Abstract
Methionine- and choline-deficient diet (MCD) is a model for nonalcoholic steatohepatitis (NASH) in rodents. However, the mechanism of NASH development by dietary methionine/choline deficiency remains undetermined. To elucidate the early metabolic changes associated with MCD-NASH, serum metabolomic analysis was performed using mice treated with MCD and control diet for three days and one week, revealing significant increases in oleic and linoleic acids after MCD treatment. These increases were correlated with reduced body weight and white adipose tissue (WAT) mass, increased phosphorylation of hormone-sensitive lipase, and up-regulation of genes encoding carboxylesterase 3 and β2-adrenergic receptor in WAT, indicating accelerated lipolysis in adipocytes. The changes in serum fatty acids and WAT by MCD treatment were reversed by methionine supplementation, and similar alterations were detected in mice fed a methionine-deficient diet (MD), thus demonstrating that dietary methionine deficiency enhances lipolysis in WAT. MD treatment decreased glucose and increased fibroblast growth factor 21 in serum, thus exhibiting a similar metabolic phenotype as the fasting response. Comparison between MCD and choline-deficient diet (CD) treatments suggested that the addition of MD-induced metabolic alterations, such as WAT lipolysis, to CD-induced hepatic steatosis promotes liver injury. Collectively, these results demonstrate an important role for dietary methionine deficiency and WAT lipolysis in the development of MCD-NASH.
Keywords: fasting response, linoleic acid, lipolysis, metabolomics, oleic acid, choline deficiency
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), defined by the presence of hepatic steatosis regardless of no ethanol consumption, is a common chronic liver disease that is increasing worldwide [1]. NAFLD is classified into two categories, simple steatosis (SS) and nonalcoholic steatohepatitis (NASH), according to the liver histology [2]. The clinical course and outcome differ between SS and NASH, where SS has a benign clinical course, while NASH can develop into liver cirrhosis, hepatic failure, and hepatocellular carcinoma [3]. Several studies to clarify the pathogenesis of NASH using animal models and clinical trials regarding the treatment of NASH have been published [4–7]. The pathogenesis of NASH as a two-hit model was proposed in 1998 and has been widely accepted [8]. The first hit is singular triglyceride (TG) accumulation in hepatocytes resulting in hepatic steatosis and increasing the sensitivity of the liver to the second hits that causes hepatocyte damage, inflammation, and fibrosis. Considering clear differences in the clinical features between steatosis and steatohepatitis, it is of great value to clarify the mechanism of the progression from steatosis to steatohepatitis.
Methionine- and choline-deficient diet (MCD) is a conventional and useful model to induce NASH in rodents. In 1942, Gyorgy and Goldblatt reported that supplementation of choline or methionine reduced the incidence of rats having hepatic steatosis, hepatocyte necrosis, and cirrhosis induced by low casein diet, showing an important role of these nutrients in the pathogenesis of nutritional liver injury [9]. Mice treated with MCD show macrovesicular steatosis, hepatitis, hepatocyte ballooning, and enhancement of pro-inflammatory cytokines and oxidative stress in the liver within a few weeks, and the longer treatment results in hepatic fibrosis [10]. These pathologies are similar to those found in human NASH. Although this model is not accompanied by obesity and insulin resistance, such close similarities prompt the use of MCD for determining the mechanism of NASH. It is recognized that choline-deficient diet (CD) treatment causes hepatic steatosis in mice, mainly due to impaired secretion of very-low-density lipoprotein (VLDL) from the liver [11]. However, the mechanism on how dietary methionine/choline deficiency contributes to the NASH development is not fully understood.
Metabolomics using ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOFMS) is a useful method to detect global metabolic alterations in the physiological/pathological conditions in an unbiased manner [12]. Previous studies revealed alterations in phospholipid and bile acid metabolism in mouse models of NASH as a result of enhanced inflammatory signaling [10]. However, the early metabolic changes related to the occurrence of MCD-NASH were not determined. In this study, serum metabolomic analysis of mice treated with MCD for three days and one week was conducted using UPLC-ESI-QTOFMS. Serum metabolomics uncovered significant increases in oleic and linoleic acids after the MCD treatment due to enhanced lipolysis in white adipose tissue (WAT). Increased WAT lipolysis was caused by dietary methionine deficiency. Comparison between MCD and CD treatments suggested that the addition of metabolic changes caused by dietary methionine deficiency, such as enhanced WAT lipolysis, to CD-induced hepatic steatosis promotes liver injury. Induction of lipolysis by a 36-hour fasting to CD-treated mice resulted in the increases in serum alanine aminotransferase (ALT) levels, an indicator of hepatocyte injury. These findings provide new insight into the role of dietary methionine deficiency and WAT lipolysis in the progression to MCD-NASH.
2. Methods
2.1. Mice and treatment
All studies were conducted according to Institute of Laboratory Animal Resource guidelines and approved by the National Cancer Institute Animal Care and Use Committee. The mice were housed in a specific pathogen-free environment controlled for temperature and light (25 °C, 12-hour light/dark cycle) and maintained with NIH31 regular chow and tap water ad libitum. In all experiments, male C57BL/6NCr wild-type mice at 8–12 weeks of age were used. The MCD, CD, and methionine-deficient diet (MD) were purchased from Dyets Inc. (#518810, #518753, and #518896, respectively; Bethlehem, PA) and methionine- and choline-supplemented MCD diet (MCS, #518754; Dyets) was used as a control diet. The composition of these diets was shown in Supplementary Table 1. Before starting the experiments, the NIH31 chow was replaced with MCS for acclimatization. After acclimatization for three to five days, mice were moved to new cages and the respective diet was given. For the analysis of changes in serum metabolites and phenotypes after 3-day and 1-week MCD treatment, sera, epididymal white adipose tissues (eWAT), and liver tissues obtained from the mice reported previously [10] were used (n = 5/group). To assess the effect of MCD treatment on other WAT depots, mice were treated with MCS or MCD for one week (n = 7–8/group) and inguinal WAT, perirenal WAT, and mesentery were harvested. To examine the effect of methionine or choline supplementation on MCD treatment, mice were treated with (1) MCS with drinking deionized water; (2) MCD with drinking deionized water; (3) MCD with drinking deionized water containing choline bitartrate (30 mg/mL; Sigma-Aldrich, St. Louis, MO); and (4) MCD with drinking deionized water containing L-methionine (4 mg/mL; Sigma-Aldrich) for two weeks as described previously [10] (n = 5/group). To assess the influence of dietary deficiency of methionine or choline on the pathogenesis of MCD-NASH, mice were treated with MCD, CD, MD, or control MCS diet for two weeks (n = 4–7/group). For the fasting study, mice (n = 20) were treated with CD or MCS and randomly divided into two groups at two weeks after commencing the treatment. Mice in one group were fasted for 36 hours before killing and mice in the other group were continuously fed CD or MCS (n = 5/group). Throughout all experiments, mice were weighed and killed after a 6-hour fasting. Blood was collected using Serum Separator Tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and centrifuged for 10 minutes at 8,000 g at 4 °C to isolate serum. Liver and WAT were isolated, weighed, and divided into the two parts. One part of the liver tissue and WAT from each mouse was immediately soaked in 10% neutral formalin for histological examination. Sera and the remaining liver and WAT were immediately frozen in liquid nitrogen and kept at −80 °C until use.
2.2. UPLC-ESI-QTOFMS analysis
Serum (6 μL) was diluted with 114 μL of 66% acetonitrile containing 5 μM of chlorpropamide as an internal standard and centrifuged twice at 18,000 g for 25 min at 4 °C to eliminate precipitated proteins. The eluted sample (5 μL/injection) was introduced by electrospray ionization into the mass spectrometer [Q-TOF Premier® (Waters Corp., Milford, MA)] operating in either negative or positive electrospray ionization modes. All samples were analyzed in a randomized fashion to avoid complications due to artifacts related to injection order and changes in instrument efficiency. MassLynx software (Waters) was used to acquire the chromatogram and mass spectrometric data [10].
Centroided and integrated chromatographic mass data were processed by MarkerLynx (Waters) to generate a multivariate data matrix. Pareto-scaled MarkerLynx matrices including information on sample identity were analyzed by principal component analysis (PCA) and supervised orthogonal projection to latent structure (OPLS) analysis using SIMCA-P +12 (Umetrics, Kinnelon, NJ). The OPLS loading scatter S-plot was used to determine the ions that contributed significantly to the separation between MCD- and MCS-treated mice. The identity of ions with a correlation of 0.8 or higher to the model was further investigated. The identity of ions was confirmed by tandem mass spectrometry MS/MS fragmentation patterns.
2.3. Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from liver tissue and WAT using a TRIzol Reagent (Invitrogen, Carlsbad, CA) and cDNA was generated from 1 μg RNA with a SuperScript II™ Reverse Transcriptase kit and random oligonucleotides (Invitrogen). Quantitative PCR was performed using SYBR green PCR master mix and ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The primer pairs were designed using qPrimerDepot (http://mouseprimerdepot.nci.nih.gov/) and listed as Supplementary Table 2. Measured mRNA levels were normalized to those of 18S ribosomal RNA and expressed as fold change relative to those of control mice.
2.4. Histological analysis
Small pieces of liver tissue and WAT were fixed in 10% neutral formalin, dehydrated by serial ethanol/xylene, and embedded in paraffin. Sections (4 μm thick) were stained by the hematoxylin and eosin method. At least three discontinuous sections were evaluated for each mouse.
2.5. Biochemical analysis
Serum levels of ALT, non-esterified fatty acid (NEFA), and glucose were measured with assay kits for ALT (Catachem, Bridgeport, CT), NEFA-HR(2) reagent (Wako Chemicals USA, Inc., Richmond, VA), and glucose (HK) (Sigma-Aldrich), respectively. Serum fibroblast growth factor 21 (FGF21) levels were assayed by use of the Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN). Hepatic TG contents were quantified using Wako TG kit (Wako Chemicals USA Inc.) as described elsewhere [13]. Measurement of protein concentrations was carried out using BSA™ protein assay kit (Thermo Scientific, Rockford, IL).
2.6. Immunoblot analysis
Approximately 40 mg of WAT was homogenized in RIPA buffer containing a proteinase inhibitor cocktail. The homogenates were centrifuged at 10,000 g for 10 min at 4 °C to obtain lipid-free cytosolic extracts. Cytosolic extracts (30 μg of protein) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% bovine serum albumin or skim milk and incubated overnight with primary antibodies against hormone-sensitive lipase (HSL; Cell Signaling Technology, Inc., Danvers, MA, #4107, 1:1000 dilution), phosphorylated HSL (Cell Signaling, #4139, 1:1000 dilution), and adipose TG lipase (ATGL; Cell Signaling, #2439, 1:1000 dilution). The antibody against β-actin (abcam, Cambridge, MA. ab8227, 1:10000 dilution), was used to measure β-actin as the loading control. After three-times washing, the blots were incubated with peroxidase-conjugated goat anti-rabbit IgG (Cell Signaling, #7074, 1:3000 dilution) and scanned.
2.7. Statistical analysis
Quantitative data were expressed as mean ± standard deviation. Statistical analyses were performed using the two-tailed Student’s t-test between the two groups and the one-way ANOVA test with Bonferroni’s correction between the multiple groups, respectively. A p value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Serum metabolomic analysis reveals significant increases in oleic and linoleic acids in early stage of MCD-NASH
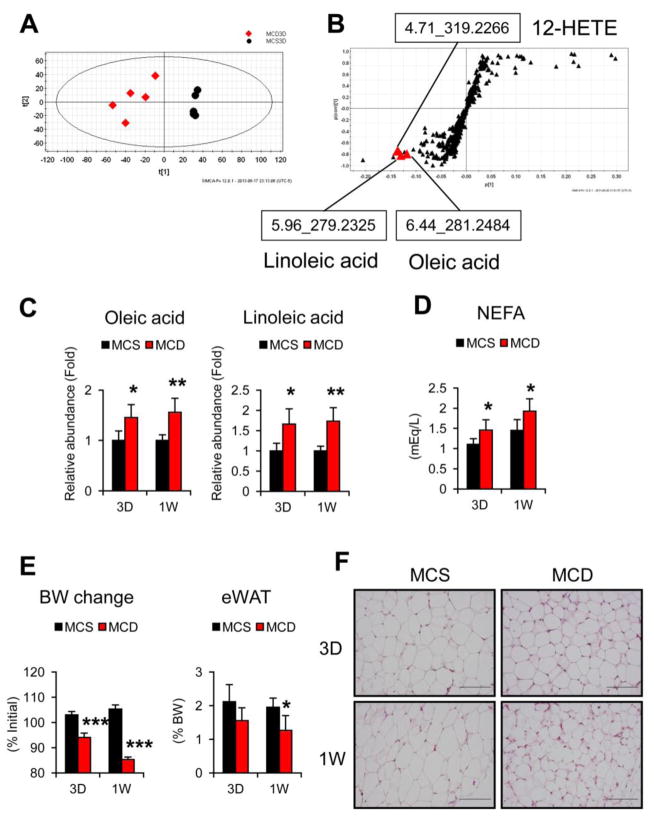
Mice treated with MCD for three days and one week showed minimal to mild hepatic steatosis and inflammatory cell infiltration, and the mRNAs encoding F4/80 (EGF-like module containing, mucin-like, hormone receptor-like sequence 1, Emr1) and integrin alpha M (Itgam), which are expressed in macrophages and infiltrated inflammatory cells, were increased (Supplementary Fig. 1). Hepatic TG contents and serum ALT levels were increased even after a short 3-day MCD treatment [10]. To examine the initial metabolic changes associated with the occurrence of MCD-NASH, a metabolomic analysis was carried out using sera obtained from the mice treated with MCD or MCS for three days and one week. PCA of UPLC-ESI-QTOFMS negative mode data demonstrated clear discrimination between the MCD and MCS groups (Fig. 1A and Supplementary Fig. 2A). OPLS analysis also showed separation between the two groups (data not shown) that was further examined with S-plot loading scatter contribution analysis. This revealed three ions that were significantly increased after both 3-day and 1-week MCD treatment: linoleic acid (m/s 279.2325 with retention time 5.96 s), oleic acid (m/s 281.2484 with retention time 6.44 s), and 12-hydroxyeicosatetraenoic acid (12-HETE) (m/s 281.2484 with retention time 6.44 s) (Fig. 1B and Supplementary Fig. 2B). Because the increases in 12-HETE in sera of MCD-fed mice were reported previously [10], the present study focused on the changes in serum oleic and linoleic acids. The identities of oleic and linoleic acids were confirmed by MS/MS analysis (Supplementary Fig. 2C). Quantification of serum oleic and linoleic acid levels and measurement of NEFA concentrations revealed significant increases in these fatty acids (FA) in early stage of MCD-NASH (Fig. 1C and D).
Fig. 1. Serum metabolomic analysis reveals significant increases in oleic and linoleic acids in early stage of MCD-NASH.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were fed a methionine- and choline-deficient diet (MCD) or control methionine- and choline-supplemented MCD diet (MCS) for three days or one week (n = 5/group).
(A) PCA of serum metabolites between mice treated with 3-day MCD (red diamond) and MCS (black circle).
(B) S-plot of OPLA analysis using the same data as (A). Retention time and molecular mass were indicated.
(C) Serum levels of oleic and linoleic acids. Values were normalized to those of MCS-treated mice in each time point and were expressed as relative abundance.
(D) Serum levels of NEFA.
(E) Body weight (BW) change and epididymal WAT (eWAT) weight. BW was measured just prior to killing and BW changes were expressed as the percentage relative to BW just before commencing the MCD or MCS treatment.
(F) Histology of epididymal WAT. Hematoxylin and eosin staining, Bar = 100 μm. Statistical analysis was performed using the Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs. MCS-treated mice in the same time point.
3.2. Increases in serum oleic and linoleic acids are associated with enhanced FA release from WAT
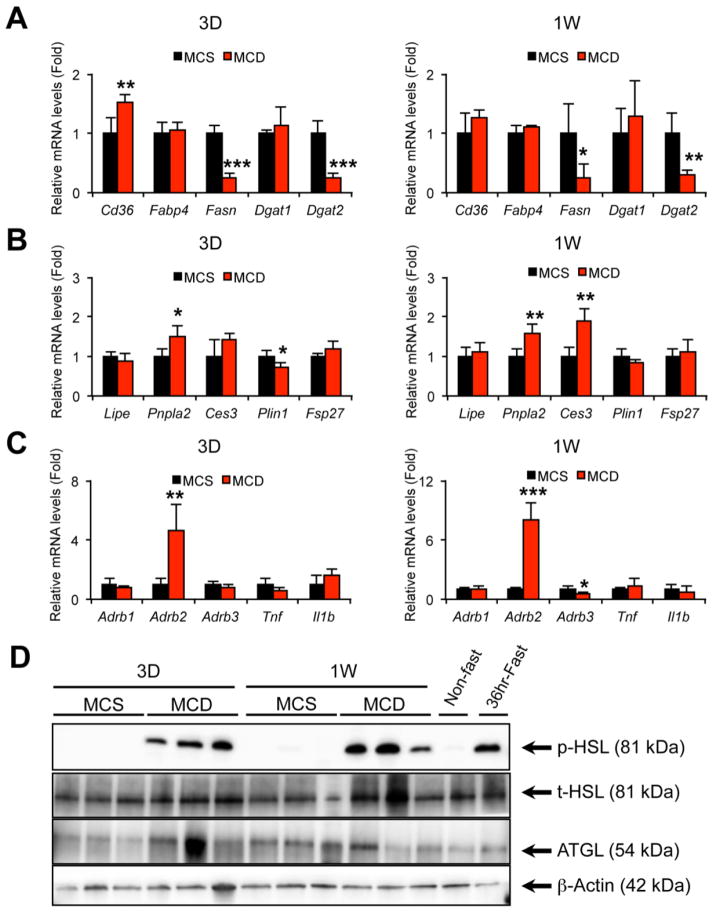
Since WAT is a major source of FA in serum, early changes in WAT after MCD treatment were examined. Body weight and eWAT weight were markedly decreased (Fig. 1E) and histological appearance of eWAT demonstrated a reduction in adipocyte size with no apparent inflammatory cell infiltration and adipocyte browning (Fig. 1F). In order to assess the mechanism of changes in WAT caused by MCD treatment, the mRNA levels of genes associated with lipid metabolism were measured. The mRNAs encoding genes related to lipogenesis, such as fatty acid synthase (Fasn) and diacylglycerol O-acyltransferase 2 (Dgat2), were suppressed in mice fed a MCD (Fig. 2A). In addition, the mRNAs encoding ATGL (patatin-like phospholipase domain containing 2, Pnpla2) and carboxylesterase 3 (Ces3), involved in lipolysis, were significantly increased (Fig. 2B), and immunoblot analysis revealed marked increases in phosphorylated HSL in these mice (Fig. 2D). These results indicate acceleration of adipose lipolysis by MCD feeding. The lipolytic activity in WAT is enhanced by multiple factors, such as β-adrenergic receptor activation and inflammatory signaling, such as tumor necrosis factor α (TNFα) and interleukin 1β (Il-1β)[14]. The mRNAs encoding β2-adrenergic receptor (Adrb2) gene were up-regulated by MCD treatment, while Tnf and Il1b mRNA levels were not changed (Fig. 2C).
Fig. 2. Quantitative PCR analysis of the genes associated with lipid metabolism and immunoblot analysis of lipolytic enzymes in the epididymal WAT of mice treated with MCD for 3 days or 1 week.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with MCS or MCD for three days or one week (n = 5/group) and epididymal WAT was subjected to qPCR and immunoblot analyses.
(A–C) qPCR analysis. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice. Statistical analysis was performed using the Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs. MCS-treated mice in the same time point. Full terms of the gene names were listed in Supplementary Table 2.
(D) Immunoblot analysis of phosphorylated and total hormone-sensitive lipase (p-HSL and t-HSL, respectively) and adipose triglyceride lipase (ATGL). Cytosolic extracts of WAT (30 μg of protein) were loaded in each well. The band of β-actin was used as a loading control. Epididymal WAT isolated from a 36 hour-fasted mouse was used for detecting the true position of HSL and ATGL bands.
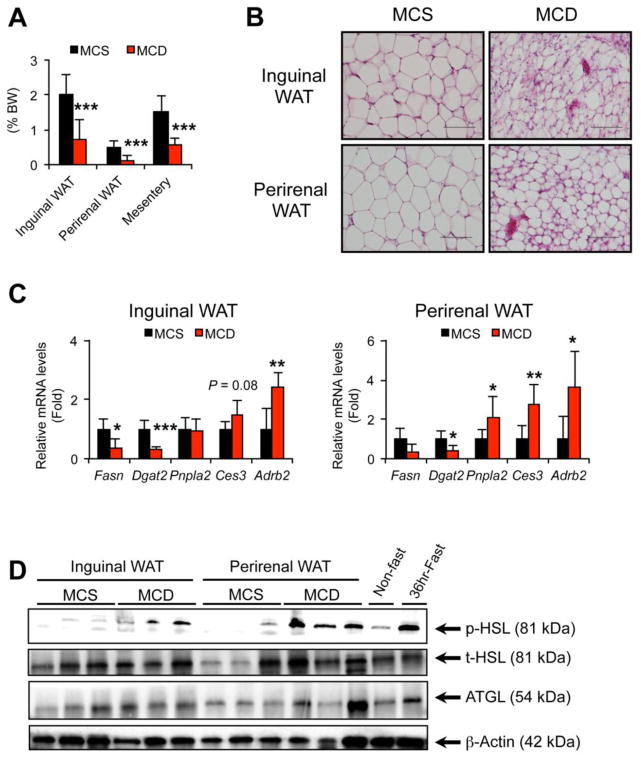
The effect of MCD treatment on other WAT depots was also examined. The weights of inguinal and perirenal WAT and mesentery were significantly decreased after 1-week MCD treatment (Fig. 3A) and histological appearance of inguinal and perirenal WAT revealed a marked reduction in adipocyte size (Fig. 3B). The mRNAs encoding Fasn, Dgat2, Pnpla2, Ces3, and Adrb2 in inguinal and perirenal WAT showed similar changes to those in eWAT of the MCD-fed mice (Fig. 3C). Additionally, phosphorylation of HSL was significantly enhanced in inguinal and perirenal WAT of MCD-treated mice (Fig. 3D). Overall, these results clearly demonstrate that WAT lipolysis is markedly enhanced by MCD treatment and that serum FA increases in early stages of MCD-NASH are correlated with increased lipolytic activity in WAT.
Fig. 3. The effect of MCD treatment on other WAT depots.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with MCS or MCD for one week (n = 7–8/group) and inguinal WAT, perirenal WAT, and mesentery were harvested.
(A) The weights of inguinal and perirenal WAT and mesentery.
(B) Histology of inguinal and perirenal WAT. Hematoxylin and eosin staining, Bar = 100 μm.
(C) qPCR analysis. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice. Statistical analysis was performed using the Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs. MCS-treated mice.
(D) Immunoblot analysis of p-HSL, t-HSL, and ATGL. Cytosolic extracts of WAT (30 μg of protein) were loaded in each well. The band of β-actin was used as a loading control.
3.3. Increases in serum oleic and linoleic acids and changes in WAT by MCD treatment are due to dietary methionine deficiency
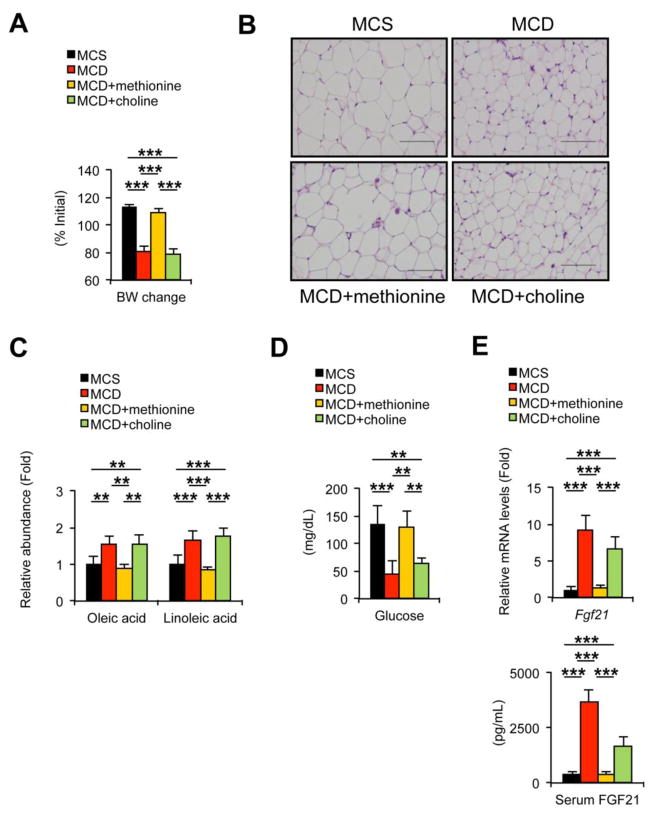
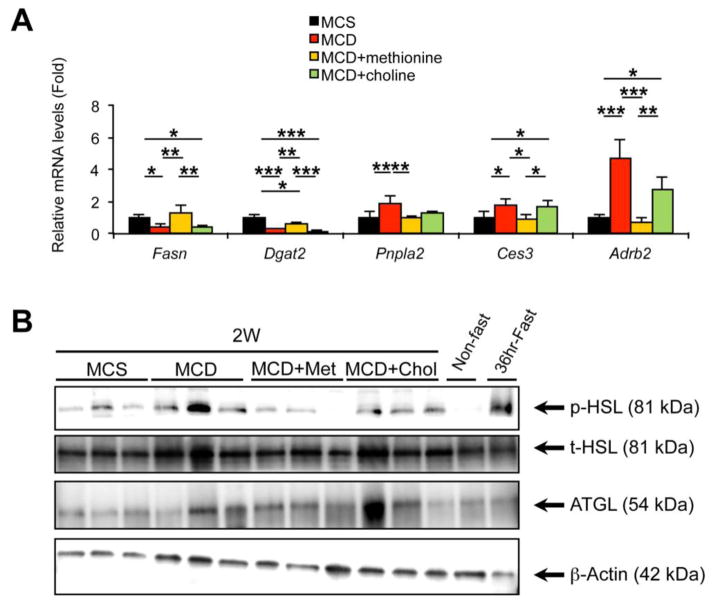
To determine the contribution of dietary deprivation of methionine and choline to the changes in serum FA and WAT following MCD treatment, methionine or choline was supplemented to MCD-fed mice as described previously [10]. No significant differences in food consumption were noted between the groups (Supplementary Fig. 3A). Mice fed the MCD for two weeks showed histologically-confirmed steatohepatitis (Supplementary Fig. 3B and C) and similar changes in WAT and serum FA to 3-day and 1-week MCD feeding, i.e., decreased body weight, eWAT weight, and adipocyte size, increased serum oleic and linoleic acids, and increased Ces3 and Adrb2 mRNAs and HSL phosphorylation in WAT; (Fig. 4A–C, Fig. 5, and Supplementary Fig. 3D). These changes in serum FA and WAT following the MCD treatment were completely reversed by supplementation of methionine, but not choline (Fig. 4A–C, Fig. 5, and Supplementary Fig. 3D). Additionally, choline supplementation to MCD-fed mice, which mimics the condition of dietary methionine deficiency, markedly attenuated steatohepatitis, as evidenced by liver histology and hepatic Emr1 and Itgam mRNA expression (Supplementary Fig. 3B and C), but it could not normalize the changes in serum FA and WAT (Fig. 4A–C). Hydrolysis of phospholipids and chylomicron/lipoproteins is another possible source of FA in serum [15]. Although the mRNAs encoding lecithin cholesterol acyltransferase (Lcat) and lipoprotein lipase (Lpl) were increased by 3-day and 1-week MCD treatment, respectively (Supplementary Fig. 4A), there was no meaningful association between the increases in serum oleic and linoleic acids and hepatic mRNAs encoding Lcat and Lpl (Supplementary Fig. 4B). Overall, these findings reveal a relationship between increased serum FA and enhanced WAT lipolysis upon MCD treatment. Also, these results suggest that the changes in serum FA and WAT following MCD treatment are due to dietary methionine deprivation, and not choline deprivation and hepatic/adipose inflammation.
Fig. 4. Increases in serum oleic and linoleic acids and changes in WAT by MCD treatment are reversed by methionine supplementation.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with MCS with drinking deionized water, MCD with drinking deionized water, MCD with drinking deionized water containing L-methionine (4 mg/mL, MCD+methionine), or MCD with drinking deionized water containing choline bitartrate (30 mg/mL, MCD+choline) for two weeks (n = 5/group) and serum, liver, and epididymal WAT were collected.
(A) Body weight (BW) change. Values were expressed as the percentage relative to BW just before commencing the MCD or MCS treatment.
(B) Histology of epididymal WAT. Hematoxylin and eosin staining, Bar = 100 μm.
(C) Serum levels of oleic and linoleic acids. Values were normalized to those of MCS-treated mice and were expressed as relative abundance.
(D) Serum glucose concentrations.
(E) Hepatic Fgf21 mRNA levels and serum FGF21 concentrations. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice.
Statistical analysis was performed using the one-way ANOVA test with Bonferroni’s correction. *, P<0.05; **, P<0.01; ***, P<0.001.
Fig. 5. The effect of supplementation of methionine or choline on gene/protein expression in the WAT of MCD-treated mice.
Epididymal WAT isolated from mice shown in Fig. 4 was subjected to qPCR and immunoblot analyses.
(A) qPCR analysis of the indicated genes. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice. Statistical analysis was performed using the one-way ANOVA test with Bonferroni’s correction. *, P<0.05; **, P<0.01; ***, P<0.001.
(B) Immunoblot analysis of p-HSL, t-HSL, and ATGL. Cytosolic extracts of WAT (30 μg of protein) were loaded in each well. The band of β-actin was used as a loading control. Met, methionine; Chol, choline.
3.4. MD treatment induces WAT lipolysis similar to MCD treatment
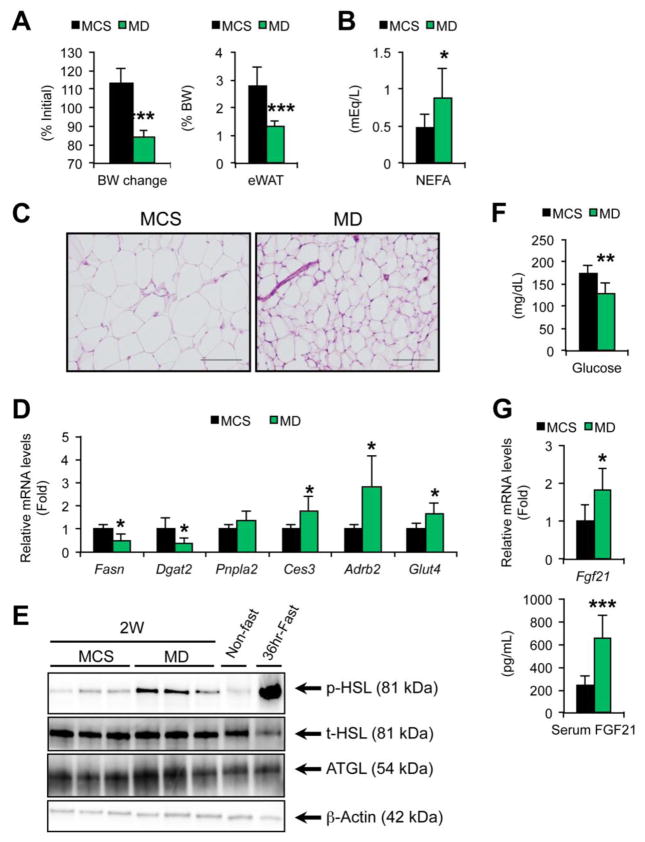
To verify whether dietary methionine deficiency induces WAT lipolysis and increases serum FA, mice were treated with MD or control MCS diet for two weeks. The body weight and eWAT mass were markedly decreased and serum NEFA concentrations were increased by MD treatment (Fig. 6A and B). Histological appearance of eWAT showed a marked decrease in adipocyte size without inflammatory cell infiltration (Fig. 6C). The levels of Ces3 and Adrb2 mRNAs and phosphorylated HSL were significantly increased in WAT of MD-fed mice (Fig. 6D and E). These results corroborate that dietary methionine deficiency enhances WAT lipolysis.
Fig. 6. MD treatment causes similar metabolic alterations to MCD treatment.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with methionine-deficient diet (MD) or control MCS diet for two weeks (n = 7/group) and serum, liver, and epididymal WAT (eWAT) were harvested.
(A) Body weight (BW) change and eWAT weight. BW change values were expressed as the percentage relative to BW just before commencing the MD or MCS treatment.
(B) Serum NEFA levels.
(C) Histology of eWAT. Hematoxylin and eosin staining, Bar = 100 μm.
(D) The mRNA levels of indicated genes in eWAT. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice.
(E) Immunoblot analysis of p-HSL, t-HSL, and ATGL. Cytosolic extracts of WAT (30 μg of protein) were loaded in each well. The band of β-actin was used as a loading control.
(F) Serum glucose concentrations.
(G) Hepatic Fgf21 mRNA levels and serum FGF21 concentrations. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice.
Statistical analysis was performed using the Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs. MCS-treated mice.
3.5. MD treatment decreases serum glucose concentrations and increases FGF21 levels
In addition to decreased body weight and WAT mass and increased serum FA, significant decreases in serum glucose and increases in hepatic Fgf21 mRNA and serum FGF21 were noted in mice with MCD treatment that were all reversed by methionine supplementation (Fig. 4D and E). Similar changes in serum glucose and FGF21 were also detected in MD-treated mice (Fig. 6F and G). The mRNA encoding glucose transporter type 4 (Glut4, also called solute carrier family 2, member 4, Slc2a4) was increased in WAT of mice on MD (Fig. 6D) that might be associated with a reduction in serum glucose concentrations in these mice. These results suggest that MD treatment causes similar metabolic changes as the fasting response.
3.6. Role of MD in the progression of MCD-NASH
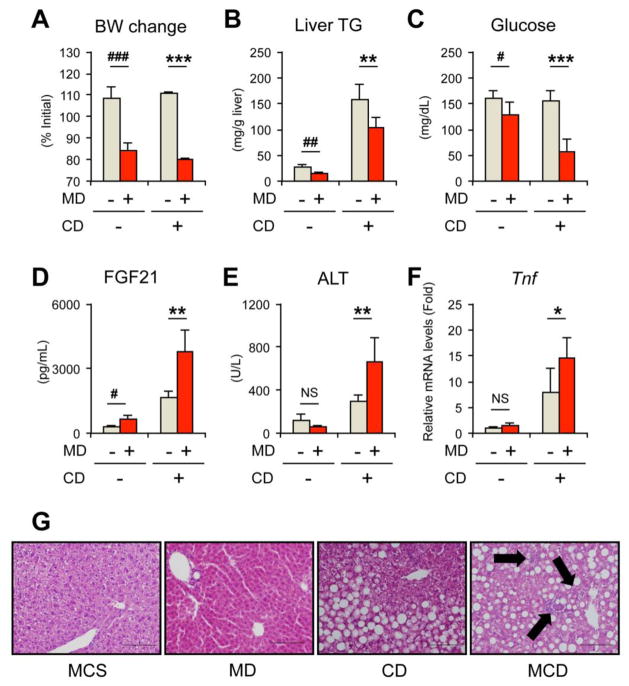
To understand the significance of dietary methionine deficiency and the following WAT lipolysis in the pathogenesis of MCD-NASH, liver phenotypes were compared between mice treated with MCS and MD, and between mice treated with CD and MCD for two weeks. CD treatment did not singularly affect body weight, but induced hepatic steatosis (Fig. 7A and B). MCD treatment markedly reduced body weight and serum glucose and increased serum FGF21 compared with CD treatment (Fig. 7A, C, and D). Although serum ALT and hepatic expression of Tnf and Itgam mRNAs were not increased by MD treatment compared with control MCS treatment, these parameters were significantly increased by MCD treatment compared with CD treatment (Fig. 7E and F, Supplementary Fig. 5). Consistent with serum ALT and hepatic Tnf/Itgam expression, hepatocyte degeneration and inflammatory cell infiltration were not seen in the livers of MD-treated mice (Fig. 7G). Macrovesicular steatosis was seen in CD-fed mice, and hepatocyte damage and mild-to-moderate focal inflammation in addition to steatosis were detected in MCD-fed mice (Fig. 7G). Therefore, these results suggest that adding dietary methionine deficiency to CD-induced steatosis promotes liver injury that may lead to steatohepatitis. This unfavorable effect of additional dietary methionine deficiency under the CD-fed condition is not due to the direct hepatotoxicity caused by dietary methionine deprivation, but likely due to MD-induced metabolic alterations, such as increased adipose lipolysis and enhanced FA flux.
Fig. 7. The effect of dietary methionine deficiency on liver pathology.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with MCD (designated as MD+ CD+), choline-deficient diet (CD, designated as MD− CD+), methonine-deficient diet (MD, designated as MD+ CD−), or control MCS diet (designated as MD− CD−) for two weeks (n = 4–7/group) and serum and liver were collected.
(A) Body weight (BW) change. Values were expressed as the percentage relative to BW just before commencing the MCD, CD, MD, or MCS treatment.
(B) Liver TG contents.
(C–E) Serum levels of glucose (C), FGF21 (D), and ALT (E).
(F) Hepatic Tnf mRNA levels. The mRNA levels were normalized to those of 18S ribosomal mRNA and subsequently normalized to those of MCS-treated mice.
(G) Representative liver histology. Hematoxylin and eosin staining, Bar = 100 μm. Arrows in MCD photograph indicate inflammatory foci.
Statistical analysis was performed using the Student’s t-test. #P<0.05, ##P<0.01, ###P<0.001 vs. MCS-treated mice (MD− CD−); *P<0.05, **P<0.01, ***P<0.001 vs. CD-treated mice (MD− CD+); NS, not significant.
3.7. Role of CD in the steatogenesis of MCD-NASH
The impact of dietary choline deficiency on hepatic fat accumulation was also addressed. CD treatment did not affect the mRNAs encoding genes involved in de novo lipogenesis, such as ATP citrate lyase (Acly), acetyl-CoA carboxylase α (Acaca), and Fasn (Supplementary Fig. 6). Stearoyl-CoA desaturase 1 (Scd1) mRNA was decreased in CD- and MCD-fed mice. The mRNAs encoding genes associated with VLDL secretion, microsomal TG transfer protein (Mttp) and apolipoprotein B (Apob), were significantly altered by both treatments (Supplementary Fig. 6), supporting the previous findings that dietary choline deficiency disrupts VLDL secretion leading to hepatosteatosis [11].
3.8. Enhancing WAT lipolysis by fasting exacerbates liver injury in the presence of dietary choline deficiency
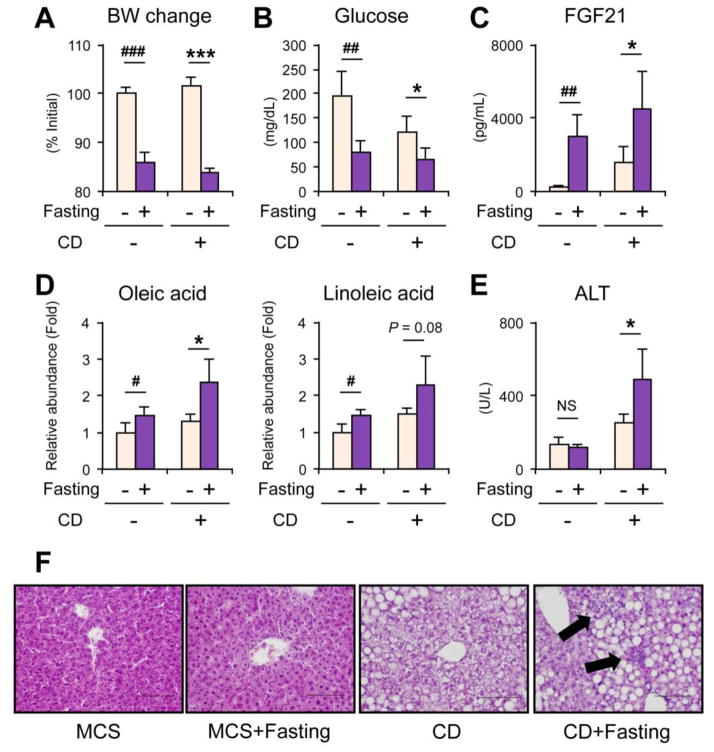
The addition of methionine deficiency to CD-induced steatosis aggravated liver injury and dietary methionine deficiency caused similar metabolic changes to fasting, i.e., acceleration of adipose lipolysis. Lastly, to explore the possibility that the MD-like metabolic alterations can exacerbate liver damage upon CD state, mice were treated with CD or MCS for two weeks and killed after a 36-hour fasting. A 36-hour fasting caused decreased body weight and serum glucose, increased serum FGF21 and oleic and linoleic acids in CD- and MCS-treated mice, which were similar to changes found in the MD-treated mice (Fig. 8A–D). Although the mRNAs encoding Tnf and Itgam did not increase (data not shown), serum ALT levels were significantly increased by fasting only in the CD-treated group (Fig. 8E). Similar to MD-treated mice, hepatocyte degeneration and inflammatory cell infiltration were not observed in fasted control mice (Fig. 8F). However, some inflammatory cells were seen in CD-treated mice with fasting (Fig. 8F). These results suggest a detrimental effect of MD-like metabolic changes, such as WAT lipolysis, to the progression of liver injury in the presence of dietary choline deficiency.
Fig. 8. Enhanced WAT lipolysis by 36-hour fasting exacerbates liver injury in the presence of dietary choline deficiency.
Male C57BL/6NCr wild-type mice at 8–12 weeks of age were treated with choline-deficient diet (CD, designated as CD+) or control diet (MCS, designated as CD−) for two weeks and randomly divided into fasting or non-fasting group (n = 5/group). Mice in one group were fasted for 36 hours before killing (fasting+) and mice in the other group were continuously fed CD or MCS (fasting−). Serum and liver were harvested.
(A) Body weight (BW) change. Values were expressed as the percentage relative to BW at 36 hours before killing.
(B–E) Serum levels of glucose (B), FGF21 (C), oleic and linoleic acids (D), and ALT (E). Serum oleic and linoleic acid levels were normalized to those of non-fasted MCS-treated mice and were expressed as relative abundance.
(F) Representative liver histology. Hematoxylin and eosin staining, Bar = 100 μm. Arrows indicate focal inflammation.
Statistical analysis was performed using the Student’s t-test. #P<0.05, ##P<0.01, ###P<0.001 vs. non-fasted MCS-treated mice; *P<0.05, **P<0.01, ***P<0.001 vs. non-fasted CD-treated mice; NS, not significant.
4. Discussion
The present study focused on serum metabolite changes in the early stages of MCD-NASH in order to explore the metabolic disruption associated with occurrence of NASH. Serum metabolomic analysis in mice treated with MCD for three days uncovered significant increases in oleic and linoleic acids early during the development of MCD-NASH, which was due to enhanced lipolysis in WAT. The changes in serum FA and WAT after the MCD treatment were due to dietary methionine deficiency. When MCD treatment was compared with CD treatment, the methionine deficiency-induced metabolic changes, such as WAT lipolysis, aggravated liver injury. Collectively, these results indicate an important role for dietary methionine deficiency and WAT lipolysis in the development of MCD-NASH.
Serum FA levels are mainly influenced by dietary intake, storage and release from WAT, and hydrolysis of phospholipids and chylomicron/lipoproteins. Increased serum FA by MCD treatment were correlated with decreased body weight, WAT mass, and adipocyte size and elevated Ces3 and Adrb2 mRNA levels and phosphorylation of HSL in WAT, but not with changes in hepatic expression of Lcat and Lpl mRNAs, indicating a major contribution of WAT lipolysis to augmentation of serum FA. In humans having NAFLD, approximately 60% of hepatic TG was reported to originate from FA released from WAT [16]. Additionally, recent studies demonstrated that the overall TG hydrolysis activity was significantly increased in the WAT of MCD-treated mice, but not in the liver [17, 18], thus corroborating the present observations. Since storing FA as stable lipid droplets is a crucial role of WAT in vivo, inappropriate increases in lipolysis activity in WAT may be harmful to other FA-utilizing organs, such as liver. Notably, in steatotic hepatocytes under the CD state, FA overload from WAT causes excess reactive oxygen species (ROS) generation mainly in mitochondria. In response to the increased ROS, glutathione (GSH), a potent endogenous antioxidant, is consumed to detoxify ROS. Since methionine and choline can be precursors of GSH, addition of dietary methionine deficiency to the CD state might gradually reduce mitochondrial GSH and further lower the capacity to detoxify ROS [19]. These metabolic alterations enhance hepatic oxidative stress and sensitize steatotic hepatocytes to apoptosis and necrosis [20, 21], leading to the progression to steatohepatitis. It was reported that some patients developed NASH following massive weight loss e.g., after bariatric surgery [22] or pancreaticoduodenectomy [23]. The mechanism to develop NASH caused by malnutrition is considered to be similar to that of MCD-NASH. Furthermore, enhanced lipolysis in WAT and the predominance of FA use in the liver were also documented in humans having insulin resistance and NAFLD [16, 21]. The findings in the present study offer one of the potential mechanisms on how WAT lipolysis contributes to the development and progression of NASH/NAFLD.
Dietary methionine restriction is known to reduce adiposity [24]. This study strengthened the view that dietary methionine deficiency causes reduction in WAT mass, but the precise mechanism remains unclear. One possibility is induction of FGF21 in the liver. FGF21 is a hepatokine that improves insulin sensitivity and its overexpression in mice yields a lean phenotype, increased lipolysis in WAT, and resistance to diet-induced obesity [25]. FGF21 treatment to 3T3-L1 adipocytes was reported to induce lipolysis, suggesting the direct effect of FGF21 on WAT lipolysis [25]. Additionally, FGF21 was shown to stimulate glucose uptake in 3T3-L1 adipocytes [26] and FGF21 administration caused a marked decrease in plasma glucose in diabetic rhesus monkeys [27]. Therefore, dietary methionine deficiency can induce FGF21 synthesis in the liver, enhance glucose uptake in peripheral tissues, such as WAT, and lower serum glucose. As a consequence, sympathetic nerve activation occurs and WAT lipolysis is enhanced. Increased Adrb2 and Glut4 expression in WAT by MD treatment supports this hypothesis. Since elevated serum/liver FGF21 levels are reported in human NAFLD/NASH [28, 29], further studies are needed to clarify the contribution of FGF21 to the pathogenesis of NAFLD/NASH.
Another intriguing finding in the present study was up-regulation of Ces3 (also termed triacylglycerol hydrolase, Tgh) in WAT by MCD and MD treatments. Although HSL and ATGL are the main lipolytic enzymes in WAT, multiple enzymes and several lipid droplet-coating proteins also regulate WAT lipolysis [14]. Ces3 is one of the major lipases in adipocytes [30] and is induced by fasting in WAT [31]. The WAT of Ces3/Tgh-null mice showed a 50% decrease in total TG hydrolysis activity, a 70% increase in WAT mass, and significant decreases in serum FA and glycerol [32], suggesting a major contribution of Ces3 in the regulation of overall lipolysis activity of WAT. Therefore, increased Ces3 expression in WAT may, at least in part, be associated with enhanced WAT lipolysis by MCD and MD treatments. It may be of value to examine whether disruption of adipose Ces3 can modulate the severity of MCD-NASH.
Although it is difficult to determine the relative contributions of HSL, ATGL, CES3, and other lipases/proteins to the MCD- and MD-induced lipolysis, the percentage of HSL and ATGL to the overall TG hydrolysis activity is estimated as approximately 70% and 20% in WAT of 2-week MCD-treated mice, respectively, based on the results of a recent study [17]. Phosphorylation of HSL and up-regulation of Adrb2 appeared even after a 3-day MCD treatment, thus indicating significant sympathetic nerve activation and HSL phosphorylation in early stages of MCD-NASH. On the contrary, the levels of ATGL protein seemed to be gradually increased. This observation is partially in agreement with the published finding that ATGL protein was increased in ob/ob mice treated with MCD for five weeks [18]. The present study could not assess the levels of phosphorylated ATGL and actual ATGL activity in 3-day or 1-week MCD-fed mice, while the previous study indicated up-regulation of ATGL activity in WAT of 2-week MCD-fed mice [17].
The finding in this study that WAT lipolysis may aggravate liver injury in the presence of CD-induced steatosis seems to be not entirely consistent with a previous observation that ATGL plays a protective role in MCD-induced hepatic steatosis and inflammation [17]. However, ATGL is a major lipase not only in WAT but also in liver that regulates peroxisome proliferator-activated receptor α (PPARα) signaling. In fact, suppression of ATGL in hepatocytes by injecting adenovirus encoding ATGL short hairpin RNA to mice led to marked down-regulation of PPARα and its downstream targets [33]. Since PPARα plays a critical role in attenuating hepatic TG accumulation and inflammation [34], hepatic ATGL may protect hepatocytes from lipotoxicity and inflammatory signaling through modulating PPARα. To address the precise role of adipose ATGL in the context of several liver diseases, mice with adipocyte-specific disruption of ATGL may be of value to examine in future studies.
In the present study, MD treatment did not cause liver injury, which was different from a previous report [35]. Although the reason of this discrepancy was unclear, MD treatment did increase hepatic mRNA levels of DNA-damage inducible transcript 3, a typical marker of endoplasmic reticulum (ER) stress. Since persistent ER stress can lead to mitochondrial damage and hepatocyte apoptosis [36], longer MD treatment might induce liver damage to some degrees.
Recently, others reported significant increases in linoleic acid in the serum and liver of ob/ob mice treated with the MCD for five weeks [18], a finding that is in agreement with the results in the present study. Linoleic acid is an unsaturated and not saturated, such as palmitic and stearic acids. Although direct toxicity of palmitic acid was shown in isolated hepatocytes [36], linoleic acid is auto-oxidized in vivo and can produce several lipid peroxides, such as 4-hydroxynonenal and malondialdehyde [37]. Increased release of linoleic acid from WAT and its influx into the liver may further amplify lipotoxicity and trigger the progression to NASH.
In conclusion, the present study revealed an important role of WAT lipolysis in the development of MCD-NASH. These findings provide the possibility that modulating WAT lipolysis may be a novel therapeutic target of NASH/NAFLD. Elucidating changes in WAT functions in patients having NASH might lead to further comprehensive understanding of the pathogenesis of NASH.
Supplementary Material
Highlights.
Significant increases in fatty acids were observed in early stages of MCD-NASH.
The increases in fatty acids were due to enhanced adipose lipolysis.
These changes were derived from dietary methionine deficiency, but not hepatitis.
We report an important role for adipose lipolysis in the development of MCD-NASH.
Acknowledgments
Financial support: Supported by the National Cancer Institute Intramural Research Program and U54 ES16015 (F.J.G)
We thank Linda G. Byrd and John Buckley for providing technical assistance with the mouse studies. This study was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, U554 ES16015 and 1R01ES022186-01, National Institutes of Health.
Abbreviations
- Acaca
acetyl-coenzyme A carboxylase alpha
- Acly
ATP citrate lyase
- Adrb
β-adrenergic receptor
- ALT
alanine aminotransferase
- Apob
apolipoprotein B
- ATGL
adipose triglyceride lipase
- CD
choline-deficient diet
- Ces3
carboxylesterase 3
- Dgat
diacylglycerol O-acyltransferase
- Emr1
EGF-like module containing, mucin-like, hormone receptor-like sequence 1
- ER
endoplasmic reticulum
- eWAT
epididymal white adipose tissue
- FA
fatty acid
- Fasn
fatty acid synthase
- FGF
fibroblast growth factor
- Glut4
glucose transporter type 4
- GSH
glutathione
- HETE
hydroxyeicosatetraenoic acid
- HSL
hormone-sensitive lipase
- IL
interleukin
- Itgam
integrin alpha M
- Lcat
lecithin cholesterol acyltransferase
- Lpl
lipoprotein lipase
- MCD
methionine- and choline-deficient diet
- MCS
methionine- and choline-supplemented MCD diet
- MD
methionine-deficient diet
- Mttp
microsomal triglyceride transfer protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NEFA
non-esterified fatty acid
- OPLS
orthogonal projection to latent structure
- PCA
principal component analysis
- Pnpla2
patatin-like phospholipase domain containing 2
- PPAR
peroxisome proliferator-activated receptor
- qPCR
quantitative polymerase chain reaction
- ROS
reactive oxygen species
- Scd1
stearoyl-coenzyme A desaturase 1
- SS
simple steatosis
- TG
triglyceride
- TNF
tumor necrosis factor
- UPLC-ESI-QTOFMS
ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry
- VLDL
very-low-density lipoprotein
- WAT
white adipose tissue
Footnotes
Potential conflict of interest: The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insight. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 6.Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver. 2012;6:149–171. doi: 10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Day CP, James OFW. Steatohepatitis: a tale of two “hit”? Gastroenterology. 1998;114:824–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 9.Gyorgy O, Goldblatt H. Observations on the conditions of dietary hepatic injury (necrosis, cirrhosis) in rats. J Exp Med. 1942;75:355–368. doi: 10.1084/jem.75.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 12.Idle JR, Gonzalez FJ. Metabolomics. Cell Metab. 2007;6:348–351. doi: 10.1016/j.cmet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsubara T, Tanaka N, Krausz KW, Manna SK, Kang DW, Anderson ER, Luecke H, Patterson AD, Shah YM, Gonzalez FJ. Metabolomics identifies an inflammatory cascade involved in dioxin- and diet-induced steatohepatitis. Cell Metab. 2012;16:634–644. doi: 10.1016/j.cmet.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Nilsson A. Source of eicosanoid precursor fatty acid pools in tissues. J Lipid Res. 2001;42:1521–1542. [PubMed] [Google Scholar]
- 16.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jha P, Claudel T, Baghdasaryan A, Mueller M, Halilbasic E, Das SK, Lass A, Zimmermann R, Zechner R, Hoefler G, Trauner M. Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59:858–869. doi: 10.1002/hep.26732. [DOI] [PubMed] [Google Scholar]
- 18.Jha P, Knopf A, Koefeler H, Mueller M, Lackner C, Hoefler G, Claudel T, Trauner M. Role of adipose tissue in methionine-choline-deficient model of non-alcoholic steatohepatitis (NASH) Biochim Biophys Acta. 2014;1842:959–970. doi: 10.1016/j.bbadis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Montfort C, Matias N, Fernandez A, Fucho R, Conde de la Rosa L, Martinez-Chantar ML, Mato JM, Machida K, Tsukamoto H, Murphy MP, Mansouri A, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. J Hepatol. 2012;57:852–859. doi: 10.1016/j.jhep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008;118:683–694. doi: 10.1172/JCI33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm IS, Schindler W, Haluszka O. Steatohepatitis and fatal hepatic failure after biliopancreatic diversion. Am J Gastroenterol. 1992;87:775–779. [PubMed] [Google Scholar]
- 23.Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S, Aoyama T, Tanaka E. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758–768. doi: 10.1007/s00535-011-0370-5. [DOI] [PubMed] [Google Scholar]
- 24.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One. 2012;7:e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Dong K, Fang Q, Hou X, Zhou M, Bao Y, Xiang K, Xu A, Jia W. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. J Hepatol. 2013;58:557–563. doi: 10.1016/j.jhep.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Soni KG, Lehner R, Metalnikov P, O’Donnell P, Semache M, Gao W, Ashman K, Pshezhetsky AV, Mitchell GA. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J Biol Chem. 2004;279:40683–40689. doi: 10.1074/jbc.M400541200. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki H, Igarashi M, Nishi M, Tajima M, Sekiya M, Okazaki S, Yahagi N, Ohashi K, Tsukamoto K, Amemiya-Kudo M, Matsuzaka T, Shimano H, Yamada N, Aoki J, Morikawa R, Takanezawa Y, Arai H, Nagai R, Kadowaki T, Osuga J, Ishibashi S. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes. 2006;55:2091–2097. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]
- 32.Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JR, Mitchell G, Korbutt GS, Lehner R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab. 2010;11:183–193. doi: 10.1016/j.cmet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 35.Caballero F, Fernández A, Matías N, Martínez L, Fucho R, Elena M, Caballeria J, Morales A, Fernández-Checa JC, García-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazanave SC, Mott JL, Bronk SF, Werneburg NW, Fingas CD, Meng XW, Finnberg N, El-Deiry WS, Kaufmann SH, Gores GJ. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286:39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiteller G. Peroxidation of linoleic acid and its relation to aging and age dependent diseases. Mech Ageing Dev. 2001;122:617–657. doi: 10.1016/s0047-6374(01)00220-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.