Abstract
Streptococcal collagen-like protein 1 (Scl-1) is one of the most highly expressed proteins in the invasive M1T1 serotype group A Streptococcus (GAS), a globally disseminated clone associated with higher risk of severe invasive infections. Previous studies using recombinant Scl-1 protein suggested a role in cell attachment and binding and inhibition of serum proteins. Here, we studied the contribution of Scl-1 to the virulence of the M1T1 clone in the physiological context of the live bacterium by generating an isogenic strain lacking the scl-1 gene. Upon subcutaneous infection in mice, wild-type bacteria induced larger lesions than the Δscl mutant. However, loss of Scl-1 did not alter bacterial adherence to or invasion of skin keratinocytes. We found instead that Scl-1 plays a critical role in GAS resistance to human and murine phagocytic cells, allowing the bacteria to persist at the site of infection. Phenotypic analyses demonstrated that Scl-1 mediates bacterial survival in neutrophil extracellular traps (NETs) and protects GAS from antimicrobial peptides found within the NETs. Additionally, Scl-1 interferes with myeloperoxidase (MPO) release, a prerequisite for NET production, thereby suppressing NET formation. We conclude that Scl-1 is a virulence determinant in the M1T1 GAS clone, allowing GAS to subvert innate immune functions that are critical in clearing bacterial infections.
INTRODUCTION
Group A Streptococcus (GAS) (Streptococcus pyogenes) is among the top 10 human pathogens worldwide and is responsible for considerable infection-associated morbidity and mortality (1). GAS manifests in a broad array of diseases ranging from self-limiting infections, such as impetigo and pharyngitis, to life-threatening conditions, such as necrotizing fasciitis and toxic shock syndrome (2). Overall, GAS annually causes about 700 million infections, including 650,000 severe invasive infections (1), yet no commercial vaccines to protect against GAS disease are currently available.
Infectious disease outcome is determined in large part by the functions of the host innate immune response. In the setting of acute bacterial infection, the first innate immune cells recruited to the site of infection are neutrophils, which employ three basic mechanisms to clear the pathogen: phagocytosis, exposure to antimicrobial content stored in granules, and extracellular killing via phagocyte extracellular traps, particularly neutrophil extracellular traps (NETs) (3). To combat these defenses, GAS has evolved several strategies to evade and counteract the innate phagocyte immune response (2). A significant portion of the global burden of invasive GAS disease is attributable to the prevalent M1T1 clone (4, 5), which encodes multiple immune evasion genes, including factors that impede neutrophil recruitment, limit phagocytosis (6, 7), resist antimicrobial peptides (8, 9), and degrade NETs (10, 11). These multiple immune evasion mechanisms may allow GAS to resist the innate immune defenses of the host and allow the bacteria to produce systemic infections, including infections in previously healthy individuals.
Among known and potential virulence factors of GAS, the gene encoding streptococcal collagen-like 1 protein (Scl-1, also denoted SclA) has been shown to be one of the most highly upregulated genes in strains causing invasive infections (12) and in whole-blood infections (13). Scl-1 encodes a surface protein bearing a collagen-like domain that is conserved among GAS strains and a hypervariable N-terminal domain (14, 15). In other, less virulent GAS serotype strains, Scl-1 has been shown to participate in biofilm formation (16, 17), attachment to extracellular matrix proteins (18), and attachment to lung epithelial cells (15, 19, 20) but not pharyngeal cells (14). Studies using recombinant Scl-1 have shown that the protein, via its serotype-hypervariable N terminus, can bind a variety of host proteins, including serum lipoproteins (21, 22), integrins (23, 24), the complement inhibitors FH and FH-related protein 1 (25, 26), and the thrombin-activatable fibrinolysis inhibitor (27). While Scl-1 proteins of different GAS serotypes bind a variety of host proteins, the results suggest a common role for Scl-1 in perturbing the functions of the innate immune system.
Despite numerous detailed studies using recombinant Scl-1 protein, very few studies have examined the role of Scl-1 at physiological expression levels and in the context of the living pathogen. Of those studies using isogenic mutants and various infection models, differences in virulence between strains possessing and lacking Scl-1 have been relatively mild (14, 15, 17, 18), which may be in part due to the overall moderate virulence characteristics of the bacterial strains studied. The current work explores the importance of this protein in the context of a highly virulent M1T1 clone. Our analysis reveals that Scl-1 plays an important role in M1T1 GAS neutrophil resistance and innate immune evasion in vitro and in vivo.
MATERIALS AND METHODS
Cell culture.
THP-1 (ATCC TIB-202), HaCaT (28), and J774 (ATCC TIB-67) cells were maintained in RPMI or Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Invitrogen). Neutrophils were isolated from healthy donors (with use and procedures approved by the University of California San Diego Human Research Protections Program) using the PolyMorphPrep kit (Fresenius Kabi) as previously described (29).
Bacterial strains and mutant construction.
Wild-type (WT) GAS strain M1T1 5448 was originally isolated from a patient with necrotizing fasciitis and toxic shock syndrome (30) and was cultivated in Todd-Hewitt broth (THB) at 37°C. A precise, in-frame allelic replacement of scl-1 with the chloramphenicol transferase (cat) gene was created in M1T1 GAS strain 5448 as described previously (31). The primer sets used to PCR amplify DNA fragments directly upstream and downstream of scl-1 from the M1T1 GAS genome were scl downF (5′-ATGTTGTTCTCTCTTTCTTTATTAG-3′ with a 30-bp 5′ extension matching the 5′ end of the cat gene) plus scl downR (5′-CCATGATGACCTGGGCTAACCGCG-3′) and scl upF (5′-CCATGGTTTCGCGCGAAATGCGC-3′) plus scl upR (5′-TTCTCTGAATTGAGAGACCTTAC-3′ with a 30-bp 5′ extension matching the 3′ end of the cat gene). The mutant strain was denoted Δscl, and allelic replacement was verified by PCR and sequencing. To complement the mutation, the scl-1 gene was amplified from the M1T1 genome by PCR with primers scl1F (5′-TTAGTTGTTTTCTTTGCGTTTTGT-3′) and scl1R (5′-ATGTTGACATCAAAGCACCAT-3′), cloned into pCR2.1-TOPO (Invitrogen), and subcloned into shuttle expression vector pDCerm (31). The plasmid carrying the scl-1 gene (pscl) was introduced via electroporation into the Δscl mutant, and the resulting strain was cultured in THB supplemented with 2 μg/ml erythromycin to maintain the plasmid. To express green fluorescent protein (GFP) in GAS, GFP was subcloned into the shuttle expression vector pDCerm and introduced via electroporation into the WT and Δscl strains. The resulting strains were cultured in THB supplemented with 2 μg/ml erythromycin to maintain the plasmid. Bacteria were grown to log phase in THB for all experiments. Growth of all bacterial strains in THB was not significantly different (data not shown).
Mouse infection models.
All animal use and procedures were approved by the UCSD Institutional Animal Care and Use Committee. Eight- to 10-week-old C57BL/6 mice (Charles River Laboratories) were infected with 2 × 108 CFU subcutaneously. Lesion sizes were monitored over a 3-day period by photography from a fixed camera height; a ruler was included to calibrate the length/pixel ratio. Surface area of lesions was measured in mm2 using ImageJ software. On day three postinfection, skin lesions were excised with approximately 2-mm margins and homogenized in phosphate-buffered saline (PBS) with 1.5-mm zirconium beads. The bacterial load in the lesions was determined by enumeration of lesion homogenates on Todd-Hewitt agar (THA) plates.
Cell adherence and invasion assays.
Adherence and invasion assays using HaCaT keratinocytes were performed as previously described (32). Briefly, HaCaT cells were plated at 2 × 105 cells/well in a 24-well plate 1 day prior to the assay. Log-phase bacteria were used to infect cells at a multiplicity of infection (MOI) of 10 bacteria to 1 cell. Plates were centrifuged for 5 min at 500 × g to initiate bacterial contact and incubated for 30 min at 37°C in 5% CO2 (adherence) or 2 h (invasion). For invasion assays, cells were washed three times with PBS, fresh medium containing 10 μg/ml penicillin plus 100 μg/ml gentamicin was added, and cells were incubated for an additional 2 h. After incubation, cells were washed 6 times with PBS, trypsinized and lysed with 0.025% Triton X-100, and plated on THA for enumeration. Bacterial adherence and invasion were calculated as a percentage of the initial inoculum.
Cell killing experiments.
For neutrophil assays, freshly isolated cells were stimulated with 25 nM phorbol 12-myristate 13-acetate (PMA) (Sigma) for 20 min prior to the addition of bacteria. THP-1 cells were seeded 1 day prior to infection in 24-well plates at 5 × 105 cells per well with a final concentration of 10 nM PMA to differentiate cells to macrophages (33). J774 cells were seeded at 2.5 × 105 cells/well in 24-well plates 1 day prior to infection. Bone-marrow derived macrophages (BMDMs) were isolated from 8- to 10-week-old C57BL/6 WT or cathelicidin-related antimicrobial peptide (CRAMP)-deficient mice as previously described (34) and seeded at 5 × 105 cells/well in 24-well plates 1 day prior to infection. Prior to bacterial infection, cells were treated with 10 μg/ml cytochalasin D (cyt D) (Sigma) for 10 min, 100 nM butylated hydroxyanisole (BHA) (Sigma) for 15 min, 0.2 to 0.5 kU/ml catalase (Sigma) for 20 min, or 100 μM 4-aminobenzoic acid hydrazide (ABAH) (Fisher Scientific) for 20 min. Appropriate vehicle controls were included. Log-phase bacteria were added to cells at an MOI of 1 bacterium/cell, centrifuged for 5 min at 500 × g to initiate bacterial contact, and incubated for 90 min at 37°C in 5% CO2. Cells were lysed with a final concentration of 0.025% Triton X-100 and enumerated on THA plates. Survival was calculated as the percentage of the initial inoculum, and results were compared with those for the wild-type strain to account for donor-dependent variability.
Phagocytosis assay.
Polymorphonuclear leukocytes (PMNs) (2 × 106) were stimulated with PMA for 20 min in RPMI with 2% FBS and then infected at an MOI of 10 with the nonopsonized log-phase WT or Δscl mutant strain transformed with a plasmid encoding GFP. Cells were incubated for 20 min in the presence or absence of 10 μg/ml cyt D and then incubated for 10 min with 100 U/ml penicillin and 100 μg/ml streptomycin to kill extracellular bacteria. Cells were washed three times with PBS, fixed with 4% paraformaldehyde, and analyzed by flow cytometry. Neutrophils were gated by side and forward scatters, and the fluorescence intensity was measured for a total of 10,000 cells. Uninfected PMNs were used as a negative control. Flow cytometry data were analyzed with FlowJo v. 9.4.10 (Tree Star, Inc.). To visualize extracellular bacteria by fluorescence microscopy, nonpermeabilized cells were incubated with 0.01 mg/ml isolated rabbit IgG (Sigma) for 1 h at 37°C, followed by incubation with goat anti-rabbit Alexa 568 antibody (Invitrogen). Cells were counterstained with DAPI (4′,6′-diamidino-2-phenylindole) and imaged on a fluorescence microscope (Zeiss).
NET killing and induction experiments.
NET killing experiments were performed as previously described (35). Briefly, neutrophils were stimulated with 25 nM PMA for 4 h, washed to remove remaining cells, and infected with log-phase bacteria at an MOI of 0.1 for 20 min. As a control, NETs were degraded by the addition of 500 mU/ml micrococcal nuclease (MNase) (Sigma) prior to infection. CFU were enumerated by plating onto THA. Survival was calculated as the percentage of the initial inoculum, and results were compared with those for the wild-type strain to account for donor-dependent variability.
NET induction assays were performed as previously described (35). Briefly, 2 × 105 neutrophils were seeded per well into a 96-well plate and infected with log-phase bacteria at an MOI of 1. Control PMNs were stimulated with 25 nM PMA (positive control) or untreated (negative control). Cells were incubated for 3 h at 37°C, centrifuged, and washed to remove cells, and NET DNA was collected in medium containing 500 mU/ml MNase for 10 min to release NETs from the wells, followed by inactivation of MNase with 5 mM EDTA. The supernatant was incubated with Quant-iT PicoGreen reagent (Invitrogen) for 5 min at room temperature (RT), and fluorescence was quantified on a SpectraMax M3 plate reader at 480-nm excitation and 520-nm emission wavelengths using SoftMax Pro software.
LL-37 and CRAMP susceptibility.
Bacteria were grown in RPMI plus 5% THB to log phase, adjusted to 2 × 105 CFU/ml in RPMI plus 5% THB, and incubated with 8 μM murine-derived CRAMP or 4 μM human-derived LL-37 (AnaSpec). Samples were taken every 30 min for 120 min, and the CFU were enumerated from THA plates. Survival was calculated as the percentage of the initial inoculum.
FITC-labeled poly-l-lysine and TAMRA-labeled LL-37 binding assay.
For poly-l-lysine binding, bacteria were grown to log phase, washed twice with 20 mM HEPES (pH 7.24), and adjusted to 2 × 108 CFU/ml. Fluorescein isothiocyanate (FITC)-labeled poly-l-lysine (Sigma) was added at 100 μg/ml and incubated for 30 min at 37°C with shaking in the dark. For 6-carboxytetramethylrhodamine (TAMRA)-labeled LL-37 binding, bacteria were grown to log phase in RPMI without phenol red plus 5% THB. Bacteria were labeled with 5 μM TAMRA-labeled LL-37 (AnaSpec) with rotation at 37°C in the dark for 30 min. Following incubation with FITC–poly-l-lysine or TAMRA–LL-37, bacteria were washed three times with PBS and analyzed by flow cytometry. Bacteria were gated by side and forward scatters, and the fluorescence intensity was measured for total of 10,000 cells. Unlabeled bacteria were used as a negative control. Flow cytometry data were analyzed with FlowJo v. 9.4.10 (Tree Star, Inc.).
Peroxide production assays.
THP-1 cells were seeded in 96-well plates at 2 × 105 cells with 10 nM PMA 1 day prior to infection. Cells were washed and incubated in medium containing 50 μM Amplex Red (Invitrogen) and 0.1 U/ml horseradish peroxidase (Invitrogen). Log-phase bacteria were added at an MOI of 1, and plates were centrifuged to initiate bacterial contact and incubated in a fluorescent plate reader at 37°C. Fluorescence was measured every 15 min on a Beckman-Coulter DTX 880 plate reader at 535-nm excitation and 595-nm emission wavelengths using Multimode Analysis Software.
MPO activity assays.
Neutrophils were infected with bacteria at an MOI of 1 as described above. Myeloperoxidase (MPO) release into the supernatant was quantified by incubating cell-free supernatant with developing reagent containing 0.5% hexadecyl trimethyl ammonium bromide (Sigma), 0.167 mg/ml o-dinanisidine dihydrochloride (Sigma), and 100 μM hydrogen peroxide in 50 mM sodium phosphate buffer (pH 6.0) at RT for 10 min. Total neutrophil MPO content was measured from unstimulated neutrophils lysed with 0.025% Triton X-100. The absorbance was measured with a SpectraMax M3 plate reader at 450 nm for MPO using SoftMax Pro software.
Statistical analysis.
All data were collected from at least three independent experiments in triplicate. Experiments using neutrophils were performed with a minimum of three different healthy volunteers. The data were combined, normalized, and expressed as mean ± standard error of the mean (SEM) except where indicated. All assay data were analyzed by one-way analysis of variance (ANOVA) with Tukey's multiple-comparison posttest or by two-way ANOVA with the Bonferroni posttest. All statistical tests were performed using GraphPad Prism version 5.0 (GraphPad Software Inc.). P values of <0.05 were considered statistically significant.
RESULTS
Scl-1 is important for bacterial persistence in a mouse model of subcutaneous infection.
To determine the role of Scl-1 in the physiological context of the globally disseminated M1T1 GAS clone, we constructed a mutant strain lacking the scl-1 gene (designated Δscl) by precise in-frame allelic exchange mutagenesis as previously described (10). To satisfy molecular Koch's postulates, the gene was restored in the mutant background on a heterologous expression plasmid (designated Δscl + pscl). Construction of the mutant strain was verified by sequencing, and quantitative PCR confirmed that the WT and complemented strains appropriately transcribed the scl-1 gene and that the allelic replacement mutant lacked detectable transcript (data not shown). Immunofluorescence analysis revealed that Scl-1 was appropriately expressed on the surface of the WT and complemented strains and absent on the surface of the mutant strain (see Fig. S1 in the supplemental material). Additionally, we demonstrated that there were no differences in other surface molecules, such as M protein and hyaluronic capsule, and that SpeB activity was similar in all strains (see Fig. S1 in the supplemental material). These data further verify that the mutant strain serves as a proper vehicle for assessment of the role of Scl-1 in the M1T1 clone.
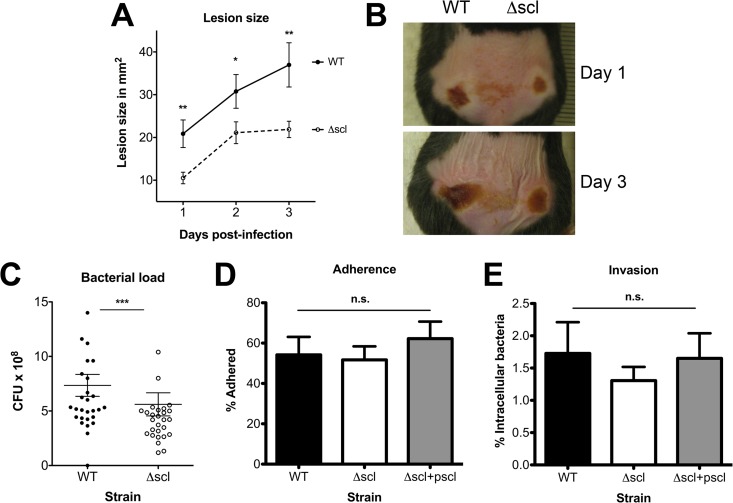
Scl-1 is an LP(X)TG motif protein anchored to the GAS cell wall, and in studies utilizing recombinant protein, Scl-1 has been shown to interact with extracellular matrix proteins and host cells (15, 18–20). We first tested whether Scl-1 contributed to M1T1 GAS virulence using a mouse model of localized subcutaneous infection. Animals were infected with either WT or Δscl mutant bacteria in opposing flanks, and lesion sizes were monitored over a 3-day time course (Fig. 1A and B). Infection with the WT strain produced larger lesions at every time point postinfection than infection with the Δscl mutant (Fig. 1A and B). Furthermore, a higher bacterial load was recovered from the larger WT-infected lesions at 3 days postinfection compared than from Δscl-infected lesions (Fig. 1C). Similar findings in a skin infection model have been previously observed for a different M1 strain (15). To determine whether our in vivo findings resulted from Scl-1-mediated differences in cellular adhesion and invasion, we performed in vitro assays with a human keratinocyte cell line (HaCaT), but we identified no difference among our bacterial strains in either adhesion (Fig. 1D) or invasion (Fig. 1E) of HaCaT keratinocytes. These results suggested that the differences in lesion size and bacterial recovery in our mouse subcutaneous infection model (Fig. 1A and B) were less likely to reflect differences in cell attachment or entry but rather involve a role for Scl-1 in GAS resistance to host innate immune clearance.
FIG 1.
Scl-1 increases GAS persistence in a subcutaneous infection model but is not involved in adherence or invasion of skin keratinocytes. (A) Lesion size development was monitored over a 3-day time course. (B) Representative images of infected mice demonstrate differences in lesion development. (C) Total bacteria recovered from lesions collected at 3 days postinfection. For panels A to C, a total of 24 mice were analyzed in two independent experiments and results were analyzed by Student's t test (***, P < 0.001; **, P < 0.01; *, P < 0.05). (D and E) Adherence (D) and invasion (E) of the WT, Δscl mutant, and complemented strains to HaCaT keratinocytes. For panels D and E, data from three independent experiments were combined, and the results are given as average ± SEM and were analyzed by one-way ANOVA with Tukey's multiple posttest. n.s., not significant.
Scl-1 protects bacteria from killing by phagocytic cells.
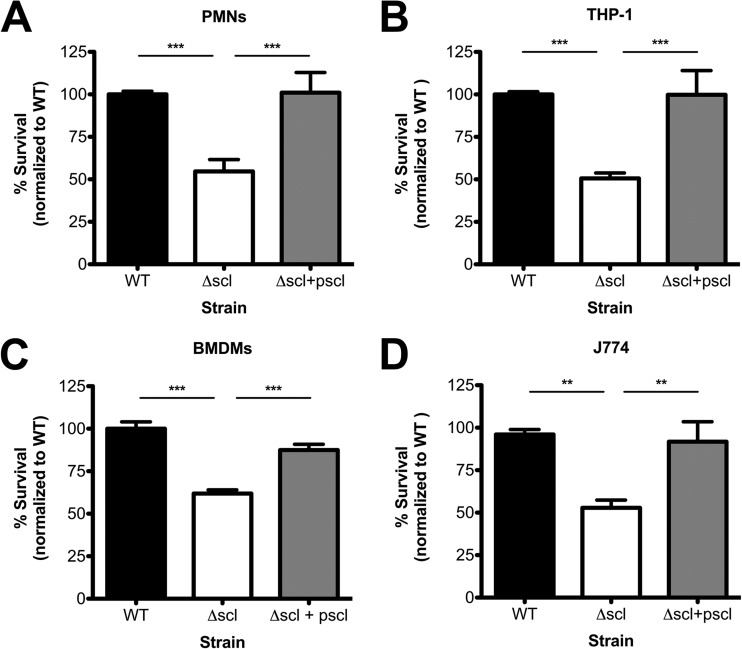
Neutrophils are the predominant innate immune cell type that migrates to the site of infection to combat invading pathogens. Resident macrophages also play an important role in sensing and destroying pathogens. To determine whether the reduced persistence of the Δscl mutant strain observed in the mouse infection model was due to increased susceptibility to phagocytic cell killing, we studied the contribution of Scl-1 to bacterial survival in total killing assays with human and murine phagocytic cells in vitro. When incubated with isolated human neutrophils (polymorphonuclear cells [PMNs]), Δscl mutant bacteria were more susceptible to killing than either the WT or complemented strain (Fig. 2A). We observed similar trends when we expanded our studies to other phagocytic cell types, including cultured human monocytes (THP-1 cells) (Fig. 2B), murine bone marrow-derived macrophages (Fig. 2C), and cultured murine macrophages (J774 cells, Fig. 2D). Scl-1 was also important for survival in freshly isolated human whole blood (see Fig. S2 in the supplemental material), though the differences between the WT and Δscl mutant strains were not statistically significant in the context of the complemented strain, which survived extremely well in blood (see Fig. S2 in the supplemental material). These data support our hypothesis that the reduced persistence and virulence of the Δscl mutant strain observed in our in vivo infection model (Fig. 1) were due to reduced resistance to phagocytic cell killing.
FIG 2.
Scl-1 enhances bacterial resistance to phagocytic killing. The survival of the WT, Δscl, and complemented strains in total killing assays was analyzed using human neutrophils (PMNs) (A), cultured human monocytes (THP-1) (B), murine bone marrow-derived macrophages (BMDMs) (C), and cultured murine macrophages (J774) (D). Data are normalized to the survival of the WT strain, and data from at least three independent experiments were combined. Results are given as average ± SEM and were analyzed by one-way ANOVA with Tukey's multiple posttest (***, P < 0.001; **, P < 0.01).
Scl-1 protects bacteria from a neutrophil extracellular killing mechanism.
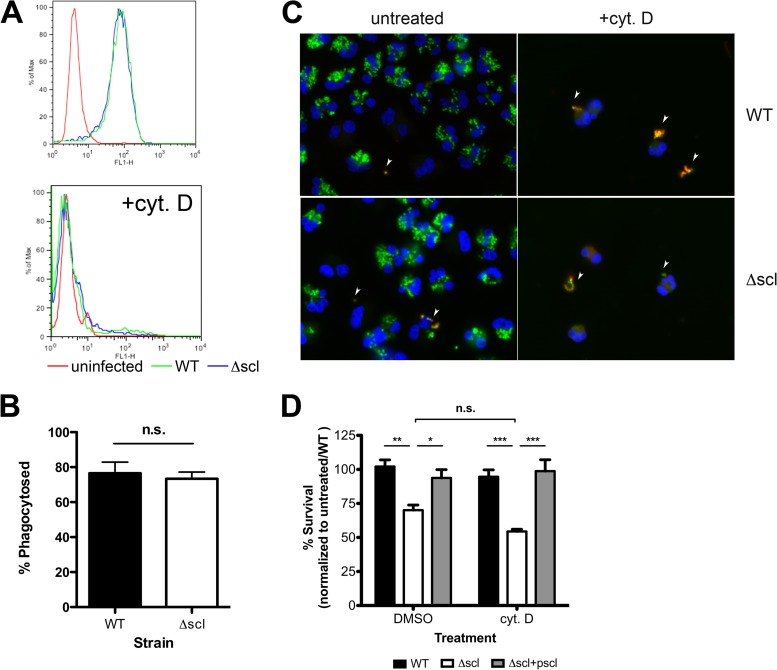
Because Scl-1 is a surface protein, a potential immune evasion function it could serve is to act as an inhibitor of phagocytosis, either by sterically hindering phagocytic cell receptors or by cloaking molecules that would otherwise be recognized by phagocytic receptors. To study phagocytosis, we introduced a green fluorescent protein (GFP) expression construct into the WT and Δscl M1T1 GAS strains and analyzed neutrophil phagocytosis of the fluorescent bacteria by flow cytometry (Fig. 3A, top panel). To prevent the possibility of serum proteins binding to Scl-1 (21, 22, 25–27) and affecting phagocytosis, bacteria were not preopsonized or incubated in the presence of human serum during phagocytosis assays or subsequent in vitro cell-based assays. Control treatment of the neutrophils with cyt D to prevent phagocytosis effectively eliminated the fluorescent signal and validated our assay specificity (Fig. 3A, bottom panel). We found no measurable differences in uptake of the WT strain compared to the Δscl mutant strain by human neutrophils (Fig. 3A and B). These findings were confirmed by fluorescence microscopy and direct visualization of neutrophils infected with GFP-expressing strains (Fig. 3C). Furthermore, when phagocytosis was pharmacologically inhibited by cyt D in the in vitro cell killing experiments, a significant difference in survival of the Δscl strain was still detected (Fig. 3D). Thus, Scl-1 does not appear to function as an antiphagocytic molecule. Furthermore, because the survival of strains in the presence of cyt D-treated cells was not significantly different from the survival of strains incubated with vehicle-treated cells (Fig. 3D), the results further suggest that Scl-1 protects the bacteria from an extracellular killing mechanism of phagocytic cells.
FIG 3.
Scl-1 is not an antiphagocytic molecule. (A) WT and Δscl strains expressing GFP were incubated with PMNs in the presence or absence of cyt D and analyzed by flow cytometry to assess phagocytosis of bacteria. (B) The combined results of phagocytosis assays were quantified. (C) Immunofluorescence assays qualitatively confirmed no differences in phagocytosis and that the fluorescent signals originated solely from internalized bacteria. Extracellular bacteria stained with red fluorescent antibody and are indicated by arrowheads. Representative images are shown at a magnification of ×100. (D) Total killing assay with THP-1 cells treated with dimethyl sulfoxide (DMSO) (vehicle control) or cyt D to block phagocytosis. Data are normalized to the survival of the WT strain with untreated cells. For panels A and C, representative data from three independent experiments are shown. For panels B and D, data from at least three independent experiments were combined. Results are given as average ± SEM and were analyzed by Student's t test (B) or one-way ANOVA with Tukey's multiple posttest (D) (n.s., not significant; ***, P < 0.001; **, P < 0.01; *, P < 0.05).
Scl-1 protects against NET-mediated killing and antimicrobial peptides.
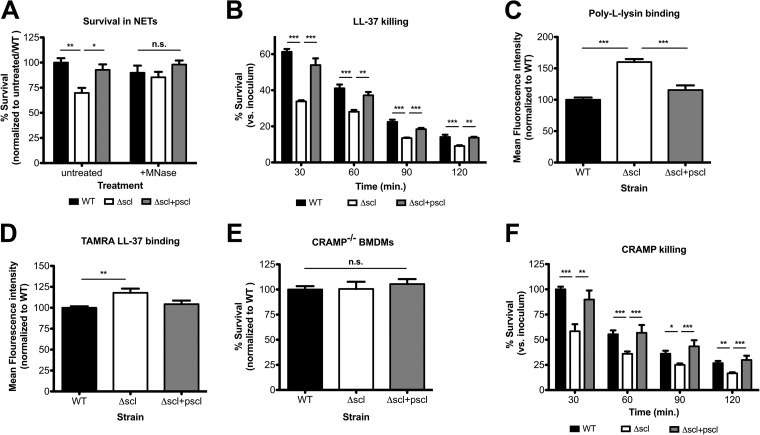
NETs are a relatively recently discovered killing strategy of neutrophils (36) and are one mechanism by which neutrophils can kill bacteria extracellularly even in the presence of cyt D (37). To determine whether Scl-1 provided protection against NET-mediated killing, we performed pure NET killing assays. PMA was used to stimulate maximum NET production, and bacterial strains were incubated with a cell-free accumulation of NETs. Induced NETs were able to eliminate the Δscl mutant strain more efficiently than its WT parent strain (Fig. 4A). Control treatments with micrococcal nuclease (MNase), an exonuclease derived from Staphylococcus aureus that was used to dismantle the DNA backbone, confirmed that intact NET structures were required for the observed differences in WT compared to Δscl mutant bacterial survival (Fig. 4A).
FIG 4.
Scl-1 confers resistance to NETs by providing protection from cathelicidin. (A) Survival of WT, Δscl mutant, and complement strains incubated with pure NETs in the presence or absence of micrococcal nuclease (MNase) to degrade DNA structures. (B) Bacterial survival in the presence of recombinant LL-37. (C and D) Binding of FITC-labeled poly-l-lysine (C) or TAMRA-labeled LL-37 (D) to live bacteria quantified by flow cytometry. (E) Survival of strains incubated with BMDMs isolated from CRAMP-deficient mice. (F) Bacterial survival in the presence of recombinant CRAMP. For panels A, C, D, and E, data are normalized to the WT strain, and for panels B and F, data are compared with the initial inoculum. For all panels, data from at least three independent experiments were combined, and results are given as average ± SEM and were analyzed by one-way ANOVA with Tukey's multiple posttest (***, P < 0.001; **, P < 0.01; *, P < 0.05; n.s., not significant).
NETs contain many bactericidal components, including antimicrobial peptides, granule proteases, and histones (38). The human cathelicidin antimicrobial peptide LL-37 is a major component of NETs and contributes to GAS killing (37, 38). Incubation of the strains with recombinant LL-37 in vitro demonstrated that the presence of Scl-1 delayed the killing kinetics of the WT and complemented strains compared with the Δscl mutant strain (Fig. 4B). These data support that it may specifically be the LL-37 found in NETs that is responsible for the more effective elimination of the Δscl strain (Fig. 4A). LL-37 is a cationic molecule that exerts its bactericidal activity through pore formation initiated upon LL-37 binding to negatively charged bacterial surfaces (39). Bacterial binding studies with positively charged FITC-labeled poly-l-lysine demonstrated that the presence of Scl-1 reduces the binding of positively charged molecules to the bacteria (Fig. 4C). Binding studies with TAMRA-labeled LL-37 (Fig. 4D) corroborated this observation and support a mechanism in which increased LL-37 binding to the mutant bacterial cell surface contributes to the accelerated killing kinetics seen in Fig. 4B.
The murine cathelicidin-related antimicrobial peptide (CRAMP) similarly localizes to murine phagocyte extracellular traps (40, 41). To expand on our data examining the sensitivity of our mutant strain to antimicrobial peptides in extracellular traps, we used macrophages isolated from mice genetically deficient in CRAMP in bacterial killing experiments. In contrast to experiments performed with macrophages isolated from WT mice (Fig. 2C), we observed that the survival of the Δscl mutant was equivalent to that of Scl-1-expressing strains when incubated with CRAMP-deficient macrophages (Fig. 4E). Similarly, Scl-1 conferred slower killing kinetics by recombinant CRAMP in vitro (Fig. 4F). Thus, our data show that reducing binding and susceptibility to cathelicidin antimicrobial peptides in the Scl-1-expressing WT M1T1 strain may contribute to its resistance to phagocyte extracellular trap-mediated killing.
Scl-1 suppresses MPO activity and subsequent NET formation.
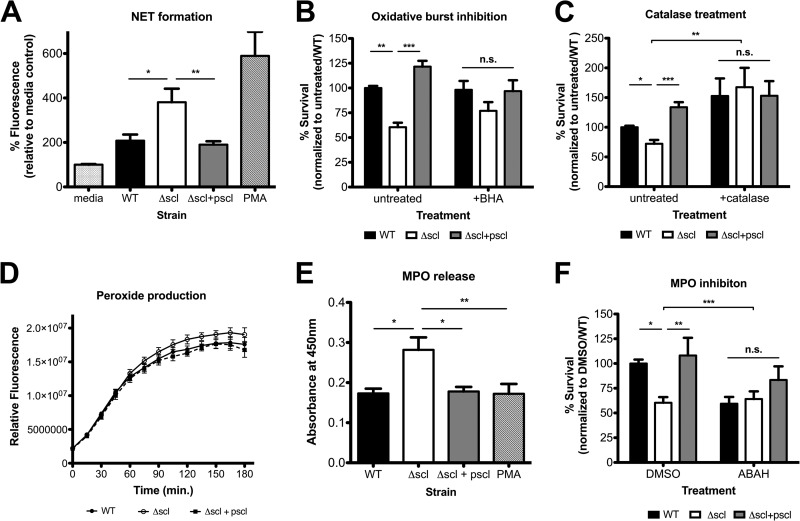
Previous research by our lab has demonstrated that the immunodominant GAS surface protein virulence factor M1 increases bacterial survival in NETs by capturing LL-37 but simultaneously binds fibrinogen in proinflammatory complexes to activate neutrophils and generate NETs (37, 42). We asked whether the Scl-1-dependent differences in phagocyte survival (Fig. 2) were influenced not only by relative susceptibility to antimicrobial LL-37 found in NETs (Fig. 4) but also modulation of the abundance of NETs formed. Indeed, we found that the Δscl mutant strain induced the formation of significantly more NETs, indicating that in the WT and complemented strains, Scl-1 appears to suppress NET formation (Fig. 5A).
FIG 5.
Scl-1 suppresses NET formation by preventing MPO release. (A) NET induction by PMNs in response to the indicated stimuli. (B and C) Bacterial survival in neutrophil killing assays in the presence of ROS scavenger BHA (B) or catalase (C). (D) Hydrogen peroxide production by cells incubated with the different strains. (E) MPO release into the supernatant after incubation of the strains with neutrophils. (F) Neutrophil killing assays in the presence of DMSO (vehicle control) or ABAH (MPO inhibitor). For all panels, data from at least three independent experiments were combined, and results are given as average ± SEM and were analyzed by one-way ANOVA with Tukey's multiple posttest (***, P < 0.001; **, P < 0.01; *, P < 0.05; n.s., not significant).
The production of reactive oxygen species (ROS) through the oxidative burst is a critical stimulatory pathway promoting the generation of NETs (3). When neutrophils were treated with a general oxidative burst inhibitor (butylated hydroxyanisole [BHA]) or when catalase, a peroxide-degrading enzyme, was included in neutrophil killing experiments, differences in strain survival were abolished, compared to the enhanced survival of the WT over the Δscl mutant seen under control conditions (Fig. 5B and C). However, when we examined the production of the ROS hydrogen peroxide by cells in response to the different strains, we found no differences in the amount of oxidants produced (Fig. 5D). Thus, limiting NET formation via inhibition of the oxidative burst response generally decreases the ability of the cells to kill the Δscl strain, but Scl-1 does not aid in suppressing the generation of ROS.
Following ROS exposure, granule and nuclear membranes break down and initiate the mixing of granule contents with the nucleus and begin the NET formation process (3). Myeloperoxidase (MPO) is a major enzyme stored in neutrophil azurophilic granules, and release of MPO into the environment and subsequent localization to NETs are a hallmark of NET formation (38). Therefore, we measured the release of MPO from neutrophils in response to the different strains as a gauge of NET generation. After 90 min of infection and over a 45-min time course, the Δscl mutant strain induced greater release of MPO by neutrophils into the medium (Fig. 5E), supporting that Scl-1 in the WT and complemented strains prevents NET induction (Fig. 5A) by inhibiting MPO release. Indeed, when MPO activity was blocked using the pharmacological agent 4-aminobenzoic acid hydrazide (ABAH), we abrogated the protective function of Scl-1 in bacterial resistance to neutrophil killing (Fig. 5F). Based on these findings, we conclude that Scl-1 blunts the release of MPO and that Scl-1 not only can directly protect bacteria from the cathelicidin LL-37 deployed in NETs (Fig. 4) but also suppresses the quantity of NETs produced (Fig. 5).
DISCUSSION
Surface proteins of GAS are critical mediators of bacterial resistance to host innate immunity. A particularly well-characterized example is M protein, which mediates adhesion to host cells, provides resistance to opsonophagocytosis, and improves survival in NETs (2, 43). The multiple roles that M protein plays in immune resistance are likely to contribute to the increased virulence in subcutaneous mouse models of infection and in human blood (2, 43). Similarly, Scl-1 in other GAS serotypes has been reported to be important for host cell adhesion and survival in blood and serum (15, 19–22, 25, 27). The hypervariable N-terminal domain of the Scl-1 protein is largely responsible for binding various serum proteins, while the collagen-like domain has been shown to bind integrins to facilitate interactions with host cells (15, 19–22, 25, 27). Collagen-like proteins in other pathogenic bacteria, including Streptococcus pneumoniae (44), Bacillus anthracis (45), and Legionella pneumophila (46), similarly aid in bacterial adhesion to and invasion of host cells. In our studies of the Scl-1 protein in the physiological context of the predominant M1T1 GAS clone, we found that Scl-1 was not important for adhesion to keratinocytes, nor did it affect phagocytosis of bacteria in the absence of serum proteins, but rather it represented an important virulence determinant in vitro and in vivo by increasing resistance to phagocyte killing mechanisms. With neutrophils, a critical innate immune cell, Scl-1 appeared to both limit the formation of NETs and provide resistance to cathelicidins found within the NETs. These resistance mechanisms can be exclusively attributed to Scl-1 of M1T1 GAS, as there are no differences in other surface molecules of our bacterial strains (see Fig. S1 in the supplemental material). Furthermore, the role of Scl-1 in immune resistance is likely due to the N-terminal domain of M1 serotype Scl-1, as the collagen-like domain appears to chiefly play a role in bacterial adhesion to host cells (19, 20). Sequence analysis of the N-terminal domain of M1 Scl-1 did not reveal significant homology to other proteins of known function (data not shown). Although many studies of Scl-1 in non-M1 GAS strains have not previously demonstrated a role for the protein in phagocyte resistance (15, 19–22, 25, 27), a recent study similarly described a role for Scl-1 in a non-M1 strain in whole-blood survival and neutrophil resistance (13), consistent with our observations of M1T1 GAS with neutrophils and multiple macrophage cell lines (Fig. 2) and whole blood (see Fig. S2 in the supplemental material). Another interesting recent study suggests that Scl-1 could also be important for GAS persistence and delayed wound healing by binding to the EDA domain of cellular fibronectin (16). We hypothesize that the larger skin lesions induced by WT Scl-1-expressing M1T1 GAS in vivo (Fig. 1) could reflect a combined result of resistance to phagocytic killing at the site of infection and interference with the wound healing process. This may also explain the different outcomes observed in previous mouse infection studies, where strains lacking Scl-1 resulted in differences in skin infection but not systemic infection (data not shown) (15).
We found that Scl-1 in M1T1 serotype GAS confers resistance to NET-mediated killing, which may be in large part due to the antimicrobial peptides found within the NETs (Fig. 4). Although we have focused our studies primarily on extracellular traps generated by neutrophils, extracellular traps can be generated by a number of innate immune cell types (38), including macrophages (40, 41). Thus, our findings can be extended to the other phagocytic cell types used in our studies (Fig. 2 and 4), though susceptibility of the mutant strain to antimicrobial peptides in these experiments may not necessarily be limited to those found in extracellular traps. Susceptibility of the mutant strain to extracellular trap antimicrobial peptides may in part be explained by altered surface charge (Fig. 4). In addition to Scl-1, other surface proteins of GAS, such as the surface protein M1 in the GAS M1T1 clone, similarly promote survival against NET-mediated killing by conferring protection from cathelicidin (37).
Our data further show that Scl-1 has an additional role in suppressing the release of MPO, which ultimately limits the production of NETs (Fig. 5). A similar effect is observed in GBS infections, where engagement of the immunosuppressive receptor Siglec-9 by surface glycans results in decreased NET formation and increased bacterial survival in NETs (47). Scl-1 likely does not engage an immunosuppressive receptor, as the phagocytosis of the strains (Fig. 3) and the oxidative burst response (Fig. 5) are intact in cells infected with Scl-1-expressing strains. However, the exact molecular mechanism by which Scl-1 prevents MPO release remains to be elucidated and warrants further study. Thus, Scl-1 appears to have a multifunctional role in providing protection from extracellular traps and antimicrobial peptides while simultaneously suppressing release of azurophilic granule contents and limiting extracellular trap formation. The diverse effects of Scl-1 are difficult to explain and will be explored in future work.
As Scl-1 is an important virulence factor for the prevalent M1T1 clone of GAS, it may prove to be an effective therapeutic target. A study by Åkesson and colleagues demonstrated that patients with invasive GAS infection have significantly lower levels of antibodies against Scl-1 than patients with mild infections (48). This study suggests that the production of anti-Scl-1 antibodies is important for the outcome of invasive GAS infections (48). Because our data suggest that the hypervariable N-terminal domain of M1T1 Scl-1 is responsible for phagocyte resistance, this portion of the protein may prove to be a useful target for vaccine development, circumventing the possibility of cross-reactivity with human collagen. A multivalent strategy to target the hypervariable region of M1T1 Scl-1, similar to the approach applied to the development of an M protein vaccine (49), can be promising for the development of a GAS-specific therapeutic treatment. This strategy of designing a therapeutic for a specific bacterial virulence factor is particularly attractive in the face of rapidly growing numbers of antibiotic-resistant bacteria.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided National Institutes of Health grants AI077780 and AR052728 to V.N., UCSD/SDSU IRACDA Postdoctoral Fellowship Program grant GM068524 to C.Y.M.O., a grant from the Deutschlandstipendium of the Technische Universität Braunschweig to S.D., and grants from the Undergraduate Research Center of Occidental College to N.E., H.N., and J.S.
We gratefully acknowledge Magnus Rasmussen, Jason Cole, and the lab of Roberta Pollock for the use of several reagents and protocols.
Footnotes
Published ahead of print 14 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01921-14.
REFERENCES
- 1. Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 2. Cole JN, Barnett TC, Nizet V, Walker MJ. 2011. Molecular insight into invasive group A streptococcal disease. Nat. Rev. Microbiol. 9:724–736. 10.1038/nrmicro2648 [DOI] [PubMed] [Google Scholar]
- 3. Papayannopoulos V, Zychlinsky A. 2009. NETs: a new strategy for using old weapons. Trends Immunol. 30:513–521. 10.1016/j.it.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 4. Aziz RK, Kotb M. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511–1517. 10.3201/eid1410.071660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maamary PG, Ben Zakour NL, Cole JN, Hollands A, Aziz RK, Barnett TC, Cork AJ, Henningham A, Sanderson-Smith M, McArthur JD, Venturini C, Gillen CM, Kirk JK, Johnson DR, Taylor WL, Kaplan EL, Kotb M, Nizet V, Beatson SA, Walker MJ. 2012. Tracing the evolutionary history of the pandemic group A streptococcal M1T1 clone. FASEB J. 26:4675–4684. 10.1096/fj.12-212142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cole JN, Pence MA, von Köckritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, Nizet V. 2010. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. mBio 1(4):e00191-10. 10.1128/mBio.00191-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. 2008. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe 4:170–178. 10.1016/j.chom.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pence MA, Rooijakkers SH, Cogen AL, Cole JN, Hollands A, Gallo RL, Nizet V. 2010. Streptococcal inhibitor of complement promotes innate immune resistance phenotypes of invasive M1T1 group A Streptococcus. J. Innate Immun. 2:587–595. 10.1159/000317672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hollands A, Gonzalez D, Leire E, Donald C, Gallo RL, Sanderson-Smith M, Dorrestein PC, Nizet V. 2012. A bacterial pathogen co-opts host plasmin to resist killing by cathelicidin antimicrobial peptides. J. Biol. Chem. 287:40891–40897. 10.1074/jbc.M112.404582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400. 10.1016/j.cub.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 11. Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, Deleo FR, Musser JM. 2005. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 102:1679–1684. 10.1073/pnas.0406641102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2:e5. 10.1371/journal.ppat.0020005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsatsaronis JA, Hollands A, Cole JN, Maamary PG, Gillen CM, Ben Zakour NL, Kotb M, Nizet V, Beatson SA, Walker MJ, Sanderson-Smith ML. 2013. Streptococcal collagen-like protein A and general stress protein 24 are immunomodulating virulence factors of group A Streptococcus. FASEB J. 27:2633–2643. 10.1096/fj.12-226662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rasmussen M, Edén A, Björck L. 2000. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect. Immun. 68:6370–6377. 10.1128/IAI.68.11.6370-6377.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lukomski S, Nakashima K, Abdi I, Cipriano VJ, Ireland RM, Reid SD, Adams GG, Musser JM. 2000. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect. Immun. 68:6542–6553. 10.1128/IAI.68.12.6542-6553.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oliver-Kozup H, Martin KH, Schwegler-Berry D, Green BJ, Betts C, Shinde AV, Van De Water L, Lukomski S. 2013. The group A streptococcal collagen-like protein-1, Scl1, mediates biofilm formation by targeting the extra domain A-containing variant of cellular fibronectin expressed in wounded tissue. Mol. Microbiol. 87:672–689. 10.1111/mmi.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliver-Kozup HA, Elliott M, Bachert BA, Martin KH, Reid SD, Schwegler-Berry DE, Green BJ, Lukomski S. 2011. The streptococcal collagen-like protein-1 (Scl1) is a significant determinant for biofilm formation by group a Streptococcus. BMC Microbiol. 11:262. 10.1186/1471-2180-11-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caswell CC, Oliver-Kozup H, Han R, Lukomska E, Lukomski S. 2010. Scl1, the multifunctional adhesin of group A Streptococcus, selectively binds cellular fibronectin and laminin, and mediates pathogen internalization by human cells. FEMS Microbiol. Lett. 303:61–68. 10.1111/j.1574-6968.2009.01864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen SM, Tsai YS, Wu CM, Liao SK, Wu LC, Chang CS, Liu YH, Tsai PJ. 2010. Streptococcal collagen-like surface protein 1 promotes adhesion to the respiratory epithelial cell. BMC Microbiol. 10:320. 10.1186/1471-2180-10-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caswell CC, Lukomska E, Seo NS, Höök M, Lukomski S. 2007. Scl1-dependent internalization of group A Streptococcus via direct interactions with the α2β1 integrin enhances pathogen survival and re-emergence. Mol. Microbiol. 64:1319–1331. 10.1111/j.1365-2958.2007.05741.x [DOI] [PubMed] [Google Scholar]
- 21. Han R, Caswell CC, Lukomska E, Keene DR, Pawlowski M, Bujnicki JM, Kim JK, Lukomski S. 2006. Binding of the low-density lipoprotein by streptococcal collagen-like protein Scl1 of Streptococcus pyogenes. Mol. Microbiol. 61:351–367. 10.1111/j.1365-2958.2006.05237.x [DOI] [PubMed] [Google Scholar]
- 22. Gao Y, Liang C, Zhao R, Lukomski S, Han R. 2010. The Scl1 of M41-type group A Streptococcus binds the high-density lipoprotein. FEMS Microbiol. Lett. 309:55–61. 10.1111/j.1574-6968.2010.02013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Humtsoe JO, Kim JK, Xu Y, Keene DR, Höök M, Lukomski S, Wary KK. 2005. A streptococcal collagen-like protein interacts with the α2β1 integrin and induces intracellular signaling. J. Biol. Chem. 280:13848–13857. 10.1074/jbc.M410605200 [DOI] [PubMed] [Google Scholar]
- 24. Caswell CC, Barczyk M, Keene DR, Lukomska E, Gullberg DE, Lukomski S. 2008. Identification of the first prokaryotic collagen sequence motif that mediates binding to human collagen receptors, integrins α2β1 and α11β1. J. Biol. Chem. 283:36168–36175. 10.1074/jbc.M806865200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caswell CC, Han R, Hovis KM, Ciborowski P, Keene DR, Marconi RT, Lukomski S. 2008. The Scl1 protein of M6-type group A Streptococcus binds the human complement regulatory protein, factor H, and inhibits the alternative pathway of complement. Mol. Microbiol. 67:584–596. 10.1111/j.1365-2958.2007.06067.x [DOI] [PubMed] [Google Scholar]
- 26. Reuter M, Caswell CC, Lukomski S, Zipfel PF. 2010. Binding of the human complement regulators CFHR1 and factor H by streptococcal collagen-like Protein 1 (Scl1) via their conserved C termini allows control of the complement cascade at multiple levels. J. Biol. Chem. 285:38473–38485. 10.1074/jbc.M110.143727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Påhlman LI, Marx PF, Mörgelin M, Lukomski S, Meijers JC, Herwald H. 2007. Thrombin-activatable fibrinolysis inhibitor binds to Streptococcus pyogenes by interacting with collagen-like proteins A and B. J. Biol. Chem. 282:24873–24881. 10.1074/jbc.M610015200 [DOI] [PubMed] [Google Scholar]
- 28. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761–771. 10.1083/jcb.106.3.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. 2005. d-alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 187:6719–6725. 10.1128/JB.187.19.6719-6725.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatellier S, Ihendyane N, Kansal RG, Khambaty F, Basma H, Norrby-Teglund A, Low DE, McGeer A, Kotb M. 2000. Genetic relatedness and superantigen expression in group A Streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68:3523–3534. 10.1128/IAI.68.6.3523-3534.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. 2003. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J. Bacteriol. 185:1208–1217. 10.1128/JB.185.4.1208-1217.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Timmer AM, Kristian SA, Datta V, Jeng A, Gillen CM, Walker MJ, Beall B, Nizet V. 2006. Serum opacity factor promotes group A streptococcal epithelial cell invasion and virulence. Mol. Microbiol. 62:15–25. 10.1111/j.1365-2958.2006.05337.x [DOI] [PubMed] [Google Scholar]
- 33. Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. 2007. Optimized Thp-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 56:45–50. 10.1007/s00011-007-6115-5 [DOI] [PubMed] [Google Scholar]
- 34. Kuang E, Okumura CY, Sheffy-Levin S, Varsano T, Shu VC, Qi J, Niesman IR, Yang HJ, López-Otín C, Yang WY, Reed JC, Broday L, Nizet V, Ronai ZA. 2012. Regulation of ATG4B stability by RNF5 limits basal levels of autophagy and influences susceptibility to bacterial infection. PLoS Genet. 8:e1003007. 10.1371/journal.pgen.1003007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Köckritz-Blickwede M, Chow O, Ghochani M, Nizet V. 2010. Visualization and functional evaluation of phagocyte extracellular traps. Methods Microbiol. 37:139–160. 10.1016/S0580-9517(10)37007-3 [DOI] [Google Scholar]
- 36. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 37. Lauth X, von Köckritz-Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B, Ghosh P, Gallo RL, Nizet V. 2009. M1 protein allows group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J. Innate Immun. 1:202–214. 10.1159/000203645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Köckritz-Blickwede M, Nizet V. 2009. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. (Berl.) 87:775–783. 10.1007/s00109-009-0481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 280:22–35. 10.1016/j.cellimm.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 40. Jann N, Schmaler M, Kristian S, Radek K, Gallo R, Nizet V, Peschel A, Landmann R. 2009. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J. Leukoc. Biol. 86:1159–1169. 10.1189/jlb.0209053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. 2010. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8:445–454. 10.1016/j.chom.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oehmcke S, Mörgelin M, Herwald H. 2009. Activation of the human contact system on neutrophil extracellular traps. J. Innate Immun. 1:225–230. 10.1159/000203700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oehmcke S, Shannon O, Mörgelin M, Herwald H. 2010. Streptococcal M proteins and their role as virulence determinants. Clin. Chim. Acta 411:1172–1180. 10.1016/j.cca.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 44. Paterson GK, Nieminen L, Jefferies JM, Mitchell TJ. 2008. PclA, a pneumococcal collagen-like protein with selected strain distribution, contributes to adherence and invasion of host cells. FEMS Microbiol. Lett. 285:170–176. 10.1111/j.1574-6968.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 45. Bozue J, Moody KL, Cote CK, Stiles BG, Friedlander AM, Welkos SL, Hale ML. 2007. Bacillus anthracis spores of the bclA mutant exhibit increased adherence to epithelial cells, fibroblasts, and endothelial cells but not to macrophages. Infect. Immun. 75:4498–4505. 10.1128/IAI.00434-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vandersmissen L, De Buck E, Saels V, Coil DA, Anné J. 2010. A Legionella pneumophila collagen-like protein encoded by a gene with a variable number of tandem repeats is involved in the adherence and invasion of host cells. FEMS Microbiol. Lett. 306:168–176. 10.1111/j.1574-6968.2010.01951.x [DOI] [PubMed] [Google Scholar]
- 47. Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. 2009. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113:3333–3336. 10.1182/blood-2008-11-187302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Åkesson P, Rasmussen M, Mascini E, von Pawel-Rammingen E, Janulczyk R, Collin M, Olsén A, Mattsson E, Olsson ML, Björck L, Christensson B. 2004. Low antibody levels against cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. J. Infect. Dis. 189:797–804. 10.1086/381982 [DOI] [PubMed] [Google Scholar]
- 49. Dale JB, Penfound T, Chiang EY, Long V, Shulman ST, Beall B. 2005. Multivalent group A streptococcal vaccine elicits bactericidal antibodies against variant M subtypes. Clin. Diagn. Lab. Immunol. 12:833–836. 10.1128/CDLI.12.7.833-836.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.