Abstract
A novel New Delhi metallo-β-lactamase variant, NDM-12, was identified in a carbapenem-resistant Escherichia coli clinical isolate obtained from a urine sample from a patient in Nepal. NDM-12 differed from NDM-1 by two amino acid substitutions (M154L and G222D). The enzymatic activities of NDM-12 against β-lactams were similar to those of NDM-1, although NDM-12 showed lower kcat/Km ratios for all β-lactams tested except doripenem. The blaNDM-12 gene was located in a plasmid of 160 kb.
TEXT
Metallo-β-lactamases (MBLs) usually confer reduced susceptibility to carbapenems, cephalosporins, and penicillins but not monobactams (1). Acquired MBLs are produced by Gram-negative bacteria, including Acinetobacer spp., Pseudomonas aeruginosa, and several Enterobacteriaceae (1). MBLs are categorized by their amino acid sequences into various types (2–4), including AIM (5), DIM (6), FIM (7), GIM (8), IMPs (9), KHM (10), NDMs (11), SMB (12), SIM (13), SPM (14), TMBs (15), and VIMs (16). The most prevalent types of MBLs are IMP-, VIM-, and NDM-type enzymes (1, 2, 17). NDM-1 was initially isolated from Klebsiella pneumoniae and Escherichia coli in 2008 in Sweden (11). Subsequently, at least 11 NDM variants (www.lahey.org/studies) have been reported in several countries (4, 18–29).
This study was ethically reviewed and approved by the Institutional Review Board of the Institute of Medicine at Tribhuvan University (reference 6-11-E) and the Biosafety Committee at the National Center for Global Health and Medicine (approval no. 26-D-088 and 26-D-089).
E. coli IOMTU388.1 was isolated from a urine sample obtained from a patient in 2013 in a university hospital in Nepal. The isolate was phenotypically identified, and the species identification was confirmed by 16S rRNA sequencing (30). E. coli DH5α (TaKaRa Bio, Shiga, Japan) and E. coli BL21-CodonPlus(DE3)-RIP (Agilent Technologies, Santa Clara, CA) were used as hosts for recombinant plasmids and for expression of blaNDM-1 and blaNDM-12, respectively.
MICs were determined using the broth microdilution method as recommended by the Clinical and Laboratory Standards Institute (31). The MICs of β-lactams for E. coli IOMTU388.1 are shown in Table 1, and the MICs of other antibiotics were as follows: amikacin, >1,024 μg/ml; arbekacin, >1,024 μg/ml; ciprofloxacin, 128 μg/ml; colistin, ≤0.125 μg/ml; fosfomycin, 8 μg/ml; gentamicin, >1,024 μg/ml; kanamycin, >1,024 μg/ml; levofloxacin, 32 μg/ml; minocycline, 8 μg/ml; tigecycline, ≤0.125 μg/ml; and tobramycin, >1,024 μg/ml. PCR analysis was performed to detect the MBL genes blaDIM, blaGIM, blaIMP, blaNDM, blaSIM, blaSPM, and blaVIM (32, 33). The isolate was PCR positive for blaNDM but negative for the other MBL genes tested. The DNA sequence of the PCR product revealed that the isolate had blaNDM-12. Multilocus sequence typing (MLST) of IOMTU388.1 typed it as ST635 (E. coli MLST Database; http://www.pasteur.fr/recherche/genopole/PF8/mlst/EColi.html). blaNDM-1 obtained from P. aeruginosa IOMTU9 (29) was used as a reference gene.
TABLE 1.
MICs of various β-lactams for E. coli IOMTU388.1 and E. coli DH5α transformed with plasmids encoding NDM-12 or NDM-1
| Antibiotic(s) | MIC (μg/ml) for strain: |
|||
|---|---|---|---|---|
| IOMTU388.1 | DH5α(pHSG398/NDM-1) | DH5α(pHSG398/NDM-12) | DH5α(pHSG398) | |
| Ampicillin | >1,024 | 256 | 512 | 4 |
| Ampicillin-sulbactam | >1,024 | 128 | 128 | 2 |
| Aztreonam | 64 | ≤0.063 | ≤0.063 | ≤0.063 |
| Cefepime | 512 | 0.5 | 1 | ≤0.063 |
| Cefoselis | 1,024 | 16 | 8 | 1 |
| Cefotaxime | >1,024 | 8 | 16 | ≤0.063 |
| Cefoxitin | >1,024 | 64 | 16 | ≤0.063 |
| Cefpirome | 512 | 2 | 2 | ≤0.063 |
| Ceftazidime | >1,024 | 256 | 256 | ≤0.063 |
| Ceftriaxone | >1,024 | 16 | 16 | ≤0.063 |
| Cefradine | >1,024 | 512 | 256 | 16 |
| Doripenem | 32 | 0.063 | 0.063 | ≤0.063 |
| Imipenem | 16 | 0.5 | 0.25 | ≤0.063 |
| Meropenem | 64 | 0.25 | 0.125 | ≤0.063 |
| Moxalactam | >1,024 | 16 | 4 | 0.125 |
| Penicillin G | >1,024 | 256 | 256 | 32 |
The blaNDM-12 sequence had 2 amino acid substitutions (M154L and G222D) compared with blaNDM-1 (accession no. JF798502) and one substitution (G222D) compared with NDM-4 (accession no. JQ348841).
The blaNDM-1 and blaNDM-12 genes were cloned into the corresponding sites of pHSG398 (TaKaRa, Shiga, Japan) using the primer set EcoRI-NDM-F (5′-GGGAATTCATGGAATTGCCCAATATTATG-3′) and PstI-NDM-R (5′-AACTGCAGTCAGCGCAGCTTGTCGGCCAT-3′). E. coli DH5α was transformed with pHSG398-NDM-1 or pHSG398-NDM-12.
The open reading frames of NDM-1 and NDM-12 without signal peptide regions were cloned into the pET28a expression vector (Novagen, Inc., Madison, WI) using the primer set BamHI-TEV-NDM-F (5′-ATGGATCCGAAAACCTGTATTTCCAAGGCCAGCAAATGGAAACTGGCGAC-3′) and XhoI-NDM-R (5′-ATCTCGAGTCAGCGCAGCTTGTCGGCCATG-3′). The resulting plasmids were transformed into E. coli BL21-CodonPlus(DE3)-RIP (Agilent Technologies, Santa Clara, CA). Both recombinant NDM-1 and NDM-12 were purified simultaneously using Ni-nitrilotriacetic acid (NTA) agarose according to the manufacturer's instruction (Qiagen, Hilden, Germany). His tags were removed by digestion with TurboTEV protease (Accelagen, San Diego, CA) and untagged proteins were purified by an additional passage over the Ni-NTA agarose. The purities of NDM-1 and NDM-12, which were estimated by SDS-PAGE, were greater than 90%. During the purification procedure, the presence of β-lactamase activity was monitored using nitrocefin (Oxoid, Ltd., Basingstoke, United Kingdom). Initial hydrolysis rates were determined in 50 mM Tris-HCl buffer (pH 7.4) containing 0.3 M NaCl and 5 μM Zn(NO3)2 at 37°C, using a UV-visible spectrophotometer (V-530; Jasco, Tokyo, Japan). The Km and kcat values and the kcat/Km ratio were determined by analyzing β-lactam hydrolysis with a Lineweaver-Burk plot. Wavelengths and extinction coefficients for β-lactam substrates have been reported previously (34–36). The Km and kcat values (means ± standard deviations) were obtained from three individual experiments. The enzymatic activities of NDM-1 were measured in parallel with those of NDM-12.
The plasmid harboring blaNDM-12 was extracted (37) and sequenced using MiSeq (Illumina, San Diego, CA). The size of the plasmid harboring blaNDM-12 was determined using pulsed-field gel electrophoresis (PFGE) and Southern hybridization. A probe for blaNDM-12 from IOMTU388.1 was amplified by PCR using the primer sets for EcoRI-NDM-F and PstI-NDM-R. Signal detection was carried out using the digoxigenin (DIG) High Prime DNA labeling and detection starter kit II (Roche Applied Science, Indianapolis, IN).
Mating-out assays between the parental strain IOMTU388.1 and the chloramphenicol-resistant E. coli strain BL21 were performed in LB broth using a 1:4 donor/recipient ratio for 3 h at 37°C. Transconjugants were selected on Muller-Hinton agar plates containing ceftazidime (100 μg/ml) and chloramphenicol (30 μg/ml). Selected transconjugants harboring blaNDM-12 were confirmed by PCR with the primer set EcoRI-NDM-F and PstI-NDM-R.
E. coli DH5α harboring blaNDM-1 or blaNDM-12 showed reduced susceptibility to moxalactam and all penicillins, cephalosporins, and carbapenems tested compared with DH5α harboring a vector control (Table 1). The MICs of the β-lactams cefoxitin and moxalactam for DH5α harboring blaNDM-12 were 4-fold less than those for DH5α harboring blaNDM-1 (Table 1).
As shown in Table 2, recombinant NDM-1 and NDM-12 hydrolyzed all β-lactams tested except for aztreonam. The profiles of enzymatic activities of NDM-12 against β-lactams tested were similar to those of NDM-1, although NDM-12 had lower kcat/Km ratios for all β-lactams tested except for doripenem. The lower kcat/Km ratios were likely to be caused by the lower kcat values of NDM-12 compared with those of NDM-1, as the values of NDM-12 were 11.4 to 73.6% of those of NDM-1 (Table 2). The profiles of enzymatic activities of NDM-1 except for cefoxitin were similar to those of NDM-1 that we reported previously (29). The kcat/Km ratio for cefoxitin in Table 2 was 10-fold higher than that in our previous study (see Table 2 in reference 29). The difference between the ratios may be explained by the use of different buffer solutions in the kinetics assays (Tris buffer and phosphate buffer, respectively). It was reported that phosphate ions affected the enzymatic activities of metallo-β-lactamase IMP-1 (38). Phosphate ions may affect the enzymatic activities of NDM-1 against cefoxitin.
TABLE 2.
Kinetic parameters of the NDM-1 and NDM-12 enzymesa
| β-Lactam | NDM-1b |
NDM-12b |
||||
|---|---|---|---|---|---|---|
| Km (μM) | kcat (s−1)b | kcat/Km (μM−1 s−1) | Km (μM) | kcat (s−1)b | kcat/Km (μM−1 s−1) | |
| Ampicillin | 231 ± 33 | 249 ± 22 | 1.1 | 126 ± 4 | 136 ± 2 | 1.1 |
| Aztreonam | NHc | NH | NH | NH | NH | NH |
| Cefepime | 162 ± 7 | 31 ± 1 | 0.19 | 103 ± 6 | 11.1 ± 0.2 | 0.11 |
| Cefotaxime | 102 ± 16 | 137 ± 7 | 1.1 | 45 ± 4 | 38 ± 1 | 0.84 |
| Cefoxitin | 13 ± 1 | 6.7 ± 0.1 | 0.50 | 26 ± 2 | 0.66 ± 0.01 | 0.02 |
| Ceftazidime | 202 ± 7 | 56 ± 1 | 0.28 | 53 ± 4 | 5.7 ± 0.1 | 0.11 |
| Cefradine | 27 ± 3 | 72 ± 1 | 2.7 | 57 ± 4 | 16 ± 1 | 0.28 |
| Doripenem | 201 ± 27 | 114 ± 9 | 0.57 | 88 ± 2 | 53 ± 1 | 0.60 |
| Imipenem | 249 ± 43 | 44 ± 2 | 0.34 | 125 ± 22 | 22 ± 2 | 0.18 |
| Meropenem | 81 ± 10 | 139 ± 10 | 1.7 | 91 ± 8 | 53 ± 2 | 0.58 |
| Moxalactam | 4.5 ± 2.3 | 7.6 ± 0.3 | 2.0 | 67 ± 5 | 6.0 ± 0.2 | 0.09 |
| Penicillin G | 67 ± 6 | 104 ± 1 | 1.6 | 64 ± 8 | 42 ± 2 | 0.66 |
The proteins were initially modified by a His tag, which was removed after purification.
The Km and kcat values shown represent the means from 3 independent experiments ± standard deviations.
NH, no hydrolysis was detected under conditions with substrate concentrations up to 1 mM and enzyme concentrations up to 700 nM.
The MBL gene blaNDM-12 in E. coli IOMTU388.1 was detected in a plasmid, pIOMTU388-NDM (accession no. AB926431), with a size of 160 kb (Fig. 1). The sequence surrounding blaNDM-12 was blaNDM-12-bleMBL-trpF-dsbC-tnpA-sulI-qacEΔI. This plasmid showed more than 99.9% identity at the nucleotide sequence level to the sequence located from bp 70978 to 77904 in the pGUE-NDM plasmid (accession no. JQ364967) from E. coli strain GUE, which was isolated in India (39), and also showed 99.9% identity at the nucleotide sequence level to the sequence located from bp 372 to 7298 in the pEC77-NDM plasmid (accession no.AB898038) from E. coli strain NCGM77, which was isolated in Japan (40). The plasmid harboring blaNDM-12 belonged to the IncF incompatibility group and was conjugated from IOMTU388.1 to E. coli BL21 at a conjugative frequency of 1.63 × 10−3.
FIG 1.
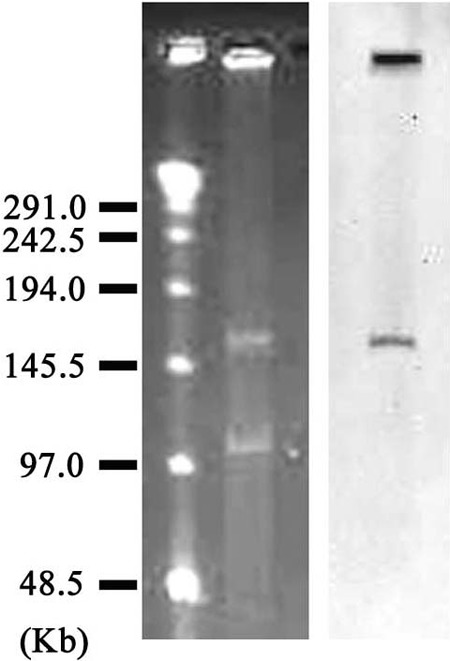
Localization of blaNDM-12 on a plasmid from E. coli strain IOMTU388.1 separated by PFGE. Left lane, MidRange PFG marker (New England BioLabs, Tokyo, Japan); middle lane, plasmids from E. coli strain IOMTU388.1; right lane, hybridization of the plasmid with a probe specific for blaNDM-12.
The 2 substitutions M154L and G222D in NDM-12 (compared with NDM-1) affected the activity of the enzyme (Table 2). Nordmann et al. (24) reported that a mutant containing M154L (NDM-4) possessed increased hydrolytic activity toward carbapenems and several cephalosporins compared to NDM-1. Unexpectedly, NDM-12, which contains the M154L substitution, did not show an increase in hydrolytic activities. The substitution at position 222 found in NDM-12 has been not reported in other variants, to our knowledge. Although we did not directly compare the enzymatic activity of NDM-12 with those of NDM-4, the substitution of G222D in NDM-12 may be associated with a decrease in hydrolytic activities toward these antibiotics (Table 2). Position 222 is located in loop L10 of NDM-1, which forms the active site of NDM-1 with L3 at the bottom of a shallow groove (41–44). Among all known 11 NDM-1 variants, amino acid substitutions were found at 13 amino acid positions, including positions 28, 32, 36, 69, 74, 88, 95, 130, 152, 154, 200, and 233. Positions 28, 32, and 36 were in the signal peptide region. Positions 95, 130, and 154 have been reported to affect β-lactam-hydrolyzing activities, although whether the activities are affected by the other 6 substitutions has not been reported. Residue 95 is located in α1 on the protein surface, and the amino acid substitution at position 95 affected the kcat values of NDM-3 (40). The substitution at position 130 (Met to Leu) showed increased hydrolytic activity toward carbapenems and several cephalosporins compared to NDM-1 (24, 29).
This is the first report describing NDM-12-producing E. coli in Nepal. NDMs seem to evolve rapidly; therefore, careful monitoring of NDM-producing pathogens is required.
Nucleotide sequence accession number.
The plasmid sequence including blaNDM-12 has been deposited in GenBank under accession no. AB926431.
ACKNOWLEDGMENTS
This study was supported by grants from International Health Cooperation Research (24-S-5 and 26-A-103) and a grant (H24-Shinko-ippan-010) from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print 4 August 2014
REFERENCES
- 1.Bush K. 2001. New beta-lactamases in Gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085–1089. 10.1086/319610 [DOI] [PubMed] [Google Scholar]
- 2.Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-beta-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306–325. 10.1128/CMR.18.2.306-325.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54:969–976. 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect. Dis. 11:381–393. 10.1016/S1473-3099(11)70056-1 [DOI] [PubMed] [Google Scholar]
- 5.Yong D, Toleman MA, Bell J, Ritchie B, Pratt R, Ryley H, Walsh TR. 2012. Genetic and biochemical characterization of an acquired subgroup B3 metallo-beta-lactamase gene, blaAIM-1, and its unique genetic context in Pseudomonas aeruginosa from Australia. Antimicrob. Agents Chemother. 56:6154–6159. 10.1128/AAC.05654-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogalski TM, Gilbert MM, Devenport D, Norman KR, Moerman DG. 2003. DIM-1, a novel immunoglobulin superfamily protein in Caenorhabditis elegans, is necessary for maintaining bodywall muscle integrity. Genetics 163:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollini S, Maradei S, Pecile P, Olivo G, Luzzaro F, Docquier JD, Rossolini GM. 2013. FIM-1, a new acquired metallo-beta-lactamase from a Pseudomonas aeruginosa clinical isolate from Italy. Antimicrob. Agents Chemother. 57:410–416. 10.1128/AAC.01953-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castanheira M, Toleman MA, Jones RN, Schmidt FJ, Walsh TR. 2004. Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase. Antimicrob. Agents Chemother. 48:4654–4661. 10.1128/AAC.48.12.4654-4661.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. 1994. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiguchi J, Morita K, Kitao T, Watanabe N, Okazaki M, Miyoshi-Akiyama T, Kanamori M, Kirikae T. 2008. KHM-1, a novel plasmid-mediated metallo-beta-lactamase from a Citrobacter freundii clinical isolate. Antimicrob. Agents Chemother. 52:4194–4197. 10.1128/AAC.01337-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachino J, Yoshida H, Yamane K, Suzuki S, Matsui M, Yamagishi T, Tsutsui A, Konda T, Shibayama K, Arakawa Y. 2011. SMB-1, a novel subclass B3 metallo-beta-lactamase, associated with ISCR1 and a class 1 integron, from a carbapenem-resistant Serratia marcescens clinical isolate. Antimicrob. Agents Chemother. 55:5143–5149. 10.1128/AAC.05045-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, Rossolini GM, Chong Y. 2005. Novel acquired metallo-beta-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob. Agents Chemother. 49:4485–4491. 10.1128/AAC.49.11.4485-4491.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zavascki AP, Gaspareto PB, Martins AF, Goncalves AL, Barth AL. 2005. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-β-lactamase in a teaching hospital in southern Brazil. J. Antimicrob. Chemother. 56:1148–1151. 10.1093/jac/dki390 [DOI] [PubMed] [Google Scholar]
- 15.El Salabi A, Borra PS, Toleman MA, Samuelsen O, Walsh TR. 2012. Genetic and biochemical characterization of a novel metallo-beta-lactamase, TMB-1, from an Achromobacter xylosoxidans strain isolated in Tripoli, Libya. Antimicrob. Agents Chemother. 56:2241–2245. 10.1128/AAC.05640-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, Rossolini GM. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby GA, Munoz-Price LS. 2005. The new beta-lactamases. N. Engl. J. Med. 352:380–391. 10.1056/NEJMra041359 [DOI] [PubMed] [Google Scholar]
- 18.Pillai DR, McGeer A, Low DE. 2011. New Delhi metallo-beta-lactamase-1 in Enterobacteriaceae: emerging resistance. CMAJ 183:59–64. 10.1503/cmaj.101487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaase M, Nordmann P, Wichelhaus TA, Gatermann SG, Bonnin RA, Poirel L. 2011. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 66:1260–1262. 10.1093/jac/dkr135 [DOI] [PubMed] [Google Scholar]
- 20.Espinal P, Fugazza G, Lopez Y, Kasma M, Lerman Y, Malhotra-Kumar S, Goossens H, Carmeli Y, Vila J. 2011. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob. Agents Chemother. 55:5396–5398. 10.1128/AAC.00679-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. 2012. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 56:1087–1089. 10.1128/AAC.05620-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghazawi A, Sonnevend A, Bonnin RA, Poirel L, Nordmann P, Hashmey R, Rizvi TA, Hamadeh MB, Pal T. 2012. NDM-2 carbapenemase-producing Acinetobacter baumannii in the United Arab Emirates. Clin. Microbiol. Infect. 18:E34–E36. 10.1111/j.1469-0691.2011.03726.x [DOI] [PubMed] [Google Scholar]
- 23.Rogers BA, Sidjabat HE, Silvey A, Anderson TL, Perera S, Li J, Paterson DL. 2013. Treatment options for New Delhi metallo-beta-lactamase-harboring Enterobacteriaceae. Microb. Drug Resist. 19:100–103. 10.1089/mdr.2012.0063 [DOI] [PubMed] [Google Scholar]
- 24.Nordmann P, Boulanger AE, Poirel L. 2012. NDM-4 metallo-beta-lactamase with increased carbapenemase activity from Escherichia coli. Antimicrob. Agents Chemother. 56:2184–2186. 10.1128/AAC.05961-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornsey M, Phee L, Wareham DW. 2011. A novel variant, NDM-5, of the New Delhi metallo-beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 55:5952–5954. 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, Woodhouse R, Mowat E, Dyet K, Paterson DL, Blackmore T, Burns A, Heffernan H. 2012. Identification and molecular characterisation of New Delhi metallo-beta-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int. J. Antimicrob. Agents 39:529–533. 10.1016/j.ijantimicag.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 27.Cuzon G, Bonnin RA, Nordmann P. 2013. First identification of novel NDM carbapenemase, NDM-7, in Escherichia coli in France. PLoS One 8:e61322. 10.1371/journal.pone.0061322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottig S, Hamprecht AG, Christ S, Kempf VA, Wichelhaus TA. 2013. Detection of NDM-7 in Germany, a new variant of the New Delhi metallo-beta-lactamase with increased carbapenemase activity. J. Antimicrob. Chemother. 68:1737–1740. 10.1093/jac/dkt088 [DOI] [PubMed] [Google Scholar]
- 29.Tada T, Miyoshi-Akiyama T, Dahal RK, Sah MK, Ohara H, Kirikae T, Pokhrel BM. 2013. NDM-8 metallo-beta-lactamase in a multidrug-resistant Escherichia coli strain isolated in Nepal. Antimicrob. Agents Chemother. 57:2394–2396. 10.1128/AAC.02553-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki MT, Taylor LT, DeLong EF. 2000. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 66:4605–4614. 10.1128/AEM.66.11.4605-4614.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2013. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed. Approved standard M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 32.Ellington MJ, Kistler J, Livermore DM, Woodford N. 2007. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J. Antimicrob. Chemother. 59:321–322. 10.1093/jac/dkl481 [DOI] [PubMed] [Google Scholar]
- 33.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70:119–123. 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Boschi L, Mercuri PS, Riccio ML, Amicosante G, Galleni M, Frere JM, Rossolini GM. 2000. The Legionella (Fluoribacter) gormanii metallo-beta-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 beta-lactamases. Antimicrob. Agents Chemother. 44:1538–1543. 10.1128/AAC.44.6.1538-1543.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowder MW, Walsh TR, Banovic L, Pettit M, Spencer J. 1998. Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 42:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Queenan AM, Shang W, Flamm R, Bush K. 2010. Hydrolysis and inhibition profiles of beta-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob. Agents Chemother. 54:565–569. 10.1128/AAC.01004-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarczynski MC, Meyer WJ, Min JJ, Wood KA, Hellwig RJ. 1994. Two-minute miniprep method for plasmid DNA isolation. Biotechniques 16:514–519 [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Kuroki T, Yasuzawa H, Higashi T, Jin W, Kawanami A, Yamagata Y, Arakawa Y, Goto M, Kurosaki H. 2005. Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic studies, and X-ray crystallography. J. Biol. Chem. 280:20824–20832. 10.1074/jbc.M414314200 [DOI] [PubMed] [Google Scholar]
- 39.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. 10.1371/journal.pone.0034752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tada T, Miyoshi-Akiyama T, Shimada K, Kirikae T. 2014. Biochemical analysis of metallo-beta-lactamase NDM-3 from a multidrug-resistant Escherichia coli strain isolated in Japan. Antimicrob. Agents Chemother. 58:3538–3540. 10.1128/AAC.02793-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green VL, Verma A, Owens RJ, Phillips SE, Carr SB. 2011. Structure of New Delhi metallo-beta-lactamase 1 (NDM-1). Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 67:1160–1164. 10.1107/S1744309111029654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y, Tesar C, Mire J, Jedrzejczak R, Binkowski A, Babnigg G, Sacchettini J, Joachimiak A. 2011. Structure of apo- and monometalated forms of NDM-1—a highly potent carbapenem-hydrolyzing metallo-beta-lactamase. PLoS One 6:e24621. 10.1371/journal.pone.0024621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King D, Strynadka N. 2011. Crystal structure of New Delhi metallo-beta-lactamase reveals molecular basis for antibiotic resistance. Protein Sci. 20:1484–1491. 10.1002/pro.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Hao Q. 2011. Crystal structure of NDM-1 reveals a common beta-lactam hydrolysis mechanism. FASEB J. 25:2574–2582. 10.1096/fj.11-184036 [DOI] [PubMed] [Google Scholar]


