Abstract
Objectives
To assess coronary artery calcification in patients of age ≥ 10 years with a history of Kawasaki Disease (KD).
Background
Patients with a history of KD and coronary artery aneurysms are at risk for late morbidity from coronary artery events. It is unknown whether KD patients with acutely normal or transiently dilated coronary arteries also have increased risk of late coronary artery complications. Coronary calcium scoring by non-contrast computed tomography (CT) is a well-established tool for risk stratifying patients with atherosclerotic coronary artery disease, but there are limited data on its role in evaluating patients with a history of KD.
Methods
We performed coronary artery calcium (CAC) volume scoring using a low radiation dose CT protocol on 70 subjects (median age: 20.0 years) with a remote history of KD (median interval from acute KD to imaging: 14.8 years): 44 (63%) had no history of coronary dilation, 12 (17%) had a history of transient dilation, and 14 (20%) had coronary aneurysms.
Results
All of the subjects with normal coronary artery internal diameter during the acute phase of KD and 11 of 12 subjects with transient dilatation had CAC scores of zero. Coronary calcification was observed in 10 of the 14 subjects with coronary aneurysms, with the degree of calcification ranging from mild to severe and occurring years after the subjects’ acute KD.
Conclusions
Coronary calcification was not observed in subjects with a history of KD and normal coronary arteries during the acute phase. Therefore, CAC scanning may be a useful tool to screen patients with a remote history of KD or suspected KD and unknown coronary artery status. Coronary calcification, which may be severe, occurs late in patients with coronary aneurysms. The pathophysiology and clinical implications of coronary calcification in patients with aneurysms are currently unknown and warrant further study.
Keywords: Calcium Scoring, Kawasaki Disease, Aneurysm, Computed Tomography
Introduction
Kawasaki disease (KD), the most common cause of acquired heart disease in children, is an acute vasculitis associated with the formation of coronary aneurysms in 25% of untreated cases and 5% of patients treated with intravenous immunoglobulin(1–3). Patients with a history of KD and aneurysm formation are at risk for coronary artery thrombosis, stenosis, myocardial infarction, and death(2,4–7). Patients with aneurysms are known to deposit calcium in the arterial wall in sites of antecedent inflammation and such dramatic calcifications can be appreciated on chest radiographs decades after the acute disease(8). Those with a history of KD without aneurysm formation have a benign course in the short term, but whether they are at increased risk for coronary and myocardial complications later in life is currently unknown(9). As a result, there is a need for better tools to identify patients at increased risk and to help risk stratify patients for appropriate medical treatments such as anti-platelet, anticoagulation, and lipid-lowering therapies, and invasive treatments such as percutaneous coronary intervention and coronary artery bypass surgery (CABG)(5,6).
Coronary artery calcium (CAC) scoring by computed tomography (CT) has a proven role in detecting and managing atherosclerotic coronary artery disease (CAD), and is highly predictive of mortality(10–12). This examination uses a relatively low radiation dose, requires no contrast, is inexpensive, and provides a single score that can be useful in patient management(10,13). For atherosclerotic coronary artery disease, the CT CAC score risk stratifies patients more accurately than traditional risk factors(11).
Thus far, the role of CAC scoring in the management of patients with a history of KD has not been systematically studied. In one small study of 18 young patients with a history of KD investigated using electron-beam CT, elevated CAC scores were only observed in those patients with coronary aneurysms, and the patient with the highest CAC score suffered sudden cardiac death(14). In another study of 79 patients with KD and a history of abnormal coronary arteries acutely, electron-beam CT was used to assess for the presence of qualitative calcification (15). We report here the results of CAC scoring using multi-slice CT in a diverse population of patients with a distant history of KD both with and without coronary aneurysms.
Methods
Study Population
A total of 70 subjects were enrolled from 2 separate cohorts. We enrolled 44 patients (age ≥ 10 years) recruited from those originally diagnosed, treated, and followed by the Kawasaki Disease Research Center at the University of California, San Diego Medical Center and Rady Children’s Hospital (Cohort 1). In addition, we enrolled 26 subjects who were self-referred for our study or responded to recruiting efforts (Cohort 2), the majority of whom became aware of it via word-of-mouth and locally held informational symposia. For Cohort 2, 23 of the subjects had a known history of KD. In the remaining 3 subjects, the diagnosis of KD was based on the presence of proximal coronary artery aneurysms diagnosed by CT or coronary angiography independently reviewed by two cardiologists, and a history of a KD-compatible illness obtained through interview of the subject or his or her parents. This study was approved by the Intuitional Review Board of the University of California and all subjects or their parents provided written informed consent.
The coronary artery status at the time of subject’s acute KD presentation was determined from the initial evaluation by echocardiography, and by repeat imaging with echocardiography, invasive coronary angiography or CT angiography (CTA) where available. For those subjects with coronary artery abnormalities, subsequent coronary artery status was determined by the most recent clinically indicated CTA or invasive coronary angiography. Data from one of these two tests was available for all of the subjects with coronary aneurysms. Subjects were grouped into four categories: 1) no history of coronary artery dilation (American Heart Association Risk Level I)(9), 2) transient coronary artery dilation that subsequently resolved, (American Heart Association Risk Level 2), 3) persistent coronary artery aneurysms < 8 mm in diameter, 4) persistent giant coronary aneurysms ≥ 8 mm in diameter. Major adverse cardiac events (MACE) were defined as a history of CABG, myocardial infarction, or percutaneous coronary intervention.
Risk Factors for Coronary Artery Disease
All subjects completed a standardized questionnaire that documented current medications and assessed risk factors for atherosclerotic CAD: physician-diagnosed hypertension, diabetes mellitus, hyperlipidemia, family history of early atherosclerotic CAD (defined as disease diagnosed at age < 55 years in a first degree relative), and current smoking. Height and weight were obtained at the time of CT scanning, and body mass index (BMI, kg/m2) was computed.
CT Scanning Protocol
Subjects were scanned during a single breath-hold on a 64-slice Discovery CT750 HD scanner (GE Healthcare, Milwaukee, WI) with a customized protocol designed to minimize the administered radiation dose. All but three of the subjects were scanned using a tube energy of 100 kVp (instead of the tube energy of 120 kVp traditionally used for calcium scoring) in gated axial mode. The tube current was adjusted based on the BMI to give adequate signal to noise while minimizing the net current, using the empirically determined formula: tube current (mA) = 11 × BMI. The time of imaging during the cardiac cycle was adjusted based on the heart rate (HR), imaging at 75% of the R-R interval for HR < 75 beats per minute, and at 50% of the R-R interval for HR ≥ 75 beats per minutes. The slice thickness was 2.5 mm, the gantry rotational period was 0.35 seconds, and the scan length was adjusted from the scout images to encompass the entire heart. The median radiation dose-length product (DLP) was 42 mGy-cm, which, using a conversion factor of 0.014 mSv/mGy-cm, corresponds to a median effective radiation dose of 0.59 mSv(16). The minimum, 1st quartile, 3rd quartile, and maximum doses were 0.32, 0.49, 0.68, and 1.46 mSv respectively (of note, the maximum dose was for one of the three subjects scanned using 120 kVp). The minimum, 1st quartile, median, 3rd quartile, and maximum effective radiation doses divided by body mass were 0.0064, 0.0086, 0.0095, 0.011, and 0.020 mSv/kg respectively.
CT angiography was performed in selected patients when clinically indicated using the scanner described above. Oral and/or intravenous metoprolol was administered as needed to decrease the patients’ heart rate, with a goal heart rate of less than 60 beats per minute. A timing bolus with 10 ml of iodinated contrast and subsequent sequential imaging and analysis of a single slice at the level of the left main coronary artery was used to determine the optimal imaging time. Iodinated contrast was injected with a triple phase technique (contrast, contrast/saline mix, saline) with the dose, mixture and flow rate adjusted for the patients’ BMI. The tube voltage and current were also adjusted for the patients’ BMI. Images were obtained during a single breath-hold using prospective gating with imaging centered at 75% of the R-R interval and a slice thickness of 0.625 mm.
Image Analysis
Images for CAC scoring were reconstructed using a 512 × 512 matrix and analyzed offline using a Ziostation workstation (Ziosoft, Inc., Redwood City, CA). For each subject the total coronary calcium volume was calculated using a minimum threshold of 147 Hounsfield units (HU), following the work of Nakazatu et al. to adjust for the lower tube energy, and a minimum volume of 2.5 mm3(17). For the three subjects scanned using 120 kVp, the traditional 130 HU threshold was used(18). Regions of calcification of the coronary arteries were manually identified on the workstation and for each subject the total volume of coronary calcification was calculated. Regions of coronary arteries with stents were excluded from scoring due to artifacts from the stents. CT angiography images were viewed, reconstructed, and analyzed using a Vitrea workstation (Vital Images, Inc., Minnetonka, MN).
Statistical Analysis
For each group of subjects, we calculated medians and quartiles for all continuous parameters studied. Spearman rank-order correlation was used to assess correlation between continuous variables. For all tests a two-sided p <0.05 was considered statistically significant.
Results
Demographics
Subjects ranged in age from 10.3 to 59.8 years with a median age of 20.0 years. The median interval from the onset of KD to the time of imaging was 14.8 years. Of the 70 subjects, 44 (63%) had no history of coronary dilation, 12 (17%) had a history of transient dilation, 14 (20%) had aneurysms, and of these, 6 (9%) had giant (>8 mm in diameter) aneurysms (Table 1).
Table 1.
Patient Characteristics
| Cohort 1 N=44 |
Cohort 2 N=26 |
Total N=70 |
|
|---|---|---|---|
| Males (%) | 27 (61%) | 15 (58%) | 42 (60%) |
| Age at onset of KD, yrs: 1st quartile, median, 3rd quartile | 1.2, 2.4, 5.9 | 3.0, 4.5, 7.5 | 1.4, 3.4, 6.5 |
| Age at time of CT, yrs: 1st quartile, median, 3rd quartile | 13.4, 16.7, 20.5 | 21.6, 27.7, 32.4 | 14.7, 20.0, 24.8 |
| Interval from onset of KD to CT scan, years: 1st quartile, median, 3rd quartile | 10.5, 13.4, 16.0 | 13.0, 24.5, 28.3 | 10.9, 14.8, 23.8 |
| Coronary artery status | |||
| No dilation | 26 (59%) | 18 (69%) | 44 (63%) |
| Transiently dilated | 11 (25%) | 1 (4%) | 12 (17%) |
| Aneurysm < 8mm in diameter | 5 (11%) | 3 (12%) | 8 (11%) |
| Giant aneurysm (≥ 8mm in diameter) | 2 (5%) | 4 (15%) | 6 (9%) |
| Cardiovascular Risk Factors | |||
| Hypertension | 2 (5%) | 2 (8%) | 4 (6%) |
| Diabetes Mellitus | 1 (2%) | 1 (4%) | 3 (4%) |
| Current Smoking | 3 (7%) | 2 (8%) | 5 (7%) |
| Hyperlipidemia | 5 (11%) | 6 (23%) | 11 (16%) |
| Family History of Early CAD (< 55 yrs) | 14 (32%) | 11 (42%) | 25 (36%) |
| Body Mass Index (kg/m2): 1st quartile, median, 3rd quartile | 19, 21, 24 | 20, 22, 24 | 19, 22, 24 |
| Medications | |||
| Statin medication | 2 (5%) | 3 (12%) | 5 (7%) |
| Niacin | 1 (2%) | 1 (4%) | 2 (3%) |
| ACE-I or ARB | 3 (7%) | 3 (12%) | 6 (9%) |
| Beta blocker | 2 (5%) | 2 (12%) | 5 (7%) |
| Calcium channel blocker | 2 (5%) | 0 (0%) | 2 (3%) |
| Warfarin or Dabigatran | 3 (7%) | 3 (12%) | 6 (9%) |
| Aspirin | 5 (11%) | 10 (38%) | 15 (21%) |
| Clopidogrel | 2 (5%) | 2 (8%) | 4 (6%) |
Key: Cohort 1: Patients originally diagnosed, treated, and followed by the Kawasaki Disease Research Center at the University of California, San Diego Medical Center and Rady Children’s Hospital
Cohort 2: All other patients
ACE-I: Angiotensin-converting enzyme inhibitors
ARB: Angiotensin II receptor blockers
Diabetes mellitus, hypertension, and current smoking were each present in fewer than 10% of the subjects. A history of hyperlipidemia was present in 16% of the subjects, and 36% of the subjects had a family history of early CAD. The majority (38 subjects, 54%) had no risk factors for coronary artery disease, 23 (33%) had 1 risk factor, and 9 (13%) had 2 or more risk factors. Of the 38 subjects with 1 coronary risk factor, 25 (66%) had a family history of early CAD. Five subjects were on statin medications and two subjects were on niacin. Six of the subjects were on systemic anticoagulation: 5 were on warfarin and one was on dabigatran.
CAC Volume Scores
Table 2 shows the CAC scores as a function of coronary artery status. No calcification was detected in any of the subjects whose coronary artery internal diameter was always assessed as normal. Of the subjects with transient coronary dilation, only one had calcification. For subjects with aneurysms < 8 mm in diameter and for those with giant aneurysms, a wide range of calcium scores was observed, with scores ranging from zero to greater than 8000 mm3, indicative of severe calcification.
Table 2.
Calcium Score Results
| Coronary Artery Status | Calcium Volume (mm3) | |||
|---|---|---|---|---|
|
| ||||
| 0 | 1–100 | 101–1000 | >1000 | |
| No dilation | 44 | 0 | 0 | 0 |
| Transiently Dilated | 11 | 0 | 1 | 0 |
| Aneurysm < 8 mm | 3 | 3 | 0 | 2 |
| Giant Aneurysm | 1 | 0 | 2 | 3 |
Overall, 59 (84%) of the subjects studied had no coronary calcification and 11 (16%) had calcification. The majority of the subjects with calcification (8 out of 11) had ≤ 1 cardiac risk factor: 4 had none and 4 had only 1 risk factor. Of these 11 subjects with calcification, 9 had a history of MACE (6 had undergone CABG, 2 had myocardial infarctions, and 1 had undergone percutaneous coronary intervention with stent placement). Of the 14 subjects with coronary aneurysms, 9 had MACE, all but one of whom had a positive CAC score. The one subject with a MACE and a CAC score of zero was a 19 year old man with giant coronary aneurysms who suffered a myocardial infarction 6 years after KD onset while off of anticoagulation. Of the 5 subjects on warfarin, all had aneurysms and 4 of them had positive CAC scores. The one subject on warfarin without calcification was studied 6 years after his episode of acute KD.
The CAC scan from the one subject with transient coronary dilation during his acute KD and a positive CAC score is shown in Figure 1(a). This 33 year old man with no cardiac risk factors and a history of KD at age 5 years presented with unstable angina. Invasive angiography demonstrated a 90% left main coronary artery stenosis which was treated with urgent CABG.
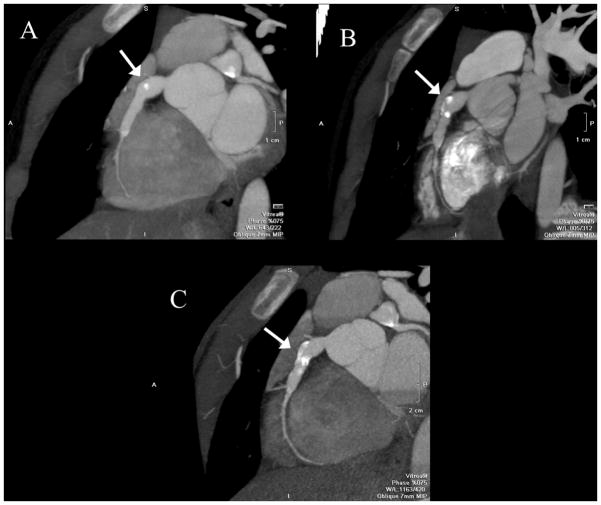
Figure 1. CT Coronary artery calcium (CAC) scans.
(A) 33 year old man with a history of Kawasaki Disease (KD) at age 4, a history of transient coronary dilation, and significant coronary calcification of the left anterior descending artery (arrows) and (B) 44 year old woman with a history of KD at age 13, giant coronary aneurysms, and a CAC scan demonstrating severe coronary calcification of the right coronary (arrowhead) and left anterior descending arteries (arrows).
An analysis of the CAC scores as a function of the time since the subject’s episode of acute KD revealed that all subjects with positive CAC scores had their acute KD at least 10 years prior to their scan (Figure 4). However, the degree of calcification did not correlate with the time since acute KD (rs = 0.05, p=0.88),
Figure 4.
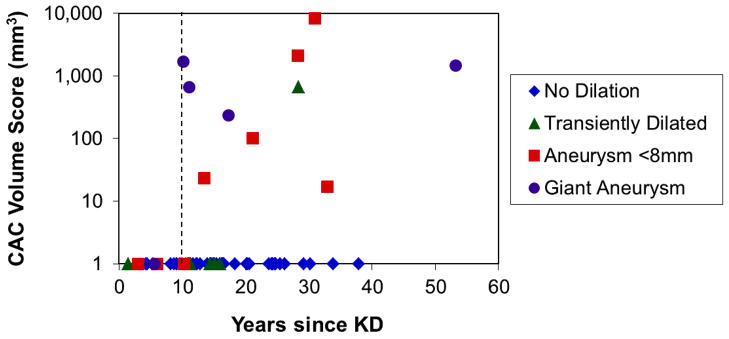
CT calcium volume scores (on a logarithmic scale) as a function of the time since the patients’ acute Kawasaki Disease (KD), coded by coronary artery status. The vertical dotted line indicates 10 years post-onset of KD.
Discussion
Patients with no acute coronary abnormalities
Every subject who had normal-appearing coronary arteries in the acute KD phase had no coronary calcification, consistent with the previous findings of Dadlani et. al(14). Subclinical pathology in such patients has been assessed by measuring coronary flow reserve, yielding mixed results with some studies showing impaired coronary flow reserve(19,20) and others showing no difference from controls(21,22). The absence of calcification in our subjects does not exclude the possibility of impaired endothelial function as well as structural damage to the arterial wall and hence some subclinical pathology. Autopsy studies have raised concerns that abnormal remodeling of the arterial wall can occur in the absence of documented changes during the acute illness(23,24). Long-term, longitudinal studies of patients with normal echocardiograms during the acute illness are needed to address this question.
Subjects with transient coronary dilation
In this study the number of subjects with transient coronary artery dilation was relatively small. Only one of these subjects had coronary calcification. However, this was associated with a significant clinical outcome requiring urgent CABG. While it is conceivable that this subject’s CAD was not due to his KD, this was a relatively young man with no risk factors for CAD, and hence this seems unlikely. Had he undergone calcium scoring, this subject could have been identified as a high risk patient and had earlier, elective intervention. In the previous study by Dadlani et. al., in which the subjects were younger and imaged sooner (mean age: 12.8 years; mean elapsed time since acute KD: 8.6 years), there were only four patients with transient dilation and none had coronary calcification(14). Given the relatively small number of patients studied, and the presence of significant calcification in one subject, this is a category that merits further study, and for which the utility and implications of CT CAC scoring remain unclear.
Subjects with Aneurysms
From our data and consistent with previous work, it seems clear that patients with aneurysms have a high likelihood of developing calcification of their coronary arteries(8,14,15). Our data suggest that it may take approximately ten years for significant calcification to develop. By comparison, in the study by Kaichi et. al., for coronary arteries with initial diameters of 6 mm or greater, the prevalence of coronary calcification was 12% at 5 years, 44% at 10 years, and 94% at 20 years(15). Once calcification does develop, in our data the CAC volumes did not correlate with the elapsed times since the initial episodes of KD.
Radiation Dose from CAC scoring
While the effects of radiation at small doses are unknown with certainty, minimizing radiation dose is desirable and considered good medical practice. Radiation doses are of particular concern in younger patients, in whom there may be greater risk from radiation(25). We used a customized protocol specifically designed to minimize the radiation administered while still providing diagnostic data. The median radiation dose of 0.59 mSv used in this study is much lower than that previously reported for CAC scoring using multidetector CT(13). By comparison, the average annual radiation dose from natural sources in the United States is approximately 3 mSv(26). Hence, on average, this examination is equivalent to just 2.4 months of natural radiation exposure, and imparts a lower dose than invasive angiography, CT angiography, and cardiac nuclear stress testing(26).
Mechanism of Calcification
The underlying mechanisms causing coronary arteries to calcify in KD patients are currently unclear; possibilities include inflammatory, immunologic, treatment-related, or hemodynamic factors, or some combination of these. Calcification may be the long-term result of damage from the initial inflammatory insult associated with coronary aneurysm formation. Alternatively, recent patient-specific modeling of hemodynamics in a patient with giant aneurysms demonstrated that the presence of aneurysms resulted in abnormal flow patterns with markedly increased flow recirculation times and reduced wall shear stress within the aneurysms(27). Correlating regions of hemodynamic abnormalities with those that develop increased calcification may help clarify the possible connection. Recently, Zhu et. al. have shown that vascular calcification is associated with up-regulation of alkaline phosphatase and the sodium-dependent phosphate transporter PiT-1, known osteocytic molecules(28). What role genetics may play and whether upregulation of these proteins is occurring in some KD patients is currently unknown. Vascular calcification has also been associated with warfarin use(29). In our study, only 5 patients were on warfarin. Although 4 of these 5 had positive CAC scores, these were also patients with aneurysms for whom 10 or more years had elapsed since their episode of acute KD. Hence, due to the small number of patients on warfarin, and the presence of confounding factors, no clear conclusions regarding the effects of warfarin use can be drawn from our study data.
Clinical Implications
The use of CT calcium scoring with a low-radiation protocol may contribute to the management of patients with a history of KD and unknown coronary artery status. In such patients, the finding of coronary calcification suggests significant coronary pathology and should prompt further clinical evaluation, typically with CT angiography to evaluate for the presence of coronary aneurysms and stenoses.
For patients with aneurysms who develop severe calcification, the clinical implications are currently unclear. In the case of atherosclerotic heart disease, high calcium scores identify patients at increased risk of cardiovascular events and mortality, probably because these patients have more extensive vascular pathology(12). However, for a given coronary lesion, CT data have shown that the lack of calcification or the presence of spotty calcification are associated with increased events, possibly because these plaques have higher lipid content and hence are more prone to rupture(30–33).
The coronary lesions in KD have a different pathophysiology than coronary atherosclerosis and their natural history into adulthood is largely unknown(34). Postmortem case report literature has documented that extensive arterial wall dystrophic calcification occurs in coronary artery aneurysms late after KD(35). A frequent cause of coronary events in KD patients is thrombus formation within aneurysms and subsequent coronary occlusion. In addition, patients may experience stenoses at the distal portions of the aneurysms. Future longitudinal clinical study will be required to determine whether the degree of calcification in patients with a history of KD is a risk factor for events, or is protective. Hence, using CT CAC scoring to assess the degree of calcification may be useful in risk stratifying patients with KD and aneurysms. However, due to the time required for calcification to develop, our data suggest that its potential utility would probably be restricted to patients for whom approximately 10 or more years have elapsed since having acute KD.
Study Strengths and Limitations
Strengths of this study include our study population, which included subjects with a range of KD pathology, ages, and elapsed time since their acute KD occurred. Also, the CT protocol used a very low radiation dose while still providing diagnostic data in all subjects. This study also has several limitations. The subjects in Cohort 2 were self-referred to this study or responded to recruiting efforts and hence may not be representative of KD patients overall. In addition, one of the subjects had stents in his coronary arteries. Because these regions are likely to be among the most diseased segments and were necessarily excluded from calcium scoring, this likely resulted in an under-estimation of the calcium scores for this subject. Finally, the relatively small sample size, particularly for the transiently dilated subjects, limits our ability to draw conclusions or make recommendations about the use of CAC scoring in this group of subjects.
Conclusion
In this study we used low dose non-contrast CT scanning to assess coronary calcification in subjects with a remote history of KD. Our primary findings were that subjects with no coronary artery dilation had no calcification, and that those with aneurysms developed significant calcification approximately ten or more years after their episode of acute KD. The pathophysiology and clinical implications of coronary calcification in this population will require further study.
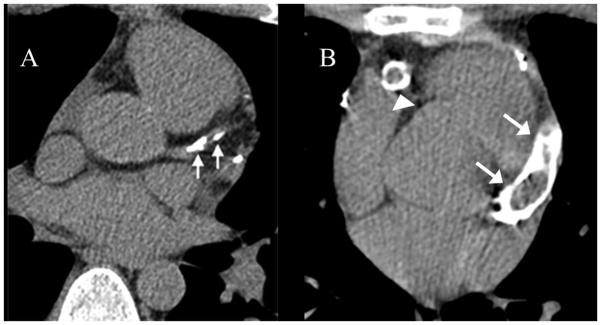
Figure 2. Serial CT angiograms in a subject with a history of Kawasaki Disease.
Serial CT angiograms in a subject with a history of Kawasaki Disease at age 3 performed at (A) 7 years (B) 8 years and (C) 10 years after KD onset. Studies show progressively increasing calcification of the right coronary artery (arrows).
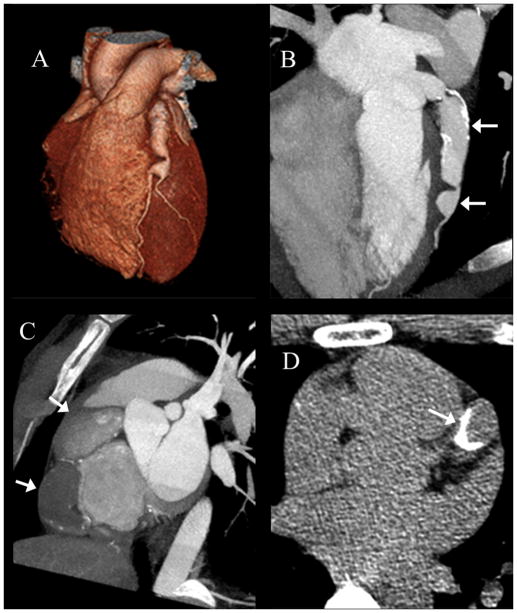
Figure 3.
CT angiogram and calcium scan on a Vietnamese male from Cohort 2 with no cardiac risk factors and a history of a Kawasaki Disease-compatible illness at age 6 years, who presented at the age of 22 years with an acute myocardial infarction and thrombotic occlusion of the right coronary artery. CT angiography performed 4 years later showed large sequential aneurysms of the left anterior descending artery (LAD) seen in 3D reconstruction (A) and oblique cross-section (B, arrows), and a 28 mm aneurysm (arrows) of the right coronary artery (C) with occlusive thrombus. (D) Shows an image from his calcium scan demonstrating significant calcification of the LAD (arrow).
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health, Heart, Lung, and Blood Institute to JCB (RO1-HL69413), a grant from the American Heart Association National Affiliate (09SDG2010231) to LBD, and a grant from the Macklin Foundation. The Ziostation workstation was provided to the University of California, San Diego, without charge, via a research agreement with Ziosoft, Inc.
Abbreviations List
- CAC
Coronary Artery Calcium
- CT
Computed Tomography
- KD
Kawasaki Disease
- CABG
Coronary Artery Bypass Surgery
- CAD
Coronary Artery Disease
- MACE
Major adverse cardiac events
- CTA
Computed Tomography angiography
- BMI
Body Mass Index
- DLP
Dose-Length Product
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119:279–82. doi: 10.1016/s0022-3476(05)80742-5. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 4.Tatara K, Kusakawa S, Itoh K, et al. Long-term prognosis of Kawasaki disease patients with coronary artery obstruction. Heart Vessels. 1989;5:47–51. doi: 10.1007/BF02058358. [DOI] [PubMed] [Google Scholar]
- 5.Senzaki H. Long-term outcome of Kawasaki disease. Circulation. 2008;118:2763–72. doi: 10.1161/CIRCULATIONAHA.107.749515. [DOI] [PubMed] [Google Scholar]
- 6.Gordon JB, Kahn AM, Burns JC. When children with kawasaki disease grow up myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiraishi I, Onouchi Z, Hayano T, Hamaoka K, Kiyosawa N. Asymptomatic myocardial infarction in Kawasaki disease: long-term prognosis. Pediatr Cardiol. 1991;12:78–82. doi: 10.1007/BF02238407. [DOI] [PubMed] [Google Scholar]
- 8.Lapierre C, Bitsch A, Guerin R, Garel L, Miro J, Dahdah N. Follow-up chest X-ray in patients with Kawasaki disease: the significance and clinical application of coronary artery macro-calcification. Pediatr Cardiol. 2010;31:56–61. doi: 10.1007/s00246-009-9548-5. [DOI] [PubMed] [Google Scholar]
- 9.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 10.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 12.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–94. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadlani GH, Gingell RL, Orie JD, et al. Coronary artery calcifications in the long-term follow-up of Kawasaki disease. Am Heart J. 2005;150:1016. doi: 10.1016/j.ahj.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Kaichi S, Tsuda E, Fujita H, et al. Acute coronary artery dilation due to Kawasaki disease and subsequent late calcification as detected by electron beam computed tomography. Pediatr Cardiol. 2008;29:568–73. doi: 10.1007/s00246-007-9144-5. [DOI] [PubMed] [Google Scholar]
- 16.Halliburton SS, Abbara S, Chen MY, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 5:198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakazato R, Dey D, Gutstein A, et al. Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr. 2009;3:394–400. doi: 10.1016/j.jcct.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Muzik O, Paridon SM, Singh TP, Morrow WR, Dayanikli F, Di Carli MF. Quantification of myocardial blood flow and flow reserve in children with a history of Kawasaki disease and normal coronary arteries using positron emission tomography. J Am Coll Cardiol. 1996;28:757–62. doi: 10.1016/0735-1097(96)00199-4. [DOI] [PubMed] [Google Scholar]
- 20.Hauser M, Bengel F, Kuehn A, et al. Myocardial blood flow and coronary flow reserve in children with “normal” epicardial coronary arteries after the onset of Kawasaki disease assessed by positron emission tomography. Pediatr Cardiol. 2004;25:108–12. doi: 10.1007/s00246-003-0472-9. [DOI] [PubMed] [Google Scholar]
- 21.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–11. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimura T, Kato H, Inoue O, Takagi J, Fukuda T, Sato N. Vasodilatory response of the coronary arteries after Kawasaki disease: evaluation by intracoronary injection of isosorbide dinitrate. J Pediatr. 1992;121:684–8. doi: 10.1016/s0022-3476(05)81893-1. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Oharaseki T, Naoe S. Pathological study of postcoronary arteritis in adolescents and young adults: with reference to the relationship between sequelae of Kawasaki disease and atherosclerosis. Pediatr Cardiol. 2001;22:138–42. doi: 10.1007/s002460010180. [DOI] [PubMed] [Google Scholar]
- 24.Naoe S, Takahashi K, Masuda H, Tanaka N. Coronary findings post Kawasaki disease in children who died of other causes. Prog Clin Biol Res. 1987;250:341–6. [PubMed] [Google Scholar]
- 25.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 26.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation BoRER, Division on Earth and Life Studies, National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation, BEIR VII, Phase 2. Washington D.C: National Academies Press; 2006. [PubMed] [Google Scholar]
- 27.Sengupta DK, AM, Burns JC, Sankaran S, Shadden S, Marsden AL. Image-based modeling of hemodynamics in coronary artery aneurysms caused by Kawasaki disease. 2011. unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu D, Mackenzie NC, Millan JL, Farquharson C, Macrae VE. The Appearance and Modulation of Osteocyte Marker Expression during Calcification of Vascular Smooth Muscle Cells. PLoS One. 6:e19595. doi: 10.1371/journal.pone.0019595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennenberg RJ, van Varik BJ, Schurgers LJ, et al. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 115:5121–3. doi: 10.1182/blood-2010-01-264598. [DOI] [PubMed] [Google Scholar]
- 30.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 31.Vancraeynest D, Pasquet A, Roelants V, Gerber BL, Vanoverschelde JL. Imaging the vulnerable plaque. J Am Coll Cardiol. 2011;57:1961–79. doi: 10.1016/j.jacc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Pundziute G, Schuijf JD, Jukema JW, et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J. 2008;29:2373–81. doi: 10.1093/eurheartj/ehn356. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa T, Yamamoto H, Horiguchi J, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2:153–60. doi: 10.1016/j.jcmg.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Miyagawa-Tomita S, Komatsu K, et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation. 2000;101:2935–41. doi: 10.1161/01.cir.101.25.2935. [DOI] [PubMed] [Google Scholar]
- 35.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J. 1979;43:633–43. doi: 10.1253/jcj.43.633. [DOI] [PMC free article] [PubMed] [Google Scholar]