Abstract
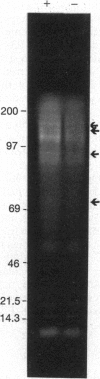
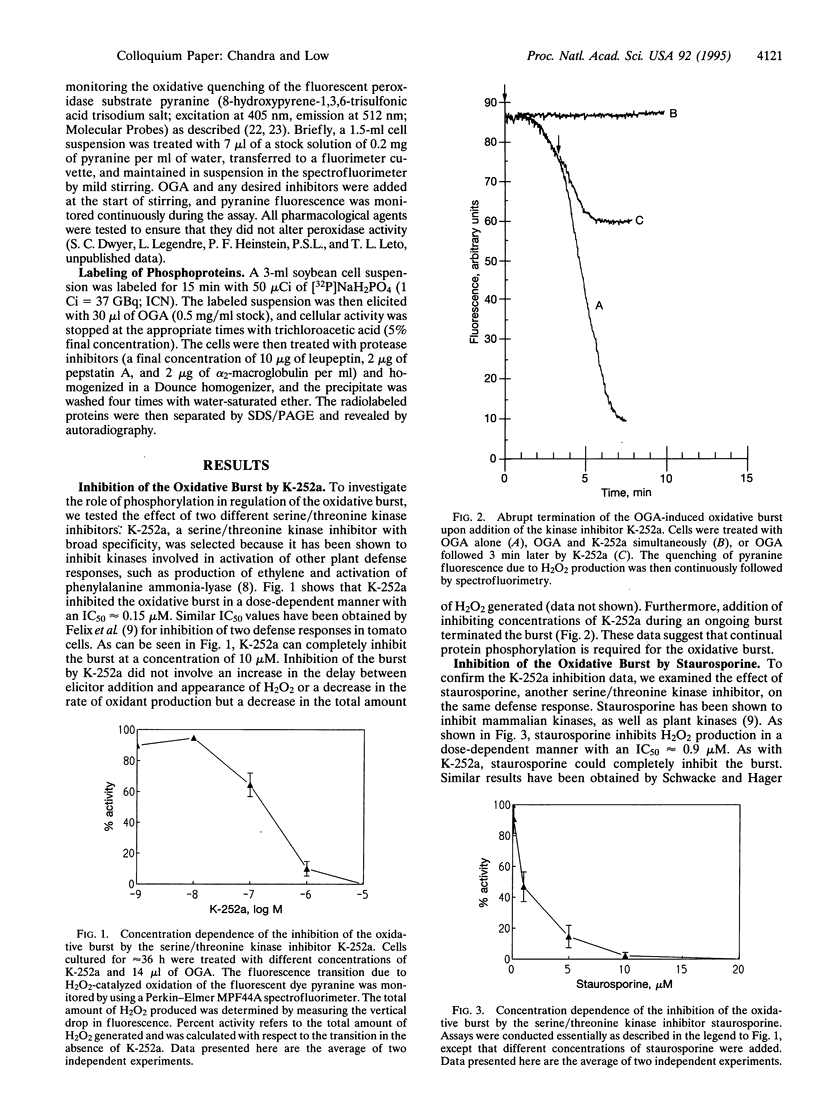
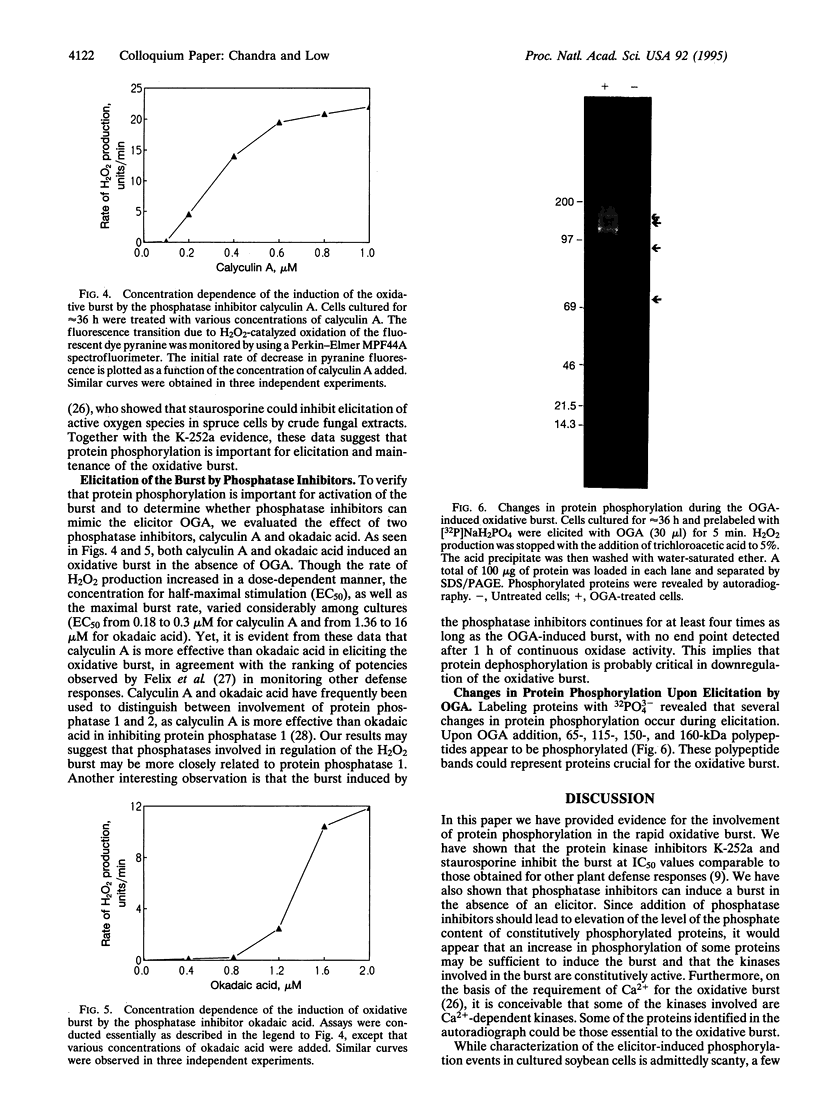
The oxidative burst is likely the most rapid defense response mounted by a plant under pathogen attack, and the generated oxidant species may be essential to several subsequent defense responses. In our effort to characterize the signal-transduction pathways leading to rapid H2O2/O2- biosynthesis, we have examined the role of protein phosphorylation in this resistance mechanism. K-252a and staurosporine, two protein-kinase inhibitors, were found to block the oxidative burst in a concentration-dependent manner. When added during H2O2 generation, the burst was observed to rapidly terminate, suggesting that continuous phosphorylation was essential for its maintenance. Importantly, phosphatase inhibitors (calyculin A and okadaic acid) were found to induce the oxidative burst in the absence of any additional stimulus. This may suggest that certain kinases required for the burst are constitutively active and that stabilization of the phosphorylated forms of their substrates is all that is required for burst activity. In autoradiographs of elicited and unstimulated cells equilibrated with 32PO4(3-), several phosphorylated polypeptide bands were revealed that could represent proteins essential for the burst.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderberg R. J., Walker-Simmons M. K. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10183–10187. doi: 10.1073/pnas.89.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Kjellbom P., Lamb C. J. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992 Jul 10;70(1):21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Conrath U., Jeblick W., Kauss H. The protein kinase inhibitor, K-252a, decreases elicitor-induced Ca2+ uptake and K+ release, and increases coumarin synthesis in parsley cells. FEBS Lett. 1991 Feb 11;279(1):141–144. doi: 10.1016/0014-5793(91)80269-9. [DOI] [PubMed] [Google Scholar]
- Coughlan S., Hind G. Phosphorylation of thylakoid proteins by a purified kinase. J Biol Chem. 1987 Jun 15;262(17):8402–8408. [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Drøbak B. K. Phosphoinositides and protein phosphorylation in plant signal transduction. Semin Cell Biol. 1993 Apr;4(2):123–130. doi: 10.1006/scel.1993.1015. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Moloshok T. D., Saxton M. J., Ryan C. A. Oligosaccharide signaling in plants. Specificity of oligouronide-enhanced plasma membrane protein phosphorylation. J Biol Chem. 1991 Feb 15;266(5):3140–3145. [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Regenass M., Spanu P., Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskopf D. G., Felix G., Boller T. K-252a inhibits the response of tomato cells to fungal elicitors in vivo and their microsomal protein kinase in vitro. FEBS Lett. 1990 Nov 26;275(1-2):177–180. doi: 10.1016/0014-5793(90)81466-2. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kieber J. J., Rothenberg M., Roman G., Feldmann K. A., Ecker J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993 Feb 12;72(3):427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Legendre L., Heinstein P. F., Low P. S. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem. 1992 Oct 5;267(28):20140–20147. [PubMed] [Google Scholar]
- Legendre L., Rueter S., Heinstein P. F., Low P. S. Characterization of the Oligogalacturonide-Induced Oxidative Burst in Cultured Soybean (Glycine max) Cells. Plant Physiol. 1993 May;102(1):233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L., Yueh Y. G., Crain R., Haddock N., Heinstein P. F., Low P. S. Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem. 1993 Nov 25;268(33):24559–24563. [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Lu D. J., Takai A., Leto T. L., Grinstein S. Modulation of neutrophil activation by okadaic acid, a protein phosphatase inhibitor. Am J Physiol. 1992 Jan;262(1 Pt 1):C39–C49. doi: 10.1152/ajpcell.1992.262.1.C39. [DOI] [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993 Nov 26;262(5138):1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C. Active Oxygen Species in Plant Defense against Pathogens. Plant Physiol. 1994 Jun;105(2):467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K., Leube M. P., Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994 Jun 3;264(5164):1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Reymond P., Short T. W., Briggs W. R., Poff K. L. Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1992 May;89(10):4718–4721. doi: 10.1073/pnas.89.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J. L., Rivin C. J., Sessions R. A., Feldmann K. A., Zambryski P. C. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell. 1993 Dec 3;75(5):939–950. doi: 10.1016/0092-8674(93)90537-z. [DOI] [PubMed] [Google Scholar]
- Stein J. C., Howlett B., Boyes D. C., Nasrallah M. E., Nasrallah J. B. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]