa/Dr DAEC, and it has been found that pks-positive E. coli strains belonging to the phylogenic B2 group are prevalently present in biopsy specimens of patients with intestinal cancers and induce DNA double-strand breaks in intestinal cells as well as trigger chromosomal instability, gene mutations, and cell transformation (22, 240, 246, 544). Afa/Dr DAEC infection is accompanied by the transepithelial migration of PMNLs, and in the intestinal tract migration of PMNLs across the epithelial lining is a hallmark of IBD and can create a deleterious inflammatory situation (455) and preneoplastic conditions, since transmigrated PMNLs exert a direct cytotoxic effect by releasing products such as oxidative reagents and elastase (545). Moreover, after Afa/Dr DAEC-induced transmigration of PMNLs across a monolayer of intestinal cells, the epithelial cells displayed deregulated expression of membrane-associated hDAF with altered functions, some of which are implicated in carcinogenesis (546). On the other hand, it should be noted that hCEACAMs, the receptors of Afa/Dr adhesins, are known to be implicated in carcinogenesis (275). In addition, the EMT and angiogenesis observed to be triggered by intestinal Afa/Dr DAEC are indicative that these bacteria play a role in carcinogenesis (480). An important function of autophagy in cancer is the limitation of inflammation, tissue damage, and genome instability, and when the autophagy process is blocked at the degradative autophagosome step, cells accumulate cytotoxic material, thus promoting DNA mutation and carcinogenesis (547, 548). Consequently, the observed blockade of autophagosome maturation triggered by the Afa/Dr DAEC Sat toxin may play a role in carcinogenesis. It is noteworthy that this deleterious effect of autophagy has been observed for H. pylori in relation to its role in the development of gastric cancer (549), since the vacuolating cytotoxin VacA induces autophagy and disrupts autophagosome maturation after prolonged exposure (550). UPEC-induced cell exfoliation in turn induces the upregulation of urothelial turnover, and it has been postulated that it may play a role in patients' predisposition to bladder cancer (504, 551, 552). It is tempting to hypothesize that Afa/Dr DAEC is involved in the development of bladder cancer, given the observed cell exfoliation effect triggered by the Afa/Dr DAEC Sat toxin. Collectively, these results provide a group of facts but do not amount to evidence of a possible link between Afa/Dr DAEC infection and intestinal or bladder carcinogenesis.
ACKNOWLEDGMENTS
I express my earnest thanks to Marie-Françoise Bernet-Camard, Julie Guignot, Isabelle Peiffer, Cedric N. Berger, Imad Kansau, Sylvie Chollet-Martin, Anne-Béatrice Blanc-Potard, Sylvie Hudault, Clémence Rougeaux, Sophie Kerneis, and Vanessa Liévin-Le Moal for their outstanding contributions that have illuminated the understanding of the cellular and molecular mechanisms of pathogenesis of Afa/Dr DAEC (Research Teams Inserm and University Paris-Sud, CJF 94.07 and Units 510 and 756 at the University Paris-Sud, Faculty of Pharmacy, Châtenay-Malabry, France). Particular thanks go to Steve L. Moseley and Natalia Korotkova (University of Washington, Seattle, WA, USA), Bogdan Nowicki (Meharry Medical College, Nashville, TN, USA), Steve Matthews (Imperial College London, London, United Kingdom), and Paul Hofman and Valérie Vouret-Craviari (Centre Hospitalo-Universitaire de Nice and Institute of Research on Cancer and Ageing, University of Nice, Nice, France), Chantal Le Bouguénec (Pasteur Institute, Paris, France), Oliver Billker (Wellcome Trust Sanger Institute, Cambridge, United Kingdom), Doug Lublin (Washington University School of Medicine, St. Louis, MO, USA), and Jean-Philippe Nougayrède and Eric Oswald (Inserm, UMR1043, Centre de Physiopathologie de Purpan, Université de Toulouse, Toulouse, France) for their invaluable collaborative contributions.
Biography

Alain L. Servin received his Doctorat d'Université (University Paris-Sud) in 1973 and Doctorat d'Etat es Sciences (University Paris VI) in 1987. In 1980 he joined the French National Institute of Health and Medical Research (Inserm), working on Biophysic and Cellular Pharmacology (Inserm Institute Saint Antoine Hospital, Paris, and Unit 178 Paul Brousse Hospital, Villejuif). He was research director and head of research teams Inserm and University Paris-Sud (CJF 94.07, Units 510 and 756, Federative Research Institute 75) between 1990 and 2010 at the Faculty of Pharmacy Châtenay-Malabry. He began his interest in Microbiology in 1990, and with his collaborators he focuses his research on the pathogenesis of Afa/Dr DAEC and rotavirus. He also studies the roles of members of the intestinal and vaginal microbiota in controlling enteric and vaginal infection. He mentored 15 doctoral and postdoctoral fellows who continued their careers in academia, Inserm, CNRS, Anses, and industry. Dr. Servin's homepage can be found at http://cvscience.aviesan.fr/cv/827/alain-l-servin.
Footnotes
This article is dedicated to the memory of Arlette Darfeuille-Michaud (UMR 1071 “Microbes, Intestine, Inflammation and Host Susceptibility,” Inserm, Inra, and Université d'Auvergne, Clermont-Ferrand, France).
REFERENCES
- 1.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 2.Johnson TJ, Nolan LK. 2009. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 73:750–774. 10.1128/MMBR.00015-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. 2013. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 26:822–880. 10.1128/CMR.00022-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements A, Young JC, Constantinou N, Frankel G. 2012. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 3:71–87. 10.4161/gmic.19182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405–1413. 10.1016/S0016-5085(98)70019-8 [DOI] [PubMed] [Google Scholar]
- 7.Barnich N, Darfeuille-Michaud A. 2007. Adherent-invasive Escherichia coli and Crohn's disease. Curr. Opin. Gastroenterol. 23:16–20. 10.1097/MOG.0b013e3280105a38 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd AL, Henderson TA, Vigil PD, Mobley HL. 2009. Genomic islands of uropathogenic Escherichia coli contribute to virulence. J. Bacteriol. 191:3469–3481. 10.1128/JB.01717-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokady D, Gophna U, Ron EZ. 2005. Virulence factors of septicemic Escherichia coli strains. Int. J. Med. Microbiol. 295:455–462. 10.1016/j.ijmm.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Kim KS. 2008. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 6:625–634. 10.1038/nrmicro1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Servin AL. 2005. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin. Microbiol. Rev. 18:264–292. 10.1128/CMR.18.2.264-292.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benz I, Schmidt MA. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beinke C, Laarmann S, Wachter C, Karch H, Greune L, Schmidt MA. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect. Immun. 66:528–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laarmann S, Schmidt MA. 2003. The Escherichia coli AIDA autotransporter adhesin recognizes an integral membrane glycoprotein as receptor. Microbiology 149:1871–1882. 10.1099/mic.0.26264-0 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt MA. 2010. LEEways: tales of EPEC, ATEC and EHEC. Cell. Microbiol. 12:1544–1552. 10.1111/j.1462-5822.2010.01518.x [DOI] [PubMed] [Google Scholar]
- 16.Hernandes RT, Elias WP, Vieira MA, Gomes TA. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 297:137–149. 10.1111/j.1574-6968.2009.01664.x [DOI] [PubMed] [Google Scholar]
- 17.Le Bouguenec C, Servin AL. 2006. Diffusely adherent Escherichia coli strains expressing Afa/Dr adhesins (Afa/Dr DAEC): hitherto unrecognized pathogens. FEMS Microbiol. Lett. 256:185–194. 10.1111/j.1574-6968.2006.00144.x [DOI] [PubMed] [Google Scholar]
- 18.Czeczulin JR, Whittam TS, Henderson IR, Navarro-Garcia F, Nataro JP. 1999. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261–272. 10.1086/315217 [DOI] [PubMed] [Google Scholar]
- 20.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8:207–217. 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- 21.Nowrouzian FL, Adlerberth I, Wold AE. 2006. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 8:834–840. 10.1016/j.micinf.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 22.Nowrouzian FL, Oswald E. 2012. Escherichia coli strains with the capacity for long-term persistence in the bowel microbiota carry the potentially genotoxic pks island. Microb. Pathog. 53:180–182. 10.1016/j.micpath.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 23.Nowrouzian FL, Wold AE, Adlerberth I. 2005. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J. Infect. Dis. 191:1078–1083. 10.1086/427996 [DOI] [PubMed] [Google Scholar]
- 24.Labigne-Roussel A, Schmidt MA, Walz W, Falkow S. 1985. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J. Bacteriol. 162:1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labigne-Roussel AF, Lark D, Schoolnik G, Falkow S. 1984. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect. Immun. 46:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalioui L, Jouve M, Gounon P, Le Bouguenec C. 1999. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia coli strains associated with diarrhea or septicemia in calves. Infect. Immun. 67:5048–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bouguenec C, Garcia MI, Ouin V, Desperrier JM, Gounon P, Labigne A. 1993. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect. Immun. 61:5106–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia MI, Gounon P, Courcoux P, Labigne A, Le Bouguenec C. 1996. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol. Microbiol. 19:683–693. 10.1046/j.1365-2958.1996.394935.x [DOI] [PubMed] [Google Scholar]
- 29.Nowicki B, Barrish JP, Korhonen T, Hull RA, Hull SI. 1987. Molecular cloning of the Escherichia coli O75X adhesin. Infect. Immun. 55:3168–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham TQ, Goluszko P, Popov V, Nowicki S, Nowicki BJ. 1997. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect. Immun. 65:4309–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilge SS, Apostol JM, Jr, Fullner KJ, Moseley SL. 1993. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol. Microbiol. 7:993–1006. 10.1111/j.1365-2958.1993.tb01191.x [DOI] [PubMed] [Google Scholar]
- 32.Bilge SS, Clausen CR, Lau W, Moseley SL. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki B, Moulds J, Hull R, Hull S. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki B, Selvarangan R, Nowicki S. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl 1):S24–S27. 10.1086/318846 [DOI] [PubMed] [Google Scholar]
- 35.Scaletsky IC, Silva ML, Trabulsi LR. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nataro JP, Scaletsky IC, Kaper JB, Levine MM, Trabulsi LR. 1985. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 48:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walz W, Schmidt MA, Labigne-Roussel AF, Falkow S, Schoolnik G. 1985. AFA-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur. J. Biochem. 152:315–321 [DOI] [PubMed] [Google Scholar]
- 38.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki BJ. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176:158–167. 10.1086/514018 [DOI] [PubMed] [Google Scholar]
- 39.Goluszko P, Selvarangan R, Nowicki BJ, Nowicki S, Hart A, Pawelczyk E, Nguyen K. 2001. Rapid receptor-clustering assay to detect uropathogenic and diarrheal Escherichia coli isolates bearing adhesins of the Dr family. J. Clin. Microbiol. 39:2317–2320. 10.1128/JCM.39.6.2317-2320.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goluszko P, Selvarangan R, Popov V, Pham T, Wen JW, Singhal J. 1999. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67:3989–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guignot J, Peiffer I, Bernet-Camard MF, Lublin DM, Carnoy C, Moseley SL, Servin AL. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554–3563. 10.1128/IAI.68.6.3554-3563.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jouve M, Garcia MI, Courcoux P, Labigne A, Gounon P, Le Bouguenec C. 1997. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 65:4082–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girardeau JP, Lalioui L, Said AM, De Champs C, Le Bouguenec C. 2003. Extended virulence genotype of pathogenic Escherichia coli isolates carrying the afa-8 operon: evidence of similarities between isolates from humans and animals with extraintestinal infections. J. Clin. Microbiol. 41:218–226. 10.1128/JCM.41.1.218-226.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lalioui L, Le Bouguenec C. 2001. afa-8 gene cluster is carried by a pathogenicity island inserted into the tRNA(Phe) of human and bovine pathogenic Escherichia coli isolates. Infect. Immun. 69:937–948. 10.1128/IAI.69.2.937-948.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Bouguenec C, Lalioui L, du Merle L, Jouve M, Courcoux P, Bouzari S, Selvarangan R, Nowicki BJ, Germani Y, Andremont A, Gounon P, Garcia MI. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 39:1738–1745. 10.1128/JCM.39.5.1738-1745.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piva IC, Pereira AL, Ferraz LR, Silva RS, Vieira AC, Blanco JE, Blanco M, Blanco J, Giugliano LG. 2003. Virulence markers of enteroaggregative Escherichia coli isolated from children and adults with diarrhea in Brasilia, Brazil. J. Clin. Microbiol. 41:1827–1832. 10.1128/JCM.41.5.1827-1832.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller R, Ordonez JG, de Oliveira RR, Trabulsi LR, Baldwin TJ, Knutton S. 2002. Afa, a diffuse adherence fibrillar adhesin associated with enteropathogenic Escherichia coli. Infect. Immun. 70:2681–2689. 10.1128/IAI.70.5.2681-2689.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scaletsky IC, Pedroso MZ, Morais MB, Carvalho RL, Silva RM, Fabbricotti SH, Fagundes-Neto U. 1999. Association of patterns of Escherichia coli adherence to HEp-2 cells with acute and persistent diarrhea. Arq. Gastroenterol. 36:54–60 [PubMed] [Google Scholar]
- 49.Jallat C, Darfeuille-Michaud A, Rich C, Joly B. 1994. Survey of clinical isolates of diarrhoeogenic Escherichia coli: diffusely adhering E. coli strains with multiple adhesive factors. Res. Microbiol. 145:621–632. 10.1016/0923-2508(94)90079-5 [DOI] [PubMed] [Google Scholar]
- 50.Echeverria P, Serichantalerg O, Changchawalit S, Baudry B, Levine MM, Orskov F, Orskov I. 1992. Tissue culture-adherent Escherichia coli in infantile diarrhea. J. Infect. Dis. 165:141–143. 10.1093/infdis/165.1.141 [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Kaneko M, Changchawalit S, Serichantalergs O, Ijuin S, Echeverria P. 1994. Actin accumulation associated with clustered and localized adherence in Escherichia coli isolated from patients with diarrhea. Infect. Immun. 62:2917–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantey JR, Moseley SL. 1991. HeLa cell adherence, actin aggregation, and invasion by nonenteropathogenic Escherichia coli possessing the eae gene. Infect. Immun. 59:3924–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Archambaud M, Courcoux P, Labigne-Roussel A. 1988. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann. Inst. Pasteur Microbiol. 139:575–588. 10.1016/0769-2609(88)90156-1 [DOI] [PubMed] [Google Scholar]
- 54.Le Bouguenec C, Archambaud M, Labigne A. 1992. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 30:1189–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowicki B, Svanborg-Eden C, Hull R, Hull S. 1989. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect. Immun. 57:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foxman B, Zhang L, Palin K, Tallman P, Marrs CF. 1995. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 171:1514–1521. 10.1093/infdis/171.6.1514 [DOI] [PubMed] [Google Scholar]
- 57.Stapleton A, Moseley S, Stamm WE. 1991. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J. Infect. Dis. 163:773–779. 10.1093/infdis/163.4.773 [DOI] [PubMed] [Google Scholar]
- 58.Spano LC, Sadovsky AD, Segui PN, Saick KW, Kitagawa SM, Pereira FE, Fagundes-Neto U, Scaletsky IC. 2008. Age-specific prevalence of diffusely adherent Escherichia coli in Brazilian children with acute diarrhoea. J. Med. Microbiol. 57:359–363. 10.1099/jmm.0.47660-0 [DOI] [PubMed] [Google Scholar]
- 59.Campos LC, Vieira MA, Trabulsi LR, da Silva LA, Monteiro-Neto V, Gomes TA. 1999. Diffusely adhering Escherichia coli (DAEC) strains of fecal origin rarely express F1845 adhesin. Microbiol. Immunol. 43:167–170. 10.1111/j.1348-0421.1999.tb02388.x [DOI] [PubMed] [Google Scholar]
- 60.Blanc-Potard AB, Tinsley C, Scaletsky I, Le Bouguenec C, Guignot J, Servin AL, Nassif X, Bernet-Camard MF. 2002. Representational difference analysis between Afa/Dr diffusely adhering Escherichia coli and nonpathogenic E. coli K-12. Infect. Immun. 70:5503–5511. 10.1128/IAI.70.10.5503-5511.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escobar-Paramo P, Clermont O, Blanc-Potard AB, Bui H, Le Bouguenec C, Denamur E. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085–1094. 10.1093/molbev/msh118 [DOI] [PubMed] [Google Scholar]
- 62.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin DM, Nowicki BJ. 1995. dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect. Immun. 63:1663–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Germani Y, Begaud E, Duval P, Le Bouguenec C. 1996. Prevalence of enteropathogenic, enteroaggregative, and diffusely adherent Escherichia coli among isolates from children with diarrhea in new Caledonia. J. Infect. Dis. 174:1124–1126. 10.1093/infdis/174.5.1124 [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Foxman B, Tallman P, Cladera E, Le Bouguenec C, Marrs CF. 1997. Distribution of drb genes coding for Dr binding adhesins among uropathogenic and fecal Escherichia coli isolates and identification of new subtypes. Infect. Immun. 65:2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto S, Terai A, Yuri K, Kurazono H, Takeda Y, Yoshida O. 1995. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 12:85–90. 10.1111/j.1574-695X.1995.tb00179.x [DOI] [PubMed] [Google Scholar]
- 66.Barletta F, Ochoa TJ, Cleary TG. 2013. Multiplex real-time PCR (MRT-PCR) for diarrheagenic. Methods Mol. Biol. 943:307–314. 10.1007/978-1-60327-353-4_21 [DOI] [PubMed] [Google Scholar]
- 67.Barletta F, Ochoa TJ, Ecker L, Gil AI, Lanata CF, Cleary TG. 2009. Validation of five-colony pool analysis using multiplex real-time PCR for detection of diarrheagenic Escherichia coli. J. Clin. Microbiol. 47:1915–1917. 10.1128/JCM.00608-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. 2008. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J. Clin. Microbiol. 46:1752–1757. 10.1128/JCM.02341-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith HR, Scotland SM, Willshaw GA, Rowe B, Cravioto A, Eslava C. 1994. Isolates of Escherichia coli O44:H18 of diverse origin are enteroaggregative. J. Infect. Dis. 170:1610–1613. 10.1093/infdis/170.6.1610 [DOI] [PubMed] [Google Scholar]
- 70.Baudry B, Savarino SJ, Vial P, Kaper JB, Levine MM. 1990. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J. Infect. Dis. 161:1249–1251. 10.1093/infdis/161.6.1249 [DOI] [PubMed] [Google Scholar]
- 71.Gomes TA, Vieira MA, Abe CM, Rodrigues D, Griffin PM, Ramos SR. 1998. Adherence patterns and adherence-related DNA sequences in Escherichia coli isolates from children with and without diarrhea in Sao Paulo city, Brazil. J. Clin. Microbiol. 36:3609–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snelling AM, Macfarlane-Smith LR, Fletcher JN, Okeke IN. 2009. The commonly-used DNA probe for diffusely-adherent Escherichia coli cross-reacts with a subset of enteroaggregative E. coli. BMC Microbiol. 9:269. 10.1186/1471-2180-9-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins C, Chart H, Willshaw GA, Cheasty T, Tompkins DS. 2007. Association of putative pathogenicity genes with adherence characteristics and fimbrial genotypes in typical enteroaggregative Escherichia coli from patients with and without diarrhoea in the United Kingdom. Eur. J. Clin. Microbiol. Infect. Dis. 26:901–906. 10.1007/s10096-007-0388-z [DOI] [PubMed] [Google Scholar]
- 74.Monteiro BT, Campos LC, Sircili MP, Franzolin MR, Bevilacqua LF, Nataro JP, Elias WP. 2009. The dispersin-encoding gene (aap) is not restricted to enteroaggregative Escherichia coli. Diagn. Microbiol. Infect. Dis. 65:81–84. 10.1016/j.diagmicrobio.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 75.Bernier C, Gounon P, Le Bouguenec C. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 70:4302–4311. 10.1128/IAI.70.8.4302-4311.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, Navarro-Garcia F, Nataro JP. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nataro JP, Deng Y, Maneval DR, German AL, Martin WC, Levine MM. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. 2008. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect. Immun. 76:3281–3292. 10.1128/IAI.01646-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estrada-Garcia T, Navarro-Garcia F. 2012. Enteroaggregative Escherichia coli pathotype: a genetically heterogeneous emerging foodborne enteropathogen. FEMS Immunol. Med. Microbiol. 66:281–298. 10.1111/j.1574-695X.2012.01008.x [DOI] [PubMed] [Google Scholar]
- 80.Harrington SM, Dudley EG, Nataro JP. 2006. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 254:12–18. 10.1111/j.1574-6968.2005.00005.x [DOI] [PubMed] [Google Scholar]
- 81.Navarro-Garcia F, Elias WP. 2011. Autotransporters and virulence of enteroaggregative E. coli. Gut Microbes 2:13–24 [DOI] [PubMed] [Google Scholar]
- 82.Nowicki B, Nowicki S. 2013. DAF as a therapeutic target for steroid hormones: implications for host-pathogen interactions. Adv. Exp. Med. Biol. 735:83–96. 10.1007/978-1-4614-4118-2_5 [DOI] [PubMed] [Google Scholar]
- 83.Zalewska-Piatek BM. 2011. Urinary tract infections of Escherichia coli strains of chaperone-usher system. Pol. J. Microbiol. 60:279–285 [PubMed] [Google Scholar]
- 84.Labigne-Roussel A, Falkow S. 1988. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect. Immun. 56:640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arthur M, Johnson CE, Rubin RH, Arbeit RD, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daigle F, Harel J, Fairbrother JM, Lebel P. 1994. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can. J. Microbiol. 40:286–291. 10.1139/m94-046 [DOI] [PubMed] [Google Scholar]
- 87.D'Orazio SE, Collins CM. 1998. Molecular pathogenesis of urinary tract infections. Curr. Top. Microbiol. Immunol. 225:137–164 [DOI] [PubMed] [Google Scholar]
- 88.Usein CR, Damian M, Tatu-Chitoiu D, Capusa C, Fagaras R, Mircescu G. 2003. Comparison of genomic profiles of Escherichia coli isolates from urinary tract infections. Roum. Arch. Microbiol. Immunol. 62:137–154 [PubMed] [Google Scholar]
- 89.Usein CR, Damian M, Tatu-Chitoiu D, Capusa C, Fagaras R, Tudorache D, Nica M, Le Bouguenec C. 2001. Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell. Mol. Med. 5:303–310. 10.1111/j.1582-4934.2001.tb00164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hart A, Nowicki BJ, Reisner B, Pawelczyk E, Goluszko P, Urvil P, Anderson G, Nowicki S. 2001. Ampicillin-resistant Escherichia coli in gestational pyelonephritis: increased occurrence and association with the colonization factor Dr adhesin. J. Infect. Dis. 183:1526–1529. 10.1086/320196 [DOI] [PubMed] [Google Scholar]
- 91.Hart A, Pham T, Nowicki S, Whorton EB, Jr, Martens MG, Anderson GD, Nowicki BJ. 1996. Gestational pyelonephritis-associated Escherichia coli isolates represent a nonrandom, closely related population. Am. J. Obstet. Gynecol. 174:983–989. 10.1016/S0002-9378(96)70337-X [DOI] [PubMed] [Google Scholar]
- 92.Foxman B, Zhang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs CF. 1995. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J. Infect. Dis. 172:1536–1541. 10.1093/infdis/172.6.1536 [DOI] [PubMed] [Google Scholar]
- 93.Rice JC, Peng T, Kuo YF, Pendyala S, Simmons L, Boughton J, Ishihara K, Nowicki S, Nowicki BJ. 2006. Renal allograft injury is associated with urinary tract infection caused by Escherichia coli bearing adherence factors. Am. J. Transplant. 6:2375–2383. 10.1111/j.1600-6143.2006.01471.x [DOI] [PubMed] [Google Scholar]
- 94.Goluszko P, Niesel D, Nowicki B, Selvarangan R, Nowicki S, Hart A, Pawelczyk E, Das M, Urvil P, Hasan R. 2001. Dr operon-associated invasiveness of Escherichia coli from pregnant patients with pyelonephritis. Infect. Immun. 69:4678–4680. 10.1128/IAI.69.7.4678-4680.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nowicki B. 2002. Urinary tract infection in pregnant women: old dogmas and current concepts regarding pathogenesis. Curr. Infect. Dis. Rep. 4:529–535. 10.1007/s11908-002-0041-z [DOI] [PubMed] [Google Scholar]
- 96.Nowicki B, Martens M, Hart A, Nowicki S. 1994. Gestational age-dependent distribution of Escherichia coli fimbriae in pregnant patients with pyelonephritis. Ann. N. Y. Acad. Sci. 730:290–291. 10.1111/j.1749-6632.1994.tb44268.x [DOI] [PubMed] [Google Scholar]
- 97.Sledzinska A, Mielech A, Krawczyk B, Samet A, Nowicki B, Nowicki S, Jankowski Z, Kur J. 2011. Fatal sepsis in a pregnant woman with pyelonephritis caused by Escherichia coli bearing Dr and P adhesins: diagnosis based on postmortem strain genotyping. BJOG 118:266–269. 10.1111/j.1471-0528.2010.02732.x [DOI] [PubMed] [Google Scholar]
- 98.Zhang L, Foxman B. 2003. Molecular epidemiology of Escherichia coli mediated urinary tract infections. Front. Biosci. 8:e235–e244. 10.2741/1007 [DOI] [PubMed] [Google Scholar]
- 99.Forestier C, Meyer M, Favre-Bonte S, Rich C, Malpuech G, Le Bouguenec C, Sirot J, Joly B, De Champs C. 1996. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J. Clin. Microbiol. 34:2897–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishitoya S, Yamamoto S, Kanamaru S, Kurazono H, Habuchi T, Ogawa O, Terai A. 2003. Distribution of afaE adhesins in Escherichia coli isolated from Japanese patients with urinary tract infection. J. Urol. 169:1758–1761. 10.1097/01.ju.0000057968.53213.b1 [DOI] [PubMed] [Google Scholar]
- 101.Szemiako K, Krawczyk B, Samet A, Sledzinska A, Nowicki B, Nowicki S, Kur J. 2013. A subset of two adherence systems, acute pro-inflammatory pap genes and invasion coding dra, fim, or sfa, increases the risk of Escherichia coli translocation to the bloodstream. Eur. J. Clin. Microbiol. Infect. Dis. 32:1579–1582. 10.1007/s10096-013-1913-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guignot J, Breard J, Bernet-Camard MF, Peiffer I, Nowicki BJ, Servin AL, Blanc-Potard AB. 2000. Pyelonephritogenic diffusely adhering Escherichia coli EC7372 harboring Dr-II adhesin carries classical uropathogenic virulence genes and promotes cell lysis and apoptosis in polarized epithelial Caco-2/TC7 cells. Infect. Immun. 68:7018–7027. 10.1128/IAI.68.12.7018-7027.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nimmich W, Voigt W, Seltmann G. 1997. Characterization of urinary Escherichia coli O75 strains. J. Clin. Microbiol. 35:1112–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie J, Foxman B, Zhang L, Marrs CF. 2006. Molecular epidemiologic identification of Escherichia coli genes that are potentially involved in movement of the organism from the intestinal tract to the vagina and bladder. J. Clin. Microbiol. 44:2434–2441. 10.1128/JCM.00397-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nowicki B, Sledzinska A, Samet A, Nowicki S. 2011. Pathogenesis of gestational urinary tract infection: urinary obstruction versus immune adaptation and microbial virulence. BJOG 118:109–112. 10.1111/j.1471-0528.2010.02706.x [DOI] [PubMed] [Google Scholar]
- 106.Tacket CO, Moseley SL, Kay B, Losonsky G, Levine MM. 1990. Challenge studies in volunteers using Escherichia coli strains with diffuse adherence to HEp-2 cells. J. Infect. Dis. 162:550–552. 10.1093/infdis/162.2.550 [DOI] [PubMed] [Google Scholar]
- 107.Meraz IM, Jiang ZD, Ericsson CD, Bourgeois AL, Steffen R, Taylor DN, Hernandez N, DuPont HL. 2008. Enterotoxigenic Escherichia coli and diffusely adherent E. coli as likely causes of a proportion of pathogen-negative travelers' diarrhea—a PCR-based study. J. Travel Med. 15:412–418. 10.1111/j.1708-8305.2008.00249.x [DOI] [PubMed] [Google Scholar]
- 108.Fujihara S, Arikawa K, Aota T, Tanaka H, Nakamura H, Wada T, Hase A, Nishikawa Y. 2009. Prevalence and properties of diarrheagenic Escherichia coli among healthy individuals in Osaka City, Japan. Jpn. J. Infect. Dis. 62:318–323 [PubMed] [Google Scholar]
- 109.Lopes LM, Fabbricotti SH, Ferreira AJ, Kato MA, Michalski J, Scaletsky IC. 2005. Heterogeneity among strains of diffusely adherent Escherichia coli isolated in Brazil. J. Clin. Microbiol. 43:1968–1972. 10.1128/JCM.43.4.1968-1972.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vargas M, Gascon J, Gallardo F, Jimenez De Anta MT, Vila J. 1998. Prevalence of diarrheagenic Escherichia coli strains detected by PCR in patients with travelers' diarrhea. Clin. Microbiol. Infect. 4:682–688. 10.1111/j.1469-0691.1998.tb00652.x [DOI] [PubMed] [Google Scholar]
- 112.Schultsz C, Moussa M, van Ketel R, Tytgat GN, Dankert J. 1997. Frequency of pathogenic and enteroadherent Escherichia coli in patients with inflammatory bowel disease and controls. J. Clin. Pathol. 50:573–579. 10.1136/jcp.50.7.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kang G, Mathan MM, Mathan VI. 1995. Evaluation of a simplified HEp-2 cell adherence assay for Escherichia coli isolated from south Indian children with acute diarrhea and controls. J. Clin. Microbiol. 33:2204–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morelli R, Baldassarri L, Falbo V, Donelli G, Caprioli A. 1994. Detection of enteroadherent Escherichia coli associated with diarrhoea in Italy. J. Med. Microbiol. 41:399–404. 10.1099/00222615-41-6-399 [DOI] [PubMed] [Google Scholar]
- 115.Brook MG, Smith HR, Bannister BA, McConnell M, Chart H, Scotland SM, Sawyer A, Smith M, Rowe B. 1994. Prospective study of verocytotoxin-producing, enteroaggregative and diffusely adherent Escherichia coli in different diarrhoeal states. Epidemiol. Infect. 112:63–67. 10.1017/S0950268800057423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen MB, Nataro JP, Bernstein DI, Hawkins J, Roberts N, Staat MA. 2005. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J. Pediatr. 146:54–61. 10.1016/j.jpeds.2004.08.059 [DOI] [PubMed] [Google Scholar]
- 117.Giron JA, Jones T, Millan-Velasco F, Castro-Munoz E, Zarate L, Fry J, Frankel G, Moseley SL, Baudry B, Kaper JB, et al. 1991. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J. Infect. Dis. 163:507–513. 10.1093/infdis/163.3.507 [DOI] [PubMed] [Google Scholar]
- 118.Levine MM, Ferreccio C, Prado V, Cayazzo M, Abrego P, Martinez J, Maggi L, Baldini MM, Martin W, Maneval D, et al. 1993. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am. J. Epidemiol. 138:849–869 [DOI] [PubMed] [Google Scholar]
- 119.Meraz IM, Arikawa K, Nakamura H, Ogasawara J, Hase A, Nishikawa Y. 2007. Association of IL-8-inducing strains of diffusely adherent Escherichia coli with sporadic diarrheal patients with less than 5 years of age. Braz. J. Infect. Dis. 11:44–49. 10.1590/S1413-86702007000100012 [DOI] [PubMed] [Google Scholar]
- 120.Scaletsky IC, Fabbricotti SH, Carvalho RL, Nunes CR, Maranhao HS, Morais MB, Fagundes-Neto U. 2002. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. J. Clin. Microbiol. 40:645–648. 10.1128/JCM.40.2.645-648.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosa AC, Mariano AT, Pereira AM, Tibana A, Gomes TA, Andrade JR. 1998. Enteropathogenicity markers in Escherichia coli isolated from infants with acute diarrhoea and healthy controls in Rio de Janeiro, Brazil. J. Med. Microbiol. 47:781–790. 10.1099/00222615-47-9-781 [DOI] [PubMed] [Google Scholar]
- 122.Gomez-Duarte OG, Arzuza O, Urbina D, Bai J, Guerra J, Montes O, Puello M, Mendoza K, Castro GY. 2010. Detection of Escherichia coli enteropathogens by multiplex polymerase chain reaction from children's diarrheal stools in two Caribbean-Colombian cities. Foodborne Pathog. Dis. 7:199–206. 10.1089/fpd.2009.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ochoa TJ, Mercado EH, Durand D, Rivera FP, Mosquito S, Contreras C, Riveros M, Lluque A, Barletta F, Prada A, Ruiz J. 2011. Frequency and pathotypes of diarrheagenic Escherichia coli in Peruvian children with and without diarrhea. Rev. Peru Med. Exp. Salud Publica 28:13–20. 10.1590/S1726-46342011000100003 [DOI] [PubMed] [Google Scholar]
- 124.Ochoa TJ, Ruiz J, Molina M, Del Valle LJ, Vargas M, Gil AI, Ecker L, Barletta F, Hall E, Cleary TG, Lanata CF. 2009. High frequency of antimicrobial drug resistance of diarrheagenic Escherichia coli in infants in Peru. Am. J. Trop. Med. Hyg. 81:296–301 [PMC free article] [PubMed] [Google Scholar]
- 125.Ochoa TJ, Rivera FP, Bernal M, Meza R, Ecker L, Gil AI, Cepeda D, Mosquito S, Mercado E, Maves RC, Hall ER, Svennerholm AM, McVeigh A, Savarino S, Lanata CF. 2010. Detection of the CS20 colonization factor antigen in diffuse-adhering Escherichia coli strains. FEMS Immunol. Med. Microbiol. 60:186–189. 10.1111/j.1574-695X.2010.00730.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riveros L, Barletta F, Cabello M, Durand D, Mercado EH, Contreras C, Rivera FP, Mosquito S, Lluque Á, Ochoa TJ. 2011. Patrones de adherencia de cepas de Escherichia coli difusamente adherente (DAEC) provenientes de ninos con y sin diarrhea. Rev. Peru Med. Exp. Salud Publica 28:21–28. 10.1590/S1726-46342011000100004 [DOI] [PubMed] [Google Scholar]
- 127.Riveros M, Barletta F, Cabello M, Durand D, Mercado EH, Contreras C, Rivera FP, Mosquito S, Lluque A, Ochoa TJ. 2011. Adhesion patterns in diffusely adherent Escherichia coli (DAEC) strains isolated from children with and without diarrhea. Rev. Peru Med. Exp. Salud Publica 28:21–28. 10.1590/S1726-46342011000100004 [DOI] [PubMed] [Google Scholar]
- 128.Quiroga M, Oviedo P, Chinen I, Pegels E, Husulak E, Binztein N, Rivas M, Schiavoni L, Vergara M. 2000. Asymptomatic infections by diarrheagenic Escherichia coli in children from Misiones, Argentina, during the first twenty months of their lives. Rev. Inst. Med. Trop. Sao Paulo 42:9–15 [DOI] [PubMed] [Google Scholar]
- 129.Baqui AH, Sack RB, Black RE, Haider K, Hossain A, Alim AR, Yunus M, Chowdhury HR, Siddique AK. 1992. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J. Infect. Dis. 166:792–796. 10.1093/infdis/166.4.792 [DOI] [PubMed] [Google Scholar]
- 130.Germani Y, Begaud E, Duval P, Le Bouguenec C. 1997. An Escherichia coli clone carrying the adhesin-encoding afa operon is involved in both diarrhoea and cystitis in twins. Trans. R. Soc. Trop. Med. Hyg. 91:573. 10.1016/S0035-9203(97)90031-6 [DOI] [PubMed] [Google Scholar]
- 131.Valentiner-Branth P, Steinsland H, Fischer TK, Perch M, Scheutz F, Dias F, Aaby P, Molbak K, Sommerfelt H. 2003. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J. Clin. Microbiol. 41:4238–4245. 10.1128/JCM.41.9.4238-4245.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okeke IN. 2009. Diarrheagenic Escherichia coli in sub-Saharan Africa: status, uncertainties and necessities. J. Infect. Dev. Ctries. 3:817–842 [DOI] [PubMed] [Google Scholar]
- 133.Okeke IN, Lamikanra A, Steinruck H, Kaper JB. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Knutton S, Shaw R, Phillips AD, Smith HR, Willshaw GA, Watson P, Price E. 2001. Phenotypic and genetic analysis of diarrhea-associated Escherichia coli isolated from children in the United Kingdom. J. Pediatr. Gastroenterol. Nutr. 33:32–40. 10.1097/00005176-200107000-00006 [DOI] [PubMed] [Google Scholar]
- 135.Poitrineau P, Forestier C, Meyer M, Jallat C, Rich C, Malpuech G, De Champs C. 1995. Retrospective case-control study of diffusely adhering Escherichia coli and clinical features in children with diarrhea. J. Clin. Microbiol. 33:1961–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jallat C, Livrelli V, Darfeuille-Michaud A, Rich C, Joly B. 1993. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J. Clin. Microbiol. 31:2031–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guignot J, Chaplais C, Coconnier-Polter MH, Servin AL. 2007. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 9:204–221. 10.1111/j.1462-5822.2006.00782.x [DOI] [PubMed] [Google Scholar]
- 138.Martinez-Medina M, Aldeguer X, Lopez-Siles M, Gonzalez-Huix F, Lopez-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil LJ, Darfeuille-Michaud A. 2009. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm. Bowel Dis. 15:872–882. 10.1002/ibd.20860 [DOI] [PubMed] [Google Scholar]
- 139.Martinez-Medina M, Mora A, Blanco M, Lopez C, Alonso MP, Bonacorsi S, Nicolas-Chanoine MH, Darfeuille-Michaud A, Garcia-Gil J, Blanco J. 2009. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J. Clin. Microbiol. 47:3968–3979. 10.1128/JCM.01484-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, Knight P, Codling C, Marchesi JR, Winstanley C, Hall N, Rhodes JM, Campbell BJ. 2014. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63:581–594. 10.1136/gutjnl-2013-304739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muller D, Greune L, Heusipp G, Karch H, Fruth A, Tschape H, Schmidt MA. 2007. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 73:3380–3390. 10.1128/AEM.02855-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kline KA, Falker S, Dahlberg S, Normark S, Henriques-Normark B. 2009. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5:580–592. 10.1016/j.chom.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 143.Hayward RD, Leong JM, Koronakis V, Campellone KG. 2006. Exploiting pathogenic Escherichia coli to model transmembrane receptor signalling. Nat. Rev. Microbiol. 4:358–370. 10.1038/nrmicro1391 [DOI] [PubMed] [Google Scholar]
- 144.Gerlach RG, Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 297:401–415. 10.1016/j.ijmm.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 145.Thanassi DG, Bliska JB, Christie PJ. 2012. Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function. FEMS Microbiol. Rev. 36:1046–1082. 10.1111/j.1574-6976.2012.00342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Waksman G, Hultgren SJ. 2009. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 7:765–774. 10.1038/nrmicro2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zav'yalov V, Zavialov A, Zav'yalova G, Korpela T. 2010. Adhesive organelles of Gram-negative pathogens assembled with the classical chaperone/usher machinery: structure and function from a clinical standpoint. FEMS Microbiol. Rev. 34:317–378. 10.1111/j.1574-6976.2009.00201.x [DOI] [PubMed] [Google Scholar]
- 148.Soto GE, Hultgren SJ. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fronzes R, Remaut H, Waksman G. 2008. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 27:2271–2280. 10.1038/emboj.2008.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holland IB, Schmitt L, Young J. 2005. Type 1 protein secretion in bacteria, the ABC-transporter dependent pathway. Mol. Membr. Biol. 22:29–39. 10.1080/09687860500042013 [DOI] [PubMed] [Google Scholar]
- 151.Lai Y, Rosenshine I, Leong JM, Frankel G. 2013. Intimate host attachment: enteropathogenic and enterohaemorrhagic Escherichia coli. Cell. Microbiol. 15:1796–1808. 10.1111/cmi.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Voth DE, Broederdorf LJ, Graham JG. 2012. Bacterial type IV secretion systems: versatile virulence machines. Future Microbiol. 7:241–257. 10.2217/fmb.11.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692–744. 10.1128/MMBR.68.4.692-744.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Elias WP, Suzart S, Trabulsi LR, Nataro JP, Gomes TA. 1999. Distribution of aggA and aafA gene sequences among Escherichia coli isolates with genotypic or phenotypic characteristics, or both, of enteroaggregative E. coli. J. Med. Microbiol. 48:597–599. 10.1099/00222615-48-6-597 [DOI] [PubMed] [Google Scholar]
- 155.Vaisanen-Rhen V. 1984. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect. Immun. 46:401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abe K, Miyazaki M, Koji T, Furusu A, Ozono Y, Harada T, Sakai H, Nakane PK, Kohno S. 1998. Expression of decay accelerating factor mRNA and complement C3 mRNA in human diseased kidney. Kidney Int. 54:120–130. 10.1046/j.1523-1755.1998.00961.x [DOI] [PubMed] [Google Scholar]
- 157.Korotkova N, Le Trong I, Samudrala R, Korotkov K, Van Loy CP, Bui AL, Moseley SL, Stenkamp RE. 2006. Crystal structure and mutational analysis of the DaaE adhesin of Escherichia coli. J. Biol. Chem. 281:22367–22377. 10.1074/jbc.M604646200 [DOI] [PubMed] [Google Scholar]
- 158.Anderson KL, Billington J, Pettigrew D, Cota E, Simpson P, Roversi P, Chen HA, Urvil P, du Merle L, Barlow PN, Medof ME, Smith RA, Nowicki B, Le Bouguenec C, Lea SM, Matthews S. 2004. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15:647–657. 10.1016/j.molcel.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 159.Anderson KL, Cota E, Simpson P, Chen HA, Du Merle L, Bouguenec CL, Matthews S. 2004. Complete resonance assignments of a ‘donor-strand complemented' AfaE: the afimbrial adhesin from diffusely adherent E. coli. J. Biomol. NMR 29:409–410. 10.1023/B:JNMR.0000032498.94441.08 [DOI] [PubMed] [Google Scholar]
- 160.Korotkova N, Cota E, Lebedin Y, Monpouet S, Guignot J, Servin AL, Matthews S, Moseley SL. 2006. A subfamily of Dr adhesins of Escherichia coli bind independently to decay-accelerating factor and the N-domain of carcinoembryonic antigen. J. Biol. Chem. 281:29120–29130. 10.1074/jbc.M605681200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pettigrew D, Anderson KL, Billington J, Cota E, Simpson P, Urvil P, Rabuzin F, Roversi P, Nowicki B, du Merle L, Le Bouguenec C, Matthews S, Lea SM. 2004. High resolution studies of the Afa/Dr adhesin DraE and its interaction with chloramphenicol. J. Biol. Chem. 279:46851–46857. 10.1074/jbc.M409284200 [DOI] [PubMed] [Google Scholar]
- 162.Pettigrew DM, Roversi P, Davies SG, Russell AJ, Lea SM. 2009. A structural study of the interaction between the Dr haemagglutinin DraE and derivatives of chloramphenicol. Acta Crystallogr. Section D Biol. Cryst. 65:513–522. 10.1107/S0907444909005113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garcia MI, Labigne A, Le Bouguenec C. 1994. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J. Bacteriol. 176:7601–7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bilge SS, Apostol JM, Jr, Aldape MA, Moseley SL. 1993. mRNA processing independent of RNase III and RNase E in the expression of the F1845 fimbrial adhesin of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 90:1455–1459. 10.1073/pnas.90.4.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Loomis WP, Moseley SL. 1998. Translational control of mRNA processing in the F1845 fimbrial operon of Escherichia coli. Mol. Microbiol. 30:843–853. 10.1046/j.1365-2958.1998.01117.x [DOI] [PubMed] [Google Scholar]
- 166.Cota E, Chen HA, Anderson KL, Simpson P, Du Merle L, Bernier-Febreau C, Piaatek R, Zalewska B, Nowicki B, Kur J, Le Bouguenec C, Matthews S. 2004. Complete resonance assignments of the ‘donor-strand complemented' AfaD: the afimbrial invasin from diffusely adherent E. coli. J. Biomol. NMR 29:411–412. 10.1023/B:JNMR.0000032499.61022.45 [DOI] [PubMed] [Google Scholar]
- 167.Cota E, Jones C, Simpson P, Altroff H, Anderson KL, du Merle L, Guignot J, Servin A, Le Bouguenec C, Mardon H, Matthews S. 2006. The solution structure of the invasive tip complex from Afa/Dr fibrils. Mol. Microbiol. 62:356–366. 10.1111/j.1365-2958.2006.05375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garcia MI, Jouve M, Nataro JP, Gounon P, Le Bouguenec C. 2000. Characterization of the AfaD-like family of invasins encoded by pathogenic Escherichia coli associated with intestinal and extra-intestinal infections. FEBS Lett. 479:111–117. 10.1016/S0014-5793(00)01898-6 [DOI] [PubMed] [Google Scholar]
- 169.Gounon P, Jouve M, Le Bouguenec C. 2000. Immunocytochemistry of the AfaE adhesin and AfaD invasin produced by pathogenic Escherichia coli strains during interaction of the bacteria with HeLa cells by high-resolution scanning electron microscopy. Microbes Infect. 2:359–365. 10.1016/S1286-4579(00)00331-2 [DOI] [PubMed] [Google Scholar]
- 170.Van Loy CP, Sokurenko EV, Moseley SL. 2002. The major structural subunits of Dr and F1845 fimbriae are adhesins. Infect. Immun. 70:1694–1702. 10.1128/IAI.70.4.1694-1702.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pichon C, du Merle L, Caliot ME, Trieu-Cuot P, Le Bouguenec C. 2012. An in silico model for identification of small RNAs in whole bacterial genomes: characterization of antisense RNAs in pathogenic Escherichia coli and Streptococcus agalactiae strains. Nucleic Acids Res. 40:2846–2861. 10.1093/nar/gkr1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pichon C, du Merle L, Lequeutre I, Le Bouguenec C. 2013. The AfaR small RNA controls expression of the AfaD-VIII invasin in pathogenic Escherichia coli strains. Nucleic Acids Res. 41:5469–5482. 10.1093/nar/gkt208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gerardin J, Lalioui L, Jacquemin E, Le Bouguenec C, Mainil JG. 2000. The afa-related gene cluster in necrotoxigenic and other Escherichia coli from animals belongs to the afa-8 variant. Vet. Microbiol. 76:175–184. 10.1016/S0378-1135(00)00234-0 [DOI] [PubMed] [Google Scholar]
- 174.Piatek R, Bruzdziak P, Wojciechowski M, Zalewska-Piatek B, Kur J. 2010. The noncanonical disulfide bond as the important stabilizing element of the immunoglobulin fold of the Dr fimbrial DraE subunit. Biochemistry 49:1460–1468. 10.1021/bi901896b [DOI] [PubMed] [Google Scholar]
- 175.Piatek R, Bruzdziak P, Zalewska-Piatek B, Kur J, Stangret J. 2009. Preclusion of irreversible destruction of Dr adhesin structures by a high activation barrier for the unfolding stage of the fimbrial DraE subunit. Biochemistry 48:11807–11816. 10.1021/bi900920k [DOI] [PubMed] [Google Scholar]
- 176.Piatek R, Zalewska B, Bury K, Kur J. 2005. The chaperone-usher pathway of bacterial adhesin biogenesis—from molecular mechanism to strategies of anti-bacterial prevention and modern vaccine design. Acta Biochim. Pol. 52:639–646 [PubMed] [Google Scholar]
- 177.Piatek R, Zalewska B, Kolaj O, Ferens M, Nowicki B, Kur J. 2005. Molecular aspects of biogenesis of Escherichia coli Dr fimbriae: characterization of DraB-DraE complexes. Infect. Immun. 73:135–145. 10.1128/IAI.73.1.135-145.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zalewska-Piatek B, Kur M, Wilkanowicz S, Piatek R, Kur J. 2010. The DraC usher in Dr fimbriae biogenesis of uropathogenic E. coli Dr(+) strains. Arch. Microbiol. 192:351–363. 10.1007/s00203-010-0564-x [DOI] [PubMed] [Google Scholar]
- 179.Zalewska B, Piatek R, Cieslinski H, Nowicki B, Kur J. 2001. Cloning, expression, and purification of the uropathogenic Escherichia coli invasin DraD. Protein Expr. Purif. 23:476–482. 10.1006/prep.2001.1536 [DOI] [PubMed] [Google Scholar]
- 180.Piatek RJ, Bruzdziak P, Zalewska-Piatek BM, Wojciechowski MA, Namiesnik JM, Kur JW. 2011. Analysis of the unique structural and physicochemical properties of the DraD/AfaD invasin in the context of its belonging to the family of chaperone/usher type fimbrial subunits. BMC Struct. Biol. 11:25. 10.1186/1472-6807-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jedrzejczak R, Dauter Z, Dauter M, Piatek R, Zalewska B, Mroz M, Bury K, Nowicki B, Kur J. 2006. Structure of DraD invasin from uropathogenic Escherichia coli: a dimer with swapped beta-tails. Acta Crystallogr. D Biol. Cryst. 62:157–164. 10.1107/S0907444905036747 [DOI] [PubMed] [Google Scholar]
- 182.Zalewska B, Piatek R, Bury K, Samet A, Nowicki B, Nowicki S, Kur J. 2005. A surface-exposed DraD protein of uropathogenic Escherichia coli bearing Dr fimbriae may be expressed and secreted independently from DraC usher and DraE adhesin. Microbiology 151:2477–2486. 10.1099/mic.0.28083-0 [DOI] [PubMed] [Google Scholar]
- 183.Zalewska-Piatek B, Bury K, Piatek R, Bruzdziak P, Kur J. 2008. Type II secretory pathway for surface secretion of DraD invasin from the uropathogenic Escherichia coli Dr+ strain. J. Bacteriol. 190:5044–5056. 10.1128/JB.00224-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weissman SJ, Moseley SL, Dykhuizen DE, Sokurenko EV. 2003. Enterobacterial adhesins and the case for studying SNPs in bacteria. Trends Microbiol. 11:115–117. 10.1016/S0966-842X(03)00010-6 [DOI] [PubMed] [Google Scholar]
- 185.Carnoy C, Moseley SL. 1997. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol. Microbiol. 23:365–379. 10.1046/j.1365-2958.1997.2231590.x [DOI] [PubMed] [Google Scholar]
- 186.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen TK. 1989. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol. Microbiol. 3:329–337. 10.1111/j.1365-2958.1989.tb00178.x [DOI] [PubMed] [Google Scholar]
- 187.Korotkova N, Chattopadhyay S, Tabata TA, Beskhlebnaya V, Vigdorovich V, Kaiser BK, Strong RK, Dykhuizen DE, Sokurenko EV, Moseley SL. 2007. Selection for functional diversity drives accumulation of point mutations in Dr adhesins of Escherichia coli. Mol. Microbiol. 64:180–194. 10.1111/j.1365-2958.2007.05648.x [DOI] [PubMed] [Google Scholar]
- 188.Korotkova N, Yarova-Yarovaya Y, Tchesnokova V, Yazvenko N, Carl MA, Stapleton AE, Moseley SL. 2008. Escherichia coli DraE adhesin-associated bacterial internalization by epithelial cells is promoted independently by decay-accelerating factor and carcinoembryonic antigen-related cell adhesion molecule binding and does not require the DraD invasin. Infect. Immun. 76:3869–3880. 10.1128/IAI.00427-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guignot J, Hudault S, Kansau I, Chau I, Servin AL. 2009. Human decay-accelerating factor and CEACAM receptor-mediated internalization and intracellular lifestyle of Afa/Dr diffusely adhering Escherichia coli in epithelial cells. Infect. Immun. 77:517–531. 10.1128/IAI.00695-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zalewska-Piatek B, Wilkanowicz S, Bruzdziak P, Piatek R, Kur J. 2013. Biochemical characteristic of biofilm of uropathogenic Escherichia coli Dr(+) strains. Microbiol. Res. 168:367–378. 10.1016/j.micres.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 191.Ahrens R, Ott M, Ritter A, Hoschutzky H, Buhler T, Lottspeich F, Boulnois GJ, Jann K, Hacker J. 1993. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coli uropathogen. Infect. Immun. 61:2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Loomis WP, Koo JT, Cheung TP, Moseley SL. 2001. A tripeptide sequence within the nascent DaaP protein is required for mRNA processing of a fimbrial operon in Escherichia coli. Mol. Microbiol. 39:693–707. 10.1046/j.1365-2958.2001.02241.x [DOI] [PubMed] [Google Scholar]
- 193.White-Ziegler CA, Villapakkam A, Ronaszeki K, Young S. 2000. H-NS controls pap and daa fimbrial transcription in Escherichia coli in response to multiple environmental cues. J. Bacteriol. 182:6391–6400. 10.1128/JB.182.22.6391-6400.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van der Woude MW, Low DA. 1994. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol. Microbiol. 11:605–618. 10.1111/j.1365-2958.1994.tb00340.x [DOI] [PubMed] [Google Scholar]
- 195.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6:455–465. 10.1038/nrmicro1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Minamino T, Imada K, Namba K. 2008. Molecular motors of the bacterial flagella. Curr. Opin. Struct. Biol. 18:693–701. 10.1016/j.sbi.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 197.Josenhans C, Suerbaum S. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605–614. 10.1078/1438-4221-00173 [DOI] [PubMed] [Google Scholar]
- 198.Patrick JE, Kearns DB. 2012. Swarming motility and the control of master regulators of flagellar biosynthesis. Mol. Microbiol. 83:14–23. 10.1111/j.1365-2958.2011.07917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 36:616–648. 10.1111/j.1574-6976.2012.00339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lindberg S, Xia Y, Sonden B, Goransson M, Hacker J, Uhlin BE. 2008. Regulatory interactions among adhesin gene systems of uropathogenic Escherichia coli. Infect. Immun. 76:771–780. 10.1128/IAI.01010-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simms AN, Mobley HL. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J. Bacteriol. 190:3747–3756. 10.1128/JB.01870-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pichon C, Hechard C, du Merle L, Chaudray C, Bonne I, Guadagnini S, Vandewalle A, Le Bouguenec C. 2009. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell. Microbiol. 11:616–628. 10.1111/j.1462-5822.2008.01278.x [DOI] [PubMed] [Google Scholar]
- 203.Arikawa K, Meraz IM, Nishikawa Y, Ogasawara J, Hase A. 2005. Interleukin-8 secretion by epithelial cells infected with diffusely adherent Escherichia coli possessing Afa adhesin-coding genes. Microbiol. Immunol. 49:493–503. 10.1111/j.1348-0421.2005.tb03754.x [DOI] [PubMed] [Google Scholar]
- 204.Ruiz-Perez F, Nataro JP. 2014. Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence. Cell. Mol. Life Sci. 71:745–770. 10.1007/s00018-013-1355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guyer DM, Henderson IR, Nataro JP, Mobley HL. 2000. Identification of sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53–66. 10.1046/j.1365-2958.2000.02110.x [DOI] [PubMed] [Google Scholar]
- 207.Guyer DM, Kao JS, Mobley HL. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 66:4411–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Parham NJ, Pollard SJ, Chaudhuri RR, Beatson SA, Desvaux M, Russell MA, Ruiz J, Fivian A, Vila J, Henderson IR. 2005. Prevalence of pathogenicity island II CFT073 genes among extraintestinal clinical isolates of Escherichia coli. J. Clin. Microbiol. 43:2425–2434. 10.1128/JCM.43.5.2425-2434.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Welch RA, Burland V, Plunkett G, III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024. 10.1073/pnas.252529799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olesen B, Hansen DS, Nilsson F, Frimodt-Moller J, Leihof RF, Struve C, Scheutz F, Johnston B, Krogfelt KA, Johnson JR. 2013. Prevalence and characteristics of the epidemic multiresistant Escherichia coli ST131 clonal group among extended-spectrum beta-lactamase-producing E. coli isolates in Copenhagen, Denmark. J. Clin. Microbiol. 51:1779–1785. 10.1128/JCM.00346-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ruiz J, Simon K, Horcajada JP, Velasco M, Barranco M, Roig G, Moreno-Martinez A, Martinez JA, Jimenez de Anta T, Mensa J, Vila J. 2002. Differences in virulence factors among clinical isolates of Escherichia coli causing cystitis and pyelonephritis in women and prostatitis in men. J. Clin. Microbiol. 40:4445–4449. 10.1128/JCM.40.12.4445-4449.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vila J, Simon K, Ruiz J, Horcajada JP, Velasco M, Barranco M, Moreno A, Mensa J. 2002. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J. Infect. Dis. 186:1039–1042. 10.1086/342955 [DOI] [PubMed] [Google Scholar]
- 213.Guyer DM, Gunther NWt Mobley HL. 2001. Secreted proteins and other features specific to uropathogenic Escherichia coli. J. Infect. Dis. 183(Suppl 1):S32–S35. 10.1086/318854 [DOI] [PubMed] [Google Scholar]
- 214.Parham NJ, Pollard SJ, Desvaux M, Scott-Tucker A, Liu C, Fivian A, Henderson IR. 2005. Distribution of the serine protease autotransporters of the Enterobacteriaceae among extraintestinal clinical isolates of Escherichia coli. J. Clin. Microbiol. 43:4076–4082. 10.1128/JCM.43.8.4076-4082.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abreu AG, Bueris V, Porangaba TM, Sircili MP, Navarro-Garcia F, Elias WP. 2013. Autotransporter protein-encoding genes of diarrheagenic Escherichia coli are found in both typical and atypical enteropathogenic E. coli strains. Appl. Environ. Microbiol. 79:411–414. 10.1128/AEM.02635-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. 2009. High prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am. J. Trop. Med. Hyg. 80:294–301 [PMC free article] [PubMed] [Google Scholar]
- 217.Kotlowski R, Bernstein CN, Sepehri S, Krause DO. 2007. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 56:669–675. 10.1136/gut.2006.099796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Taddei CR, Moreno AC, Fernandes Filho A, Montemor LP, Martinez MB. 2003. Prevalence of secreted autotransporter toxin gene among diffusely adhering Escherichia coli isolated from stools of children. FEMS Microbiol. Lett. 227:249–253. 10.1016/S0378-1097(03)00688-8 [DOI] [PubMed] [Google Scholar]
- 219.Roy S, Thanasekaran K, Dutta Roy AR, Sehgal SC. 2006. Distribution of Shigella enterotoxin genes and secreted autotransporter toxin gene among diverse species and serotypes of shigella isolated from Andaman Islands, India. Trop. Med. Int. Health 11:1694–1698. 10.1111/j.1365-3156.2006.01723.x [DOI] [PubMed] [Google Scholar]
- 220.Ruiz J, Navia MM, Vila J, Gascon J. 2002. Prevalence of the Sat gene among clinical isolates of Shigella spp. causing travelers' diarrhea: geographical and specific differences. J. Clin. Microbiol. 40:1565–1566. 10.1128/JCM.40.4.1565-1566.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Taddei CR, Fasano A, Ferreira AJ, Trabulsi LR, Martinez MB. 2005. Secreted autotransporter toxin produced by a diffusely adhering Escherichia coli strain causes intestinal damage in animal model assays. FEMS Microbiol. Lett. 250:263–269. 10.1016/j.femsle.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 222.Mansan-Almeida R, Pereira AL, Giugliano LG. 2013. Diffusely adherent Escherichia coli strains isolated from children and adults constitute two different populations. BMC Microbiol. 13:22. 10.1186/1471-2180-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Goluszko P, Nowicki S, Kaul AK, Pham T, Nowicki BJ. 1995. Dr fimbriae coding region associated hemolytic activity of Escherichia coli. FEMS Microbiol. Lett. 130:13–17. 10.1016/0378-1097(95)00177-7 [DOI] [PubMed] [Google Scholar]
- 224.Rasko DA, Phillips JA, Li X, Mobley HL. 2001. Identification of DNA sequences from a second pathogenicity island of uropathogenic Escherichia coli CFT073: probes specific for uropathogenic populations. J. Infect. Dis. 184:1041–1049. 10.1086/323602 [DOI] [PubMed] [Google Scholar]
- 225.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Dobrindt U. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 186:5432–5441. 10.1128/JB.186.16.5432-5441.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sabate M, Moreno E, Perez T, Andreu A, Prats G. 2006. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin. Microbiol. Infect. 12:880–886. 10.1111/j.1469-0691.2006.01461.x [DOI] [PubMed] [Google Scholar]
- 227.Hancock V, Vejborg RM, Klemm P. 2010. Functional genomics of probiotic Escherichia coli Nissle 1917 and 83972, and UPEC strain CFT073: comparison of transcriptomes, growth and biofilm formation. Mol. Genet. Genomics 284:437–454. 10.1007/s00438-010-0578-8 [DOI] [PubMed] [Google Scholar]
- 228.Krause DO, Little AC, Dowd SE, Bernstein CN. 2011. Complete genome sequence of adherent invasive Escherichia coli UM146 isolated from ileal Crohn's disease biopsy tissue. J. Bacteriol. 193:583. 10.1128/JB.01290-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bernier-Febreau C, du Merle L, Turlin E, Labas V, Ordonez J, Gilles AM, Le Bouguenec C. 2004. Use of deoxyribose by intestinal and extraintestinal pathogenic Escherichia coli strains: a metabolic adaptation involved in competitiveness. Infect. Immun. 72:6151–6156. 10.1128/IAI.72.10.6151-6156.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang Z, Aboulwafa M, Smith MH, Saier MH., Jr 2003. The ascorbate transporter of Escherichia coli. J. Bacteriol. 185:2243–2250. 10.1128/JB.185.7.2243-2250.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Martinez-Jehanne V, Pichon C, du Merle L, Poupel O, Cayet N, Bouchier C, Le Bouguenec C. 2012. Role of the vpe carbohydrate permease in Escherichia coli urovirulence and fitness in vivo. Infect. Immun. 80:2655–2666. 10.1128/IAI.00457-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bower JM, Eto DS, Mulvey MA. 2005. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic 6:18–31. 10.1111/j.1600-0854.2004.00251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zalewska-Piatek BM, Wilkanowicz SI, Piatek RJ, Kur JW. 2009. Biofilm formation as a virulence determinant of uropathogenic Escherichia coli Dr+ strains. Pol. J. Microbiol. 58:223–229 [PubMed] [Google Scholar]
- 234.Rana T, Hasan RJ, Nowicki S, Venkatarajan MS, Singh R, Urvil PT, Popov V, Braun WA, Popik W, Goodwin JS, Nowicki BJ. 2014. Complement protective epitopes and CD55-microtubule complexes facilitate the invasion and intracellular persistence of uropathogenic Escherichia coli. J. Infect. Dis. 209:1066–1076. 10.1093/infdis/jit619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Prashar A, Bhatia S, Gigliozzi D, Martin T, Duncan C, Guyard C, Terebiznik MR. 2013. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol. 203:1081–1097. 10.1083/jcb.201304095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Horvath DJ, Jr, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, Justice SS. 2011. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 13:426–437. 10.1016/j.micinf.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Oswald E, Nougayrede JP, Taieb F, Sugai M. 2005. Bacterial toxins that modulate host cell-cycle progression. Curr. Opin. Microbiol. 8:83–91. 10.1016/j.mib.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 238.Nougayrede JP, Taieb F, De Rycke J, Oswald E. 2005. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 13:103–110. 10.1016/j.tim.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 239.Homburg S, Oswald E, Hacker J, Dobrindt U. 2007. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol. Lett. 275:255–262. 10.1111/j.1574-6968.2007.00889.x [DOI] [PubMed] [Google Scholar]
- 240.Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. 2006. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313:848–851. 10.1126/science.1127059 [DOI] [PubMed] [Google Scholar]
- 241.Nougayrede JP, Oswald E. 2011. Microbiota and colorectal cancer: genotoxic bacteria in the intestinal tract. Bull. Acad. Natl. Med. 195:1295–1304 [PubMed] [Google Scholar]
- 242.Johnson JR, Johnston B, Kuskowski MA, Nougayrede JP, Oswald E. 2008. Molecular epidemiology and phylogenetic distribution of the Escherichia coli pks genomic island. J. Clin. Microbiol. 46:3906–3911. 10.1128/JCM.00949-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Putze J, Hennequin C, Nougayrede JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U. 2009. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 77:4696–4703. 10.1128/IAI.00522-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. 2012. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338:120–123. 10.1126/science.1224820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Dechelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. 2014. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 20:859–867. 10.1158/1078-0432.CCR-13-1343 [DOI] [PubMed] [Google Scholar]
- 246.Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R. 2013. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 8:e56964. 10.1371/journal.pone.0056964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dubois D, Delmas J, Cady A, Robin F, Sivignon A, Oswald E, Bonnet R. 2010. Cyclomodulins in urosepsis strains of Escherichia coli. J. Clin. Microbiol. 48:2122–2129. 10.1128/JCM.02365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jacobi CA, Malfertheiner P. 2011. Escherichia coli Nissle 1917 (Mutaflor): new insights into an old probiotic bacterium. Dig. Dis. 29:600–607. 10.1159/000333307 [DOI] [PubMed] [Google Scholar]
- 249.Olier M, Marcq I, Salvador-Cartier C, Secher T, Dobrindt U, Boury M, Bacquie V, Penary M, Gaultier E, Nougayrede JP, Fioramonti J, Oswald E. 2012. Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut Microbes 3:501–509. 10.4161/gmic.21737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rhen M, Vaisanen-Rhen V, Saraste M, Korhonen TK. 1986. Organization of genes expressing the blood-group-M-specific hemagglutinin of Escherichia coli: identification and nucleotide sequence of the M-agglutinin subunit gene. Gene 49:351–360. 10.1016/0378-1119(86)90371-9 [DOI] [PubMed] [Google Scholar]
- 251.Lublin DM. 2005. Cromer and DAF: role in health and disease. Immunohematology 21:39–47 [PubMed] [Google Scholar]
- 252.Kuttner-Kondo L, Hourcade DE, Anderson VE, Muqim N, Mitchell L, Soares DC, Barlow PN, Medof ME. 2007. Structure-based mapping of DAF active site residues that accelerate the decay of C3 convertases. J. Biol. Chem. 282:18552–18562. 10.1074/jbc.M611650200 [DOI] [PubMed] [Google Scholar]
- 253.Coyne KE, Hall SE, Thompson S, Arce MA, Kinoshita T, Fujita T, Anstee DJ, Rosse W, Lublin DM. 1992. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J. Immunol. 149:2906–2913 [PubMed] [Google Scholar]
- 254.Hudault S, Spiller OB, Morgan BP, Servin AL. 2004. Human diffusely adhering Escherichia coli expressing Afa/Dr adhesins that use human CD55 (decay-accelerating factor) as a receptor does not bind the rodent and pig analogues of CD55. Infect. Immun. 72:4859–4863. 10.1128/IAI.72.8.4859-4863.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nowicki B, Truong L, Moulds J, Hull R. 1988. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am. J. Pathol. 133:1–4 [PMC free article] [PubMed] [Google Scholar]
- 256.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. 1990. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect. Immun. 58:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nowicki B, Hart A, Coyne KE, Lublin DM, Nowicki S. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115–2121. 10.1084/jem.178.6.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Queval CJ, Nicolas V, Beau I. 2011. Role of Src kinases in mobilization of glycosylphosphatidylinositol-anchored decay-accelerating factor by Dr fimbria-positive adhering bacteria. Infect. Immun. 79:2519–2534. 10.1128/IAI.01052-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Selvarangan R, Goluszko P, Popov V, Singhal J, Pham T, Lublin DM, Nowicki S, Nowicki B. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391–1399. 10.1128/IAI.68.3.1391-1399.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hasan RJ, Pawelczyk E, Urvil PT, Venkatarajan MS, Goluszko P, Kur J, Selvarangan R, Nowicki S, Braun WA, Nowicki BJ. 2002. Structure-function analysis of decay-accelerating factor: identification of residues important for binding of the Escherichia coli Dr adhesin and complement regulation. Infect. Immun. 70:4485–4493. 10.1128/IAI.70.8.4485-4493.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Van Loy CP, Sokurenko EV, Samudrala R, Moseley SL. 2002. Identification of amino acids in the Dr adhesin required for binding to decay-accelerating factor. Mol. Microbiol. 45:439–452. 10.1046/j.1365-2958.2002.03022.x [DOI] [PubMed] [Google Scholar]
- 262.Swanson TN, Bilge SS, Nowicki B, Moseley SL. 1991. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect. Immun. 59:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shafren DR, Bates RC, Agrez MV, Herd RL, Burns GF, Barry RD. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bergelson JM, Mohanty JG, Crowell RL, St John NF, Lublin DM, Finberg RW. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shafren DR, Dorahy DJ, Ingham RA, Burns GF, Barry RD. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Karnauchow TM, Tolson DL, Harrison BA, Altman E, Lublin DM, Dimock K. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Clarkson NA, Kaufman R, Lublin DM, Ward T, Pipkin PA, Minor PD, Evans DJ, Almond JW. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:5497–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stuart AD, McKee TA, Williams PA, Harley C, Shen S, Stuart DI, Brown TD, Lea SM. 2002. Determination of the structure of a decay accelerating factor-binding clinical isolate of echovirus 11 allows mapping of mutants with altered receptor requirements for infection. J. Virol. 76:7694–7704. 10.1128/JVI.76.15.7694-7704.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Williams P, Chaudhry Y, Goodfellow IG, Billington J, Powell R, Spiller OB, Evans DJ, Lea S. 2003. Mapping CD55 function. The structure of two pathogen-binding domains at 1.7 A. J. Biol. Chem. 278:10691–10696. 10.1074/jbc.M212561200 [DOI] [PubMed] [Google Scholar]
- 270.Spiller OB, Goodfellow IG, Evans DJ, Almond JW, Morgan BP. 2000. Echoviruses and coxsackie B viruses that use human decay-accelerating factor (DAF) as a receptor do not bind the rodent analogues of DAF. J. Infect. Dis. 181:340–343. 10.1086/315210 [DOI] [PubMed] [Google Scholar]
- 271.Krautkramer E, Zeier M. 2008. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 82:4257–4264. 10.1128/JVI.02210-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.O'Brien DP, Israel DA, Krishna U, Romero-Gallo J, Nedrud J, Medof ME, Lin F, Redline R, Lublin DM, Nowicki BJ, Franco AT, Ogden S, Williams AD, Polk DB, Peek RM., Jr 2006. The role of decay-accelerating factor as a receptor for Helicobacter pylori and a mediator of gastric inflammation. J. Biol. Chem. 281:13317–13323. 10.1074/jbc.M601805200 [DOI] [PubMed] [Google Scholar]
- 273.O'Brien DP, Romero-Gallo J, Schneider BG, Chaturvedi R, Delgado A, Harris EJ, Krishna U, Ogden SR, Israel DA, Wilson KT, Peek RM., Jr 2008. Regulation of the Helicobacter pylori cellular receptor decay-accelerating factor. J. Biol. Chem. 283:23922–23930. 10.1074/jbc.M801144200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52:963–983. 10.1111/j.1365-2958.2004.04033.x [DOI] [PubMed] [Google Scholar]
- 275.Beauchemin N, Arabzadeh A. 2013. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 32:643–671. 10.1007/s10555-013-9444-6 [DOI] [PubMed] [Google Scholar]
- 276.Hammarstrom S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9:67–81. 10.1006/scbi.1998.0119 [DOI] [PubMed] [Google Scholar]
- 277.Sundberg U, Obrink B. 2002. CEACAM1 isoforms with different cytoplasmic domains show different localization, organization and adhesive properties in polarized epithelial cells. J. Cell Sci. 115:1273–1284 [DOI] [PubMed] [Google Scholar]
- 278.Sundberg U, Beauchemin N, Obrink B. 2004. The cytoplasmic domain of CEACAM1-L controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J. Cell Sci. 117:1091–1104. 10.1242/jcs.00944 [DOI] [PubMed] [Google Scholar]
- 279.Chan CH, Camacho-Leal P, Stanners CP. 2007. Colorectal hyperplasia and dysplasia due to human carcinoembryonic antigen (CEA) family member expression in transgenic mice. PLoS One 2:e1353. 10.1371/journal.pone.0001353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. 2007. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 7:2. 10.1186/1471-2407-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hammarstrom S, Baranov V. 2001. Is there a role for CEA in innate immunity in the colon? Trends Microbiol. 9:119–125. 10.1016/S0966-842X(01)01952-7 [DOI] [PubMed] [Google Scholar]
- 282.Duxbury MS, Ito H, Ashley SW, Whang EE. 2004. CEACAM6 cross-linking induces caveolin-1-dependent, Src-mediated focal adhesion kinase phosphorylation in BxPC3 pancreatic adenocarcinoma cells. J. Biol. Chem. 279:23176–23182. 10.1074/jbc.M402051200 [DOI] [PubMed] [Google Scholar]
- 283.Duxbury MS, Ito H, Ashley SW, Whang EE. 2004. c-Src-dependent cross-talk between CEACAM6 and alphavbeta3 integrin enhances pancreatic adenocarcinoma cell adhesion to extracellular matrix components. Biochem. Biophys. Res. Commun. 317:133–141. 10.1016/j.bbrc.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 284.Korotkova N, Yang Y, Le Trong I, Cota E, Demeler B, Marchant J, Thomas WE, Stenkamp RE, Moseley SL, Matthews S. 2008. Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol. Microbiol. 67:420–434. 10.1111/j.1365-2958.2007.06054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C. 1991. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect. Immun. 59:2051–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Leusch HG, Hefta SA, Drzeniek Z, Hummel K, Markos-Pusztai Z, Wagener C. 1990. Escherichia coli of human origin binds to carcinoembryonic antigen (CEA) and non-specific crossreacting antigen (NCA). FEBS Lett. 261:405–409. 10.1016/0014-5793(90)80603-G [DOI] [PubMed] [Google Scholar]
- 287.Sauter SL, Rutherfurd SM, Wagener C, Shively JE, Hefta SA. 1991. Binding of nonspecific cross-reacting antigen, a granulocyte membrane glycoprotein, to Escherichia coli expressing type 1 fimbriae. Infect. Immun. 59:2485–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. 2007. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 117:1566–1574. 10.1172/JCI30504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Virji M. 2009. Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7:274–286. 10.1038/nrmicro2097 [DOI] [PubMed] [Google Scholar]
- 290.Voges M, Bachmann V, Kammerer R, Gophna U, Hauck CR. 2010. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol. 10:117. 10.1186/1471-2180-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Virji M, Evans D, Griffith J, Hill D, Serino L, Hadfield A, Watt SM. 2000. Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol. Microbiol. 36:784–795. 10.1046/j.1365-2958.2000.01885.x [DOI] [PubMed] [Google Scholar]
- 292.Hill DJ, Virji M. 2003. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol. Microbiol. 48:117–129. 10.1046/j.1365-2958.2003.03433.x [DOI] [PubMed] [Google Scholar]
- 293.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22:929–939. 10.1046/j.1365-2958.1996.01548.x [DOI] [PubMed] [Google Scholar]
- 294.Dveksler GS, Dieffenbach CW, Cardellichio CB, McCuaig K, Pensiero MN, Jiang GS, Beauchemin N, Holmes KV. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 67:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Nedellec P, Dveksler GS, Daniels E, Turbide C, Chow B, Basile AA, Holmes KV, Beauchemin N. 1994. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J. Virol. 68:4525–4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liévin-Le Moal V, Beau I, Rougeaux C, Kansau I, Fabrega S, Brice C, Korotkova N, Moseley SL, Servin AL. 2011. Apical expression of human full-length hCEACAM1-4L protein renders the Madin Darby canine kidney cells responsive to lipopolysaccharide leading to TLR4-dependent Erk1/2 and p38 MAPK signalling. Cell. Microbiol. 13:764–785. 10.1111/j.1462-5822.2011.01575.x [DOI] [PubMed] [Google Scholar]
- 297.Lu R, Pan H, Shively JE. 2012. CEACAM1 negatively regulates IL-1beta production in LPS activated neutrophils by recruiting SHP-1 to a SYK-TLR4-CEACAM1 complex. PLoS Pathog. 8:e1002597. 10.1371/journal.ppat.1002597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Yurchenco PD. 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3:a004911. 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Turner JR. 2009. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9:799–809. 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- 300.Westerlund B, Korhonen TK. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687–694. 10.1111/j.1365-2958.1993.tb01729.x [DOI] [PubMed] [Google Scholar]
- 301.Selvarangan R, Goluszko P, Singhal J, Carnoy C, Moseley S, Hudson B, Nowicki S, Nowicki B. 2004. Interaction of Dr adhesin with collagen type IV is a critical step in Escherichia coli renal persistence. Infect. Immun. 72:4827–4835. 10.1128/IAI.72.8.4827-4835.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schmick M, Bastiaens PIH. 2014. The interdependence of membrane shape and cellular signal processing. Cell 156:1132–1138. 10.1016/j.cell.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 303.Shenoy-Scaria AM, Gauen LK, Kwong J, Shaw AS, Lublin DM. 1993. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol. Cell. Biol. 13:6385–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. 1992. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 149:3535–3541 [PubMed] [Google Scholar]
- 305.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. 1997. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene 14:783–790. 10.1038/sj.onc.1200888 [DOI] [PubMed] [Google Scholar]
- 306.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. 1999. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem. 274:335–344. 10.1074/jbc.274.1.335 [DOI] [PubMed] [Google Scholar]
- 307.Obrink B, Sawa H, Scheffrahn I, Singer BB, Sigmundsson K, Sundberg U, Heymann R, Beauchemin N, Weng G, Ram P, Iyengar R. 2002. Computational analysis of isoform-specific signal regulation by CEACAM1—a cell adhesion molecule expressed in PC12 cells. Ann. N. Y. Acad. Sci. 971:597–607. 10.1111/j.1749-6632.2002.tb04536.x [DOI] [PubMed] [Google Scholar]
- 308.Kuespert K, Pils S, Hauck CR. 2006. CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18:565–571. 10.1016/j.ceb.2006.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lea S. 2002. Interactions of CD55 with non-complement ligands. Biochem. Soc. Trans. 30:1014–1019 [DOI] [PubMed] [Google Scholar]
- 310.Lingwood D, Kaiser HJ, Levental I, Simons K. 2009. Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 37:955–960. 10.1042/BST0370955 [DOI] [PubMed] [Google Scholar]
- 311.Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327:46–50. 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- 312.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. 2006. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 103:329–334. 10.1073/pnas.0509885103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Langhorst MF, Reuter A, Stuermer CA. 2005. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell. Mol. Life Sci. 62:2228–2240. 10.1007/s00018-005-5166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Langhorst MF, Solis GP, Hannbeck S, Plattner H, Stuermer CA. 2007. Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 581:4697–4703. 10.1016/j.febslet.2007.08.074 [DOI] [PubMed] [Google Scholar]
- 315.Morrow IC, Parton RG. 2005. Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic 6:725–740. 10.1111/j.1600-0854.2005.00318.x [DOI] [PubMed] [Google Scholar]
- 316.Chichili GR, Rodgers W. 2009. Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 66:2319–2328. 10.1007/s00018-009-0022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kansau I, Berger C, Hospital M, Amsellem R, Nicolas V, Servin AL, Bernet-Camard MF. 2004. Zipper-like internalization of Dr-positive Escherichia coli by epithelial cells is preceded by an adhesin-induced mobilization of raft-associated molecules in the initial step of adhesion. Infect. Immun. 72:3733–3742. 10.1128/IAI.72.7.3733-3742.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rougeaux C, Berger CN, Servin AL. 2008. hCEACAM1-4L downregulates hDAF-associated signalling after being recognized by the Dr adhesin of diffusely adhering Escherichia coli. Cell. Microbiol. 10:632–654. 10.1111/j.1462-5822.2007.01072.x [DOI] [PubMed] [Google Scholar]
- 319.Das M, Hart-Van Tassell A, Urvil PT, Lea S, Pettigrew D, Anderson KL, Samet A, Kur J, Matthews S, Nowicki S, Popov V, Goluszko P, Nowicki BJ. 2005. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect. Immun. 73:6119–6126. 10.1128/IAI.73.9.6119-6126.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, Danielsen EM. 2003. Microvillar membrane microdomains exist at physiological temperature. Role of galectin-4 as lipid raft stabilizer revealed by “superrafts.” J. Biol. Chem. 278:15679–15684. 10.1074/jbc.M211228200 [DOI] [PubMed] [Google Scholar]
- 321.Danielsen EM, Hansen GH. 2006. Lipid raft organization and function in brush borders of epithelial cells. Mol. Membr. Biol. 23:71–79. 10.1080/09687860500445604 [DOI] [PubMed] [Google Scholar]
- 322.Patel KP, Coyne CB, Bergelson JM. 2009. Dynamin- and lipid raft-dependent entry of decay-accelerating factor (DAF)-binding and non-DAF-binding coxsackieviruses into nonpolarized cells. J. Virol. 83:11064–11077. 10.1128/JVI.01016-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Stuart AD, Eustace HE, McKee TA, Brown TD. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307–9322. 10.1128/JVI.76.18.9307-9322.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Muenzner P, Bachmann V, Kuespert K, Hauck CR. 2008. The CEACAM1 transmembrane domain, but not the cytoplasmic domain, directs internalization of human pathogens via membrane microdomains. Cell. Microbiol. 10:1074–1092. 10.1111/j.1462-5822.2007.01106.x [DOI] [PubMed] [Google Scholar]
- 325.Schmitter T, Pils S, Weibel S, Agerer F, Peterson L, Buntru A, Kopp K, Hauck CR. 2007. Opa proteins of pathogenic neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect. Immun. 75:4116–4126. 10.1128/IAI.01835-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Knutton S, Baldwin T, Williams PH, McNeish AS. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Finlay BB, Ruschkowski S, Dedhar S. 1991. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 99:283–296 [DOI] [PubMed] [Google Scholar]
- 328.Billker O, Popp A, GrayOwen SD, Meyer TF. 2000. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 8:258–260 (Discussion, 8:260–251.) [DOI] [PubMed] [Google Scholar]
- 329.Billker O, Popp A, Brinkmann V, Wenig G, Schneider J, Caron E, Meyer TF. 2002. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 21:560–571. 10.1093/emboj/21.4.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.McCaw SE, Liao EH, Gray-Owen SD. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72:2742–2752. 10.1128/IAI.72.5.2742-2752.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Guignot J, Bernet-Camard MF, Pous C, Plancon L, Le Bouguenec C, Servin AL. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for alpha5beta1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856–1868. 10.1128/IAI.69.3.1856-1868.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Plancon L, Du Merle L, Le Friec S, Gounon P, Jouve M, Guignot J, Servin A, Le Bouguenec C. 2003. Recognition of the cellular beta1-chain integrin by the bacterial AfaD invasin is implicated in the internalization of afa-expressing pathogenic Escherichia coli strains. Cell. Microbiol. 5:681–693. 10.1046/j.1462-5822.2003.00308.x [DOI] [PubMed] [Google Scholar]
- 333.Norambuena A, Schwartz MA. 2011. Effects of integrin-mediated cell adhesion on plasma membrane lipid raft components and signaling. Mol. Biol. Cell 22:3456–3464. 10.1091/mbc.E11-04-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.van Zanten TS, Cambi A, Koopman M, Joosten B, Figdor CG, Garcia-Parajo MF. 2009. Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc. Natl. Acad. Sci. U. S. A. 106:18557–18562. 10.1073/pnas.0905217106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yoon J, Terada A, Kita H. 2007. CD66b regulates adhesion and activation of human eosinophils. J. Immunol. 179:8454–8462. 10.4049/jimmunol.179.12.8454 [DOI] [PubMed] [Google Scholar]
- 336.Fang Z, Takizawa N, Wilson KA, Smith TC, Delprato A, Davidson MW, Lambright DG, Luna EJ. 2010. The membrane-associated protein, supervillin, accelerates F-actin-dependent rapid integrin recycling and cell motility. Traffic 11:782–799. 10.1111/j.1600-0854.2010.01062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Runz S, Mierke CT, Joumaa S, Behrens J, Fabry B, Altevogt P. 2008. CD24 induces localization of beta1 integrin to lipid raft domains. Biochem. Biophys. Res. Commun. 365:35–41. 10.1016/j.bbrc.2007.10.139 [DOI] [PubMed] [Google Scholar]
- 338.Mierke CT, Bretz N, Altevogt P. 2011. Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion. J. Biol. Chem. 286:34858–34871. 10.1074/jbc.M111.245183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lipscomb EA, Mercurio AM. 2005. Mobilization and activation of a signaling competent alpha6beta4integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 24:413–423. 10.1007/s10555-005-5133-4 [DOI] [PubMed] [Google Scholar]
- 340.Peiffer I, Servin AL, Bernet-Camard MF. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Betis F, Brest P, Hofman V, Guignot J, Bernet-Camard MF, Rossi B, Servin A, Hofman P. 2003. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect. Immun. 71:1068–1074. 10.1128/IAI.71.3.1068-1074.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Betis F, Brest P, Hofman V, Guignot J, Kansau I, Rossi B, Servin A, Hofman P. 2003. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect. Immun. 71:1774–1783. 10.1128/IAI.71.4.1774-1783.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Diard S, Liévin-Le Moal V, Toribio AL, Boum Y, Vigier F, Servin AL, Bouvet O. 2009. Norepinephrine-dependently released Dr fimbriae of diffusely adhering Escherichia coli strain IH11128 promotes a mitogen-activated protein kinase ERK1/2-dependent production of pro-inflammatory cytokine, IL-8 in human intestinal Caco-2/TC7 cells. Microbes Infect. 11:886–894. 10.1016/j.micinf.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 344.Cane G, Ginouves A, Marchetti S, Busca R, Pouyssegur J, Berra E, Hofman P, Vouret-Craviari V. 2010. HIF-1alpha mediates the induction of IL-8 and VEGF expression on infection with Afa/Dr diffusely adhering E. coli and promotes EMT-like behaviour. Cell. Microbiol. 12:640–653. 10.1111/j.1462-5822.2009.01422.x [DOI] [PubMed] [Google Scholar]
- 345.Cane G, Liévin-Le Moal V, Pages G, Servin AL, Hofman P, Vouret-Craviari V. 2007. Up-regulation of intestinal vascular endothelial growth factor by Afa/Dr diffusely adhering Escherichia coli. PLoS One 2:e1359. 10.1371/journal.pone.0001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Semiramoth N, Gleizes A, Turbica I, Sandre C, Marin-Esteban V, Gorges R, Servin A, Chollet-Martin S. 2010. Afa/Dr-expressing, diffusely adhering Escherichia coli strain C1845 triggers F1845 fimbria-dependent phosphatidylserine externalization on neutrophil-like differentiated PLB-985 cells through an apoptosis-independent mechanism. Infect. Immun. 78:2974–2983. 10.1128/IAI.01354-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Semiramoth N, Gleizes A, Turbica I, Sandre C, Gorges R, Kansau I, Servin A, Chollet-Martin S. 2009. Escherichia coli type 1 pili trigger late IL-8 production by neutrophil-like differentiated PLB-985 cells through a Src family kinase- and MAPK-dependent mechanism. J. Leukoc. Biol. 85:310–321. 10.1189/jlb.0608350 [DOI] [PubMed] [Google Scholar]
- 348.Boulton IC, Gray-Owen SD. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229–236. 10.1038/ni769 [DOI] [PubMed] [Google Scholar]
- 349.Foxman B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7:653–660. 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- 350.Salvador E, Wagenlehner F, Kohler CD, Mellmann A, Hacker J, Svanborg C, Dobrindt U. 2012. Comparison of asymptomatic bacteriuria Escherichia coli isolates from healthy individuals versus those from hospital patients shows that long-term bladder colonization selects for attenuated virulence phenotypes. Infect. Immun. 80:668–678. 10.1128/IAI.06191-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zdziarski J, Svanborg C, Wullt B, Hacker J, Dobrindt U. 2008. Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation? Infect. Immun. 76:695–703. 10.1128/IAI.01215-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Koves B, Salvador E, Gronberg-Hernandez J, Zdziarski J, Wullt B, Svanborg C, Dobrindt U. 2014. Rare emergence of symptoms during long-term asymptomatic E. coli 83972 carriage, without altered virulence factor repertoire. J. Urol. 191:519–528. 10.1016/j.juro.2013.07.060 [DOI] [PubMed] [Google Scholar]
- 353.Wiles TJ, Kulesus RR, Mulvey MA. 2008. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 85:11–19. 10.1016/j.yexmp.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Fang L, Nowicki BJ, Urvil P, Goluszko P, Nowicki S, Young SL, Yallampalli C. 2004. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infect. Immun. 72:2907–2914. 10.1128/IAI.72.5.2907-2914.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yoshida S, Sasakawa C. 2003. Exploiting host microtubule dynamics: a new aspect of bacterial invasion. Trends Microbiol. 11:139–143. 10.1016/S0966-842X(03)00023-4 [DOI] [PubMed] [Google Scholar]
- 356.Ivetic A, Ridley AJ. 2004. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 112:165–176. 10.1111/j.1365-2567.2004.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Le Bouguenec C. 2005. Adhesins and invasins of pathogenic Escherichia coli. Int. J. Med. Microbiol. 295:471–478. 10.1016/j.ijmm.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 358.Watson KG, Holden DW. 2010. Dynamics of growth and dissemination of Salmonella in vivo. Cell. Microbiol. 12:1389–1397. 10.1111/j.1462-5822.2010.01511.x [DOI] [PubMed] [Google Scholar]
- 359.Ray K, Marteyn B, Sansonetti PJ, Tang CM. 2009. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 7:333–340. 10.1038/nrmicro2112 [DOI] [PubMed] [Google Scholar]
- 360.Huotari J, Helenius A. 2011. Endosome maturation. EMBO J. 30:3481–3500. 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Luzio JP, Pryor PR, Bright NA. 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell. Biol. 8:622–632. 10.1038/nrm2217 [DOI] [PubMed] [Google Scholar]
- 362.Eto DS, Sundsbak JL, Mulvey MA. 2006. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 8:704–717. 10.1111/j.1462-5822.2006.00691.x [DOI] [PubMed] [Google Scholar]
- 363.He C, Klionsky DJ. 2009. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43:67–93. 10.1146/annurev-genet-102808-114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lamb CA, Dooley HC, Tooze SA. 2013. Endocytosis and autophagy: shared machinery for degradation. Bioessays 35:34–45. 10.1002/bies.201200130 [DOI] [PubMed] [Google Scholar]
- 365.Deretic V, Saitoh T, Akira S. 2013. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13:722–737. 10.1038/nri3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wang C, Mendonsa GR, Symington JW, Zhang Q, Cadwell K, Virgin HW, Mysorekar IU. 2012. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:11008–11013. 10.1073/pnas.1203952109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Chargui A, Cesaro A, Mimouna S, Fareh M, Brest P, Naquet P, Darfeuille-Michaud A, Hebuterne X, Mograbi B, Vouret-Craviari V, Hofman P. 2012. Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death. PLoS One 7:e51727. 10.1371/journal.pone.0051727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Lapaquette P, Bringer MA, Darfeuille-Michaud A. 2012. Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response. Cell. Microbiol. 14:791–807. 10.1111/j.1462-5822.2012.01768.x [DOI] [PubMed] [Google Scholar]
- 369.Lapaquette P, Glasser AL, Huett A, Xavier RJ, Darfeuille-Michaud A. 2010. Crohn's disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell. Microbiol. 12:99–113. 10.1111/j.1462-5822.2009.01381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. 2010. Bacterial interactions with the host epithelium. Cell Host Microbe 8:20–35. 10.1016/j.chom.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 371.Gutierrez MG. 2013. Functional role(s) of phagosomal Rab GTPases. Small GTPases 4:148–158. 10.4161/sgtp.25604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Abu Kwaik Y, Bumann D. 2013. Microbial quest for food in vivo: ‘nutritional virulence' as an emerging paradigm. Cell. Microbiol. 15:882–890. 10.1111/cmi.12138 [DOI] [PubMed] [Google Scholar]
- 373.Vincent JP, Fletcher AG, Baena-Lopez LA. 2013. Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell. Biol. 14:581–591. 10.1038/nrm3639 [DOI] [PubMed] [Google Scholar]
- 374.Liévin-Le Moal V, Comenge Y, Ruby V, Amsellem R, Nicolas V, Servin AL. 2011. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell. Microbiol. 13:992–1013. 10.1111/j.1462-5822.2011.01595.x [DOI] [PubMed] [Google Scholar]
- 375.Mestre MB, Fader CM, Sola C, Colombo MI. 2010. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 6:110–125. 10.4161/auto.6.1.10698 [DOI] [PubMed] [Google Scholar]
- 376.Cappello RE, Estrada-Gutierrez G, Irles C, Giono-Cerezo S, Bloch RJ, Nataro JP. 2011. Effects of the plasmid-encoded toxin of enteroaggregative Escherichia coli on focal adhesion complexes. FEMS Immunol. Med. Microbiol. 61:301–314. 10.1111/j.1574-695X.2010.00776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro JP. 1999. Cytoskeletal effects induced by pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect. Immun. 67:2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Villaseca JM, Navarro-Garcia F, Mendoza-Hernandez G, Nataro JP, Cravioto A, Eslava C. 2000. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect. Immun. 68:5920–5927. 10.1128/IAI.68.10.5920-5927.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Dhakal BK, Mulvey MA. 2012. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11:58–69. 10.1016/j.chom.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguenec C, Buzoni-Gatel D, Vandewalle A. 2006. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J. Immunol. 177:4773–4784. 10.4049/jimmunol.177.7.4773 [DOI] [PubMed] [Google Scholar]
- 381.Chassin C, Tourneur E, Bens M, Vandewalle A. 2011. A role for collecting duct epithelial cells in renal antibacterial defences. Cell. Microbiol. 13:1107–1113. 10.1111/j.1462-5822.2011.01614.x [DOI] [PubMed] [Google Scholar]
- 382.Ingersoll MA, Albert ML. 2013. From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 6:1041–1053. 10.1038/mi.2013.72 [DOI] [PubMed] [Google Scholar]
- 383.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Grone HJ, Garbi N, Kastenmuller W, Knolle PA, Kurts C, Engel DR. 2014. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156:456–468. 10.1016/j.cell.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Goluszko P, Moseley SL, Truong LD, Kaul A, Williford JR, Selvarangan R, Nowicki S, Nowicki B. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Invest. 99:1662–1672. 10.1172/JCI119329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Goluszko P, Goluszko E, Nowicki B, Nowicki S, Popov V, Wang HQ. 2005. Vaccination with purified Dr fimbriae reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect. Immun. 73:627–631. 10.1128/IAI.73.1.627-631.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Miettinen A, Westerlund B, Tarkkanen AM, Tornroth T, Ljungberg P, Renkonen OV, Korhonen TK. 1993. Binding of bacterial adhesins to rat glomerular mesangium in vivo. Kidney Int. 43:592–600. 10.1038/ki.1993.87 [DOI] [PubMed] [Google Scholar]
- 387.Guyer DM, Radulovic S, Jones FE, Mobley HL. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539–4546. 10.1128/IAI.70.8.4539-4546.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Nowicki S, Izban MG, Pawelczyk E, Agboto VK, Pratap S, Olson G, Nowicki B. 2009. Preterm labor: CD55 in maternal blood leukocytes. Am. J. Reprod. Immunol. 61:360–367. 10.1111/j.1600-0897.2009.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Pacheco LD, Hankins GD, Costantine MM, Anderson GD, Pawelczyk E, Nowicki S, Nowicki BJ. 2011. The role of human decay-accelerating factor in the pathogenesis of preterm labor. Am. J. Perinatol. 28:565–570. 10.1055/s-0031-1274510 [DOI] [PubMed] [Google Scholar]
- 390.Pratap S, Brown LE, Izban MG, Nowicki S, Nowicki BJ. 2013. Accurate preterm labor diagnosis using a CD55-TLR4 combination biomarker model. J. Biomed. Sci. Eng. 6:253–257. 10.4236/jbise.2013.63031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Pawelczyk E, Nowicki BJ, Izban MG, Pratap S, Sashti NA, Sanderson M, Nowicki S. 2010. Spontaneous preterm labor is associated with an increase in the proinflammatory signal transducer TLR4 receptor on maternal blood monocytes. BMC Pregn. Childbirth 10:66. 10.1186/1471-2393-10-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kaul A, Nagamani M, Nowicki B. 1995. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am. J. Reprod. Immunol. 34:236–240. 10.1111/j.1600-0897.1995.tb00947.x [DOI] [PubMed] [Google Scholar]
- 393.Kaul A, Nowicki BJ, Martens MG, Goluszko P, Hart A, Nagamani M, Kumar D, Pham TQ, Nowicki S. 1994. Decay-accelerating factor is expressed in the human endometrium and may serve as the attachment ligand for Dr pili of Escherichia coli. Am. J. Reprod. Immunol. 32:194–199. 10.1111/j.1600-0897.1994.tb01114.x [DOI] [PubMed] [Google Scholar]
- 394.Kaul AK, Kumar D, Nagamani M, Goluszko P, Nowicki S, Nowicki BJ. 1996. Rapid cyclic changes in density and accessibility of endometrial ligands for Escherichia coli Dr fimbriae. Infect. Immun. 64:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Nowicki B, Fang L, Singhal J, Nowicki S, Yallampalli C. 1997. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am. J. Reprod. Immunol. 38:309–312. 10.1111/j.1600-0897.1997.tb00521.x [DOI] [PubMed] [Google Scholar]
- 396.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. 1999. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect. Immun. 67:5958–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wroblewska-Seniuk K, Selvarangan R, Hart A, Pladzyk R, Goluszko P, Jafari A, du Merle L, Nowicki S, Yallampalli C, Le Bouguenec C, Nowicki B. 2005. Dra/AfaE adhesin of uropathogenic Dr/Afa+ Escherichia coli mediates mortality in pregnant rats. Infect. Immun. 73:7597–7601. 10.1128/IAI.73.11.7597-7601.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Banadakoppa M, Goluszko P, Liebenthal D, Yallampalli C. 2012. Nitric oxide induces segregation of decay accelerating factor (DAF or CD55) from the membrane lipid-rafts and its internalization in human endometrial cells. Cell Biol. Int. 36:901–907. 10.1042/CBI20110586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Riemer RK, Buscher C, Bansal RK, Black SM, He Y, Natuzzi ES. 1997. Increased expression of nitric oxide synthase in the myometrium of the pregnant rat uterus. Am. J. Physiol. 272:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- 400.Yallampalli C, Dong YL, Gangula PR, Fang L. 1998. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J. Soc. Gynecol. Invest. 5:58–67. 10.1016/S1071-5576(97)00106-8 [DOI] [PubMed] [Google Scholar]
- 401.Fang L, Nowicki BJ, Dong YL, Yallampalli C. 1999. Localized increase in nitric oxide production and the expression of nitric oxide synthase isoforms in rat uterus with experimental intrauterine infection. Am. J. Obstet. Gynecol. 181:601–609. 10.1016/S0002-9378(99)70499-0 [DOI] [PubMed] [Google Scholar]
- 402.Fang L, Nowicki B, Yallampalli C. 2001. Differential expression of uterine NO in pregnant and nonpregnant rats with intrauterine bacterial infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R1356–R1363 [DOI] [PubMed] [Google Scholar]
- 403.Nowicki B, Singhal J, Fang L, Nowicki S, Yallampalli C. 1999. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/HeJ mice. Infect. Immun. 67:2421–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wroblewska-Seniuk K, Nowicki S, Le Bouguenec C, Nowicki B, Yallampalli C. 2011. Maternal/fetal mortality and fetal growth restriction: role of nitric oxide and virulence factors in intrauterine infection in rats. Am. J. Obstet. Gynecol. 205:83e81–e87. 10.1016/j.ajog.2011.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Banadakoppa M, Goluszko P, Liebenthal D, Nowicki BJ, Nowicki S, Yallampalli C. 2014. PI3K/Akt pathway restricts epithelial adhesion of Dr+ Escherichia coli by down-regulating the expression of decay accelerating factor. Exp. Biol. Med. 239:581–594. 10.1177/1535370214522183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. 2012. Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226:380–393. 10.1002/path.3000 [DOI] [PubMed] [Google Scholar]
- 407.Barker N. 2014. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell. Biol. 15:19–33 [DOI] [PubMed] [Google Scholar]
- 408.Rodriguez-Boulan E, Macara IG. 2014. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell. Biol. 15:225–242. 10.1038/nrm3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Marchiando AM, Graham WV, Turner JR. 2010. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 5:119–144. 10.1146/annurev.pathol.4.110807.092135 [DOI] [PubMed] [Google Scholar]
- 410.Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Peiffer I, Bernet-Camard MF, Rousset M, Servin AL. 2001. Impairments in enzyme activity and biosynthesis of brush border-associated hydrolases in human intestinal Caco-2/TC7 cells infected by members of the Afa/Dr family of diffusely adhering Escherichia coli. Cell. Microbiol. 3:341–357. 10.1046/j.1462-5822.2001.00121.x [DOI] [PubMed] [Google Scholar]
- 412.Peiffer I, Guignot J, Barbat A, Carnoy C, Moseley SL, Nowicki BJ, Servin AL, Bernet-Camard MF. 2000. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68:5979–5990. 10.1128/IAI.68.10.5979-5990.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kerneis S, Bilge SS, Fourel V, Chauviere G, Coconnier MH, Servin AL. 1991. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect. Immun. 59:4013–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Kerneis S, Gabastou JM, Bernet-Camard MF, Coconnier MH, Nowicki BJ, Servin AL. 1994. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol. Lett. 119:27–32. 10.1111/j.1574-6968.1994.tb06862.x [DOI] [PubMed] [Google Scholar]
- 415.Adlerberth I, Hanson LA, Svanborg C, Svennerholm AM, Nordgren S, Wold AE. 1995. Adhesins of Escherichia coli associated with extra-intestinal pathogenicity confer binding to colonic epithelial cells. Microb. Pathog. 18:373–385. 10.1006/mpat.1995.0034 [DOI] [PubMed] [Google Scholar]
- 416.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Peterson MD, Bement WM, Mooseker MS. 1993. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 105:461–472 [DOI] [PubMed] [Google Scholar]
- 418.Peterson MD, Mooseker MS. 1993. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 105:445–460 [DOI] [PubMed] [Google Scholar]
- 419.Fanning AS, Van Itallie CM, Anderson JM. 2012. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 23:577–590. 10.1091/mbc.E11-09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Law RJ, Gur-Arie L, Rosenshine I, Finlay BB. 2013. In vitro and in vivo model systems for studying enteropathogenic Escherichia coli infections. Cold Spring Harb. Perspect. Med. 3:a009977. 10.1101/cshperspect.a009977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Liévin-Le Moal V, Servin AL. 2013. Pathogenesis of human enterovirulent bacteria: lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol. Mol. Biol. Rev. 77:380–439. 10.1128/MMBR.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Navarro-Garcia F, Serapio-Palacios A, Ugalde-Silva P, Tapia-Pastrana G, Chavez-Duenas L. 2013. Actin cytoskeleton manipulation by effector proteins secreted by diarrheagenic Escherichia coli pathotypes. Biomed. Res. Int. 2013:374395. 10.1155/2013/374395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Knutton S, Lloyd DR, McNeish AS. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Cookson ST, Nataro JP. 1996. Characterization of HEp-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb. Pathog. 21:421–434. 10.1006/mpat.1996.0073 [DOI] [PubMed] [Google Scholar]
- 425.Hall A. 2012. Rho family GTPases. Biochem. Soc. Trans. 40:1378–1382. 10.1042/BST20120103 [DOI] [PubMed] [Google Scholar]
- 426.Cherfils J, Zeghouf M. 2013. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93:269–309. 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- 427.Lemichez E, Aktories K. 2013. Hijacking of Rho GTPases during bacterial infection. Exp. Cell Res. 319:2329–2336. 10.1016/j.yexcr.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 428.McConnell RE, Tyska MJ. 2007. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J. Cell Biol. 177:671–681. 10.1083/jcb.200701144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.McConnell RE, Higginbotham JN, Shifrin DA, Jr, Tabb DL, Coffey RJ, Tyska MJ. 2009. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185:1285–1298. 10.1083/jcb.200902147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Gilbert T, Rodriguez-Boulan E. 1991. Induction of vacuolar apical compartments in the Caco-2 intestinal epithelial cell line. J. Cell Sci. 100:451–458 [DOI] [PubMed] [Google Scholar]
- 431.Gilbert T, Le Bivic A, Quaroni A, Rodriguez-Boulan E. 1991. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J. Cell Biol. 113:275–288. 10.1083/jcb.113.2.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Le Bivic A, Quaroni A, Nichols B, Rodriguez-Boulan E. 1990. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J. Cell Biol. 111:1351–1361. 10.1083/jcb.111.4.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Lisanti MP, Le Bivic A, Saltiel AR, Rodriguez-Boulan E. 1990. Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J. Membr. Biol. 113:155–167. 10.1007/BF01872889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. 1989. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J. Cell Biol. 109:2145–2156. 10.1083/jcb.109.5.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wrackmeyer U, Hansen GH, Seya T, Danielsen EM. 2006. Intelectin: a novel lipid raft-associated protein in the enterocyte brush border. Biochemistry 45:9188–9197. 10.1021/bi060570x [DOI] [PubMed] [Google Scholar]
- 436.Danielsen EM, Hansen GH. 2003. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta 1617:1–9. 10.1016/j.bbamem.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 437.Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM. 2000. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J. Biol. Chem. 275:5136–5142. 10.1074/jbc.275.7.5136 [DOI] [PubMed] [Google Scholar]
- 438.Cavet ME, Akhter S, Murtazina R, Sanchez de Medina F, Tse CM, Donowitz M. 2001. Half-lives of plasma membrane Na(+)/H(+) exchangers NHE1-3: plasma membrane NHE2 has a rapid rate of degradation. Am. J. Physiol. Cell Physiol. 281:C2039–C2048 [DOI] [PubMed] [Google Scholar]
- 439.Musch MW, Arvans DL, Wu GD, Chang EB. 2009. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am. J. Physiol. Gastrointest. Liver Physiol. 296:G202–G210. 10.1152/ajpgi.90350.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Saksena S, Tyagi S, Goyal S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. 2010. Stimulation of apical Cl(−)/HCO(3)(−)(OH(−)) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 299:G1334–1343. 10.1152/ajpgi.00039.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Nguyen HT, Charrier-Hisamuddin L, Dalmasso G, Hiol A, Sitaraman S, Merlin D. 2007. Association of PepT1 with lipid rafts differently modulates its transport activity in polarized and nonpolarized cells. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G1155–G1165. 10.1152/ajpgi.00334.2007 [DOI] [PubMed] [Google Scholar]
- 442.Viswanathan VK, Hodges K, Hecht G. 2009. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol. 7:110–119. 10.1038/nrmicro2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Harris TJ, Tepass U. 2010. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell. Biol. 11:502–514. 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- 444.Ivanov AI, Naydenov NG. 2013. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int. Rev. Cell Mol. Biol. 303:27–99. 10.1016/B978-0-12-407697-6.00002-7 [DOI] [PubMed] [Google Scholar]
- 445.Anderson JM, Van Itallie CM. 2009. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1:a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Shen L. 2012. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann. N. Y. Acad. Sci. 1258:9–18. 10.1111/j.1749-6632.2012.06613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Weber CR. 2012. Dynamic properties of the tight junction barrier. Ann. N. Y. Acad. Sci. 1257:77–84. 10.1111/j.1749-6632.2012.06528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Guttman JA, Finlay BB. 2009. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788:832–841. 10.1016/j.bbamem.2008.10.028 [DOI] [PubMed] [Google Scholar]
- 449.Peiffer I, Blanc-Potard AB, Bernet-Camard MF, Guignot J, Barbat A, Servin AL. 2000. Afa/Dr diffusely adhering Escherichia coli C1845 infection promotes selective injuries in the junctional domain of polarized human intestinal Caco-2/TC7 cells. Infect. Immun. 68:3431–3442. 10.1128/IAI.68.6.3431-3442.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Grasset E, Bernabeu J, Pinto M. 1985. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am. J. Physiol. 248:C410–C418 [DOI] [PubMed] [Google Scholar]
- 451.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. 1984. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am. J. Physiol. 247:C260–C267 [DOI] [PubMed] [Google Scholar]
- 452.Tanimoto Y, Arikawa K, Nishikawa Y. 2013. Effect of diffusely adherent Escherichia coli strains isolated from diarrhoeal patients and healthy carriers on IL-8 secretion and tight junction barrier integrity of Caco-2 cells. Vet. Immunol. Immunopathol. 152:183–188. 10.1016/j.vetimm.2012.09.031 [DOI] [PubMed] [Google Scholar]
- 453.Brest P, Betis F, Cuburu N, Selva E, Herrant M, Servin A, Auberger P, Hofman P. 2004. Increased rate of apoptosis and diminished phagocytic ability of human neutrophils infected with Afa/Dr diffusely adhering Escherichia coli strains. Infect. Immun. 72:5741–5749. 10.1128/IAI.72.10.5741-5749.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Arikawa K, Nishikawa Y. 2010. Interleukin-8 induction due to diffusely adherent Escherichia coli possessing Afa/Dr genes depends on flagella and epithelial Toll-like receptor 5. Microbiol. Immunol. 54:491–501. 10.1111/j.1348-0421.2010.00244.x [DOI] [PubMed] [Google Scholar]
- 455.Fournier BM, Parkos CA. 2012. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 5:354–366. 10.1038/mi.2012.24 [DOI] [PubMed] [Google Scholar]
- 456.Andoh A, Fujiyama Y, Sumiyoshi K, Sakumoto H, Okabe H, Bamba T. 1997. Tumour necrosis factor-alpha up-regulates decay-accelerating factor gene expression in human intestinal epithelial cells. Immunology 90:358–363. 10.1111/j.1365-2567.1997.00358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Bjorge L, Jensen TS, Matre R. 1996. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol. Immunother. 42:185–192. 10.1007/s002620050269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Nasu J, Mizuno M, Uesu T, Takeuchi K, Inaba T, Ohya S, Kawada M, Shimo K, Okada H, Fujita T, Tsuji T. 1998. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin. Exp. Immunol. 113:379–385. 10.1046/j.1365-2249.1998.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Spiller OB, Criado-Garcia O, Rodriguez De Cordoba S, Morgan BP. 2000. Cytokine-mediated up-regulation of CD55 and CD59 protects human hepatoma cells from complement attack. Clin. Exp. Immunol. 121:234–241. 10.1046/j.1365-2249.2000.01305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. 2003. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J. Exp. Med. 198:999–1010. 10.1084/jem.20030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Louis NA, Hamilton KE, Kong T, Colgan SP. 2005. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 19:950–959. 10.1096/fj.04-3251com [DOI] [PubMed] [Google Scholar]
- 462.Choy MK, Phipps ME. 2010. MICA polymorphism: biology and importance in immunity and disease. Trends Mol. Med. 16:97–106. 10.1016/j.molmed.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 463.Shao L, Kamalu O, Mayer L. 2005. Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol. Rev. 206:160–176. 10.1111/j.0105-2896.2005.00295.x [DOI] [PubMed] [Google Scholar]
- 464.Gonzalez S, Groh V, Spies T. 2006. Immunobiology of human NKG2D and its ligands. Curr. Top. Microbiol. Immunol. 298:121–138 [DOI] [PubMed] [Google Scholar]
- 465.Lanier LL. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495–502. 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Glas J, Martin K, Brunnler G, Kopp R, Folwaczny C, Weiss EH, Albert ED. 2001. MICA, MICB and C1_4_1 polymorphism in Crohn's disease and ulcerative colitis. Tissue Antigens. 58:243–249. 10.1034/j.1399-0039.2001.580404.x [DOI] [PubMed] [Google Scholar]
- 467.Tieng V, Le Bouguenec C, du Merle L, Bertheau P, Desreumaux P, Janin A, Charron D, Toubert A. 2002. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc. Natl. Acad. Sci. U. S. A. 99:2977–2982. 10.1073/pnas.032668099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Kendall MM, Sperandio V. 2007. Quorum sensing by enteric pathogens. Curr. Opin. Gastroenterol. 23:10–15. 10.1097/MOG.0b013e3280118289 [DOI] [PubMed] [Google Scholar]
- 469.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. U. S. A. 100:8951–8956. 10.1073/pnas.1537100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Diard S, Toribio AL, Boum Y, Vigier F, Kansau I, Bouvet O, Servin A. 2006. Environmental signals implicated in Dr fimbriae release by pathogenic Escherichia coli. Microbes Infect. 8:1851–1858. 10.1016/j.micinf.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 471.Marin-Esteban V, Turbica I, Dufour G, Semiramoth N, Gleizes A, Gorges R, Beau I, Servin AL, Liévin-Le Moal V, Sandre C, Chollet-Martin S. 2012. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect. Immun. 80:1891–1899. 10.1128/IAI.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Johnson JR, Skubitz KM, Nowicki BJ, Jacques-Palaz K, Rakita RM. 1995. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect. Immun. 63:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Hofman P, Piche M, Far DF, Le Negrate G, Selva E, Landraud L, Alliana-Schmid A, Boquet P, Rossi B. 2000. Increased Escherichia coli phagocytosis in neutrophils that have transmigrated across a cultured intestinal epithelium. Infect. Immun. 68:449–455. 10.1128/IAI.68.2.449-455.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. 2006. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell. Biol. 7:359–371. 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- 475.Weidemann A, Johnson RS. 2008. Biology of HIF-1alpha. Cell. Death Differ. 15:621–627. 10.1038/cdd.2008.12 [DOI] [PubMed] [Google Scholar]
- 476.Mimouna S, Goncalves D, Barnich N, Darfeuille-Michaud A, Hofman P, Vouret-Craviari V. 2011. Crohn disease-associated Escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses. Gut Microbes 2:335–346. 10.4161/gmic.18771 [DOI] [PubMed] [Google Scholar]
- 477.Shao L, Allez M, Park MS, Mayer L. 2006. Immunomodulatory roles of the carcinoembryonic antigen family of glycoproteins. Ann. N. Y. Acad. Sci. 1072:194–209. 10.1196/annals.1326.037 [DOI] [PubMed] [Google Scholar]
- 478.Pan X, Hobbs RP, Coulombe PA. 2013. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 25:47–56. 10.1016/j.ceb.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lander R, Nordin K, LaBonne C. 2011. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. J. Cell Biol. 194:17–25. 10.1083/jcb.201012085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hofman P, Vouret-Craviari V. 2012. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes 3:176–185. 10.4161/gmic.20288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 15:178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Liu JJ, Davis EM, Wine E, Lou Y, Rudzinski JK, Alipour M, Boulanger P, Thiesen AL, Sergi C, Fedorak RN, Muruve D, Madsen KL, Irvin RT. 2013. Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm. Bowel Dis. 19:912–921. 10.1097/MIB.0b013e3182807600 [DOI] [PubMed] [Google Scholar]
- 483.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. 2011. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140:1208–1218. 10.1053/j.gastro.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Martin P, Marcq I, Magistro G, Penary M, Garcie C, Payros D, Boury M, Olier M, Nougayrede JP, Audebert M, Chalut C, Schubert S, Oswald E. 2013. Interplay between siderophores and colibactin genotoxin biosynthetic pathways in Escherichia coli. PLoS Pathog. 9:e1003437. 10.1371/journal.ppat.1003437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Dechelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, Bonnet R. 21 March 2014. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 10.1136/gutjnl-2013-305257 [DOI] [PubMed] [Google Scholar]
- 486.Secher T, Samba-Louaka A, Oswald E, Nougayrede JP. 2013. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One 8:e77157. 10.1371/journal.pone.0077157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. 1996. Differentiation-associated antimicrobial functions in human colon adenocarcinoma cell lines. Exp. Cell Res. 226:80–89. 10.1006/excr.1996.0205 [DOI] [PubMed] [Google Scholar]
- 488.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. 2002. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 70:953–963. 10.1128/IAI.70.2.953-963.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Shifrin DA, Jr, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. 2012. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr. Biol. 22:627–631. 10.1016/j.sbi.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Brinkmann V, Zychlinsky A. 2012. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198:773–783. 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kaplan MJ, Radic M. 2012. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189:2689–2695. 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. 2010. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584:3193–3197. 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 493.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 7:e32366. 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Le Bouguenec C, Bertin Y. 1999. AFA and F17 adhesins produced by pathogenic Escherichia coli strains in domestic animals. Vet. Res. 30:317–342 [PubMed] [Google Scholar]
- 495.Hur J, Jeon BW, Kim YJ, Oh IG, Lee JH. 2013. Escherichia coli isolates from calf diarrhea in Korea and their virulent genetic characteristics. J. Vet. Med. Sci. 75:519–522. 10.1292/jvms.12-0378 [DOI] [PubMed] [Google Scholar]
- 496.Mainil JG, Jacquemin E, Herault F, Oswald E. 1997. Presence of pap-, sfa-, and afa-related sequences in necrotoxigenic Escherichia coli isolates from cattle: evidence for new variants of the AFA family. Can. J. Vet. Res. 61:193–199 [PMC free article] [PubMed] [Google Scholar]
- 497.Maluta RP, Stella AE, Riccardi K, Rigobelo EC, Marin JM, Carvalho MB, de Avila FA. 2012. Phenotypical characterization and adhesin identification in Escherichia coli strains isolated from dogs with urinary tract infections. Braz. J. Microbiol. 43:375–381. 10.1590/S1517-83822012000100045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 54:1855–1863. 10.1128/AAC.00014-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. 2004. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 12:424–430. 10.1016/j.tim.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 500.Anderson GG, Martin SM, Hultgren SJ. 2004. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 6:1094–1101. 10.1016/j.micinf.2004.05.023 [DOI] [PubMed] [Google Scholar]
- 501.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107. 10.1126/science.1084550 [DOI] [PubMed] [Google Scholar]
- 502.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:1333–1338. 10.1073/pnas.0308125100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572–4579. 10.1128/IAI.69.7.4572-4579.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. U. S. A. 97:8829–8835. 10.1073/pnas.97.16.8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Schilling JD, Mulvey MA, Hultgren SJ. 2001. Dynamic interactions between host and pathogen during acute urinary tract infections. Urology 57:56–61. 10.1016/S0090-4295(01)01130-X [DOI] [PubMed] [Google Scholar]
- 506.Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, Szajewska H, European Society for Paediatric Gastroenterology Hepatology, and Nutrition, European Society for Paediatric Infectious Diseases 2008. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J. Pediatr. Gastroenterol. Nutr. 46:S81–S122. 10.1097/MPG.0b013e31816f7b16 [DOI] [PubMed] [Google Scholar]
- 507.Menees S, Saad R, Chey WD. 2012. Agents that act luminally to treat diarrhoea and constipation. Nat. Rev. Gastroenterol. Hepatol. 9:661–674. 10.1038/nrgastro.2012.162 [DOI] [PubMed] [Google Scholar]
- 508.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 509.Willing BP, Russell SL, Finlay BB. 2011. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 9:233–243. 10.1038/nrmicro2536 [DOI] [PubMed] [Google Scholar]
- 510.Liévin-Le Moal V, Servin AL. 2014. Anti-infective activities of human intestinal microbiota Lactobacillus strains: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 27:167–199. 10.1128/CMR.00080-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Beceiro A, Tomas M, Bou G. 2013. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 26:185–230. 10.1128/CMR.00059-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Depardieu F, Podglajen I, Leclercq R, Collatz E, Courvalin P. 2007. Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 20:79–114. 10.1128/CMR.00015-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A. 2009. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 34:407–413. 10.1016/j.ijantimicag.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 514.Sanchez GV, Master RN, Karlowsky JA, Bordon JM. 2012. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob. Agents Chemother. 56:2181–2183. 10.1128/AAC.06060-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Koren J, Curova K, Kmetova M, Siegfried L, Janko V, Kovacs L, Hupkova H, Luha J. 2013. Involvement of virulence properties and antibiotic resistance in Escherichia coli strains causing pyelonephritis in children. Folia Microbiol. (Praha) 58:53–59. 10.1007/s12223-012-0176-8 [DOI] [PubMed] [Google Scholar]
- 516.Kachroo BB. 2001. Association between antibiotic resistance and the expression of Dr adhesin among uropathogenic Escherichia coli. Chemotherapy 47:97–103. 10.1159/000048507 [DOI] [PubMed] [Google Scholar]
- 517.Oteo J, Orden B, Bautista V, Cuevas O, Arroyo M, Martinez-Ruiz R, Perez-Vazquez M, Alcaraz M, Garcia-Cobos S, Campos J. 2009. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. J. Antimicrob. Chemother. 64:712–717. 10.1093/jac/dkp288 [DOI] [PubMed] [Google Scholar]
- 518.Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M. 2012. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J. Antimicrob. Chemother. 67:346–356. 10.1093/jac/dkr451 [DOI] [PubMed] [Google Scholar]
- 519.Starcic Erjavec M, Rijavec M, Krizan-Hergouth V, Fruth A, Zgur-Bertok D. 2007. Chloramphenicol- and tetracycline-resistant uropathogenic Escherichia coli (UPEC) exhibit reduced virulence potential. Int. J. Antimicrob. Agents 30:436–442. 10.1016/j.ijantimicag.2007.06.025 [DOI] [PubMed] [Google Scholar]
- 520.Brumbaugh AR, Mobley HL. 2012. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev. Vaccines 11:663–676. 10.1586/erv.12.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. 2008. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6:17–27. 10.1038/nrmicro1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, Calteau A, Chiapello H, Clermont O, Cruveiller S, Danchin A, Diard M, Dossat C, Karoui ME, Frapy E, Garry L, Ghigo JM, Gilles AM, Johnson J, Le Bouguenec C, Lescat M, Mangenot S, Martinez-Jehanne V, Matic I, Nassif X, Oztas S, Petit MA, Pichon C, Rouy Z, Ruf CS, Schneider D, Tourret J, Vacherie B, Vallenet D, Medigue C, Rocha EP, Denamur E. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344. 10.1371/journal.pgen.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Njoroge J, Sperandio V. 2009. Jamming bacterial communication: new approaches for the treatment of infectious diseases. EMBO Mol. Med. 1:201–210. 10.1002/emmm.200900032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Wieser A, Romann E, Magistro G, Hoffmann C, Norenberg D, Weinert K, Schubert S. 2010. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect. Immun. 78:3432–3442. 10.1128/IAI.00174-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Aberg V, Almqvist F. 2007. Pilicides—small molecules targeting bacterial virulence. Org. Biomol. Chem. 5:1827–1834. 10.1039/b702397a [DOI] [PubMed] [Google Scholar]
- 526.Chorell E, Pinkner JS, Bengtsson C, Banchelin TS, Edvinsson S, Linusson A, Hultgren SJ, Almqvist F. 2012. Mapping pilicide anti-virulence effect in Escherichia coli, a comprehensive structure-activity study. Bioorg. Med. Chem. 20:3128–3142. 10.1016/j.bmc.2012.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Piatek R, Zalewska-Piatek B, Dzierzbicka K, Makowiec S, Pilipczuk J, Szemiako K, Cyranka-Czaja A, Wojciechowski M. 2013. Pilicides inhibit the FGL chaperone/usher assisted biogenesis of the Dr fimbrial polyadhesin from uropathogenic Escherichia coli. BMC Microbiol. 13:131. 10.1186/1471-2180-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Fang S-H, Schuller S, Phillips AD. 2013. Human intestinal in vitro organ culture as a model for investigation of bacteria–host interactions. J. Exp. Clin. Med. 5:43–50. 10.1016/j.jecm.2013.02.006 [DOI] [Google Scholar]
- 529.Chow J, Tang H, Mazmanian SK. 2011. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr. Opin. Immunol. 23:473–480. 10.1016/j.coi.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Stecher B, Maier L, Hardt WD. 2013. ‘Blooming' in the gut: how dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 11:277–284. 10.1038/nrmicro2989 [DOI] [PubMed] [Google Scholar]
- 531.Hentges DJ, Freter R. 1962. In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. I. Correlation between various tests. J. Infect. Dis. 110:30–37 [DOI] [PubMed] [Google Scholar]
- 532.Freter R. 1962. In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. II. The inhibitory mechanism. J. Infect. Dis. 110:38–46 [DOI] [PubMed] [Google Scholar]
- 533.Macutkiewicz C, Carlson G, Clark E, Dobrindt U, Roberts I, Warhurst G. 2008. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect. 10:424–431. 10.1016/j.micinf.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 534.Zareie M, Riff J, Donato K, McKay DM, Perdue MH, Soderholm JD, Karmali M, Cohen MB, Hawkins J, Sherman PM. 2005. Novel effects of the prototype translocating Escherichia coli, strain C25 on intestinal epithelial structure and barrier function. Cell. Microbiol. 7:1782–1797. 10.1111/j.1462-5822.2005.00595.x [DOI] [PubMed] [Google Scholar]
- 535.Michalsky MP, Deitch EA, Ding J, Lu Q, Huang Q. 1997. Interleukin-6 and tumor necrosis factor production in an enterocyte cell model (Caco-2) during exposure to Escherichia coli. Shock 7:139–146. 10.1097/00024382-199702000-00010 [DOI] [PubMed] [Google Scholar]
- 536.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. 2005. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 128:1258–1267. 10.1053/j.gastro.2005.01.046 [DOI] [PubMed] [Google Scholar]
- 537.Cho JH. 2008. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 8:458–466. 10.1038/nri2340 [DOI] [PubMed] [Google Scholar]
- 538.Nagalingam NA, Lynch SV. 2012. Role of the microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 18:968–984. 10.1002/ibd.21866 [DOI] [PubMed] [Google Scholar]
- 539.Pineton de Chambrun G, Colombel JF, Poulain D, Darfeuille-Michaud A. 2008. Pathogenic agents in inflammatory bowel diseases. Curr. Opin. Gastroenterol. 24:440–447. 10.1097/MOG.0b013e3283023be5 [DOI] [PubMed] [Google Scholar]
- 540.Weitzman MD, Weitzman JB. 2014. What's the damage? The impact of pathogens on pathways that maintain host genome integrity. Cell Host Microbe 15:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Schwabe RF, Jobin C. 2013. The microbiome and cancer. Nat. Rev. Cancer 13:800–812. 10.1038/nrc3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Sears CL, Garrett WS. 2014. Microbes, microbiota, and colon cancer. Cell Host Microbe 15:317–328. 10.1016/j.chom.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Hatakeyama M. 2014. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe 15:306–316. 10.1016/j.chom.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 544.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrede JP. 2010. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 107:11537–11542. 10.1073/pnas.1001261107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Hofman PM. 2010. Pathobiology of the neutrophil-intestinal epithelial cell interaction: role in carcinogenesis. World J. Gastroenterol. 16:5790–5800. 10.3748/wjg.v16.i46.5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Spendlove I, Ramage JM, Bradley R, Harris C, Durrant LG. 2006. Complement decay accelerating factor (DAF)/CD55 in cancer. Cancer Immunol. Immunother. 55:987–995. 10.1007/s00262-006-0136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Lorin S, Hamai A, Mehrpour M, Codogno P. 2013. Autophagy regulation and its role in cancer. Semin. Cancer Biol. 23:361–379. 10.1016/j.semcancer.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 548.White E. 2012. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12:401–410. 10.1038/nrc3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Greenfield LK, Jones NL. 2013. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 21:602–612. 10.1016/j.tim.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 550.Terebiznik MR, Raju D, Vazquez CL, Torbricki K, Kulkarni R, Blanke SR, Yoshimori T, Colombo MI, Jones NL. 2009. Effect of Helicobacter pylori's vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy 5:370–379. 10.4161/auto.5.3.7663 [DOI] [PubMed] [Google Scholar]
- 551.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148–1155. 10.4049/jimmunol.166.2.1148 [DOI] [PubMed] [Google Scholar]
- 552.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412–7419. 10.1074/jbc.M110560200 [DOI] [PubMed] [Google Scholar]