Abstract
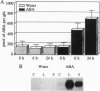
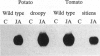
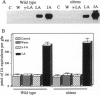
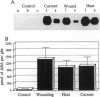
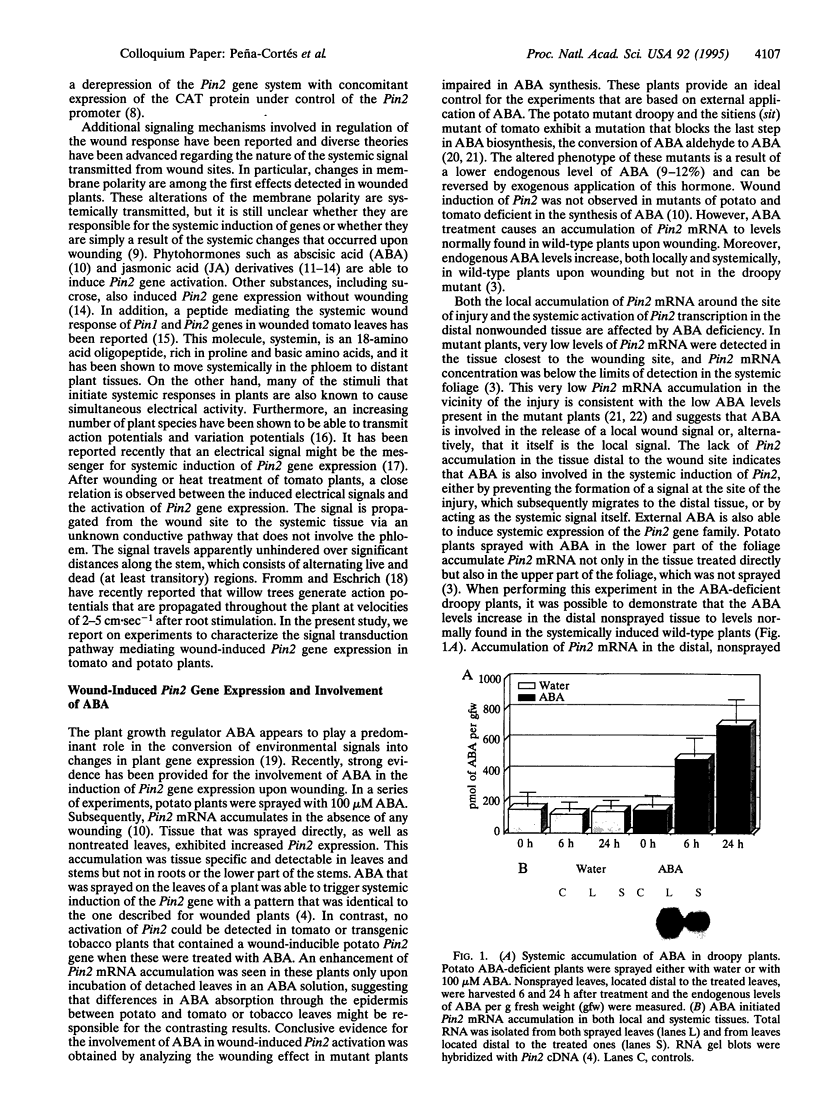
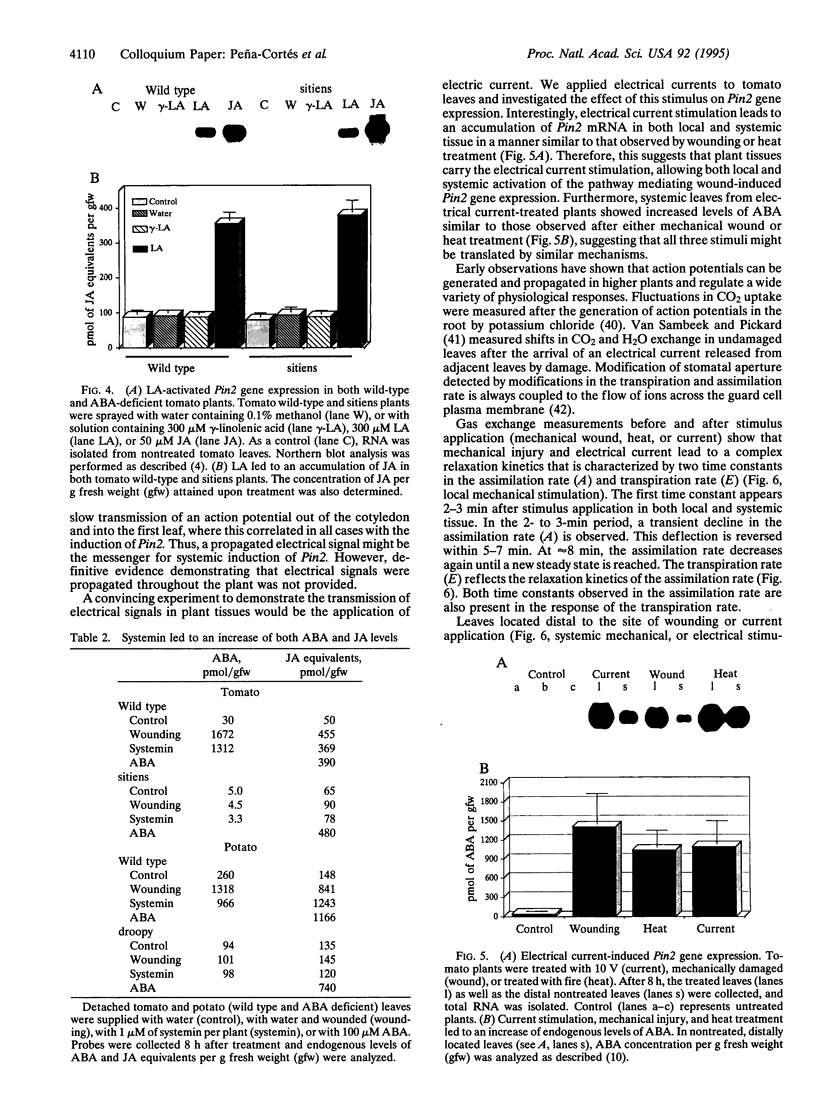
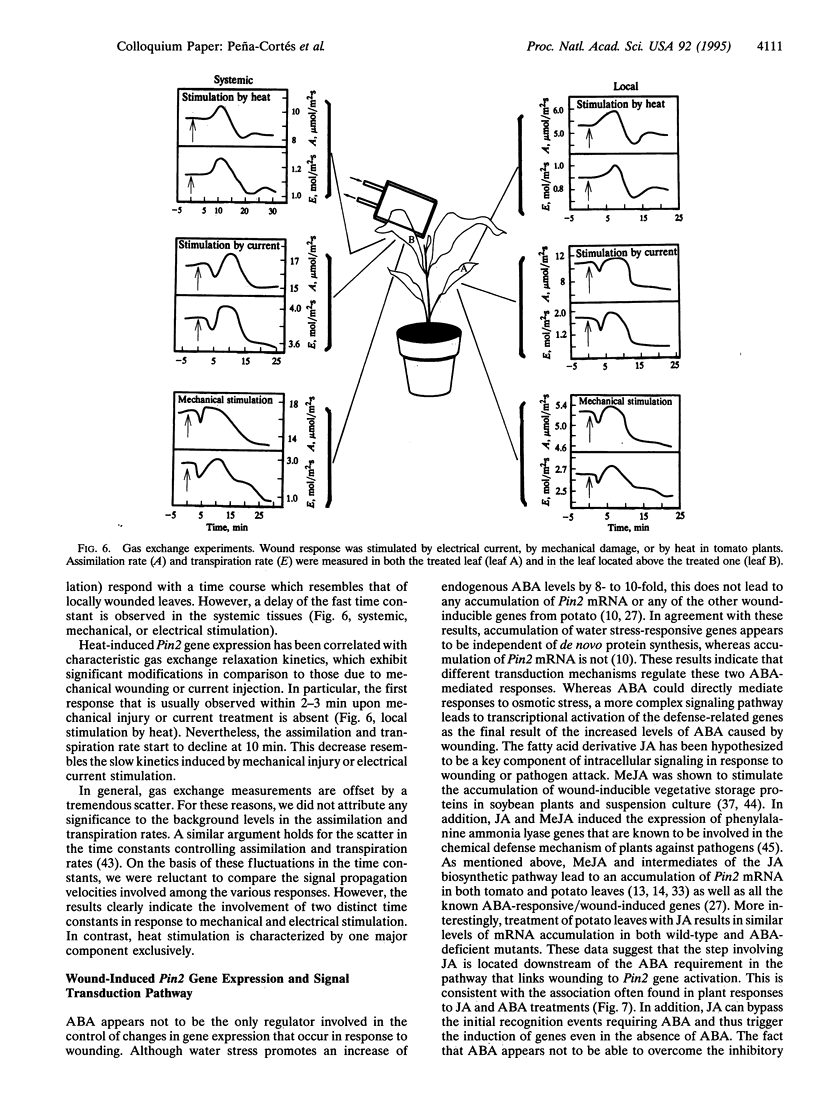
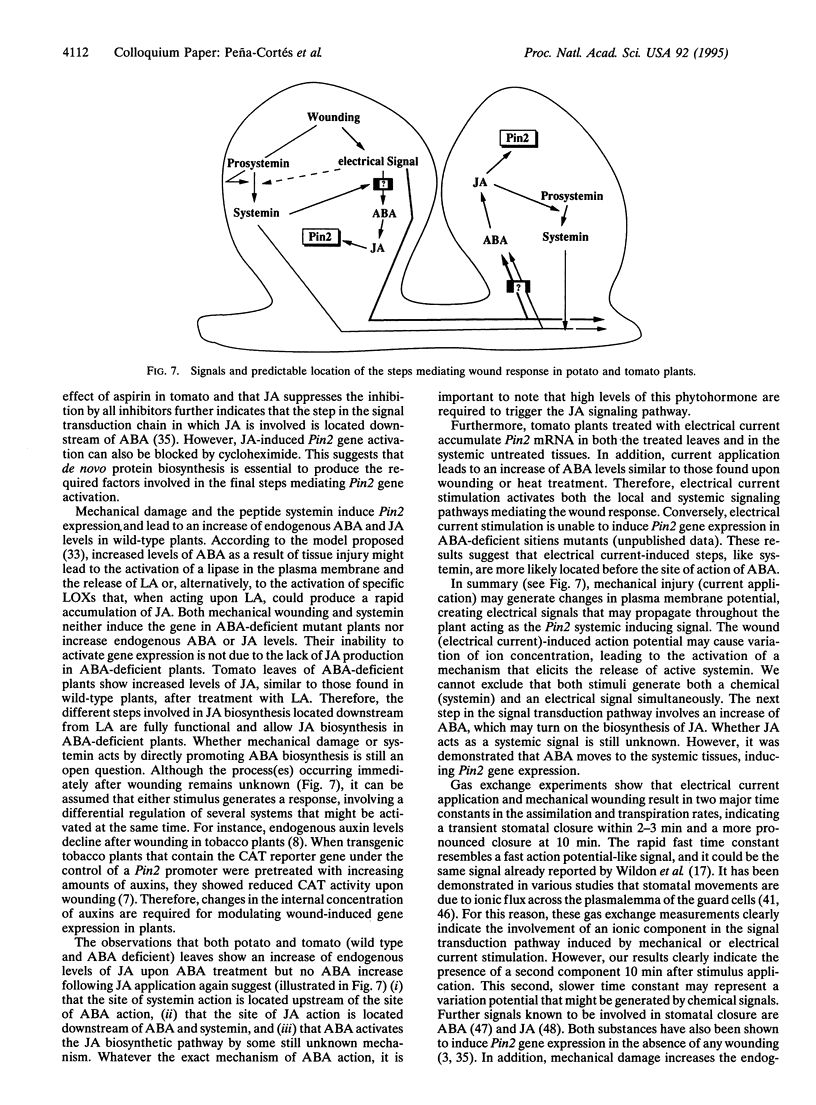
Chemical and physical signals have been reported to mediate wound-induced proteinase inhibitor II (Pin2) gene expression in tomato and potato plants. Among the chemical signals, phytohormones such as abscisic acid (ABA) and jasmonic acid (JA) and the peptide systemin represent the best characterized systems. Furthermore, electrical and hydraulic mechanisms have also been postulated as putative Pin2-inducing systemic signals. Most of the chemical agents are able to induce Pin2 gene expression without any mechanical wounding. Thus, ABA, JA, and systemin initiate Pin2 mRNA accumulation in the directly treated leaves and in the nontreated leaves (systemic) that are located distal to the treated ones. ABA-deficient tomato and potato plants do not respond to wounding by accumulation of Pin2 mRNA, therefore providing a suitable model system for analysis of the signal transduction pathway involved in wound-induced gene activation. It was demonstrated that the site of action of JA is located downstream to the site of action of ABA. Moreover, systemin represents one of the initial steps in the signal transduction pathway regulating the wound response. Recently, it was reported that heat treatment and mechanical injury generate electrical signals, which propagate throughout the plant. These signals are capable of inducing Pin2 gene expression in the nontreated leaves of wounded plants. Furthermore, electrical current application to tomato leaves leads to an accumulation of Pin2 mRNA in local and systemic tissues. Examination of photosynthetic parameters (assimilation and transpiration rate) on several types of stimuli suggests that heat-induced Pin2 gene expression is regulated by an alternative pathway from that mediating the electrical current and mechanical wound response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann S. M. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Broekaert I., Lee H. I., Kush A., Chua N. H., Raikhel N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc Natl Acad Sci U S A. 1990 Oct;87(19):7633–7637. doi: 10.1073/pnas.87.19.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero M. J., Raventós D., San Segundo B. Expression of a maize proteinase inhibitor gene is induced in response to wounding and fungal infection: systemic wound-response of a monocot gene. Plant J. 1994 Aug;6(2):141–150. doi: 10.1046/j.1365-313x.1994.6020141.x. [DOI] [PubMed] [Google Scholar]
- Creelman R. A., Tierney M. L., Mullet J. E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Johnson R. R., Ryan C. A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic Acid. Plant Physiol. 1992 Mar;98(3):995–1002. doi: 10.1104/pp.98.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildmann T., Ebneth M., Peña-Cortés H., Sánchez-Serrano J. J., Willmitzer L., Prat S. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell. 1992 Sep;4(9):1157–1170. doi: 10.1105/tpc.4.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M., Sánchez-Serrano J. J., Willmitzer L. Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J. 1989 May;8(5):1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan A., Thornburg R. W. Auxin Levels Regulate the Expression of a Wound-Inducible Proteinase Inhibitor II-Chloramphenicol Acetyl Transferase Gene Fusion in Vitro and in Vivo. Plant Physiol. 1989 Sep;91(1):73–78. doi: 10.1104/pp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J., Pearce G., Ryan C. A., Browse J. Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol Gen Genet. 1993 Dec;241(5-6):595–601. doi: 10.1007/BF00279902. [DOI] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Pena-Cortes H., Willmitzer L., Sanchez-Serrano J. J. Abscisic Acid Mediates Wound Induction but Not Developmental-Specific Expression of the Proteinase Inhibitor II Gene Family. Plant Cell. 1991 Sep;3(9):963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E., Kolattukudy P. E. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet. 1989 Jun;217(2-3):223–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Jasmonate, genes, and fragrant signals. Plant Physiol. 1992 Jul;99(3):804–807. doi: 10.1104/pp.99.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. R., Twomey L. T. Age changes in lumbar zygapophyseal joints. Observations on structure and function. Spine (Phila Pa 1976) 1986 Sep;11(7):739–745. doi: 10.1097/00007632-198609000-00014. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., Li X. Wounding Nicotiana tabacum Leaves Causes a Decline in Endogenous Indole-3-Acetic Acid. Plant Physiol. 1991 Jul;96(3):802–805. doi: 10.1104/pp.96.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]
- Xu D., McElroy D., Thornburg R. W., Wu R. Systemic induction of a potato pin2 promoter by wounding, methyl jasmonate, and abscisic acid in transgenic rice plants. Plant Mol Biol. 1993 Jul;22(4):573–588. doi: 10.1007/BF00047399. [DOI] [PubMed] [Google Scholar]