Abstract
Nonhomologous end joining (NHEJ) is a recently described bacterial DNA double-strand break (DSB) repair pathway that has been best characterized for mycobacteria. NHEJ can religate transformed linear plasmids, repair ionizing radiation (IR)-induced DSBs in nonreplicating cells, and seal I-SceI-induced chromosomal DSBs. The core components of the mycobacterial NHEJ machinery are the DNA end binding protein Ku and the polyfunctional DNA ligase LigD. LigD has three autonomous enzymatic modules: ATP-dependent DNA ligase (LIG), DNA/RNA polymerase (POL), and 3′ phosphoesterase (PE). Although genetic ablation of ku or ligD abolishes NHEJ and sensitizes nonreplicating cells to ionizing radiation, selective ablation of the ligase activity of LigD in vivo only mildly impairs NHEJ of linearized plasmids, indicating that an additional DNA ligase can support NHEJ. Additionally, the in vivo role of the POL and PE domains in NHEJ is unclear. Here we define a LigD ligase-independent NHEJ pathway in Mycobacterium smegmatis that requires the ATP-dependent DNA ligase LigC1 and the POL domain of LigD. Mycobacterium tuberculosis LigC can also support this backup NHEJ pathway. We also demonstrate that, although dispensable for efficient plasmid NHEJ, the activities of the POL and PE domains are required for repair of IR-induced DSBs in nonreplicating cells. These findings define the genetic requirements for a LigD-independent NHEJ pathway in mycobacteria and demonstrate that all enzymatic functions of the LigD protein participate in NHEJ in vivo.
INTRODUCTION
Chromosomal DNA is under constant attack from clastogens that threaten the coding capacity and stability of the genome. Among the wide variety of DNA lesions that the cell must process, double strand breaks (DSBs) of DNA represent a particularly lethal form of DNA damage that must be repaired for chromosome replication and transcription to proceed. The sources of DSBs include ionizing radiation (IR), replication across a single-stranded nick (1), DNA processing enzymes that generate DSBs, such as topoisomerases, and embedded ribonucleotides in chromosomal DNA (2–5). As such, the repair of DSBs is a critical function in all living organisms, including bacteria. The most intensely studied and universally distributed pathway of DSB repair is RecA-dependent homologous recombination (HR), which uses an intact chromosomal template to repair the DSB without mutation. RecA-dependent HR is the dominant and sole DSB repair pathway in Escherichia coli. However, additional pathways of DSB repair operate in other bacteria, including mycobacteria, such as Mycobacterium smegmatis and M. tuberculosis. M. smegmatis elaborates three genetically distinct DNA repair pathways: RecA-dependent HR, single-strand annealing (SSA), and nonhomologous end joining (NHEJ) (6). All mycobacterial HR uses the RecA strand exchange protein, whereas two subpathways utilize the AdnAB helicase-nuclease (6, 7) or RecO (8), respectively. The SSA pathway requires RecBCD (6), which does not participate in HR in mycobacteria, and RecO (8).
Mycobacterial NHEJ can recircularize transformed linear plasmids (9–14), repair IR-induced DSBs in late-stationary-phase cells (15, 16), protect mycobacteria from desiccation (15), and seal homing endonuclease-induced DSBs (6, 16). In all of these experimental systems, the NHEJ pathway requires the DNA end binding protein Ku and the polyfunctional DNA ligase LigD. Genetic ablation of ku or ligD reduces the efficiency of plasmid NHEJ by approximately 500-fold (10). Similarly, M. smegmatis ΔKu or ΔligD bacteria in late stationary phase but not log phase are sensitized to IR (16) and cannot repair I-SceI-induced chromosomal breaks by NHEJ (6). Ablation of ku or ligD also leads to reciprocal upregulation of the HR pathway in the I-SceI system, suggesting that the NHEJ and HR pathways compete for repair of DSBs in log-phase cells (6).
The LigD protein has three autonomous enzymatic domains: polymerase (POL), phosphoesterase (PE), and ligase (LIG). LigD-POL is a primase-like polymerase that can add both templated and nontemplated deoxynucleoside triphosphates (dNTPs) or ribonucleoside triphosphates (rNTPs) to DNA substrates (13, 17). Alanine substitution mutations at the diaspartate metal binding site of the polymerase (D136A/D138A) abolish polymerase activity in vitro (11). M. smegmatis expressing LigD-D136/138A cannot add nontemplated nucleotides to blunt end linear plasmid substrates, resulting in a rise in the fidelity of NHEJ with little change in the overall efficiency (10, 13). However, templated fill-in of 5′-overhang NHEJ substrates is not abolished by ablation of POL activity, implicating additional polymerases in these end modifications. The LigD PE domain is a 3′-end-processing enzyme that can resect a tract of ribonucleotides on a primer template, leaving a single ribonucleotide (18, 19). PE also displays phosphodiesterase and monoesterase activities that can process 3′-phosphate-terminated DNA ends to 3′ OH. However, ablation of LigD-PE has no measurable effect on the efficiency or fidelity of plasmid NHEJ (10). Finally, LigD-LIG is an ATP-dependent DNA ligase with a relatively poor nick ligation activity in vitro (12). LigD activity is stimulated by the presence of a single ribonucleotide, suggesting that the ribonucleotide addition and processing activities of the POL and PE domains may process the ends to facilitate LigD ligation (20). Interestingly, this ribonucleotide-stimulated nick ligation activity is also exhibited by ligase C (20), an ATP dependent ligase without POL or PE domains whose role in vivo is not clear.
Surprisingly, ablation of LigD ligase activity in vivo (either through the K484A active site substitution or deletion of the LIG domain) did not reduce plasmid NHEJ substantially (9, 10), in contrast to the severe NHEJ decrement observed with the ΔligD strain. The LigD-independent NHEJ pathway that is evident in the LigD-K484A strain is low fidelity and characterized by frequent deletions at the break site. Deletion of the three additional ATP-dependent DNA ligases in combination (LigB, LigC1, and LigC2) abolishes this backup NHEJ pathway (10), but the responsible ligase has not been further defined.
Using the plasmid-based assay for NHEJ, these prior studies documented the importance of LigD in NHEJ and revealed the existence of a backup NHEJ pathway. However, multiple questions remained unanswered. First, the DSBs in the plasmid assay have “clean” 5′-PO4/3′-OH ends that do not require end modification for religation. This feature of the plasmid NHEJ assay may obscure the participation of end processing functions of the NHEJ machinery (such as POL and PE), which would be required to repair “dirty” DSBs generated by clastogens that may have 3′ PO4 and/or require gap filling by a polymerase. Additionally, the molecular participants in the low-fidelity LigD-independent NHEJ pathway, including the DNA ligase, are not known. In this study, we used two assays of chromosomal DSB repair, late-stationary-phase IR sensitivity and I-SceI-induced DSBs, to define the contribution of the LigD-POL and PE domains in repair, define the molecular constituents of the LigD-ligase-independent NHEJ pathway, and examine the expression of NHEJ components in late-stationary-phase mycobacterial cells.
MATERIALS AND METHODS
Strains, plasmids, and oligonucleotides.
Bacterial strains are listed in Table S1, plasmids in Table S2, and oligonucleotides in Table S3 in the supplemental material.
NHEJ plasmid assay.
The plasmid assay for NHEJ was executed as previously described (10) with blunt-end and 5′-overhang ends. To determine NHEJ efficiency, three replicate transformations were performed, and average values for each were calculated. Kanamycin-resistant transformants that had recircularized the linear plasmid were recovered on agar medium containing kanamycin (20 μg/ml) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (50 μg/ml). The numbers of blue and white colonies were enumerated for linear and circular transformations. Efficiency of linear transformation was calculated as the number of transformants per ng of the linear plasmid divided by the number of transformants per ng of the circular plasmid. Efficiency for a given strain was normalized to the efficiency of the wild-type (WT) strain or another reference strain (as appropriate) and expressed as a percentage. Percent fidelity was calculated as number of kanamycin-resistant blue transformants/total number of kanamycin-resistant transformants.
Late-stationary-phase ionizing radiation treatment.
The various strains were grown in Difco Middlebrook 7H9 broth supplemented with 0.5% glycerol, 0.5% dextrose, and 0.05% Tween 80 in 200 ml nonbaffled flasks until the optical density at 600 nm (OD600) stabilized in stationary phase. Forty-eight hours later, bacteria were resuspended in phosphate-buffered saline (PBS) with 0.05% Tween 80 and subjected to ionizing radiation (IR) treatment at 600- and 1,200-Gy dosages from a 137Cs source that irradiates at the rate of 10.3 Gy/min in an irradiation chamber equipped with a rotating platform to equalize dosages between samples. Serial dilutions in PBS–0.05% Tween 80 were spotted onto Difco 7H10 plates containing 0.5% glycerol and 0.5% dextrose. Surviving colonies were counted 3 days after incubating at 37°C, and fraction survival was calculated in comparison to unexposed control cells from the same strain. The absolute fractional survival in these assays is somewhat variable between experiments within a 10-fold range, likely due to experimental variability within the radiation chamber and the long exposure times. To control for this variability, we have included relevant control strains in every exposure experiment. An unpaired parametric t test was used to compare fraction sensitivity at 1,200 Gy using GraphPad Prism 6.
Chromosomal I-SceI assays.
The M. smegmatis wild type or the various strains harboring the chromosomally integrated lacZ reporter construct (see Table S1 in the supplemental material) were subjected to the plasmid transformation-based I-SceI DSB repair assay as described previously (6). Briefly, equivalent molar amounts of the I-SceI plasmid or control vector plasmid were transformed to determine the frequency of HR, SSA, and NHEJ repair in these strains. For each strain, the experiment was performed at least thrice using different batches of competent cells, and results are expressed as mean values. Since the observed percent survival was similar for all the strains (0.030 [±0.002]), frequencies of different repair outcomes are compared by calculating the relative repair frequency, as follows: relative HR frequency = (HR events/blue events) × (% blue); relative SSA frequency = (SSA events/blue events) × (% blue). Among the blue colonies, the HR and SSA events were distinguished by scoring for kanamycin resistance, whereby the kanamycin-resistant colonies represent HR and kanamycin-sensitive colonies denote SSA. To ascertain mean values for the relative HR and SSA frequencies in different genetic backgrounds, large number of blue colonies (ranging from 60 to 100) were analyzed in separate repeat experiments. To determine NHEJ outcomes in the white colonies, repair junctions were analyzed by PCR amplification and sequencing as described previously (6).
ligC1, ligC2, ligB, and ligCTB complementation constructs.
Complementation constructs expressing M. smegmatis ligC1, ligC2, or ligB under their putative native promoters were constructed in the vector pDB60. pDB60 is an attB- and L5 integrase-containing vector that integrates into the mycobacterial attB site in the chromosome. M. smegmatis ligC1 and ligC2 are divergently transcribed in M. smegmatis chromosome (see Fig. S1 in the supplemental material). The predicted intergenic region between the LigC1 and LigC2 translation initiation codons is 59 nucleotides (nt) in length. The presumed native promoter of ligC1 was cloned to contain these 59 nt plus 61 nt from the ligC2 open reading frame using the primers ligC1_3′ and ligC5′ (see Fig. S1 in the supplemental material). To amplify ligC2 with its presumed native promoter, 47 nt from the ligC1 open reading frame were included, in addition to the 59 nt from the intergenic region using the primers ligC3′ and ligC2_5′ (see Fig. S1). ligB was cloned with 151 nt upstream of its open reading frame to include its native promoter using the primers LigB_pro_for and LigB_pro_rev. The PCR products containing ligC1, ligC2, or ligB were inserted into the shuttle vector pMSG234 and, after DNA sequencing to exclude mutations, were subcloned into pDB60 to give the plasmids pHB1 (ligC1), pHB2 (ligC2), and pHB3 (ligB). ligC2 under the constitutive mycobacterial optimized promoter (MOP) was inserted into pDB60 to give the plasmid pHB4. ligC1 under its native promoter and ligC2 under its native promoter and the MOP were tagged with a hemagglutinin (HA) tag at the C terminus using the primers “HA tag to LigC2” and “HA into ligC1”and also inserted into pDB60. ligC from M. tuberculosis, tagged with HA at the C terminus and expressed from the M. smegmatis ligC1 promoter, was constructed by overlap extension PCR with the primers ligC1_3′, ligCTBwithC1pF, ligCTBwithC1pR, and ligCTBHAtag (see Fig. S1). The PCR product was inserted into pMSG234 and, after DNA sequencing to exclude mutations, inserted into pDB60 to give pHB8. The plasmids pHB1 to -8 were transformed into ligD-(K484A) ΔligC1-ligC2 ΔligB (MGM 810) and ΔligD ΔligC1-ligC2 ΔligB (MGM 808) strains to yield the strains listed in Table S1.
Immunoblotting.
For the determination of the protein levels of LigC isoforms, the appropriate strains were grown to an OD600 of 0.4. The cells were collected by centrifugation at 3,700 × g and treated with 1 mg/ml lysozyme in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at 37°C for 45 min. The sample was then boiled in SDS-PAGE loading buffer with dithiothreitol (DTT) for 10 min, and proteins were separated by SDS-PAGE in 4 to 12% Bis-Tris acrylamide gels at 200 V for 45 min in morpholinepropanesulfonic acid (MOPS)-SDS running buffer. After transfer to nitrocellulose membrane (Protran; PerkinElmer), HA epitope-tagged proteins were identified by immunoblotting with anti-HA (1:10,000; Covance) and horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody (1:20,000 dilution; Zymed). Nitrocellulose membranes were stripped with 2% SDS, 6.25 mM Tris-HCl, and 0.8% β-mercaptoethanol at pH 6.8 for 30 min at 37°C and reprobed with anti-CarD monoclonal antibodies and HRP-conjugated anti-mouse secondary antibody as a loading control.
RT-qPCR.
To measure mRNA expression, RNA was extracted from bacteria grown to mid-log (0.5 O.D600) or late stationary phase (maximum OD600 for 12 h) in LB medium containing 0.5% glycerol, 0.5% dextrose, and 0.05% Tween 80. Cells were lysed in TRIzol reagent using Zirconia beads in a Mini-Beadbeater instrument (Biospec). Residual genomic DNA contamination was removed using a Turbo DNA-free kit (Ambion). RNA was additionally purified using the RNeasy minikit (Qiagen). SuperScript III reverse transcriptase (RT; Invitrogen) was used to reverse transcribe the RNA to cDNA using random hexamer primers. Quantitative PCR was carried out with cDNA samples using SYBR green and an Opticon2 real-time fluorescence detector (MJ Research) in biologic triplicates. Amplification of a single product from the quantitative PCR (qPCR) was confirmed by melting-curve analysis. The cycle threshold value (CT) from qPCR from a given primer set was subtracted from CT values from primers that amplify the sigA gene. The relative level of each mRNA normalized to sigA was calculated using the formula 2CT – CT(sigA). Control reactions lacking RT were executed with sigA primers and yielded CT values more than 10 cycles higher than those of reactions with RT.
C2FDG β-galactosidase assay.
The primers “Ku promoter 5′” and “Ku promoter 3′” were used to amplify the putative ku promoter and first 12 nt of the ku coding sequence from M. smegmatis chromosomal DNA. This PCR product was inserted into pJEM13 (21) to create pMSG335, which contains 721 nt upstream of ku, and the coding sequence for the first four amino acids of Ku fused to the lacZ reading frame. Wild-type M. smegmatis strains transformed with pMSG335 or pJEM13 (promoterless lacZ) were collected at an OD600 of 0.5, and aliquots were removed, normalized to 106 CFU/well by OD600 (using a factor of OD600 of 1 = 5 × 108 CFU/ml), and added to a black 96-well plate. 5-Acetylaminofluorescein di-β-d-galactopyranoside (C2FDG) was added to each well to a final concentration of 30 μM. PBS was added to control wells instead of C2FDG to enable calculation of background fluorescence. For each culture, 3 experimental wells and 3 background wells were used. Plates were incubated at 37°C for 3 h, and fluorescence was determined using a Wallace 1420 plate reader with the temperature control set to 37°C and the following filter set: excitation/emission (Ex/Em), 485 ± 20 nm/530 ± 25 nm.
Strain construction.
polD1 was deleted in the ligD-(K484A) background as described in reference 14.
RESULTS
Role of the LigD-LIG domain in NHEJ-mediated repair of chromosomal damage.
Our prior investigations into the role of each LigD domain in NHEJ efficiency and fidelity used a plasmid recircularization assay in which NHEJ is the only pathway that can repair the plasmid and the ends are compatible due to their generation through restriction endonuclease cleavage. Our prior work has also shown that mycobacterial NHEJ becomes important for IR resistance in late-stationary-phase cells (16), but the role of each LigD structural domain or domain activity in this chromosomal repair phenotype is unknown. To clarify the role of each LigD activity (ligase, phosphoesterase, and polymerase) in chromosomal repair, we measured the stationary-phase IR resistance of strains encoding full-length LigD proteins with inactivating mutations of each LigD domain.
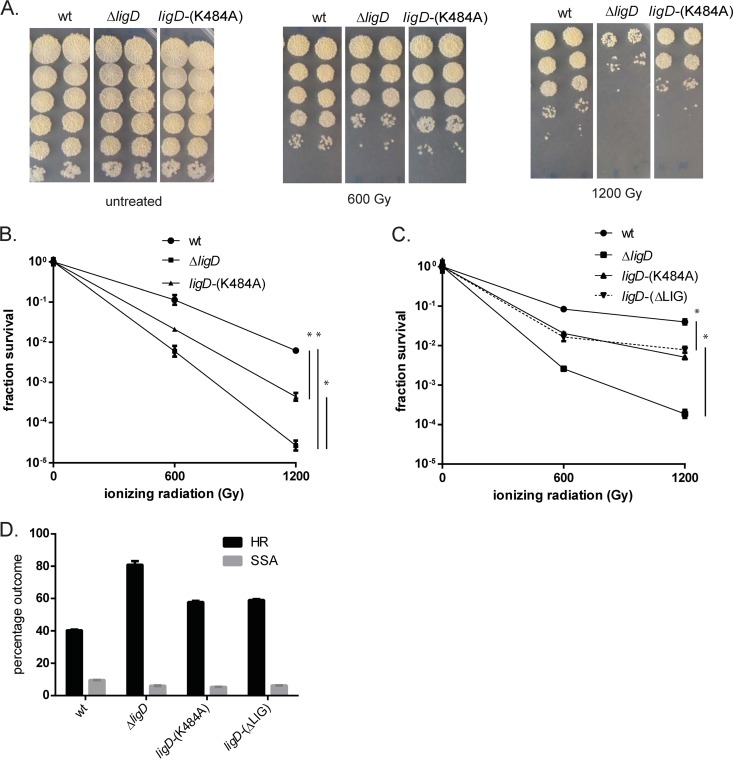
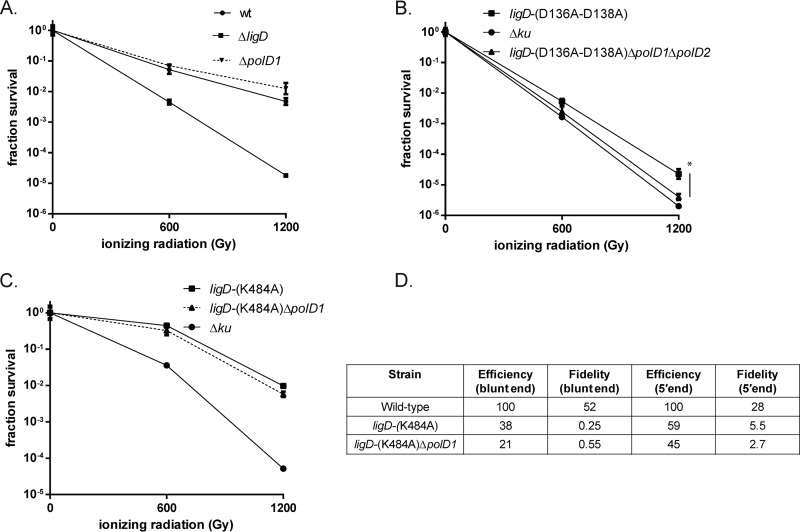
We first investigated the role of the catalytic activity of the LigD-LIG domain in IR resistance by testing M. smegmatis wild-type, ΔligD, and ligD-(K484A) strains in late stationary phase, the conditions in which NHEJ becomes important for survival. The protein encoded by the ligD-(K484A) allele lacks all ligase activity (9, 10, 12). When wild-type M. smegmatis was subjected to 600 and 1,200 Gy IR, we observed 0.9- and 2.2-log killing (Fig. 1A and B), whereas the same dosages of IR resulted in 2.2 and 4.8 logs of killing in the ΔligD strain (Fig. 1A and B), confirming our prior findings that LigD is required for DSB repair in late stationary phase (16). The ligD-(K484A) strain displays a phenotype intermediate between those of the wild-type and ΔligD strains (Fig. 1A and B), and loss of the entire ligase domain [ligD-(ΔLIG)] phenocopied ligD-(K484A) (Fig. 1B and C). Specifically, at a dose of 1,200 Gy, the ligD-(K484A) and ligD-(ΔLIG) strains are 0.7 to 0.9 logs more sensitive than the wild type, whereas the ΔligD strain is about 2.3 logs more sensitive (Fig. 1C). Taken together, these results indicate that loss of the LigD ligase activity, either through active site mutation or domain deletion, does not recapitulate the IR sensitivity observed with loss of the entire LigD protein, implying that an additional ligase can compensate for the loss of LigD ligase activity, provided that the LigD protein is present.
FIG 1.
A LigD-LIG-independent NHEJ pathway participates in chromosomal repair. (A) M. smegmatis ligD-(K484A) (MGM 803) and M. smegmatis ligD-(ΔLIG) (MGM 805) were subjected to 600 and 1,200 Gy of IR after 2 days in late stationary phase in 7H9 minimal medium alongside wild-type and ΔligD (MGM 140) strains. The surviving cells were enumerated as 10-fold serial dilutions. Treatment was carried out on biologic triplicates, and each treatment was spotted in duplicate. (B) Fraction survival of wild-type (wt), ligD-(K484A), and ΔligD strains was plotted in log scale with the IR dose on the x axis. Statistical significance between strains is indicated by a vertical line, with “*” indicating P < 0.05. (C) The fraction survival of wild-type, ligD-(ΔLIG), ligD-(K484A), and ΔligD strains. (D) The relative frequency of homologous recombination (HR) and single-stranded annealing (SSA) in the chromosomal I-SceI assay for the indicated strains.
To glean additional insight into the effect of the LIG domain inactivation and deletion on relative frequencies of NHEJ, HR, and SSA, an I-SceI-based assay was employed. This assay can simultaneously assay all three DSB repair pathways in mycobacteria (HR, NHEJ, and SSA), and we have previously shown that loss of NHEJ through genetic ablation of ku or ligD leads to an upregulation of HR frequency (6). Accordingly, we assayed the frequency of HR in the ligD-(K484A) and ligD-(ΔLIG) strains. The frequency of HR in the ΔligD strain was 80.9%, a 2-fold increase above that of the WT, as previously reported (6). The ligD-(K484A) and ligD-(ΔLIG) strains showed 57.8 and 59.0% HR events, respectively, a level intermediate between those of the WT and ΔligD strains (Fig. 1D). To determine the molecular outcomes of NHEJ events in these strains, we amplified the repair junctions from surviving white colonies, which represent a mixture of NHEJ-mediated repair and unmodified sites due to inactivation of the I-SceI enzyme (6). Out of the 42 white colonies of the ligD-(K484A) mutant analyzed by PCR amplification and sequencing, 23 showed intact chromosomal loci with unmodified I-SceI sites (∼1,350-bp amplification product), whereas 12 events entailed deletions (<2 kb) with the characteristic NHEJ outcomes (bidirectional/unidirectional deletions and cross-break fill-in) that we have previously observed in the wild-type strain (6), indicating that even in the absence of an active LigD ligase domain, the backup NHEJ pathway can function to repair DSBs (see Fig. S2A in the supplemental material). For the remaining 7 repair events, even using primers that anneal farther away from the I-SceI site, PCR products could not be obtained. We surmise that these events reflect large deletions that could not be mapped by the primer pairs used.
Role of LigD-POL domain in NHEJ-mediated repair of chromosomal damage.
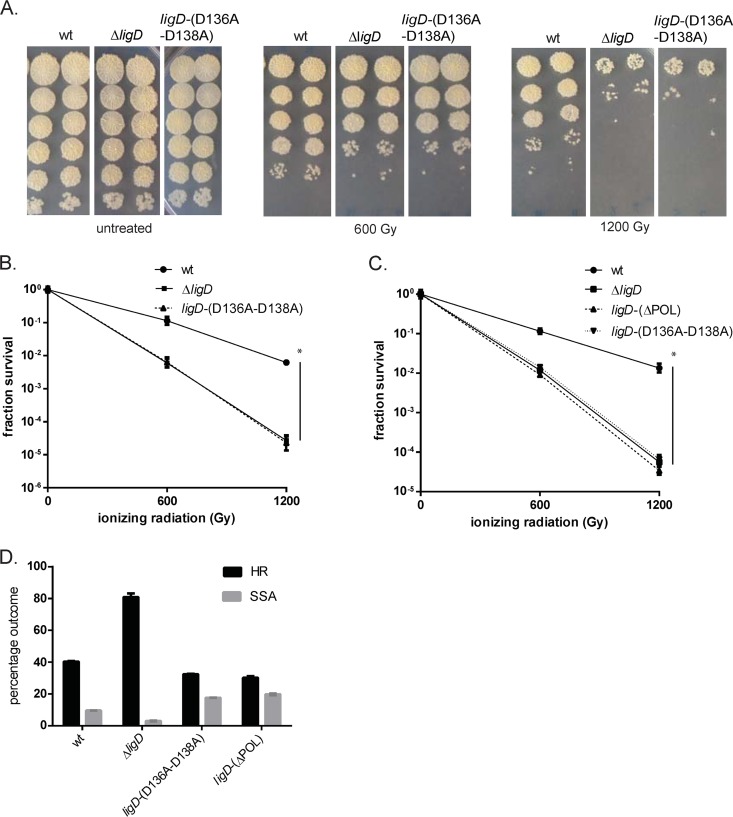
In our prior plasmid recircularization assays, inactivation of LigD-POL polymerase activity (through the D136A-D138A substitutions) did not affect the efficiency of NHEJ, although the fidelity was raised due to loss of end modifications, particularly at blunt ends, catalyzed by LigD-POL (10, 13, 22). In contrast, deletion of the POL domain from LigD reduced NHEJ to levels of the ΔligD strain, implying a structural role for the POL domain that is independent of its polymerization activity. It is likely that IR-induced DSBs frequently have incompatible ends that must be modified before ligation, potentially requiring the activity of LigD-POL or other polymerases. To test the role of the POL domain in chromosomal DSB repair, we tested the ligD-(D136A-D138A) and ligD-(ΔPOL) strains for late-stationary-phase IR sensitivity. Surprisingly, M. smegmatis ligD-(D136A-D138A) was as sensitive as the ΔligD strain (Fig. 2A and B). Both the ligD-(D136A-D138A) and ΔligD strains were 1.3 and 2.4 logs more sensitive than the wild type at 600 and 1,200 Gy, respectively. A similar phenotype was observed for the ΔPOL strain, which was 1.1 and 2.6 logs more sensitive than the wild type at 600 and 1,200 Gy, a sensitization similar to that seen for the ΔligD strain (Fig. 2C). These results implicate the polymerization activity of LigD-POL in repairing IR-induced DSBs and stand in contrast to our prior plasmid assays, in which POL activity was largely dispensable for NHEJ efficiency, likely due to the presence of compatible DSB ends.
FIG 2.
LigD POL activity is required for NHEJ-mediated repair of chromosomal DSBs. (A and B) The wild-type (wt), ΔligD (MGM 140), or ligD-(D136A-D138A) (MGM 801) strain was subjected to IR in late stationary phase, duplicate serial 10-fold dilutions were cultured (A), and fraction survival was calculated and plotted in log scale (B). Statistical significance between strains is indicated by a vertical line, with “*” indicating P < 0.05. (C) Fraction survival of the ligD-(ΔPOL) strain (MGM 802) at 600 and 1,200 Gy IR was plotted alongside that of the wild-type, ligD-(D136A-D138A), and ΔligD strains. (D) Relative frequencies of homologous recombination (HR) and single-stranded annealing (SSA) in the chromosomal I-SceI assay for the indicated strains.
To further examine the role of LigD-POL in the repair of chromosomal DNA breaks with incompatible ends, we tested the ligD-(D136A-D138A) and ligD-(ΔPOL) strains in the I-SceI assay. Both ligD-(D136A-D138A) and ligD-(ΔPOL) strains demonstrated a mild decrement in relative HR frequency and a mild increase in SSA frequency compared to those of the wild type (Fig. 2D). We next characterized the repair junctions of white colonies surviving I-SceI induction in the ligD-(D136A-D138A) strain. Only 1 out of 40 junctions was found to have a short deletion and cross-break priming (see Fig. S2B in the supplemental material). The majority of the junctions (24/40) could not be amplified, suggesting longer deletions, and the remaining 15 events showed intact I-SceI sites, indicating I-SceI inactivation. These results confirm that LigD-POL is required for efficient NHEJ of incompatible I-SceI-generated breaks.
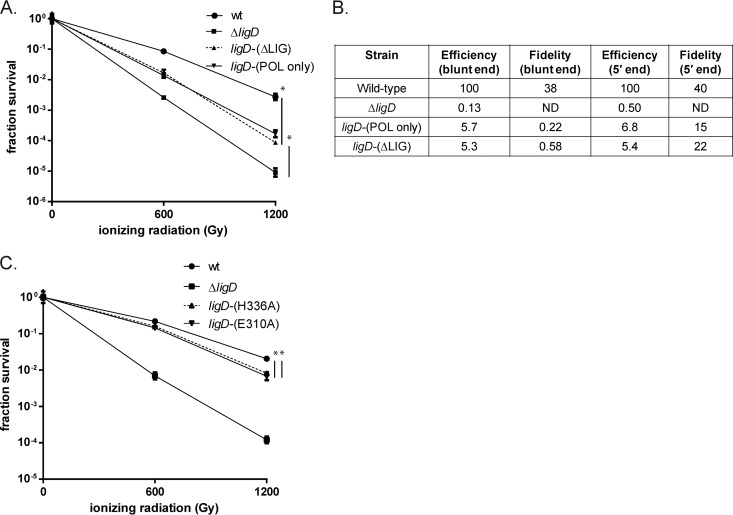
The ability of the LigD-K484A strain but not the ΔligD or ΔPOL strains to support NHEJ implies that the POL domain is necessary for the NHEJ complex to function. To define whether the POL domain is sufficient to support NHEJ-mediated repair of chromosomal breaks independent of the PE and LIG domains, we created a strain that expresses only LigD-POL but lacks both PE and LIG [LigD-(POL only)] and tested its NHEJ proficiency. The ligD-(POL only) strain was 0.8 and 1.2 logs more sensitive than the wild type at 600 and 1,200 Gy, a phenotype that recapitulates the ligD-(ΔLIG) phenotype (Fig. 3A). We next tested the ability of the POL domain to support NHEJ-mediated plasmid recircularization. NHEJ efficiencies of blunt- and 5′-end linear plasmids were 5.7 and 6.8% of that of the wild type in the ligD-(POL only) strain, similar to the efficiency observed for the ligD-ΔLIG strain (Fig. 3B). The NHEJ pathway supported by the POL domain was a low-fidelity repair pathway, with fidelity values similar to those previously reported for the backup NHEJ pathway that operates independently of LigD ligase activity (9, 10) (Fig. 3B). These data demonstrate that the POL domain is sufficient to support the LigD-LIG-independent NHEJ pathway.
FIG 3.
Role of the PE and POL domains in NHEJ. (A) Wild-type (wt), ligD-(POL only) (MGM 812), ligD-(ΔLIG) (MGM 805), and ΔligD (MGM 140) strains were treated with IR in late stationary phase, and fraction survival for the strains is plotted. Statistical significance between strains is indicated by a vertical line, with “*” indicating P < 0.05. (B) Fidelity and efficiency of plasmid NHEJ for blunt and 5′ ends in the wt, ΔligD, ligD-(POL only), and ligD-(ΔLIG) strains. (C) Strains carrying inactivating mutations in the PE domain, ligD-(E310A) (MGM 806) and ligD-(H336A) (MGM 807), were subjected to ionizing radiation at 600 Gy and 1,200 Gy. Fraction survival was plotted alongside that of the wild-type and ΔligD controls.
Role of LigD-PE domain in NHEJ-mediated repair of chromosomal damage.
Our prior plasmid assays failed to reveal a role for the PE domain in plasmid NHEJ (10). To determine the role of the LigD-PE domain in the repair of chromosomal damage, we studied M. smegmatis carrying LigD with inactivating mutations in the LigD-PE domain, LigD-E310A and -H336A. The E310A substitution abolishes only phosphomonoesterase activity, whereas the H336A substitution abolishes both phosphomonoesterase and phosphodiesterase activities (19, 23, 24). Stationary-phase IR experiments indicated that PE inactivation through either mutation conferred a small but statistically significant sensitization to IR of approximately 5-fold at 1,200 Gy (Fig. 3C). These data demonstrate an in vivo function for the PE domain in repair of chromosomal DNA damage.
LigC1 is the ligase of the LigD-independent backup NHEJ pathway.
Our prior studies have shown that the residual plasmid NHEJ observed in the ligD-(K484A) strain is abolished by deletion of the three additional mycobacterial ATP-dependent ligases in combination (encoded by ligC1, ligC2, and ligB) (10). This result indicates that one or more of the three ATP-dependent ligases, LigC1, LigC2, or LigB, can sustain a backup pathway of NHEJ when the LigD ligase domain is inactivated. To further define which ligase supports the backup NHEJ pathway, we complemented M. smegmatis ligD-(K484A) ΔligC1-ligC2 ΔligB individually with ligC1, ligC2, or ligB under the control of their native promoters and measured NHEJ by plasmid recircularization. As previously reported, NHEJ in the ligD-(K484A) strain is efficient but low fidelity. NHEJ efficiency is 38 and 59% for blunt-end and 5′-end NHEJ, respectively, compared to that of the wild type (Table 1). The fidelity was 0.25% for blunt-end ligation and 5.5% for 5′-end ligation. NHEJ efficiency dropped to 0.08 and 0.13 when ligC1, ligC2, and ligB were deleted in the ligD-(K484A) strain, as previously reported (Table 1). Complementation by ligC1 restored NHEJ efficiency to the level of the ligD-(K484A) strain, indicating that LigC1 provides the backup ligation activity when LigD-LIG is inactivated (Table 1). This LigC1-mediated NHEJ pathway also was low fidelity, with fidelity of 0.31% (blunt ends) or 4.2% (5′ end), similar to that observed in the ligD-(K484A) strain. In contrast, complementation with ligC2 or ligB had no measurable effect on the efficiency of NHEJ in the ligD-(K484A) ΔligC1-ligC2 ΔligB strain (Table 1).
TABLE 1.
Plasmid assay with 5′ overhang and blunt ends for the wild-type strain and variantsa
| Genotype | NHEJ analysisb |
|||
|---|---|---|---|---|
| Blunt end |
5′ overhang end |
|||
| % efficiency | % fidelity | % efficiency | % fidelity | |
| Wild type | 100 | 52 | 100 | 28 |
| ligD-(K484A) | 38 | 0.25 | 59 | 5.5 |
| ligD-(K484A) ΔligB-ligC | 0.08 | ND | 0.13 | ND |
| ligD-(K484A) ΔligB-ligC attB::ligC1 | 23.3 | 0.31 | 22 | 4.2 |
| ligD-(K484A) ΔligB-ligC attB::ligC2 | 0.04 | ND | 0.03 | ND |
| ligD-(K484A) ΔligB-ligC attB::ligB | 0.08 | ND | 0.06 | ND |
Wild-type, ligD-(K484A) (MGM 835h), ligD-(K484A) ΔligC1-ligC2 ΔligB (MGM 832h), ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC1 (MGM 839h), ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC2 (MGM 840h), and ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligB (MGM 833h) strains were analyzed.
Efficiency and fidelity of recircularization of linear plasmids with 5′ overhang ends and blunt ends were calculated as described in Materials and Methods. ND, not determined.
To determine whether the molecular outcomes in the ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC1 strain (where nat_ligC1 indicates the native promoter for ligC1) are similar to those observed in the ligD-(K484A) strain, we determined the nucleotide sequences of religated plasmids from unfaithful (white) NHEJ events in these two strains. Eight out of nine junctions in the ligD-(K484A) strain and 10 out of 12 junctions in the ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC1 strain showed deletions at the blunt end (see Fig. S3 in the supplemental material). Only two events from each strain had nontemplated additions (see Fig. S3). Sequence analysis of 5′-end junctions revealed that all the unfaithful NHEJ events from the ligD-(K484A) and ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC1 strains comprised deletions at the junction (see Fig. S4). Only 2 out of 8 repair events in the ligD-(K484A) strain show templated addition, whereas 6 out of 9 ligD-(K484A) ΔligC1-ligC2 ΔligB attB::nat_ligC1 clones show templated additions (see Fig. S4). These results demonstrate that LigC1 is the only ATP-dependent ligase capable of compensating for the loss of LigD ligase activity and that the LigC1-mediated backup NHEJ pathway is competent for plasmid NHEJ, albeit at low fidelity.
LigC1 can substitute for LigD-LIG in chromosomal repair.
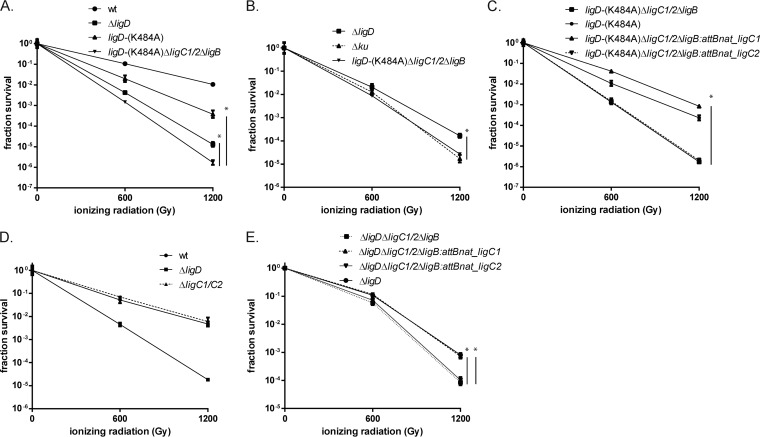
To assess the role of the LigC1-mediated NHEJ pathway in the repair of chromosomal damage, the ligD-(K484A) ΔligC1-ligC2 ΔligB strain was subjected to IR in late stationary phase along with the wild-type, ΔligD, and ligD-(K484A) strains. The ligD-(K484A) ΔligC1-ligC2 ΔligB strain was not only more sensitive than the ligD-(K484A) strain but was also more sensitive than the ΔligD strain (Fig. 4A). The late-stationary-phase IR sensitivity of the ligD-(K484A) ΔligC1-ligC2 ΔligB strain was similar to that of the ΔKu strain (Fig. 4B). To test whether ligC1 or ligC2 is sufficient to support chromosomal repair, we tested the ligD-(K484A) ΔligC1-ligC2 ΔligB strain with either ligC1 or ligC2 and observed that ligC1, but not ligC2, restored IR resistance to the level of that of the ligD-(K484A) strain (Fig. 4C). To test whether ligC1 or ligC2 contributes to chromosomal repair in a cell with wild-type LigD, we tested the ΔligC1-ligC2 strain and observed no sensitization (Fig. 4D), confirming that LigC functions only in a backup role when LigD ligase is nonfunctional.
FIG 4.
LigC1 is the ligase of the LigD-independent NHEJ pathway. Late-stationary-phase IR sensitivity of the wild-type, ΔligD (MGM 140), ligD-(K484A) ΔligC1-ligC2 ΔligB (MGM 810), and ligD-(K484A) (MGM 803) strains (A), Δku (MGM 154), ΔligD, and ligD-(K484A) ΔligC1-ligC2 ΔligB strains (B), ligD-(K484A) (MGM 835h) and ligD-(K484A) ΔligC1-ligC2 ΔligB (MGM 832h) strains, the ligD-(K484A) ΔligC1-ligC2 ΔligB strain complemented with ligC1 (MGM 839h), or the ligC2 (MGM 840h) strain (C), wild-type, ΔligD, and ΔligC1-ligC2 (MGM 139) strains (D), or the ΔligD (MGM 858h) or ΔligD ΔligC ΔligB (MGM 859h) strain, the ΔligD ΔligC ΔligB strain complemented with ligC1 (MGM 860h), or the ligC2 (MGM 861h) strain (E) is shown. Statistical significance between strains is indicated by a vertical line, with “*” indicating P < 0.05.
The experiments above indicate that LigC1 can compensate for the loss of the LigD ligase when the LigD protein is still present. To test whether ligC1 or ligC2 has a function in repair when LigD is completely absent, we tested the ΔligD ΔligC1-ligC2 ΔligB, ΔligD ΔligC1-ligC2 ΔligB attB::nat_ligC1, ΔligD ΔligC ΔligB::nat_ligC2, and ΔligD strains. The ΔligD ΔligC1-ligC2 ΔligB strain was 0.3 and 1.0 logs more sensitive than the ΔligD strain at 600- and 1,200-Gy dosages of IR, indicating a role for ligB or ligC in late-stationary-phase IR resistance in the absence of LigD. Complementation with ligC1 restored sensitivity of the ΔligD ΔligC1-ligC2 ΔligB strain to ΔligD strain levels, but complementation with ligC2 did not, indicating that LigC1 mediates clastogen resistance when LigD is absent (Fig. 4E). Taken together, these results indicate that LigC1 functions in chromosomal repair and can function in cells lacking the entire LigD protein.
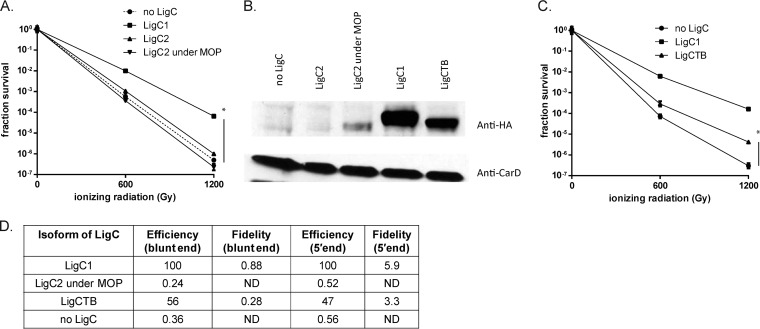
The differential ability of ligC1 and ligC2 to support NHEJ is surprising insofar as the LigC1 and LigC2 proteins are highly homologous. There is 49% amino acid sequence identity between LigC1 and LigC2 (see Fig. S5 in the supplemental material). There is only one LigC protein in M. tuberculosis, encoded by the rv3731 gene. M. tuberculosis LigC is more homologous to LigC2 and shows 73% sequence identity to LigC2 versus 49% identity to LigC1 (see Fig. S5). To investigate whether the failure of LigC2 to support NHEJ is due to differences in expression, we generated ligC1 and ligC2 complementing alleles encoding hemagglutinin epitope-tagged proteins at their C termini. We also placed the expression of ligC2 under control of the strong constitutive MOP. LigC1-HA complemented the IR sensitivity phenotype of the ligD-(K484A) ΔligC1-ligC2 ΔligB strain, but neither native LigC2-HA nor MOP-LigC2-HA was able to restore IR resistance (Fig. 5A). We compared the levels of LigC1-HA and LigC2-HA by immunoblotting and found no detectable LigC2 when expressed from its native promoter, whereas LigC1-HA was easily detectable (Fig. 5B). MOP-LigC2 was detectable but at a lower level than the LigC1 protein (Fig. 5B, lane 3).
FIG 5.
M. tuberculosis LigC can support LigD-independent NHEJ. (A and C) Late-stationary-phase IR sensitivities of the ligD-(K484A) ΔligC1-ligC2 ΔligB strain complemented with vector (no LigC) (MGM 832h), M. smegmatis LigC1-HA (MGM 865h), M. smegmatis LigC2-HA (MGM 863h), M. smegmatis MOP-LigC2-HA (MGM 864h), or M. tuberculosis LigC-HA (MGM 866h) strains. Statistical significance between strains is indicated by a vertical line, with “*” indicating P < 0.05. (B) Relative expression of the indicated HA-tagged LigC proteins was assessed using anti-HA antibodies and anti-CarD antibody as loading control. (D) Plasmid assay of backup NHEJ in the same strains used in panels A to C, with LigC1 set to 100%.
LigC from M. tuberculosis can substitute for LigD in NHEJ repair.
M. tuberculosis has only one LigC protein, which is more similar to M. smegmatis LigC2 than LigC1 (see Fig. S5 in the supplemental material), possibly suggesting that M. tuberculosis LigC does not substitute for LigC1 in the NHEJ pathway. To test this idea, we complemented the ligD-(K484A) ΔligC1-ligC2 ΔligB strain with M. tuberculosis LigC-HA expressed from the M. smegmatis LigC1 promoter. LigCTB expressed from the ligC1 promoter was able to partially restore resistance to IR in late stationary phase (Fig. 5C), although not to the level achieved by LigC1 complementation (Fig. 5C). We next tested whether LigC from M. tuberculosis could support the backup pathway of plasmid NHEJ. LigC2-HA expressed under a MOP did not support plasmid NHEJ via the backup pathway (Fig. 5D). However, LigCTB-HA expressed from the ligC1 promoter was able to restore NHEJ almost to the level of that supported by LigC1 (56% and 47% blunt-end and 5′-end ligation efficiencies [Fig. 5D]). The blunt-end and 5′-end fidelity values were 0.28 and 3.3%, compared to 0.88 and 5.9% for the LigC1-HA-complemented strain (Fig. 5D). LigCTB-HA was expressed at levels higher than LigC2 under a MOP but not as well as LigC1-HA (Fig. 5B, lane 5). RT-qPCR experiments confirmed that all three LigC genes (ligC1, ligC2, and M. tuberculosis ligC) are expressed, indicating that the lack of the LigC2 protein is due to posttranscriptional or posttranslational regulation (see Fig. S6A, B, and C in the supplemental material). These results demonstrate that M. tuberculosis LigC is capable of supporting the mycobacterial LigD-independent NHEJ pathway. We cannot determine with certainty whether the noncomplementation by LigC2 is due to poor expression or is an intrinsic property of the LigC2 ligase, but the similarity of M. tuberculosis LigC to LigC2 and the higher expression of M. tuberculosis LigC suggest that the low expression level of the LigC2 protein may be responsible for its inactivity in vivo.
A role for PolD1 and PolD2 in chromosomal repair.
LigC1 is encoded in an apparent operon with polD1, encoding a freestanding LigD-POL-like polymerase. We have previously characterized the PolD1 and PolD2 proteins and demonstrated that their biochemical activities are similar to those of LigD-POL (14), but deletion of PolD1 and PolD2 in combination did not affect plasmid NHEJ (14). However, given our findings that M. smegmatis ligD-(D136A-D138A) is highly sensitized to IR, we readdressed the possibility that PolD1 and PolD2 participate in NHEJ-mediated repair of chromosomal breaks. The ΔpolD1 strain showed no more IR sensitivity than the wild type (Fig. 6A). We next tested the ligD-(D136A-D138A) ΔpolD1 ΔpolD2 strain alongside the ligD-(D136A-D138A) and Δku strains and observed that the ligD-(D136A-D138A) ΔpolD1 ΔpolD2 strain was 0.3 and 0.8 logs more sensitive than the ligD-(D136A-D138A) strain at 600 and 1,200 Gy (P < 0.05) and phenocopied the Δku strain (Fig. 6B). PolD1 did not contribute to NHEJ in the LigD-K484A strain, in which the backup NHEJ pathway is supporting NHEJ (Fig. 6C and D).
FIG 6.
Role of PolD1 and PolD2 in NHEJ. Late-stationary-phase IR sensitivity of wild-type, ΔligD, and ΔpolD1 (MGM 843) strains. (B to D) ligD-(D136A-D138A) (MGM 801), ligD-(D136A-D138A) ΔpolD1 ΔpolD2 (MGM 846), and Δku (MGM 154) strains (B), ligD-(K484A) (MGM 803), ligD-(K484A) ΔpolD1 (MGM 844h), and Δku (MGM 154) strains (C), or plasmid NHEJ assay with 5′-overhang and blunt-end plasmids (D). Statistical significance between strains is indicated, with “*” indicating P < 0.05.
Relative levels of ku, ligD, ligC1, and ligC2 mRNA in mid-log and late stationary phases.
Since NHEJ is required for DSB repair in late stationary phase, we hypothesized that the critical mediators of the NHEJ pathway may be upregulated during this growth phase. We used RT-qPCR to measure the mRNA levels of ku, ligD, ligC1, and ligC2 (all normalized to sigA) in mid-log- and late-stationary-phase M. smegmatis. The mRNA encoding Ku was upregulated 8-fold in stationary phase compared to levels in log phase, whereas ligC1 was unchanged and ligD and ligC2 were upregulated 2-fold (see Fig. S7A to D in the supplemental material). We confirmed the upregulation of the ku promoter using a ku promoter-lacZ fusion. ku promoter activity was substantially upregulated when the cells were left in late stationary phase for more than 12 h (see Fig. S7E).
DISCUSSION
A LigD-independent pathway of NHEJ in mycobacteria.
Our studies further define an NHEJ mechanism in M. smegmatis that operates independently of the LigD ligase. Our prior studies indicated that the efficient NHEJ observed in the absence of LigD ligase was mediated by one of the three additional ATP-dependent DNA ligases in the M. smegmatis proteome. In this work, we identified LigC1 as the additional NHEJ ligase. Importantly, the LigC1 pathway also requires the LigD-POL domain, which can support NHEJ as an isolated domain independent of LIG or PE, and Ku. The model that emerges from these data is that Ku, LigD-POL, and LigC1 are capable of forming a functional NHEJ complex. Ku interacts directly with LigD-POL (25), and this interaction has been hypothesized to recruit LigD to the broken ends. Structural data also implicate LigD-POL as a synaptic factor that can bridge broken DNA ends, further supporting its central role in nucleating the NHEJ complex (26). Our data would suggest that LigC1 can interact directly with Ku or LigD-POL, although our attempts to identify LigC interactors have not identified either of these two proteins (H. Bhattarai and M. S. Glickman, unpublished data).
Substantial biochemical data complement our genetic findings implicating LigC in NHEJ. In contrast to LigD proteins, LigC proteins are minimized DNA ligases without the additional domains present in LigD. M. smegmatis LigC1 is a poor nick-sealing enzyme in vitro (12). However, the ligation activity of LigC from Agrobacterium is stimulated by Ku, as is that of LigD (27), suggesting that LigC and Ku form a physical complex which is functionally important. Agrobacterium LigC and LigD are both stimulated by the presence of a single ribonucleotide at the break, strongly suggesting that the ribonucleotides added by LigD-POL and trimmed by PE stimulate ligation (20). However, as mentioned above, the LigC protein does not contain POL or PE domains, and therefore its stimulation by a ribonucleotide rationalizes the functional interaction with LigD-POL documented here.
Requirement for PE and POL in chromosomal repair.
Our data also identify important functions for the LigD polymerase and PE domains in chromosomal repair. Our prior efforts to document a function for these LigD enzymatic modules in NHEJ had not revealed a role for the PE domain. This lack of phenotype was likely due to the irrelevance of a 3′-end processing activity for a restriction endonuclease-generated 5′ overhang or blunt end. The PE domain acts on 3′-phosphate-terminated ends to regenerate a 3′ hydroxyl which can be ligated by ligase or further extended by polymerase. This activity is stimulated by a 5′ single-stranded tail, suggesting that PE acts at recessed 3′ ends (18). PE activity is thus likely to be important during NHEJ-mediated repair of clastogen-induced DSBs that may generate 3′ phosphate but would be irrelevant for restriction endonuclease-generated ends, which contain 5′ phosphates. Inactivation of the PE domain impaired the survival of M. smegmatis under late-stationary-phase IR exposure, although the degree of sensitization was less dramatic (5-fold) than loss of LigD or the POL domain (see below). The mild phenotype of PE inactivation is logical insofar as only a minority of IR-induced breaks will have recessed 3′ phosphates. Approximately half of the 3′ termini generated by IR treatment of DNA in vitro have 3′ phosphates (28), confirming that only a subset of the IR-induced breaks would require PE for processing.
The data presented here also strongly revise our view of the importance of the POL domain in NHEJ. In prior experiments, inactivation of the POL domain did change the fidelity of plasmid NHEJ but had minimal effects on NHEJ efficiency. In contrast, LigD-POL activity is absolutely required for IR resistance in late stationary phase, such that the ligD-(D136A-D138A) strain is as sensitive as the ΔligD strain. This observation suggests that all LigD-mediated repair of IR-induced chromosomal damage requires polymerization by LigD-POL, which is consistent with the complexity of the damage induced by this clastogen. Our experiments also indicate a role for the PolD enzymes in IR resistance when LigD-POL is inactivated, confirming that these enzymes participate in DSB repair. The LigD-POL-like enzymes PolD1 and PolD2, are biochemically similar to LigD-POL (14) in their ability to add templated and nontemplated nucleotides and their preference for ribonucleotides over dNTPs. Our experiments indicate substantial redundancy between LigD-POL and PolD1/PolD2, such that the combined loss of PolD1/PolD2 impairs NHEJ only when LigD-POL is inactive. The central role of LigD-POL in NHEJ-mediated repair of IR-induced chromosomal damage also suggests that ribonucleotide incorporation into the chromosome is a frequent event during NHEJ-mediated repair. Ribonucleotides are more abundant than dNTPs even in log-phase cells, and recent literature indicates that replicative polymerases frequently incorporate rNTPs into chromosomal DNA, the removal of which by RNase H2 is required to maintain genomic integrity (3, 4, 29–31). Our data suggest that such rNTP incorporation may also be a frequent event during mycobacterial NHEJ and suggest that a system to remove embedded ribonucleotides may cooperate with the NHEJ system.
Comparison to ligase IV-independent NHEJ in eukaryotes.
Our finding that an alternative DNA ligase, LigC, can substitute for LigD is conceptually reminiscent of the situation in yeast and mammalian NHEJ. The primary NHEJ ligase in eukaryotic cells is DNA ligase IV (32–34). However, cells lacking LigIV can still execute end joining, including class switch recombination in B cells (35–37). Similarly, yeast carrying an inactivating lysine mutation in LigIV exhibits a backup NHEJ pathway which may be mediated by DNA ligase 1 (38). This LigIV-independent pathway, variously termed alt-NHEJ, nc-NHEJ, or backup NHEJ (b-NHEJ) (1), is characterized by large deletions and frequent use of microhomology at the junctions. The DNA ligase that supports the alternative NHEJ pathway of mammalian cells is not completely clear but likely is a combination of DNA ligase III and DNA ligase I (39–41). The LigD-independent NHEJ pathway examined here is similar in its use of an alternative DNA ligase and its low fidelity with frequent deletions. However, LigD-independent NHEJ in mycobacteria is dependent on Ku and also requires the POL domain of LigD and is therefore apparently distinct, in both its genetic requirements and overall efficiency of repair, from the Ku- and LigD-independent NHEJ of plasmid substrates we have previously described (10). It remains unclear, both in the mycobacterial system demonstrated here and in eukaryotic NHEJ systems, whether the ability of alternative DNA ligases to support NHEJ in the absence of the primary NHEJ ligase represents a true alternative “pathway” that operates in wild-type cells or a “substitution” of a ligation activity supplied by an alternative ligase (42). Regardless of the nomenclature chosen, our results indicate that LigC1 can substantially replace LigD ligase in cells that retain both the LigD-POL domain and Ku.
In summary, we have further elucidated the genetic requirements for NHEJ in mycobacteria. We find that all enzymatic activities of the LigD protein are required for NHEJ. We also find that there is a fully competent NHEJ mechanism that is active in cells lacking LigD-LIG and that this pathway requires LigC1, Ku, and the POL domain of LigD.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant AI64693 (to M.S.G.) and P30 CA 008748.
Footnotes
Published ahead of print 23 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01832-14.
REFERENCES
- 1.Lieber MR. 2010. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79:181–211. 10.1146/annurev.biochem.052308.093131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nick McElhinny SA, Kumar D, Clark AB, Watt DL, Watts BE, Lundstrom EB, Johansson E, Chabes A, Kunkel TA. 2010. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 6:774–781. 10.1038/nchembio.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JS, Smith DJ, Marjavaara L, Lujan SA, Chabes A, Kunkel TA. 2013. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol. Cell 49:1010–1015. 10.1016/j.molcel.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazzaro F, Novarina D, Amara F, Watt DL, Stone JE, Costanzo V, Burgers PM, Kunkel TA, Plevani P, Muzi-Falconi M. 2012. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol. Cell 45:99–110. 10.1016/j.molcel.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reijns MA, Rabe B, Rigby RE, Mill P, Astell KR, Lettice LA, Boyle S, Leitch A, Keighren M, Kilanowski F, Devenney PS, Sexton D, Grimes G, Holt IJ, Hill RE, Taylor MS, Lawson KA, Dorin JR, Jackson AP. 2012. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149:1008–1022. 10.1016/j.cell.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Barkan D, Redelman-Sidi G, Shuman S, Glickman MS. 2011. Mycobacteria exploit three genetically distinct DNA double-strand break repair pathways. Mol. Microbiol. 79:316–330. 10.1111/j.1365-2958.2010.07463.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha KM, Unciuleac M-CC, Glickman MS, Shuman S. 2009. AdnAB: a new DSB-resecting motor-nuclease from mycobacteria. Genes Dev. 23:1423–1437. 10.1101/gad.1805709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta R, Ryzhikov M, Koroleva O, Unciuleac M, Shuman S, Korolev S, Glickman MS. 2013. A dual role for mycobacterial RecO in RecA-dependent homologous recombination and RecA-independent single-strand annealing. Nucleic Acids Res. 41:2284–2295. 10.1093/nar/gks1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akey D, Martins A, Aniukwu J, Glickman MS, Shuman S, Berger JM. 2006. Crystal structure and nonhomologous end-joining function of the ligase component of Mycobacterium DNA ligase D. J. Biol. Chem. 281:13412–13423. 10.1074/jbc.M513550200 [DOI] [PubMed] [Google Scholar]
- 10.Aniukwu J, Glickman MS, Shuman S. 2008. The pathways and outcomes of mycobacterial NHEJ depend on the structure of the broken DNA ends. Genes Dev. 22:512–527. 10.1101/gad.1631908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong C, Bongiorno P, Martins A, Stephanou NC, Zhu H, Shuman S, Glickman MS. 2005. Mechanism of nonhomologous end-joining in mycobacteria: a low-fidelity repair system driven by Ku, ligase D and ligase C. Nat. Struct. Mol. Biol. 12:304–312. 10.1038/nsmb915 [DOI] [PubMed] [Google Scholar]
- 12.Gong C, Martins A, Bongiorno P, Glickman M, Shuman S. 2004. Biochemical and genetic analysis of the four DNA ligases of mycobacteria. J. Biol. Chem. 279:20594–20606. 10.1074/jbc.M401841200 [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Nandakumar J, Aniukwu J, Wang LK, Glickman MS, Lima CD, Shuman S. 2006. Atomic structure and nonhomologous end-joining function of the polymerase component of bacterial DNA ligase D. Proc. Natl. Acad. Sci. U. S. A. 103:1711–1716. 10.1073/pnas.0509083103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Bhattarai H, Yan HG, Shuman S, Glickman MS. 2012. Characterization of Mycobacterium smegmatis PolD2 and PolD1 as RNA/DNA polymerases homologous to the POL domain of bacterial DNA ligase D. Biochemistry 51:10147–10158. 10.1021/bi301202e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitcher RS, Green AJ, Brzostek A, Korycka-Machala M, Dziadek J, Doherty AJ. 2007. NHEJ protects mycobacteria in stationary phase against the harmful effects of desiccation. DNA Repair 6:1271–1276. 10.1016/j.dnarep.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 16.Stephanou NC, Gao F, Bongiorno P, Ehrt S, Schnappinger D, Shuman S, Glickman MS. 2007. Mycobacterial nonhomologous end joining mediates mutagenic repair of chromosomal double-strand DNA breaks. J. Bacteriol. 189:5237–5246. 10.1128/JB.00332-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright D, DeBeaux A, Shi R, Doherty AJ, Harrison L. 2010. Characterization of the roles of the catalytic domains of Mycobacterium tuberculosis ligase D in Ku-dependent error-prone DNA end joining. Mutagenesis 25:473–481. 10.1093/mutage/geq029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H, Shuman S. 2006. Substrate specificity and structure-function analysis of the 3′-phosphoesterase component of the bacterial NHEJ protein, DNA ligase D. J. Biol. Chem. 281:13873–13881. 10.1074/jbc.M600055200 [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Shuman S. 2005. Novel 3′-ribonuclease and 3′-phosphatase activities of the bacterial non-homologous end-joining protein, DNA ligase D. J. Biol. Chem. 280:25973–25981. 10.1074/jbc.M504002200 [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Shuman S. 2008. Bacterial nonhomologous end joining ligases preferentially seal breaks with a 3′-OH monoribonucleotide. J. Biol. Chem. 283:8331–8339. 10.1074/jbc.M705476200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timm J, Lim EM, Gicquel B. 1994. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J. Bacteriol. 176:6749–6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitcher RS, Brissett NC, Picher AJ, Andrade P, Juarez R, Thompson D, Fox GC, Blanco L, Doherty AJ. 2007. Structure and function of a mycobacterial NHEJ DNA repair polymerase. J. Mol. Biol. 366:391–405. 10.1016/j.jmb.2006.10.046 [DOI] [PubMed] [Google Scholar]
- 23.Nair PA, Smith P, Shuman S. 2010. Structure of bacterial LigD 3′-phosphoesterase unveils a DNA repair superfamily. Proc. Natl. Acad. Sci. U. S. A. 107:12822–12827. 10.1073/pnas.1005830107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Wang LK, Shuman S. 2005. Essential constituents of the 3′-phosphoesterase domain of bacterial DNA ligase D, a nonhomologous end-joining enzyme. J. Biol. Chem. 280:33707–33715. 10.1074/jbc.M506838200 [DOI] [PubMed] [Google Scholar]
- 25.Pitcher RS, Tonkin LM, Green AJ, Doherty AJ. 2005. Domain structure of a NHEJ DNA repair ligase from Mycobacterium tuberculosis. J. Mol. Biol. 351:531–544. 10.1016/j.jmb.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 26.Brissett NC, Pitcher RS, Juarez R, Picher AJ, Green AJ, Dafforn TR, Fox GC, Blanco L, Doherty AJ. 2007. Structure of a NHEJ polymerase-mediated DNA synaptic complex. Science 318:456–459. 10.1126/science.1145112 [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Shuman S. 2007. Characterization of Agrobacterium tumefaciens DNA ligases C and D. Nucleic Acids Res. 35:3631–3645. 10.1093/nar/gkm145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henner WD, Grunberg SM, Haseltine WA. 1982. Sites and structure of gamma radiation-induced DNA strand breaks. J. Biol. Chem. 257:11750–11754 [PubMed] [Google Scholar]
- 29.Nick McElhinny SA, Watts BE, Kumar D, Watt DL, Lundstrom EB, Burgers PM, Johansson E, Chabes A, Kunkel TA. 2010. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl. Acad. Sci. U. S. A. 107:4949–4954. 10.1073/pnas.0914857107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparks JL, Chon H, Cerritelli SM, Kunkel TA, Johansson E, Crouch RJ, Burgers PM. 2012. RNase H2-initiated ribonucleotide excision repair. Mol. Cell 47:980–986. 10.1016/j.molcel.2012.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiller B, Achleitner M, Glage S, Naumann R, Behrendt R, Roers A. 2012. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 209:1419–1426. 10.1084/jem.20120876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo SH, Jackson SP. 1997. Identification of Saccharomyces cerevisiae DNA ligase IV: involvement in DNA double-strand break repair. EMBO J. 16:4788–4795. 10.1093/emboj/16.15.4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson TE, Grawunder U, Lieber MR. 1997. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature 388:495–498. 10.1038/41365 [DOI] [PubMed] [Google Scholar]
- 34.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. 1998. DNA ligase IV is essential for V(D)J. recombination and DNA double-strand break repair in human precursor lymphocytes. Mol. Cell 2:477–484. 10.1016/S1097-2765(00)80147-1 [DOI] [PubMed] [Google Scholar]
- 35.Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, Rajewsky K, Alt FW. 2007. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 449:478–482. 10.1038/nature06020 [DOI] [PubMed] [Google Scholar]
- 36.Soulas-Sprauel P, Le Guyader G, Rivera-Munoz P, Abramowski V, Olivier-Martin C, Goujet-Zalc C, Charneau P, de Villartay J-P 2007. Role for DNA repair factor XRCC4 in immunoglobulin class switch recombination. J. Exp. Med. 204:1717–1727. 10.1084/jem.20070255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han L, Yu K. 2008. Altered kinetics of nonhomologous end joining and class switch recombination in ligase IV-deficient B cells. J. Exp. Med. 205:2745–2753. 10.1084/jem.20081623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiruvella KK, Liang Z, Birkeland SR, Basrur V, Wilson TE. 2013. Saccharomyces cerevisiae DNA ligase IV supports imprecise end joining independently of its catalytic activity. PLoS Genet. 9:e1003599. 10.1371/journal.pgen.1003599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simsek D, Brunet E, Wong SY-W, Katyal S, Gao Y, McKinnon PJ, Lou J, Zhang L, Li J, Rebar EJ, Gregory PD, Holmes MC, Jasin M. 2011. DNA ligase III promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS Genet. 7:e1002080. 10.1371/journal.pgen.1002080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul K, Wang M, Mladenov E, Bencsik-Theilen A, Bednar T, Wu W, Arakawa H, Iliakis G. 2013. DNA ligases I and III cooperate in alternative non-homologous end-joining in vertebrates. PLoS One 8:e59505. 10.1371/journal.pone.0059505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boboila C, Oksenych V, Gostissa M, Wang JH, Zha S, Zhang Y, Chai H, Lee C-S, Jankovic M, Saez L-MA, Nussenzweig MC, McKinnon PJ, Alt FW, Schwer B. 2012. Robust chromosomal DNA repair via alternative end-joining in the absence of X-ray repair cross-complementing protein 1 (XRCC1). Proc. Natl. Acad. Sci. U. S. A. 109:2473–2478. 10.1073/pnas.1121470109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pannunzio NR, Li S, Watanabe G, Lieber MR. 2014. Non-homologous end joining often uses microhomology: implications for alternative end joining. DNA Repair (Amst.) 17:74–80. 10.1016/j.dnarep.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.