Abstract
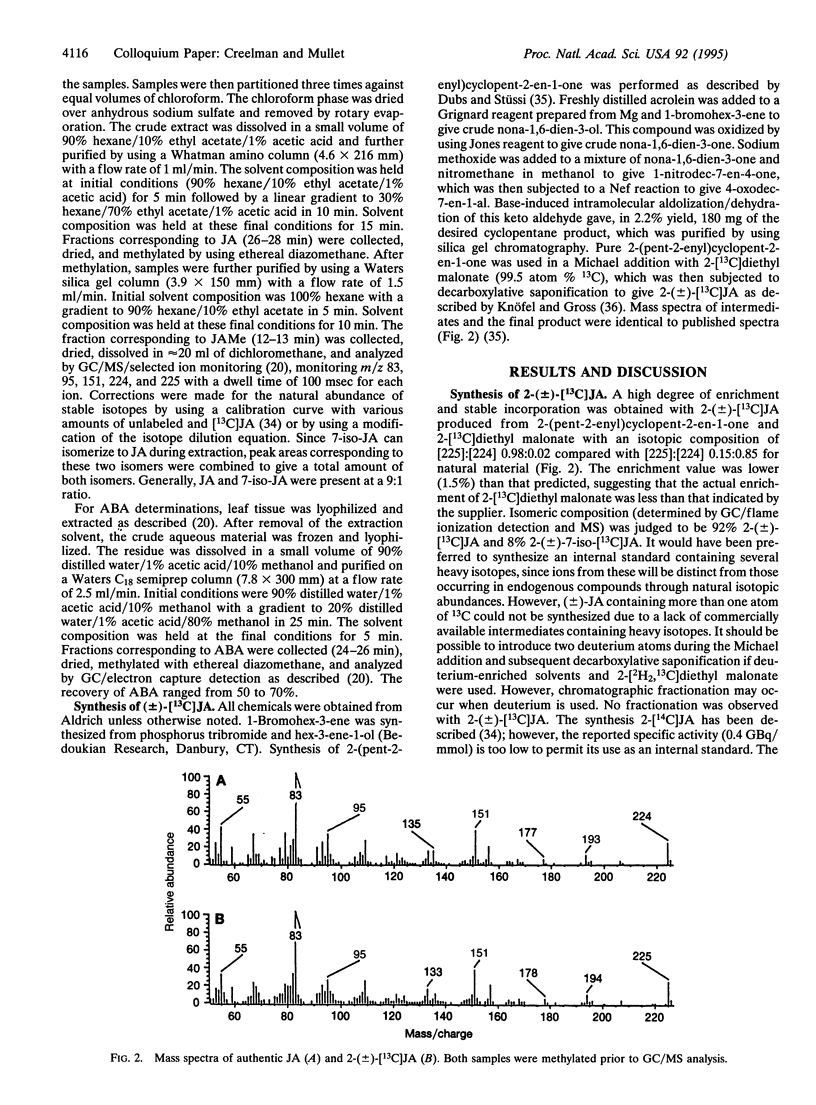
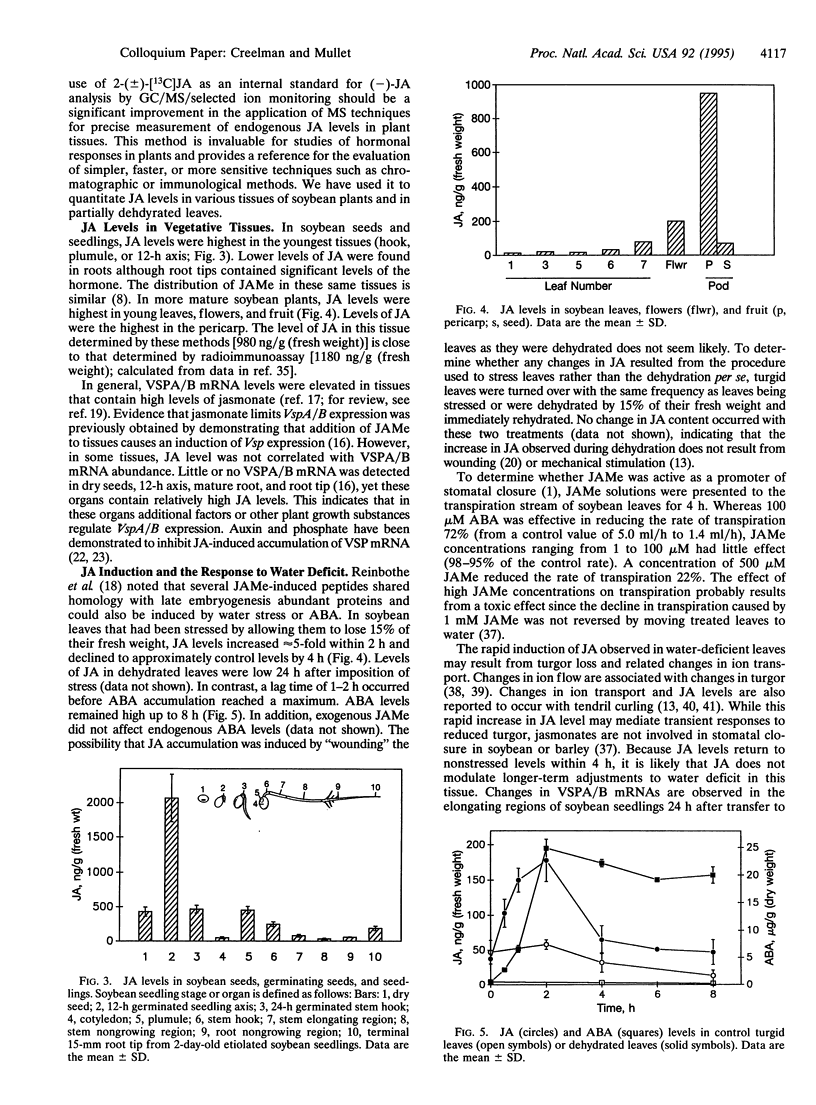
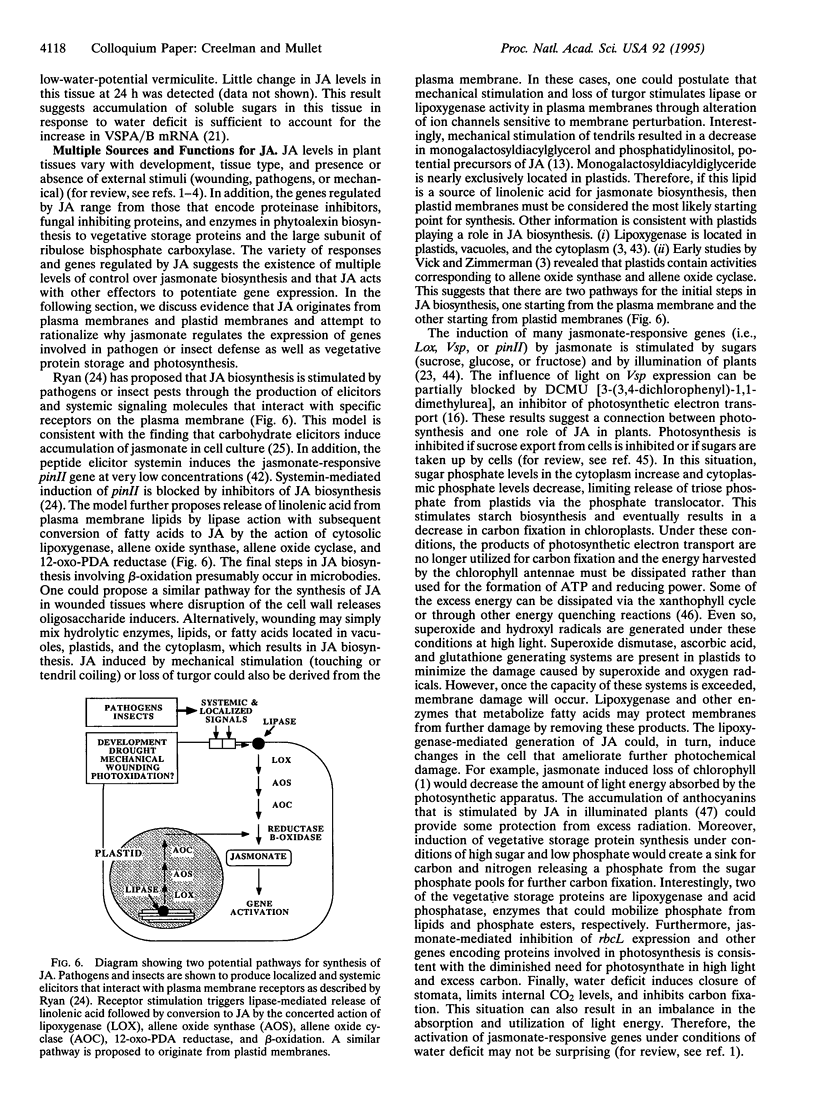
Jasmonic acid (JA) is a naturally occurring growth regulator found in higher plants. Several physiological roles have been described for this compound (or a related compound, methyl jasmonate) during plant development and in response to biotic and abiotic stress. To accurately determine JA levels in plant tissue, we have synthesized JA containing 13C for use as an internal standard with an isotopic composition of [225]:[224] 0.98:0.02 compared with [225]:[224] 0.15:0.85 for natural material. GC analysis (flame ionization detection and MS) indicate that the internal standard is composed of 92% 2-(+/-)-[13C]JA and 8% 2-(+/-)-7-iso-[13C]JA. In soybean plants, JA levels were highest in young leaves, flowers, and fruit (highest in the pericarp). In soybean seeds and seedlings, JA levels were highest in the youngest organs including the hypocotyl hook, plumule, and 12-h axis. In soybean leaves that had been dehydrated to cause a 15% decrease in fresh weight, JA levels increased approximately 5-fold within 2 h and declined to approximately control levels by 4 h. In contrast, a lag time of 1-2 h occurred before abscisic acid accumulation reached a maximum. These results will be discussed in the context of multiple pathways for JA biosynthesis and the role of JA in plant development and responses to environmental signals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen I., Becker W., Schlüter K., Burges J., Parthier B., Apel K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare). Plant Mol Biol. 1992 May;19(2):193–204. doi: 10.1007/BF00027341. [DOI] [PubMed] [Google Scholar]
- Bell E., Mullet J. E. Lipoxygenase gene expression is modulated in plants by water deficit, wounding, and methyl jasmonate. Mol Gen Genet. 1991 Dec;230(3):456–462. doi: 10.1007/BF00280303. [DOI] [PubMed] [Google Scholar]
- Choi D., Bostock R. M., Avdiushko S., Hildebrand D. F. Lipid-derived signals that discriminate wound- and pathogen-responsive isoprenoid pathways in plants: methyl jasmonate and the fungal elicitor arachidonic acid induce different 3-hydroxy-3-methylglutaryl-coenzyme A reductase genes and antimicrobial isoprenoids in Solanum tuberosum L. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2329–2333. doi: 10.1073/pnas.91.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Hedrich R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta. 1991 Dec;186(1):143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]
- Creelman R. A., Tierney M. L., Mullet J. E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWald D. B., Sadka A., Mullet J. E. Sucrose Modulation of Soybean Vsp Gene Expression Is Inhibited by Auxin. Plant Physiol. 1994 Feb;104(2):439–444. doi: 10.1104/pp.104.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes H. D., Koetje D. S., Franceschi V. R. Expression, activity, and cellular accumulation of methyl jasmonate-responsive lipoxygenase in soybean seedlings. Plant Physiol. 1992 Sep;100(1):433–443. doi: 10.1104/pp.100.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Gardner H. W. Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochim Biophys Acta. 1992 Nov 11;1165(1):1–18. doi: 10.1016/0005-2760(92)90069-8. [DOI] [PubMed] [Google Scholar]
- Horton R. F. Methyl jasmonate and transpiration in barley. Plant Physiol. 1991 Aug;96(4):1376–1378. doi: 10.1104/pp.96.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J., Galston A. W. Physiological studies on pea tendrils. I. Growth and coiling following mechanical stimulation. Plant Physiol. 1966 Jun;41(6):1014–1025. doi: 10.1104/pp.41.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Ryan C. A. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990 Apr;14(4):527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Koda Y. The role of jasmonic acid and related compounds in the regulation of plant development. Int Rev Cytol. 1992;135:155–199. doi: 10.1016/s0074-7696(08)62040-9. [DOI] [PubMed] [Google Scholar]
- Mason H. S., DeWald D. B., Mullet J. E. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell. 1993 Mar;5(3):241–251. doi: 10.1105/tpc.5.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Dewald D. B., Creelman R. A., Mullet J. E. Coregulation of soybean vegetative storage protein gene expression by methyl jasmonate and soluble sugars. Plant Physiol. 1992 Mar;98(3):859–867. doi: 10.1104/pp.98.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Peever T. L., Higgins V. J. Electrolyte Leakage, Lipoxygenase, and Lipid Peroxidation Induced in Tomato Leaf Tissue by Specific and Nonspecific Elicitors from Cladosporium fulvum. Plant Physiol. 1989 Jul;90(3):867–875. doi: 10.1104/pp.90.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S., Reinbothe C., Heintzen C., Seidenbecher C., Parthier B. A methyl jasmonate-induced shift in the length of the 5' untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J. 1993 Apr;12(4):1505–1512. doi: 10.1002/j.1460-2075.1993.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. The search for the proteinase inhibitor-inducing factor, PIIF. Plant Mol Biol. 1992 May;19(1):123–133. doi: 10.1007/BF00015610. [DOI] [PubMed] [Google Scholar]
- Sadka A., DeWald D. B., May G. D., Park W. D., Mullet J. E. Phosphate Modulates Transcription of Soybean VspB and Other Sugar-Inducible Genes. Plant Cell. 1994 May;6(5):737–749. doi: 10.1105/tpc.6.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. C., Brash A. R. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science. 1991 Aug 16;253(5021):781–784. doi: 10.1126/science.1876834. [DOI] [PubMed] [Google Scholar]
- Staswick P. E., Su W., Howell S. H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Chang PFL., Liu D., Narasimhan M. L., Raghothama K. G., Hasegawa P. M., Bressan R. A. Plant Defense Genes Are Synergistically Induced by Ethylene and Methyl Jasmonate. Plant Cell. 1994 Aug;6(8):1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]



