Abstract
The ERK1/2 MAP kinase pathway is an evolutionarily conserved signaling module that controls many fundamental physiological processes. Deregulated activity of ERK1/2 MAP kinases is associated with developmental syndromes and several human diseases. Despite the importance of this pathway, a comprehensive picture of the natural substrate repertoire and biochemical mechanisms regulated by ERK1/2 is still lacking. In this study, we used large-scale quantitative phosphoproteomics and bioinformatics analyses to identify novel candidate ERK1/2 substrates based on their phosphorylation signature and kinetic profiles in epithelial cells. We identified a total of 7936 phosphorylation sites within 1861 proteins, of which 155 classify as candidate ERK1/2 substrates, including 128 new targets. Candidate ERK1/2 substrates are involved in diverse cellular processes including transcriptional regulation, chromatin remodeling, RNA splicing, cytoskeleton dynamics, cellular junctions and cell signaling. Detailed characterization of one newly identified substrate, the transcriptional regulator JunB, revealed that ERK1/2 phosphorylate JunB on a serine adjacent to the DNA-binding domain, resulting in increased DNA-binding affinity and transcriptional activity. Our study expands the spectrum of cellular functions controlled by ERK1/2 kinases.
Keywords: bioinformatics, cell signaling, MAP kinases, phosphoproteomics, phosphorylation dynamics
Introduction
The ubiquitously expressed serine/threonine protein kinases extracellular signal-regulated kinase 1 (ERK1) and 2 are effector components of the prototypical ERK1/2 mitogen-activated protein (MAP) kinase signaling pathway. ERK1 and ERK2 transduce signals from a wide variety of mitogens, cytokines and differentiation factors to regulate biological processes such as cell proliferation, cell-fate specification, survival, morphogenesis and immune responses (Pearson et al, 2001). Their activation is catalyzed by the dual-specificity MAP kinase kinases MEK1 and MEK2, which are themselves phosphorylated and activated by upstream MAP kinase kinase kinases such as Raf (Cobb and Goldsmith, 1995; Cuevas et al, 2007). ERK1 and ERK2 are the only known physiological substrates of MEK1/2 (Pearson et al, 2001). Deregulated activity of ERK1/2 is causally associated with neuro–cardio–facial–cutaneous developmental syndromes and with a range of human diseases including cancer, inflammatory disorders and neurodegenerative diseases (Schubbert et al, 2007; Lawrence et al, 2008; Tartaglia and Gelb, 2010). Consequently, small molecule inhibitors of the ERK1/2 pathway are currently being evaluated clinically for cancer therapy and other indications ( http://clinicaltrials.gov).
ERK1/2 are multifunctional protein kinases that phosphorylate target substrates within the minimal consensus motif Ser/Thr–Pro, with a preference for proline at −2 position (Gonzalez et al, 1991; Songyang et al, 1996). Although many substrates of ERK1/2 have been reported to date (Yoon and Seger, 2006), only a fraction of these have been adequately validated biochemically. More recent phosphoproteomics studies have led to the identification of additional candidate ERK1/2 substrates (Kosako et al, 2009; Old et al, 2009; Pan et al, 2009; Carlson et al, 2011), but yielded limited information on the dynamic changes in phosphorylation of the corresponding substrates. Moreover, very limited overlap of candidate ERK1/2 substrates was observed between these studies, indicating that these analyses are far from saturation. Thus, despite the physiological importance of the ERK1/2 MAP kinase pathway and its pharmacological relevance, a comprehensive picture of the substrate repertoire and biochemical reactions regulated by ERK1/2 protein kinases is still lacking.
In a systematic effort to identify novel substrates of ERK1/2, we have capitalized on advances in mass spectrometry (MS) instrumentation, quantitative phosphoproteomics and bioinformatics to measure the dynamic changes in phosphorylation of ERK1/2 consensus sequences in response to cell stimulation and pharmacological inhibition of MEK1/2 in intestinal epithelial cells. This approach enabled the identification of 155 candidate ERK1/2 substrates, of which only 12 proteins correspond to previously validated ERK1/2 phosphorylation sites. A subset of these putative substrates was selected and directly phosphorylated by ERK1 in vitro on the predicted sites, thereby validating our analysis. Candidate ERK1/2 substrates are involved in a broader than appreciated range of pathways and biological processes. Among the newly identified ERK1/2 substrates is the transcriptional factor JunB. We show that ERK1 phosphorylates JunB on Ser256 close to the DNA-binding domain, resulting in increased DNA-binding affinity and transcriptional activity of JunB/c-Fos-activating protein-1 (AP-1) complexes. Our findings considerably expand the spectrum of cellular functions under control of the ERK1/2 MAP kinase pathway.
Results
Global proteomic analysis of dynamic phosphorylation profiles
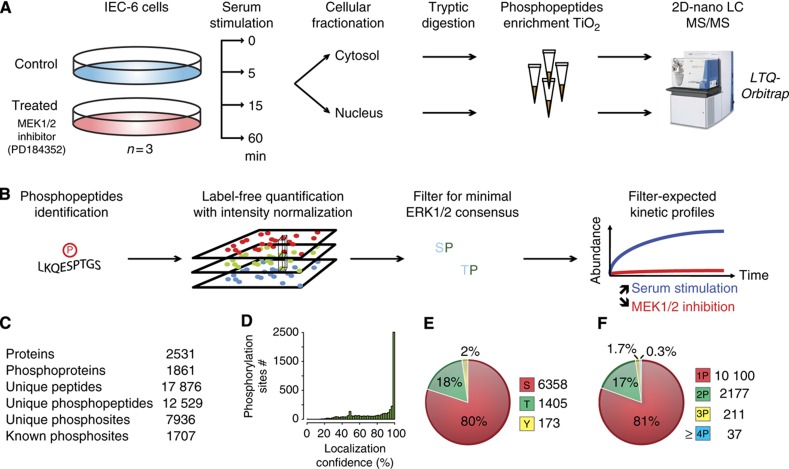
We used a quantitative phosphoproteomics approach to measure the dynamics of site-specific phosphorylation on a global scale (Figure 1A). To specifically profile phosphorylation events downstream of ERK1/2 kinases, biological triplicates from two populations of rat intestinal epithelial cells (IEC-6) were stimulated for 0, 5, 15 or 60 min with fetal bovine serum in the presence or absence of PD184352. PD184352 is a potent, ATP non-competitive inhibitor of MEK1/2 that displays extremely high selectivity over a large panel of protein kinases (Bain et al, 2007). When used at a low concentration of 2 μM, PD184352 inhibits MEK1/2 without affecting the activity of the related MKK5 kinase (Mody et al, 2001). Under these conditions, PD184352 almost completely inhibits serum-stimulated ERK1/2 activation (Supplementary Figure S1). Proteins from cytosolic and nuclear fractions were digested with trypsin and phosphopeptides were enriched on TiO2 microcolumns. Phosphopeptides were then separated into five different SCX fractions and analyzed by LC-MS/MS on a hybrid LTQ-Orbitrap mass spectrometer. Label-free quantitation was used to profile the abundance of phosphopeptides that were checked manually for accuracy (Marcantonio et al, 2008; Trost et al, 2009). To identify candidate ERK1/2 substrates, additional filtering was applied to the list of phosphopeptides to select sites within ERK1/2 consensus motifs that display statistically significant changes in abundance upon cell stimulation and MEK1/2 inhibition (Figure 1B).
Figure 1.
Experimental workflow and data processing for the identification of candidate ERK1/2 substrates. (A) Experimental workflow for sample processing and MS analysis. (B) Data analysis for the selection of candidate ERK1/2 substrates. (C) Statistics on the number of identified phosphopeptides. (D) Distribution of site-localization confidence data. (E) Distribution of phosphorylated amino acids. (F) Number of phosphorylation sites per peptide.
We identified 7936 unique phosphorylation sites on 1861 proteins, of which two-third represent high-confidence assignment with a localization probability of at least 0.75 (Figure 1C and D and Supplementary Table S1). All identifications are available online in ProteoConnections (Courcelles et al, 2011). The distribution of pS, pT and pY sites was 80, 18, 2%, respectively (Figure 1E), and peptides were predominantly singly and doubly phosphorylated (Figure 1F), consistent with previous reports (Pan et al, 2008). We obtained the dynamic profile of 3015 and 5222 phosphopeptides from the cytosol and nuclear fractions, respectively (Supplementary Table S2). The reproducibility of abundance measurements from our label-free quantitative procedure was evaluated to determine a fold-change cutoff above technical and biological variability. The s.d. of these measurements was 37, and 95% of phosphopeptides showed less than twofold change across biological replicates (Supplementary Figure S2). Using a cutoff of twofold change with a P-value of 0.05 (two-tailed Student’s t-test), 2510 phosphopeptides displayed a significant change in abundance for at least one time point of stimulation. We next summed the abundance changes of the four time points using Σlog10(PD184352/control) to determine the overall impact of PD184352 on all quantifiable substrates. A normal distribution centered on zero was obtained for both cytosolic and nuclear extracts, indicating that MEK1/2 inhibition resulted in an even up- and downregulation of phosphorylated proteins (Supplementary Figure S3). Many phosphopeptides bearing the minimal ERK1/2 consensus motif were downregulated by PD184352 and thus represent candidate ERK1/2 substrates (see below).
Phosphoproteome dynamics identify novel candidate ERK1/2 substrates
We identified 2296 high-confidence phosphosites on 987 proteins that contained the minimal ERK1/2 consensus motif. Selection of phosphopeptides whose relative abundance is regulated positively upon serum stimulation and negatively following MEK1/2 inhibition resulted in a list of 155 candidate ERK1/2 substrates (232 phosphorylation sites) (Supplementary Table S3 and Figure 2A). Representative stimulation and inhibition temporal profiles of regulated phosphopeptides were grouped using fuzzy c-means clustering into six groups (arbitrary chosen number) to graphically report the general trends of the selected candidates (Supplementary Figure S4). Of these 232 phosphorylation sites, 49 sites (21%) contained the optimal P-X-(S/T)-P ERK1/2 phosphorylation consensus motif. Bioinformatics analyses revealed that 94% of the sites with their neighboring proline are conserved in either human or mouse.
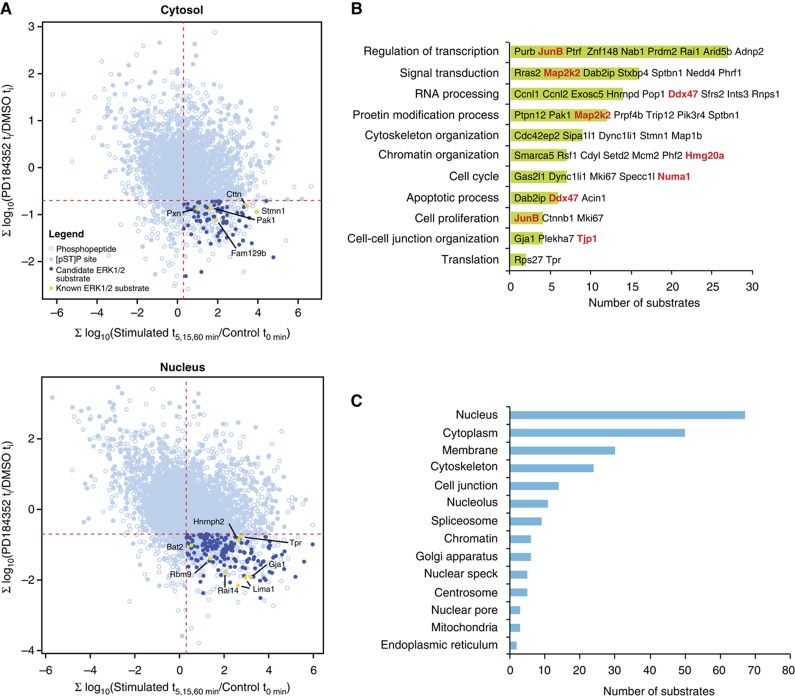
Figure 2.
Dynamic changes of protein phosphorylation identify ERK1/2 substrates from cytosolic and nuclear compartments. (A) Two-dimensional representation of quantitative changes in protein phosphorylation following serum stimulation and PD184352 treatment. Each point corresponds to a different phosphopeptide. Candidate ERK1/2 substrates are shown as dark blue circles in the bottom right quadrant and represent phosphopeptides displaying increase in phosphorylation upon serum stimulation and decrease upon PD184352 treatment (two-tailed t-test). Note that a few peptides with (pS/T)-P sites in this quadrant did not have significant abundance change and were not retained as ERK1/2 candidates. Yellow circles correspond to previously known ERK1/2 substrates. (B, C) GO analysis. Candidate ERK1/2 substrates were annotated and classified according to (B) biological process or (C) cellular component GO terms. P is the P-value of Fisher’s exact test and E is the calculated odds ratio for category enrichment. Only E-values above 1 are presented. A subset of proteins belonging to each category is shown. Substrates validated by in vitro kinase assays are shown in red.
Binding to docking sites such as the D or DEF domain confers additional specificity to ERK1/2 kinases (Jacobs et al, 1999; Tanoue et al, 2000). We found that 22 of the potential ERK1/2 substrates (14%) contain a D domain, whereas one contains a DEF domain (0.6%) (Supplementary Table S3). In comparison, 17% (278) and 0.4% (11) of all the phosphoproteins identified in this study have a D or DEF domain, respectively, within 20 amino acids of the phosphorylated site. This indicates that the putative ERK1/2 substrates are not enriched for these docking domains.
The filtered list included 13 previously identified and biochemically validated ERK1/2 phosphorylation sites present on 12 substrates: Bat2, Cttn, Fam129b, Gja1, Hnrnph2, Lima1, Pak1, Pxn, Rai14, Rbm9, Stmn1 and Tpr (Supplementary Figure S5). A further 15 proteins have been reported as ERK1/2 substrates but either the phosphorylation site was not mapped, or a distinct site was identified from these studies (Supplementary Figure S6). This analysis provided a first level of validation of our approach. We next performed gene ontology (GO) analyses on candidate ERK1/2 substrates to identify functional terms previously reported for these proteins (Supplementary Table S4, Figure 2B and C). Several proteins were found with related GO terms: regulation of transcription (27 proteins, GO:0006355), nucleic acid metabolic process (24 proteins, GO:00090304), signal transduction (16 proteins, GO:0007165), RNA processing (14 proteins, GO:0006396), cytoskeleton organization (9 proteins, GO:0007010), chromatin organization (8 proteins, GO:0006325), apoptotic process (6 proteins, GO:0006915) and cell cycle (7 proteins, GO:0007049). The analysis of cellular components ontology showed that ERK1/2 substrates are enriched by 4.5-fold for the term cell junction (14 proteins, GO:0030054) and 10.5-fold for nuclear pore (3 proteins, GO:0005643). Interestingly, we also noted a significant enrichment (>10-fold) for two related categories associated with nuclear speckles (5 proteins, GO:0016607) and spliceosomes (9 proteins, GO:0005681). Closer inspection of the data indicated that these two categories comprise members of the SR (serine/arginine-rich) protein family associated with pre-mRNA splicing. Splicing factors are regulated extensively by phosphorylation (Stamm, 2008), and our results suggest that ERK1/2 kinases contribute to this regulation.
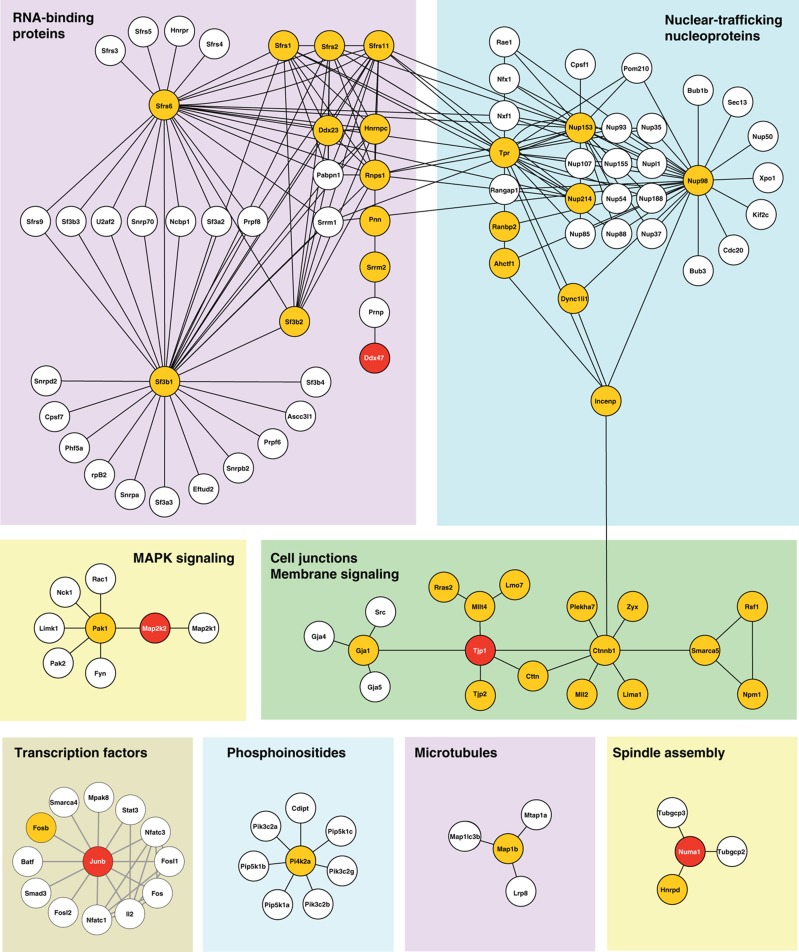
We used our phosphorylation data to generate a protein-interaction network of the putative ERK1/2 substrates using the STRING database that integrates known and predicted interactions from different sources. We combined interactions found in rat and their human orthologs, resulting in a network of 146 proteins (nodes) and 242 connections (edges). This interaction network together with analyses of functional protein annotations highlighted different subsets of proteins associated with RNA binding, nuclear trafficking, MAPK signaling cell junction membrane, spindle assembly and signaling transcription factors (Figure 3). Interacting proteins identified in this study are highlighted in orange while red circles represent substrates validated in subsequent biochemical assays (see below). Interestingly, we identified several RNA-binding proteins that were regulated in response to serum activation and inhibition with PD184352, including Hnrnp C, Ddx47 and different Ser/Arg-rich splicing factors involved in the early steps of spliceosome assembly and pre-mRNA splicing. Several potential substrates modulated by ERK1/2 were also involved in cell junction membrane signaling such as Mllt4, Tjp1, Tjp2 and Cttn that have important roles in tight and adherens junctions and in the regulation of cell migration. One notable feature of this network is that out of the 155 candidate ERK1/2 substrates, 34 (22%) are directly interconnected in the STRING network. This high degree of connectivity suggests that ERK1/2 are not phosphorylating proteins randomly in the cell but often regulate members of the same functional pathway or protein complexes.
Figure 3.
Networks of ERK substrates. Identified substrates were overlaid on interaction network from the STRING database ( http://string.embl.de). Only high-confidence interaction from experiments or databases were extracted for rat and complemented with human data set. Orange and red circles represent substrates identified and validated in the study, respectively. Major subnetworks concern splicing, nuclear trafficking and nucleoproteins, MAPK signaling, cell junctions and membrane signaling. Binary interactions were removed. Candidate ERK1/2 substrates show a high degree of connectivity (34/155 are interconnected).
We also noted that changes in the phosphorylation status of several kinases and regulators acting upstream of ERK1/2, such as Pak1, Raf1, Map3k1, MEK1, MEK2, Ksr1, Rras2 and Ywhae (14-3-3 epsilon), were detected in cells treated with PD184352 (Figure 4). For example, we observed an increase in the activation loop phosphorylation of MEK1 and MEK2, and in Ser621 phosphorylation of Raf1. This observation is consistent with the known participation of ERK1/2 in multiple negative feedback regulatory loops in stimulated cells (Ramos, 2008; Fritsche-Guenther et al, 2011). Deciphering the complex network of signaling events and feedback loops that modulate the activity of upstream ERK1/2 regulators upon pharmacological inhibition of the pathway will be important for understanding the underlying mechanisms of resistance to Raf and MEK1/2 inhibitors in the clinic.
Figure 4.
Phosphorylation changes in the upstream regulators of ERK1/2 MAP kinases. Identified phosphoproteins (round circles) were mapped on the interaction network from the STRING database. A color gradient is used to represent the modulation (summed log fold change) of each individual phosphorylation site following treatment with PD184352. Protein nodes with multiple phosphorylation sites are represented using a pie chart where each slice corresponds to the abundance change of a unique phosphorylated site.
Validation of the site-specific phosphorylation of candidate substrates by ERK1 in vitro
To further validate the list of candidate ERK1/2 substrates, we selected six unrelated proteins of various functions (Supplementary Table S5 and Figure 5A) that were purified as recombinant GST-fusion proteins from bacteria for subsequent in vitro kinase assays. In the case of MEK2, the catalytically inactive GST-MEK2K101E mutant was used to prevent autophosphorylation of the kinase. All selected proteins were efficiently phosphorylated by recombinant active ERK1 in vitro (Figure 5B). In each case, alanine substitution of the identified phosphorylation residue markedly reduced phosphorylation, confirming the site-specific assignment. The identity of the in vitro phosphorylated residue was further confirmed by MS analysis (Figure 5C). Additional studies revealed that Numa1 is also phosphorylated on Thr2015 by ERK1. Together, these results validate the predictive potential of our quantitative phosphoproteomics approach to identify novel ERK1/2 substrates.
Figure 5.
Validation of ERK1/2 substrates. (A) Phosphorylation kinetic profiles of selected ERK1/2 substrates in serum-stimulated cells treated (red) or not (blue) with the MEK1/2 inhibitor PD184352. (B) In vitro kinase assays of wild-type and alanine mutant candidate substrates with recombinant active ERK1. (C) MS/MS analysis of selected ERK1/2 substrates phosphorylated by recombinant active ERK1 in vitro. Source data for this figure is available on the online supplementary information page.
ERK1/2 phosphorylate JunB on Ser256 in vitro and in vivo
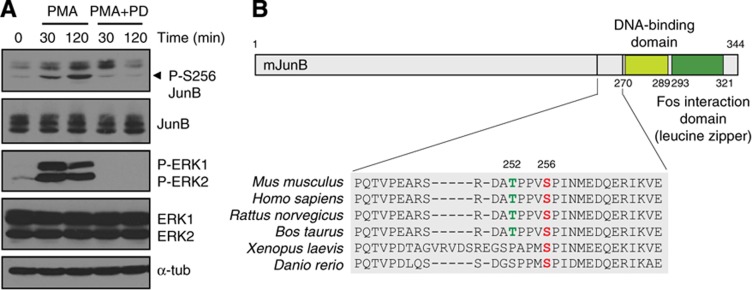
One candidate of interest was JunB, a component of the AP-1 family of transcription factors (Angel and Karin, 1991). The phosphorylation of Ser256, a residue that lies in a full ERK1/2 consensus sequence, was markedly downregulated after PD184352 treatment (Figure 5A). In vitro kinase assays confirmed that ERK1 directly phosphorylates JunB on Ser256 (Figure 4B and C). Notably, Thr252, which is contained in the same tryptic peptide and also lies in a minimal ERK1/2 motif, was not phosphorylated by ERK1 in vitro, highlighting the specificity of these kinases. To determine whether Ser256 of JunB is phosphorylated by ERK1/2 in vivo, we used a commercial phospho-specific antibody. We first ensured that the antibody was specific for Ser256 (Supplementary Figure S7). Stimulation of cells with the ERK1/2 activator phorbol 12-myristate 13-acetate (PMA) induced the phosphorylation of endogenous JunB on Ser256, and this signal was abolished by treatment with PD184352 (Figure 6A).
Figure 6.
ERK1/2 phosphorylate JunB on Ser256 in vivo. (A) NIH 3T3 cells were stimulated with PMA in the presence or absence of PD184352. Lysates were analyzed by immunoblotting with a specific anti-JunB (Ser256) antibody. (B) Schematic representation of mouse JunB structure. Source data for this figure is available on the online supplementary information page.
ERK1/2 phosphorylation of JunB potentiates DNA binding of c-Fos/JunB heterodimers
We next investigated the impact of Ser256 phosphorylation on JunB regulation. Ser256 is conserved among vertebrates, consistent with a functional role (Figure 6B). The regulation of AP-1 factors is complex and occurs through multiple mechanisms (Karin et al, 1997). We first examined whether Ser256 phosphorylation could modulate the affinity of JunB for its heterodimeric partner c-Fos. No apparent difference in the binding of JunB phosphorylation-site mutants to c-Fos was observed in reciprocal co-immunoprecipitation experiments (Supplementary Figure S8). Mutations of Ser256 and Thr252 also did not affect the nuclear localization of JunB (Supplementary Figure S9). Interestingly, a recent study has suggested that phosphorylation of human JunB on Ser259 (equivalent to mouse/rat Ser256) by an unidentified kinase primes the phosphorylation of Ser251 and Thr255 (mouse/rat Thr252) by GSK3β, to create a Fbxw7 phospho-degron that targets JunB for degradation in G2 phase (Perez-Benavente et al, 2012). However, cycloheximide-chase experiments failed to reveal any impact of Ser256 and Thr252 phosphorylation on the stability of JunB in exponentially proliferating NIH 3T3 cells (Supplementary Figure S10).
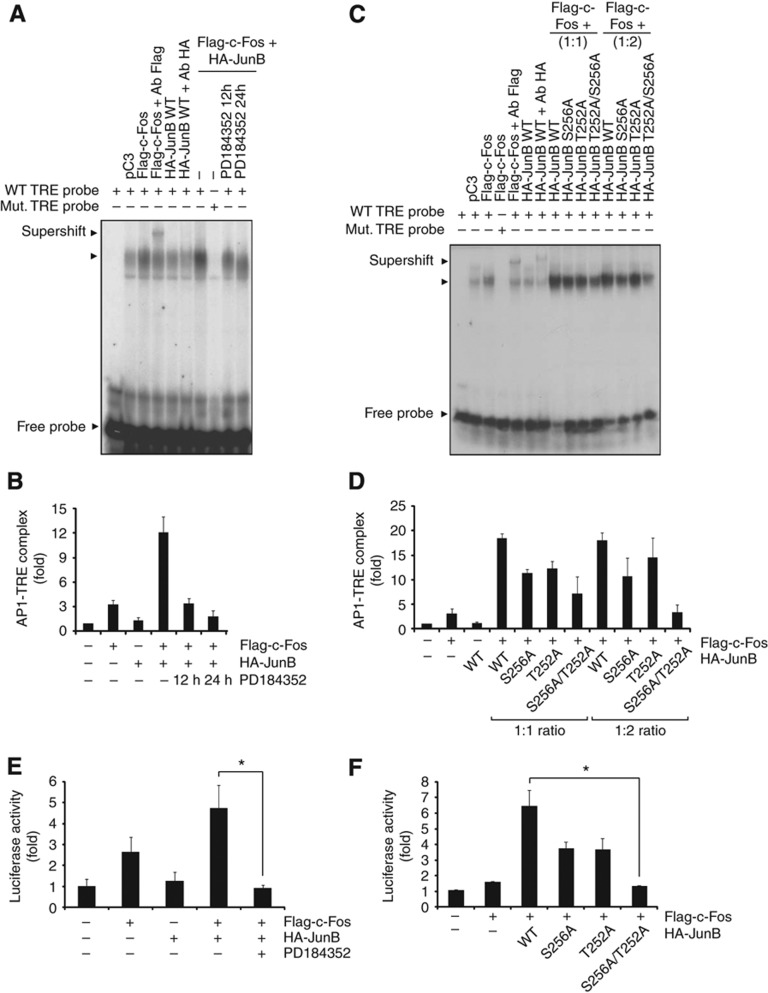
Ser256 is adjacent to the DNA-binding domain of JunB (Figure 6B). To assess whether phosphorylation of Ser256 could affect the DNA-binding properties of JunB-containing AP-1 complexes, we measured the binding of JunB/c-Fos heterodimer to TPA-response elements (TREs) and cAMP-responsive elements (CREs) by electrophoretic mobility shift assay (EMSA). As previously documented (Nakabeppu et al, 1988), we found that JunB expressed alone binds weakly to the TRE, though its affinity for DNA is greatly enhanced by co-expression of c-Fos (Figure 7 A–D). Inhibition of MEK1/2 activity by PD184352 markedly impaired the binding of AP-1 complexes to their target sequences, suggesting a role of ERK1/2 signaling in regulating the binding of JunB/c-Fos complexes to DNA (Figure 7A and B). Notably, mutation of Ser256 to alanine in JunB significantly decreased the DNA binding of JunB/c-Fos dimer. As Thr252 is also located in the vicinity of the JunB DNA-binding domain, we further tested the impact of a Thr-to-Ala substitution, alone or in combination with the Ser-to-Ala mutation at position 256. Whereas the single mutation of Thr252 had a weak effect, the double mutation severely compromised the DNA-binding activity of the AP-1 complex in a concentration-dependent manner (Figure 7C and D). Essentially similar results were obtained using a CRE DNA probe (Supplementary Figure S11).
Figure 7.
ERK1/2 phosphorylation of JunB on Ser256 promotes cooperative DNA binding of JunB/c-Fos heterodimers. (A, C) HEK 293 cells were transfected with the indicated constructs, and nuclear extracts were analyzed by EMSA using a radiolabeled TRE probe. When indicated, cells were treated with PD184352 for 12 h or 24 h (A). Supershift analyses were performed by incubating nuclear extracts with Flag or HA antibody. Results are representative of three experiments. (B, D) Quantification of EMSA results in A and C by densitometric analysis. Results are normalized to control cells transfected with empty pcDNA3 and represent the mean±s.e.m. of three independent experiments. (E) HEC-1B cells were co-transfected with the indicated constructs and the -73-Col-Luc reporter and pRL-TK control plasmids. After 24 h, the cells were treated or not for 24 h with PD184352. Luciferase activity was measured using the Dual-Luciferase assay. *P<0.05. (F) HEC-1B cells were co-transfected with the indicated JunB constructs and -73-Col-Luc reporter activity was measured as above. Luciferase results are expressed as fold increase over the reporter alone and are representative of at least three independent experiments performed in triplicate. *P<0.05. All of the results are expressed as the mean and the s.e.m. of at least triplicate measurements. Student’s t-test was used to analyze the data. Source data for this figure is available on the online supplementary information page.
We next evaluated the impact of Ser256 phosphorylation on the transcriptional activity of JunB complexes using the -73-Col-Luc reporter plasmid. As reported previously (Chiu et al, 1989), expression of JunB alone failed to activate the collagenase promoter, while c-Fos slightly increased reporter activity (Figure 7E). However, co-expression of JunB with c-Fos potentiated its transcriptional activity, consistent with the increase in DNA-binding affinity. This effect was completely abolished by treatment with the MEK1/2 inhibitor PD184352 (Figure 7E), arguing for a key role of ERK1/2 signaling in the transcriptional activation of JunB/c-Fos heterodimers. Importantly, alanine substitution of Ser256 and Thr252 impaired the stimulatory effect of JunB on c-Fos transactivation, whereas the single mutants had weaker and more variable effects (Figure 7F). Taken together, these results strongly suggest that ERK1/2 phosphorylation of JunB on Ser256 is necessary for the full transcriptional activity of JunB/c-Fos AP-1 complexes.
Discussion
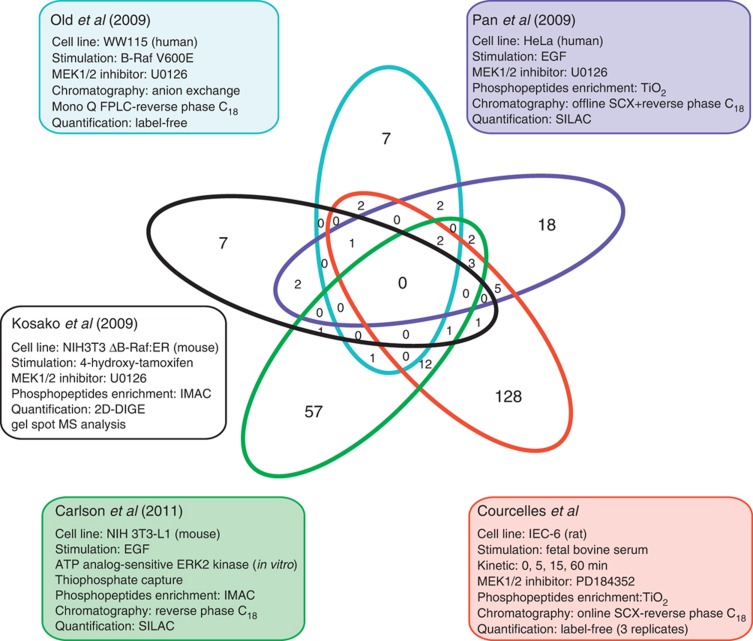
The involvement of the ERK1/2 MAP kinase signaling pathway in a myriad of cellular processes suggests that many more ERK1/2 substrates remain to be discovered. Recent phosphoproteomics studies of variable scales have used label-free (Old et al, 2009), stable isotope labeling with amino acids in cell culture (SILAC) (Pan et al, 2009), two-dimensional-difference gel electrophoresis (Kosako et al, 2009) or analog-sensitive ERK2 kinase (Carlson et al, 2011) technologies to identify candidate ERK1/2 substrates. Noticeably, a comparative analysis of these studies has revealed very little overlap in potential ERK1/2 substrates (Figure 8). This can be explained in part by the selection of cell lines with distinct histological origins and by the use of different proteomics approaches. However, this also indicates that these analyses have not exhaustively mined the repertoire of ERK1/2 substrates, as only a small fraction of them has been identified so far.
Figure 8.
Comparative analysis of phosphoproteomics studies. Comparative analysis of the list of candidate ERK1/2 substrates identified from four previous phosphoproteomics experiments of variable scale with the present study. The comparison was done at the protein level, as the data set of Kosako et al (2009) did not report the position of the phosphorylated sites.
Here, we have used a robust quantitative phosphoproteomics approach to comprehensively profile dynamic changes in protein phosphorylation on a global scale. The selection of phosphopeptides with a consensus ERK1/2 phosphorylation motif that display statistically significant changes in phosphorylation upon stimulation and inhibition of ERK1/2 signaling enabled the identification of 155 candidate ERK1/2 substrates, of which 128 represent new targets. This represents the largest list of ERK1/2 substrates identified in a single study (Figure 8). Notably, we could associate each substrate identified to a specific dynamic profile of phosphorylation by ERK1/2 kinases that will facilitate future studies by other investigators to analyze the role of these phosphorylation events (Supplementary Table 3). Interestingly, these profiles were quite variable depending on the nature of the substrate itself (Supplementary Figure S4). In addition to the known ERK1/2 substrates, we have validated that a selected subset of candidates are directly phosphorylated by ERK1 in vitro on the assigned site, confirming the predictive value of our data set to identify bona fide ERK1/2 substrates.
The list of candidate substrates considerably expands the spectrum of biological functions regulated by the ERK1/2 MAP kinase pathway. For example, the identification of several proteins associated with pre-mRNA splicing, including SR family members, heterogeneous nuclear ribonucleoproteins, Prpf4b and Pnn, suggests an important and hitherto underestimated role of ERK1/2 signaling in this process. Several regulators of chromatin organization, such as the high-mobility group protein Hmg20a, the ISWI chromatin remodeling subunit Rsf-1 and the H3K56 methyltransferase Setd2 were identified. Other potential ERK1/2 substrates are involved in cellular junction assembly, mitotic spindle maintenance, or the DNA damage response. Additional validation efforts will be required to demonstrate that these proteins are physiological substrates of ERK1/2 and to define the biochemical consequence of these phosphorylation events.
In this study, we focused on the regulation of the AP-1 factor JunB. The AP-1 transcription factor is a dimeric basic region-leucine zipper (bZIP) complex that comprises members of the Jun (c-Jun, JunB, JunD), Fos, Atf and Maf protein families (Angel and Karin, 1991; Chinenov and Kerppola, 2001). Jun can form homodimers or heterodimers with other AP-1 factors to recognize different response elements in the enhancers of target genes. Jun proteins have been implicated in a wide variety of biological functions, including cell proliferation, differentiation, survival, embryonic development and tumorigenesis (Jochum et al, 2001; Shaulian and Karin, 2002). The regulation of Jun-containing AP-1 factors is complex and occurs at multiple levels: transcription of AP-1 genes, protein stability, transcriptional activation by phosphorylation and dimer composition (Karin et al, 1997; Eferl and Wagner, 2003). While the mechanisms of activation of c-Jun have been extensively studied, much less is known about the regulation of JunB. JunB is phosphorylated at Thr102 and Thr104 by the c-Jun N-terminal kinases subfamily of MAP kinases, which promotes its transcriptional synergy with c-Maf to activate the interleukin 4 promoter (Li et al, 1999). It has also been reported that JunB phosphorylation by cyclin B-Cdk1 on Ser23, Thr150 and Ser186 triggers its degradation in mitosis (Bakiri et al, 2000). A more recent study has proposed that phosphorylation of Ser259 (Ser256 equivalent) primes the phosphorylation of Ser251 and Thr255 to target JunB for degradation in G2 by a Fbxw7-dependent mechanism (Perez-Benavente et al, 2012). The kinase responsible for the phosphorylation of Ser256 was not identified in that study. Here, we validated that Ser256 of JunB is a bona fide physiological target of ERK1/2. Although we could not document any impact of Ser256 phosphorylation on the stability of JunB in cycling cells, we provide strong evidence that Ser256 regulates the cooperative binding of JunB/c-Fos heterodimers to DNA. Alanine substitution of Ser256 and Thr252 markedly reduced the binding of JunB/c-Fos heterodimers to TRE and CRE sequences, and prevented the synergistic transactivation of the collagenase promoter by JunB and c-Fos. Accordingly, pharmacological inhibition of ERK1/2 signaling with PD184352 abolished the transcriptional activity of JunB/c-Fos heterodimers. Our results add another layer to the complex and fine regulation of AP-1 activity.
This contribution presents the first phosphoproteome dynamic study of the ERK1/2 MAP kinase signaling pathway. Taken together, our data provide one of the most comprehensive phosphoproteomics study to identify ERK1/2 substrates, and represent a unique resource to investigate new areas of ERK1/2 MAP kinase biology.
Materials and methods
Cell culture
IEC-6 were grown to confluence in 150 mm Petri dishes, and made quiescent by serum starvation for 24 h. The cells were then pre-treated for 1 h with DMSO (control) or 2 μM PD184352 before stimulation with 10% fetal bovine serum for 0, 5, 15 or 60 min.
Reagents, antibodies and plasmids
PD184352 was a gift from Pfizer. Commercial antibodies were obtained from the following suppliers: anti-ERK1/2 CT from Upstate Biotechnology; anti-phospho-ERK1/2(Thr202/Tyr204) and anti-JunB from Cell Signaling Technology; anti-phospho-JunB(Ser256) and anti-Hsc70 from Santa Cruz Biotechnology; anti-HA from Covance; anti-Flag and anti-α-tubulin from Sigma.
pcDNA3-Flag-c-Fos was obtained from Trang Hoang (Université de Montréal). The pGEX-KG-JunB vector was constructed by subcloning the mouse JunB sequence from pBabe-JunB (obtained from Philippe Roux, Université de Montréal) into EcoRI/XhoI sites of pGex-KG vector. The pcDNA3-HA-JunB vector was constructed by subcloning mouse JunB sequence into EcoRI/XhoI sites of pcDNA3-HA. The pGEX-KG-DDX47 vector was kindly provided by Takeshi Sekiguchi (Kyushu University, Japan). The pGEX-KG-Numa1 (amino acids 1605–2016 of human protein) plasmid was a kind gift of Nai-Wen Chi (La Jolla, California). The pGEX-KG-MEK2(K101E) vector was constructed by subcloning the human MEK2 sequence into the EcoRI site of pGEX-KG. The kinase-dead K101E mutant of MEK2 was used in the in vitro kinase assays to avoid autophosphorylation of MEK2. The pGEX-KG-Hmg20a vector was constructed by subcloning the human Hmg20a sequence from pCMV6-XL5-HMG20A (Origene) into XbaI/XhoI sites of pGEX vector. The pGEX-KG-Tjp1 was constructed by subcloning the first 510 amino acids of human Tjp1 from pEGFP-C1-ZO1 (kind gift of Alan Fanning, University of North Carolina) into EcoRI/XhoI sites of pGEX-KG. pRL-TK and -73 Col-Luc were obtained from J Hiscott (McGill University, Montreal). All mutations and PCR products were verified by DNA sequencing. Sequence of primers used for PCR and details about cloning strategies are available upon request.
Cellular fractionation and protein extraction
Biological triplicates were generated for MS analysis. Cells (5 × 108) were washed twice with ice-cold PBS, collected by scrapping, and lysed in lysis buffer (10 mM Tris–HCl, pH 8.4, 140 mM NaCl, 1.5 mM MgCl2, 0.5% NP-40 (Calbiochem), 1 mM dithiothreitol (DTT), protease and phosphatase inhibitor added freshly). After centrifugation at 1000 g for 3 min at 4 °C, supernatants were transferred to separate tubes (cytoplasmic fraction). The pellets were then resuspended in lysis buffer plus 1/10 of detergent stock (3.3% w/v sodium deoxycholate, 6.6% Tween 40), vortexed at slow speed, incubated on ice for 5 min, and centrifuged at 1000 g for 3 min at 4 °C. The supernatant was discarded again and the pellet, containing the nuclei, was rinsed with lysis buffer and lysed in extraction buffer (20 mM Hepes, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol) by sonication. Benzonase nuclease HC (Novagen) was added to digest nucleic acids and obtain the nuclear fraction. Proteins were precipitated overnight with cold acetone (−20 °C) and resuspended in a solution of 1% SDS and 50 mM ammonium bicarbonate. Cytosolic and nuclear protein extracts were reduced for 20 min at 37 °C with 0.5 mM tris(2-carboxyethyl)phosphine (TCEP) (Pierce) and then alkylated with 50 mM iodoacetamide for 20 min at 37 °C. The excess of iodoacetamide was neutralized by the addition of 50 mM DTT. Proteins were quantified with Micro BCA Protein Assay (Pierce), diluted 10 times with 50 mM ammonium bicarbonate, and digested with sequencing-grade trypsin (1:100) (Promega) overnight at 37 °C with agitation. Tryptic digests were acidified below pH 4.0 with trifluoroacetic acid (TFA) to inactivate trypsin and dried in a SpeedVac apparatus (Thermo).
Phosphopeptides enrichment and MS
Phosphopeptides (1 mg/replicate) were enriched as described (Thingholm et al, 2006) on home-made TiO2 affinity columns (1.25 mg Titansphere, 5 μm, GL Sciences) and eluted with 30 μl of 1% ammonium hydroxide. Eluates were acidified with 1 μl TFA, desalted using 30 mg HLB cartridge (Waters), dried and resuspended in 2% acetonitrile (ACN)/0.2% formic acid before analysis. Phosphopeptides were separated online by 2D-nanoLC (Eksigent). Peptides were first fractionated on a Opti-Guard 1 mm cation SCX column (Optimize Technologies) using five ammonium acetate salt fractions (0, 0.25, 0.5, 1 and 2 M) in 2% CAN at pH 3.0. Each fraction was then loaded on a reverse-phase precolumn (4 mm length, 360 μm i.d.) and separated on a reverse-phase analytical column (10 cm length, 150 μm i.d.) (Jupiter C18, 3 μm, 300 Å, Phenomenex). Both columns were packed manually. A gradient from 2 to 33% ACN over 53 min followed by a gradient from 33 to 60% ACN over 10 min with a flow rate of 600 nl/min was used to elute the peptides to the MS system with the nanoelectrospray source voltage set to 1.7 kV. MS analysis was done on a LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific). MS spectra were acquired with a resolution of 60 000 in FTMS using lock mass. CID MS/MS spectra were acquired in data-dependent mode for the three most abundant multiply charged ions with intensity above 10 000 counts. A dynamic exclusion window was set to 90s.
MS/MS processing for peptide and protein identifications
MS/MS spectra peak lists were extracted from Xcalibur raw data files (Thermo Fischer Scientific) and preprocessed using Mascot Distiller v2.1.1 (Matrix Science) using the configuration file for low-resolution MS/MS for the LTQ-Orbitrap. MGF peak lists were searched with Mascot 2.1 on a concatenated target/decoy IPI rat database v3.54 (39 928 protein sequences) (Kersey et al, 2004) using the following parameters: peptide mass tolerance±10 p.p.m., fragment mass tolerance±0.5 Da, trypsin with two missed cleavages, and the variable modifications carbamidomethyl (C), deamidation (NQ), oxidation (M), phosphorylation (STY). All search results were then transferred to ProteoConnections, our in-house bioinformatics platform dedicated to phosphoproteomics analysis (Courcelles et al, 2011). Identifications and MS/MS spectra are available online in ProteoConnections ( http://www.thibault.iric.ca/proteoconnections). From there, a 1% FDR cutoff was applied to peptides assigned to proteins with a P<0.05 significance threshold. Phosphorylation-site-localization confidence was assigned by ProteoConnections as previously proposed (Olsen et al, 2006). All data files are obtainable at http://www.peptideatlas.org/PASS/PASS00138.
Data processing for label-free peptide quantification
Peptides detection for all raw files was done using our in-house peptide-detection software. The software retrieves peak intensity value, mass-to-charge ratio, retention time and charge state for each peptide. An intensity threshold of 10 000 counts was selected to detect peptides above the noise level. Detected peptides were then aligned with an m/z tolerance of 15 p.p.m., retention time window of ±1 min and same charge state to get abundance values for all conditions and replicates. A median normalization procedure was applied to minimize experimental variability of the peptides population abundance. Since the peptides population size is not equal between experimental conditions, only reproducibly detected peptides were used to calculate the median of samples.
Soft clustering of phosphopeptide kinetic profiles was done using fuzzy c-means clustering in MFuzz R package (number of clusters centroid c=6, parameter m=1.5). The number of clusters was chosen arbitrarily to 6 to show diverse phosphorylation change patterns. Grouping is done by minimizing the Euclidian distance between the phosphopeptide kinetic fold-change profiles with a weighted square error function. Fuzzy c-means clustering is a soft-clustering algorithm that distinguishes itself from hard-clustering algorithm by providing a membership probability value to each member of the clusters. This information indicates how similar a profile is compared with the rest of the cluster members.
Selection of candidate ERK1/2 substrates
An algorithm, implemented in Perl, was written to select putative ERK1/2 substrates using the following criteria. First, high-confidence phosphorylation sites (⩾75%) with the minimal ERK1/2 consensus (pS/T)-P motif were selected using ProteoConnections. Second, phosphorylation level must increase after serum stimulation. Phosphopeptides were filtered with a cutoff of Σlog10(stimulated t5-60/control t0) ⩾0.3, a value clearly above our biological replica measurements. Third, a decrease in phosphorylation-site abundance is observed after treatment with the MEK1/2 inhibitor PD184352. To select downregulated phosphopeptides, a cutoff of Σlog10(PD184352 treated/control) ⩽−0.7 was used, a value 2.5 time above fold change found between replicates. Furthermore, at least one time point of the kinetics must show a significant decrease in abundance with a two-tailed t-test (P-value⩽0.05). Finally, manual inspection of the abundance profiles was performed on potential candidates to fix potential peak selection errors of the label-free quantification software.
Bioinformatics analyses
For GO analysis, enrichment and depletion of categories were calculated using odds ratios. Ratios are calculated as: (number of proteins with the GO term in the data set/number of proteins in the data set)/(number of proteins with the GO term in the proteome/number of proteins in the proteome). P-values were calculated using Fisher's exact test. To generate protein interactions network, we first used ProteoConnections to map the list of candidate ERK1/2 substrates to the STRING interactions database. We retained only the highest confidence (>0.9) interactions extracted from experiments and databases found in rat. For all candidates found, we gathered extra interactors one level deeper (white node). Since the rat interactome is not well studied, we chose to expand our network by including interactions from human orthologs. After converting rat gene identifiers to human, we extracted mapped interaction from STRING as above. We manually edited the human network to keep interactions that extended our rat network (connectable components) and removed white node (unless needed to connect two subnetworks). Finally, fusion of the rat and the remaining human network was made using Cytoscape. This software was also used to organize spatially the network for Figure 3. Binary interactions were removed to generate the figure. Identified phosphorylation sites were compared with Swissprot v15.53 (Boeckmann et al, 2003), Phospho.ELM v8.2 (Diella et al, 2008) and PhosphositePlus v2.0 (Hornbeck et al, 2004) database to report the fraction of novel sites. ProteoConnections was used to indicate the presence of potential docking sequences DEF (motif F-X-[FY]-P) or D domain (motif [KR]2-5-X1-6-[LIV]-X-[LIV]) in the protein sequence of putative ERK1/2 substrates.
In vitro kinase assays
Recombinant GST-tagged candidate substrates were produced in E. coli and purified on glutathione-Sepharose beads. Recombinant proteins were incubated in kinase buffer (20 mM Tris–HCl, pH 7.4, 20 mM NaCl, 10 mM MgCl2, 1 mM DTT) supplemented with 50 μM ATP and 5 μCi [γ-32P]ATP for 20 min at 30 °C in the presence of 30 ng recombinant active ERK1 protein (Millipore). The reaction products were analyzed by SDS-gel electrophoresis, autoradiography and MS/MS.
Immunoblot analysis and immunofluorescence microscopy
Cell lysis and immunoblot analysis were performed as described previously (Servant et al, 2000). Staining of cells for immunofluorescence microscopy was performed as described (Julien et al, 2003) and the cells were examined on a Zeiss LSM510 confocal microscope. At least 150 cells were scored for each coverslip.
Electrophoretic mobility shift assay
HEK 293 cells were washed once with ice-cold PBS and lysed by scraping in lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl and 1.5 mM MgCl2) containing 0.1% Nonidet P-40, 0.5 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. The nuclei were collected by centrifugation (12 000 g, 5 min, 4 °C) and washed with lysis buffer without detergent. Nuclear proteins were extracted by suspending the nuclei in ice-cold hypertonic buffer A (420 mM NaCl, 20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM DTT and protease inhibitors) for 10 min on ice. After centrifugation (12 000 × g, 5 min, 4 °C), the supernatant was transferred to another tube and the nuclear extract was diluted immediately with 1.5 volumes of buffer B (50 mM KCl, 20 mM HEPES, pH 7.9, 0.2 mM EDTA, 20% glycerol, 0.5 mM DTT, and protease inhibitors). The samples were then frozen at −80 °C.
For EMSA, two complementary oligonucleotides containing AP-1 element (TRE or CRE) were end-labeled with [γ-32P]ATP and purified on a G-25 spin column. The 32P-labeled oligonucleotide probe was incubated in gel shift buffer (4 mM Tris–HCl, pH 7.9, 25 mM KCl, 5 mM MgCl2, 0.4 mM EDTA, 5% glycerol, 0.4 mM DTT) with 10 μg nuclear proteins and 50 μg/ml poly(dI-dC) for 20 min at room temperature. DNA–protein complexes were separated on 5% native polyacrylamide gels, transferred to Whatman 3 MM paper, and exposed to X-ray films. The specificity of binding was assessed using mutant TRE and CRE oligonucleotides. For supershift experiments, the reaction mixture was pre-incubated for 20 min with 1 μg of antibody against HA (Covance) or Flag (Sigma). The sequences (5′→3′) of the oligonucleotides used in the EMSA are: TRE wt (5′-TTCCGGCTGACTCATCAAGCG-3′); TRE mutant (5′-TTCCGGCTGACTTGTCAAGCG-3′); CRE wt (5′-TTCCGGCTGACGTCATCAAGCG-3′); and CRE mutant (5′-TTCCGGCTGACGTTGTCAAGCG-3′).
Luciferase reporter assay
HEC-1B cells were seeded in 48-well plates and co-transfected by the calcium phosphate method with AP-1 expression plasmids (50–250 ng), 100 ng -73 Col-Luc (collagenase I promoter with single TRE site) firefly luciferase reporter plasmid and 25 ng Renilla luciferase internal control pRL-TK. The total amount of transfected DNA was adjusted with pcDNA3. After 48 h, cells were lysed in Passive Lysis Buffer (Promega) and lysates transferred to 96-well luminometer plates. Firefly and Renilla luciferase activities were assayed using the Dual-Luciferase Reporter System (Promega) on a MicroBeta 1450 luminometer (PerkinElmer).
Supplementary Material
Legends to Supplementary tables S1-5, Supplementary figures S1-11
Annotated phosphorylation sites
Kinetic profiles of phosphorylated peptides
Kinetic profiles of candidate ERK substrates
GO analysis of ERK substrates
Validated ERK substrates
Acknowledgments
We thank Éric Bonneil for technical assistance in MS analyses and Ivan Topisirovic for the cellular fractionation protocol. MC is recipient of studentships from the Canadian Institutes for Health Research (CIHR), BiT program and the Fonds de recherche sur la nature et les technologies du Québec (FQRNT). CF is recipient of fellowships from the Cole foundation, the French Association pour la Recherche contre le Cancer (ARC) and the Fonds de la recherche en santé du Québec (FRSQ). PT and SM hold the Canada Research Chairs in Proteomics and Bioanalytical Spectrometry and Cellular Signaling, respectively. This work was supported by operating grants from the National Science and Engineering Research Council (NSERC) to PT, and the CIHR and Cancer Research Society to SM. IRIC is supported in part by the Canadian Center of Excellence in Commercialization and Research, the Canada Foundation for Innovation and the FRSQ.
Author Contributions: MC, CF, LV, SM and PT designed research; MC, CF and LV performed research; MC, CF, LV, SL, SM and PT analyzed data; and MC, CF, SM and PT wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Angel P, Karin M (1991) The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157 [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M (2000) Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J 19: 2056–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O'Donovan C, Phan I, Pilbout S, Schneider M (2003) The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res 31: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Chouinard CR, Labadorf A, Lam CJ, Schmelzle K, Fraenkel E, White FM (2011) Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci Signal 4: rs11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov Y, Kerppola TK (2001) Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20: 2438–2452 [DOI] [PubMed] [Google Scholar]
- Chiu R, Angel P, Karin M (1989) Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell 59: 979–986 [DOI] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ (1995) How MAP kinases are regulated. J Biol Chem 270: 14843–14846 [DOI] [PubMed] [Google Scholar]
- Courcelles M, Lemieux S, Voisin L, Meloche S, Thibault P (2011) ProteoConnections: a bioinformatics platform to facilitate proteome and phosphoproteome analyses. Proteomics 11: 2654–2671 [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Johnson GL (2007) Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 26: 3159–3171 [DOI] [PubMed] [Google Scholar]
- Diella F, Gould CM, Chica C, Via A, Gibson TJ (2008) Phospho.ELM: a database of phosphorylation sites--update 2008. Nucleic Acids Res 36: D240–D244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868 [DOI] [PubMed] [Google Scholar]
- Fritsche-Guenther R, Witzel F, Sieber A, Herr R, Schmidt N, Braun S, Brummer T, Sers C, Bluthgen N (2011) Strong negative feedback from Erk to Raf confers robustness to MAPK signalling. Mol Syst Biol 7: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Raden DL, Davis RJ (1991) Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J Biol Chem 266: 22159–22163 [PubMed] [Google Scholar]
- Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B (2004) PhosphoSite: a bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 4: 1551–1561 [DOI] [PubMed] [Google Scholar]
- Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K (1999) Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev 13: 163–175 [PMC free article] [PubMed] [Google Scholar]
- Jochum W, Passegue E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20: 2401–2412 [DOI] [PubMed] [Google Scholar]
- Julien C, Coulombe P, Meloche S (2003) Nuclear export of ERK3 by a CRM1-dependent mechanism regulates its inhibitory action on cell cycle progression. J Biol Chem 278: 42615–42624 [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246 [DOI] [PubMed] [Google Scholar]
- Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R (2004) The International Protein Index: an integrated database for proteomics experiments. Proteomics 4: 1985–1988 [DOI] [PubMed] [Google Scholar]
- Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S (2009) Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol 16: 1026–1035 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, Osborne J, McGlynn K, Stippec S, Earnest S, Chen W, Cobb MH (2008) The roles of MAPKs in disease. Cell Res 18: 436–442 [DOI] [PubMed] [Google Scholar]
- Li B, Tournier C, Davis RJ, Flavell RA (1999) Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J 18: 420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio M, Trost M, Courcelles M, Desjardins M, Thibault P (2008) Combined enzymatic and data mining approaches for comprehensive phosphoproteome analyses: application to cell signaling events of interferon-gamma-stimulated macrophages. Mol Cell Proteomics 7: 645–660 [DOI] [PubMed] [Google Scholar]
- Mody N, Leitch J, Armstrong C, Dixon J, Cohen P (2001) Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett 502: 21–24 [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Ryder K, Nathans D (1988) DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell 55: 907–915 [DOI] [PubMed] [Google Scholar]
- Old WM, Shabb JB, Houel S, Wang H, Couts KL, Yen CY, Litman ES, Croy CH, Meyer-Arendt K, Miranda JG, Brown RA, Witze ES, Schweppe RE, Resing KA, Ahn NG (2009) Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma. Mol Cell 34: 115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648 [DOI] [PubMed] [Google Scholar]
- Pan C, Gnad F, Olsen JV, Mann M (2008) Quantitative phosphoproteome analysis of a mouse liver cell line reveals specificity of phosphatase inhibitors. Proteomics 8: 4534–4546 [DOI] [PubMed] [Google Scholar]
- Pan C, Olsen JV, Daub H, Mann M (2009) Global effects of kinase inhibitors on signaling networks revealed by quantitative phosphoproteomics. Mol Cell Proteomics 8: 2796–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Perez-Benavente B, Garcia JL, Rodriguez MS, Pineda-Lucena A, Piechaczyk M, Font de Mora J, Farras R (2012) GSK3-SCF(FBXW7) targets JunB for degradation in G2 to preserve chromatid cohesion before anaphase. Oncogene 32: 2189–2199 [DOI] [PubMed] [Google Scholar]
- Ramos JW (2008) The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol 40: 2707–2719 [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G (2007) Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 7: 295–308 [DOI] [PubMed] [Google Scholar]
- Servant MJ, Coulombe P, Turgeon B, Meloche S (2000) Differential regulation of p27(Kip1) expression by mitogenic and hypertrophic factors: involvement of transcriptional and posttranscriptional mechanisms. J Cell Biol 148: 543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136 [DOI] [PubMed] [Google Scholar]
- Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC (1996) A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol 16: 6486–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm S (2008) Regulation of alternative splicing by reversible protein phosphorylation. J Biol Chem 283: 1223–1227 [DOI] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E (2000) A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol 2: 110–116 [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD (2010) Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci 1214: 99–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thingholm TE, Jorgensen TJ, Jensen ON, Larsen MR (2006) Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc 1: 1929–1935 [DOI] [PubMed] [Google Scholar]
- Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P (2009) The phagosomal proteome in interferon-gamma-activated macrophages. Immunity 30: 143–154 [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24: 21–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legends to Supplementary tables S1-5, Supplementary figures S1-11
Annotated phosphorylation sites
Kinetic profiles of phosphorylated peptides
Kinetic profiles of candidate ERK substrates
GO analysis of ERK substrates
Validated ERK substrates