Abstract
Background
Nrf2, a master regulator of the antioxidant host defense, maintains the cellular redox homeostasis.
Methods
This study was designed to investigate the role and molecular mechanisms by which Nrf2 regulates TLR4-driven inflammation response in a mouse model of hepatic warm ischemia (90min) and reperfusion (6h) injury (IRI).
Results
Activation of Nrf2 after preconditioning of WT mouse recipients with cobalt protoporphyrin (CoPP) ameliorated liver IRI, evidenced by improved hepatocellular function (sALT levels) and preserved tissue architecture (histology Suzuki’s score). In marked contrast, ablation of Nrf2 signaling exacerbated IR-induced liver inflammation and damage in Nrf2 KO hosts irrespective of adjunctive CoPP treatment. Nrf2 activation reduced macrophage/neutrophil trafficking, pro-inflammatory cytokine programs, and hepatocellular necrosis/apoptosis, while increasing anti-apoptotic functions in IR-stressed livers. At the molecular level, Nrf2 activation augmented HO-1 expression and Stat3 phosphorylation, promoted PI3K/Akt while suppressing Foxo1 signaling. In contrast, Nrf2 deficiency diminished PI3K/Akt and enhanced Foxo1 expression in the ischemic livers. In parallel in vitro studies, Nrf2 knockdown in LPS-stimulated bone marrow-derived macrophages (BMMs) decreased HO-1 and PI3K/Akt yet increased Foxo1 transcription, leading to enhanced expression of TLR4 proinflammatory mediators. Moreover, pretreatment of BMMs with PI3K inhibitor (LY294002) activated Foxo1 signaling, which in turn enhanced TLR4-driven innate responses in vitro.
Conclusions
Activation of Nrf2 promoted PI3K/Akt, and inhibited Foxo1 activity in IR-triggered local inflammation response. By identifying a novel integrated Nrf2/Akt/Foxo1 signaling network in PI3K-dependent regulation of TLR4-driven innate immune activation, this study provides the rationale for refined therapeutic approaches to manage liver inflammation and IRI in transplant recipients.
Keywords: Ischemia-Reperfusion Injury, Nrf2, TLR4, FOXO1, Innate Immunity
Introduction
Hepatic ischemia-reperfusion injury (IRI), an innate immunity-dominated local inflammation response, remains the leading cause of organ dysfunction and failure in liver transplantation. IR activates Kupffer cells, which generate reactive oxygen species (ROS) to facilitate proinflammatory cytokine/chemokine release, and cell apoptosis (1). We and others have documented TLR4-dependence of cytokine/chemokine programs required for inflammation and ultimate tissue damage in liver IRI immune cascade (2, 3).
The nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of the antioxidant cell defense system (4), is held under normal conditions in the cytoplasm through binding to Keap1 (Kelch-lke ECH-associated protein 1), and leads to its degradation by the ubiquitin proteasome pathway (5). Activation of Nrf2 promotes cell growth/survival, and contributes to cytoprotection in oxidative stress-induced injury, whereas disruption of Nrf2 enhances hyperoxia-induced acute lung injury and cell damage in oxidative stress-mediated neuroinflammation (6, 7). Moreover, disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells and antioxidant gene expression leading to cardiac hypertrophy, myocardial fibrosis and apoptosis in response to hemodynamic stress (8). Thus, Nrf2 is fundamental to the defense against oxidative stress and may be crucial in regulating inflammatory responses against oxidant stress-induced organ injury.
Forkhead box O (Foxo) family regulates multiple transcriptional targets in cell cycle, proliferation, survival and apoptosis (9). Indeed, Foxos are essential in resistance to oxidative stress through regulation of cell survival (10). The phosphorylation of Foxo by Akt blocks Foxo DNA binding domain, leading to inhibition of Foxo1 transcriptional activity (11, 12). In turn, dephosphorylation of Foxo increases its nuclear accumulation/activity, which enhances target gene expression and apoptosis (13, 14). Increasing Foxo1 activity negatively regulated cardiomyocyte proliferation (15), whereas phosphorylation of Foxo1 increased cell survival and inhibited cell apoptosis in response to oxidative stress (16). Moreover, disruption of Foxo1 reduced the expression of innate immune factors such as antimicrobial peptides and proinflammatory cytokines (17). Although these studies suggest an essential regulatory role of AKt/Foxo1 in a variety of cell functions, little is known about molecular mechanisms by which Nrf2-mediated Akt/Foxo1 signaling may regulate innate immune responses in liver IRI pathology.
Here, we identify the Nrf2/Akt/Foxo1 axis as a regulator of inflammation response in IR-stressed liver. Activation of Nrf2 decreased macrophage/neutrophil sequestration, enhanced antiapoptotic functions, and increased HO-1/Stat3 while inhibiting Foxo1 signaling, with resultant amelioration of liver IRI. This study reveals a novel PI3K-dependent crosstalk between integrated Nrf2/Akt/Foxo1 signaling network and TLR4-driven innate responses.
Results
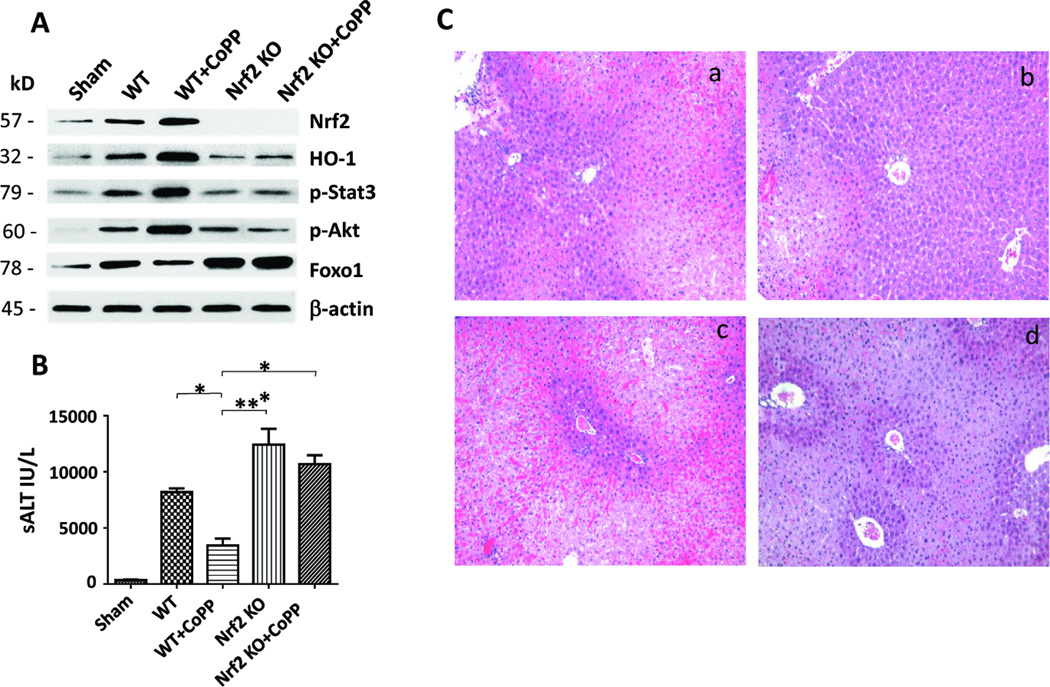
We employed an established model of hepatocellular damage in mouse livers subjected to 90min of warm ischemia and 6h reperfusion (18). First, as shown in Figure 1A, we found deletion of Nrf2 in IR-stressed livers decreased HO-1 expression (AU) as compared with WT mice (0.28±0.05 vs. 0.84±0.07, p<0.0005). Unlike in WT controls, Nrf2 deficiency reduced the Stat3 and Akt phosphorylation (0.35±0.02 vs. 0.81±0.04, p<0.0005 and 0.51±0.04 vs. 0.83±0.08, p<0.01) but increased Foxo1 expression (1.79±0.11 vs. 1.26±0.08, p<0.005). Moreover, we confirmed that preconditioning of WT mice with CoPP enhanced the expression of Nrf2 and HO-1 in IR-stressed livers as compared with untreated WT mice (1.08±0.04 vs. 0.46±0.12, p<0.005 and 1.28±0.07 vs. 0.84±0.07, p<0.0005). In contrast, Nrf2 deficiency, irrespective of adjunctive CoPP treatment, depressed HO-1 expression (0.42±0.11 vs. 1.08±0.04, p<0.0005) in IR-stressed livers. Furthermore, preconditioning with CoPP increased p-Stat3 (1.5±0.05 vs. 0.52±0.13, p<0.0005) and p-Akt (1.64±0.06 vs. 0.25±0.05, p<0.0001) but diminished nuclear total Foxo1 (0.78±0.06 vs. 1.94±0.09, p<0.0001) expression, as compared with Nrf2 deficiency with concomitant CoPP in IR-stressed livers (Fig. 1A).
Figure 1. Activation of Nrf2 promotes Akt/Foxo1 signaling, and ameliorates liver IRI (6h of reperfusion after 90min of warm ischemia).
(A) Western-assisted expression of Nrf2, HO-1, phosphorylated Stat3 (p-Stat3), p-Akt, and Foxo1 in CoPP-preconditioned WT, Nrf2-deficient and CoPP-preconditioned Nrf2-deficient livers. β-actin served as an internal control. Data representative of three experiments. (B) The hepatocellular function in distict animal groups evaluated by sALT (IU/L) levels. Mean±SD; n=4–6 mice/group. *p<0.01, **p<0.005. (C) Representative H&E staining (magnification ×100) of IR-stressed livers. (a) WT; (b) WT+CoPP; (c) Nrf2 KO; (d) Nrf2 KO+CoPP; Mean±SD; n=4–6 mice/group.
Symbols used: sham (  ); WT (
); WT (  ); WT+CoPP (
); WT+CoPP (  ); Nrf2 KO (
); Nrf2 KO (  ); Nrf2 KO+CoPP (
); Nrf2 KO+CoPP (  ).
).
CoPP-preconditioning decreased sALT levels (IU/L), compared with untreated WT controls (3450±605 vs. 9195±425, p<0.01), Nrf2 KO or Nrf2 KO+CoPP treated mice (11929±1378 and 10679±807, p<0.005; Fig. 1B). We then evaluated the severity of hepatic IRI by Suzuki’s histological grading (Fig. 1C). Unlike WT controls, which showed moderate to severe sinusoidal congestion, cytoplasmic vacuolization, and hepatocellular necrosis (Panel a; score=3.33±0.33), those pretreated with CoPP had well preserved hepatic architecture (Panel b; score=1.5±0.29, p<0.01). In marked contrast, Nrf2-deficient IR-stressed livers showed significant edema, severe sinusoidal congestion/cytoplasmic vacuolization, and extensive (30–50%) necrosis (Panel c; score=3.75±0.25, p<0.005). Similar findings were recorded in Nrf2-deficient IR-stressed livers despite CoPP-preconditioning (Panel d; score=3.4±0.25, p<0.01).
Nrf2 activation regulates macrophage/neutrophil trafficking in liver IRI
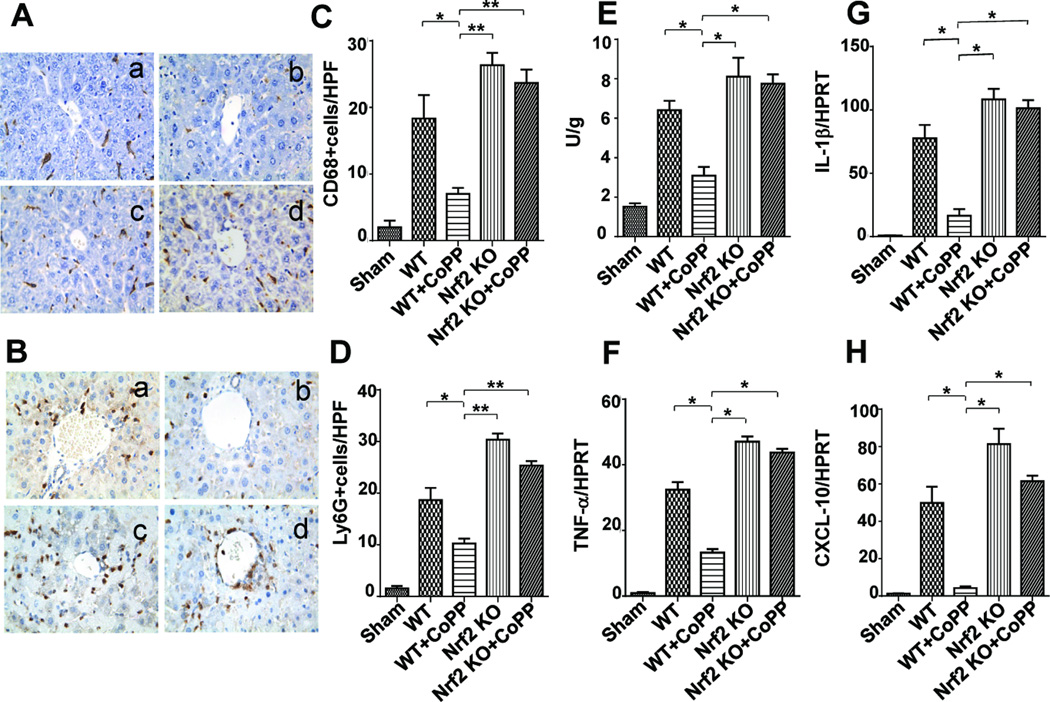
To determine whether Nrf2 activation may regulate cell trafficking, we performed immunohistochemical staining of CD68+ macrophages and Ly6G+ neutrophils in livers at 6h of reperfusion after 90 min ischemia. Indeed, Nrf2 activation decreased macrophage infiltration (Fig. 2A/C, Panel b; 7±0.91) as compared to WT controls (Panel a: 18.33±3.53, p<0.05), whereas Nrf2 deficiency augmented hepatic cell sequestration (Panel c: 26.33±1.86, p<0.001; Panel d: 23.67±2.03, p<0.001). Furthermore, CoPP-preconditioned livers showed decreased neutrophil accumulation (Fig. 2B/D, Panel b; 11.25±0.85) compared to WT (Panel a: 22±2.08, p<0.01) or Nrf2-deficient livers (Panel c: 30.33±1.2, p<0.001; Panel d: 25.33±0.88, p<0.001). The MPO assay (Fig. 2E) has revealed comparable hepatic neutrophil activity (U/g) pattern in CoPP-treated vs. WT vs. Nrf2 KO livers, respectively (3.09±0.44 vs. 6.41±0.47, p<0.005; vs. 8.11±0.06, p<0.005; vs. 7.75±0.48, p<0.005). Consistent with the immunostaining data, the mRNA levels coding for liver IRI signature genes, i.e., IL-1β, TNF-α, and CXCL-10 were consistently reduced in Nrf2-overexpressing, as compared with untreated WT or Nrf2-deficient livers (Fig. 2F–H).
Figure 2. Activation of Nrf2 regulates intrahepatic macrophage/neutrophil trafficking and inflammatory programs in liver IRI (6h of reperfusion after 90min of warm ischemia).
Immunohistochemical staining for CD68+ cells (A/C) and LY6G+ cells (B/D): (a) WT; (b) WT+CoPP; (c) Nrf2 KO; (d) Nrf2 KO+CoPP; Mean±SD; Representative of 4 mice/group; magnification ×400; (C): *p<0.05; **p<0.001; (D): *p<0.01; **p<0.001. (E) Neutrophil MPO activity (U/gm). Mean�SD (n=4–6 samples/group). *p<0.005 (F–H) Quantitative RT-PCR-assisted detection of cytokine/chemokine gene expression; Mean�SD; n=3–4/group; *p<0.005.
Symbols used: sham (  ); WT (
); WT (  ); WT+CoPP (
); WT+CoPP (  ); Nrf2 KO (
); Nrf2 KO (  ); Nrf2 KO+CoPP (
); Nrf2 KO+CoPP (  ).
).
Nrf2 activation promotes resistance to IR-hepatocellular apoptosis
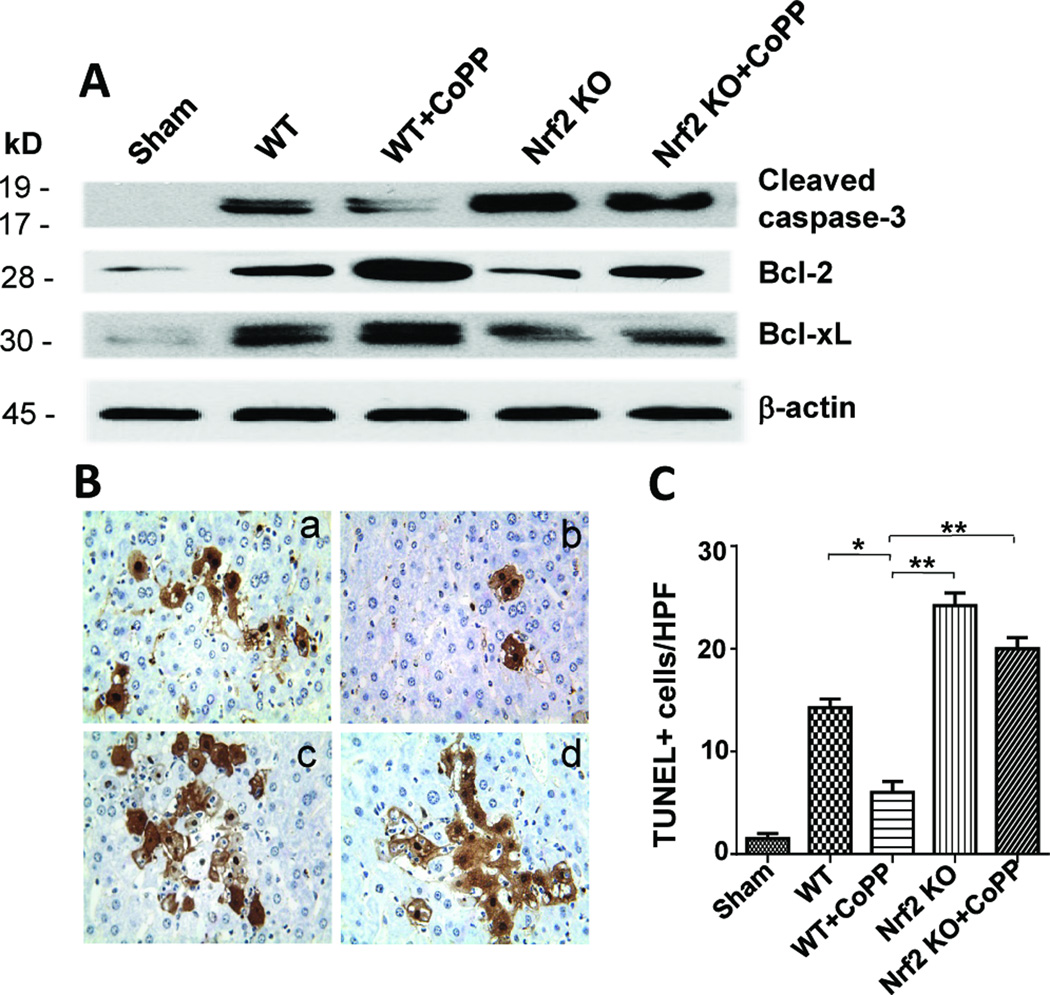
We used Western blots to analyze whether Nrf2 signaling regulates cell apoptosis in IR-stressed livers (Fig. 3A). Indeed, CoPP-induced Nrf2 activation markedly increased hepatic expression (AU) of Bcl-2 and Bcl-xl, as compared with Nrf2-deficient (1.98±0.04 vs. 0.46±0.05, p<0.001 and 1.75±0.1 vs. 0.83±0.1, p<0.001) or Nrf2-deficient + CoPP (0.52±0.19 and 0.84±0.1, respectively, p<0.0005) groups. Moreover, Nrf2 deficiency, irrespective of adjunctive CoPP, consistently augmented the expression of cleaved caspase-3 (1.38±0.16 and 1.32±0.08), compared with WT controls (0.85±0.1, p<0.01).
Figure 3. Activation of Nrf2 reduces hepatocellular apoptosis in liver IRI (6h of reperfusion after 90min of warm ischemia).
(A) Western-assisted analysis of cleaved caspase-3, Bcl-2/Bcl-xl. Representative of three experiments. (B/C) TUNEL staining. (a) WT; (b) WT+CoPP; (c) Nrf2 KO; (d) Nrf2 KO+CoPP; Mean±SD; Representative of 4 mice/group; magnification ×400 *p<0.005; **p<0.001.
Symbols used: sham (  ); WT (
); WT (  ); WT+CoPP (
); WT+CoPP (  ); Nrf2 KO (
); Nrf2 KO (  ); Nrf2 KO+CoPP (
); Nrf2 KO+CoPP (  ).
).
We further analyzed liver cell apoptosis by TUNEL staining. As shown in Fig. 3B/C, CoPP preconditioning decreased the frequency of TUNEL+ cells in ischemic liver lobes (Panel b: 6.00±1.08), as compared with WT (Panel a: 14.33±1.2, p<0.005) or Nrf2-deficient groups, which consistently showed increased numbers of TUNEL+ cells (Panel c: 22.67±1.45, p<0.001; and Panel d: 20.01±1.08, p<0.001).
Nrf2 activation promotes Akt/Foxo1 to suppress TLR4 response in vitro
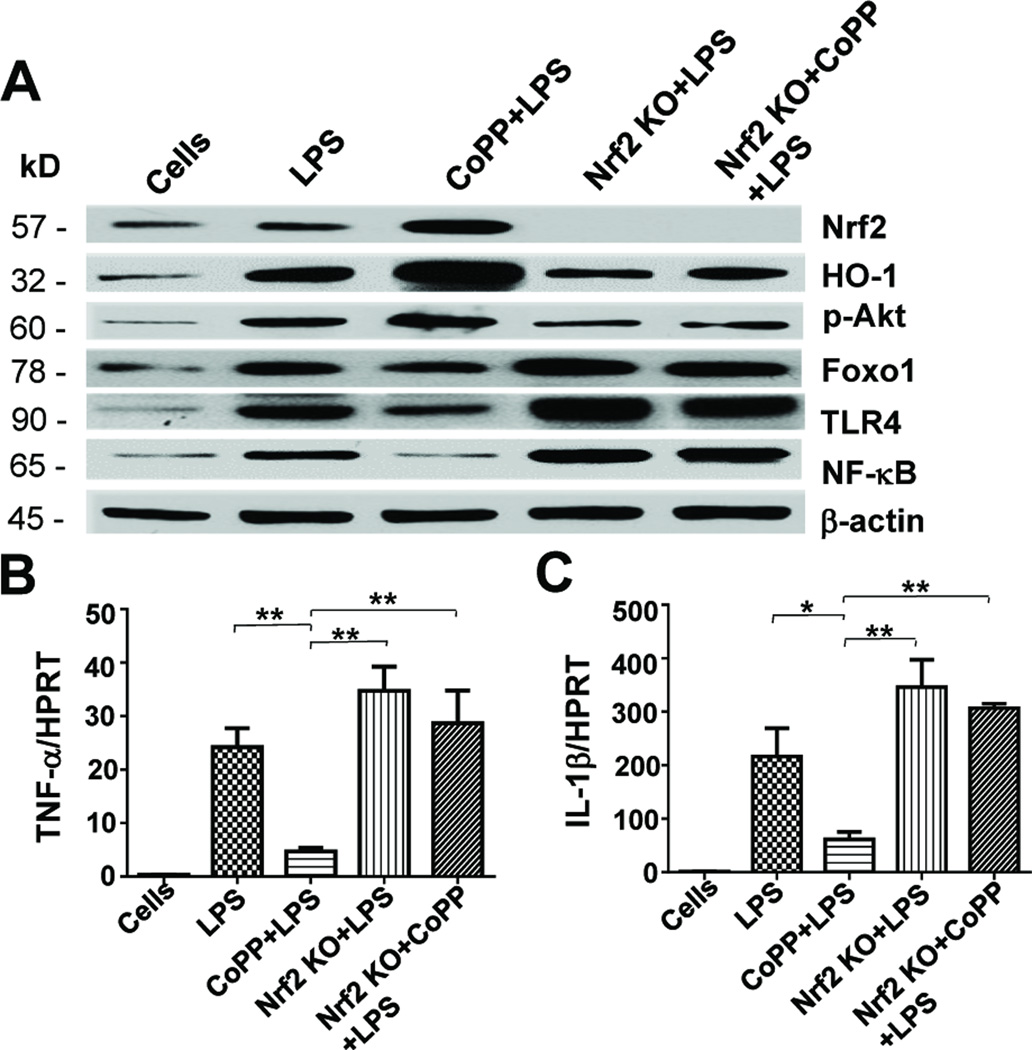
Our in vivo data shows that Nrf2 signaling activated hepatic Akt/Foxo1, which in turn diminished liver IR-inflammation. We then assessed LPS-stimulated BMM cell cultures by Western blots to test a hypothesis that Nrf2 regulates TLR4 through an Akt/Foxo1 pathway (Fig. 4A). Indeed, compared with LPS-stimulated WT BMMs, CoPP treatment induced Nrf2 activation (1.24±0.08 vs. 0.52±0.09, p<0.005) and up-regulated the expression (AU) of HO-1 (2.32±0.08 vs. 1.22±0.08, p<0.0001) and p-Akt (1.16±0.14 vs. 0.65±0.06, p<0.005) while depressing Foxo1 (0.87±0.05 vs. 1.18±0.03, p<0.001), TLR4 (0.49±0.03 vs. 1.2±0.14, p<0.001) and NF-κB (0.15±0.16 vs. 0.79±0.09, p<0.0005) levels. In contrast, Nrf2 deficiency, irrespective of adjunctive CoPP, depressed HO-1 (0.63±0.08 and 0.56±0.06, p<0.005), p-Akt (0.38±0.11 and 0.39±0.04, p<0.05) yet enhanced Foxo1 (1.48±0.02 and 1.45±0.09, p<0.05), TLR4 (1.83±0.2 and 1.72±0.2, p<0.05) and NF-κB (1.21±0.01 and 1.19±0.04, p<0.005) as compared with LPS-stimulated WT BMMs. Furthermore, the expression of both TNF-α and IL-1β markedly increased in LPS-stimulated Nrf2-deficient as compared with CoPP-conditioned WT cells (Fig. 4B–C).
Figure 4. Activation of Nrf2 regulates Akt/Foxo1 signaling and TLR4-driven innate immune activation in vitro.
Murine BMM cultures were supplemented for 4h with with LPS (100ng/ml) +/− CoPP (50μM). (A) Western-assisted expression of Nrf2, HO-1, p-Akt, Foxo1, TLR4 and NF-κB. Representative of three experiments. (B–C) Quantitative RT-PCR-assisted detection of mRNA coding for TNF-α and IL-1β. Data were normalized to HPRT gene expression. Mean±SD; n=3–4/group; *p<0.05, **p<0.005.
Symbols used: Cells (  ); LPS (
); LPS (  ); CoPP+LPS (
); CoPP+LPS (  ); Nrf2 KO+LPS (
); Nrf2 KO+LPS (  ); Nrf2 KO+CoPP+LPS (
); Nrf2 KO+CoPP+LPS (  ).
).
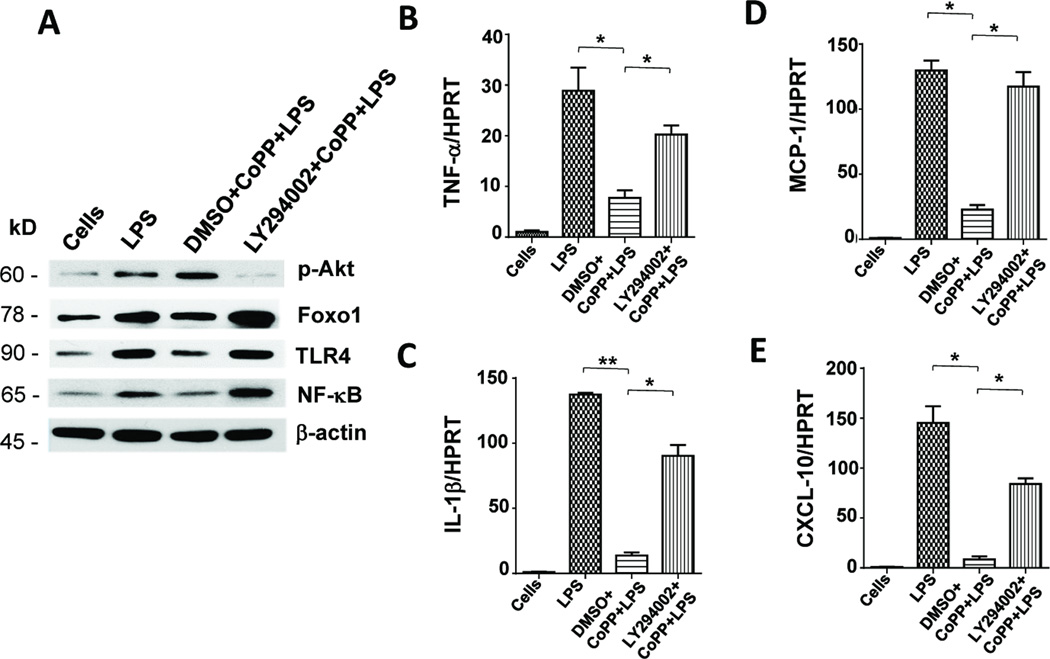
Inhibition of PI3K disrupts Akt/Foxo1 and enhances TLR4 response in vitro
As Nrf2 activation promoted Akt/Foxo1 signaling in IR-stressed livers, we then asked as to whether and how PI3K/Akt modulates Foxo1-mediated TLR4 function in vitro. LPS-stimulated BMMs were treated first with a PI3K inhibitor (LY294002) or DMSO, followed by CoPP. As shown in Figure 5A, Western blot analysis has revealed that unlike CoPP-conditioned WT cells, adjunctive inhibition of PI3K abolished the expression (AU) of p-Akt (0.1–0.2 vs. 0.5–0.6) while increasing Foxo1 (1.5–1.6 vs. 0.8–0.9), TLR4 (1.5–1.6 vs. 0.8–0.9) and NF-κB (0.8–1.0 vs. 0.2–0.3). These findings were consistent with increased TNF-α/IL-1β and MCP-1/CXCL-10 levels in BMMs subjected to PI3K blockade (Fig. 5B–E).
Figure 5. Inhibition of PI3K disrupts Akt/Foxo1 signaling and enhances TLR4-innate activation in vitro.
BMMs from WT mice were pretreated with PI3K inhibitor LY294002 or DMSO, followed by LPS +/− CoPP. (A) Western-assisted expression of Nrf2, p-Akt, Foxo1, TLR4 and NF-κB. β-actin served as an internal control. Representative of three experiments. (B–E) Quantitative RT-PCR-assisted detection of TNF-α, IL-1β, MCP-1 and CXCL-10. Mean�SD; n=3–4/group; *p<0.05, **p<0.005.
Symbols used: Cells (  ); LPS (
); LPS (  ); DMSO+CoPP+LPS (
); DMSO+CoPP+LPS (  ); LY294002+CoPP +LPS (
); LY294002+CoPP +LPS (  ).
).
Discussion
In this study, we have identified molecular mechanisms by which Nrf2 signaling modulates innate immune responses in mouse livers subjected to 90min warm ischemia and 6h reperfusion. Notably, our in vivo and in vitro findings document that CoPP-induced Nrf2 activation down-regulated TLR4-mediated inflammation response via an integrated Akt/Foxo1 signaling network in PI3K-dependent manner.
Since liver IR triggers macrophage activation and neutrophil recruitment leading to local inflammation, Nrf2 regulatory mechanisms encompass multiple immune signaling pathways. Consistent with the ability of CoPP to upregulate Nrf2 protein in human liver cells by post-transcriptional site of action (19), we found that Nrf2 activation following CoPP conditioning in vivo, increased Stat3 and Akt phosphorylation but inhibited Foxo1, consistent with diminished Stat3/Akt yet enhanced Foxo1 protein levels in Nrf2-deficient livers. It is important to note that CoPP-mediated effects in our present study were Nrf2-dependent, as global knockdown of Nrf2 consistently triggered fulminant liver IRI phenotype irrespective of adjunctive CoPP conditioning.
Foxo1 signaling is known to promote proinflammatory cytokine gene programs (20), and to regulate innate immune functions in respiratory epithelial cells (17). Moreover, we have recently shown the ability of PTEN-mediated Foxo1 to regulate inflammatory response by enhancing TLR4 activation in liver IRI (21). Our current results show that activation of Nrf2 signaling, unlike its deficiency, promoted hepatic Akt/Foxo1 signaling while decreasing macrophage/neutrophil hepatic sequestration, which further implies that Nrf2 regulates innate immune functions in IR-stressed livers. This finding complements our recent report in which hepatocyte-specific Keap1 deficiency ameliorated IRI by facilitating Nrf2 nuclear translocation and activating panels of cytoprotective genes in cold-preserved mouse liver transplants (22).
We found that Nrf2 activation in ischemic livers decreased caspase-3 but increased Bcl-2/Bcl-xL expression. In fact, the emerging evidence suggests involvement of Foxo transcription factors in intracellular apoptosis pathways (23). As downstream target of the serine/threonine protein kinase B (PKB)/Akt, phosphorylation of Foxo1 by Akt reduces its DNA-binding capacity and exports Foxo1 from nucleus to cytoplasm, where it degrades after ubiquitinylation, leading to cell survival (24). Thus, by regulating Akt/Foxo1 transcription, activation of Nrf2 promotes hepatocellular survival. Our TUNEL assay provided further evidence for increased frequency of apoptotic cells in Nrf2-deficient livers (−/+ CoPP) while decreased apoptotic cell death was readily detectable in Nrf2 overexpressing WT livers.
The innate immune response, initiated via pathogen-associated molecular patterns (PAMPs) signaling through TLRs expressed on host innate immune cells (25) can be influenced by the cellular redox state (26, 27). Nrf2 is critical for induction of hepatic glutathione S-transferase (GST) and NAD(P)H:quinine oxidoreductase (NQO1) in response to oxidative stress (28). Moreover, while increased Nrf2 activity was shown to ameliorate chronic kidney disease-associated inflammation (29), disruption of Nrf2 resulted in increased susceptibility to bacterial infection and enhanced inflammatory cytokine programs during acute lung injury (30). In agreement with the regulatory function of Nrf2 in ROS-mediated TLR4 activation in sepsis (31), our current in vivo data highlights the essential role of Nrf2 in the modulation of TLR4-driven inflammatory responses in IR-stressed livers,
Given our findings on Nrf2-mediated regulation of inflammation in IR-stressed livers, we next turned to well-controlled cell culture system to explore putative molecular mechanisms by which Nrf2 signaling may affect innate immune activation. Indeed, we have confirmed that Nrf2 activation was critically required for increased HO-1 expression in CoPP-conditioned LPS-stimulated BMMs. The immunomodulatory role of HO-1 associates with cell type-specific functions in myeloid cells (macrophages/monocytes), pivotal for inflammatory responses (32). As stress-dependent HO-1 induction is primarily mediated through the cap’n’ collar (CNC) Nrf2 (33), activation of the latter is controlled by the cytosolic inhibitor Keap1, which permits subsequent nuclear translocation of Nrf2 (34). We have recently shown the essential role of Keap1/Nrf2 axis in preventing hepatic IRI in mouse liver transplants subjected to prolonged cold storage (22). Thus, in agreement with others (35), we now document that Nrf2-dependent HO-1 induction represents an important component of anti-inflammatory innate immune network in IR-stressed livers.
Activation of nuclear Foxo1 increases the expression of antimicrobial peptide (AMP), an important class of innate effector molecules that modulate an array of defense inflammatory responses (36). Our data shows that Nrf2 activation increased Akt phosphorylation and inhibited Foxo1, TLR4, and NF-κB expression. In contrast, ablation of Nrf2 signaling depressed p-Akt, yet enhanced Foxo1, TLR4 and NF-κB, along with proinflammatory cytokine programs. Indeed, increasing Nrf2 activation regulated antioxidant myeloid leukocyte functions, and improved septic survival by balancing inflammatory responses via redox regulation of TLR4 signaling while preserving antibacterial defenses (37). Activation of Nrf2 was also found to attenuate pulmonary inflammation by regulating NF-κB activation and proinflammatory cytokine programs (38).
To further elucidate the regulatory mechanisms of Nrf2-mediated Akt/Foxo1 signaling in innate inflammation in vitro, we blocked PI3K/Akt pathway in LPS-stimulated BMMs. We found that pretreatment of BMMs with Ly294002 enhanced Foxo1 and TLR4/NF-κB activation, leading to increased proinflammatory profile. Akt is a serine-threonine kinase that is regulated via activation of the second message PI3K (39). The PI3K/Akt signaling regulates cell proliferation/ survival in part by phosphorylating Foxo (24). Increasing Foxo1 transcriptional activity enhances IL-1β/IL-2 production after LPS stimulation, whereas activation of NF-κB increases Foxo1 binding to IL-1β promoter (20), suggesting NF-κB dependence of Foxo1-mediated regulation of proinflammatory cytokines. Moreover, by binding to multiple enhancer-like elements within the TLR4 gene, Foxo1 triggers macrophage TLR4 activation (40).
In conclusion, Nrf2 activation promotes PI3K/Akt and inactivates Foxo1 signaling, which in turn inhibits TLR4 activation in the mechanism of liver IRI (Suppl. Fig. 1). By identifying a novel PI3K-dependent crosstalk between integrated Nrf2/Akt/Foxo1 signaling network and TLR4-driven innate immune activation, this study provides the rationale for refined therapeutic approaches to manage liver inflammation and IRI in transplant recipients. Of note, our present results are important in the context of conflicting recent findings on the biological effects of Nrf2 induction in the liver, i.e., benefits of Nrf2-dependent cytoprotection against IRI (41), as in our study; yet impaired hepatic regeneration in CCL4-injured livers despite constitutive Nrf2 hepatocyte overexpression (42).
Materials and Methods
Animals
Wild-type (WT) male mice (C57BL/6) at 6–8 weeks of age were used (Jackson Laboratory, Bar Harbor, ME). The Nrf2-deficient (Nrf2−/− ; KO) breeding pairs (C57BL/6) were kindly provided by Dr. T. Kensler (Johns Hopkins University, Baltimore, MD). Animals were housed in UCLA animal facility under specific pathogen-free conditions, received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
Mouse liver IRI model
We used an established mouse model of warm hepatic ischemia followed by reperfusion (18). Groups of WT and Nrf2 KO mice were injected with heparin (100U/kg) and an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the cephalad liver lobes. After 90min the clip was removed, and mice were sacrificed at 6h of reperfusion. Additional groups of WT and Nrf2 KO animals were treated with Cobalt protoporphyrin (CoPP; 5mg/kg, i.p.; Frontier Scientific Porphyrin Products, Logan, UT) 24h prior to the ischemia.
Hepatocellular function assay
Serum alanine aminotransferase (sALT) levels, an indicator of hepatocellular injury, were measured by IDEXX Laboratories (Westbrook, ME).
Histology and immunohistochemistry
Liver sections (5µm) were stained with hematoxylin and eosin (H&E). The severity of IRI was graded using Suzuki’s criteria on a scale from 0–4 (43). Liver macrophages and neutrophils were detected using primary rat anti-mouse CD68 (AbD Serotec, Raleigh, NC) and Ly6G (BD Biosciences, San Jose, CA) mAb. The secondary, biotinylated goat anti-rat IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
TUNEL assay
The Klenow-FragEL DNA Detection Kit (EMD Chemicals, Gibbstown, NJ) was used to detect the DNA fragmentation characteristic of apoptosis in formalin-fixed paraffin-embedded liver sections (44). The results were scored semi-quantitatively by averaging the number of apoptotic cells/HPF (400×magnification). Ten fields were evaluated per tissue sample.
Quantitative RT-PCR analysis
Quantitative real-time PCR was performed using the DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA). In a final reaction volume of 25µl, the following were added: 1× SuperMix (Platinum SYBR Green qPCR Kit; Invitrogen, San Diego, CA) cDNA and 10µM of each primer. Amplification conditions were: 50°C (2min), 95°C (5min), followed by 40 cycles of 95°C (15sec) and 60°C (30sec). Primers used to amplify specific gene fragments are shown in Supplementary Table 1.
Western blot analysis
Proteins (30µg/sample) from cell cultures or liver samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Monoclonal rabbit anti-mouse phos-Akt, phos-Stat3, Foxo1, NF-κB, Bcl-2, Bcl-xl, cleaved caspase-3, and β-actin Abs (Cell Signaling Technology, Danvers, MA), Polyclonal rabbit anti-mouse Nrf2 (Santa Cruz Biotechnology, Santa Cruz, CA), HO-1 (StressGen Biotech, Victoria, BC, Canada), and TLR4 (Imgenex, San Diego, CA) were used. Relative quantities of protein were determined using a densitometer and are expressed in absorbance units (AU).
Cell isolation and in vitro cultures
Murine bone marrow-derived macrophages (BMM) were generated, as described (44). Bone marrow cells were removed from the femurs and tibias of WT and Nrf2 KO mice, cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FCS and 15% L929-conditioned medium. Cells (1×106/well) cultured for 7 days were treated with CoPP (50μM) and LPS (100ng/ml) for 4h. In some experiments, cells were pretreated with PI3K inhibitor (LY294002; 10μM [Calbiochem]) or DMSO (6.5µl/ml) for 1h.
Statistical analysis
Data are expressed as mean±SD. Statistical comparisons were analyzed by Student’s t-test. Differences were statistically significant at the p-value of <0.05.
Supplementary Material
Schematic diagram of molecular mechanisms by which Nrf2-mediated Akt/Foxo1 signaling regulates innate immune responses in liver IRI. See text for details.
Acknowledgements
This work was supported by NIH Grant DK 062357; The W.M. Keck, Diann Kim and Dumont Research Foundations.
List of Abbreviations
- BMMs
bone marrow derived-macrophages
- CoPP
cobalt protoporphyrin IX
- Foxo
Forkhead box O
- HO-1
hemeoxygenase-1
- Keap1
Kelch-lke ECH-associated protein 1
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PI3K
phosphoinositide 3-kinase
- sALT
serum alanine aminotransferase
- Stat3
Signal transducer and activator of transcription 3
- TLR4
Toll-like receptor 4
- TUNEL
terminal deoxyribonucleotidyl transferase (TdT)-mediated dUTP-digoxigenin Nick End Labeling
Footnotes
Authors Contributions:
Jing Huang - participated in research design; performed most of molecular experimental testing, wrote first draft of the manuscript.
Shi Yue – performed all in vivo liver IRI, assisted with molecular testing, performed real-time PCR.
Bibo Ke – participated in research design, data analysis, and assisted with gene transfection.
Jianjun Zhu – assisted with cell culture and Western blots.
Xiu-da Shen - assisted with in vivo liver IRI experiments.
Yuan Zhai – participated in data analysis.
Masayuki Yamamoto – senior discussant; provided Nrf2 knockout mice.
Ronald W. Busuttil – senior discussant; provided partial funding.
Jerzy W. Kupiec-Weglinski – study concept, participated in research design, finalized the manuscript, obtained funding for the project.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Suppl. Table 1: Primers used in qRT-PCR.
References
- 1.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11(8):1563. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 3.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175(11):7661. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 4.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2- regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374(Pt 2):337. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278(24):21592. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 6.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol. 2009;182(11):7264. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29(11):1843. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 9.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 10.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 11.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 12.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398(6728):630. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2(1):81. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 14.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419(6904):316. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 15.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102(6):686. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286(9):7468. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler F, Hellberg J, Lepper PM, Kamyschnikow A, Herr C, Bischoff M, et al. FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J Immunol. 2013;190(4):1603. doi: 10.4049/jimmunol.1200596. [DOI] [PubMed] [Google Scholar]
- 18.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74(3):315. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in upregulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20(14):2651. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 20.Su D, Coudriet GM, Hyun Kim D, Lu Y, Perdomo G, Qu S, et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes. 2009;58(11):2624. doi: 10.2337/db09-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamo N, Ke B, Busuttil RW, Kupiec-Weglinski JW. PTEN-mediated Akt/beta-catenin/Foxo1 signaling regulates innate immune responses in mouse liver ischemia/reperfusion injury. Hepatology. 2013;57(1):289. doi: 10.1002/hep.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue S, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59(6):1200. doi: 10.1016/j.jhep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27(7):352. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 26.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11(4):397. doi: 10.1016/j.coph.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy NM, Suryanarayana V, Kalvakolanu DV, et al. Innate immunity against bacterial infection following hyperoxia exposure is impaired in NRF2-deficient mice. J Immunol. 2009;183(7):4601. doi: 10.4049/jimmunol.0901754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2- deficient mice. J Immunol. 2010;185(1):569. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206(5):1167. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274(37):26071. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 34.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287(40):33720. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, et al. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463(7279):369. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 37.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184(8):928. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang T, Tian F, Zheng H, Whitman SA, Lin Y, Zhang Z, et al. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-kappaB-mediated inflammatory response. Kidney Int. 2013 doi: 10.1038/ki.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 40.Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29(24):4223. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudoh K, Uchinami H, Yoshioka M, Seki E, Yamamoto Y. Nrf2 Activation Protects the Liver From Ischemia/Reperfusion Injury in Mice. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohler UA, Kurinna S, Schwitter D, Marti A, Schafer M, Hellerbrand C, et al. Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cell cycle control and apoptosis. Hepatology. 2013 doi: 10.1002/hep.26964. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55(6):1265. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56(2):359. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic diagram of molecular mechanisms by which Nrf2-mediated Akt/Foxo1 signaling regulates innate immune responses in liver IRI. See text for details.