Abstract
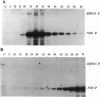
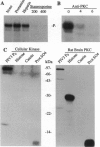
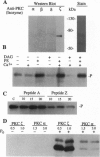
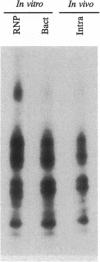
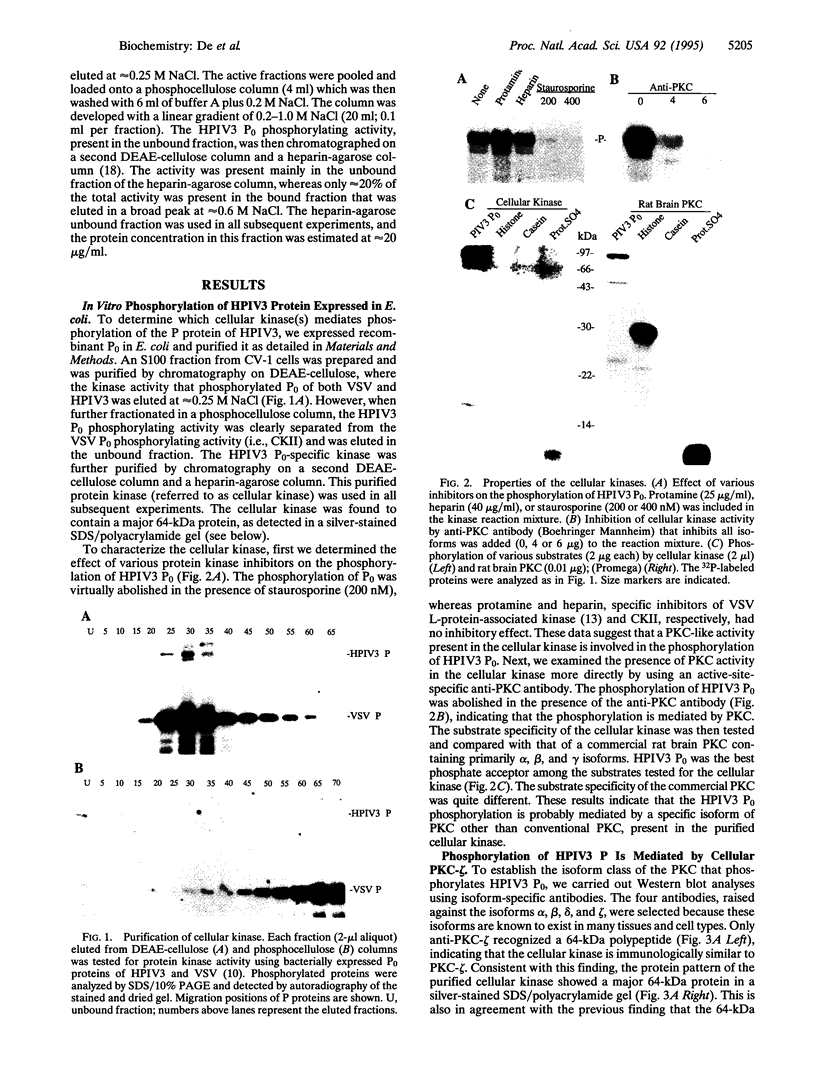
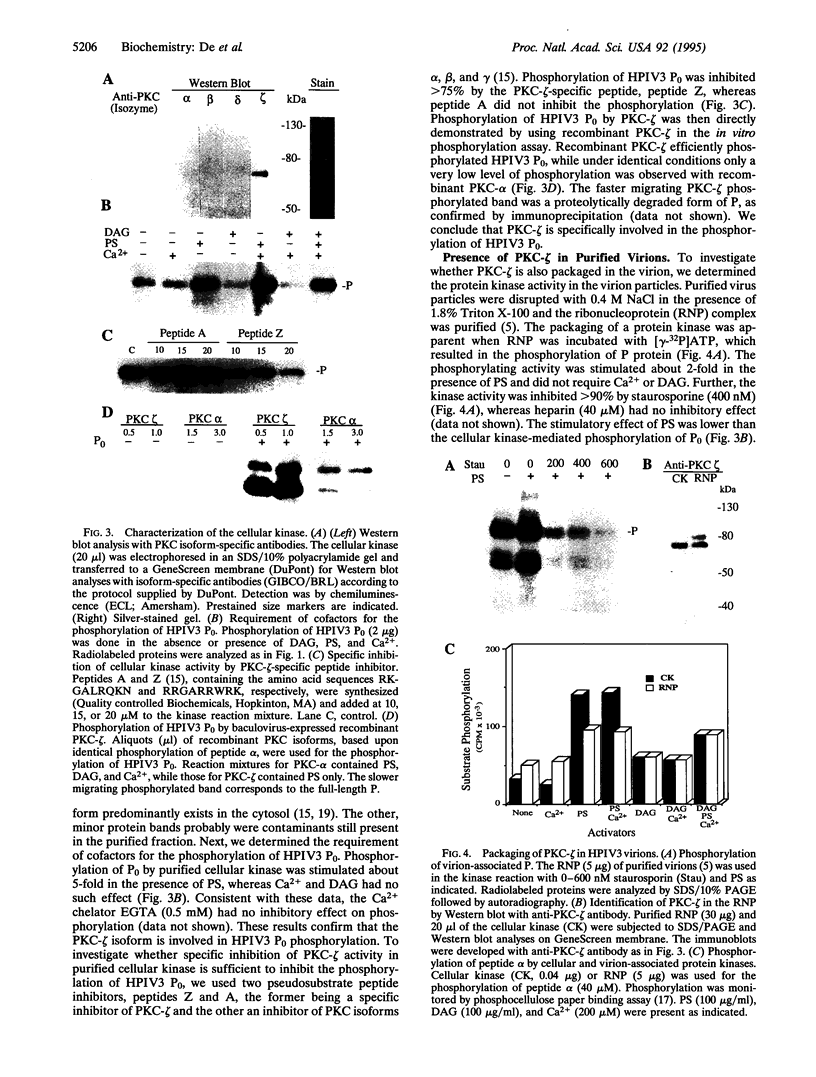
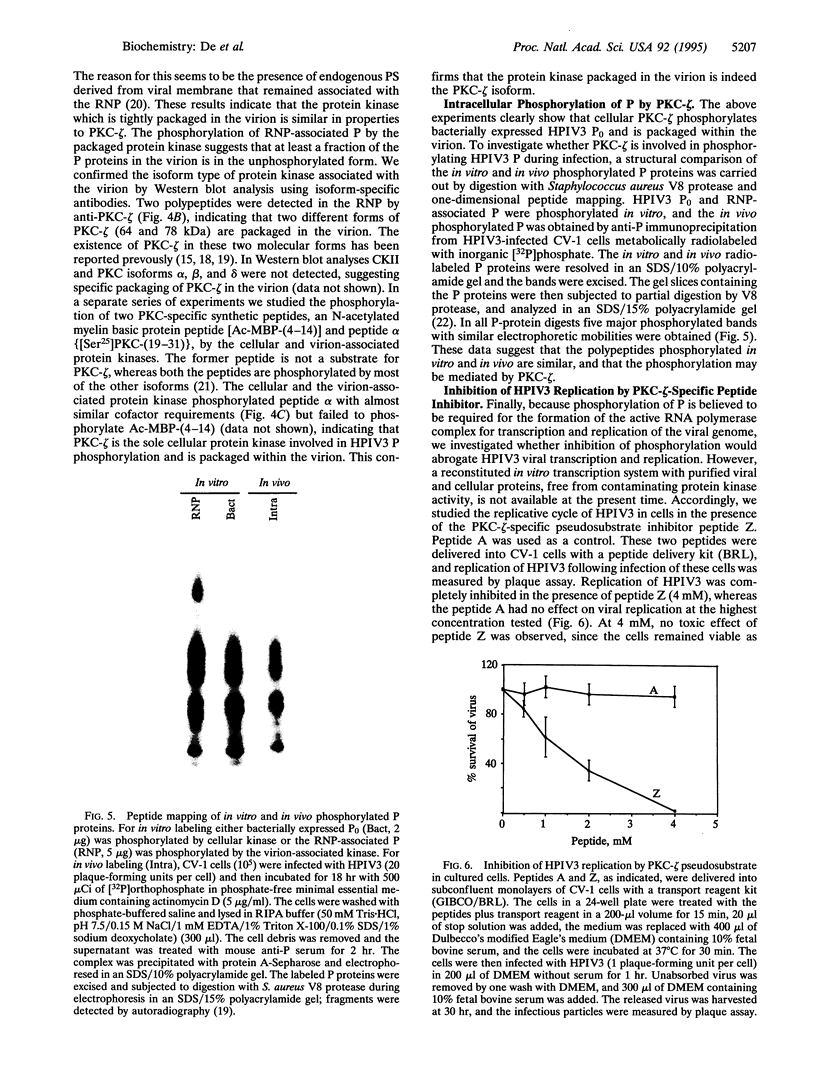
Phosphorylation of the P proteins of nonsegmented negative-strand RNA viruses is critical for their function as transactivators of the viral RNA polymerases. Using unphosphorylated P protein of human parainfluenza virus type 3 (HPIV3) expressed in Escherichia coli, we have shown that the cellular protein kinase that phosphorylates P in vitro is biochemically and immunologically indistinguishable from cellular protein kinase C isoform zeta (PKC-zeta). Further, PKC-zeta is specifically packaged within the progeny HPIV3 virions and remains tightly associated with the ribonucleoprotein complex. The P protein seems also to be phosphorylated intracellularly by PKC-zeta, as shown by the similar protease digestion pattern of the in vitro and in vivo phosphorylated P proteins. The growth of HPIV3 in CV-1 cells is completely abrogated when a PKC-zeta-specific inhibitor pseudosubstrate peptide was delivered into cells. These data indicate that PKC-zeta plays an important role in HPIV3 gene expression by phosphorylating P protein, thus providing an opportunity to develop antiviral agents against an important human pathogen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Barik S., De B. P. Gene expression of nonsegmented negative strand RNA viruses. Pharmacol Ther. 1991;51(1):47–70. doi: 10.1016/0163-7258(91)90041-j. [DOI] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Cloning and expression of the vesicular stomatitis virus phosphoprotein gene in Escherichia coli: analysis of phosphorylation status versus transcriptional activity. J Virol. 1991 Apr;65(4):1719–1726. doi: 10.1128/jvi.65.4.1719-1726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Sequential phosphorylation of the phosphoprotein of vesicular stomatitis virus by cellular and viral protein kinases is essential for transcription activation. J Virol. 1992 Feb;66(2):1109–1118. doi: 10.1128/jvi.66.2.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Casnellie J. E. Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Methods Enzymol. 1991;200:115–120. doi: 10.1016/0076-6879(91)00133-h. [DOI] [PubMed] [Google Scholar]
- Curran J., Marq J. B., Kolakofsky D. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology. 1992 Aug;189(2):647–656. doi: 10.1016/0042-6822(92)90588-g. [DOI] [PubMed] [Google Scholar]
- De B. P., Burdsall A. L., Banerjee A. K. Role of cellular actin in human parainfluenza virus type 3 genome transcription. J Biol Chem. 1993 Mar 15;268(8):5703–5710. [PubMed] [Google Scholar]
- De B. P., Lesoon A., Banerjee A. K. Human parainfluenza virus type 3 transcription in vitro: role of cellular actin in mRNA synthesis. J Virol. 1991 Jun;65(6):3268–3275. doi: 10.1128/jvi.65.6.3268-3275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Berra E., Municio M. M., Sanz L., Lozano J., Dominguez I., Diaz-Golpe V., Lain de Lera M. T., Alcamí J., Payá C. V. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993 Aug;13(8):4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I., Diaz-Meco M. T., Municio M. M., Berra E., García de Herreros A., Cornet M. E., Sanz L., Moscat J. Evidence for a role of protein kinase C zeta subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992 Sep;12(9):3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G. Peptide mapping in gels. Methods Enzymol. 1983;100:424–430. doi: 10.1016/0076-6879(83)00071-3. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Das T., Banerjee A. K. Casein kinase II is the P protein phosphorylating cellular kinase associated with the ribonucleoprotein complex of purified vesicular stomatitis virus. J Gen Virol. 1995 Feb;76(Pt 2):365–372. doi: 10.1099/0022-1317-76-2-365. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Yoshida T., Nishikawa K., Naruse H., Nagai Y. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex. Virology. 1983 Jul 15;128(1):105–117. doi: 10.1016/0042-6822(83)90322-7. [DOI] [PubMed] [Google Scholar]
- Kochs G., Hummel R., Meyer D., Hug H., Marmé D., Sarre T. F. Activation and substrate specificity of the human protein kinase C alpha and zeta isoenzymes. Eur J Biochem. 1993 Sep 1;216(2):597–606. doi: 10.1111/j.1432-1033.1993.tb18179.x. [DOI] [PubMed] [Google Scholar]
- Mazumder B., Barik S. Requirement of casein kinase II-mediated phosphorylation for the transcriptional activity of human respiratory syncytial viral phosphoprotein P: transdominant negative phenotype of phosphorylation-defective P mutants. Virology. 1994 Nov 15;205(1):104–111. doi: 10.1006/viro.1994.1624. [DOI] [PubMed] [Google Scholar]
- Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993 Mar 25;268(9):6090–6096. [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Pfeffer L. M. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Villanueva N., Navarro J., Méndez E., García-Albert I. Identification of a protein kinase involved in the phosphorylation of the C-terminal region of human respiratory syncytial virus P protein. J Gen Virol. 1994 Mar;75(Pt 3):555–565. doi: 10.1099/0022-1317-75-3-555. [DOI] [PubMed] [Google Scholar]