Abstract
The androgen receptor (AR) in stromal cells contributes significantly to the development and growth of prostate during fetal stages as well as during prostate carcinogenesis and cancer progression. During prostate development, stromal AR induces and promotes epithelial cell growth, as observed from tissue recombinant and mouse knockout studies. During prostate carcinogenesis and progression, the stromal cells begin to lose AR expression as early as at the stage of high-grade prostatic intraepithelial neoplasia. The extent of loss of stromal AR is directly proportional to the degree of differentiation (Gleason grade) and progression of prostate cancer (PCa). Co-culture studies suggested that stromal AR inhibits the growth of malignant epithelial cells, possibly through expression of certain paracrine factors in the presence of androgens. This functional reversal of stromal AR, from growth promotion during fetal prostate development to mediating certain growth-inhibiting effects in cancer, explains to some extent the reason that loss of AR expression in stromal cells may be crucial for development of resistance to androgen ablation therapy for PCa. From a translational perspective, it generates the need to re-examine the current therapeutic options and opens a fundamental new direction for therapeutic interventions, especially in advanced PCa.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Prostate cancer (PCa) is the most common non-skin malignancy in the male population within the United States and is the second most common cancer in men worldwide.1 It is also one of the leading causes of cancer-related deaths in males in the United States.
Normal human prostate is composed of an epithelial tissue and an adjacent stroma. The epithelium is composed of two principal cell types, the tall columnar secretory luminal cells that line the glandular ducts and the flattened basal cells surrounding them. In addition, some rare neuroendocrine cells are also present. Often, the terms mesenchyme and stroma are loosely used. Herein, mesenchyme refers to the mesodermal-derived fetal or newborn tissues with instructive induction potential. The word stroma describes the tissues surrounding the prostatic epithelium, later in development. In the adult human prostate, the stroma is composed mainly of smooth muscle cells. However, it also includes some fibroblasts, nerves, blood vessels, and various infiltrating immune and inflammatory cell types.
Circulating androgens mediate the development and function of prostate by stimulating the androgen receptor (AR). Rat studies have shown that in stroma, AR is expressed in mesenchymal cells of the urogenital sinus (UGS), especially those adjacent to the epithelium, concurrent with the formation of prostatic buds.2,3 As the prostate develops and the mesenchymal cells differentiate to form smooth muscle, AR expression is widespread, but not universal, throughout the muscle. In the past, investigators have mainly focused on studying epithelial AR function in prostate. Relatively limited data are available to describe the expression and function of stromal AR in prostate development2–14 and cancer. Stromal AR is involved in both prostate development and prostate carcinogenesis, with distinct functions in these two processes. We examine the current knowledge and understanding of stromal AR function, including its translational significance.
Stromal AR in Prostate Development
The role of stromal cells in prostate development, function, and maintenance of tissue differentiation is well established.15–17 The principal stromal cells in human prostate are fibroblasts and smooth muscle cells, which contribute to the synthesis of the extracellular matrix. Together, the stromal cells and extracellular matrix generate a microenvironment that regulates the growth of the adjacent epithelial cells.
AR plays a critical role in prostate development through stage-specific regulation of androgenic effects on epithelial cells. Much of this activity is believed to be mediated by the release of paracrine factors, which are independent of the presence of epithelial AR.10 The profiles of AR expression in mouse and rat have been well described and show a predominance of mesenchymal AR expression during the fetal period, whereas epithelial AR is expressed on gland maturation2,8,18 during the post-natal period.
Stromal-Epithelial Interactions in Fetal Prostate
Interactions between mesenchymal and epithelial cells are obligatory for prostate development from the embryonic UGS.5 Androgen-dependent inductive signals from UGS mesenchyme direct the overall development process.19 The UGS mesenchyme induces the prostatic epithelium to bud from the UGS and form solid cords that grow, arborize, and canalize, developing an epithelium characterized by differentiation into tall columnar luminal cells interspersed by basal cells on the basement membrane.20,21 Concurrent with the epithelial differentiation, the loose mesenchymal cells differentiate to form smooth muscle immediately adjacent to the epithelium.2,18 The role of the epithelial cells in this process is underlined by the observation that the patterning of the smooth muscle sheets is determined by the species of the epithelial cells, rather than the species of the mesenchymal cells.2,18,22
A series of elegant experiments by Cunha and coworkers,5,23 using tissues from testicular feminized mice, established the importance of stromal and epithelial AR. Testicular feminized mice, which have a functional null mutation in the AR, have a female phenotype and do not develop prostates. By using tissue recombination experiments, it was demonstrated that mesenchymal AR drives the proliferation and differentiation of the epithelial cells, whereas epithelial AR plays a role in the differentiated function of the gland, specifically in the expression of various secreted proteins.5,23 Thus, the stromal-epithelial interactions are reciprocal in the development of mature prostatic tissue in which the epithelium expresses a highly differentiated secretory phenotype with specific secretory proteins, whereas the mature stroma is predominantly composed of smooth muscle cells. This clearly implies that the nature of the paracrine signals expressed by fetal mesenchyme and adult prostatic smooth muscle in response to androgens is different and results in contrasting effects on the epithelium. The development of the female genital tract shows a similar pattern, where estrogen and progesterone receptors in the stroma control the epithelial proliferation and differentiation. However, the differentiated epithelial function is regulated by epithelial receptors.24
Mediators of AR Expression in Prostate Development
It is important to understand that, unlike many other organs, the stromal-epithelial interactions in prostate development are androgen dependent. Several investigators7,8 have demonstrated that before and during prostatic development, the mesenchymal (stromal) cells express high levels of AR (Figure 1), whereas AR is initially undetectable in the epithelium. The fact that epithelial AR appears in the later phases of prostate development has been confirmed through the analysis of developing prostates, organ cultures, and tissue recombinants,10,25,26 reinforcing the idea that androgenic effects on epithelium in early development are independent of epithelial AR.
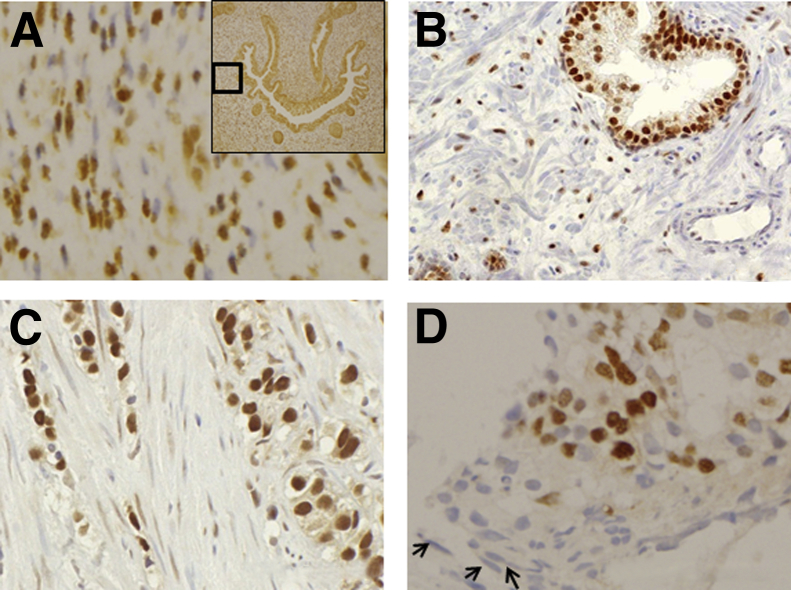
Figure 1.
Stromal AR expression in developing prostate and cancer progression. Stromal AR expression in a 13-week developing prostate (A), benign prostate (B), primary PCa (C), and bone metastatic PCa (D).
Although androgens can act directly on the epithelium via epithelial AR to elicit differentiated function,27 there are many instances where the androgens appear to act indirectly via stromal AR28 and by elaboration of paracrine mediators, such as epithelial growth factor and fibroblast growth factor family members. Other important growth factors include nerve growth factor and insulin-like growth factor.29–33 In addition, inflammatory chemokines have been implicated as potential players in prostatic development.34 These mediators can diffuse from the stromal compartment to epithelial cells and influence epithelial growth and differentiation.29–31 More recently, stromal AR knockout mice, either with AR knockout in fibroblasts or in smooth muscle or a double knockout in both fibroblasts and smooth muscle, showed suppressed prostate epithelial cell growth and development,35,36 further suggesting the importance of stromal AR in prostate development.
In a recent study (E.S., unpublished data) using human fetal tissues, the pattern of AR expression during early prostate development was found to be broadly similar to observations in rodent studies. However, there were some interesting variations that perhaps reflected the much longer gestational period in humans and the greater amount of ductal development that occurs in human fetal prostate. By using immunohistochemistry (IHC), tissue sections, representing all regions of the human fetal prostate at a gestational age between 7 and 22 weeks, were stained using anti-AR antibodies. At a gestational age of 7 weeks, AR expression was mainly in the peripheral stroma, cephalic (anterior) to the ejaculatory ducts (EDs), and in the epithelium lining the UGS. By 9 weeks, AR expression was seen throughout the stroma and in the suprabasal cells of the UGS. It was also seen exclusively in the basal cells of central dorsal UGS, caudal (posterior) to the EDs. The stroma, especially in the periphery, showed increased AR staining by 11 to 13 weeks. Epithelial AR expression increased from the base to the apex and throughout the cell layers of the UGS, ducts, and acini. Basal cells of the UGS and ducts anterior to the EDs did not express AR until this period. By 22 weeks, AR expression was throughout the gland, with maximum staining in the epithelium and the posterior and posterolateral peripheral stroma. These findings illustrate crucial differences between human prostate and the rodent models on which most of our knowledge is based.
Stromal Cells in Prostate Carcinogenesis
Normal adult prostate stromal cells have been characterized as maintaining growth quiescence within prostatic tissue. For many years, investigators studied the role of stromal cells in the initiation and promotion of carcinogenesis. The recognition of the similarities to the stromal microenvironment of a wound site resulted in Dvorak's famous description of cancer as a wound that does not heal.37 Tumor stroma, variously known as cancer-associated stroma (CAS), cancer-associated fibroblasts, tumor-associated stroma, or reactive stroma, is often different from the normal stroma.38–41 Animal studies have demonstrated that when normal or benign stromal cells are associated with malignant epithelial cells, there is a decrease in the proliferation rate42–44 and an apparent loss of former malignant properties of the epithelial cells.44,45 Some investigators have also shown a restriction of growth in epithelial cells and their induction into a more differentiated phenotype.46 Recombination studies using Dunning rat prostate adenocarcinoma revealed that the normal stromal environment may override the effects of oncogenic mutations in tumor cells.44 Normal stromal cells retain the properties of growth control and can possibly prevent the proliferation of cells undergoing neoplastic transformation.
Morphological Modifications in Stroma
Modification of the stromal environment is common in carcinogenesis, and it is adequately evident on observation of stroma immediately adjacent to PCa cells.40,47–49 During carcinogenesis, the composition of the prostate stroma often changes with the emergence of a stromal phenotype characterized by a loss of well-differentiated smooth muscle, the presence of myofibroblasts, and an increase in the amount of extracellular collagen.40,49,50 Morphologically and on the basis of cytoskeletal protein expression, myofibroblasts are an intermediate between fibroblasts and smooth muscle cells,51 characterized mainly by a prominent rough endoplasmic reticulum (as in fibroblasts) and contractile myofilaments (smooth muscle cells), with increased expression of vimentin and α-actin and decreased expression of calponin and smooth muscle myosin.52,53 Myofibroblasts have been widely implicated as the cancer-promoting cells in the stroma.38,54,55 Molecular changes to the prostate tumor stroma include changes in the expression of cell signaling and immunomodulatory proteins.56,57 There is a suggestion that the tumor stroma has the potential to become a target for therapy, leading to preclinical development of some innovative approaches, such as the activation of bee venom by proteases expressed in breast and prostate cancer stroma.58
Factors Promoting the Stromal Modification
There are several possible factors that promote the modification of normal stromal cells into CAS. Some signals from cancer epithelium to surrounding stromal cells have been shown to alter the function of stromal cells and extracellular matrix production. These include transforming growth factor-β1, which induces stromal secretion of versican, an extracellular chondroitin sulfate proteoglycan.59 By using a prostate cell line, transforming growth factor-β1 has been shown to cause the formation of larger tumors and extensive metastatic disease.60 In a hormone-sensitive cell model, variations in extracellular matrix have been shown to regulate stromal cell phenotype.61 Although it has been suggested that there are genetic modifications seen in the CAS,62 possibly as a result of epithelial-to-mesenchymal transitions of previously genetically abnormal epithelial cells, these results may reflect the technological limitations at the time the study was performed. More recent work has shown that the stromal cells seem to have limited, if any, genetic damage, suggesting that previous studies may reflect experimental artifacts, such as limiting and poor-quality DNA, followed by highly multiplexed PCR-based analysis.63 However, there is substantial evidence that the differences in gene expression between normal stroma and CAS are heritable within a cell lineage, indicating the possible operation of mechanisms, such as epigenetic changes,64–66 in maintaining this phenotype. In an analysis by Dakhova et al,67 when compared with normal stroma, a total of 544 unique genes were significantly higher and 606 unique genes were lower in the reactive stroma. Gene ontology analysis revealed significant alterations in several novel processes in PCa-reactive stroma, including neurogenesis, axon genesis, and the DNA damage/repair pathways, as well as evidence of increases in stem cells in PCa-reactive stroma. Changes in gene expression profiles (both positive and negative) associated with tumor stroma have been shown to be sufficient to mediate tumor promotion.68,69
Wound Repair as an Analogy for Carcinogenesis of Stroma
In the reactive stroma hypothesis,50 the stroma of the prostate has been correlated with the granulation tissue in wound repair, with reference to similar biological responses. Wound healing and generation of tumor stroma share many important properties. Both begin with the infiltration of plasma proteins, including fibrinogen, fibronectin, and plasminogen. In both the processes, the extravascular fibrin-fibronectin clot serves as a provisional stroma, providing a matrix for the migration of macrophages, fibroblasts, and new capillaries.37 As in any wound repair situation, the microenvironment would be expected to be growth promoting, which correlates with the promotion of survival and proliferation of carcinoma cells by stroma in prostatic carcinogenesis. Tissue recombination studies have demonstrated that human prostatic CAS can promote carcinogenesis in genetically initiated human prostate epithelial cells.38,70 To investigate whether these intra–tumor-reactive stromal cells in human PCa are predictive of survival, tumor stroma volume and specific stroma markers were quantitated by Ayala et al49 using tissue microarrays. Statistical analysis of the survival data set showed that the presence and quality of reactive stroma in the tumor was a significant predictor of disease-free survival. Stroma volume was also seen to be an independent predictor of progression in tumors with a Gleason score 7, a group that represents the interface between high and low risk of progression. Outcomes for patients with Gleason score 7 tumors are hard to predict using traditional methods, and scoring stromal data may refine the accuracy of such prognostications.
Progressive Loss of AR Expression in Stromal Cell Transition from Benign to Cancer and during its Progression
Numerous studies have focused on AR expression in epithelial cells during the genesis and the progression of PCa from primary to metastatic and from hormone-sensitive to castration-resistant PCa (CRPC). It has also been established that the function of epithelial AR changes with disease progression so that the proproliferative response to androgens becomes, at least to some degree, cell autonomous in cancer epithelium,71 rather than completely mediated through the stroma, as occurs in development. Increased epithelial AR expression in patient tumors has been associated with aggressive disease and decreased progression-free survival.72 However, the expression and function of stromal AR may be distinct from epithelial AR. As a result of the structural and genomic modifications64–67 of the stromal cells, there are functional modifications in tumor-associated stroma. Stromal AR expression progressively decreases during the transition from benign tissue to in situ cancer lesion and during progression of PCa from low to high grade, primary to metastatic, and hormone sensitive to CRPC.73–77
Transition from Benign to Neoplasia
Compared with normal prostate, stromal AR expression was found to decline starting as early in the disease process as high-grade prostatic intraepithelial neoplasia.74 Analysis of stromal tissue adjacent to PCa from patients undergoing transurethral resection of prostate or radical prostatectomy showed that loss of stromal AR expression increased linearly with higher histological grades. AR expression was absent in 67% of peri-epithelial stromal tissue in well-differentiated PCa (Gleason score, 2 to 4), 91% in moderately differentiated PCa (Gleason score, 5 to 7), and 94% in poorly differentiated PCa (Gleason score, 8 to 10).74 We have shown a statistically significant decrease in stromal AR expression (P < 0.001) in the areas surrounding PCa compared with benign prostate, with up to a 6% decrease in stromal AR expression.77 When stratified with Gleason score, we also established a trend of greater decrease of AR-positive stromal cells in cancerous areas compared with benign areas with increased tumor grade.77 Other investigators have also demonstrated that the loss of stromal AR is directly correlated with advances in pathological stage and with higher Gleason scores75 using AR IHC of transurethral resection of prostate specimens obtained from patients with varying Gleason scores and pathological stages. This difference was notable (P < 0.05) in tumor specimens of stage T2 and tumors with a Gleason score of 7, whereas it was more statistically significant (P < 0.01) in tumor stages T3 and T4 and in specimens with Gleason scores of 8 to 10.
Progression from Castration-Sensitive to Castration-Resistant PCa
Decreased stromal AR expression also correlates with disease progression, including metastasis (Figure 1D) and castrate resistance. Wikstrom et al75 showed that specimens with metastatic disease displayed significantly lower (P < 0.01) stromal AR expression compared with specimens with primary disease. Only 1.6% of cells stained positive for AR in metastatic tumor stroma compared with 18% of cells in normal stroma. In the primary prostate tumor specimens, 13% of tumor stroma cells stained positive for AR compared with 48% in normal stroma, which was equivalent to a 3.5-fold loss of expression in CAS.
We also demonstrated that, during transition of PCa from hormone naive to castration resistance, there is a significant decrease in stromal AR expression. We determined AR levels in prostate stroma of 44 cases of androgen-dependent PCa and in 22 cases of CRPC by IHC analysis using affinity-purified polyclonal AR antibodies. The levels of stromal AR expression were measured as an average percentage of AR-positive stromal cells. When comparing androgen-dependent and CRPC tumors, we observed a statistically significant, threefold decrease from 12% to 4%, of AR-positive stromal cells that were associated with CRPC.77
Understanding the Mechanisms of Loss of AR Expression
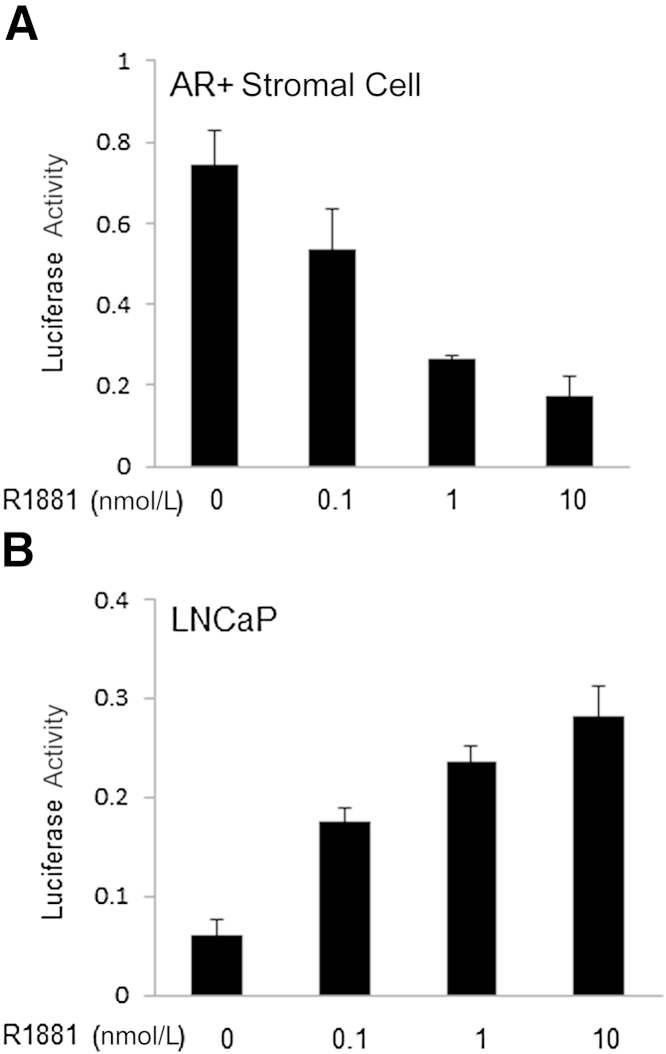
It is of great interest to determine the function of stromal AR target genes in PCa. It is well established that stromal AR regulates its target genes differentially than prostate epithelial cells. For example, most androgen-regulated genes in prostatic epithelium require the presence of the transcription factor Fox a1, which is not expressed in the stroma.78–81 It is likely that different combinations of transcription factors, likely involving alternative Fox family members, are involved in androgen-regulated gene expression in the stroma. It is also possible that these relationships change as the differentiation status of the stroma is altered from smooth muscle to a more myofibroblastic phenotype. By using proteomics pathway array analysis, we reported that androgen and AR inhibit the proliferation of stromal cells through transcriptional suppression of CNNB1, a target gene of AR in prostate stromal cells, confirmed with luciferase assays (Figure 2A).82 The negative regulation of CNNB1 by stromal AR is mediated through switching between transcription factors E2F1 and E2F4 on CNNB1 promoter. However, CNNB1 is positively regulated by AR in cancer epithelial cells (Figure 2B). It has also been shown that the activation of the estrogen-inducible cell cycle promoter CNND1 in normal prostatic stroma allows the stromal cells to behave more like cancer-associated fibroblasts.83
Figure 2.
Differential transcriptional activation and repression of cyclin B1 by AR in prostate epithelial and stromal cells. A: AR-mediated transcriptional repression of cyclin B1 in immortalized myofibroblastic stromal cells with stable AR expression by luciferase assay using 100 ng 200-bp LUC reporter containing 200-bp cyclin B1 promoter. B: AR-mediated transcriptional activation of cyclin B1 in PC-3 cells with luciferase assay. The 200-bp LUC reporter (100 ng) and pcDNA-AR (50 ng) were cotransfected to PC-3 cells.
The mechanism behind the loss of AR expression in the peri-epithelial stroma is not well established. It has been suggested that, during malignant transformation of epithelial cells, there is a shift in AR axis from stromal cell–dependent paracrine pathways to autocrine-dependent pathways.71 When these cancer cells shift to an autocrine mechanism of proliferation, it appears that epithelial AR regulates a new series of genes for survival and proliferation, not normally expressed by prostate epithelial cells.29 The consequence may be that malignant epithelial cells no longer depend on stromal-epithelial interactions and stromal AR-mediated growth factors for their survival and proliferation. It is also notable that during development, the androgen-regulated paracrine signals produced by the stroma contribute initially to epithelial proliferation that becomes progressively more organized as the stroma gradually differentiates into AR-expressing smooth muscle. As cancer progresses, the stroma becomes progressively less differentiated, suggesting a loss of signaling that normally restricts epithelial growth and maintains a largely growth quiescent differentiated form, a concept reviewed elsewhere.84–86
PCa has been characterized as more aggressive in African American (AA) patients compared with Caucasian (CA) patients. AR in cancer epithelial cell expression has been shown to be increased in AA PCa in comparison to CA patients.87 In contrast, stromal AR in AA PCa decreased more when compared with that in CA.82
Stromal AR Inhibits Cancer Epithelial Cell Growth, Causing a Functional Reversal
We have previously demonstrated in experiments using well-characterized AR-positive and AR-negative stromal cell lines, both by co-culture in vitro and coxenograft in vivo, that in the presence of androgen, stromal cells expressing AR decrease the growth and invasive ability of PCa epithelial cells.77 It was hypothesized that this distinct effect of AR in stromal cells is due to the involvement of paracrine factors/mechanisms regulated by both epithelial and stromal cells.
To establish this analysis, a well-characterized prostate stromal cell line, morphologically similar to the tumor stroma, was used. We constructed an immortalized stromal cell line termed PShTert from a prostate with benign prostatic hyperplasia. The cells stably expressed the human telomerase catalytic subunit, hTert. Morphologically, the cells showed typical characteristics of myofibroblasts. IHC showed diffuse, strongly positive staining for vimentin, strong smooth muscle actin staining in 25% of cells, and negative staining for desmin. Together, these data support the myofibroblastic phenotype of the PShTert stromal cells. Western blot analysis confirmed the absence of AR in these cell lines. We transduced this cell line with the pBabe-AR retroviral vector and selected stable clonal cell lines expressing AR, termed PShTertAR. Further Western blot analysis, performed with nuclear and cytoplasmic extracts, showed AR expression in cytoplasmic and nuclear fractions in the absence and presence of androgen, respectively. Ensuring the functionality of the ectopic AR in the nucleus is crucial to model the AR-expressing section of tumor stroma, and was confirmed by dual-luciferase assay eliciting ligand-dependent transcriptional activation in the presence of androgens.77
For in vitro analysis, transwell indirect co-culture assays using these two stromal cells with PC-3 cells were performed. In the presence of androgens, co-culture with PShTertAR resulted in inhibition of PC-3 cell growth compared with PC-3 cell proliferation when cultured alone (P = 0.045). In contrast, co-culture with AR-negative PShTert cells resulted in enhancement of growth rate of PC-3 cells compared with PC-3 cells grown alone (P = 0.03). Flow cytometric analysis revealed that PC-3 cells co-cultured with PShTertAR showed a decrease from 27% to 20% of cells in S-phase compared with cells co-cultured with PShTert cells. Expression of cell cycle genes, including CNNA1, CNNB1, cyclin-dependent kinase inhibitor 1A (p21), cyclin-dependent kinase inhibitor 1B (p27), and S-phase kinase-associated protein 2 (Skp2), was examined. We showed decreased expression of Skp2 in PC-3 cells co-cultured with PShTertAR compared with PC-3 cells co-cultured with PShTert cells. Expression of other genes was similar for both the co-cultures. In co-cultures with androgen-free media, both PShTert and PShTertAR cells stimulated the growth of PC-3 cells.77
Similarly, experiments performed by co-injecting PC-3 cells with PShTert cells s.c. in the flank region of male nude mice resulted in development of tumors twice as large as in control mice with PC-3 cells alone. On the other side, co-injection of PC-3 cells and the PShTertAR cell line resulted in statistically significant reductions of tumor growth and size.77
There were two important observations made from this analysis. First, consistent with previous observations, both AR-negative and AR-positive stromal cells promote growth of PCa epithelial cells in the absence of androgen by secretion of a paracrine factor, which is independent of AR.88–90 Second, AR-positive stromal cells secrete another paracrine factor, which is growth inhibitory for PCa epithelial cells and is dependent on androgen/AR presence.91
Furthermore, media from the androgen-treated PShTertAR cells were used to culture PC-3 cells. Contrary to the co-culture, there was an increase in the growth of PC-3 cells.77 This signifies that such an AR-dependent growth-inhibitory factor is dependent on the presence of stromal cells for its expression, strongly supporting a role for active two-way intercellular communication. Further investigation and understanding of this two-way paracrine regulation of growth in metastasis and androgen independence are necessary and may lead to development of novel therapeutic interventional targets for PCa.
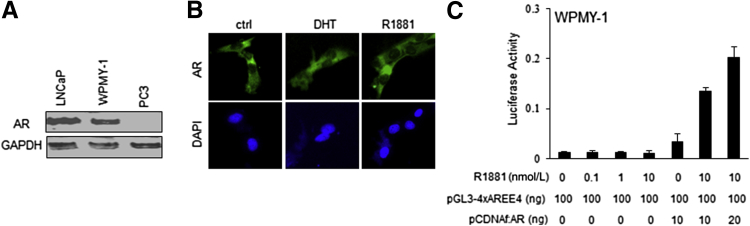
Interestingly, in an animal model experiment using recombination of human prostate stromal cell line (WPMY-1) with human PCa epithelial cell line (PC-3), Niu et al92 have indicated that stromal AR may play a more dominant role than epithelial AR in promoting primary tumor proliferation at earlier stages. One caveat is that the endogenous AR expressed in the WPMY-1 cell line is nonfunctional. By using immunofluorescence, we demonstrated that, in the WPMY-1 cell line, AR is exclusively expressed in the cytoplasm, regardless of the presence of androgen (Figure 3). Furthermore, the endogenous AR expressed in this cell line showed no transcriptional activation by luciferase reporter assay in the presence of androgen (Figure 3). Together, these results demonstrate that WPMY-1 may not be an appropriate cell line model for characterization of AR-positive PCa stromal cells. Considering nonfunctional endogenous AR expression as equivalent to absence of AR expression, the results of the study by Li et al77 are consistent with our analysis. More recently, the group of Yu et al93 showed stromal AR function in promoting cancer epithelial cell growth using immortalized cancer-associated fibroblast cells from prostate. It will be important to clarify this situation.
Figure 3.
AR expression, localization, and activity in WPMY-1 cells. A: AR expression in WPMY-1 cells. B: Cytoplasmic AR localization regardless of R1881 or dihydrotestosterone (DHT) stimulation. C: Luciferase assay with the reporter pGL3-4xAREE4 (containing 4xARE upstream E4 promoter) indicates that the endogenous AR fails to show any activation, whereas ectopic AR increases transactivation on R1881 stimulation. Ctrl, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Need to Re-Evaluate the Role of Continued ADT in CRPC and Future Directions
A normal androgen-responsive prostate stroma has previously been shown to suppress tumor cell growth.44 These data are consistent with our hypothesis that AR-positive stromal cells normally inhibit the growth of tumors. Apart from the case of the PC-3 cells in data described above, we also analyzed and found similar results using AR-positive LNCaP cells. However, the magnitude of growth inhibition by AR-positive stromal cells was lower in LNCaP cells compared with PC-3 cells.77 One of the explanations for this observation may be that, because androgen and AR enhance the growth of LNCaP cells, the end result is due to inhibition of epithelial (LNCaP) cells through effects mediated by stromal AR and additional stimulation of epithelial (LNCaP) cells by androgen and epithelial AR. This illustrates that stromal AR-mediated growth inhibition of cancer epithelial cells is more evident in the CRPC/metastatic PCa disease model (PC-3) compared with the androgen-dependent PCa model (LNCaP). Relevant to this observation is a recent description of the forced re-expression of proteins associated with the differentiation of normal prostatic stroma in tumor stromal cells where they have been lost. In this study, consistent with the reported observations on AR, tumor growth was suppressed by expression of either delta-like 1 homolog (Dlk1) or signal peptide, CUB domain, epithelial growth factor–like 1 (SCUBE1) in tumor stroma.94
The role of continued androgen deprivation therapy (ADT) during progression to CRPC has been under evaluation for some years.95,96 Recent clinical trials that compared the use of intermittent with continued ADT have not been able to demonstrate any conclusive results.97 It may be reasonable to accept that with the onset of androgen-independent epithelial tumor cells, the therapeutic efficacy and potency of ADT begin to wane. However, with the progressive loss of stromal AR, there may be a loss of growth-inhibitory stromal effects that are also androgen mediated. Consistently, it supports high-dose exogenous testosterone therapy in patients with CRPC, initiated in a clinical trial setting.98
Summary
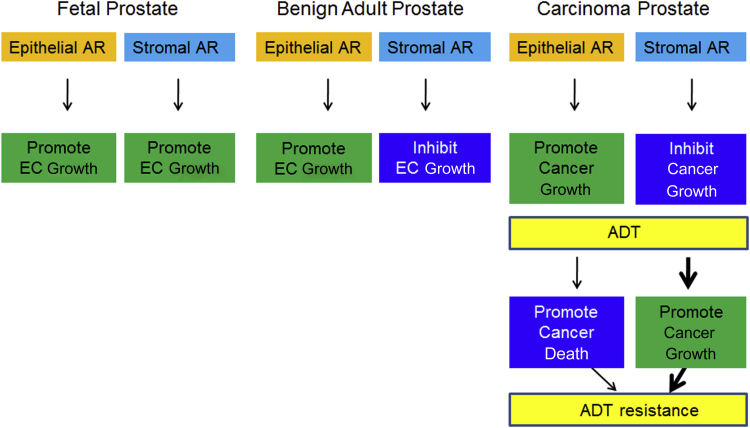
A close observation of the behavior of stromal AR chronologically from the fetal/pubertal development up to the stage of poorly differentiated/metastatic PCa illustrates a change from promoting growth and differentiation in the developing prostate to suppressing growth and maintaining differentiation in the normal adult organ. In cancer, the role of androgens becomes more complex, with direct growth promotion in the epithelium and stromal cell state–specific responses to androgenic stimulation of the stroma that can range from promoting to suppressing growth (Figure 4). Studies exploring changes in the signaling pathways in stromal cells should be encouraged.99 More investigations are required to understand the mechanism of this functional versatility in stromal AR for its utility in PCa prognosis and treatment.
Figure 4.
Distinct function of stromal AR in fetal, adult, and cancerous prostate and its therapeutic implication in PCa. ADT, androgen deprivation therapy; EC, epithelial cell.
Footnotes
Supported by NIH grants 1U01CA149556-01A1 and 3U01CA149556-01S1 (P.L.), Department of Defense Prostate Cancer Research Program grants PC080010 and PC111624 (P.L.), and VA Merit grants 1I01BX001505-01 (P.L.) and 1U01 CA151924-01A1 (S.W.H.).
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development).
Disclosures: None declared.
Contributor Information
Simon W. Hayward, Email: simon.hayward@vanderbilt.edu.
Peng Lee, Email: peng.lee@nyumc.org.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hayward S.W., Baskin L.S., Haughney P.C., Foster B.A., Cunha A.R., Dahiya R., Prins G.S., Cunha G.R. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat (Basel) 1996;155:94–103. doi: 10.1159/000147794. [DOI] [PubMed] [Google Scholar]
- 3.Takeda H., Mizuno T., Lasnitzki I. Autoradiographic studies of androgen-binding sites in the rat urogenital sinus and postnatal prostate. J Endocrinol. 1985;104:87–92. doi: 10.1677/joe.0.1040087. [DOI] [PubMed] [Google Scholar]
- 4.Heinlein C.A., Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 5.Cunha G.R., Chung L.W. Stromal-epithelial interactions–I: induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 6.Cunha G.R. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol. 1976;47:137–194. doi: 10.1016/s0074-7696(08)60088-1. [DOI] [PubMed] [Google Scholar]
- 7.Cooke P.S., Young P., Cunha G.R. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128:2867–2873. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- 8.Takeda H., Chang C. Immunohistochemical and in situ hybridization analysis of androgen receptor expression during the development of the mouse prostate gland. J Endocrinol. 1991;129:83–89. doi: 10.1677/joe.0.1290083. [DOI] [PubMed] [Google Scholar]
- 9.Cunha G., Lung B. The possible influences of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–194. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 10.Cunha G.R., Alarid E.T., Turner T., Donjacour A.A., Boutin E.L., Foster B.A. Normal and abnormal development of the male urogenital tract: role of androgens, mesenchymal-epithelial interactions and growth factors. J Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- 11.Lai K.P., Yamashita S., Vitkus S., Shyr C.R., Yeh S., Chang C. Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor. Mol Endocrinol. 2012;26:52–66. doi: 10.1210/me.2011-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda I., Lasnitzki I., Mizuno T. Analysis of prostatic bud induction by brief androgen treatment in the fetal rat urogenital sinus. J Endocrinol. 1986;110:467–470. doi: 10.1677/joe.0.1100467. [DOI] [PubMed] [Google Scholar]
- 13.Shannon J.M., Cunha G.R. Autoradiographic localization of androgen binding in the developing mouse prostate. Prostate. 1983;4:367–373. doi: 10.1002/pros.2990040406. [DOI] [PubMed] [Google Scholar]
- 14.Cai G., Huang H., Shapiro E., Zhou H., Yeh S., Melamed J., Greco M.A., Lee P. Expression of androgen receptor associated protein 55 (ARA55) in the developing human fetal prostate. J Urol. 2005;173:2190–2193. doi: 10.1097/01.ju.0000158119.34126.70. [DOI] [PubMed] [Google Scholar]
- 15.Marker P.C., Donjacour A.A., Dahiya R., Cunha G.R. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 16.Thomson A.A., Timms B.G., Barton L., Cunha G.R., Grace O.C. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. [DOI] [PubMed] [Google Scholar]
- 17.Donjacour A.A., Cunha G.R. Induction of prostatic morphology and secretion in urothelium by seminal vesicle mesenchyme. Development. 1995;121:2199–2207. doi: 10.1242/dev.121.7.2199. [DOI] [PubMed] [Google Scholar]
- 18.Hayward S.W., Baskin L.S., Haughney P.C., Cunha A.R., Foster B.A., Dahiya R., Prins G.S., Cunha G.R. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anat (Basel) 1996;155:81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- 19.Cunha G.R., Donjacour A.A., Cooke P.S., Mee S., Bigsby R.M., Higgins S.J., Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 20.Cunha G.R., Fujii H., Neubauer B.L., Shannon J.M., Sawyer L., Reese B.A. Epithelial-mesenchymal interactions in prostatic development, I: morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha G.R., Sekkingstad M., Meloy B.A. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differentiation. 1983;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 22.Cunha G.R., Battle E., Young P., Brody J., Donjacour A., Hayashi N., Kinbara H. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1992;1:76–83. [PubMed] [Google Scholar]
- 23.Donjacour A.A., Cunha G.R. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- 24.Kurita T., Lee K.J., Cooke P.S., Taylor J.A., Lubahn D.B., Cunha G.R. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 25.Hayward S.W., Haughney P.C., Lopes E.S., Danielpour D., Cunha G.R. The rat prostatic epithelial cell line NRP-152 can differentiate in vivo in response to its stromal environment. Prostate. 1999;39:205–212. doi: 10.1002/(sici)1097-0045(19990515)39:3<205::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Hayward S.W., Wang Y., Cao M., Hom Y.K., Zhang B., Grossfeld G.D., Sudilovsky D., Cunha G.R. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 27.Donjacour A.A., Cunha G.R. The effect of androgen deprivation on branching morphogenesis in the mouse prostate. Dev Biol. 1988;128:1–14. doi: 10.1016/0012-1606(88)90260-6. [DOI] [PubMed] [Google Scholar]
- 28.Cunha G.R., Bigsby R.M., Cooke P.S., Sugimura Y. Stromal epithelial interactions in adult organs. Cell Differ Dev. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- 29.Arnold J.T., Isaacs J.T. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell's fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong Y.C., Wang Y.Z. Growth factors and epithelial-stromal interactions in prostate cancer development. Int Rev Cytol. 2000;199:65–116. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- 31.Cunha G.R. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–1044. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Culig Z., Hobisch A., Cronauer M.V., Radmayr C., Hittmair A., Zhang J., Thurnher M., Bartsch G., Klocker H. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Story M.T., Hopp K.A., Meier D.A., Begun F.P., Lawson R.K. Influence of transforming growth factor beta 1 and other growth factors on basic fibroblast growth factor level and proliferation of cultured human prostate-derived fibroblasts. Prostate. 1993;22:183–197. doi: 10.1002/pros.2990220302. [DOI] [PubMed] [Google Scholar]
- 34.Jerde T.J., Bushman W. IL-1 induces IGF-dependent epithelial proliferation in prostate development and reactive hyperplasia. Sci Signal. 2009;2:ra49. doi: 10.1126/scisignal.2000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu S., Yeh C.R., Niu Y., Chang H.C., Tsai Y.C., Moses H.L., Shyr C.R., Chang C., Yeh S. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72:437–449. doi: 10.1002/pros.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S., Zhang C., Lin C.C., Niu Y., Lai K.P., Chang H.C., Yeh S.D., Chang C., Yeh S. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate. 2011;71:517–524. doi: 10.1002/pros.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dvorak H.F. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 38.Olumi A.F., Grossfeld G.D., Hayward S.W., Carroll P.R., Tlsty T.D., Cunha G.R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowley D.R. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 40.Tuxhorn J.A., Ayala G.E., Smith M.J., Smith V.C., Dang T.D., Rowley D.R. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 41.Bosman F.T., de Bruine A., Flohil C., van der Wurff A., ten Kate J., Dinjens W.W. Epithelial-stromal interactions in colon cancer. Int J Dev Biol. 1993;37:203–211. [PubMed] [Google Scholar]
- 42.DeCosse J.J., Gossens C., Kuzma J.F., Unsworth B.R. Embryonic inductive tissues that cause histologic differentiation of murine mammary carcinoma in vitro. J Natl Cancer Inst. 1975;54:913–922. [PubMed] [Google Scholar]
- 43.Hayashi N., Cunha G.R., Wong Y.C. Influence of male genital tract mesenchymes on differentiation of Dunning prostatic adenocarcinoma. Cancer Res. 1990;50:4747–4754. [PubMed] [Google Scholar]
- 44.Hayashi N., Cunha G.R. Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 1991;51:4924–4930. [PubMed] [Google Scholar]
- 45.Cooper M., Pinkus H. Intrauterine transplantation of rat basal cell carcinoma as a model for reconversion of malignant to benign growth. Cancer Res. 1977;37:2544–2552. [PubMed] [Google Scholar]
- 46.Arnold J.T., Lessey B.A., Seppala M., Kaufman D.G. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res. 2002;62:79–88. [PubMed] [Google Scholar]
- 47.Howlett A.R., Bissell M.J. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- 48.Ronnov-Jessen L., Petersen O.W., Bissell M.J. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 49.Ayala G., Tuxhorn J.A., Wheeler T.M., Frolov A., Scardino P.T., Ohori M., Wheeler M., Spitler J., Rowley D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- 50.Tuxhorn J.A., Ayala G.E., Rowley D.R. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 51.Gabbiani G., Hirschel B.J., Ryan G.B., Statkov P.R., Majno G. Granulation tissue as a contractile organ: a study of structure and function. J Exp Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majno G., Gabbiani G., Hirschel B.J., Ryan G.B., Statkov P.R. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173:548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- 53.Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994;190:851–853. doi: 10.1016/S0344-0338(11)80988-X. [DOI] [PubMed] [Google Scholar]
- 54.Bhowmick N.A., Chytil A., Plieth D., Gorska A.E., Dumont N., Shappell S., Washington M.K., Neilson E.G., Moses H.L. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 55.Desmouliere A., Guyot C., Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 56.Henke A., Grace O.C., Ashley G.R., Stewart G.D., Riddick A.C., Yeun H., O'Donnell M., Anderson R.A., Thomson A.A. Stromal expression of decorin, Semaphorin6D, SPARC, Sprouty1 and Tsukushi in developing prostate and decreased levels of decorin in prostate cancer. PLoS One. 2012;7:e42516. doi: 10.1371/journal.pone.0042516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H., Peehl D.M. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69:991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBeau A.M., Brennen W.N., Aggarwal S., Denmeade S.R. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8:1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakko A.J., Ricciardelli C., Mayne K., Tilley W.D., Lebaron R.G., Horsfall D.J. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 2001;61:926–930. [PubMed] [Google Scholar]
- 60.Steiner M.S., Barrack E.R. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992;6:15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- 61.Arnold J.T., Kaufman D.G., Seppala M., Lessey B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 62.Macintosh C.A., Stower M., Reid N., Maitland N.J. Precise microdissection of human prostate cancers reveals genotypic heterogeneity. Cancer Res. 1998;58:23–28. [PubMed] [Google Scholar]
- 63.Campbell I., Polyak K., Haviv I. Clonal mutations in the cancer-associated fibroblasts: the case against genetic coevolution. Cancer Res. 2009;69:6765–6768. doi: 10.1158/0008-5472.CAN-08-4253. discussion 6769. [DOI] [PubMed] [Google Scholar]
- 64.Haviv I., Polyak K., Qiu W., Hu M., Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. doi: 10.4161/cc.8.4.7669. [DOI] [PubMed] [Google Scholar]
- 65.Hu M., Yao J., Cai L., Bachman K.E., van den Brule F., Velculescu V., Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 66.Fiegl H., Millinger S., Goebel G., Muller-Holzner E., Marth C., Laird P.W., Widschwendter M. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 67.Dakhova O., Ozen M., Creighton C.J., Li R., Ayala G., Rowley D., Ittmann M. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ao M., Franco O.E., Park D., Raman D., Williams K., Hayward S.W. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 69.Franco O.E., Jiang M., Strand D.W., Peacock J., Fernandez S., Jackson R.S., 2nd, Revelo M.P., Bhowmick N.A., Hayward S.W. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barclay W.W., Woodruff R.D., Hall M.C., Cramer S.D. A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer. Endocrinology. 2005;146:13–18. doi: 10.1210/en.2004-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao J., Arnold J.T., Isaacs J.T. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 72.Li R., Wheeler T., Dai H., Frolov A., Thompson T., Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–934. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 73.Henshall S.M., Quinn D.I., Lee C.S., Head D.R., Golovsky D., Brenner P.C., Delprado W., Stricker P.D., Grygiel J.J., Sutherland R.L. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61:423–427. [PubMed] [Google Scholar]
- 74.Olapade-Olaopa E.O., MacKay E.H., Taub N.A., Sandhu D.P., Terry T.R., Habib F.K. Malignant transformation of human prostatic epithelium is associated with the loss of androgen receptor immunoreactivity in the surrounding stroma. Clin Cancer Res. 1999;5:569–576. [PubMed] [Google Scholar]
- 75.Wikstrom P., Marusic J., Stattin P., Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- 76.Wang L.G., Johnson E.M., Kinoshita Y., Babb J.S., Buckley M.T., Liebes L.F., Melamed J., Liu X.M., Kurek R., Ossowski L., Ferrari A.C. Androgen receptor overexpression in prostate cancer linked to Pur alpha loss from a novel repressor complex. Cancer Res. 2008;68:2678–2688. doi: 10.1158/0008-5472.CAN-07-6017. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Li C.X., Ye H., Chen F., Melamed J., Peng Y., Liu J., Wang Z., Tsou H.C., Wei J., Walden P., Garabedian M.J., Lee P. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12:2790–2798. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao N., Ishii K., Mirosevich J., Kuwajima S., Oppenheimer S.R., Roberts R.L., Jiang M., Yu X., Shappell S.B., Caprioli R.M., Stoffel M., Hayward S.W., Matusik R.J. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 79.Mirosevich J., Gao N., Matusik R.J. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62:339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 80.DeGraff D.J., Grabowska M.M., Case T.C., Yu X., Herrick M.K., Hayward W.J., Strand D.W., Cates J.M., Hayward S.W., Gao N., Walter M.A., Buttyan R., Yi Y., Kaestner K.H., Matusik R.J. FOXA1 deletion in luminal epithelium causes prostatic hyperplasia and alteration of differentiated phenotype. Lab Invest. 2014;94:726–739. doi: 10.1038/labinvest.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu X., Gupta A., Wang Y., Suzuki K., Mirosevich J., Orgebin-Crist M.C., Matusik R.J. Foxa1 and foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann N Y Acad Sci. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- 82.Li Y., Zhang D.Y., Ren Q., Ye F., Zhao X., Daniels G., Wu X., Dynlacht B., Lee P. Regulation of a novel androgen receptor target gene, the cyclin B1 gene, through androgen-dependent E2F family member switching. Mol Cell Biol. 2012;32:2454–2466. doi: 10.1128/MCB.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Y., Franco O.E., Jiang M., Williams K., Love H.D., Coleman I.M., Nelson P.S., Hayward S.W. Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression. Cancer Res. 2007;67:8188–8197. doi: 10.1158/0008-5472.CAN-07-0418. [DOI] [PubMed] [Google Scholar]
- 84.Cunha G.R., Hayward S.W., Dahiya R., Foster B.A. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel) 1996;155:63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- 85.Hayward S.W., Cunha G.R., Dahiya R. Normal development and carcinogenesis of the prostate: a unifying hypothesis. Ann N Y Acad Sci. 1996;784:50–62. doi: 10.1111/j.1749-6632.1996.tb16227.x. [DOI] [PubMed] [Google Scholar]
- 86.Hayward S.W., Rosen M.A., Cunha G.R. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(Suppl 2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- 87.Mohler J.L., Gaston K.E., Moore D.T., Schell M.J., Cohen B.L., Weaver C., Petrusz P. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J Urol. 2004;171:2277–2280. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]
- 88.Chang S.M., Chung L.W.K. Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology. 1989;125:2719–2727. doi: 10.1210/endo-125-5-2719. [DOI] [PubMed] [Google Scholar]
- 89.Gleave M., Hsieh J.T., Gao C.A., von E.A., Chung L.W. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753–3761. [PubMed] [Google Scholar]
- 90.Gleave M.E., Hsieh J.T., von Eschenbach A.C., Chung L.W. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol. 1992;147:1151–1159. doi: 10.1016/s0022-5347(17)37506-7. [DOI] [PubMed] [Google Scholar]
- 91.Tenniswood M. Role of epithelial-stromal interactions in the control of gene expression in the prostate: an hypothesis. Prostate. 1986;9:375–385. doi: 10.1002/pros.2990090407. [DOI] [PubMed] [Google Scholar]
- 92.Niu Y., Altuwaijri S., Yeh S., Lai K.P., Yu S., Chuang K.H., Huang S.P., Lardy H., Chang C. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci U S A. 2008;105:12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu S., Xia S., Yang D., Wang K., Yeh S., Gao Z., Chang C. Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med Oncol. 2013;30:674. doi: 10.1007/s12032-013-0674-9. [DOI] [PubMed] [Google Scholar]
- 94.Orr B., Grace O.C., Brown P., Riddick A.C., Stewart G.D., Franco O.E., Hayward S.W., Thomson A.A. Reduction of pro-tumorigenic activity of human prostate cancer-associated fibroblasts using Dlk1 or SCUBE1. Dis Model Mech. 2013;6:530–536. doi: 10.1242/dmm.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koivisto P., Kononen J., Palmberg C., Tammela T., Hyytinen E., Isola J., Trapman J., Cleutjens K., Noordzij A., Visakorpi T., Kallioniemi O.P. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 96.Eisenberger M.A., Blumenstein B.A., Crawford E.D., Miller G., McLeod D.G., Loehrer P.J., Wilding G., Sears K., Culkin D.J., Thompson I.M., Bueschen A.J., Lowe B.A. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 97.Hussain M., Tangen C.M., Berry D.L., Higano C.S., Crawford E.D., Liu G., Wilding G., Prescott S., Sundaram S.K., Small E.J., Dawson N.A., Donnelly B.J., Venner P.M., Vaishampayan U.N., Schellhammer P.F., Quinn D.I., Raghavan D., Ely B., Moinpour C.M., Vogelzang N.J., Thompson I.M. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris M.J., Huang D., Kelly W.K., Slovin S.F., Stephenson R.D., Eicher C., Delacruz A., Curley T., Schwartz L.H., Scher H.I. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur Urol. 2009;56:237–244. doi: 10.1016/j.eururo.2009.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Azoulay S., Terry S., Chimingqi M., Sirab N., Faucon H., Gil Diez de Medina S., Moutereau S., Maille P., Soyeux P., Abbou C., Salomon L., Vacherot F., de La Taille A., Loric S., Allory Y. Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J Pathol. 2008;216:460–470. doi: 10.1002/path.2427. [DOI] [PubMed] [Google Scholar]