Abstract
Cyclin-dependent kinase 7 (CDK7) activates cell cycle CDKs and is a member of the general transcription factor TFIIH. Although there is substantial evidence for an active role of CDK7 in mRNA synthesis and associated processes, the degree of its influence on global and gene-specific transcription in mammalian species is unclear. In the current study, we utilize two novel inhibitors with high specificity for CDK7 to demonstrate a restricted but robust impact of CDK7 on gene transcription in vivo and in in vitro-reconstituted reactions. We distinguish between relative low- and high-dose responses and relate them to distinct molecular mechanisms and altered physiological responses. Low inhibitor doses cause rapid clearance of paused RNA polymerase II (RNAPII) molecules and sufficed to cause genome-wide alterations in gene expression, delays in cell cycle progression at both the G1/S and G2/M checkpoints, and diminished survival of human tumor cells. Higher doses and prolonged inhibition led to strong reductions in RNAPII carboxyl-terminal domain (CTD) phosphorylation, eventual activation of the p53 program, and increased cell death. Together, our data reason for a quantitative contribution of CDK7 to mRNA synthesis, which is critical for cellular homeostasis.
INTRODUCTION
Cyclin-dependent kinases (CDKs) form the enzymatic components of a group of heterodimeric serine/threonine kinases that have important roles in multiple cellular processes (1). CDK7/KIN28 was originally identified as a critical regulator of mRNA transcription in Saccharomyces cerevisiae (2–5). In vertebrates CDK7 has a dual function, influencing cell cycle progression and RNA polymerase II (RNAPII) transcription (6). Specifically, CDK7 forms the CDK-activating kinase (CAK) with two other TFIIH subunits, cyclin H and MAT1. The CAK activates downstream cell cycle CDKs, including cdc-2/CDK1, CDK2, CDK4, and CDK6, by phosphorylating key threonine residues in a process known as T-loop activation (7, 8). In transcription, as RNAPII begins to lose contact with many of the general transcription factors (GTFs) during promoter escape, CDK7, functioning as part of the TFIIH complex, phosphorylates RNAPII and allows the elongation complex to move downstream away from the transcription start site (TSS) (reviewed in reference 9). Specifically, CDK7 directly targets the carboxyl-terminal domain (CTD) of the Rpb1 subunit of RNAPII, which is comprised of 52 heptad repeats (Y1S2P3T4S5P6S7) in humans. While the serine 2 (Ser2), serine 5 (Ser5), and serine 7 (Ser7) residues are all subject to phosphorylation, CDK7 preferentially targets Ser5 and Ser7 (10–17). Phosphorylation patterning of the CTD is important as it influences the association of numerous nuclear factors with RNAPII (18, 19), as was recently demonstrated in yeast, where KIN28-driven phosphorylation of Ser5 residues was shown to trigger dissociation of the coactivator Mediator (20). In mammals, the exact mechanisms linking CTD phosphorylation (CTD-P) with transcription are yet to be fully elucidated. Indeed, whether CDK7 actually influences RNAPII transcription is a controversial issue in itself. On the one hand, recent studies have shown that inhibition of CDK7 caused gene-specific reduction of RNAPII promoter occupancy and abrogated TFIIE/DSIF exchange, resulting in attenuated pausing and delayed elongation (14, 21). Knockout of the CAK subunit MAT1 caused loss of TFIIH kinase function (reduced Ser5 phosphorylation) and resulted in decreased transcription of nascent RNA and impaired mRNA capping (22). On the other hand, knockout of CDK7 in mice altered the mRNA levels of only a small subset of genes and did not affect global levels of Ser5 phosphorylation, leading to the conclusion that CDK7 is not required for global RNAPII transcription (7). Regardless, knockout studies are limited in their ability to differentiate between direct and indirect effects.
In the current study, we introduce and characterize two novel specific inhibitors of CDK7 and then utilize these compounds to interrogate CDK7 function. We demonstrate robust effects of CDK7 on RNAPII-driven transcription both in vitro and in vivo. Short inhibitor treatment results in altered expression of hundreds of coding and noncoding RNAs, as well as impaired nascent RNA synthesis in vivo with both low and high concentrations. Specifically, inhibition of CDK7 does not completely block transcription but, instead, quantitatively determines the output, suggesting that CDK7 is an important but not an essential factor. These effects correlate with the observed RNAPII alterations, where higher inhibitor concentrations are required to strongly impair phosphorylation of Ser5 of the RNAPII CTD (CTD Ser5-P) globally and at multiple gene promoters. Longer periods of inhibition maintain an altered state of hypophosphorylation that may ultimately contribute to the observed physiological consequences, which include cell cycle delays and increased cell death that are independent of the p53 response.
MATERIALS AND METHODS
Antibodies for Western blot analysis.
The following antibodies were used: MED15 (clone 1H7), RNAPII phosphorylated at Ser2 (Ser2-P) (clone 3E10), RNAPII Ser5-P (clone 3E8), and RNAPII Ser7-P (clone 4E12) were gifts from D. Eick; α-tubulin (catalog number sc-23948), CDK7 (sc-7344), phosphorylated CDK7 (CDK7-P) (Thr170; sc-130185), MED26 (sc-48776), RNAPII (Rpb1; N-20; sc-899), RNAPII (F-12; sc-55492), retinoblastoma protein (Rb; sc-74562), phosphorylated Jun N-terminal protein kinase (JNK-P; Thr183/185; sc-6254), p53 (sc-126), TATA-binding protein (TBP; sc-273), TFIIB (sc-225), and TFIIH (p89; sc-293) were from Santa Cruz Biotechnology; cdc2-P (Thr161; catalog number 9114), H2B ubiquitin (Ub) (Lys120; 5546), H2A Ub (Lys119; 8240), p53-acetyl (Lys382; 2525), p53-P (Ser15; 9284), p53-P (Ser33; 2526), and Rb-P (Ser780; 3590) were from Cell Signaling; NELF-A (catalog number A301-910A) was from Bethyl Laboratories, Inc.; Spt5 (catalog number BD611106) was from BD Transduction Laboratories; histone H3 trimethylated at lysine 4 (H3K4me3; catalog number 39159) was from Active Motif.
In vitro enzymatic kinase assay for CDKs.
The 50% inhibitory concentrations (IC50s) for CDK inhibitors were determined using a fluorescence resonance energy transfer (FRET)-based Lance Ultra KinaSelect Ser/Thr kit (PerkinElmer), and kinase activity and inhibition were measured according to the manufacturer's instructions and as previously described (23).
In vitro kinase assay.
An in vitro kinase assay was performed as previously described (16). In brief, recombinant human trimeric CDK7/cyclin H/MAT1 complex (ProQinase) was added to a kinase reaction buffer containing 10 mM Tris-HCl, pH 7.3, 10 mM HEPES, pH 8.2, 50 mM KCl, 5 mM MgCl2, 5% glycerol, 0.01% Igepal, 0.01 mg/ml bovine serum albumin (BSA), 100 mM dithiothreitol (DTT), 100 μM ATP, and 1 ng of glutathione S-transferase-tagged RNAPII CTD (GST-CTD) that was expressed in and purified from Escherichia coli. Kinase reactions were allowed to occur for 30 min at 25°C and 800 rpm before the supernatants were recovered, supplemented with 6× SDS buffer (see above) to a final concentration of 1×, and analyzed by gel electrophoresis and Western blotting.
Nuclear extract preparation.
Nuclear extracts of HeLa and A549 cells were prepared as previously described (24). HEK 293T and Raji nuclear extracts were produced using a low-salt extraction protocol (up to 210 mM NaCl). Extracts were dialyzed against BC0 (20 mM Tris-HCl, pH 7.3, 20% [vol/vol] glycerol, 0.2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 5 mM DTT), and the KCl concentration was increased in a stepwise fashion up to a final concentration of 100 mM.
ITAs.
Immobilized template assays (ITAs) were conducted according to a previously described protocol (16). Preinitiation complexes (PICs) were formed by the addition of nuclear extract to 300 ng of the purified DNA template (coupled to 10 μg of beads). PIC reactions were halted after 60 min by the addition of 20 mM EDTA, pH 8. The supernatant was then removed, and the beads were washed once with 50 μl of transcription (Tx) wash buffer (25 mM HEPES, pH 8.2, 5 mM EDTA, 1 mM DTT, 0.2 mM EDTA, pH 8.0, 0.01% Igepal, 70 mM potassium glutamate, 10% glycerol). For elution of bound proteins, beads were resuspended in 20 μl of 2× SDS buffer (100 mM Tris-HCl, pH 6.8 at room temperature [RT], 200 mM DTT, 4% [wt/vol] SDS, 0.17% bromphenol blue, 20% [vol/vol] glycerol), and samples were then heated for 10 min at 70°C before gel electrophoresis and subsequent Western blot analysis.
RNA preparation.
For RNA analysis, cells were harvested and resuspended in TRIzol reagent (Invitrogen). Briefly, DNA was sheared with a 23-gauge needle and purified by successive chloroform and isopropanol addition. Pellets were collected and washed in ethanol (EtOH) before resuspension in RNase-free water and storage at −80°C.
Microarray analysis.
Total RNA was prepared from three biological replicates using TRIzol. Microarray analysis was conducted using high-density oligonucleotide arrays (GeneChip Human Gene 2.0 ST array) by an authorized Affymetrix service provider (KFB, Regensburg, Germany). Labeling of samples, hybridization, scanning, extraction of raw data, and robust multiarray average (RMA) analysis of probe set values (background adjusted, normalized, log transformed) were conducted by KFB. Regulated genes were classified as those with a fold change of ≥1.5 relative to control-treated cells and with P value of <0.05. The program DAVID was used for gene ontology (GO) analysis of regulated genes (http://david.abcc.ncifcrf.gov).
RT-qPCR.
One microgram of RNA per sample was used to generate cDNA using a Quantitect reverse transcription kit (Qiagen) with random hexamers or using a cDNA synthesis kit (TaKaRa). Real-time reverse transcription-quantitative PCR (RT-qPCR) was conducted using cDNA samples in conjunction with a SYBR green PCR Master kit (Applied Biosystems), according to the manufacturer's protocol. A Step One Plus PCR machine (Applied Biosystems) was used for analysis, and relative transcript levels were calculated by the ΔΔCT (where CT is threshold cycle) method. Primers were designed using Primer3 (http://primer3.wi.mit.edu/) and are available on request.
Luciferase assay.
HeLa cells carrying an expression cassette containing the luciferase gene under the control of a minimal cytomegalovirus (CMV) promoter (25) were induced with doxycycline (2 μg/ml) for 30 min. Cells were harvested in TRIzol, and the purified RNA was then used for cDNA synthesis.
In vitro transcription assay.
In vitro transcription reactions were performed using an immobilized (bead-coupled) DNA template comprising the adenovirus major late core promoter. As previously described (16, 26), DNA templates were amplified by PCR using a 5′ biotin-labeled primer and then conjugated to paramagnetic streptavidin beads (Promega). Elongation was conducted under standard nucleotide (nucleoside triphosphate [NTP]) concentrations (100 μM ATP, 100 μM CTP, 100 μM GTP, and 2.5 μM UTP) unless otherwise stated.
ChIP.
Chromatin immunoprecipitation (ChIP) was conducted according to a previously published protocol (27). Briefly, 1 μg of antibodies for RNAPII (N-20; catalog number sc-899), RNAPII CTD (Ser5; sc-47701), TFIIEα (sc-237), TFIIB (p33; sc-225), TFIIH (p89; sc-293), TBP (ab28175; Abcam), Spt5 (monoclonal rat antibody, clone 6F1, produced by E. Kremmers [unpublished data]), and control IgG (ab46540; Abcam) was conjugated to 20 μl of Dynabeads protein G solution (Invitrogen), and volumes of chromatin extracts corresponding to 3 × 106 cells were added to antibody-conjugated beads before overnight incubation for ChIP assembly. Following reversal of cross-linking and RNase and proteinase K treatment, DNA was purified (QIAquick PCR purification kit; Qiagen), and samples were then analyzed by RT-qPCR as described above. Primers used for analysis are available on request.
Immunodepletion.
293T nuclear extracts were immunodepleted for CDK7 in a two-step repeated process as previously described (16). Briefly, 10 μl of Dynabeads protein G (Invitrogen) per sample was washed with phosphate-buffered saline (PBS) containing 0.01% Tween and blocked in the same solution containing 5 mg/ml BSA for 30 min at 4°C. After two washes in 0.01% Tween–PBS, antibodies were conjugated to beads overnight at 4°C. Two different antibodies for CDK7 and a control antibody were used, namely, 10 μg of anti-CDK7 rabbit (sc-529), 15 μg of anti-CDK7 mouse (sc-7344), and 15 μg of anti-YY1 mouse (sc-7341). After overnight incubation, beads were once again washed in PBS-Tween and then washed twice in depletion buffer (150 mM potassium glutamate, 20 mM Tris-HCl, pH 7.3 [RT], 0.2 mM EDTA, 10% glycerol, 0.1% Igepal, 0.2 mM PMSF). 293T nuclear extracts were adjusted to contain 150 mM potassium glutamate, 0.1% Igepal, and 0.2 mM PMSF and then added to the beads for 2.5 h at 4°C. These single-depletion extracts were then added to fresh antibody-coated beads, and the depletion incubation step was repeated. Supernatants were then collected and stored at −80°C.
Cell cycle analysis.
Approximately 50,000 harvested cells were collected in 50 μl of PBS and added to 950 μl of 4′,6′-diamidino-2-phenylindole (DAPI) solution to be analyzed by flow cytometry (Cyflow; Partec). FlowJo software (Treestar, Inc.) was used for cell cycle analysis, and the percentage of total cells in each phase was calculated using the Dean-Jett-Fox algorithm.
Apoptosis analysis.
Apoptosis was measured by flow cytometry by staining cells with annexin-fluorescein and propidium iodide using an annexin V-FITC (fluorescein isothiocyanate) apoptosis detection kit I (BD Biosciences).
RESULTS
Two novel small molecule inhibitors, LDC3140 and LDC4297, with high specificity for CDK7.
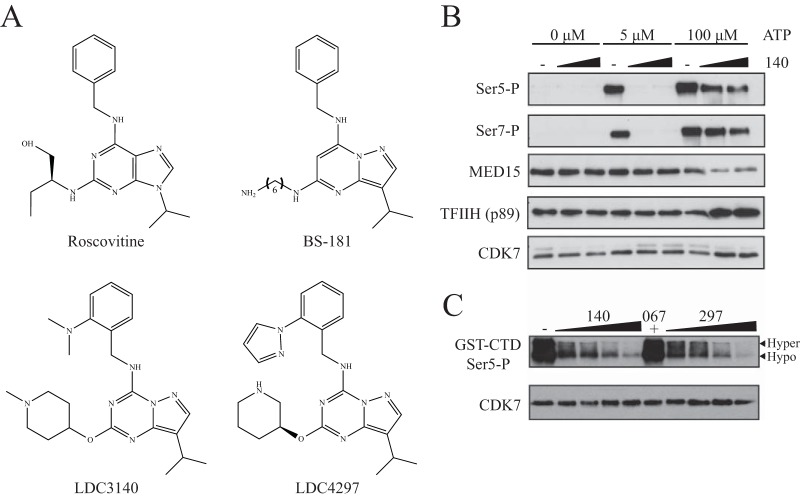
Considering the current restrictions in the specificity and potency of commonly used CDK7 inhibitors, we aimed to design a novel specific small molecule that could be widely used in in vitro and in vivo applications with a view toward therapeutic use. Our initial intention was to develop compounds with greater specificity for CDK7 than roscovitine and BS-181 (28). The human CDK7/cyclin H/MAT1 complex (ProQinase, Freiburg, Germany) was used to screen a kinase-biased library centered on BS-181 and other known ATP-competitive kinase inhibitory scaffolds in several iterative cycles. Following optimization by medicinal chemistry, from a total of 600 substances the inhibitors LDC3140 and LDC4297 (referred to as compounds 140 and 297, respectively) were identified as two lead compounds (Fig. 1A) (J. Eickhoff, G. Zischinsky, and U. Koch, 6 September 2013, international patent application WO2013128028A1).
FIG 1.
Structure and specificity of novel CDK7 inhibitors. (A) Molecular structure of the novel compound LDC3140 and LDC4297 in comparison to roscovitine and BS-181. (B) Immunoblot of eluted samples from immobilized template assay (ITA) for phosphorylated RNAPII Ser5 (Ser5-P) and Ser7 (Ser7-P) residues. Formation of PICs was conducted with a HeLa nuclear extract in the presence of increasing compound 140 concentrations (3 μM, 10 μM) with increasing ATP conditions (5 μM, 100 μM) before a washing step and elution from beads. (C) In vitro kinase assay using GST-CTD fragment of RNAPII. Recombinant CDK7/cyclin H/MAT1 was added to a solution containing a GST-tagged RNAPII CTD fragment and either dimethyl sulfoxide (DMSO; solvent control), compound 140 (0.75 μM, 1.5 μM, 3 μM, or 6 μM), compound 297 (62.5 nM, 125 nM, 250 nM, or 500 nM), or compound 067 (10 μM). Reactions were allowed to proceed for 30 min before eluted samples were analyzed by immunoblotting using an antibody targeting the phosphorylated Ser5 residue of RNAPII CTD (GST-CTD Ser5-P).
To further demonstrate the specificity of these compounds for CDK7, inhibition of CDKs was measured using an in vitro enzymatic kinase assay, where substrate phosphorylation was analyzed using a fluorescence resonance energy transfer (FRET)-based assay at 3 μM ATP (Table 1). As indicated by the half-maximal inhibitory concentrations (IC50), CDK7 was inhibited by compound 140 to a far greater extent (at least 3 orders of magnitude) than the other tested CDKs. In comparison to compound 140, compound 297 showed a lower specificity for CDK7 relative to the other CDKs, particularly CDK2. Nevertheless, analysis conducted at higher ATP concentrations that are more representative of in vivo levels showed a strong preference of this compound toward CDK7 (Table 2). Both compounds 140 and 297 are highly specific for the class of CDKs and failed to inhibit a panel of some 150 representative non-CDK kinases, as will be published elsewhere.
TABLE 1.
In vitro enzymatic kinase assay
| Compound name | IC50 (μM)a |
|||||
|---|---|---|---|---|---|---|
| CDK1 | CDK2 | CDK4 | CDK6 | CDK7 | CDK9 | |
| Flavopiridol | <0.005 | 0.0147 ± 0.00 | 0.0376 ± 0.01 | 0.305 ± 0.02 | 0.1031 ± 0.02 | <0.005 |
| BS-181 | >10* | 4.58 ± 2.90† | >10 | >10 | 0.0571 ± 0.04† | 1.94 ± 0.58 |
| LDC043140 | >10 | 3.897 ± 0.37 | >10 | >10 | <0.005 | 7.45 ± 3.14* |
| LDC044297 | 0.0537 ± 0.01 | 0.0064 ± 0.00* | >10 | >10 | <0.005 | 1.7113 ± 0.12 |
In all cases except as noted, the number of replicates (n) = 3. *, n = 2; †, n = 4.
TABLE 2.
ATP competition assay
| Compound name | EC50 (μM) with competitora |
|||
|---|---|---|---|---|
| 3.5 mM ATP |
Km ATPb |
|||
| CDK7 | CDK2 | CDK7 | CDK2 | |
| BS-181 | 1.544 ± 0.08 | >10 | 0.166 ± 0.03 | 8.057 ± 0.21 |
| LDC043140 | 0.2639 ± 0.05 | >10 | 0.0091 ± 0.00 | 7.204 ± 0.53 |
| LDC044297 | 0.0074 ± 0.01 | 2.127 ± 0.17 | <0.005 | 0.0256 ± 0.00 |
EC50, 50% effective concentration. In all cases, n = 4, where n is the number of replicates.
Km ATP, ATP concentration corresponding to the Michaelis constant for the indicated kinases.
CDK7 inhibitors block RNAPII Ser5 and Ser7 phosphorylation in vitro.
Using an immobilized template assay (ITA) and subsequent immunoblotting, we examined the effect of our inhibitors on phosphorylation of the RNAPII CTD during preinitiation complex formation in vitro. Application of compound 140 resulted in decreased phosphorylation at the Ser5 and Ser7 residues (Fig. 1B), and a similar effect was seen for compound 297 (data not shown). Interestingly, stronger inhibition was observed at lower ATP concentrations for both compounds, indicating an ATP-competitive binding mode.
To further establish the specificity of these novel compounds, an in vitro kinase assay using the GST-tagged RNAPII CTD was conducted in the presence of CDK7 and CDK9 inhibitors (Fig. 1C). In this assay recombinant purified CDK7/cyclin H/MAT1 complex was used to phosphorylate the CTD fragment at the serine 5 residue. Both novel inhibitors caused a stark impairment of Ser5 phosphorylation while a specific CDK9 inhibitor, compound 067 (23), had no noticeable effect. These data are in agreement with previous data describing CDK7 as a CTD Ser5 and Ser7 kinase in vitro (10–17).
CDK7 affects gene expression at a global level.
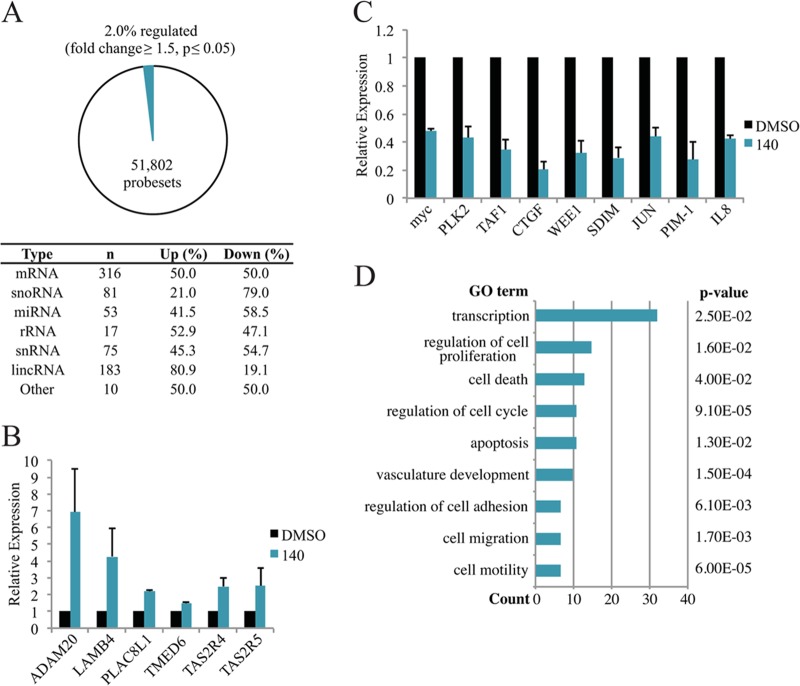
We sought to explore the influence of CDK7 inhibition in cells on a genome-wide level. Although compound 297 displays higher affinity for CDK7 and presents as a better candidate for biomedical applications, we used limited concentrations of compound 140 for this analysis due to its having specificity for CDK7 superior to that of CDK2. We conducted gene expression arrays after 90 min of treatment with 5 μM compound 140 in A549 cells using the GeneChip Human Gene 2.0 ST Array. Samples were taken at this time point to decrease the likelihood of measuring indirectly regulated genes. Of the 51,802 probe sets tested, approximately 2% of these were regulated (fold change of ≥1.5; P < 0.05), including probes for mRNA, small nucleolar RNA (snoRNA), microRNA (miRNA), rRNA, small nuclear RNA (snRNA), long intergenic noncoding RNA (lincRNA), and other noncoding RNAs (Fig. 2A). Approximately 50% of the affected mRNAs were upregulated, and 50% were downregulated. Interesting downregulated genes include the transcription factor genes MYC and JUN, the serine/threonine protein kinase gene PLK2, the G2 checkpoint cell cycle kinase gene WEE1, and the cytokine gene IL-8 (for interleukin-8). In addition, all three members of the NR4A subfamily of ligand-independent transcription factor genes, namely, NR4A1, NR4A2, and NR4A3, showed significantly decreased expression. A similar upregulated/downregulated ratio was measured for miRNAs, rRNAs, and snRNAs. Expression of microRNA 21 (miR-21), an oncomir involved in oncogenic initiation, transformation, invasion, and metastasis (reviewed in reference 29), was reduced by approximately 1.9-fold following CDK7 inhibition. The miR-17-92 cluster host gene (also known as the oncomir-1 gene), which has well-characterized roles in cell proliferation and suppression of apoptosis (reviewed in reference 51), was similarly repressed with inhibitor treatment (approximately 2.1-fold). Alternatively, Let-7d, a proposed tumor suppressor in multiple cancers (30, 31), presents as an interesting factor that was activated by CDK7 inhibition. Notably, of the 81 affected snoRNAs, 21% were upregulated, and 79% were downregulated. Conversely, close to 81% of the 183 affected lincRNAs were upregulated, while approximately 19% were downregulated.
FIG 2.
Microarray analysis of compound 140-treated A549 cells. (A) Statistics of regulated genes from microarray analysis. (B) RT-qPCR confirmation of selected genes shown to be upregulated in the gene expression array. (C) RT-qPCR confirmation of selected genes shown to be downregulated in the gene expression array. (D) Gene ontology (GO) analysis of mRNAs that were downregulated in the expression array.
The validity of the array was confirmed by RT-qPCR, where multiple reported upregulated genes showed increased mRNA levels, including ADAM20, LAMB4, and PLAC8L1 (Fig. 2B), and a number of reported downregulated genes were decreased, including PLK2, WEE1, JUN, and IL-8 (Fig. 2C). We focused on the downregulated gene set for further analysis. Gene ontology (GO) analysis of this group demonstrated an effect of CDK7 inhibition on multiple regulatory functions, with affected processes including transcription, regulation of cell proliferation, and regulation of the cell cycle (Fig. 2D). Further examination uncovered a striking characteristic of the downregulated gene set. Comparison of mRNA half-lives estimated from an experimental database (32) using a Mann-Whitney U test identified a significant difference (P < 0.001) between the means calculated for the entire database (763 min) and for those downregulated genes with defined half-lives (83 genes; 450 min). This suggests that the genes downregulated by CDK7 inhibition had a generally shorter mRNA half-life than the average gene. We therefore conclude that inhibition of CDK7, like that of CDK9 (23), first affects primarily short-lived genes with high mRNA turnover rates.
Inhibition of CDK7 causes a concentration-dependent reduction in de novo mRNA synthesis.
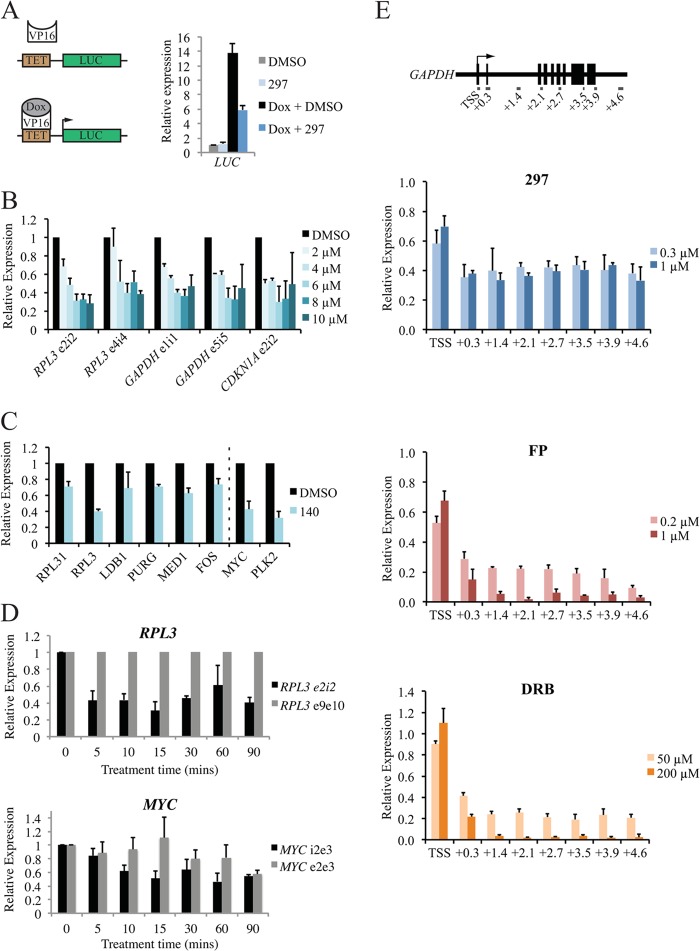
To examine the role of CDK7 in RNAPII-driven transcription in cells, we initially used a tetracycline-inducible luciferase reporter gene system under the control of a minimal CMV promoter in HeLa cells (16, 25). Addition of doxycycline caused a strong induction of luciferase expression (Fig. 3A), while treatment with compound 297 caused a reduction in activation of approximately 50%, suggesting that CDK7 inhibition prevents RNAPII transcription in an inducible-gene setting.
FIG 3.
CDK7 inhibition reduces de novo transcription by RNA polymerase II. (A) Analysis of luciferase (LUC) reporter gene expression in stably transfected HeLa cells. Pretreatment for 5 min with 0.5 μM compound 297 or dimethyl sulfoxide (DMSO) was followed by induction of expression for 30 min by addition of doxycycline (Dox). Induction was measured by RT-qPCR using a primer set targeting a region within the LUC gene (approximately 1.2 kb downstream of the TSS). LUC expression was driven by a minimal CMV promoter. TET, tetracycline. (B) RT-qPCR analysis of nascent RNA (determined using primers targeting exon/intron junctions) following a 30-min treatment with increasing concentrations of compound 140 (up to 10 μM). Values were normalized to stable RPL3 mRNA (primers located in exon 9 and exon 10). (C) De novo transcript levels analyzed for genes that were not regulated (left of dotted line) compared to levels for genes that were significantly downregulated (right of dotted line) in the expression array. Primers were designed to span exon-intron junctions or intronic regions. (D) Analysis of unspliced de novo transcripts for RPL3 and MYC after short treatment with compound 140. MYC e2e3 represents steady-state MYC mRNA. Values shown are relative to the 0-min time point. (E) Unspliced transcript levels across the GAPDH gene for 30 min with different concentrations of compound 297 (0.3 μM or 1.0 μM), FP (0.2 μM or 1.0 μM), and DRB (50 μM or 200 μM). Values shown are relative to the DMSO control. Gene locations are relative to the transcription start site (TSS) and are shown in kb.
We then examined the effect of the inhibitors on formation of nascent RNA at endogenous cellular genes. A549 cells were treated with increasing concentrations of compound 140 (up to 10 μM) for 30 min and were then harvested for subsequent RNA purification and cDNA preparation. Because of the rapid action of the splicing machinery in comparison to the relatively long half-lives of mature mRNAs, primers targeting specific exon-intron (e-i) junctions or intronic regions (i-i) allowed quantitative analysis of newly synthesized pre-mRNAs (33, 34). RT-qPCR was used to analyze mRNA levels at multiple junctions (e-i) for the RPL3 and GAPDH (for glyceraldehyde-3-phosphate dehydrogenase) housekeeping genes and at the p21 gene (Fig. 3B). A primer set targeting a region of RPL3 corresponding to exons 9 and 10 was used as the housekeeping control. Regarding specific inhibitor concentrations, 2 μM compound 140 was sufficient to cause noticeable decreases in the majority of e-i regions tested, and maximal inhibition was obtained with 6 to 10 μM, depending on the gene region. These results are consistent with data observed for other human cancer cell lines, including 293T cells (data not shown). Similar reductions were observed at e-i and i-i regions of RPL3 in A549 cells treated with 300 nM 297 (data not shown). The effect was not limited to the genes regulated in the microarray analysis, such as MYC and PLK2; unspliced de novo transcripts of unaffected genes were also affected by CDK7 inhibition (Fig. 3C). This implies an inherent experimental bias for the expression array toward genes with short half-lives.
We investigated the dynamics of transcription inhibition by analyzing newly synthesized RNA levels at shorter time points up to 90 min (Fig. 3D). Nascent RNA for RPL3 was reduced within 5 min of inhibitor treatment, and this level of reduction was maintained after 90 min, demonstrating the rapid action of the inhibitor and indicating fast dynamics on gene promoters. The latter appears to differ from one gene to another: equivalent measurements for the MYC gene showed a more gradual reduction, with maximal effects first observed after approximately 15 min of treatment. While steady-state mRNA levels of RPL3 were unaffected, equivalent MYC mRNA, which has a short half-life, was reduced after 90 min, explaining the difference observed in the microarray data.
To further investigate the importance of CDK7 in this context, the effects of inhibitors for CDK7 and CDK9 were compared across the GAPDH gene (Fig. 3E). Treatment with the CDK7 inhibitor compound 297 for 30 min reduced transcript levels for all regions tested in a concentration-independent manner; treatment with high and low doses caused similar effects, with a maximal inhibition of approximately 65%. Conversely, targeting CDK9 using flavopiridol (FP) or DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole) caused far stronger reductions in nascent transcript levels. Strikingly, high concentrations of FP and DRB resulted in almost complete loss of newly synthesized transcripts downstream of the first e-i region. That higher concentrations of our CDK7 inhibitor did not achieve comparable reductions suggests that these two targets have distinct impacts on transcription.
CDK7 regulates RNAPII transcription in vitro.
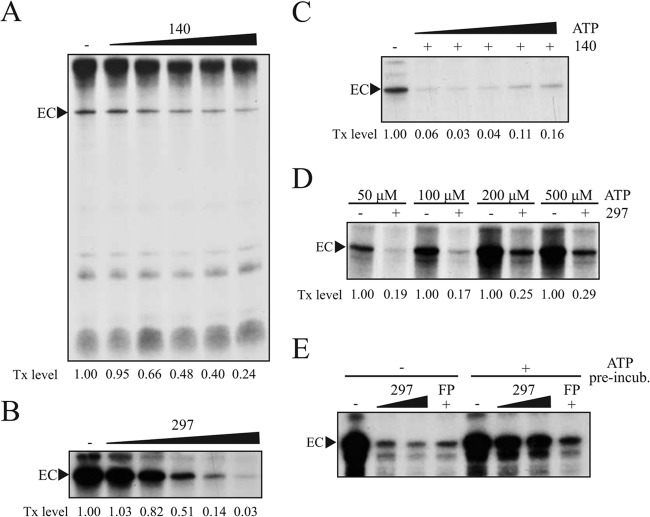
We then tested our CDK7 inhibitors in a well-established in vitro transcription system, which facilitates RNAPII transcription on a synthetic DNA template under the control of the adenovirus major late promoter. CDK7 inhibitors were initially titrated against an A549 or 293T nuclear extract (Fig. 4A and B). Concentrations as low as 2.5 μM (compound 140) and 300 nM (compound 297) strongly inhibited transcription in this context, as measured by levels of elongated transcript complexes (EC). As shown for RNAPII CTD phosphorylation in vitro (Fig. 1), the inhibitory effect of compounds 140 and 297 on transcription in vitro was ATP dependent, with EC formation recovering with increasing ATP concentrations (Fig. 4C and D). Of note, however, increasing ATP up to physiological levels (1 to 2 mM) did not cause complete rescue of transcription (data not shown). EC formation was maximally reduced to 50%, consistent with effects seen on endogenous genes (Fig. 3). Importantly, preincubation of preinitiation complexes with ATP alone before the inhibitor treatment limited the effect of compound 297 on transcription (Fig. 4E), suggesting that CDK7 could in principle elicit at least part of its role prior to transcription initiation, i.e., via phosphorylation of the RNAPII CTD or another target. These results add further evidence to support an important role for CDK7 in RNAPII-mediated transcription.
FIG 4.
CDK7 inhibition reduces in vitro transcription by RNA polymerase II. (A) Titration of compound 140. Reactions were performed using nuclear extracts obtained from A549 cells. Concentrations applied were 1 μM, 3 μM, 5 μM, 10 μM, and 20 μM compound 140. The transcription (Tx) level was determined using ImageJ and represents signal intensity of elongated complexes (EC). (B) Titration of compound 297 using 293T extract. Concentrations applied were 0.05 μM, 0.1 μM, 0.3 μM, 0.5 μM, and 1 μM 297. The Tx level was determined using ImageJ. (C) ATP dependency of compound 140. Compound 140 (10 μM) was added before PIC formation with 293T nuclear extract. Increasing concentrations of ATP (0, 50 μM, 100 μM, 250 μM, or 500 μM) were added with the standard concentrations of other NTPs before elongation occurred. Tx level was determined using ImageJ. (D) ATP dependency of compound 297. Compound 297 at 2 μM was added before PIC formation with 293T nuclear extract. Increasing concentrations of ATP were added with the standard concentrations of other nucleotides before elongation occurred. Tx level was determined using QuantityOne software (Bio-Rad) to compensate for background signals. (E) Influence of ATP on compound 297-mediated transcription inhibition. Following PIC formation with A549 nuclear extract, samples were preincubated with 100 μM ATP for 5 min before addition of compound 297 (0.4 μM or 2 μM) or FP (1 μM) and standard NTP concentrations to allow elongation.
CDK7 inhibition influences RNAPII distribution and PIC formation.
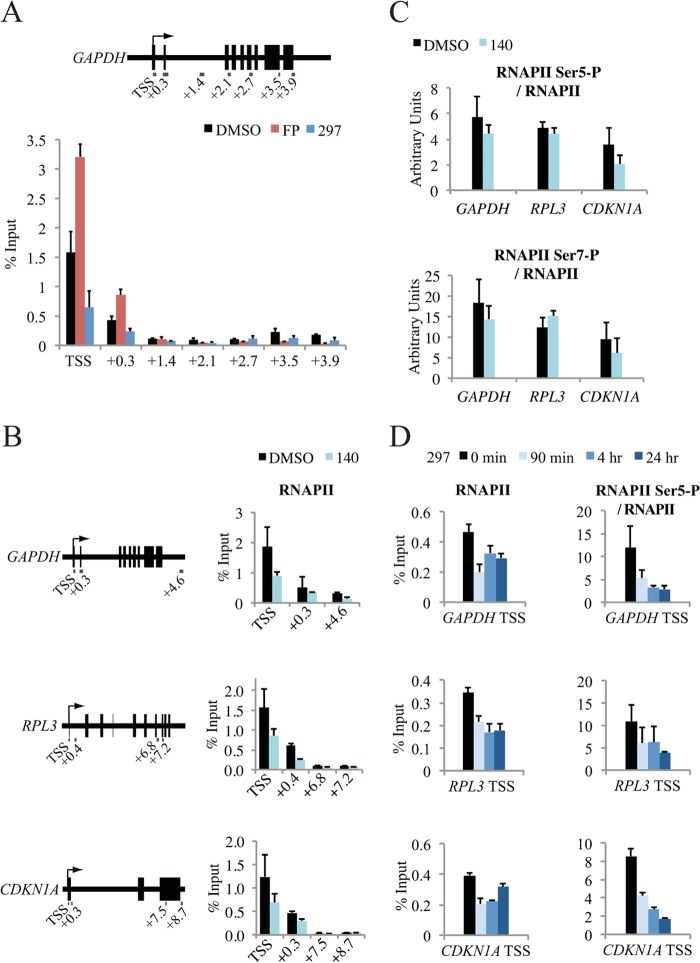
To further probe the role of CDK7 in transcription, we examined the effect of specific inhibition on RNAPII distribution and PIC assembly. A549 cells were treated for 30 min and 90 min, and RNAPII levels were determined by chromatin immunoprecipitation (ChIP) across the GAPDH gene (Fig. 5A). As observed in previous studies (14, 21), inhibition of CDK7 resulted in decreased cross-linking of RNAPII, which was most obvious at the start of the gene (TSS). A comparable effect was observed with compound 140 treatment for multiple genes, providing initial evidence for a global effect (Fig. 5B). We suggest that loss of RNAPII is due to rapid clearance of promoter-proximal paused complexes. As expected from previous results (23), inhibition of CDK9 with FP (Fig. 5A) and with another established inhibitor, DRB (data not shown), resulted in the opposite effect, namely, an increase of RNAPII near the TSS.
FIG 5.
Inhibition of CDK7 leads to alterations in RNAPII promoter occupancy. (A) Distribution of RNAPII across the GAPDH gene was determined by ChIP following a 90-min treatment of A549 cells with dimethyl sulfoxide (DMSO), compound 297 (0.3 μM), or FP (1 μM). Gene locations are relative to the transcription start site (TSS) and are shown in kb. (B) Distribution of RNAPII across the GAPDH, RPL3, and CDKN1A genes after a 30-min treatment with 5 μM compound 140. (C) Distribution of RNAPII Ser5-P and Ser7-P at the GAPDH, RPL3, and CDKN1A promoters (TSS) after a 30-min treatment with 5 μM compound 140. Values were normalized to total RNAPII levels and are shown in arbitrary units. (D) Distribution of RNAPII and RNAPII Ser5-P at the GAPDH, RPL3, and CDKN1A promoters after 2 μM compound 297 treatment for 90 min, 4 h, or 24 h.
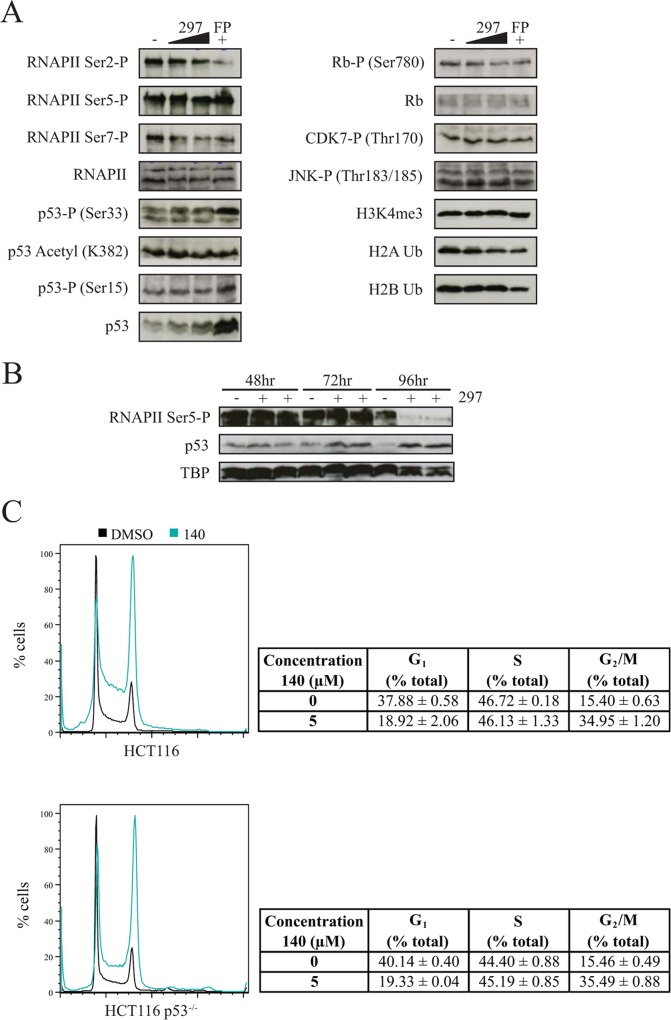
CTD Ser5-P levels were reduced in the presence of compound 140 on all genes tested; however, the effects were moderate when normalized to RNAPII (Fig. 5C). Given that these effects were within the regions of error and taking into account the observed ATP dependency of CDK7 inhibitors (Fig. 1), we next tested a higher concentration of the high-affinity inhibitor compound 297 for prolonged time periods. In this setting, levels of Ser5-P (normalized to total RNAPII) steadily decreased up to the 24-h time point, with large reductions up to 70 to 80% detected at multiple gene promoters (Fig. 5D). Although strong changes were measured on specific genes, global levels of Ser5-P and Ser7-P remained largely unchanged in MCF7 and A549 cells over days (see Fig. 8A and B), indicating a slow turnover and/or the action of other Ser5-P kinases (35–37). A loss of Ser5-P was visible only after 4 days of inhibitor treatment in A549 cells, upon which time the cells exhibited clear signs of stress, including p53 activation (see Fig. 8B). Collectively, the data confirm the well-established impact of CDK7 on RNAPII and CTD-P and further demonstrate that maintained inhibition induces a state of hypophosphorylation.
FIG 8.
CDK7 inhibition does not activate a rapid p53 response. (A) Analysis of inhibitor effect on protein expression and phosphorylation in MCF7 whole-cell lysates as determined by Western blotting. Cells were treated with 0.3 μM or 1 μM 297 or 1 μM FP for 3 h. (B) RNAPII Ser5-P and p53 levels in A549 nuclear fractions as determined by Western blotting. Cells were treated for 48, 72, and 96 h with 0.3 μM compound 297 before nuclear extracts were prepared. (C) Cell cycle analysis in HCT116 wild-type and p53−/− cells. Cells were treated for 64 h with 5 μM 140 before analysis by flow cytometry.
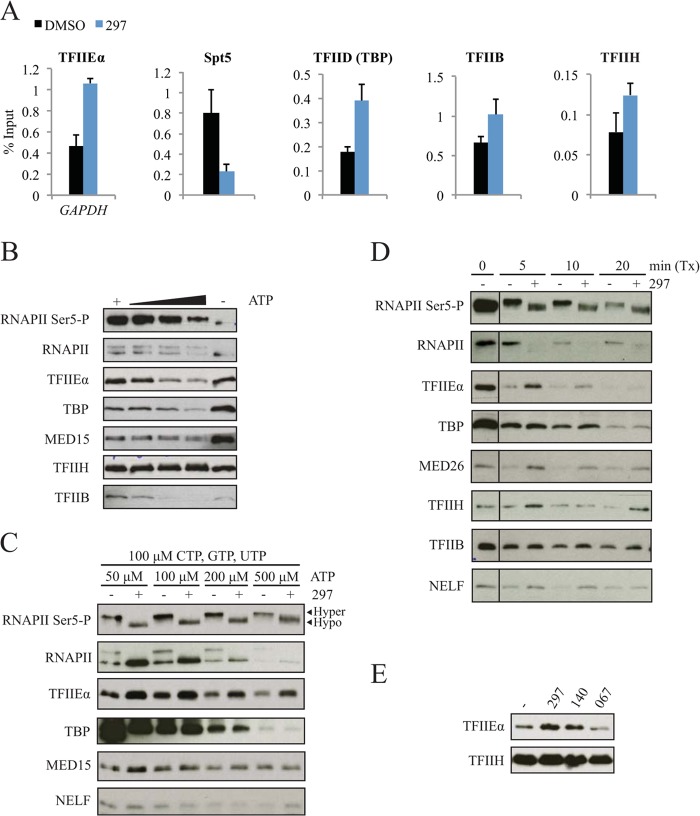
We next asked whether factors involved in the early stages of transcription were also affected by inhibited CDK7 function. As expected, occupancy of TFIIE was increased at the GAPDH promoter (Fig. 6A) and on other genes (data not shown) with a concomitant decrease in Spt5 (14, 21). Interestingly, TBP, TFIIB, and TFIIH were also retained, suggesting that preinitiation complex dynamics can be influenced by CDK7. We further investigated the dynamics of transcription complexes using immobilized template assays that allow dissection of PIC formation and transcription. Increasing concentrations of ATP, CTP, and GTP caused dissociation of RNAPII, multiple GTFs, and Mediator from immobilized DNA templates (Fig. 6B and C). In this assay, stable preinitiation complexes are formed by extended preincubation of templates with nuclear extracts. Transcription is subsequently initiated with the addition of NTPs. Consistent with the in vivo ChIP data, loss of CDK7 function during PIC formation led to retention of multiple factors involved in early transcription, including TFIIE and Mediator (Fig. 6C; also data not shown). In this setting RNAPII phosphorylation was effectively inhibited by compound 297 although RNAPII itself appeared to be retained. A more dynamic setting was required to observe retention of TBP and TFIIH and reduction of RNAPII (Fig. 6D). Specifically, reducing the incubation time with nuclear extract prevented saturation of PIC binding prior to transcription initiation. Simultaneous addition of inhibitor and extract allowed the effects of impaired CDK7 function to be observed prior to complete PIC saturation. In addition, retention of TFIIE was seen with CDK7 but not CDK9 inhibitors (Fig. 6E), which is in line with the concept that CDK7 functions before RNAPII reaches the promoter-proximal pause position. Together, the data suggest that inhibition of CDK7 leads to rapid clearance/dissociation of RNAPII complexes from promoter-proximal regions, with a concomitant prolongation of the half-life of preinitiation and/or early elongation complexes.
FIG 6.
CDK7 inhibition leads to alterations in preinitiation complex (PIC) formation. (A) Occupancy of TFIIEα, DSIF, TFIID, TFIIB, and TFIIH was determined for the GAPDH promoter region following a 90-min treatment with dimethyl sulfoxide (DMSO) and compound 297 (0.3 μM) by ChIP using antibodies targeting the p57, Spt5, TBP, p33, and p89 subunits, respectively. (B) Immunoblot of eluted samples from ITA using HeLa extract. PIC formation was performed for 60 min with increasing concentrations of NTPs as follows: lane 1, 100 μM ATP; lane 2, 60 μM ATP, 50 μM CTP and GTP, and 2.5 μM UTP; lane 3, 100 μM ATP, 100 μM CTP and GTP, and 2.5 μM UTP; lane 4, 500 μM ATP, 100 μM CTP and GTP, and 2.5 μM UTP; lane 5, 0 μM ATP, CTP, GTP, and UTP. (C) Immunoblot of eluted samples from ITA with 293T extract. Formation of PICs was conducted in the presence of 2 μM compound 297 with increasing ATP concentrations and constant concentration of other NTPs. Hyper- and hypophosphorylated forms of RNAPII were differentiated by molecular weight. (D) Immunoblot of eluted samples from modified ITA. Formation of PICs for 10 min was followed by transcription (Tx) with NTPs (400 μM ATP and 200 μM GTP, CTP, and UTP). Compound 297 at 2 μM was added initially with the 293T nuclear extract before PIC formation. (E) Immunoblot of eluted samples from ITA for TFIIEα and TFIIH. PICs were formed in the presence of standard NTP concentrations using HeLa extracts with 1 μM 297, 5 μM 140, or 10 μM compound 067.
Inhibition of CDK7 induces apoptosis and causes a cell-type-specific G1 or G2/M delay.
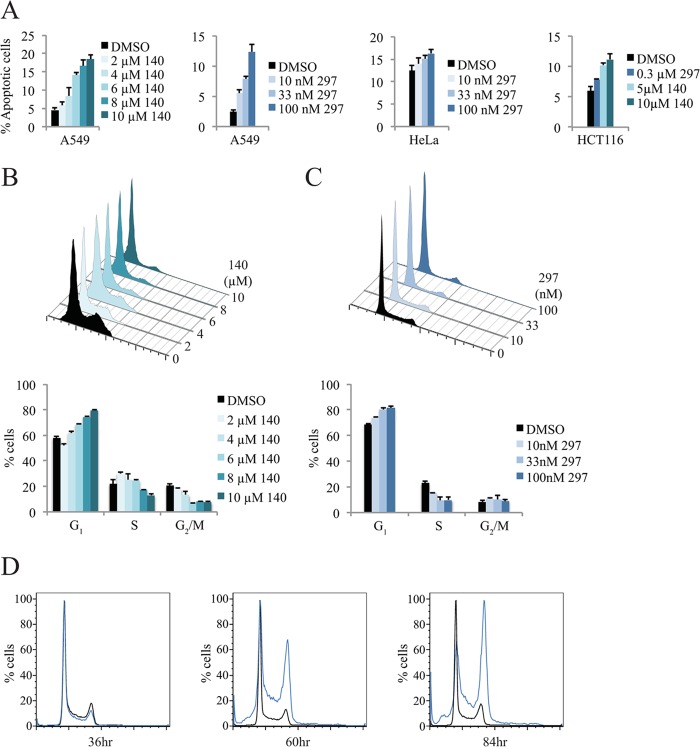
Given the reported role of CDK7 on downstream CDKs, we decided to further investigate the effects of our CDK7 inhibitors on programmed cell death and the cell cycle. Treatment with compound 140 or 297 induced a clear apoptotic response in multiple tumor cell lines (Fig. 7A). The strength of induction appeared to be cell type specific, with A549 cells responding to a higher degree than HeLa and HCT116 cells. No appreciable effect was observed with treatment of primary mouse embryonic fibroblasts (data not shown). Together, these data suggest that the inhibitors may be more suited for use in certain types of cell lines and tumor entities. Alterations in the cell cycle were also observed for both inhibitors. A549 cells were treated with increasing concentrations of compounds 140 and 297 (Fig. 7B and C). Both compounds caused an increase in G1-phase cells and a decrease in S-phase cells that was visible after 24 h of treatment. Nevertheless, the effect of blocking CDK7 function was not consistent across all tested cell lines (data not shown). In HCT116 colon cancer cells, the cell cycle was altered only after longer incubation times: a strong G2/M delay was observed after 60 h of compound 297 treatment (Fig. 7D), indicative of indirect effects. In agreement with this, we did not observe a noticeable effect of our inhibitors on T-loop phosphorylation of either CDK1 (Thr161) or CDK2 (Thr160) in several independent asynchronously proliferating tumor cells (data not shown).
FIG 7.
Inhibition of CDK7 causes tumor cell death and cell-type-dependent cell cycle delay. (A) Induction of apoptosis in multiple cell lines following CDK7 treatment. Measurements were obtained using annexin V-FITC staining, with the percentage of apoptotic cells identified as annexin V positive and propidium iodide negative (quadrant 4). (B and C) Titration of compounds 140 and 297 in A549 cells. Cells were treated for 24 h with increasing concentrations of compounds 140 and 297 with dimethyl sulfoxide (DMSO) as a solvent control. Harvested cells were analyzed by flow cytometry, and the percentage of total cells in each phase was calculated using the Dean-Jett-Fox algorithm. (D) Cell cycle analysis in compound 297-treated HCT116 colon cancer cells. Cells were treated for 36, 60, and 84 h with 0.3 μM compound 297 or DMSO before analysis by flow cytometry.
Loss of CDK7 function does not activate a rapid p53 response.
Blockage of RNAPII transcription elongation has been previously shown to induce recruitment and activation of the ATR kinase, which subsequently stabilizes and activates p53, as indicated by increased Ser15-P levels (38). Known inhibitors of transcription, namely, actinomycin D and α-amanitin, cause accumulation and activation of p53 as a result of reduced mRNA synthesis (38, 39). Given the impairment of nascent RNA synthesis seen with both CDK7 and CDK9 inhibitors (Fig. 3E), we next asked whether inhibiting CDK7 leads to a similar stress response. As expected from previous observations with DRB (40, 41), blocking CDK9 with FP led to a strong accumulation of p53 (Fig. 8A). Contrastingly, only relatively slight increases were observed with limiting CDK7 inhibitor concentrations. Previous studies have identified multiple phosphorylation sites on p53 that can be targeted by CDK7, including Ser33 (42, 43). However, in the current setting Ser33-P was not specifically diminished by CDK7 inhibition (Fig. 8A). Furthermore, inhibiting CDK7 had little to no effect on p53 acetylation (K382), JNK phosphorylation (Thr183/185), and multiple histone modifications (H3K4me3 and H2B Ub) although a modest reduction in phosphorylation of the Rb cell cycle regulator (Ser780) and in H2B ubiquitination was observed at higher concentrations. Nonetheless, the p53 stabilization detected after prolonged inhibitor treatment may be significant (Fig. 8B and data not shown). The observed increase in RNAPII levels at the CDKN1A gene promoter after 24 h of compound 297 treatment is consistent with such a delayed p53 response (Fig. 5D). Further, comparison of HCT116 wild-type and HCT116 p53−/− cells demonstrated that cell cycle effects can occur independently of p53 with limiting concentrations of CDK7 inhibitors (Fig. 8C). Collectively, these data suggest that restricted reductions of transcription activity are insufficient to activate an immediate p53-dependent stress response although long-term treatment can cause activation potentially as an indirect consequence of altered gene expression.
Overall, our results suggest that the reduction of CDK7 activity affects the stability of preinitiation complexes and causes loss of RNAPII from promoters, with a concomitant reduction in Ser5 phosphorylation in the CTD. This in turn leads to a robust but ultimately limited effect on mRNA synthesis rates and an altered state of transcription inside cells, which may eventually contribute to the observed cell cycle delay and reduced survival of tumor cells.
DISCUSSION
Using specific inhibitors of CDK7, we have identified a large number of genes encoding short-lived mRNAs that are positively controlled by CDK7. Unexpectedly, approximately 50% of the regulated protein-coding genes and up to 80% of noncoding RNA genes showed increased expression in the microarray analysis. It is unclear whether this is a result of direct negative control or whether it is an indirect effect relating to genes encoding short-lived RNA and proteins that are under the positive control of CDK7. The ratio of upregulated/downregulated genes is unlike that seen with previous analyses using another inhibitor of transcription, namely, the specific CDK9 inhibitor, compound 067, where 94% of affected mRNAs showed reduced expression after 90 min of treatment (23). The similarity between the data sets of compounds 140 and 067 was restricted to 21.0% of downregulated genes and 2.5% of upregulated genes, arguing against similar gene targets of CDK7 and CDK9. Indeed, blocking CDK9 caused a more dramatic reduction of mRNA synthesis and led to accumulation rather than loss of RNAPII molecules at the TSS, illustrating the differential functions of these two kinases.
Importantly, multiple genes with unaffected steady-state mature mRNAs were responsive to CDK7 inhibition at the level of nascent RNA. Genes that were analyzed at this level included housekeeping genes, oncogenes, and cell cycle inhibitors. In all cases, reductions were rapid and robust in the range of 30 to 70%. We assume that these effects on newly synthesized RNA are representative of a larger population of untested genes and therefore are in line with the original concept of CDK7 as a global regulator of transcription (2–5, 44). In vitro analyses under controlled conditions using a synthetic DNA template demonstrated the potency of CDK7 inhibitors to almost completely block transcription; however, even high concentrations of the more potent compound 297 were unable to fully attenuate nascent RNA synthesis, in stark contrast to the CDK9 inhibitors FP and DRB. Consistently, promoter occupancy of RNAPII, which is also commonly used as a measure of transcription in vivo, was affected in a similar way: compound 297 treatment caused a strong and sustained but nevertheless limited reduction of RNAPII levels. These data lead to the conclusion that CDK7 is an important but ultimately nonessential factor for RNAPII-driven transcription in the cell.
Our molecular observations are mostly in agreement with previous findings, initially arguing for an authentic and CDK7-specific response of the novel chemical inhibitors. Specifically, RNAPII promoter clearance/dissociation, stabilization of TFIIB and TFIIE, and a concomitant decline of Spt5 are observed in response to CDK7 inhibition and are consistent with earlier data (14, 21). The authors of these studies concluded that CDK7 functions to regulate promoter-proximal pausing of RNAPII by controlling an initiation-to-elongation switch modulated by TFIIE/Spt5 and P-TEFb. Our data also suggest that attenuated RNAPII pausing is a result of loss of CDK7 function; however, this may involve more factors than initially thought. The transcription coactivator Mediator was retained under conditions of CDK7 inhibition (Fig. 6D), consistent with a recent study in yeast (20). We also observed modest retention of NELF, which is not in agreement with previous studies (21) and warrants deeper investigation. The dissociation of TBP was also blocked with CDK7 inhibition at promoters and at a global level. This effect was seen only in the dynamic context of transcription (Fig. 6D), where preinitiation complexes are constantly formed and disassembled, suggesting that CDK7 may influence both its dissociation from and recruitment to promoters. Currently, such an effect cannot be explained mechanistically.
CDK7 presents as a critical Ser5 and Ser7 kinase when assayed with our chemical inhibitors, which is consistent with a multitude of earlier studies in yeast and in vertebrates (10–17). Our data show that CTD Ser5-/Ser7-P and transcription in vitro are essentially abolished at sufficiently high concentrations of inhibitors, stressing the potential importance of CTD phosphorylation in this context. In vivo, however, low doses of inhibitor caused decreases in nascent RNA synthesis and RNAPII occupancy but only minimal reductions in RNAPII Ser5-P levels. In quantitative terms, the reduction of newly synthesized RNA and of RNAPII occupancy, as well as the increase in GTFs, greatly exceeds the relative changes in CTD phosphorylation at gene and global levels. We are unable to conclusively state that broad generic Ser5 phosphorylation is the sole and perhaps even the major effector of CDK7 function in human cells, as there are multiple potential explanations for our observations: (i) blocking CDK7 causes small reductions in total Ser5-P or causes only alterations in patterning across the CTD that are nonetheless sufficient to attenuate nascent RNA synthesis, (ii) the effect of CDK7 inhibition on TFIIE and Spt5 is responsible for reduced transcription, or (iii) CDK7 interacts with PIC members or even an as yet unidentified factor(s) to regulate this process.
Previous characterization of CDK7, which identified TFIID, TFIIF, and TFIIE as substrates (21, 45), as well as data from our in vitro analyses raises the possibility that CDK7 acts at several steps in initiation and/or in early elongation. Blocking CDK7 after ATP-induced preactivation caused more moderate reductions than without preactivation (Fig. 4E), suggesting that CDK7 could function at least in part before initiation. On the other hand, it is known that the GTFs are dissociated during the transcription process as RNAPII moves from the TSS through proximal regions, for example, as shown with early in vitro data where TFIIE dissociation takes place prior to position +10 downstream of the TSS (46). Our in vivo data indicate effects prior to or shortly after elongation (Fig. 6A). The fact that TFIIE is retained with CDK7 but not CDK9 inhibitors (Fig. 6E) suggests that the underlying control occurs prior to RNAPII reaching the pause site. It likely requires a detailed genetic analysis to quantitatively assess how important the CDK7-mediated phosphorylation of this general factor is to its eventual eviction.
Additionally, as stalled RNAPII is recognized by replication protein A and ATR/ATM kinases, which then activate p53 (38), the lack of a rapid p53 response indirectly argues for a function of CDK7 prior to pause release, where CDK9 is the main effector (47–50). However, another possible explanation could be the overall limited effect on transcription.
Relevant to therapeutic applications, CDK7 inhibitors induce cell cycle delays and apoptosis. We find it noteworthy that under the conditions of limited CDK7 inhibition, effects on CDK1 and CDK2 T-loop phosphorylation were not detectable in asynchronously growing tumor cells. We reason that such effects may be detectable only in cells released from serum starvation (8). This finding, together with the late cell cycle response to the CDK7 inhibitors (after several days), suggests that the cell-type-specific alterations result at least in part from alterations in the gene expression program, which may eventually also dictate tumor cell survival.
Overall, our novel chemical inhibitors have illuminated novel aspects of the molecular mechanisms underlying CDK7 function and, specifically, regarding control of mRNA synthesis. They represent valuable tools for further functional analyses toward fully understanding the function of CDK7 and lead substances for future biomedical applications.
ACKNOWLEDGMENTS
This work was supported by the German Ministry for Research and Technology (BMBF; grant number 0313860D), followed by an internal grant of the IZKF of Faculty of Medicine of the Westfalian Wilhelms University (WWU) Muenster to M.M. and the Max Planck Foundation, Munich, Germany, to the Lead Discovery Center. We are grateful to the IZKF of the Faculty of Medicine of the WWU Muenster for support. We also thank the Excellency initiative Cells in Motion and the Cell Dynamics and Disease graduate school for supporting M.M. and T.W.R.K., respectively.
We further thank the members of the M.M. laboratory for support during the experimental phase and for helpful discussions.
Footnotes
Published ahead of print 21 July 2014
REFERENCES
- 1.Malumbres M, Barbacid M. 2005. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30:630–641. 10.1016/j.tibs.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Akoulitchev S, Mäkelä TP, Weinberg RA, Reinberg D. 1995. Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature 377:557–560. 10.1038/377557a0 [DOI] [PubMed] [Google Scholar]
- 3.Cismowski MJ, Laff GM, Solomon MJ, Reed SI. 1995. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol. Cell. Biol. 15:2983–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valay JG, Simon M, Dubois MF, Bensaude O, Facca C, Faye G. 1995. The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. J. Mol. Biol. 249:535–544. 10.1006/jmbi.1995.0316 [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Kung C, Fishburn J, Ansari AZ, Shokat KM, Hahn S. 2004. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol. Cell. Biol. 24:1721–1735. 10.1128/MCB.24.4.1721-1735.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wohlbold L, Larochelle S, Liao JC-F, Livshits G, Singer J, Shokat KM, Fisher RP. 2006. The cyclin-dependent kinase (CDK) family member PNQALRE/CCRK supports cell proliferation but has no intrinsic CDK-activating kinase (CAK) activity. Cell Cycle 5:546–554. 10.4161/cc.5.5.2541 [DOI] [PubMed] [Google Scholar]
- 7.Ganuza M, Sáiz-Ladera C, Cañamero M, Gómez G, Schneider R, Blasco MA, Pisano D, Paramio JM, Santamaría D, Barbacid M. 2012. Genetic inactivation of Cdk7 leads to cell cycle arrest and induces premature aging due to adult stem cell exhaustion. EMBO J. 31:2498–2510. 10.1038/emboj.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schachter MM, Merrick KA, Larochelle S, Hirschi A, Zhang C, Shokat KM, Rubin SM, Fisher RP. 2013. A Cdk7-Cdk4 T-loop phosphorylation cascade promotes G1 progression. Mol. Cell 50:250–260. 10.1016/j.molcel.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roeder RG. 2005. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579:909–915. 10.1016/j.febslet.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 10.Roy R, Adamczewski JP, Seroz T, Vermeulen W, Tassan JP, Schaeffer L, Nigg EA, Hoeijmakers JH, Egly JM. 1994. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell 79:1093–1101. 10.1016/0092-8674(94)90039-6 [DOI] [PubMed] [Google Scholar]
- 11.Trigon S, Serizawa H, Conaway JW, Conaway RC, Jackson SP, Morange M. 1998. Characterization of the residues phosphorylated in vitro by different C-terminal domain kinases. J. Biol. Chem. 273:6769–6775. 10.1074/jbc.273.12.6769 [DOI] [PubMed] [Google Scholar]
- 12.Pinhero R, Liaw P, Bertens K, Yankulov K. 2004. Three cyclin-dependent kinases preferentially phosphorylate different parts of the C-terminal domain of the large subunit of RNA polymerase II. Eur. J. Biochem. 271:1004–1014. 10.1111/j.1432-1033.2004.04002.x [DOI] [PubMed] [Google Scholar]
- 13.Akhtar MS, Heidemann M, Tietjen JR, Zhang DW, Chapman RD, Eick D, Ansari AZ. 2009. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 34:387–393. 10.1016/j.molcel.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover-Cutter K, Larochelle S, Erickson B, Zhang C, Shokat K, Fisher RP, Bentley DL. 2009. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol. Cell. Biol. 29:5455–5464. 10.1128/MCB.00637-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Suh H, Cho E-J, Buratowski S. 2009. Phosphorylation of the yeast Rpb1 C-terminal domain at serines 2, 5, and 7. J. Biol. Chem. 284:26421–26426. 10.1074/jbc.M109.028993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boeing S, Rigault C, Heidemann M, Eick D, Meisterernst M. 2010. RNA polymerase II C-terminal heptarepeat domain Ser-7 phosphorylation is established in a mediator-dependent fashion. J. Biol. Chem. 285:188–196. 10.1074/jbc.M109.046565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiekhattar R, Mermelstein F, Fisher RP, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, Reinberg D. 1995. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 374:283–287. 10.1038/374283a0 [DOI] [PubMed] [Google Scholar]
- 18.Li B, Carey M, Workman JL. 2007. The role of chromatin during transcription. Cell 128:707–719. 10.1016/j.cell.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 19.Phatnani HP, Greenleaf AL. 2006. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20:2922–2936. 10.1101/gad.1477006 [DOI] [PubMed] [Google Scholar]
- 20.Wong KH, Jin Y, Struhl K. 2014. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol. Cell 54:601–612. 10.1016/j.molcel.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larochelle S, Amat R, Glover-Cutter K, Sansó M, Zhang C, Allen JJ, Shokat KM, Bentley DL, Fisher RP. 2012. Cyclin-dependent kinase control of the initiation-to-elongation switch of RNA polymerase II. Nat. Struct. Mol. Biol. 19:1108–1116. 10.1038/nsmb.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helenius K, Yang Y, Tselykh TV, Pessa HKJ, Frilander MJ, Mäkelä TP. 2011. Requirement of TFIIH kinase subunit Mat1 for RNA Pol II C-terminal domain Ser5 phosphorylation, transcription and mRNA turnover. Nucleic Acids Res. 39:5025–5035. 10.1093/nar/gkr107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert TK, Rigault C, Eickhoff J, Baumgart K, Antrecht C, Klebl B, Mittler G, Meisterernst M. 2014. Characterisation of molecular and cellular CDK9 functions using a novel specific inhibitor. Br. J. Pharmacol. 171:55–68. 10.1111/bph.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475–1489. 10.1093/nar/11.5.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlmann T, Boeing S, Lehmbacher M, Meisterernst M. 2007. The VP16 activation domain establishes an active mediator lacking CDK8 in vivo. J. Biol. Chem. 282:2163–2173. 10.1074/jbc.M608451200 [DOI] [PubMed] [Google Scholar]
- 26.Vojnic E, Mourão A, Seizl M, Simon B, Wenzeck L, Larivière L, Baumli S, Baumgart K, Meisterernst M, Sattler M, Cramer P. 2011. Structure and VP16 binding of the Mediator Med25 activator interaction domain. Nat. Struct. Mol. Biol. 18:404–409. 10.1038/nsmb.1997 [DOI] [PubMed] [Google Scholar]
- 27.Albert TK, Grote K, Boeing S, Meisterernst M. 2010. Basal core promoters control the equilibrium between negative cofactor 2 and preinitiation complexes in human cells. Genome Biol. 11:R33. 10.1186/gb-2010-11-3-r33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali S, Heathcote DA, Kroll SHB, Jogalekar AS, Scheiper B, Patel H, Brackow J, Siwicka A, Fuchter MJ, Periyasamy M, Tolhurst RS, Kanneganti SK, Snyder JP, Liotta DC, Aboagye EO, Barrett AGM, Coombes RC. 2009. The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res. 69:6208–6215. 10.1158/0008-5472.CAN-09-0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jazbutyte V, Thum T. 2010. MicroRNA-21: from cancer to cardiovascular disease. Curr. Drug Targets 11:926–935. 10.2174/138945010791591403 [DOI] [PubMed] [Google Scholar]
- 30.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. 2004. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 64:3753–3756. 10.1158/0008-5472.CAN-04-0637 [DOI] [PubMed] [Google Scholar]
- 31.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. 2004. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. U. S. A. 101:2999–3004. 10.1073/pnas.0307323101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neff AT, Lee JY, Wilusz J, Tian B, Wilusz CJ. 2012. Global analysis reveals multiple pathways for unique regulation of mRNA decay in induced pluripotent stem cells. Genome Res. 22:1457–1467. 10.1101/gr.134312.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MC, Debrosse M, Smith M, Dey A, Huynh W, Sarai N, Heightman TD, Tamura T, Ozato K. 2013. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol. Cell. Biol. 33:2497–2507. 10.1128/MCB.01180-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh J, Padgett RA. 2009. Rates of in situ transcription and splicing in large human genes. Nat. Struct. Mol. Biol. 16:1128–1133. 10.1038/nsmb.1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebara MM, Sayre MH, Corden JL. 1997. Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J. Cell Biochem. 64:390–402. [DOI] [PubMed] [Google Scholar]
- 36.Rickert P, Corden JL, Lees E. 1999. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene 18:1093–1102. 10.1038/sj.onc.1202399 [DOI] [PubMed] [Google Scholar]
- 37.Czudnochowski N, Bösken CA, Geyer M. 2012. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat. Commun. 3:842. 10.1038/ncomms1846 [DOI] [PubMed] [Google Scholar]
- 38.Derheimer FA, O'Hagan HM, Krueger HM, Hanasoge S, Paulsen MT, Ljungman M. 2007. RPA and ATR link transcriptional stress to p53. Proc. Natl. Acad. Sci. U. S. A. 104:12778–12783. 10.1073/pnas.0705317104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arima Y, Nitta M, Kuninaka S, Zhang D, Fujiwara T, Taya Y, Nakao M, Saya H. 2005. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J. Biol. Chem. 280:19166–19176. 10.1074/jbc.M410691200 [DOI] [PubMed] [Google Scholar]
- 40.Blaydes JP, Hupp TR. 1998. DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site. Oncogene 17:1045–1052. 10.1038/sj.onc.1202014 [DOI] [PubMed] [Google Scholar]
- 41.Ljungman M, Zhang F, Chen F, Rainbow AJ, McKay BC. 1999. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene 18:583–592. 10.1038/sj.onc.1202356 [DOI] [PubMed] [Google Scholar]
- 42.Ko LJ, Shieh SY, Chen X, Jayaraman L, Tamai K, Taya Y, Prives C, Pan ZQ. 1997. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol. Cell. Biol. 17:7220–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu H, Fisher RP, Bailey P, Levine AJ. 1997. The CDK7-cycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Mol. Cell. Biol. 17:5923–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717–728. 10.1016/S0092-8674(00)81641-4 [DOI] [PubMed] [Google Scholar]
- 45.Ohkuma Y, Roeder RG. 1994. Regulation of TFIIH ATPase and kinase activities by TFIIE during active initiation complex formation. Nature 368:160–163. 10.1038/368160a0 [DOI] [PubMed] [Google Scholar]
- 46.Zawel L, Kumar KP, Reinberg D. 1995. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 9:1479–1490. 10.1101/gad.9.12.1479 [DOI] [PubMed] [Google Scholar]
- 47.Peterlin BM, Price DH. 2006. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23:297–305. 10.1016/j.molcel.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 48.Wada T, Takagi T, Yamaguchi Y, Watanabe D, Handa H. 1998. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 17:7395–7403. 10.1093/emboj/17.24.7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. 2010. C-Myc regulates transcriptional pause release. Cell 141:432–445. 10.1016/j.cell.2010.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lis JT, Mason P, Peng J, Price DH, Werner J. 2000. P-TEFb kinase recruitment and function at heat shock loci. Genes Dev. 14:792–803 [PMC free article] [PubMed] [Google Scholar]
- 51.Olive V, Jiang I, He L. 2010. Mir-17-92, a cluster of miRNAs in the midst of the cancer network. Int. J. Biochem. Cell Biol. 42:1348–1354. 10.1016/j.biocel.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]