Abstract
The nontuberculous mycobacteria are a large group of acid-fast bacteria that are very widely distributed in the environment. While Mycobacterium avium was once regarded as innocuous, its high frequency as a cause of disseminated disease in HIV-positive individuals illustrated its potential as a pathogen. Much more recently, there is growing evidence that the incidence of M. avium and related nontuberculous species is increasing in immunocompetent individuals. The same has been observed for M. abscessus infections, which are very difficult to treat; accordingly, this review focuses primarily on these two important pathogens. Like the host response to M. tuberculosis infections, the host response to these infections is of the TH1 type but there are some subtle and as-yet-unexplained differences.
INTRODUCTION
Although nontuberculous mycobacteria have long been recognized, there is far less information regarding their pathogenicity than that of their more famous relatives Mycobacterium tuberculosis and Mycobacterium leprae. It is becoming increasingly clear, however, that the incidence of certain members of the nontuberculous mycobacterial family may be increasing, an observation not related simply to better recognition, typing, and diagnosis. Of this family, the host response to M. avium is perhaps the best understood, but even here there are interesting differences in the expression of immunity that have yet to be explained.
NOMENCLATURE
One cannot begin to address this issue without considering the current nomenclature, a morass in itself. We are using here the term “nontuberculous mycobacteria” (NTM) but could easily use “atypical mycobacteria,” “environmental mycobacteria,” the subset “rapidly growing mycobacteria” (RGM), “mycobacteria other than M. tuberculosis,” or even the “quasi-too-complex” term that can be applied to M. avium and M. abscessus. NTM is perhaps the most widely used, but one has to realize that “tuberculous” refers to “not tuberculosis” not “no tubercles.”
ECOLOGY AND EPIDEMIOLOGY
NTM were suspected as potential causes of human infections in the sanatorium era, but it was not until the 1950s that direct evidence became available. Even then, NTM were initially regarded as simple saprophytes of limited, if any, virulence occurring only in people with other predisposing lung conditions (as discussed further below). This opinion, of course, changed dramatically when M. avium complex (MAC) species emerged as major opportunistic infections in patients with HIV and more recently with observations of increases in infections with other NTM such as M. abscessus in elderly patients.
Because of extensive research, including seminal work by Falkinham, we now know that NTM are widely distributed in the environment and can cause opportunistic infections in multiple mammals, fish, and birds (particularly poultry) (1). In fact, it seems that NTM can grow essentially anywhere (2) and thrive where competing microbes are destroyed, such as in chlorinated water (3). In untreated water, NTM can even parasitize amoebae (4, 5). Even when the water supply is treated, NTM persist, and a very recent study in Holland (6) found NTM in samples of drinking water from eight separate treatment plants.
The first methods for the identification of NTM were developed in the 1950s by Ernest Runyon and were based on pigmentation and growth rates, but the development of 16S rRNA gene sequencing replaced these classical methods, allowing the identification of over 150 species of NTM. Data generated through numerical taxonomy studies that were conducted from the late 1960s through the 1970s, as well as DNA-DNA hybridization analyses, established relationships among NTM strains and provided an important bridge between the purely phenotypic Runyon classification approach and 16S rRNA gene sequencing. Moreover, taxonomic classifications based on DNA-DNA hybridizations were almost always confirmed by 16S rRNA gene sequencing and later by whole-genome sequencing. Accordingly, these applications of molecular techniques allowing genetic analysis of NTM dramatically facilitated classification and subsequent epidemiological studies (7). For example, a recent study described seven examples of identification of NTM in a plumbing system that had a DNA fingerprint identical to that of an isolate from a patient living in the same household (8). Furthermore, whole-genome sequencing of M. abscessus in cystic fibrosis patients indicated human-to-human transmission of this infection (9), although it should be stressed that this study has yet to be replicated.
The highly hydrophobic cell wall of NTM may facilitate aerosolization (5), and indeed, such insights began to provide an explanation as to why HIV-positive individuals were exposed to M. avium as a primary opportunistic pathogen. NTM can adhere to surfaces, and many NTM are resistant to both antibiotics and disinfectants such as chlorine. Many are oligotrophic (10), requiring low levels of two-carbon sources and limited access to metal ions, permitting significant survival and persistence in the environment. (Interestingly, a similar picture may be emerging in the context of M. tuberculosis persisting in necrotic tissue [11, 12].)
A key ability of NTM, becoming regarded as very important, is the formation of biofilms in the environment and hypothetically in vivo (13). Moreover, biofilm development and formation can consist of several distinct types of structures. Survival in this state may allow these bacilli to persist for very lengthy periods of time in the environment.
CLINICAL ASPECTS
NTM infections in humans fall into three main categories (14). Hypersensitivity pneumonitis is thought to be triggered by inhalation of NTM in water droplets from sources such as shower water (aerosolized by the shower head), baths, and hot tubs (hot-tub lung) (15). This may have been the primary source of MAC infections in HIV patients in the United States (where people tend to shower more often than they bathe).
Cavitary (tuberculosis-like) disease can be caused by multiple NTM species, again predominantly MAC. There is a strong association with underlying lung disease, such as chronic obstructive pulmonary disease (COPD), and with smoking or prior tuberculosis. Like patients with tuberculosis, these patients tend to have upper lobe cavitary disease, as well as standard tuberculosis-like symptoms (2).
Nodular bronchiectasis is associated mostly with MAC and can sometimes occur in mixed infections with M. abscessus. It is often seen in older nonsmoking females. These ladies are often thin, hence the name “Lady Windermere syndrome.” (from Oscar Wilde's famous play; the title lady is prim and proper, but at that point, the connection to a thin lady coughing seems to completely end).
Disease can also occur in the context of preexisting conditions. NTM infections occur in patients with cystic fibrosis; in such cases, 75% of the infections are caused by MAC (16, 17). NTM (most of which were M. gordonae) were cultured from 22% of patients showing signs of acute exacerbation of COPD (18). Nosocomial NTM infections are sometimes associated with water sources such as showers, Jacuzzis, swimming pools, saunas, and hospitals (18).
Drug therapy of NTM infections can be very difficult (for an outstanding review on this specific topic, see reference 19), with therapy long and costly (14, 20). One important factor is antibiotic inactivation, with resistance mechanisms that include beta-lactamases, aminoglycoside phosphotransferases, and aminoglycoside acetyltransferases. In addition, the p55 efflux pump confers resistance to tetracyclines and aminoglycosides, and multiple other efflux pumps likely cause antimicrobial resistance and persistence (20). Lastly, the erythromycin ribosomal methylase (erm) gene found in almost all M. abscessus subsp. abscessus and M. fortuitum strains (but not in M. chelonae) results in methylation of the 23S rRNA, rendering the bacteria resistant to macrolides, a mainstay of many NTM treatment regimens. Induction of this gene is not detected by conventional susceptibility testing, as this requires extended incubation and observation for up to 2 weeks.
A further issue of concern is the fact that multiple cases of in vitro susceptibility testing do not correlate with clinical outcomes (19). One possibility here is that drug susceptibility testing performed with single-cell suspensions in nutrient broth may not actually reflect the resistance of bacteria forming biofilms in necrotic lung tissue. Indeed, our laboratory has suggested this as a basis for the prolonged period of chemotherapy needed in animal models of tuberculosis, particularly the guinea pig model, where bacilli persist in necrotic tissue by forming biofilm-like communities (necrosis-associated extracellular clusters) (11, 21).
MAC infections are usually treated with rifampin, ethambutol, and a macrolide such as azithromycin (the finding that such macrolides could kill MAC had a substantial impact on the treatment of MAC in AIDS patients). M. kansasii tends to be susceptible to standard isoniazid, rifampin, and pyrazinamide therapy, similar to M. tuberculosis, and the addition of clarithromycin can also be beneficial. For infections with M. abscessus, outcomes are much worse; in fact, this organism seems to be essentially untreatable, with only ∼50% sputum conversion seen in chemotherapy studies. If lung resection is not an option, then amikacin, cefoxitin, and imipenem are tried, and in some cases, the isolate may respond to macrolide therapy, as indicated by drug susceptibility testing. As noted, M. abscessus has subspecies; as does M. massiliense, which lacks a macrolide resistance gene and hence is fully susceptible (22). In many cases, however, given the toxicity of these second-line drugs, it may be hard for the patient to tolerate therapy.
In the mid-1990s in the United States, NTM, especially MAC, caused an alarming number of serious opportunistic infections in HIV-positive individuals, particularly those with low CD4 counts (2, 23). In these patients, these infections tended to occur as a disseminated disease involving multiple organs rather than only the lungs. A major difficulty in treatment at the time arose when it was noticed that rifampin, which often works well against MAC, accelerated the clearance of the antiviral drugs being used to inhibit HIV. In addition, the latter drugs slowed the metabolism of rifampin, letting it reach toxic levels. This problem was finally overcome when the susceptibility of MAC to the new macrolides was discovered.
INCREASING INCIDENCE OF NTM INFECTIONS
There is reason to believe that the incidence of NTM infections is rising, and this may not reflect the possibility that this just represents better detection, which has significantly improved (24). Part of the increase probably reflects better diagnostic methods and increasing recognition of the importance of NTM, but on the basis of tracking records by public health laboratories that have bothered to keep them, this increase is definitely real.
Evidence shows that the incidence of NTM is increasing worldwide, with MAC the most frequent cause (14). Certain geographic areas seem to be foci, such as Taiwan and eastern Canada (25, 26). A recent report from Taiwan (27) noted increases in NTM cases, including a surge in cases of M. abscessus infection. Although still rare, mixed infections involving M. kansasii, MAC, and M. abscessus have been seen (28).
In a huge study (29), Hoefsloot and an army of colleagues recently provided an analysis of the global distribution of NTM infections by using data from the NTM-Network European Trials framework program. These data revealed that 91 species of NTM had been identified in over 20,000 patients from 30 countries. MAC were the most prevalent, followed by M. gordonae and M. xenopi.
There were obvious geographic differences revealed by this study. MAC is prevalent in North America but much less so in South America. Cases of M. xenopi infection tended to focus in Europe and to some degree in eastern Canada. M. malmoense tends to be found in northern Europe and the United Kingdom. Of the RGM subgroup members, M. abscessus and M. fortuitum were found worldwide. Foci of M. abscessus were noted, particularly in Asia. Why these geographic distributions exist is totally unknown.
A further curiosity that has been increasingly observed is a change in human demographics. This reflects evidence indicating a gender shift from mostly males with predisposing conditions to a current 80% incidence in middle-aged to elderly females (30, 31). A similar picture is emerging in Australia (32), where increases in cases of NTM (M. intracellulare being the most prevalent) have shifted from middle-aged smoking men to older nonsmoking females. Further examination of this issue seemed to suggest that a greater susceptibility was associated with the body characteristics of these women; they tended to be taller and rather thin, suggesting a genetic trait, and indeed, a third were shown to express the cystic fibrosis-associated transmembrane conductance regulator gene CFTR (33). Leptin levels in these females may also contribute (34).
HOST IMMUNE RESPONSE TO NTM
From an immunological point of view, there was little interest in NTM until Stanford and his colleague Rook suggested in 1981 (65) that these bacteria could potentially influence the efficacy of M. bovis BCG vaccine, resulting in reduced vaccine-derived protection. Soon after, a study appeared (35) showing that NTM behaves differently in terms of growth in activated murine macrophages; while M. tuberculosis was inhibited, M. kansasii and M. avium were slowed but not killed and M. intracellulare seemed unaffected. If mice were infected with these bacilli, the animals were protected against a second infection with M. tuberculosis (36).
BCG vaccination was then shown to be protective against infection with M. kansasii or M. avium but was not effective against M. simiae or M. intracellulare (37). Studies then showed that protection was mediated by T cells, as shown by passive cell transfer (36).
At the time, resistance to mycobacterial infections was thought to be associated with a gene, Bcg (now NRAMP1). NTM did, indeed, grow differently in Bcgs and Bcgr strains, but backcross and mouse chimera studies showed this event to be multigenic (38).
The stomach was traditionally thought to be a barrier to mycobacteria, but it was shown that virulent M. avium strains could infect mice orally and be found in gut lymphoid tissues. If the beige mutant in the C57 mouse background was used, this was amplified (39, 40). Subsequent studies showed that M. avium crossed the gut epithelium via interactions with enterocytes (41). Once M. avium was in, a CD4 response was important for host immunity whereas a local CD8 or NK cell response was not.
In addition, as shown earlier, these strains of M. avium were slowed but not killed when cultured in activated macrophages. At this time, the role of TH1 cytokines began to be investigated, and an early study suggested that tumor necrosis factor alpha (TNF-α) was more effective than gamma interferon (IFN-γ) in growth inhibition (42). Soon after, it was demonstrated that a virulent colony morphotype smooth transparent (SmT) isolate induced a delayed, relatively small TNF-α response, whereas the smooth domed (SmD) equivalent induced a rapid, large TNF-α response (43). It was then found that while macrophage activation generated reactive oxygen species in response to M. avium, isolates expressing the virulent SmT form were unaffected (44). Further studies showed that, in general, SmT isolates were virulent and SmD isolates were much less so, with rough variants showing an intermediate phenotype (45). As predicted, there were large differences between the inflammatory and protective cytokine/chemokine profiles of SmT and Smd/SmO infections (46, 47).
Nitric oxide (NO) was also produced by activated macrophages, but blocking of this event did not influence growth inhibition, and subsequent studies raised the possibility that acidification of the bacterial phagosome was, in fact, more important in the control of the infection (46). In vivo, CD4 involvement was crucial for both protection and granuloma formation, and it was suggested at the time that the TH1 cytokines IFN-γ and TNF-α produced by these cells act in synergy (47). Consistent with the TH1 pathway hypothesis, depletion of interleukin-12 (IL-12) also reduced resistance to M. avium infection (48). In addition to CD4 cells, IL-12 was also implicated in TNF-α production by NK cells in M. avium infections (49). An early role for IL-6 was suggested, as was a late response involving IL-10 (50). More recently, a role for TNF-α has been illustrated by infections occurring in patients treated with anti-TNF-α biologic reagents for rheumatoid arthritis (51, 52). M. haemophilum or M. avium was seen in 52 cases, with a high rate of extrapulmonary disease.
Consistent with earlier studies, T cells harvested from mice infected with M. avium transferred protection but only if live bacteria were used (53). This was, of course, consistent with observations at the time that only live M. tuberculosis was capable of generating protective T cells (54). While CD4 T cells were the primary source of protection, some studies suggested a contribution by CD8 cells (55), although the latter were eventually shown not to be critical.
Further investigation of the beige-mouse model indicated a neutrophil defect, and depletion studies with wild-type C57 mice produced a similar effect (56). This was then shown to reflect poor cellular accumulation due to a diminished chemokine response that was subsequently shown to be directed via CXCR2 expression (57).
A 1999 study (58) provided the unexpected result that mice that lack the NOS2 gene and cannot make NO within their macrophages were more resistant than wild-type controls to M. avium (the opposite of the result obtained with M. tuberculosis), at least partially explaining why virulent M. avium strains grow in mice despite very strong TH1 responses (59), and subsequent studies also demonstrated a superior fibrotic response in the lungs of gene knockout mice and showed that the increased resistance was consistent when multiple virulent M. avium strains were used (60). A possible explanation was the observation that along with TNF-α, NO controlled granuloma integrity rather than being directly antimicrobial (61). TNF-α seemed critical, since in TNF receptor-deficient mice, M. avium generated severe, fatal necrosis (62), although a later paper suggested otherwise (63).
Dormant for more than a decade, the idea that NTM could interfere with BCG vaccination itself reactivated. Studies by Brandt et al. showed that if mice were immunized with various NTM prior to BCG vaccination, the vaccine could not proliferate to any extent and efficacy was subsequently reduced (64). An initial explanation (65), put forward by Rook et al., was that the mixture of infections unbalanced immunity and did not allow activity by regulatory T cells. This is a rather elegant idea, and recent unpublished studies in our laboratory indicate that exposure in the gut to M. avium induces regulatory T cells that then counterbalance BCG-induced effector cells in the lungs after a challenge. A subsequent review by Andersen and Doherty nicely put all of this in perspective (66) by explaining that NTM can, in some cases, block effector immunity, diminishing protection by BCG, or can add to it. It is plausible that live NTM generating T cell immunity cross-reactive to M. tuberculosis antigens can mask the effects of BCG by itself. This idea was more recently confirmed by studies showing that people exposed to NTM have IFN-γ+ T cells that recognize multiple proteins from the DosR gene complex (67).
In 1999, a paper appeared that indicated that when M. avium grew in macrophages, some bacilli grew well but a second static population was also present (68). One explanation that appeared 4 years later was the observation that M. avium can form biofilms (69). It was then found that the capacity to form these biofilms seemed to involve glycopeptidolipid (GPL) expression by the bacillus and that mutants unable to do so were far less invasive. Our recent unpublished studies showed that M. tuberculosis mutants that cannot form biofilms also cannot form pellicles in vitro and fail to persist in animals in vivo. A very recent paper (70) associated disruption of the pks12 gene in M. avium with loss of biofilm formation (preliminary [unpublished] data suggest the same for M. tuberculosis).
TH1 immunity can be triggered through Toll-like receptors (TLRs), and it was found that mice lacking TLR2 were more susceptible to M. avium infection (71, 72). Signaling via TLR2 operates via the mitogen-activated protein kinase pathway in a TNF- and TRAIL-independent manner (72). TLR9 signaling may also play a small role. Recent evidence (73) suggests that NTM initially trigger immunity via the AIM2-inflammasome whereas M. tuberculosis does so via NLRP3.
Chronic infection of mice not only can result in severe necrosis but also (unlike tuberculosis) can result in a gradual loss of T cells (lymphopenia) (74). Such observations illustrate that the pathogenesis of these diseases is still less well understood (75), with elements of the disease process in response to certain NTM infections showing clear differences (76), lymphopenia being one such example.
Although multiple virulence factors associated with the NTM have been proposed, this picture is still not fully clear. At one point, possession of a virulence plasmid was suggested but never verified. The biological activity of various cell wall GPLs (77) has also been suggested as a factor, but this also has not been verified.
IMMUNITY TO M. ABSCESSUS, AN EMERGING PATHOGEN
Although identified as a member of the 150-plus NTM organisms, M. abscessus was barely noticed until it started to become evident over the past decade or to be clinically important. Even now, however, we know little about the host response to it (34, 78).
Compared to M. avium (M. abscessus has the reverse phenotype), rough strains of M. abscessus tend to be virulent while smooth strains are less so (79). Like M. avium, the organism expresses GPL, but rough mutants have been found that do not (80). Although much less virulent than M. avium, M. abscessus can persist in vivo in recently developed animal models such as SCID, nude, and granulocyte-macrophage colony-stimulating factor gene disruption mice and cystic fibrosis models causing a progressive pulmonary infection (unpublished results). Whereas wild-type isolates generate strong TH1 responses and are easily cleared in animal models (81), GPL-defective mutants can cause rapid death (82). Given the very rapid death, within a week, this cannot be explained in terms of bacterial growth and instead suggests some sort of shock reaction (no autopsy data were provided in that report). In addition, death resulting from infection with a rough variant has been reported (83).
Induction of host immunity did not initially appear to be associated with TLR2 signaling, since both rough and smooth variants triggered a TLR2-mediated response equally (72). However, a further study described a GPL+ strain that could form a biofilm but could not trigger a TLR2-mediated response, whereas a rough variant of this was GPL− and could not form a biofilm but could trigger a TLR2-mediated response (84). This led to the hypothesis that GPL on the outer cell wall of M. abscessus allows the bacillus to initially avoid inducing immunity by avoiding TLR2 triggering, but when it is not present, this unmasks other materials, possibly phosphatidylinositol mannosides or lipoarabinomannans, that are known to be TLR2 agonists. More recently, disruption of the mmpL4b gene, which is involved in GPL biosynthesis, resulted in bacilli that could trigger TLR2 in macrophage cultures (85, 86); these mutants lack GPL but also seem to upregulate the production of cell wall lipoproteins (87). What has yet to be explained, however, is why mechanisms other than TLR2 signaling do not compensate and prevent GPL− M. abscessus from so quickly killing mice.
Other recent studies using the aerosol route of infection provided similar results, with evidence of strong TH1 responses and granuloma formation in both the mouse and guinea pig models (78); however, it was noted that relatively high doses were needed to establish any degree of infection by aerosol. Under these experimental conditions, this seems to drive a TH1 response (predominantly IFN-γ-secreting CD4 T cells) that seems capable of controlling this infection in immunocompetent mice and clearing it. How this can progress to a chronic, uncontained infection is far from clear, but it might involve mechanisms similar to those seen in M. tuberculosis infections in which T cell plasticity shifts to a less favorable TH17 type of response (as illustrated in Fig. 1). There is little evidence to go on as yet.
FIG 1.
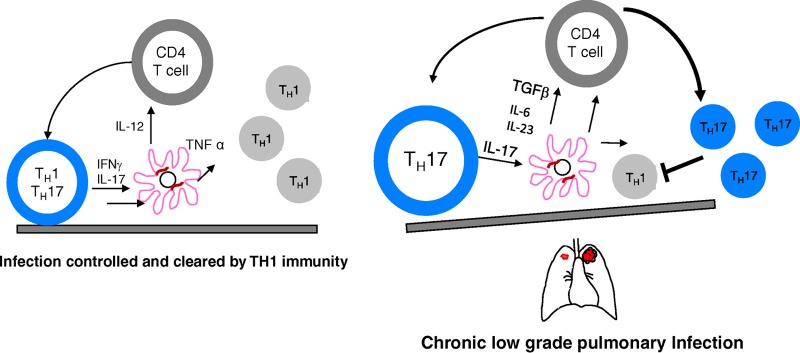
Hypothetical model explaining the persistence of M. abscessus and establishment of chronic disease. Mouse models clearly indicate that TH1 immunity predominates in situations where this pathogen can be cleared, but there is little evidence as yet explaining its survival and chronic disease. One possibility involves T cell plasticity in which a TH17 response becomes dominant, resulting in loss of a protective TH1 response and its replacement by cells that can continually drive low-grade inflammation.
KNOWLEDGE GAPS AND FUTURE DIRECTIONS
The current epidemiological data seem to indicate that the incidence of the pathogens M. avium and M. abscessus is increasing, and this information needs to be regarded with concern. The host response to these pathogens involves the TH1 response, and while this is unsurprising, there are still large gaps in our knowledge. First, there are facets of the host response, particularly to MAC, that remain enigmatic. These include the loss of reactive T cells and the apparent resistance of MAC to reactive nitrogen. Second, with regard to M. abscessus, animal models are currently very limited and host immunity and pathogenesis are difficult to measure unless very large doses of bacilli are given intravenously. If small doses are given, there is little evidence that a productive infection is even fully established. As a result, better models are needed, particularly in terms of elucidation of pathogenesis, enabling better new drug regimens for M. abscessus infections, where current therapeutic outcomes are very poor.
Biographies

Ian Orme is a University Distinguished Professor of Microbiology, Immunology and Pathology at Colorado State University. He received his B.Sc. degree in physiology and his Ph.D. in immunology from the University of London and then trained at the Trudeau Institute in Saranac Lake before joining the faculty at CSU. He has devoted his career to developing small-animal models of mycobacterial diseases.

Diane J. Ordway is currently an Associate Professor at Colorado State University Mycobacteria Research Laboratory, Department of Microbiology, Immunology and Pathology. Dr. Ordway received a full doctoral scholarship to attend the London School of Hygiene & Tropical Medicine, receiving her Ph.D. in infectious and tropical diseases. She is an immunologist with 18 years of experience devoted to host-pathogen interactions in human and animal models of infection.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Falkinham JO., III 2013. Ecology of nontuberculous mycobacteria–where do human infections come from? Semin. Respir. Crit. Care Med. 34:95–102. 10.1055/s-0033-1333568 [DOI] [PubMed] [Google Scholar]
- 2.McGrath EE, Blades Z, McCabe J, Jarry H, Anderson PB. 2010. Nontuberculous mycobacteria and the lung: from suspicion to treatment. Lung 188:269–282. 10.1007/s00408-010-9240-9 [DOI] [PubMed] [Google Scholar]
- 3.van Ingen J, Blaak H, de Beer J, de Roda Husman AM, van Soolingen D. 2010. Rapidly growing nontuberculous mycobacteria cultured from home tap and shower water. Appl. Environ. Microbiol. 76:6017–6019. 10.1128/AEM.00843-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernández-Garduño E, Elwood K. 2012. Nontuberculous mycobacteria in tap water. Emerg. Infect. Dis. 18:353. 10.3201/eid1802.110455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkinham JO., III 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J. Appl. Microbiol. 107:356–367. 10.1111/j.1365-2672.2009.04161.x [DOI] [PubMed] [Google Scholar]
- 6.van der Wielen PW, van der Kooij D. 2013. Nontuberculous mycobacteria, fungi, and opportunistic pathogens in unchlorinated drinking water in The Netherlands. Appl. Environ. Microbiol. 79:825–834. 10.1128/AEM.02748-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behr MA, Falkinham JO., III 2009. Molecular epidemiology of nontuberculous mycobacteria. Future Microbiol. 4:1009–1020. 10.2217/fmb.09.75 [DOI] [PubMed] [Google Scholar]
- 8.Falkinham JO., III 2011. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg. Infect. Dis. 17:419–424. 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkinham JO. 2010. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol. 5:951–960. 10.2217/fmb.10.53 [DOI] [PubMed] [Google Scholar]
- 11.Orme IM, Roberts AD, Furney SK, Skinner PS. 1994. Animal and cell-culture models for the study of mycobacterial infections and treatment. Eur. J. Clin. Microbiol. Infect. Dis. 13:994–999. 10.1007/BF02111500 [DOI] [PubMed] [Google Scholar]
- 12.Orme IM. 2014. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis (Edinb.) 94:8–14. 10.1016/j.tube.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MM, Yakrus MA, Arduino MJ, Cooksey RC, Crane CB, Banerjee SN, Hilborn ED, Donlan RM. 2009. Structural analysis of biofilm formation by rapidly and slowly growing nontuberculous mycobacteria. Appl. Environ. Microbiol. 75:2091–2098. 10.1128/AEM.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss CH, Glassroth J. 2012. Pulmonary disease caused by nontuberculous mycobacteria. Exp. Rev. Respir. Med. 6:597–612. 10.1586/ers.12.58 [DOI] [PubMed] [Google Scholar]
- 15.Glazer CS, Martyny JW, Lee B, Sanchez TL, Sells TM, Newman LS, Murphy J, Heifets L, Rose CS. 2007. Nontuberculous mycobacteria in aerosol droplets and bulk water samples from therapy pools and hot tubs. J. Occup. Environ. Hyg. 4:831–840. 10.1080/15459620701634403 [DOI] [PubMed] [Google Scholar]
- 16.Pinto-Powell R, Olivier KN, Marsh BJ, Donaldson S, Parker HW, Boyle W, Knowles M, Magnusson M, von Reyn CF. 1996. Skin testing with Mycobacterium avium sensitin to identify infection with M. avium complex in patients with cystic fibrosis. Clin. Infect. Dis. 22:560–562. 10.1093/clinids/22.3.560 [DOI] [PubMed] [Google Scholar]
- 17.Leung JM, Olivier KN. 2013. Nontuberculous mycobacteria in patients with cystic fibrosis. Semin. Respir. Crit. Care Med. 34:124–134. 10.1055/s-0033-1333574 [DOI] [PubMed] [Google Scholar]
- 18.Hoefsloot W, van Ingen J, Magis-Escurra C, Reijers MH, van Soolingen D, Dekhuijzen RP, Boeree MJ. 2013. Prevalence of nontuberculous mycobacteria in COPD patients with exacerbations. J. Infect. 66:542–545. 10.1016/j.jinf.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 19.Brown-Elliott BA, Nash KA, Wallace RJ., Jr 2012. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 25:545–582. 10.1128/CMR.05030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updat. 15:149–161. 10.1016/j.drup.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Ordway DJ, Shanley CA, Caraway ML, Orme EA, Bucy DS, Hascall-Dove L, Henao-Tamayo M, Harton MR, Shang S, Ackart D, Kraft SL, Lenaerts AJ, Basaraba RJ, Orme IM. 2010. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob. Agents Chemother. 54:1820–1833. 10.1128/AAC.01521-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stout JE, Floto RA. 2012. Treatment of Mycobacterium abscessus: all macrolides are equal, but perhaps some are more equal than others. Am. J. Respir. Crit. Care Med. 186:822–823. 10.1164/rccm.201208-1500ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bermudez LE, Inderlied CB, Young LS. 1992. Mycobacterium avium complex in AIDS. Curr. Clin. Top. Infect. Dis. 12:257–281 [PubMed] [Google Scholar]
- 24.Epson E, Cassidy M, Marshall-Olson A, Hedberg K, Winthrop KL. 2012. Patients with nontuberculous mycobacteria: comparison of updated and previous diagnostic criteria for lung disease. Diagn. Microbiol. Infect. Dis. 74:98–100. 10.1016/j.diagmicrobio.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 25.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR. 2010. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg. Infect. Dis. 16:294–296. 10.3201/eid1602.090675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Houqani M, Jamieson F, Chedore P, Mehta M, May K, Marras TK. 2011. Isolation prevalence of pulmonary nontuberculous mycobacteria in Ontario in 2007. Can. Respir. J. 18:19–24 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3071009/pdf/crj18019.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CY, Chen HY, Chou CH, Huang CT, Lai CC, Hsueh PR. 2012. Pulmonary infection caused by nontuberculous mycobacteria in a medical center in Taiwan, 2005-2008. Diagn. Microbiol. Infect. Dis. 72:47–51. 10.1016/j.diagmicrobio.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 28.Kurahara Y, Tachibana K, Tsuyuguchi K, Suzuki K. 2013. Mixed pulmonary infection with three types of nontuberculous mycobacteria. Intern. Med. 52:507–510. 10.2169/internalmedicine.52.8907 [DOI] [PubMed] [Google Scholar]
- 29.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Dasa R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Maugein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabo N, Thomson R, Fernandez MT, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop K, Wagner D. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: A NTM-NET collaborative study. Eur. Respir. J. 42:1604–1613. 10.1183/09031936.00149212 [DOI] [PubMed] [Google Scholar]
- 30.Cook JL. 2010. Nontuberculous mycobacteria: opportunistic environmental pathogens for predisposed hosts. Br. Med. Bull. 96:45–59. 10.1093/bmb/ldq035 [DOI] [PubMed] [Google Scholar]
- 31.Winthrop KL. 2010. Pulmonary disease due to nontuberculous mycobacteria: an epidemiologist's view. Future Microbiol. 5:343–345. 10.2217/fmb.10.13 [DOI] [PubMed] [Google Scholar]
- 32.Thomson RM. 2010. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg. Infect. Dis. 16:1576–1583. 10.3201/eid1610.091201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang MA, Kim SY, Jeong BH, Park HY, Jeon K, Kim JW, Ki CS, Koh WJ. 2013. Association of CFTR gene variants with nontuberculous mycobacterial lung disease in a Korean population with a low prevalence of cystic fibrosis. J. Hum. Genet. 58:298–303. 10.1038/jhg.2013.19 [DOI] [PubMed] [Google Scholar]
- 34.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. 2010. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am. J. Respir. Cell Mol. Biol. 43:387–393. 10.1165/rcmb.2009-0276TR [DOI] [PubMed] [Google Scholar]
- 35.Orme IM, Collins FM. 1983. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J. Immunol. 131:1452–1454 [PubMed] [Google Scholar]
- 36.Orme IM, Collins FM. 1986. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J. Exp. Med. 163:203–208. 10.1084/jem.163.1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orme IM, Collins FM. 1984. Efficacy of Mycobacterium bovis BCG vaccination in mice undergoing prior pulmonary infection with atypical mycobacteria. Infect. Immun. 44:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orme IM, Stokes RW, Collins FM. 1986. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect. Immun. 54:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young LS, Bermudez LE. 2001. Perspective on animal models: chronic intracellular infections. Clin. Infect. Dis. 33(Suppl 3):S221–S226. 10.1086/321851 [DOI] [PubMed] [Google Scholar]
- 40.Bermudez LE, Petrofsky M, Kolonoski P, Young LS. 1992. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J. Infect. Dis. 165:75–79. 10.1093/infdis/165.1.75 [DOI] [PubMed] [Google Scholar]
- 41.Sangari FJ, Goodman J, Petrofsky M, Kolonoski P, Bermudez LE. 2001. Mycobacterium avium invades the intestinal mucosa primarily by interacting with enterocytes. Infect. Immun. 69:1515–1520. 10.1128/IAI.69.3.1515-1520.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermudez LE, Young LS. 1988. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140:3006–3013 [PubMed] [Google Scholar]
- 43.Furney SK, Skinner PS, Roberts AD, Appelberg R, Orme IM. 1992. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect. Immun. 60:4410–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bermudez LE, Young LS. 1989. Oxidative and non-oxidative intracellular killing of Mycobacterium avium complex. Microb. Pathog. 7:289–298. 10.1016/0882-4010(89)90047-8 [DOI] [PubMed] [Google Scholar]
- 45.Pedrosa J, Flórido M, Kunze ZM, Castro AG, Portaels F, McFadden J, Silva MT, Appelberg R. 1994. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin. Exp. Immunol. 98:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appelberg R. 1994. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology 191:520–525. 10.1016/S0171-2985(11)80458-4 [DOI] [PubMed] [Google Scholar]
- 47.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. 1994. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect. Immun. 62:3962–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro AG, Silva RA, Appelberg R. 1995. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J. Immunol. 155:2013–2019 [PubMed] [Google Scholar]
- 49.Bermudez LE, Wu M, Young LS. 1995. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect. Immun. 63:4099–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appelberg R, Castro AG, Pedrosa J, Minoprio P. 1994. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology 82:361–364 [PMC free article] [PubMed] [Google Scholar]
- 51.Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. 2009. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg. Infect. Dis. 15:1556–1561. 10.3201/eid1510.090310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swart RM, van Ingen J, van Soolingen D, Slingerland R, Hendriks WD, den Hollander JG. 2009. Nontuberculous mycobacteria infection and tumor necrosis factor-alpha antagonists. Emerg. Infect. Dis. 15:1700–1701. 10.3201/eid1510.090110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Appelberg R, Pedrosa J. 1992. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin. Exp. Med. 87:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orme IM. 1988. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect. Immun. 56:3310–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubbard RD, Flory CM, Collins FM. 1992. T-cell immune responses in Mycobacterium avium-infected mice. Infect. Immun. 60:150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonçalves AS, Appelberg R. 2002. The involvement of the chemokine receptor CXCR2 in neutrophil recruitment in LPS-induced inflammation and in Mycobacterium avium infection. Scand. J. Immunol. 55:585–591. 10.1046/j.1365-3083.2002.01097.x [DOI] [PubMed] [Google Scholar]
- 58.Gomes MS, Flórido M, Pais TF, Appelberg R. 1999. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J. Immunol. 162:6734–6739 [PubMed] [Google Scholar]
- 59.Flórido M, Gonçalves AS, Silva RA, Ehlers S, Cooper AM, Appelberg R. 1999. Resistance of virulent Mycobacterium avium to gamma interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect. Immun. 67:3610–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lousada S, Flórido M, Appelberg R. 2006. Regulation of granuloma fibrosis by nitric oxide during Mycobacterium avium experimental infection. Int. J. Exp. Pathol. 87:307–315. 10.1111/j.1365-2613.2006.00487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehlers S, Kutsch S, Benini J, Cooper A, Hahn C, Gerdes J, Orme I, Martin C, Rietschel ET. 1999. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in Mycobacterium avium-infected mice without affecting bacterial loads. Immunology 98:313–323. 10.1046/j.1365-2567.1999.00875.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel ET, Pfeffer K. 1999. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 67:3571–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flórido M, Appelberg R. 2004. Granuloma necrosis during Mycobacterium avium infection does not require tumor necrosis factor. Infect. Immun. 72:6139–6141. 10.1128/IAI.72.10.6139-6141.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672–678. 10.1128/IAI.70.2.672-678.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. 2004. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Sem. Immunopathol. 25:237–255. 10.1007/s00281-003-0148-9 [DOI] [PubMed] [Google Scholar]
- 66.Andersen P, Doherty TM. 2005. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 3:656–662. 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 67.Lin MY, Reddy TB, Arend SM, Friggen AH, Franken KL, van Meijgaarden KE, Verduyn MJ, Schoolnik GK, Klein MR, Ottenhoff TH. 2009. Cross-reactive immunity to Mycobacterium tuberculosis DosR regulon-encoded antigens in individuals infected with environmental, nontuberculous mycobacteria. Infect. Immun. 77:5071–5079. 10.1128/IAI.00457-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bermudez LE, Wu M, Miltner E, Inderlied CB. 1999. Isolation of two subpopulations of Mycobacterium avium within human macrophages. FEMS Microbiol. Lett. 178:19–26. 10.1016/S0378-1097(99)00331-6 [DOI] [PubMed] [Google Scholar]
- 69.Carter G, Wu M, Drummond DC, Bermudez LE. 2003. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J. Med. Microbiol. 52:747–752. 10.1099/jmm.0.05224-0 [DOI] [PubMed] [Google Scholar]
- 70.Li YJ, Danelishvili L, Wagner D, Petrofsky M, Bermudez LE. 2010. Identification of virulence determinants of Mycobacterium avium that impact on the ability to resist host killing mechanisms. J. Med. Microbiol. 59:8–16. 10.1099/jmm.0.012864-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes MS, Flórido M, Cordeiro JV, Teixeira CM, Takeuchi O, Akira S, Appelberg R. 2004. Limited role of the Toll-like receptor-2 in resistance to Mycobacterium avium. Immunology 111:179–185. 10.1111/j.0019-2805.2003.01807.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sampaio EP, Elloumi HZ, Zelazny A, Ding L, Paulson ML, Sher A, Bafica AL, Shea YR, Holland SM. 2008. Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am. J. Respir. Cell Mol. Biol. 39:431–439. 10.1165/rcmb.2007-0413OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Briken V, Ahlbrand SE, Shah S. 2013. Mycobacterium tuberculosis and the host cell inflammasome: a complex relationship. Front. Cell. Infect. Biol. 3:62. 10.3389/fcimb.2013.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flórido M, Pearl JE, Solache A, Borges M, Haynes L, Cooper AM, Appelberg R. 2005. Gamma interferon-induced T-cell loss in virulent Mycobacterium avium infection. Infect. Immun. 73:3577–3586. 10.1128/IAI.73.6.3577-3586.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper AM, Appelberg R, Orme IM. 1998. Immunopathogenesis of Mycobacterium avium infection. Front. Biosci. 3:e141–e148 [DOI] [PubMed] [Google Scholar]
- 76.Appelberg R. 2006. Pathogenesis of Mycobacterium avium infection: typical responses to an atypical mycobacterium? Immunol. Res. 35:179–190. 10.1385/IR:35:3:179 [DOI] [PubMed] [Google Scholar]
- 77.Rocco JM, Irani VR. 2011. Mycobacterium avium and modulation of the host macrophage immune mechanisms. Int. J. Tuberc. Lung Dis. 15:447–452. 10.5588/ijtld.09.0695 [DOI] [PubMed] [Google Scholar]
- 78.Ordway D, Henao-Tamayo M, Smith E, Shanley C, Harton M, Troudt J, Bai X, Basaraba RJ, Orme IM, Chan ED. 2008. Animal model of Mycobacterium abscessus lung infection. J. Leukoc. Biol. 83:1502–1511. 10.1189/jlb.1007696 [DOI] [PubMed] [Google Scholar]
- 79.Byrd TF, Lyons CR. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67:4700–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, Lyons CR, Byrd TF. 2006. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152:1581–1590. 10.1099/mic.0.28625-0 [DOI] [PubMed] [Google Scholar]
- 81.Rottman M, Catherinot E, Hochedez P, Emile JF, Casanova JL, Gaillard JL, Soudais C. 2007. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 75:5898–5907. 10.1128/IAI.00014-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Catherinot E, Clarissou J, Etienne G, Ripoll F, Emile JF, Daffe M, Perronne C, Soudais C, Gaillard JL, Rottman M. 2007. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 75:1055–1058. 10.1128/IAI.00835-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Catherinot E, Roux AL, Macheras E, Hubert D, Matmar M, Dannhoffer L, Chinet T, Morand P, Poyart C, Heym B, Rottman M, Gaillard JL, Herrmann JL. 2009. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 47:271–274. 10.1128/JCM.01478-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. 2009. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J. Immunol. 183:1997–2007. 10.4049/jimmunol.0802181 [DOI] [PubMed] [Google Scholar]
- 85.Davidson LB, Nessar R, Kempaiah P, Perkins DJ, Byrd TF. 2011. Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HbetaD2 gene expression and IL-8 release. PLoS One 6(12):e29148. 10.1371/journal.pone.0029148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nessar R, Reyrat JM, Davidson LB, Byrd TF. 2011. Deletion of the mmpL4b gene in the Mycobacterium abscessus glycopeptidolipid biosynthetic pathway results in loss of surface colonization capability, but enhanced ability to replicate in human macrophages and stimulate their innate immune response. Microbiology 157:1187–1195. 10.1099/mic.0.046557-0 [DOI] [PubMed] [Google Scholar]
- 87.Roux AL, Ray A, Pawlik A, Medjahed H, Etienne G, Rottman M, Catherinot E, Coppee JY, Chaoui K, Monsarrat B, Toubert A, Daffe M, Puzo G, Gaillard JL, Brosch R, Dulphy N, Nigou J, Herrmann JL. 2011. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 13:692–704. 10.1111/j.1462-5822.2010.01565.x [DOI] [PubMed] [Google Scholar]


