Abstract
The formation of dormant endospores is a complex morphological process that permits long-term survival in inhospitable environments for many Gram-positive bacteria. Sporulation for the anaerobic gastrointestinal pathogen Clostridium difficile is necessary for survival outside of the gastrointestinal tract of its host. While the developmental stages of spore formation are largely conserved amongst endospore-forming bacteria, the genus Clostridium appears to be missing a number of conserved regulators required for efficient sporulation in other spore forming bacteria. Several recent studies have discovered novel mechanisms and distinct regulatory pathways that control the initiation of sporulation and early sporulation-specific gene expression. These differences in regulating the decision to undergo sporulation reflects the unique ecological niche and environmental conditions that C. difficile inhabits and encounters within the mammalian host.
Keywords: Spores, Endospore, Anaerobe, Sporulation, Nutrition, Phosphorylation
Introduction
Clostridium difficile is a significant gastrointestinal pathogen that infects humans and other animals and is the primary cause of antibiotic associated diarrhea (AAD). C. difficile infection (CDI) is typically precipitated by the use of antibiotics, which disrupts the native gut microbiota, providing a niche for C. difficile overgrowth and toxin production. CDI and AAD have recently become an increasing health problem in hospital and nursing home settings (O'Brien et al., 2007, Bouza, 2012, Dubberke & Olsen, 2012, Murphy et al., 2012). In addition, C. difficile has recently been recognized as an emerging pathogen and urgent public health threat by the CDC (CDC, 2013). Although C. difficile is an important pathogen, its growth and lifecycle within the host remain poorly understood.
C. difficile is a strict anaerobe that forms metabolically inactive spores within the gastrointestinal tract of mammals. These dormant spores are naturally resistant to a variety of environmental and chemical insults, including exposure to oxygen, disinfectants, desiccation and extreme temperatures (Lawley et al., 2009). The ability for C. difficile to form spores is critical for survival outside of the host and for transmission from host to host (Deakin et al., 2012). Spore formation is a factor in C. difficile resistance to traditional antibiotic therapies and also contributes to recurrent infection (Deakin et al., 2012). Further, sporulation-specific gene expression is upregulated early in the mouse model of CDI (Janoir et al., 2013). Despite the importance of sporulation in the pathogenesis of C. difficile, the triggers and molecular mechanisms that govern the initiation of spore formation are not well understood.
Our limited understanding of C. difficile sporulation is primarily based on comparisons with other spore-forming bacteria. In the model organism Bacillus subtilis, sporulation is a complex morphological event that is tightly controlled by multiple regulators, checkpoints and feedback loops. The sporulation process is demarcated into several stages based on physiological landmarks. The physiological changes within the cell are accomplished through compartmentalized transcription programs within the mother cell and the developing endospore compartments. These transcription programs are orchestrated via the four sporulation-specific sigma factors: σF, σE, σG and σK. The C. difficile genome encodes the four conserved sporulation-specific sigma factors and readily forms heat-resistant spores within mammalian intestinal tracts (Fimlaid et al., 2013, Pereira et al., 2013, Saujet et al., 2013). Although the sporulation specific sigma factors are highly conserved in C. difficile, recent global studies revealed that divergent regulatory mechanisms mediate the timing of expression and activation of these sigma factors compared to other Clostridium and Bacillus species (Fimlaid et al., 2013, Pereira et al., 2013, Saujet et al., 2013).
In B. subtilis, spore formation begins as the cells transition from exponential to stationary growth phase (Stage 0). At Stage 0, gene expression shifts to support spore formation, rather than vegetative growth. Stage 0 is defined by the post-translational activation of the transcription factor, Spo0A, which upregulates sporulation-specific gene expression and serves as the master regulator of sporulation. Because spore formation is an energy-intensive process and is irreversible at specific points in spore development, the decision to initiate sporulation involves the integration of multiple environmental signals to determine whether conditions are unfavorable to support further vegetative cell growth. In B. subtilis and other Bacillus species, Spo0A activity is tightly regulated through a phosphorylation-mediated signal transduction pathway in response to nutrient availability, cell density and other signals (Sonenshein, 2000). Importantly, the C. difficile genome lacks the B. subtilis phosphorelay orthologs, and instead, Spo0A activity appears to be controlled through at least three orphan histidine kinases and potentially other unidentified factors (Paredes et al., 2005, Underwood et al., 2009).
Genomic analyses have revealed that C. difficile does not possess many of the conserved early stage regulatory components required for Spo0A activation and efficient sporulation in other spore forming bacteria (Paredes et al., 2005, Galperin et al., 2012). In the last few years, researchers have begun to elucidate the functions of C. difficile early sporulation orthologs in controlling initiation through the regulation of spo0A transcription and Spo0A phosphorylation. Understanding the genetic pathways and environmental conditions that lead to Spo0A activation in C. difficile is key for designing targeted therapeutics to inhibit spore formation and thus, limit the spread of the disease.
Spo0A: the master regulator of sporulation
Several studies characterizing C. difficile sporulation have determined that some of the most conserved global regulators of sporulation play similar roles as found in other spore formers (Saujet et al., 2011, Antunes et al., 2012, Deakin et al., 2012). In particular, studies of Spo0A-dependent regulation have revealed that while the C. difficile Spo0A regulon has significant overlap with B. subtilis, there are differences in early sporulation gene regulation and in the role Spo0A may play in sigma factor activation and late stage gene expression (Fimlaid et al., 2013, Pereira et al., 2013, Saujet et al., 2013, Pettit et al., 2014). All sequenced C. difficile genomes contain the highly conserved Spo0A transcription factor and possess both the N-terminal phosphorylation and dimerization domain and the C-terminal DNA-binding domain. As in Bacillus species, C. difficile spo0A mutants are asporogenous and fail to activate stationary phase and early sporulation gene transcription (Heap et al., 2007, Deakin et al., 2012, Rosenbusch et al., 2012, Fimlaid et al., 2013, Pettit et al., 2014). The C. difficile Spo0A protein recognizes a consensus sequence similar to the 0A box defined in B. subtilis and directly binds with high affinity and specificity to the promoter regions of itself (spo0A), sigH (σH) and the early sporulation genes that control σF and σE activation (spoIIAA, spoIIE and spoIIGA; (Rosenbusch et al., 2012). Global transcriptional analyses have further defined the C. difficile Spo0A regulon to include additional conserved early sporulation genes, such as those encoding σF and σE, and a negative regulator of Spo0A activity, sinR (Pettit et al., 2014). The Spo0A regulon also contains genes that are unique to C. difficile, such as CD1492 and CD1579, which encode the orphan histidine kinases that directly or indirectly influence Spo0A phosphorylation (see below; (Underwood et al., 2009, Pettit et al., 2014). Unlike B. subtilis, the conditions that facilitate synchronous in vitro sporulation of C. difficile have not been identified, making it difficult to detect small changes in gene transcription during spore development (Fimlaid et al., 2013, Putnam et al., 2013, Saujet et al., 2013). As a result, there are likely many more transcripts in the Spo0A regulon that remain to be identified.
Spo0A also regulates physiological processes other than sporulation, including biofilm formation (Dawson et al., 2012, Dapa & Unnikrishnan, 2013), motility (Pettit et al., 2014), carbon metabolism (e.g. butyrate biosynthesis; (Pettit et al., 2014) and, in some cases, toxin A (TcdA) and toxin B (TcdB) production (Deakin et al., 2012, Mackin et al., 2013, Pettit et al., 2014). Interestingly, Spo0A can repress toxin production in C. difficile, but this regulation appears to occur primarily in the ribotype 027 epidemic strains and not in other evolutionarily divergent strains, such as ribotype 012 (630Δerm) or 078 (JGS6133; (Deakin et al., 2012, Rosenbusch et al., 2012, Mackin et al., 2013). Conversely, two studies report Spo0A-dependent regulation of tcdA expression in 630Δerm (Underwood et al., 2009, Pettit et al., 2014), but this effect is likely indirect as no Spo0A consensus sequences are identified upstream of tcdA (Rosenbusch et al., 2012). The inconsistencies observed in Spo0A-mediated regulation of toxin production can be partly attributed to differences in the growth medium used and the experimental conditions tested. These differences underscore the need for standard assays and growth conditions for the study of C. difficile sporulation, and highlight the need for complementation studies in this research.
Global regulators of stationary phase control expression of early sporulation-specific genes
Along with Spo0A, the transition phase sigma factor, σH, shares responsibility for upregulating expression of sporulation-related genes as well as mediating the transcriptional changes that occur during the switch from exponential to stationary phase growth. C. difficile SigH recognizes a similar consensus sequence to that in B. subtilis (Saujet et al., 2011). SigH positively regulates transcription of some early sporulation genes in C. difficile, including spo0A, a putative Spo0A histidine kinase, the putative sinRI operon as well as spo0J and soj, whose gene products are likely involved in chromosomal segregation during asymmetric division (Saujet et al., 2011). These data suggest that SigH positively contributes to sporulation initiation through multiple pathways by directly inducing spo0A gene expression and positively influencing Spo0A activity through increased transcription of the Spo0A-associated kinase, CD2492. In addition, SigH has a negative effect on expression of the app operon, which encodes a predicted oligopeptide permease (see below; (Pereira et al., 2013). Finally, the reciprocal control between SigH and Spo0A creates a positive feed-forward loop, with SigH activating spo0A transcription and Spo0A upregulating sigH gene expression. This regulatory circuitry reinforces the global transcriptional changes necessary to initiate sporulation.
There are at least two additional conserved global regulators that influence sporulation in Bacillus and Clostridium species: CcpA and CodY. Both of these transcriptional regulators share a number of regulatory targets in C. difficile, including the regulation of toxin synthesis (Dineen et al., 2007, Dineen et al., 2010, Antunes et al., 2012). Catabolite control protein A (CcpA) is a DNA-binding transcriptional regulator that governs the global response to carbon availability in low G+C Gram-positive organisms. CcpA controls expression of genes involved in sugar uptake, fermentation and amino acid metabolism in the presence of preferred carbon sources such as glucose and other phosphotransferase system (PTS) sugars (Antunes et al., 2011, Antunes et al., 2012). In B. subtilis, CcpA activity is linked to carbon availability through the direct interaction with a corepressor, HPr-Ser-P, the phosphorylated form of HPr (Fujita et al., 1995). HPr phosphorylation occurs in the presence of fructose-1,6-biphosphate (FBP) which triggers HPr serine kinase/phosphatase activity of the HprK/P protein (Deutscher & Saier, 1983). In contrast to B. subtilis, the DNA binding affinity of the C. difficile CcpA protein is enhanced by FBP in vitro, but not by the presence of HPr-Ser-P (Antunes et al., 2011). Although the molecular mechanism of this interaction is not yet understood, CcpA function in C. difficile appears to be regulated by the same carbon metabolite as in B. subtilis.
In the absence of glucose, CcpA is required for efficient sporulation in C. perfringens (Varga et al., 2004), but CcpA downregulates sporulation in C. difficile (Antunes et al., 2012), demonstrating that unique regulatory processes can control sporulation initiation in different Clostridium species. In C. difficile, CcpA binds to conserved recognition sequences known as catabolite responsive elements (creCD) and directly represses transcription of many stationary phase transcripts, including spo0A and the opp operon, which encodes a predicted oligopeptide transporter system (Antunes et al., 2012). CcpA also represses expression of the putative Spo0A histidine kinase CD1579 and the sinR transcriptional regulator, but these interactions are likely indirect as no creCD motifs are apparent in the respective promoter regions (Antunes et al. 2012). Transcription of genes involved in later stages of sporulation are also repressed by CcpA (Antunes et al., 2012). It is important to note that while CcpA-mediated catabolite repression of sporulation has been observed in Bacilli and Clostridia, glucose-mediated repression of sporulation genes occurs independently of CcpA in B. subtilis, C. difficile and C. perfringens (Moreno et al., 2001, Varga et al., 2004, Antunes et al., 2012). In B. subtilis, the primary mediator of this physiological response appears to be the transcriptional regulator ScoC, which represses sporulation gene expression in a glucose-dependent manner (see below; (Shafikhani et al., 2003).
CodY is another conserved global regulator that responds to nutrient availability in C. difficile and other Gram-positive bacteria (Sonenshein, 2005, Dineen et al., 2007, Dineen et al., 2010). CodY, along with the cofactors GTP and branched-chain amino acids (BCAAs), represses gene expression in high nutrient conditions (Dineen et al., 2007, Dineen et al., 2010). When nutrients become limiting, such as during the entry into stationary phase, the intracellular concentrations of GTP and BCAAs decreases, thereby relieving CodY-mediated repression (Sonenshein, 2007). A global analysis that combined both in vivo and in vitro methods to define the CodY regulon revealed that CodY directly binds to and regulates expression of the CD2492 histidine kinase, a Rap phosphatase ortholog (CD2123) and the opp operon (Dineen et al., 2010). While it is clear that both CcpA and CodY affect sporulation of C. difficile by regulating expression of sporulation-related genes, the intricate regulatory networks that connect CcpA and CodY with the initiation of sporulation includes hundreds of directly regulated transcripts and many more indirect targets.
Post-translational activation of Spo0A
In the genus Bacillus, the regulatory pathway controlling Spo0A phosphorylation is known as the sporulation phosphorelay. The phosphorelay is a variant of a two-component signal transduction system (TCS) comprised of five sensor kinases (KinA-E), which respond to specific ligands, allowing multiple signals to converge and influence Spo0A activity (Perego et al., 1989, Burbulys et al., 1991, Kobayashi et al., 1995, LeDeaux & Grossman, 1995, LeDeaux et al., 1995, Jiang et al., 2000). KinA-E directly phosphorylate Spo0F which subsequently transfers the phosphate to Spo0A through an additional phosphotransferase, Spo0B (Burbulys et al., 1991). Despite the conservation of Spo0A and the similarities between Spo0A targets in Bacillus and Clostridium species, the components of the Bacillus phosphorelay do not appear to be encoded within C. difficile or the other Clostridial genomes. Based on the lack of an apparent phosphorelay, the favored hypothesis is that the sporulation initiation pathway in Clostridium species functions more similarly to a traditional TCS in which sporulation-associated sensor kinases recognize specific internal or environmental signals and directly phosphorylate Spo0A (Worner et al., 2006, Steiner et al., 2011). Supporting this hypothesis, three of the five orphan histidine kinases (CD1492, CD1579 and CD2492) present in the C. difficile genome share some sequence identity with KinA-E (Underwood et al., 2009). Based on in vitro biochemical assays and in vivo sporulation studies, the CD1579 and CD2492 kinases are anticipated to directly phosphorylate Spo0A (Underwood et al., 2009). CD1579 is predicted to be a cytosolic protein and contains a degenerate heme-oxygen-sensing PAS domain ((Underwood et al., 2009, Edwards et al., 2012). The CD1579 histidine kinase was shown to directly phosphorylate Spo0A in vitro, while a CD2492 mutant exhibits a 3-fold decrease in sporulation in vivo (Underwood et al., 2009). These results strongly suggest that both of these histidine kinases influence C. difficile sporulation. CD1492 and CD2492 encode conserved autophosphorylation domains and are integral membrane proteins, suggesting that these histidine kinases may autophosphorylate upon contact with specific extracellular signals. However, no characterization of CD1492 has been published. Of the remaining two orphan histidine kinases, CD1352 has been shown to regulate a lantibiotic resistance mechanism (McBride & Sonenshein, 2011, Suarez et al., 2013), and CD1949 has no known function.
Control of sporulation initiation by limiting accumulation of Spo0A~P
In B. subtilis, Spo0A phosphorylation is further controlled through multiple phosphatases and their respective regulators. One mechanism employed by B. subtilis to restrict sporulation initiation is the dephosphorylation of the components within the phosphorelay, which in turn limits the accumulation of phosphorylated Spo0A. The Spo0E, YnzD and YisI aspartyl-phosphate phosphatases act directly on Spo0A (Perego & Hoch, 1991, Ohlsen et al., 1994, Perego, 2001), while the RapA, RapB and RapE phosphatases bind to and dephosphorylate Spo0F (Perego et al., 1994, Jiang et al., 2000). Additionally, a histidine kinase inhibitor, KipI, directly blocks the catalytic domain of KinA, preventing ATPase activity and subsequent autophosphorylation of KinA (Wang et al., 1997). Adding further complexity to these regulatory pathways, Rap activity is inhibited by specific small quorum sensing peptides, known as Phr peptides (Magnuson et al., 1994, Lazazzera et al., 1997, Perego, 1997), while KipI antikinase activity is inhibited by KipA (Wang et al., 1997). C. difficile encodes two orthologs to the Rap phosphatases and orthologs of KipI and its antagonist KipA, but no apparent Phr peptide or Spo0E, YnZD or YisI phosphatase orthologs are present (Paredes et al., 2005, Galperin et al., 2012). The Rap orthologs in C. difficile have low homology to the B. subtilis Rap proteins. The N-terminal protein binding and phosphatase domains are not conserved in C. difficile. Rather, the conserved domains present in the C. difficile orthologs are the tetratricopeptide (TPR) repeats which are responsible for direct interaction with the inhibitory Phr peptides (Diaz et al., 2012). C. difficile is also missing an apparent ortholog of sda, which encodes another histidine kinase inhibitor that prevents KinA autophosphorylation in response to defects in DNA replication initiation (Burkholder et al., 2001, Rowland et al., 2004, Paredes et al., 2005). It is not known if the Rap or Kip orthologs influence spore formation in C. difficile. Since their conserved targets within the phosphorelay are absent in C. difficile, the Rap and Kip orthologs may act on the putative histidine kinases or directly on Spo0A.
Bacillus species initiate sporulation in a cell density-dependent manner through the synthesis, export and uptake of small quorum sensing peptides known as Phr peptides. These five to six amino acid peptides are recognized and imported by two oligopeptide permeases, Spo0K (Opp) and App (Perego, 1997). Once imported, Phr peptides directly bind to and inhibit the phosphatase activity of the Rap proteins previously mentioned, initiating sporulation (Magnuson et al., 1994, Lazazzera et al., 1997, Perego, 1997). C. difficile encodes orthologs of the Opp and App transporters but is missing orthologs to the Phr peptides (Edwards et al., 2014). In contrast to B. subtilis, an opp app mutant in C. difficile increases sporulation-specific gene expression and exhibits a hypersporulation phenotype (Edwards et al., 2014). Increased sporulation appears to occur in response to a decrease in general peptide uptake mediated by these oligopeptide transporters, which corresponds with an overall decrease in CcpA and CodY activity in transporter mutants. Hence, Opp and App likely indirectly influence sporulation initiation by signaling nutrient availability through CodY- and CcpA-mediated gene regulation (Edwards et al., 2014). Because the standard methods of inducing sporulation in other spore formers, such as B. subtilis, do not increase the sporulation rate of C. difficile, it has been unclear whether C. difficile sporulates in response to nutrient starvation. The observation that Opp and App function inhibits sporulation in C. difficile is evidence that this organism initiates sporulation in nutrient limiting conditions.
Auxiliary regulators of sporulation initiation
There are several additional regulatory factors involved in sporulation initiation in B. subtilis that may also play a role in controlling sporulation in C. difficile. ScoC, a negative transcriptional regulator of sporulation in B. subtilis, downregulates both opp and app transcription (Koide et al., 1999). Overexpression of a putative scoC ortholog (CD0852), divergently transcribed from the opp operon, decreases sporulation efficiency but does not influence gene expression of the opp or app operons in C. difficile, suggesting that CD0852 may negatively influence sporulation through a unique regulatory pathway (Edwards et al., 2014). Another gene, CD3409, was suggested to function as the ScoC ortholog in C. difficile (Pettit et al., 2014); however, this gene encodes for the HPr kinase/phosphorylase mentioned previously. These genes are often confused because in B. subtilis the hpr gene encodes ScoC, while ptsH and hprK encode HPr and HPrK/P, respectively.
In B. subtilis, the AbrB protein functions as a transition state regulator and represses stationary phase and sporulation genes during exponential growth. AbrB can repress its own transcription as well as expression of both sigH (σH) and the Spo0A-phosphatase, spo0E, and acts as an activator of hpr expression (encoding ScoC; (Zuber & Losick, 1987, Strauch et al., 1989, Perego & Hoch, 1991). Expression of abrB is controlled by negative autoregulation and is repressed by activated Spo0A upon entry into transition phase (Perego et al., 1988). There are two AbrB paralogs present in B. subtilis, Abh and SpoVT, which function similarly to AbrB as DNA-binding regulators although their regulons do not fully overlap (Bagyan et al., 1996, Dong et al., 2004, Yao & Strauch, 2005). The C. difficile genome encodes two putative abrB orthologs (CD1859A and CD3120) as well as a SpoVT ortholog (CD3499). Unfortunately, no experimental evidence for any of the C. difficile AbrB orthologs is available, so the functions of these factors remain a mystery. However, recent work demonstrated that SpoVT functions slightly differently in C. difficile as compared to B. subtilis and is necessary for the formation of mature, heat-resistant spores in C. difficile (Saujet et al., 2013). Similar to B. subtilis, SpoVT does not appear to influence sporulation initiation as a C. difficile spoVT mutant forms phase dark spores (Saujet et al., 2013).
The tetrameric DNA-binding regulator SinR provides another layer of regulatory control in sporulation initiation by directly repressing spo0A gene expression (Mandic-Mulec et al., 1995). SinR repression is disrupted by the production of the antagonist, SinI, which is encoded in the same operon as sinR and forms an inactive heterodimeric complex with SinR (Bai et al., 1993). Regulation of sinIR gene expression in B. subtilis is complex as AbrB, ScoC and Spo0A all play roles in up- or downregulating transcription (Shafikhani et al., 2002). The sinRI genes are encoded in the C. difficile genome (CD2214 and CD2215), and although no characterization of these has been reported, there may be some regulatory circuitry conserved in C. difficile as Spo0A represses sinR gene expression (Pettit et al., 2014).
Finally, fidelity of DNA replication and segregation is a regulatory checkpoint for initiating sporulation in other spore formers. Together, the Soj and Spo0J proteins repress sporulation until chromosomal segregation has occurred. Soj and Spo0J are similar to the ParA/B family of proteins involved in plasmid partitioning and are required for proper chromosomal replication and segregation during vegetative growth, as well as before asymmetric division occurs during sporulation (Ireton et al., 1994). Soj prevents Spo0A-dependent transcription while Spo0J inhibits Soj activity (Ireton et al., 1994, Cervin et al., 1998, Quisel & Grossman, 2000). C. difficile contains both soj (CD3672) and spo0J (CD3671) orthologs along with an additional Spo0J-like ortholog (CD3673) encoded immediately downstream. The roles that these proteins play in DNA replication and sporulation in C. difficile have not been confirmed.
Conclusion/Outlook
The altered roles of conserved regulatory components and the apparent absence of several key factors (e.g. the phosphorelay transferases, Sda, Spo0E, YnzD, YisI and the Phr peptides) involved in early sporulation in the C. difficile genome indicate that this organism has evolved to induce sporulation specifically to survive harsh conditions within its gastrointestinal niche and outside of its host. Because of the specialized ecological niche of C. difficile, there are likely unknown unique regulators and molecular mechanisms controlling Spo0A activation. Compared to other characterized spore formers, C. difficile incorporates different environmental and intracellular signals and utilizes a novel regulatory pathway to mediate Spo0A phosphorylation and subsequent sporulation-specific gene expression. This apparently simplified phosphorylation cascade suggests that C. difficile may possess fewer checkpoints before initiating sporulation compared to Bacillus species, but it is equally plausible that there are alternative pathways and checkpoints that have yet to be discovered. With the recent advances in C. difficile genetics and whole transcriptomic and proteomic sequencing, genetic screens and global studies in this notoriously challenging organism have become feasible and are expected reveal a wealth of information about C. difficile in the coming years.
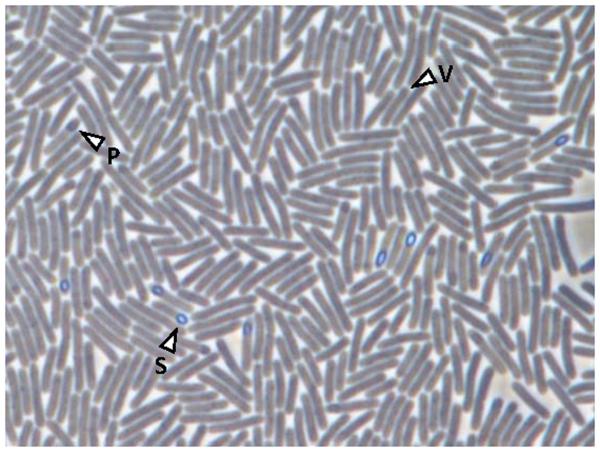
Figure 1.
Phase contrast micrograph of sporulating C. difficile R20291, an epidemic 027 ribotype strain (Stabler et al., 2009). Depicted are vegetative cells (v), phase dark prespores (p) and phase bright spores (s).
Figure 2.
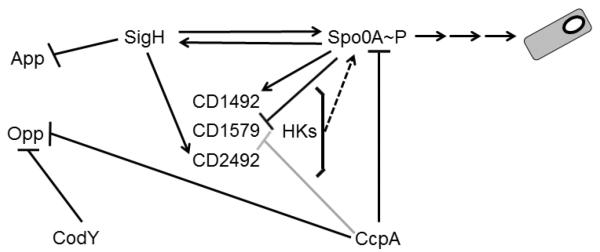
Representative schematic of the putative regulatory pathway that controls sporulation initiation in C. difficile. Solid lines represent direct transcriptional control while dashed lines indicate post-translational modifications (phosphorylation). Black lines indicate direct regulatory interactions, and gray arrows denote indirect regulation. Regulatory interactions have been demonstrated experimentally in (Underwood et al., 2009, Dineen et al., 2010, Saujet et al., 2011, Antunes et al., 2012, Pettit et al., 2014).
Table 1.
Known and predicted genes involved in early sporulation in C. difficile.
| Gene namea | Accession numberb | Known or predicted function | References |
|---|---|---|---|
| spo0A | CD1214 | master transcriptional regulator of sporulation; active when phosphorylated | (Deakin et al., 2012) |
| sigH | CD0057 | transition phase sigma factor | (Saujet et al., 2011) |
| ccpA | CD1064 | carbon catabolite control protein; transcriptional regulator that responds to fructose-1,6-biphosphate | (Antunes et al., 2011, Antunes et al., 2012) |
| codY | CD1275 | transcription regulator; requires cofactors GTP or branched chain amino acids (BCAAs) | (Dineen et al., 2007. Dineen et al., 2010) |
| CD1492 | putative Spo0A-specific histidine kinase; integral membrane protein | (Underwood et al., 2009) | |
| CD1579 | putative Spo0A-specific histidine kinase; cytosolic protein | (Underwood et al., 2009) | |
| CD2492 | putative Spo0A-specific histidine kinase; integral membrane protein | (Underwood et al., 2009) | |
| oppA-F | CD0853-57 | nonspecific oligopeptide permease; negatively influences sporulation indirectly | (Edwards et al., 2014) |
| appA-F | CD2670-74 | nonspecific oligopeptide permease; negatively influences sporulation indirectly | (Edwards et al., 2014) |
| rapA | CD2123 | putative phosphatase | |
| rapB | CD3668 | putative phosphatase | |
| sinR | CD2214 | putative transcriptional regulator | |
| sinI | CD2215 | putative inhibitor of SinR activity | |
| kipI | CD1386 | putative inhibitor of histidine kinase activity | |
| kipA | CD1387 | putative antagonist of Kipl activity | |
| hpr | CD0852 | putative ScoC transcriptional regulator | |
| spo0J | CD3671 | putative inhibitor of Soj activity | |
| soj | CD3672 | putative negative regulator of Spo0A activity | |
| spo0J2 | CD3673 | putative inhibitor of Soj activity |
If uncharacterized in C. difficile, the gene name reflects the closest ortholog present in the B. subtilis 168 genome (GenBank accession no. AL009126).
Accession number is based on aenomic annotation of C. difficile 630 (AM180355; (Monot et al. 2011).
Acknowledgments
We give special thanks to Charles Moran, Kathryn Nawrocki and members of the McBride lab for helpful criticism of this manuscript. This work was supported by the U.S. National Institutes of Health through research grants DK087763 and DK101870 to S.M.M.
References
- Antunes A, Martin-Verstraete I, Dupuy B. CcpA-mediated repression of Clostridium difficile toxin gene expression. Molecular microbiology. 2011;79:882–899. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic acids research. 2012;40:10701–10718. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagyan I, Hobot J, Cutting S. A compartmentalized regulator of developmental gene expression in Bacillus subtilis. Journal of bacteriology. 1996;178:4500–4507. doi: 10.1128/jb.178.15.4500-4507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai U, Mandic-mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-portrain. Genes & development. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(Suppl 6):5–12. doi: 10.1111/1469-0691.12064. [DOI] [PubMed] [Google Scholar]
- Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Burkholder WF, Kurtser I, Grossman AD. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell. 2001;104:269–279. doi: 10.1016/s0092-8674(01)00211-2. [DOI] [PubMed] [Google Scholar]
- CDC . In: Antibiotic Resistance Threats in the United States, 2013. Control CfD, editor. 2013. [Google Scholar]
- Cervin MA, Spiegelman GB, Raether B, Ohlsen K, Perego M, Hoch JA. A negative regulator linking chromosome segregation to developmental transcription in Bacillus subtilis. Molecular microbiology. 1998;29:85–95. doi: 10.1046/j.1365-2958.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- Dapa T, Unnikrishnan M. Biofilm formation by Clostridium difficile. Gut microbes. 2013;4 doi: 10.4161/gmic.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LF, Valiente E, Faulds-Pain A, Donahue EH, Wren BW. Characterisation of Clostridium difficile biofilm formation, a role for Spo0A. PloS one. 2012;7:e50527. doi: 10.1371/journal.pone.0050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, Wren BW, Fairweather NF, Dougan G, Lawley TD. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infection and immunity. 2012;80:2704–2711. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Saier MH., Jr. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz AR, Core LJ, Jiang M, Morelli M, Chiang CH, Szurmant H, Perego M. Bacillus subtilis RapA phosphatase domain interaction with its substrate, phosphorylated Spo0F, and its inhibitor, the PhrA peptide. Journal of bacteriology. 2012;194:1378–1388. doi: 10.1128/JB.06747-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen SS, McBride SM, Sonenshein AL. Integration of metabolism and virulence by Clostridium difficile CodY. Journal of bacteriology. 2010;192:5350–5362. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Molecular microbiology. 2007;66:206–219. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- Dong TC, Cutting SM, Lewis RJ. DNA-binding studies on the Bacillus subtilis transcriptional regulator and AbrB homologue, SpoVT. FEMS microbiology letters. 2004;233:247–256. doi: 10.1016/j.femsle.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AN, Weinert E, McBride SM. The cytosolic CD1579 histidine kinase contains a degenerate heme-oxygen sensing PAS domain. Emory University; 2012. [Google Scholar]
- Edwards AN, Nawrocki KL, McBride SM. Conserved Oligopeptide Permeases Modulate Sporulation Initiation in Clostridium difficile. 6th European Spores Conference, Royal Halloway, University of London; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimlaid KA, Bond JP, Schutz KC, Putnam EE, Leung JM, Lawley TD, Shen A. Global Analysis of the Sporulation Pathway of Clostridium difficile. PLoS genetics. 2013;9:e1003660. doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Molecular microbiology. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environmental microbiology. 2012;14:2870–2890. doi: 10.1111/j.1462-2920.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. The ClosTron: a universal gene knock-out system for the genus Clostridium. Journal of microbiological methods. 2007;70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Ireton K, Gunther NWt, Grossman AD. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. Journal of bacteriology. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoir C, Deneve C, Bouttier S, et al. Insights into the adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infection and immunity. 2013 doi: 10.1128/IAI.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Grau R, Perego M. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. Journal of bacteriology. 2000;182:303–310. doi: 10.1128/jb.182.2.303-310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Molecular microbiology. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Shoji K, Shimizu T, Nakano K, Sato T, Kobayashi Y. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. Journal of bacteriology. 1995;177:176–182. doi: 10.1128/jb.177.1.176-182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide A, Perego M, Hoch JA. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. Journal of bacteriology. 1999;181:4114–4117. doi: 10.1128/jb.181.13.4114-4117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley TD, Croucher NJ, Yu L, Clare S, Sebaihia M, Goulding D, Pickard DJ, Parkhill J, Choudhary J, Dougan G. Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. Journal of bacteriology. 2009;191:5377–5386. doi: 10.1128/JB.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzera BA, Solomon JM, Grossman AD. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell. 1997;89:917–925. doi: 10.1016/s0092-8674(00)80277-9. [DOI] [PubMed] [Google Scholar]
- LeDeaux JR, Grossman AD. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. Journal of bacteriology. 1995;177:166–175. doi: 10.1128/jb.177.1.166-175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDeaux JR, Yu N, Grossman AD. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. Journal of bacteriology. 1995;177:861–863. doi: 10.1128/jb.177.3.861-863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin KE, Carter GP, Howarth P, Rood JI, Lyras D. Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile. PloS one. 2013;8:e79666. doi: 10.1371/journal.pone.0079666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- Mandic-Mulec I, Doukhan L, Smith I. The Bacillus subtilis SinR protein is a repressor of the key sporulation gene spo0A. Journal of bacteriology. 1995;177:4619–4627. doi: 10.1128/jb.177.16.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Sonenshein AL. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infection and immunity. 2011;79:167–176. doi: 10.1128/IAI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monot M, Boursaux-Eude C, Thibonnier M, Vallenet D, Moszer I, Medigue C, Martin-Verstraete I, Dupuy B. Reannotation of the genome sequence of Clostridium difficile strain 630. Journal of medical microbiology. 2011;60:1193–1199. doi: 10.1099/jmm.0.030452-0. [DOI] [PubMed] [Google Scholar]
- Moreno MS, Schneider BL, Maile RR, Weyler W, Saier MH., Jr. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Molecular microbiology. 2001;39:1366–1381. doi: 10.1111/j.1365-2958.2001.02328.x. [DOI] [PubMed] [Google Scholar]
- Murphy CR, Avery TR, Dubberke ER, Huang SS. Frequent hospital readmissions for Clostridium difficile infection and the impact on estimates of hospital-associated C. difficile burden. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2012;33:20–28. doi: 10.1086/663209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- Ohlsen KL, Grimsley JK, Hoch JA. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1756–1760. doi: 10.1073/pnas.91.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes CJ, Alsaker KV, Papoutsakis ET. A comparative genomic view of clostridial sporulation and physiology. Nature reviews Microbiology. 2005;3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- Perego M. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8612–8617. doi: 10.1073/pnas.94.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M. A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Molecular microbiology. 2001;42:133–143. doi: 10.1046/j.1365-2958.2001.02611.x. [DOI] [PubMed] [Google Scholar]
- Perego M, Hoch JA. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. Journal of bacteriology. 1991;173:2514–2520. doi: 10.1128/jb.173.8.2514-2520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M, Spiegelman GB, Hoch JA. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Molecular microbiology. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Perego M, Cole SP, Burbulys D, Trach K, Hoch JA. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. Journal of bacteriology. 1989;171:6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M, Hanstein C, Welsh KM, Djavakhishvili T, Glaser P, Hoch JA. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. doi: 10.1016/0092-8674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Pereira FC, Saujet L, Tome AR, Serrano M, Monot M, Couture-Tosi E, Martin-Verstraete I, Dupuy B, Henriques AO. The Spore Differentiation Pathway in the Enteric Pathogen Clostridium difficile. PLoS genetics. 2013;9:e1003782. doi: 10.1371/journal.pgen.1003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit LJ, Browne HP, Yu L, et al. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC genomics. 2014;15:160. doi: 10.1186/1471-2164-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam EE, Nock AM, Lawley TD, Shen A. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. Journal of bacteriology. 2013 doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisel JD, Grossman AD. Control of sporulation gene expression in Bacillus subtilis by the chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) Journal of bacteriology. 2000;182:3446–3451. doi: 10.1128/jb.182.12.3446-3451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch KE, Bakker D, Kuijper EJ, Smits WK. C. difficile 630Deltaerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA. PloS one. 2012;7:e48608. doi: 10.1371/journal.pone.0048608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland SL, Burkholder WF, Cunningham KA, Maciejewski MW, Grossman AD, King GF. Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Molecular cell. 2004;13:689–701. doi: 10.1016/s1097-2765(04)00084-x. [DOI] [PubMed] [Google Scholar]
- Saujet L, Monot M, Dupuy B, Soutourina O, Martin-Verstraete I. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. Journal of bacteriology. 2011;193:3186–3196. doi: 10.1128/JB.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saujet L, Pereira FC, Serrano M, Soutourina O, Monot M, Shelyakin PV, Gelfand MS, Dupuy B, Henriques AO, Martin-Verstraete I. Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in Clostridium difficile. PLoS genetics. 2013;9:e1003756. doi: 10.1371/journal.pgen.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani SH, Nunez E, Leighton T. ScoC mediates catabolite repression of sporulation in Bacillus subtilis. Current microbiology. 2003;47:327–336. doi: 10.1007/s00284-002-4013-1. [DOI] [PubMed] [Google Scholar]
- Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T. Postexponential regulation of sin operon expression in Bacillus subtilis. Journal of bacteriology. 2002;184:564–571. doi: 10.1128/JB.184.2.564-571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein AL. Control of sporulation initiation in Bacillus subtilis. Current opinion in microbiology. 2000;3:561–56. doi: 10.1016/s1369-5274(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Current opinion in microbiology. 2005;8:203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sonenshein AL. Control of key metabolic intersections in Bacillus subtilis. Nature reviews Microbiology. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- Stabler RA, He M, Dawson L, et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome biology. 2009;10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M. Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Molecular microbiology. 2011;80:41–654. doi: 10.1111/j.1365-2958.2011.07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. The EMBO journal. 1989;8:1615–1621. doi: 10.1002/j.1460-2075.1989.tb03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez JM, Edwards AN, McBride SM. The Clostridium difficile cpr Locus is Regulated by a Non-contiguous Two-component System in Response to Type A and B Lantibiotics. Journal of bacteriology. 2013 doi: 10.1128/JB.00166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, Wilcox MH, Stephenson K. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. Journal of bacteriology. 2009;191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Stirewalt VL, Melville SB. The CcpA protein is necessary for efficient sporulation and enterotoxin gene (cpe) regulation in Clostridium perfringens. Journal of bacteriology. 2004;186:5221–5229. doi: 10.1128/JB.186.16.5221-5229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Grau R, Perego M, Hoch JA. A novel histidine kinase inhibitor regulating development in Bacillus subtilis. Genes & development. 1997;11:2569–2579. doi: 10.1101/gad.11.19.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worner K, Szurmant H, Chiang C, Hoch JA. Phosphorylation and functional analysis of the sporulation initiation factor Spo0A from Clostridium botulinum. Molecular microbiology. 2006;59:1000–1012. doi: 10.1111/j.1365-2958.2005.04988.x. [DOI] [PubMed] [Google Scholar]
- Yao F, Strauch MA. Independent and interchangeable multimerization domains of the AbrB, Abh, and SpoVT global regulatory proteins. Journal of bacteriology. 2005;187:6354–6362. doi: 10.1128/JB.187.18.6354-6362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. Journal of bacteriology. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]