Abstract
Background
Asthma in obese adults is typically more severe and less responsive to glucocorticoids than asthma in nonobese adults.
Objective
We sought to determine whether the clearance of apoptotic inflammatory cells (efferocytosis) by airway macrophages was associated with altered inflammation and reduced glucocorticoid sensitivity in obese asthmatic patients.
Methods
We investigated the relationship of efferocytosis by airway (induced sputum) macrophages and blood monocytes to markers of monocyte programming, in vitro glucocorticoid response, and systemic oxidative stress in a cohort of adults with persistent asthma.
Results
Efferocytosis by airway macrophages was assessed in obese (n = 14) and nonobese (n = 19) asthmatic patients. Efferocytosis by macrophages was 40% lower in obese than nonobese subjects, with a mean efferocytic index of 1.77 (SD, 1.07) versus 3.00 (SD, 1.25; P < .01). A similar reduction of efferocytic function was observed in blood monocytes of obese participants. In these monocytes there was also a relative decrease in expression of markers of alternative (M2) programming associated with efferocytosis, including peroxisome proliferator-activated receptor δ and CX3 chemokine receptor 1. Macrophage efferocytic index was significantly correlated with dexamethasone-induced mitogen-activated protein kinase phosphatase 1 expression (ρ = 0.46, P < .02) and baseline glucocorticoid receptor α expression (ρ = 0.44, P < .02) in PBMCs. Plasma 4-hydroxynonenal levels were increased in obese asthmatic patients at 0.33 ng/mL (SD, 0.15 ng/mL) versus 0.16 ng/mL (SD, 0.08 ng/mL) in nonobese patients (P = .006) and was inversely correlated with macrophage efferocytic index (ρ = −0.67, P = .02).
Conclusions
Asthma in obese adults is associated with impaired macrophage/monocyte efferocytosis. Impairment of this anti-inflammatory process is associated with altered monocyte/macrophage programming, reduced glucocorticoid responsiveness, and systemic oxidative stress.
Keywords: Asthma, obesity, inflammation, macrophage, oxidative stress, steroid
Obesity induces a systemic inflammatory state marked by increased oxidative stress1,2 and enhanced expression of inflammatory mediators (eg, TNF-α) that have been implicated in the development of metabolic syndrome and insulin resistance.3,4 Of note, oxidative stress and several of these same proinflammatory cytokines have been implicated in the initiation, maintenance, and progression of asthma, as well as important clinical phenotypes, such as the development of glucocorticoid insensitivity.5–15 Furthermore, much of the inflammation in obesity is attributed to pathologic recruitment, classical activation (M1 skewing), or both, of macrophages in adipose tissue, as well as other tissues, including liver and muscle.3,16,17 Similarly, in patients with severe or glucocorticoid-insensitive asthma, M1 skewing of blood monocytes and alveolar macrophages has been documented.18–20 These links suggest a possible intersection of obesity and asthma at the level of airway macrophage activation and function. So-called “alternatively activated” or M2 macrophages help to control and resolve inflammation, in part through the recognition and removal of dying cells in a unique phagocytic process called efferocytosis.21,22 M2 programming of macrophages is required for their expression of efferocytic receptors and bridge molecules.23–26 Notably, glucocorticoids further enhance macrophage efferocytic capability.27–29 Not only is efferocytosis important for clearance of dying cells before phlogistic disintegration,30 it leads macrophages to produce anti-inflammatory mediators, such as IL-10, TGF-β, and prostaglandin E2, which potently suppress inflammation.31–33 Impaired efferocytosis is increasingly recognized in patients with chronic airway inflammatory disorders, including severe asthma, chronic obstructive pulmonary disease, and cystic fibrosis, and is hypothesized to contribute to persistent inflammation.29,34,35 For instance, airway macrophages from patients with severe asthma have reduced ability to efferocytose apoptotic cells, a function that can be increased approximately 3-fold by glucocorticoids.29
In light of the parallels in oxidative stress, proinflammatory cytokines, and macrophage programming between obesity and glucocorticoid-insensitive or more severe asthma phenotypes, we hypothesized that airway macrophage dysfunction, specifically impaired efferocytosis, might play an important role with regard to the effect of obesity on airway inflammation in patients with asthma. This hypothesis was tested in a cross-sectional study in obese and nonobese adults with persistent asthma.
METHODS
Study population
Adults with mild-to-moderate persistent asthma36 (n = 33) were enrolled. All participants were recruited from a single site (National Jewish Health) during participation in the run-in period of 2 National Heart, Lung, and Blood Institute–sponsored asthma clinical trials.37 The inclusion and exclusion criteria and design of the common run-in period have been reported previously and are included in the Methods section in this article’s Online Repository at www.jacionline.org.37 All participants were being treated with inhaled corticosteroid (hydrofluoroalkane beclomethasone dipropionate, 80 µg twice daily). Lung function, airway hyperresponsiveness, and fraction of exhaled nitric oxide were measured by using standard techniques.38–40 Induced sputum was obtained from all participants, and cytospin preparations were prepared by using standard techniques.41 A separate healthy control population (n = 25) was comprised of nonsmoking adult participants with normal lung function. In these subjects cytologic preparations from bronchoalveolar lavage specimens42 were also prepared as above.41 Obesity was defined by using standard criteria43 as a body mass index (BMI) of 30 kg/m2 or greater. All participants provided written informed consent, and all protocols were approved by the National Jewish Health Institutional Review Board.
Efferocytosis and apoptotic cells in induced sputum
By using validated methodology,34,35 cytospin preparations of lower airway cells were viewed in triplicate for assessment of in vivo efferocytosis and apoptotic cells by using light microscopy according to a methodology described previously (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). Random fields were read in a blinded fashion by a single reader for apoptotic cells and efferocytosis until a minimum of 500 macrophages were counted. Previous measures of intraobserver and interobserver variance within the laboratory demonstrate correlations of 0.92 and 0.91, respectively. The efferocytic index, as a measure of overall clearance, was calculated by multiplying the percentage of macrophages that had phagocytosed apoptotic bodies by the average number of apoptotic bodies per macrophage.44 Apoptotic inflammatory cells were determined by the appearance of pyknotic nuclear morphology.44
Efferocytosis by blood monocytes in vitro
Blood monocytes were isolated from a representative subset of the asthmatic patients (n = 9 obese and 15 nonobese, see Table E1 in this article’s Online Repository at www.jacionline.org). Blood was phlebotomized into CPT tubes (Becton Dickinson, Franklin Lakes, NJ), followed by negative selection with magnetic beads (Miltenyi Biotec, Auburn, Calif). Monocytes (7 × 104 per well in a 96-well plate) were plated in X-VIVO 15 (10% pooled human serum; Lonza, Basel, Switzerland) for 2 hours before testing for efferocytosis. Five-micrometer carboxylated beads (Bangs Laboratories, Fishers, Ind), serving as apoptotic cell mimics, were added at a ratio of 2 beads per monocyte for 1 hour.31 After vigorous washing to remove free beads, efferocytosis of the beads was determined by means of visual inspection (see Fig E1, B). A minimum of 200 monocytes were counted, and the efferocytic index was calculated as above. Correlations of 0.95 and 0.91 for intraobserver and interobserver variance, respectively, have been documented for monocyte uptake within the laboratory.
Reverse transcribed quantitative PCR
Total RNA was extracted from freshly isolated blood monocytes and reverse transcribed to cDNA by using standard techniques. PCR was performed (7900H; Applied Biosystems, Foster City, Calif) with commercially available primers for a panel of candidate M1 and M2 programming markers and efferocytic receptors, including arginase 1, a disintegrin and metalloproteinase domain–containing protein (ADAM) 8, peroxisome proliferator-activated receptor (PPAR) δ, PPARγ, macrophage mannose receptor, CX3C chemokine receptor 1, adiponectin receptor, TGF-β receptor, and GP132 receptor.44–53 Glyceraldehyde-3-phosphate dehydrogenase or 18s RNA served as endogenous controls.
Assessment of oxidative stress
Assays for 4-hydroxynonenal (4HNE), a product of lipid peroxidation and a marker of systemic oxidative stress, were performed by using previously reported methods of gas chromatography/mass spectrometry.54
Assessment of in vitro glucocorticoid responsiveness
PBMCs were isolated and stimulated with 10−7 mol/L dexamethasone for 4 hours. Mitogen-activated protein kinase phosphatase 1 (MKP-1) expression was determined by using RT-PCR in cells treated with medium alone and medium plus dexamethasone, as described previously.55 Expression of glucocorticoid receptor (GCR) α was determined as described previously.56
Statistical methods
All data were described as means ± SDs. Between-group differences (BMI category) were evaluated by using the Mann-Whitney U test. Continuous relationships between parameters were analyzed by using unadjusted linear regression. Nonparametric Spearman ρ correlation coefficient was used to determine correlations between parameters.
RESULTS
Baseline characteristics of study participants with asthma (n = 33) are presented in Table I. Obese (n = 14) and nonobese (n = 19) asthmatic patients were well matched for demographics and markers of asthma severity and inflammation. Significant differences between the obese and nonobese asthmatic patients were demonstrated for markers associated with obesity, including leptin and high-sensitivity C-reactive protein. Cytospin preparations of induced sputum (Table II) indicated that the percentage of airway macrophages was increased in obese asthmatic patients. Airway macrophage percentages were positively correlated with BMI (Spearman ρ = 0.47, P = .01). The percentage of inflammatory cells demonstrating apoptotic features did not differ between nonobese and obese asthmatic patients and was not correlated with BMI (ρ = −0.2, P = .2). The percentages of sputum eosinophils and neutrophils did not differ between the 2 groups.
TABLE I.
Characteristics of the study population
| Nonobese subjects |
Obese subjects |
P value |
|
|---|---|---|---|
| No. | 19 | 14 | — |
| Age (y) | 39 (10) | 38 (14) | .7 |
| Female sex (no.) | 9 (48) | 5 (36) | .5 |
| African American (no.) | 4 (25) | 4 (33) | .8 |
| BMI (kg/m2) | 23.6 (2.8) | 37.2 (5.1) | <.01 |
| FEV1 (L [before albuterol]) | 2.7 (0.8) | 2.7 (0.9) | .8 |
| FEV1 (L [after albuterol]) | 3.0 (0.8) | 3.2 (0.8) | .5 |
| FVC (L [before albuterol]) | 3.8 (1.1) | 4.3 (0.9) | .9 |
| FVC (L [after albuterol]) | 4.0 (1.1) | 4.4 (0.9) | .9 |
| FEV1/FVC ratio | 0.7 (0.1) | 0.7 (0.1) | .5 |
| PC20 FEV1 (mg/mL methacholine) | 2.2 (2.4) | 1.6 (1.3) | .8 |
| Feno (ppb) | 23 (12) | 23 (21) | .3 |
| Adiponectin (µg/mL) | 8.9 (6.3) | 8.4 (4.9) | .7 |
| Leptin (ng/mL) | 8.4 (12.5) | 29.2 (11.4) | <.01 |
| IL-6 (pg/mL) | 1.4 (1.8) | 1.5 (0.8) | .06 |
| TNF-α (pg/mL) | 1.6 (1.0) | 1.5 (0.6) | .8 |
| hs-CRP (mg/mL) | 2.6 (4.5) | 4.8 (3.3) | <.01 |
Data are presented as counts (percentages of population) or means (SDs).
Feno, Fraction of exhaled nitric oxide; hs-CRP, high-sensitivity C-reactive protein.
TABLE II.
Induced sputum cell differential counts in nonobese and obese participants
| Nonobese subjects | Obese subjects | P value | |
|---|---|---|---|
| Eosinophils (%) | 3.4 (4.6) | 1.3 (2.5) | .07 |
| Neutrophil (%) | 55.6 (20.7) | 39.5 (21.9) | .06 |
| Macrophages (%) | 28.1 (15.0) | 42.1 (19.7) | .02 |
| Lymphocytes (%) | 0.8 (0.9) | 0.7 (0.7) | .90 |
| Apoptotic cells (%) | 31.6 (18.9) | 22.9 (16.6) | .26 |
Data are presented as means (SDs).
Although the percentage of airway macrophages containing 1 or more engulfed apoptotic bodies was similar in nonobese and obese asthmatic patients (2.3% [SD, 1.23%] vs 2.0% [SD, 1.6%]), the number of apoptotic bodies per macrophage was lower in the obese asthmatic patients at 1.07 (SD, 0.14) versus 1.29 (SD, 0.34) in nonobese asthmatic patients (P = .05, Table III). Represented as the efferocytic index, uptake by airway macrophages of obese asthmatic patients was 40% lower than in nonobese asthmatic patients at 1.77 (SD, 1.07) versus 3.00 (SD, 1.25; P < .01, Table III). In healthy nonasthmatic control participants an opposite trend was observed, with an increased efferocytic index in obese control subjects (n = 6; BMI, 34 kg/m2 [SD, 2.6 kg/m2]) versus nonobese control subjects (n = 19; BMI, 24.5 kg/m2 [SD, 3.0 kg/m2]) at 3.73 (0.95) and 2.42 (0.87), respectively (P = .005).
TABLE III.
Efferocytosis by airway macrophages and peripheral blood monocytes in nonobese and obese asthmatic participants
| Nonobese subjects |
Obese subjects |
P value |
|
|---|---|---|---|
| Airway macrophages | |||
| No. of apoptotic bodies per macrophage | 1.29 (0.34) | 1.07 (0.14) | .05 |
| Efferocytic index | 3.00 (1.25) | 1.77 (1.07) | <.01 |
| Apoptotic cells (%)/airway macrophage (%) ratio | 1.04 (1.07) | 0.44 (0.6) | .02 |
| Peripheral blood monocytes | |||
| No. of engulfed beads per monocyte | 3.2 (1.2) | 2.1 (0.6) | <.01 |
| Efferocytic index | 165.6 (89.2) | 98.6 (83.0) | .03 |
Data are presented as means (SDs).
When expressed as a ratio (ie, the percentage of inflammatory cells that were apoptotic divided by the percentage of inflammatory cells that were macrophages), values for nonobese asthmatic patients were higher than for obese asthmatic patients (1.04 [SD, 1.07] vs 0.44 [SD, 0.6], P = .02). However, the percentage of inflammatory cells characterized as apoptotic was not correlated with the efferocytic index (ρ = −0.07, P = .7), suggesting that the proportion of inflammatory cells undergoing apoptosis was not tightly coupled to overall clearance. When BMI was treated as a continuous variable, the efferocytic index inversely correlated with BMI (ρ = −0.51, P = .003) in asthmatic patients, suggesting a dose-response aspect to the categorical differences reported above. As with the categorical analyses above, nonasthmatic participants showed an opposite relationship between BMI and airway macrophage efferocytosis, with a positive correlation between BMI and efferocytic index (ρ = 0.46, P = .02).
We then sought to directly determine whether blood monocytes (inflammatory macrophage precursors)57 of obese asthmatic patients demonstrated impaired efferocytic capability in vitro. In a subset of asthmatic participants, 9 obese and 15 nonobese and similar in characteristics to the main cohort (group characteristics reported in Table E1), blood monocytes were isolated and tested for their ability to take up carboxylated beads (apoptotic cell mimics, see the Methods section). The percentage of monocytes taking up 1 or more carboxylated beads was similar for both groups at 49.9% (SD, 15.9%) for nonobese asthmatic patients versus 44.2% (SD, 27.8%) for obese asthmatic patients (P = .4), but substantially fewer beads were taken up by monocytes of obese asthmatic patients compared with nonobese asthmatic patients at 2.1 (SD, 0.6) versus 3.2 (SD, 1.2; P < .01, Table III). Accordingly, when expressed as the efferocytic index, efferocytosis of bead targets was significantly reduced in obese asthmatic patients relative to that seen in nonobese asthmatic patients at 98.6 (SD, 83.0) versus 165.6 (SD, 89.2; P = .03, Table III), indicating a similar 40% reduction in efferocytosis by blood monocytes. When BMI was treated as a continuous variable, a trend toward an inverse relationship of efferocytosis by blood monocytes was noted (ρ = −0.37, P = .07), but no significant correlation between efferocytosis by airway macrophages and blood monocytes was demonstrated (ρ = 0.26, P = .35).
Programming differences in blood monocytes from obese and nonobese asthmatic participants were then evaluated. Candidate markers of M2 programming were evaluated by using real-time quantitative PCR (Table IV). Of these, expressions of PPARδ and ADAM8 were inversely correlated with BMI (ρ = −0.41 and P < .05 for PPARδ; ρ = −0.61 and P < .01 for ADAM8), suggesting diminished M2 programming with increasing obesity in asthmatic patients. Expression of CX3 chemokine receptor 1, which is implicated in efferocytosis,50 was also diminished in obese asthmatic patients (ρ = −0.56, P < .05). Arginase 1 and GP132 receptor expression correlated significantly with monocyte efferocytic index. Neither PPARγ nor macrophage mannose receptor, both of which are reported to be expressed at very low levels in human monocytes before differentiation into macrophages,46 were correlated with either BMI or efferocytic index. None of the markers were significantly correlated with efferocytosis by airway macrophages. When the specific M1 programming candidate markers IL-8, monocyte chemotactic protein 1, IL-6, and TNF-α were assessed, none were found to be associated with obesity or related to the efferocytic capability of monocytes or airway macrophages. Serum leptin levels were inversely correlated with the efferocytic index of airway macrophages (ρ = −0.32, P < .05) and blood monocytes (ρ = −0.43, P < .05). However, when adjusted for BMI, these relationships with serum leptin were no longer significant.
TABLE IV.
Correlation of M2 programming/efferocytosis markers in blood monocytes with BMI and EI
| M2/efferocytic markers | ρ vs BMI | P value | ρ vs monocyte EI | P value |
|---|---|---|---|---|
| PPARδ | −0.41 | .04 | 0.38 | .06 |
| ADAM8 | −0.61 | <.01 | 0.37 | .07 |
| CX3CR1 | −0.56 | .02 | 0.43 | .07 |
| PPARγ | 0.01 | .98 | 0.29 | .16 |
| MMR | −0.29 | .18 | 0.34 | .10 |
| Arginase 1 | −0.20 | .33 | 0.42 | .04 |
| Adiponectin receptor | −0.13 | .55 | 0.38 | .06 |
| TGF-β receptor | −0.09 | .67 | 0.39 | .06 |
| GP132 receptor | −0.22 | .30 | 0.51 | .01 |
CX3CR1, CX3 chemokine receptor 1; EI, efferocytic index; MMR, macrophage mannose receptor.
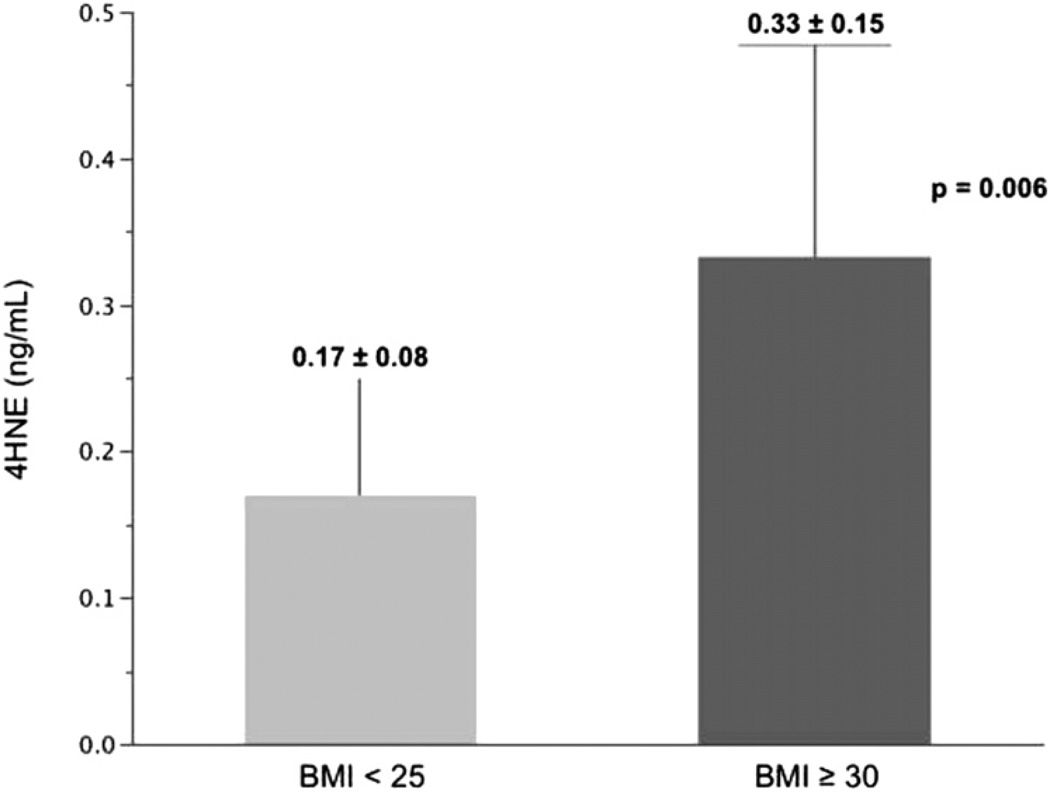
To explore the relationship between obesity, efferocytic function, and markers of oxidative stress, we assayed concentrations of plasma 4HNE, a product of lipid peroxidation and a biomarker of systemic oxidative stress. 4HNE levels were significantly increased in obese asthmatic patients (n = 10) at 0.33 ng/mL (SD, 0.15 ng/mL) versus 0.17 ng/mL (SD, 0.08 ng/mL) in nonobese asthmatic patients (n = 10, P = .006, Fig 1). 4HNE levels were significantly and inversely correlated with the efferocytic index in airway macrophages (ρ = −0.67, P = .02, n = 11, Table V) but not in blood monocytes, which demonstrated a similar trend that did not achieve statistical significance (ρ = −0.53, P = .18).
FIG 1.
4HNE concentrations in the plasma of nonobese (n = 10) and obese (n = 10) asthmatic patients.
TABLE V.
Correlation (unadjusted) of sputum efferocytic index with markers of oxidative stress and glucocorticoid response
| ρ | P value | |
|---|---|---|
| 4HNE vs EI in airway macrophages | −0.67 | .02 |
| MKP-1 expression vs EI in airway macrophages | 0.56 | .003 |
| GCRα expression vs EI in airway macrophages | 0.49 | .009 |
EI, Efferocytic index.
Because glucocorticoids are reported to enhance efferocytosis,27–29 the relationship of efferocytosis by airway macrophages to in vitro markers of glucocorticoid responsiveness was then investigated in asthmatic participants. Efferocytosis in airway macrophages was significantly correlated with both the induction of MKP-1 expression by dexamethasone (ρ = 0.56, P = .003) and GCRα expression (ρ = 0.49, P = .009) in PBMCs (Table V). When adjusted for BMI in a standard least-squares model, these relationships remained significant (r = 0.61 and P = .002 for MKP-1; r = 0.49 and P = .01 for GCRα). There was no relationship between airway macrophage efferocytosis and GCRβ, which was expressed at very low levels. No relationship between these markers of glucocorticoid response and efferocytosis in blood monocytes was demonstrated (ρ = 0.03 and P = .9 for MKP-1; ρ = −0.1 and P = .7 for GCRα).
DISCUSSION
Asthma and obesity are major public health issues, and there is increasing evidence of interaction of these 2 disorders in both adults and children.58–63 Studies suggest that asthma in obese persons can be characterized by both increased severity and reduced glucocorticoid responsiveness demonstrated both in vivo and in vitro, although not uniformly so.55,60,61,63–65 Traditional markers of TH2-type inflammation, such as fraction of exhaled nitric oxide and airway eosinophil numbers, are not increased in obese compared with nonobese patients with asthma,60 and the basis of this harder-to-control phenotype is not well understood.
In an attempt to begin to identify the underlying mechanisms, we report the first data comparing efferocytosis by airway macrophages and peripheral blood monocytes in obese and nonobese participants with asthma. This difference was not observed in subjects without asthma, suggesting that interactions between these 2 diseases result in the observed impairment in efferocytosis. The findings in obese asthmatic patients occur in the context of decreased expression of certain markers of alternative (M2) macrophage programming and are significantly related to in vitro assessment of glucocorticoid response, with the degree of impaired efferocytosis increasing as glucocorticoid responsiveness decreased. Furthermore, we demonstrate a significant relationship between efferocytic capacity and degree of systemic oxidative stress, suggesting a possible role of oxidative stress in modulating these pathways. Given these findings, we hypothesize that impaired efferocytosis by airway macrophages contributes to increased severity of asthma in the setting of obesity.
Given the increased numbers of activated macrophages found in adipose tissue and muscle in patients with obesity associated with insulin resistance,3,47,66,67 it is reasonable to hypothesize that lung macrophages might be a key inflammatory cell in patients with asthma complicated by obesity. On the basis of inflammatory cell differentials for induced sputum, the percentage of airway macrophages was increased in obese asthmatic patients. The lack of absolute macrophage quantification is a shortcoming of our current approach, although to our knowledge, no studies to date have shown that absolute numbers of airway macrophages are greater in obese patients with asthma. This question is relevant for future investigation.
Notably, there were no differences in the percentages of apoptotic cells between obese and nonobese asthmatic patients. However, there was a decrease in the percentage of apoptotic cells to the percentage of macrophages expressed as a ratio for obese asthmatic patients. Direct assessment of isolated sputum macrophages “fed” exogenous apoptotic cells will be required to determine whether impaired efferocytosis by airway macrophages of obese subjects is due to limited apoptotic target availability. Direct assessment of sputum macrophages was not performed in this study and is technically challenging. Importantly, direct assessment of blood monocyte efferocytic capacity mirrored findings in the airway macrophages, suggesting that phagocytic functional alterations are systemic in obese asthmatic patients.
Our results suggest that monocyte/macrophage programming is altered in obese asthmatic patients. We demonstrated evidence of diminished M2 programming, which is typically required for efficient efferocytosis. Monocyte mRNA expression of the M2 markers (eg, the nuclear receptor PPARδ and ADAM8) correlated inversely with BMI, and several of the target markers were positively associated with monocyte efferocytic capability (Table IV). As such, assessments of the programming and functions of monocytes, as precursors of tissue macrophages and as cells exposed to the same systemic inflammatory milieu, might serve as biomarkers, reflecting the status of macrophages in inflamed airways. Nonetheless, we acknowledge that investigation of blood monocytes as a surrogate for airway macrophages is a potential limitation of this report in that resident macrophages in tissues can demonstrate both phenotypic and functional differences when compared with circulating blood monocytes.68
There is controversy regarding M1 markers in human subjects versus murine models in obesity,16,17,69 and we were unable to demonstrate an increase in expression of candidate M1-related markers in monocytes of our obese study participants with asthma. Nonetheless, some evidence of M1 programming of airway macrophages18 and, to a limited extent, monocytes20 has been described in patients with severe steroid-resistant asthma and, in turn, associated with reduced MKP-1 induction.70 Thus it is possible that the reduced MKP-1 induction and low GCRα expression observed herein can be considered a functional readout reflective of disproportionate M1 programming, explaining significant correlation between these markers of glucocorticoids response and impaired efferocytosis by airway macrophages. In our earlier work we demonstrated that impaired glucocorticoid responsiveness in vitro required a disease-by-disease interaction; it was demonstrated only in obese asthmatic patients and not subjects with obesity alone. Our data from this disparate study population suggest that this same interaction is also required for the impairment of efferocytosis.
Finally, although not conclusive, our data also suggest that oxidative stress might be an important mechanistic link between obesity, macrophage programming and function, and glucocorticoid insensitivity in asthmatic patients. Obesity is known to be associated with increases in systemic oxidative stress,2 and increased oxidant production by macrophages inhibits the ability of macrophages to recognize, phagocytose, and clear apoptotic cells, particularly after stimulation with TNF-α.71,72 Thus the systemic proinflammatory environment in obese patients might affect asthma through proinflammatory cytokines and associated increases in oxidative stress. These, in turn, impair macrophage/monocyte function in ways that are critical to the maintenance or resolution of airway inflammation, while also altering glucocorticoid response pathways.
Taken together, these data point to the need for future investigations designed to improve our understanding of airway macrophage programming and function in asthma complicated by obesity, with the goal of identifying novel therapeutic targets. Given that the nuclear receptors PPARδ and PPARγ are both robustly implicated in M2 programming of macrophages and efferocytosis, as well as suppression of inflammation and enhanced systemic insulin sensitivity,23,44,66,67 targeting these pathways might constitute a novel and relevant clinical therapeutic approach in the treatment of obese asthmatic patients.
Supplementary Material
Key message.
Airway macrophages from obese asthmatic adults demonstrate impaired efferocytosis that is associated with increased oxidants, altered monocyte programming, and reduced glucocorticoid responsiveness.
Acknowledgments
We thank Douglas Curran-Everett, PhD, for assistance with biostatistical analyses; Ms Jenai Kailey for technical assistance; and Ms Brenda Sebern for preparation of the manuscript.
Supported by HL090982, the National Jewish Health Translational Pilot Program, HL34303, AI058228, UL1 RR025780, Catherine Kramer Scientist in Pediatric Medicine, the Max Goldenberg Foundation, the Walter S. and Lucienne B. Driskill Charitable Foundation, and the Eugene F. and Easton M. Crawford Charitable Lead Unitrust.
R. Fernandez-Boyanapalli has been supported by one or more grants from the Crawford Charitable Lead Unitrust. E. Goleva has been supported by one or more grants from the National Institutes of Health (NIH). B. Day has consultancy arrangements with Aeolus Pharmaceuticals; has received one or more grants from or has one or more grants pending with the NIH (RO1 ES017582 and U54 ES015678) and the Cystic Fibrosis Foundation; has one or more patents (planned, pending, or issued) with Saber Pharmaceuticals (US#6,231,894) and Aeolus Pharmaceuticals (US#5,994,339; #6,127,356; #6,479,477; #6,583,132; #6,916,799; #7,189,707; #7,470,677; #7,820,644; #8,217,026); and owns stock/stock options in Aeolus Pharmaceuticals. D. L. Bratton has been supported by one or more grants from the Catherine Kramer Foundation (endowed chair in pediatric medicine); has been supported by one or more grants from National Jewish Health (Translational Pilot Grant Program support for the investigation); is employed by National Jewish Health; and has received one or more payments for lecturing from or is on the speakers’ bureau for the American Thoracic Society and the American Academy of Allergy, Asthma & Immunology. E. R. Sutherland has consultancy arrangements with Forest Laboratories, GlaxoSmithKline, Merck/Schering-Plough, Novartis (inactive), Dey (inactive), and Genentech; is employed by National Jewish Health; has received one or more grants from or has one or more grants pending with Boehringer-Ingelheim, Novartis, and the National Institutes of Health; and has received one or more payments for the development of educational presentations for Genentech.
Abbreviations used
- ADAM
A disintegrin and metalloproteinase domain–containing protein
- BMI
Body mass index
- GCR
Glucocorticoid receptor
- 4HNE
4-Hydroxynonenal
- MKP-1
Mitogen-activated protein kinase phosphatase 1
- PPAR
Peroxisome proliferator-activated receptor
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keaney JF, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, et al. Obesity and systemic oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 3.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 8.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boniface S, Koscher V, Mamessier E, El Biaze M, Dupuy P, Lorec AM, et al. Assessment of T lymphocyte cytokine production in induced sputum from asthmatics: a flow cytometry study. Clin Exp Allergy. 2003;33:1238–1243. doi: 10.1046/j.1365-2222.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD41 and CD81 T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick AM, Teague WG, Burwell L, Brown MS, Brown LAS. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LAS. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. doi: 10.1016/j.jaci.2008.10.047. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-gamma as a possible target in chronic asthma. Inflamm Allergy Drug Targets. 2006;5:253–256. doi: 10.2174/187152806779010909. [DOI] [PubMed] [Google Scholar]
- 14.Litonjua AA, Sparrow D, Guevarra L, O’Connor GT, Weiss ST, Tollerud DJ. Serum interferon-gamma is associated with longitudinal decline in lung function among asthmatic patients: the Normative Aging Study. Ann Allergy Asthma Immunol. 2003;90:422–428. doi: 10.1016/S1081-1206(10)61827-3. [DOI] [PubMed] [Google Scholar]
- 15.Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, et al. Alterations of the arginine metabolome in asthma. Am J Respir Crit Care Med. 2008;178:673–681. doi: 10.1164/rccm.200710-1542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes. 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauk PJ, Goleva E, Liu AH, Hall CF, Riches DWH, Martin RJ, et al. Corticosteroid resistant (CR) asthma is associated with classical activation of airway macrophages and exposure to lipopolysaccharide (LPS) J Allergy Clin Immunol. 2006;117(suppl):S194. doi: 10.1016/j.jaci.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goleva E, Hauk PJ, Hall CF, Liu AH, Riches DW, Martin RJ, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122:550–559. doi: 10.1016/j.jaci.2008.07.007. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakonarson H, Bjornsdottir US, Halapi E, Bradfield J, Zink F, Mouy M, et al. Profiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patients. Proc Natl Acad Sci U S A. 2005;102:14789–14794. doi: 10.1073/pnas.0409904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nature medicine. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asada K, Sasaki S, Suda T, Chida K, Nakamura H. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages. Am J Respir Crit Care Med. 2004;169:195–200. doi: 10.1164/rccm.200207-740OC. [DOI] [PubMed] [Google Scholar]
- 25.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 27.Gilmour JS, Coutinho AE, Cailhier JF, Man TY, Clay M, Thomas G, et al. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:7605–7611. doi: 10.4049/jimmunol.176.12.7605. [DOI] [PubMed] [Google Scholar]
- 28.Heasman SJ, Giles KM, Rossi AG, Allen JE, Haslett C, Dransfield I. Interferon gamma suppresses glucocorticoid augmentation of macrophage clearance of apoptotic cells. Eur J Immunol. 2004;34:1752–1761. doi: 10.1002/eji.200324698. [DOI] [PubMed] [Google Scholar]
- 29.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 30.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32:350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr Biol. 2006;16:2252–2258. doi: 10.1016/j.cub.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 32.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 33.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 35.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 36.Bethesda (MD): U.S. Department of Health and Human Services; National Institutes of Health; National Heart Lung and Blood Institute; National Asthma Education and Prevention Program; 2007. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. [Google Scholar]
- 37.Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 39.Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 40.Silkoff PE, Erzurum SC, Lundberg JO, George SC, Marczin N, Hunt JF, et al. ATS workshop proceedings: exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate. Proc Am Thorac Soc. 2006;3:131–145. doi: 10.1513/pats.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 41.Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–1475. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 42.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med. 2005;172:807–816. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Global strategy on diet, physical activity and health. [Accessed April 2, 2012]; Available at: http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/. [Google Scholar]
- 44.Fernandez-Boyanapalli R, Frasch SC, Riches DW, Vandivier RW, Henson PM, Bratton DL. PPARgamma activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood. 2010;116:4512–4522. doi: 10.1182/blood-2010-02-272005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frasch SC, Fernandez-Boyanapalli RF, Berry KZ, Leslie CC, Bonventre JV, Murphy RC, et al. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J Biol Chem. 2011;286:12108–12122. doi: 10.1074/jbc.M110.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knolle MD, Owen CA. ADAM8: a new therapeutic target for asthma. Expert Opin Ther Targets. 2009;13:523–540. doi: 10.1517/14728220902889788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–208. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, et al. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic Biol Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li LB, Leung DY, Hall CF, Goleva E. Divergent expression and function of glucocorticoid receptor beta in human monocytes and T cells. J Leukoc Biol. 2006;79:818–827. doi: 10.1189/jlb.0805466. [DOI] [PubMed] [Google Scholar]
- 57.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006;43:553–558. doi: 10.1080/02770900600859123. [DOI] [PubMed] [Google Scholar]
- 59.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthmaphenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2009;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol. 2010;108:735–743. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2009;123:1328–1334. doi: 10.1016/j.jaci.2009.04.005. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutherland ER, Camargo CA, Busse WW, Meltzer EO, Ortega HG, Yancey SW, et al. Comparative effect of body mass index on response to asthma controller therapy. Allergy Asthma Proc. 2010;31:20–25. doi: 10.2500/aap.2010.31.3307. [DOI] [PubMed] [Google Scholar]
- 65.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin M, Opalek JM, Marsh CB, Wu HM. Proteome comparison of alveolar macrophages with monocytes reveals distinct protein characteristics. Am J Respir Cell Mol Biol. 2004;31:322–329. doi: 10.1165/rcmb.2004-0080OC. [DOI] [PubMed] [Google Scholar]
- 69.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Proinflammatory CD11c1CD2061 adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 71.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, et al. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 72.Borges VM, Vandivier RW, McPhillips KA, Kench JA, Morimoto K, Groshong SD, et al. TNFalpha inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L586–L595. doi: 10.1152/ajplung.90569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.