Abstract
Intracellular Mycobacterium leprae infection modifies host macrophage programming, creating a protective niche for bacterial survival. The milieu regulating cellular apoptosis in the tissue plays an important role in defining susceptible and/or resistant phenotypes. A higher density of apoptotic cells has been demonstrated in paucibacillary leprosy lesions than in multibacillary ones. However, the effect of apoptotic cell removal on M. leprae-stimulated cells has yet to be fully elucidated. In this study, we investigated whether apoptotic cell removal (efferocytosis) induces different phenotypes in proinflammatory (Mϕ1) and anti-inflammatory (Mϕ2) macrophages in the presence of M. leprae. We stimulated Mϕ1 and Mϕ2 cells with M. leprae in the presence or absence of apoptotic cells and subsequently evaluated the M. leprae uptake, cell phenotype, and cytokine pattern in the supernatants. In the presence of M. leprae and apoptotic cells, Mϕ1 macrophages changed their phenotype to resemble the Mϕ2 phenotype, displaying increased CD163 and SRA-I expression as well as higher phagocytic capacity. Efferocytosis increased M. leprae survival in Mϕ1 cells, accompanied by reduced interleukin-15 (IL-15) and IL-6 levels and increased transforming growth factor beta (TGF-β) and IL-10 secretion. Mϕ1 cells primed with M. leprae in the presence of apoptotic cells induced the secretion of Th2 cytokines IL-4 and IL-13 in autologous T cells compared with cultures stimulated with M. leprae or apoptotic cells alone. Efferocytosis did not alter the Mϕ2 cell phenotype or cytokine secretion profile, except for TGF-β. Based on these data, we suggest that, in paucibacillary leprosy patients, efferocytosis contributes to mycobacterial persistence by increasing the Mϕ2 population and sustaining the infection.
INTRODUCTION
Macrophages have remarkable plasticity, allowing them to efficiently respond to environmental signals and change their phenotype. Their physiology can be markedly altered by both innate and adaptive immune responses (1–8). Proinflammatory (Mϕ1) and anti-inflammatory (Mϕ2) macrophage polarization contributes to the resolution of inflammatory processes. The presence of the Mϕ2 macrophage population is important for maintaining a basal anti-inflammatory environment in tissues continuously exposed to exogenous agents such as skin. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) contribute to macrophage and dendritic cell development (9–12) but influence the macrophage polarization state in opposite manners. Whereas Mϕ1 polarized in the presence of GM-CSF promotes type 1 immunity, Mϕ2 polarized with M-CSF subverts type 1 immunity and thus may promote immune escape and chronic infection (13).
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, an obligate intracellular pathogen. The disease is characterized by a spectrum with two polar clinical forms. Tuberculoid or paucibacillary leprosy is characterized by a robust Th1 immune response, strong cellular immunity, low bacillary counts, and low lesion numbers. On the other hand, lepromatous or multibacillary leprosy features high levels of Th2-type cytokines, a high bacillary load, and many skin lesions (14–17).
Previous studies by our group demonstrated that M. leprae can lead to macrophage apoptosis through a mechanism involving the expression of tumor necrosis factor (TNF) and the proteasome function (18–20). In addition, in comparing lesions from multi- and paucibacillary patients, Walsh and colleagues reported that apoptosis was more frequent in paucibacillary lesions, suggesting that the activation of apoptosis could act as a containment mechanism for the multiplication and spread of bacilli (21).
Macrophages undergo dramatic molecular and functional changes upon encounter with, interaction with, and uptake of apoptotic cells during inflammation resolution. We demonstrated here that, in the presence of M. leprae, the clearance of apoptotic cells (efferocytosis) induces proinflammatory macrophage deviation toward an anti-inflammatory phenotype. Although efferocytosis has been described as an antimicrobial effector mechanism that operates during M. tuberculosis infection (22, 23), our findings suggest that, in leprosy, efferocytosis may explain the persistence of mycobacterial disease in paucibacillary patients regardless of the capacity of these patients to mount a cellular immune response by modulating the macrophage phenotype and function in cell lesions.
MATERIALS AND METHODS
Patients and clinical specimens.
The acquisition of all specimens was approved by the Oswaldo Cruz Foundation Human Ethics Committee, Rio de Janeiro, RJ, Brazil. Leprosy patients were classified according to the Ridley and Jopling classification scale (24).
Buffy coats were obtained from normal donors (healthy controls [HCs]) at the Hemotherapy Service of the Clementino Fraga Filho University Hospital, associated with the Federal University of Rio de Janeiro, RJ, Brazil, in accordance with the guidelines set down in the Declaration of Helsinki.
Immunohistochemical studies.
Leprosy patient skin biopsy specimens (from 5 borderline tuberculoid [BT] patients and 5 lepromatous leprosy [LL] patients) were obtained at diagnosis and prior to treatment. For routine histopathological analyses, all skin tissues were stained with hematoxylin and eosin (H&E) in addition to the use of the Wade method. To detect arginase (Arg), immunoperoxidase labeling of cryostat sections was performed. The cryostat sections were fixed in acetone, hydrated in Ca+2-Mg+2-free phosphate-buffered saline (PBS) (0.01 M), and incubated with hydrogen peroxide (0.3%)–PBS for 10 min to quench endogenous peroxidase activity. Unspecific binding sites were blocked with horse normal serum (kit ABC Elite; Vector Laboratories, Burlingame, CA). The mouse anti-human antibody against arginase (BD Biosciences, San Jose, CA) (1:50) was diluted in PBS (0.01 M) and incubated for 1 h at room temperature. The sections were washed three times and incubated with biotinylated horse anti-mouse immunoglobulins (kit ABC Elite; Vector Laboratories) for 1 h at room temperature. After being washed, the sections were incubated for 40 min with avidin-biotin complex (kit ABC Elite; Vector Laboratories) for signal amplification. The reaction was developed at room temperature in a solution of 3-amino-9-ethylcarbazole (AEC) for 10 min (AEC peroxidase substrate kit; Vector Laboratories). Slides were counterstained with Mayer's hematoxylin and mounted with Faramount aqueous mount medium (Dako, Thousand Oaks, CA). Images were obtained via the use of a Nikon Eclipse microscope with Infinite Capture software (Lumenera Corporation, Ottawa, Ontario, Canada).
Cell culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated under endotoxin-free conditions from buffy coats by the Ficoll-Hypaque method (Pharmacia Fine Chemicals, Piscataway, NJ). Monocytes were purified from PBMCs by magnetic cell sorting using CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). GM-CSF and M-CSF promote the acquisition of distinct morphology, pathogen susceptibility, and inflammatory functions in macrophages (13, 25–28). Therefore, although they are used interchangeably for the in vitro generation of human monocyte-derived macrophages (29), GM-CSF- and M-CSF-polarized macrophages are considered pro- and anti-inflammatory macrophages, respectively (13) and, by analogy to widely accepted nomenclature (“classical″/Mϕ1” and “alternative″/Mϕ2” macrophage polarization states), are referred to here as Mϕ1 (differentiated with GM-CSF) and Mϕ2 (differentiated with M-CSF) macrophages. To generate Mϕ1 and Mϕ2 cells, monocytes were resuspended in RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 10% fetal calf serum (FCS; Gibco BRL, Gaithersburg, MD) and containing 50 ng/ml of GM-CSF (R&D Systems, Minneapolis, MN) and 50 ng/ml of M-CSF (R&D Systems), respectively, and cultured for 6 days in 6- or 24-well plates (Costar, Cambridge, MA) at 1 × 106 cells/ml.
Cell stimulation and infection.
Irradiated armadillo-derived M. leprae whole cells subjected to probe sonication with a Sanyo sonicator to >95% breakage (NIH/NIAID Leprosy Research Support from Colorado State University) were added to the Mϕ1 and Mϕ2 cultures at 10 μg/ml. When necessary, apoptotic cells were added to the culture after 30 min and cells were incubated for 90 min or 24 h. Live M. leprae Thai-53 cells purified from hind footpads of BALB/c athymic nude mice were obtained from the National Hansen's Disease Program and the Lauro de Souza Lima Institute (Bauru, São Paulo, Brazil) and added to the cultures at a multiplicity of infection (MOI) of 10 for 24 h in the presence or absence of apoptotic cells. Bacteria were counted by acid-fast staining (BBL TB Ziehl-Neelsen Stain kit; BD Biosciences). Mycobacteria were PKH67 green labeled according to the manufacturer's instructions (PKH67 green fluorescent cell linker kit for general cell membrane labeling; Sigma-Aldrich, Saint Louis, MO). Mycobacterial viability was determined by the use of a LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Life Technologies, Carlsbad, CA), as previously described (30). In arginase inhibitory studies, Mϕ1 cells were pretreated for 30 min with 10 μM Nω-hydroxy-nor-arginine (nor-NOHA; Cayman Chemical, Ann Arbor, MI) or vehicle (0.01% dimethyl sulfoxide) before stimulation with M. leprae and apoptotic cells.
Induction of apoptosis.
Jurkat T cells were exposed to UV irradiation (254 nm) for 10 min and cultured in RPMI 1640 without serum for 3 h in 5% CO2 at 37°C. Human neutrophils were separated by Dextran sedimentation, followed by discontinuous isotonic Percoll gradient centrifugation. Human neutrophils (>95% pure) were cultured at 37°C in a 5% CO2 atmosphere at a concentration of 5 × 106/ml in Dulbecco's modified Eagle's medium (DMEM) with 10% autologous serum for 20 h to undergo apoptosis. Apoptosis of these cells was confirmed by light microscopy and annexin V and propidium iodide (PI) staining (BD Pharmingen, San Jose, CA). Apoptotic Jurkat T (ApoJ) and apoptotic neutrophil (ApoN) cells were used when approximately 80% apoptotic cells were obtained (annexin V positive [annexin V+], PI−). When necessary, a PKH26 red fluorescence cell linker kit (Sigma-Aldrich) was used to label Jurkat cells for 2 min. The reaction was halted with 10% FCS (Gibco BRL), and cells were washed twice in RPMI medium and then resuspended in PBS.
Apoptosis assay.
Phosphatidylserine (PS) externalization, an early-stage apoptotic event, was assessed by the binding of fluorescently labeled annexin V (fluorescein isothiocyanate [FITC]). Late-stage apoptosis and necrosis were measured by simultaneous staining with PI (propidium iodide) using a BD Pharmingen annexin V-FITC apoptotic detection kit according to the manufacturer's instructions. Cells were harvested by centrifugation (2,500 × g at 4°C for 5 min) after an ice-cold bath and washed three times in chilled PBS. Pellets were resuspended in 500 μl of 1× binding buffer (0.01 M HEPES, 0.14 M NaCl, and 2.5 mM CaCl2, pH 7.4). A 100-μl fraction of the cell suspension was divided into aliquots and added to flow cytometry tubes, and 5 μl of PI and 5 μl of annexin V-FITC were added. The tubes were then briefly mixed using a vortex device. The cell suspension was incubated for 15 min at room temperature (22°C) in the dark. The percentages of cells undergoing early-stage apoptosis (annexin V-FITC positive) and late-stage apoptosis/necrosis (annexin V-FITC and PI positive) were measured with excitation at 488 nm and emission in an FL1 detector (525 nm) for FITC and excitation of 536 nm and emission in an FL3 detector (610 nm) for PI using an Accuri flow cytometer (BD Biosciences). Data were collected using CFlow software, and 10,000 events were analyzed per sample.
Evaluation of Mycobacterium leprae uptake.
Prior to bacterial interaction assays, M. leprae was stained via the use of a PKH67 green fluorescence cell linker kit (Sigma-Aldrich) according to the manufacturer's instructions. Mϕ1 and Mϕ2 cells were stimulated with PKH67-labeled M. leprae (10 μg/ml), and 2 h or 24 h postinfection, the index of bacterial association was determined by flow cytometry and expressed as a percentage of PKH67-M. leprae-positive cells. To determine bacterial uptake, the fluorescent signal of extracellular bacteria was quenched with trypan blue after incubation time. The internalization of M. leprae was evaluated by flow cytometry, as previously described (31).
Fluorescence-activated cell sorter (FACS) analysis of macrophage phenotypes.
To analyze the expression of the scavenger receptors CD163 and SRA-I, Mϕ1 and Mϕ2 macrophages were collected with a cell scraper after 24 h of culture. Cells were stained for 30 min at 4°C with 1:50 allophycocyanin (APC)-conjugated anti-CD163 monoclonal antibody and 1:50 phycoerythrin (PE)-conjugated anti-SRA-I monoclonal antibody (R&D Systems). Gates were defined for collection, and 20,000 live events were analyzed on a C6 Accuri cytometer using Cflow software (BD Biosciences).
Cytokine detection by ELISA.
Supernatants from Mϕ1 and Mϕ2 cells were tested for the presence of cytokines and growth factors using commercially available enzyme-linked immunosorbent assay (ELISA) kits for interleukin-6 (IL-6), IL-10, IL-15, gamma interferon (IFN-γ), and transforming growth factor beta (TGF-β) (eBioscience, San Diego, CA) following the protocols supplied by the manufacturers.
Ultrastructural analysis.
Macrophage ultrastructures were evaluated after stimulation with M. leprae (10 μg/ml) in the presence or absence of apoptotic cells for 90 min at 37°C. Cells were washed with PBS and fixed with glutaraldehyde (2.5%)–sodium cacodylate buffer (0.1 M) (pH 7.2)–3.5% sucrose for 1 h at 4°C. Cells were then washed in the same buffer and fixed with 1% osmium tetroxide (OsO4) for 1 h at 4°C. Cells were washed in cacodylate buffer, dehydrated in serially concentrated acetone (30%, 50%, 70%, 90%, and 100%), infiltrated with a mixture of 100% acetone and resin PolyBed 812, and polymerized at 60°C for 2 days. After polymerization, ultrafine sections were made (Reichert ultramicrotome OmU3) and collected on copper grids of 300 mesh, contrasted with 5% uranyl acetate and citrate lead, and observed under a Jeol JEM-1011 transmission electronic microscope.
Molecular determination of M. leprae viability.
The viability of M. leprae was measured as previously described (32), with some modifications. Briefly, M. leprae RNA and DNA were simultaneously extracted by the TRIzol method (Life Technologies) according to the manufacturer's recommendations through single-tube homogenization using a Fast Prep FP 24 instrument (MP Biomedicals, Santa Ana, CA). Prior to reverse transcription, DNA was removed from the RNA preparations using a DNA-free Turbo kit (Ambion, Life Technologies), and RNA was reverse transcribed using random primers and SuperScript III following the manufacturer's instructions (Invitrogen-Life Technologies). M. leprae viability was estimated from the levels of 16S rRNA normalized against measured 16S DNA levels using a TaqMan-based real-time PCR assay, as previously described (32).
Real-time PCR.
TaqMan PCR was performed via the use of universal PCR master mix (2×) and specific primers and probes (Applied Biosystems, Life Technologies). PCR was performed using a ABI Prism 7000 sequence detection system (Applied Biosystems) at 50°C for 5 min, 95°C for 10 min, 50 cycles of 95°C for 15 s each cycle, and 60°C for 1 min. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an endogenous control. Arginase 1 mRNA was quantified using the 2−ΔΔCT method for the PBMC samples.
Lymphocytic stimulation test.
CD3+ T cells were isolated from PBMCs with magnetic microbeads (Miltenyi Biotec). Lymphocytes were incubated at a ratio of 1 Mϕ1 cell to 10 T cells. Mϕ1 cells were previously stimulated with M. leprae (10 μg/ml) for 24 h in the presence or absence of apoptotic cells. Cells were cocultured for 48 h, and supernatants were harvested and stored until cytokines were analyzed by ELISA.
Statistical analysis.
Results were reported as pooled data from the entire series of experiments. GraphPad Prism (GraphPad Software, La Jolla, CA) was used for all analyses, and samples were analyzed by analysis of variance (ANOVA) with a Tukey's posttest. A P value of <0.05 was deemed to represent statistical significance.
RESULTS
M. leprae stimulation did not change the phenotype of differentiated macrophages.
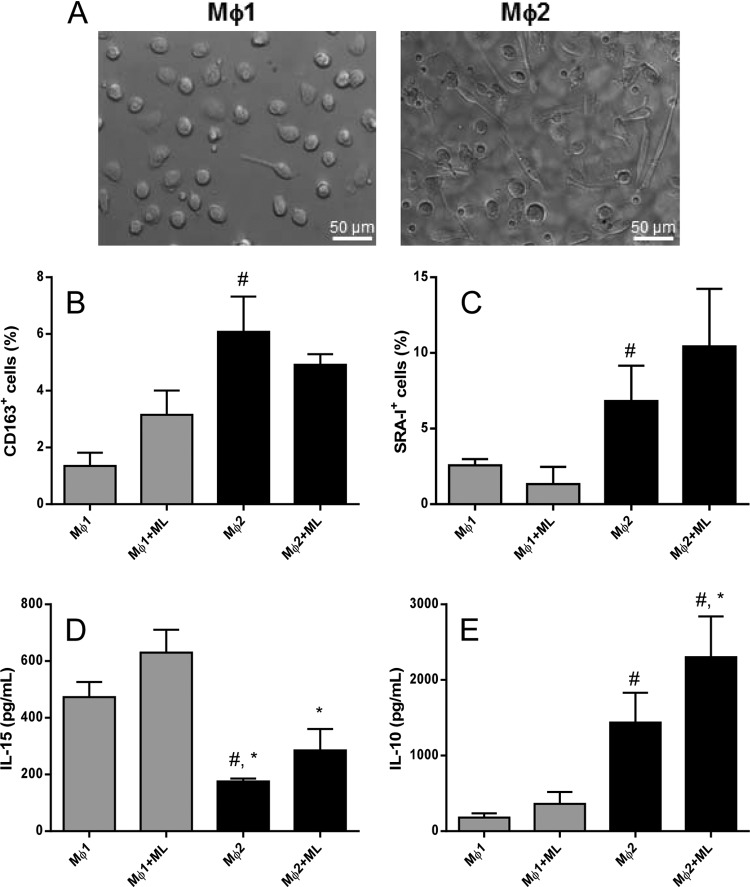
Mϕ1 and Mϕ2 cells were obtained by differentiation of purified human CD14+ monocytes in the presence of GM-CSF and M-CSF, respectively, as previously described (13, 33). It was observed that after 6 days of culture, the majority of Mϕ1 cells displayed a classical adherent “fried-egg” morphology (Fig. 1A). On the other hand, Mϕ2 cells primarily appeared as adherent cells with a stretched, spindle-like morphology (Fig. 1A). Previous results from our group have demonstrated that M. leprae is able to induce apoptosis in human monocytes by a mechanism that involves TNF and that, although necessary, M. leprae phagocytosis is not sufficient for cell death (18). Thus, we tested whether M. leprae was able to induce cell death in macrophages differentiated in vitro. M. leprae did not affect the cell viability of Mϕ2 macrophages. However, in Mϕ1 cells, M. leprae increased the percentage of apoptotic cells (annexin V+ PI−) when used at 20 μg/ml (see Fig. S1 in the supplemental material). Interestingly, M. leprae was not able to induce apoptosis in either Mϕ1 or Mϕ2 cells when used at 10 μg/ml. Since our main interest was in investigating the role of efferocytosis in the context of the immune response to M. leprae, we chose to use M. leprae at 10 μg/ml in all experiments performed in this study to avoid any influence of Mϕ1 or Mϕ2 apoptosis on the observed immune response.
FIG 1.
M. leprae stimulation did not alter the phenotype of macrophages differentiated in vitro. (A) To determine whether M. leprae may induce a different phenotype in macrophages differentiated in vitro, CD14+ cells from healthy donors were stimulated with M-CSF (50 ng/ml) or GM-CSF (50 ng/ml) for 6 days to obtain Mϕ1 or Mϕ2 macrophages, respectively. (B and C) Cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h, after which the expression of CD163-APC (B) and that of SRA-I-PE (C) were evaluated by flow cytometry. (D and E) Concentrations of the proinflammatory cytokine IL-15 (D) and the anti-inflammatory cytokine IL-10 (E) in the supernatants were evaluated by ELISA. Experiments were performed at least twice in triplicate, and data are presented as means ± standard deviations (SD). #, P < 0.05 in relation to Mϕ1;*, P < 0.05 in relation to Mϕ1+ML.
Analysis of the Mϕ2 phenotypic markers CD163 and SRA-I revealed that M-CSF-differentiated cells exhibited higher levels of these molecules than GM-CSF-differentiated cells (for CD163, Mϕ1 = 1.35 ± 0.46% versus Mϕ2 = 6.57 ± 1.13% [P < 0.05]; and for SRA-I, Mϕ1 = 2.57 ± 0.41 versus Mϕ2 = 6.83 ± 2.33 [P < 0.05]). Nevertheless, M. leprae stimulation did not alter CD163 or SRA-I expression in either type of macrophage (Fig. 1B and C).
Since previous work has demonstrated differential regulation of macrophage functional programs by IL-10 and IL-15 (34), we investigated whether macrophages polarized in vitro could be better characterized by IL-10 and IL-15 production. We found that Mϕ2 secreted less IL-15 while producing higher IL-10 levels. Our results showed that Mϕ1 macrophages produced approximately 2.7 times more IL-15 (473.1 ± 55.1 pg/ml) than Mϕ2 macrophages (175.8 ± 9.8 pg/ml), while IL-10 secretion had an inverse profile. By the same token, Mϕ2 cells also produced 7 times more IL-10 (1,436 ± 396.6 pg/ml) than Mϕ1 cells (185.1 ± 54.07 pg/ml). In the presence of M. leprae, Mϕ2 cells showed increased IL-10 production (2,302 ± 539 pg/ml) compared to Mϕ1 cells (361.8 ± 156.5 pg/ml) (Fig. 1D and E).
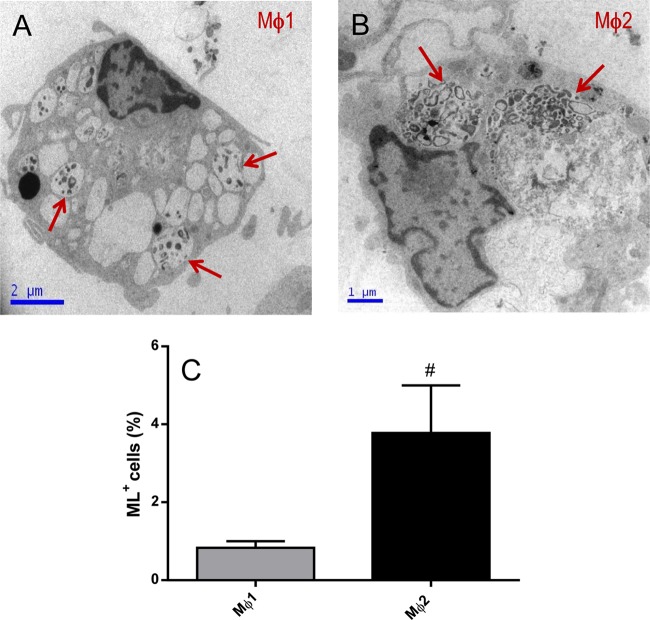
Efferocytosis increases M. leprae uptake by Mϕ1 macrophages.
Chronic evolution of infectious diseases is thought to be associated with macrophage reprogramming to shift to an Mϕ2 profile, particularly in those diseases associated with Th2 responses. Consequently, the capacity of M. leprae to be internalized by monocyte-derived macrophages was evaluated by both electron microscopy and flow cytometry. Our data showed that Mϕ2 cells internalized significantly more M. leprae than Mϕ1 cells (Mϕ1, 0.84 ± 0.2%; Mϕ2, 3.78 ± 1.2%) (Fig. 2).
FIG 2.
Mϕ2 cells differentiated in vitro are more phagocytic than Mϕ1 cells. (A) Ultrastructural analyses were performed to evaluate whether there are differences in the phagocytic capacities of these cells differentiated in vitro. Mϕ1 or Mϕ2 cells were stimulated with irradiated M. leprae at 10 μg/ml for 2 h, and M. leprae uptake was analyzed by electron microscopy (A and B) or flow cytometry (C). Experiments were performed at least three times in triplicate, and data are presented as means ± SD. Red arrows point to M. leprae in vacuoles inside Mϕ1 and Mϕ2 cells. #, P < 0.05 in relation to Mϕ1.
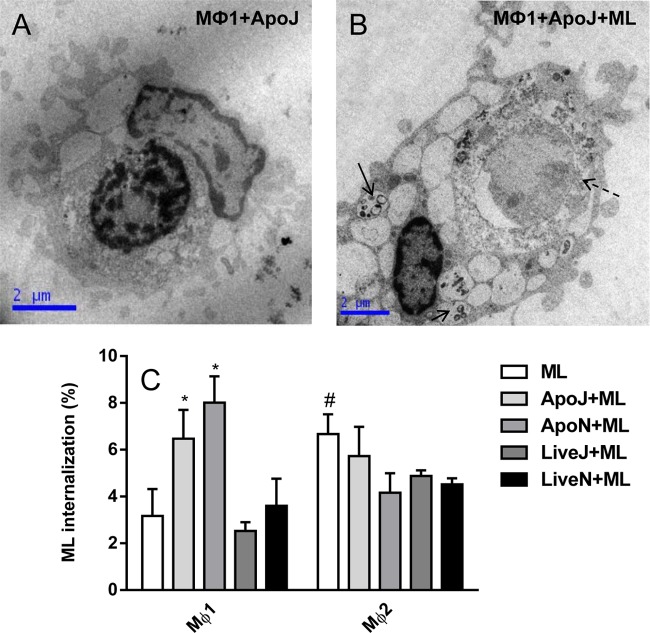
The next step involved investigating whether phagocytosis of ApoJ or ApoN cells modulates M. leprae internalization. In this context, our results showed that there was an increase in the percentage of M. leprae internalized in the presence of apoptotic cells by Mϕ1 compared with macrophages stimulated with M. leprae alone (P < 0.05) or those maintained in the presence of viable cells stimulated with the mycobacterium (P < 0.05) (Fig. 3). The presence of apoptotic cells did not affect mycobacterial uptake by Mϕ2 cells as evaluated by flow cytometry (Fig. 3). The level of uptake of M. leprae in the presence of ApoJ cells was not different from the level seen in the presence of ApoN cells (Fig. 3C).
FIG 3.
The presence of apoptotic cells increases M. leprae uptake by Mϕ1 cells. (A and B) Ultrastructural analyses were performed to evaluate M. leprae (ML) uptake by Mϕ1 and Mϕ2 cells in the presence of apoptotic cells (ApoJ, irradiated Jurkat cells). Mϕ1 or Mϕ2 cells were stimulated with irradiated M. leprae at 10 μg/ml for 2 h in the presence or absence of apoptotic and live Jurkat cells or neutrophils (1:1). (C) The percentage of M. leprae uptake was analyzed by flow cytometry. Experiments were performed at least three times in triplicate, and data are presented as means ± SD. #, P < 0.05 in relation to Mϕ1; *, P < 0.05 in relation to ML-, live-Jurkat-cell-plus-ML (LiveJ+ML)-, or live-neutrophil-plus-ML (LiveN+ML)-stimulated Mϕ1 cells. Solid arrows indicate M. leprae inside cells, whereas the dashed arrow indicates an apoptotic Jurkat cell inside Mϕ1 macrophage. ApoN, irradiated neutrophils.
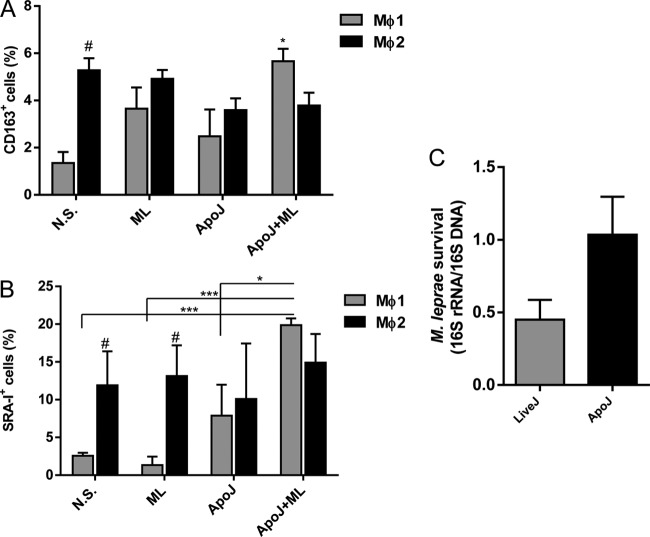
Phagocytosis of apoptotic cells in the presence of M. leprae shifts Mϕ1-polarized cells toward the Mϕ2 phenotype.
Since phagocytosis of apoptotic cells modulated M. leprae uptake in Mϕ1 cells, we evaluated whether this augmented phagocytic capacity was accompanied by phenotypic changes. We therefore evaluated the expression of CD163 and SRA-I by flow cytometry. Apoptotic cell uptake did not significantly affect CD163 and SRA-I expression in Mϕ1 cells. However, in the presence of M. leprae, apoptotic cells significantly increased the expression of both CD163 (5.20 ± 0.59 in cells stimulated with M. leprae plus apoptotic cells versus 1.35 ± 0.46 in nonstimulated cells) and SRA-I (26.53 ± 6.84 in cells stimulated with M. leprae plus apoptotic cells versus 2.57 ± 0.41 in nonstimulated cells) (Fig. 4A and B; see also Fig. S2 in the supplemental material). Besides, an increase in M. leprae viability as measured by the 16S rRNA/16S DNA ratio was detected in Mϕ1 cells (Fig. 4C). In Mϕ2 macrophages, the uptake of apoptotic cells did not change the CD163 and SRA-I patterns compared with nonstimulated cell results despite the presence of M. leprae (Fig. 4A and B; see also Fig. S2).
FIG 4.
Phagocytosis of apoptotic cells in the presence of M. leprae shifts Mϕ1 polarization toward a Mϕ2 phenotype. To determine whether M. leprae stimulation in the presence of apoptotic cells could modulate the cell phenotype, Mϕ1 and Mϕ2 cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h in the presence or absence of apoptotic Jurkat cells (1:1). (A and B) CD163-APC expression (A) and SRA-I–PE expression (B) were evaluated by flow cytometry, and the percentages of positive cells are shown. (C) M. leprae viability was determined by the ratio of 16S rRNA to 16S DNA in Mϕ1 cells stimulated or not with apoptotic cells following 24 h of infection. Experiments were performed at least three times in triplicate, and data are presented as means ± SD. *, P < 0.05 in relation to nonstimulated (N.S.) Mϕ1 cells and Mϕ1+ApoJ. ***, P < 0.001. #, P < 0.05 in relation to the N.S. and ML-stimulated Mϕ1 cells.
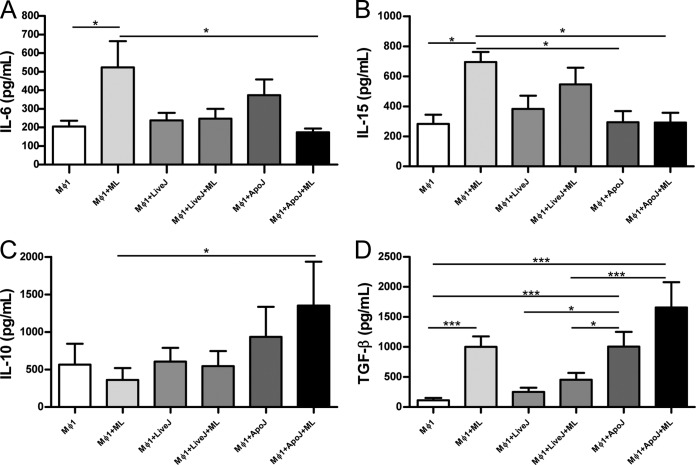
The supernatants of Mϕ1 macrophages contained significantly lower levels of IL-6 and IL-15 produced in response to M. leprae after apoptotic cell phagocytosis compared to cells stimulated with M. leprae alone (Fig. 5A and B). The production of IL-10 rose after phagocytosis of apoptotic cells in the presence of M. leprae in contrast to cells stimulated with M. leprae only. TGF-β secretion in the presence of M. leprae increased after phagocytosis of apoptotic cells compared to nonstimulated cells and those stimulated with M. leprae and live Jurkat. In Mϕ2 macrophages, neither M. leprae nor apoptotic cells affected the IL-6, IL-15, or IL-10 expression. M. leprae and apoptotic cells in the presence or absence of M. leprae, however, were able to induce increased levels of TGF-β in relation to nonstimulated Mϕ2 cells (see Fig. S3 in the supplemental material).
FIG 5.
Apoptotic cell uptake changes the cytokine secretion pattern induced by M. leprae in Mϕ1 cells. Mϕ1 cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h in the presence or absence of apoptotic or live Jurkat cells (1:1), and the concentrations of IL-6 (A), IL-15 (B), IL-10 (C), and TGF-β (D) in cell supernatants were evaluated by ELISA. Experiments were performed at least three times in triplicate, and data are presented as means ± SD. *, P < 0.05; ***, P < 0.001.
Arginase contributes to induction of the Mϕ2 phenotype in M. leprae-treated Mϕ1 cells in the presence of apoptotic cells.
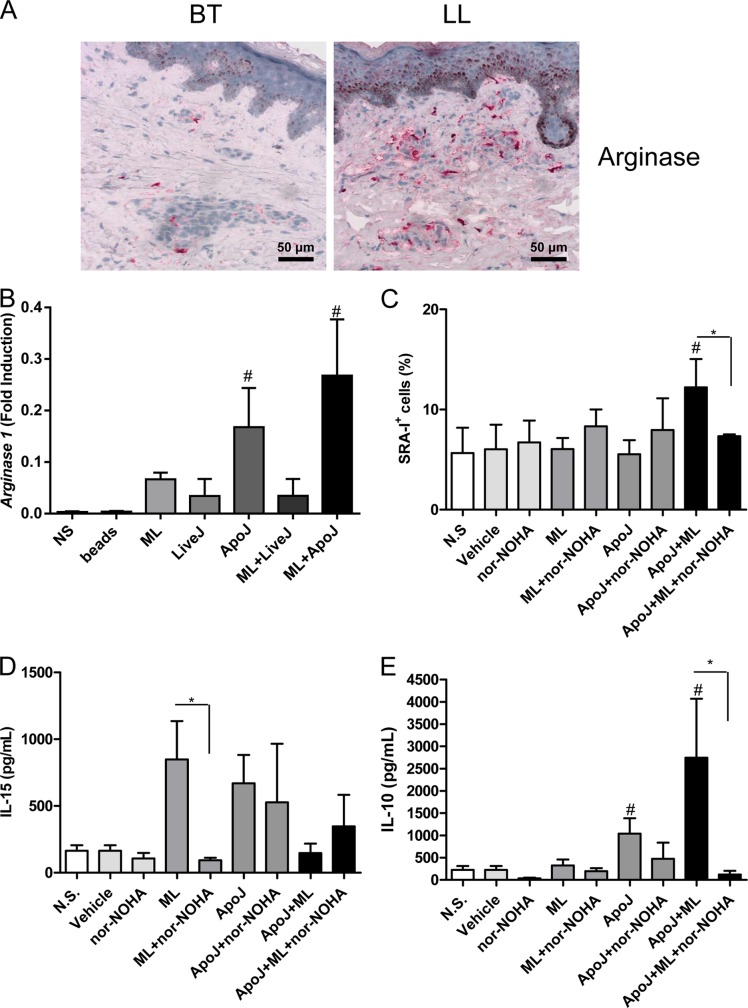
Previous reports have described arginine as the essential substrate driving macrophage polarization (35). Alterations in local l-arginine metabolism, principally mediated by the enzymes arginase (Arg) and inducible nitric oxide synthase (iNOS), have been linked to pathological wound healing. In order to investigate the activation of arginase during leprosy, we analyzed the skin lesions of patients with the polar forms of the disease. We were able to demonstrate that, in patients with multibacillary skin lesions, large numbers of macrophages express arginase. In contrast, in paucibacillary lesions, few cells express this enzyme (Fig. 6A). Over subsequent years, interest in Arg/iNOS has focused on the paradigm of the classical versus alternatively activated (Mϕ1/Mϕ2) macrophage (36, 37). We found an increase in arginase 1 mRNA expression in Mϕ1 cells stimulated with M. leprae and apoptotic cells in relation to both nonstimulated cells and those that had received these stimuli separately (Fig. 6B). We tested whether the arginase blockade could impair the Mϕ1-Mϕ2 phenotype shift by using nor-NOHA. It was seen that pretreatment with nor-NOHA impaired a rise in the percentage of SRA-I-expressing Mϕ1 cells (Fig. 6C). The expression of CD163 (data not shown) and IL-15 (Fig. 6D) was not affected by the arginase blockade, although lower IL-10 levels in nor-NOHA-pretreated cells stimulated with apoptotic cells and M. leprae were observed (Fig. 6E).
FIG 6.
The increased SRA-I expression in Mϕ1 cells stimulated with apoptotic cells and M. leprae is dependent on arginase. (A) Arginase expression in leprosy patient skin lesions (BT, n = 5; LL, n = 5) was evaluated by the use of immunoperoxidase. The images are representative of one BT patient and one LL patient. (B) Mϕ1 cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h in the presence or absence of apoptotic or live Jurkat cells (1:1), and the arginase 1 expression was evaluated by real-time PCR. #, P < 0.05 in relation to the nonstimulated (NS), bead, ML, LiveJ, and LiveJ+ML groups. (C) Mϕ1 cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h in the presence or absence of apoptotic Jurkat cells (1:1) or arginase inhibitor nor-NOHA at 10 μM. SRA-I-PE expression was evaluated by flow cytometry. #, P < 0.05 in relation to the N.S., vehicle, ML, and ApoJ groups. *, P < 0.05. (D and E) The concentrations of IL-15 (D) and IL-10 (E) in the cell supernatants were evaluated by ELISA. Experiments were performed at least three times in triplicate, and data are presented as means ± SD. #, P < 0.05 in relation to the nonstimulated (N.S), bead, ML, LiveJ, and LiveJ+ML groups. *, P < 0.05.
Effect of apoptotic cell phagocytosis and M. leprae stimulation on T cell priming by Mϕ1 cells.
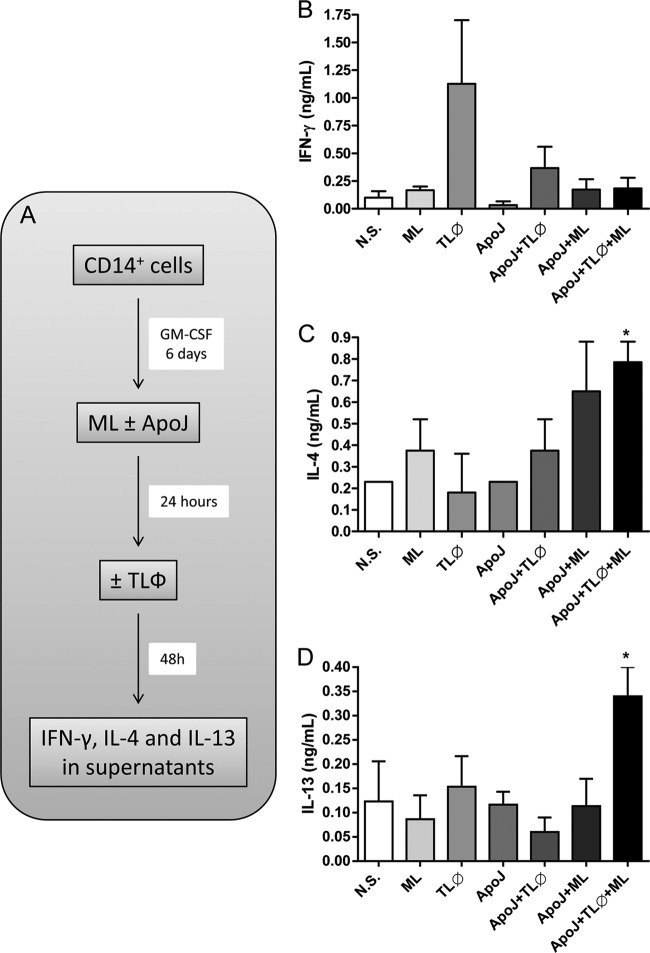
We then determined whether the phenotypic shift in Mϕ1 cells after apoptotic cell clearance in the presence of M. leprae could affect T cell priming. Mϕ1 cells were treated with M. leprae in the presence or absence of apoptotic cells for 24 h. The cell cultures were then stimulated with autologous CD3+ T cells (1 Mϕ1 cell to 10 T cells) for 48 h (Fig. 7A). The cytokine profile in the culture supernatants was subsequently evaluated, and it was found that production of the Th2 cytokines IL-4 and IL-13 increased in response to M. leprae in cultures stimulated with apoptotic cells (Fig. 7B and C) but not production of the IFN-γ cytokine (Fig. 7D).
FIG 7.
Efferocytosis leads Mϕ1 cells to induce Th2 responses to M. leprae antigens in vitro. (A) Mϕ1 cells were stimulated with irradiated M. leprae at 10 μg/ml for 24 h in the presence or absence of apoptotic Jurkat cells (1:1). Cells were incubated with CD3+ T cells (TLϕ) (1 Mϕ1 cell to 10 TLϕ cells) for 48 h, and cell supernatants were harvested for cytokine analysis. (B, C, and D) IFN-γ (B), IL-13 (C), and IL-4 (D) concentrations in cell supernatants were determined by ELISA. Experiments were performed at least three times in triplicate, and data are presented as means ± SD. *, P < 0.05 in relation to M. leprae-stimulated cells.
DISCUSSION
Macrophages are the preferred targets for infection of intracellular pathogens, including mycobacteria. This microbe-host interaction can lead to the development of protective (microbicidal) or permissive (phagocytic) host-cell programs (34), with the latter culminating in progression to active disease. In addition, macrophages can also undergo dramatic molecular and functional changes upon encounter with, interaction with, and uptake of apoptotic cells (38). The elucidation of mechanisms behind macrophage activation has recently provided important insights into various physiological and pathological conditions (39, 40).
It was shown, for example, that macrophages differentiated in vitro in the presence of GM-CSF (Mϕ1) are proinflammatory and microbicidal, promoting cellular immunity. On the other hand, macrophages differentiated in the presence of M-CSF (Mϕ2) are unable to activate CD4+ T cells, even after treatment with the CD40 ligand and IFN-γ (13, 41–43). Again, the present study investigated the role played by efferocytosis in the modulations of macrophage programs and, possibly, in the pathogenesis of leprosy.
Recent data from our group suggest that the skin lesion macrophages of multibacillary patients and Mϕ2 macrophages have similar phenotypes, with high levels of expression of CD163 and IDO (indoleamine 2,3-dioxygenase) (30, 44). This observation is reinforced here by the demonstration of intense arginase expression in lepromatous patient lesions. The phenotypes of paucibacillary patient macrophages are equivalent to the classically activated ones (Mϕ1) (30, 44) despite the fact that few positive cells for Mϕ2 markers are present in the skin lesions of these patients (30, 44), suggesting that the maintenance of lower numbers of Mϕ2 cells at the paucibacillary infection site may sustain infection in this group. Our data reinforce this hypothesis, showing that even though both Mϕ1 and Mϕ2 macrophages are able to internalize M. leprae, Mϕ2 macrophages boast a greater phagocytic capacity than Mϕ1 cells.
Previous studies have demonstrated that Mϕ2 macrophages are able to bind to the surface apoptotic cells at 4°C and to phagocytose them at 37°C at a higher percentage than Mϕ1 macrophages (45). Moreover, Verreck and colleagues demonstrated that Mϕ2 cells can internalize more Mycobacterium bovis BCG than Mϕ1 cells (13). However, Makino and colleagues reported no difference in the levels of internalization of BCG by these two types of macrophages (42). It was reported in a recent work that macrophage differentiation in the presence of M-CSF showed a greater phagocytic capacity to internalize lymphoma target cells opsonized with rituximab than was seen with GM-CSF-induced cells. Furthermore, the addition of IL-10 significantly increased while that of IL-4 greatly decreased phagocytosis in both M-CSF- and GM-CSF-differentiated macrophages (45). These findings reinforce the hypothesis that paucibacillary patients exhibit a predominance of Mϕ1-like macrophages and that, conversely, multibacillary patients exhibit a predominance of Mϕ2-like macrophages in their respective lesions.
Several studies have related the phagocytosis of apoptotic cells to the internalization of microorganisms. Apoptotic induction of lymphocytes by Trypanosoma cruzi and the phagocytosis of apoptotic cells by macrophages increase predisposition to the parasite, suggesting that the phagocytosis of apoptotic cells plays a role in disease persistence (46). Similarly, Leishmania sp. infection induces apoptosis in neutrophils, which are subsequently engulfed by macrophages. These apoptotic cells serve as a “Trojan horse,” so to speak, in that the recognition of apoptotic neutrophils prevents contact of the parasite with the macrophage receptors. As a result, Leishmania is able to reach its final host, the macrophage, culminating in the establishment of infection (47, 48). Our model found that Mϕ2 macrophages naturally phagocytose more M. leprae than Mϕ1 cells. However, in the presence of apoptotic cells, there is an increase in M. leprae uptake by Mϕ1 cells but not by Mϕ2 cells. Jurkat cells and neutrophils were used as sources of apoptotic cells, at which time similar results were observed.
In Mϕ1 macrophages, apoptotic cells and M. leprae increased the expression of scavenger receptors CD163 and SRA-I, shown to be specific markers for Mϕ2 macrophages. This suggests that stimulation with M. leprae in the presence of apoptotic cells is altering the phenotypic profile of this population. Moreover, in the presence of M. leprae, the phagocytosis of apoptotic cells by Mϕ1 macrophages resulted in reduced secretion of the proinflammatory cytokines IL-6 and IL-15 and increased production of such anti-inflammatory molecules as IL-10, TGF-β, and arginase. Arginase has been described as a marker of alternative macrophage activation, exercising a crucial host-protective function by downregulating excessive Th1-induced inflammation in different experimental models (49).
Previous studies have shown increased mRNA and protein IL-15 expression in macrophages of paucibacillary patients compared to those of multibacillary patients (34). IL-15 may represent a key cytokine involved in granuloma formation and may enhance cellular immune responses to mycobacterial antigens (15, 50). Our data showed that macrophage differentiation in vitro with GM-CSF induced increased levels of IL-15, reinforcing our hypothesis that these cells exhibit the phenotype of macrophages found in paucibacillary patients. The reduction of IL-15 levels could drive the TGF-β increase, indicating that the increased percentage of apoptotic cells in paucibacillary patients contributes to a possible reversal of the macrophage phenotype which allows the establishment of infection even in the presence of the cellular immune response. In the presence of M. leprae, stimulation with apoptotic cells increased the levels of IL-10, implying a polarization of these macrophages toward the phagocytic pathway.
Arginase has emerged as a key player in the mammalian immune system, and it is known that this enzyme is involved in various aspects of inflammation. We have found that the blockade of arginase in vitro impairs increased SRA-I and IL-10 production in Mϕ1 cells stimulated with both M. leprae and apoptotic cells. Arginase induction is not specific to M. leprae stimulation. In fact, previous studies have demonstrated that both apoptotic cells and their derivatives may alter the physiology of macrophages toward a regulatory phenotype by reducing nitric oxide production (51, 52). In addition, others have demonstrated that mycobacterium-infected macrophages produce soluble factors (i.e., IL-10), which can induce arginase expression in an autocrine-paracrine-related manner (53). The data presented here suggest that arginase not only showed increased expression in the Mϕ2 population but also was involved in Mϕ2 differentiation. Even though the molecular biology of arginase regulation in the various macrophage subsets has been poorly studied to date, a possible regulatory role for SOCS1 has been previously described (54).
Our lymphocytic stimulation assay clearly demonstrated that efferocytosis by Mϕ1 macrophages induced a Th2 response to M. leprae mediated by IL-4 and IL-13, two cytokines that may contribute to the alternative macrophage activation. It can be hypothesized that the increased TNF induced by early-stage M. leprae infection may be responsible for the higher frequency of apoptotic cells in skin lesions. Efferocytosis contributes to the maintenance of Mϕ2 cells in skin lesions, which, in turn, reinforces the maintenance of M. leprae in paucibacillary lesions. Interaction of these newer Mϕ2 cells with naive T cells tends to intensify a Th2 response that might lead, in later stages, to Mϕ2 differentiation at the infection site. Altogether, these data suggest that M. leprae-induced apoptosis or TNF or both contribute to the formation of a favorable microenvironment for the establishment of infection in paucibacillary patients, notwithstanding the presence of an effective cellular immune response.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant no. E-26/110.137/2009 from the Carlos Chagas Filho Research Support Foundation (FAPERJ) and the National Counsel of Technological and Scientific Development (CNPq).
We thank the researchers at the Rudolf Barth Platform of electronic microscopy for the use of an electron microscope, Carmen Martins Nogueira at the Hemotherapy Service of the Clementino Fraga Filho University Hospital, and Judy Grevan for editing the text.
Footnotes
Published ahead of print 14 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02194-14.
REFERENCES
- 1.Nathan CF, Murray HW, Wiebe ME, Rubin BY. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670–689. 10.1084/jem.158.3.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mues B, Langer D, Zwadlo G, Sorg C. 1989. Phenotypic characterization of macrophages in human term placenta. Immunology 67:303–307 [PMC free article] [PubMed] [Google Scholar]
- 3.Erwig LP, Kluth DC, Walsh GM, Rees AJ. 1998. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J. Immunol. 161:1983–1988 [PubMed] [Google Scholar]
- 4.Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953–964. 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 5.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. 2005. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175:342–349. 10.4049/jimmunol.175.1.342 [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. 2012. Secretory products of macrophages: twenty-five years on. J. Clin. Invest. 122:1189–1190. 10.1172/JCI62930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas SK, Chittezhath M, Shalova IN, Lim JY. 2012. Macrophage polarization and plasticity in health and disease. Immunol. Res. 53:11–24. 10.1007/s12026-012-8291-9 [DOI] [PubMed] [Google Scholar]
- 8.Guirado E, Schlesinger LS, Kaplan G. 2013. Macrophages in tuberculosis: friend or foe. Semin. Immunopathol. 35:563–583. 10.1007/s00281-013-0388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, Kim YJ, Broxmeyer HE. 2005. Macrophage colony-stimulating factor drives cord blood monocyte differentiation into IL-10 (high) IL-12 absent dendritic cells with tolerogenic potential. J. Immunol. 174:4706–4717. 10.4049/jimmunol.174.8.4706 [DOI] [PubMed] [Google Scholar]
- 11.Zhan Y, Xu Y, Lew AM. 2012. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol. Immunol. 52:30–37. 10.1016/j.molimm.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 12.van de Laar L, Coffer PJ, Woltman AM. 2012. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 119:3383–3393. 10.1182/blood-2011-11-370130 [DOI] [PubMed] [Google Scholar]
- 13.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:4560–4565. 10.1073/pnas.0400983101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, Burdick A, Sarno EN, Wagner M, Röllinghoff M, Rea TH, Colonna M, Stenger S, Bloom BR, Eisenberg D, Modlin RL. 2003. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science 301:1527–1530. 10.1126/science.1087785 [DOI] [PubMed] [Google Scholar]
- 15.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. 1997. IL-15, an immunomodulator of T cell responses in intracellular infection. J. Immunol. 158:800–806 [PubMed] [Google Scholar]
- 16.Adams LB, Krahenbuhl JL. 1996. Granulomas induced by Mycobacterium leprae. Methods 9:220–232. 10.1006/meth.1996.0029 [DOI] [PubMed] [Google Scholar]
- 17.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin R. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277–279. 10.1126/science.1925582 [DOI] [PubMed] [Google Scholar]
- 18.Hernandez MO, Neves I, Sales JS, Carvalho DS, Sarno EN, Sampaio EP. 2003. Induction of apoptosis in monocytes by Mycobacterium leprae in vitro: a possible role for tumour necrosis factor-alpha. Immunology 109:156–164. 10.1046/j.1365-2567.2003.01630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulco TO, Lopes UG, Sarno EN, Sampaio EP, Saliba AM. 2007. The proteasome function is required for Mycobacterium leprae-induced apoptosis and cytokine secretion. Immunol. Lett. 110:82–85. 10.1016/j.imlet.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 20.Hernandez MO, Fulco TO, Pinheiro RO, Pereira RM, Redner P, Sarno EN, Lopes UG, Sampaio EP. 2011. Thalidomide modulates Mycobacterium leprae-induced NF-κB pathway and lower cytokine response. Eur. J. Pharmacol. 670:272–279. 10.1016/j.ejphar.2011.08.046 [DOI] [PubMed] [Google Scholar]
- 21.Walsh DS, Lane JE, Abalos RM, Myint KSA. 2004. TUNEL and limited immunophenotypic analyses of apoptosis in paucibacillary and multibacillary leprosy lesions. FEMS Immunol. Med. Microbiol. 41:265–269. 10.1016/j.femsim.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 22.Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, Fortune SM, Remold HG, Behar SM. 2012. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12:289–300. 10.1016/j.chom.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briken V. 2012. ‘With a little help from my friends': efferocytosis as an antimicrobial mechanism. Cell Host Microbe 12:261–263. 10.1016/j.chom.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridley DS, Jopling WH. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255–273 [PubMed] [Google Scholar]
- 25.Paine R, III, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. 2000. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J. Immunol. 164:2602–2609. 10.4049/jimmunol.164.5.2602 [DOI] [PubMed] [Google Scholar]
- 26.Komuro I, Yokota Y, Yasuda S, Iwamoto A, Kagawa KS. 2003. CSF-induced and HIV-1-mediated distinct regulation of Hck and C/EBPbeta represent a heterogeneous susceptibility of monocyte-derived macrophages to M-tropic HIV-1 infection. J. Exp. Med. 198:443–453. 10.1084/jem.20022018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. 2008. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock 30:434–442. 10.1097/SHK.0b013e3181673543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. 2012. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 188:5752–5765. 10.4049/jimmunol.1103426 [DOI] [PubMed] [Google Scholar]
- 29.Akagawa KS. 2002. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int. J. Hematol. 76:27–34. 10.1007/BF02982715 [DOI] [PubMed] [Google Scholar]
- 30.Moura DF, de Mattos KA, Amadeu TP, Andrade PR, Sales JS, Schmitz V, Nery JA, Pinheiro RO, Sarno EN. 2012. CD163 favors Mycobacterium leprae survival and persistence by promoting anti-inflammatory pathways in lepromatous macrophages. Eur. J. Immunol. 42:2925–2936. 10.1002/eji.201142198 [DOI] [PubMed] [Google Scholar]
- 31.Classen A, Lloberas J, Celada A. 2009. Macrophage activation: classical versus alternative. Methods Mol. Biol. 531:29–43. 10.1007/978-1-59745-396-7_3 [DOI] [PubMed] [Google Scholar]
- 32.Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO, Williams DL. 2009. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J. Clin. Microbiol. 47:2124–2130. 10.1128/JCM.00512-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. 2006. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J. Leukoc. Biol. 79:285–293. 10.1189/jlb.0105015 [DOI] [PubMed] [Google Scholar]
- 34.Montoya D, Cruz D, Teles RM, Lee DJ, Ochoa MT, Krutzik SR, Chun R, Schenk M, Zhang X, Ferguson BG, Burdick AE, Sarno EN, Rea TH, Hewison M, Adams JS, Cheng G, Modlin RL. 2009. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe 6:343–353. 10.1016/j.chom.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhme L, Rudel T. 2009. Host cell death machinery as a target for bacterial pathogens. Microbes Infect. 11:1063–1070. 10.1016/j.micinf.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 36.Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. 2013. Local arginase 1 activity is required for cutaneous wound healing. J. Investig. Dermatol. 133:2461–2470. 10.1038/jid.2013.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills CD. 2012. M1 and M2 macrophages: oracles of health and disease. Crit. Rev. Immunol. 32:463–488. 10.1615/CritRevImmunol.v32.i6.10 [DOI] [PubMed] [Google Scholar]
- 38.Benoit M, Desnues B, Mege JL. 2008. Macrophage polarization in bacterial infections. J. Immunol. 181:3733–3739. 10.4049/jimmunol.181.6.3733 [DOI] [PubMed] [Google Scholar]
- 39.Ariel A, Serhan CN. 2012. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3:4. 10.3389/fimmu.2012.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhavsar AP, Guttman JA, Finlay BB. 2007. Manipulation of host-cell pathways by bacterial pathogens. Nature 449:827–834. 10.1038/nature06247 [DOI] [PubMed] [Google Scholar]
- 41.Locati M, Mantovani A, Sica A. 2013. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 120:163–184. 10.1016/B978-0-12-417028-5.00006-5 [DOI] [PubMed] [Google Scholar]
- 42.Makino M, Maeda Y, Fukutomi Y, Mukai T. 2007. Contribution of GM-CSF on the enhancement of the T cell-stimulating activity of macrophages. Microbes Infect. 9:70–77. 10.1016/j.micinf.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sica A, Locati M. 2005. Macrophage polarization comes of age. Immunity 23:344–346. 10.1016/j.immuni.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 44.de Souza Sales J, Lara FA, Amadeu TP, de Oliveira Fulco T, da Costa Nery JA, Sampaio EP, Pinheiro RO, Sarno EN. 2011. The role of indoleamine 2, 3-dioxygenase in lepromatous leprosy immunosuppression. Clin. Exp. Immunol. 165:251–263. 10.1111/j.1365-2249.2011.04412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. 2009. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J. Immunol. 182:4415–4422. 10.4049/jimmunol.0713732 [DOI] [PubMed] [Google Scholar]
- 46.Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, Dos Reis GA, Lopes MF. 2000. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403:199–203. 10.1038/35003208 [DOI] [PubMed] [Google Scholar]
- 47.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. 2004. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 173:6521–6525. 10.4049/jimmunol.173.11.6521 [DOI] [PubMed] [Google Scholar]
- 48.Ritter U, Frischknecht F, van Zandbergen G. 2009. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 25:505–510. 10.1016/j.pt.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 49.Munder M. 2009. Arginase: an emerging key player in the mammalian immune system. Br. J. Pharmacol. 158:638–651. 10.1111/j.1476-5381.2009.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeurer M, Seliger B, Trinder P, Gerdes J, Seitzer U. 1999. Interleukin-15 in mycobacterial infection of antigen-presenting cells. Scand. J. Immunol. 50:280–288. 10.1046/j.1365-3083.1999.00593.x [DOI] [PubMed] [Google Scholar]
- 51.Johann AM, Barra V, Kuhn AM, Weigert A, von Knethen A, Brüne B. 2007. Apoptotic cells induce arginase II in macrophages, thereby attenuating NO production. FASEB J. 21:2704–2712. 10.1096/fj.06-7815com [DOI] [PubMed] [Google Scholar]
- 52.Barra V, Kuhn AM, von Knethen A, Weigert A, Brüne B. 2011. Apoptotic cell-derived factors induce arginase II expression in murine macrophages by activating ERK5/CREB. Cell. Mol. Life Sci. 68:1815–1827. 10.1007/s00018-010-0537-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. 2010. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci. Signal. 3:ra62. 10.1126/scisignal.2000955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briken V, Mosser DM. 2011. Switching on arginase in M2 macrophages. J. Leukoc. Biol. 90:839–841. 10.1189/jlb.0411203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.