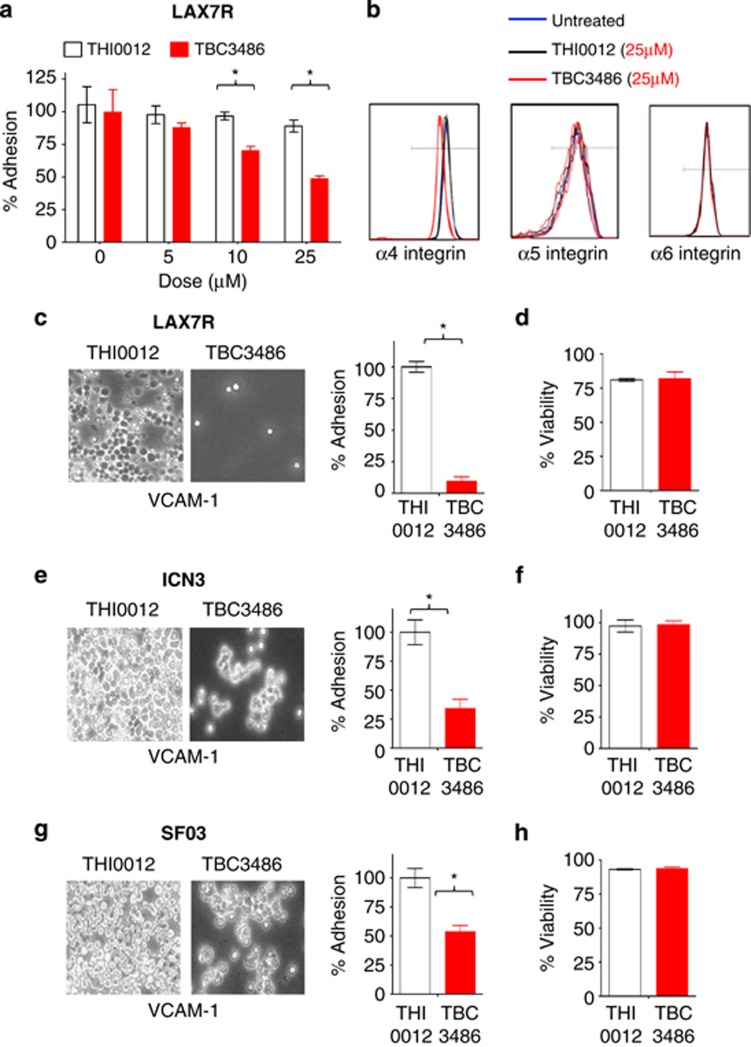
The treatment of patients with chemotherapy-resistant leukemia remains a challenge. A role of the microenvironment for drug resistance of leukemia cells has been proposed.1 We have identified the adhesion molecule integrin α4 as a central mediator of drug resistance of pre-B-cell acute lymphoblastic leukemia (ALL).2 We thus demonstrated that chemotherapy-resistant pre-B-ALL cells can be eradicated in a xenograft model by concurrent blockade of α4 using natalizumab, a humanized anti-α4 antibody in clinical use against multiple sclerosis,3 and Crohn's Disease.2 Here, we extended our studies to an alternative α4 inhibitor, the non-peptidic small molecule TBC3486. Previous in vitro assays and molecular modeling studies indicated that TBC3486 behaves as a ligand mimetic, competing with VCAM-1 for the MIDAS site of integrin α4.4 As such, the compound has shown efficacy in integrin α4-dependent models of inflammatory and autoimmune disease4 and has shown efficacy in mice with autoimmune encephalomyelitis, a model for multiple sclerosis.5 As opposed to natalizumab, which will inhibit both members of the α4 integrin family, α4β1 and α4β7, TBC3486 is 200-fold more potent in inhibiting α4β1 than α4β7. In addition, it is completely inactive against all other integrins tested, including members of the β2, β3 as well as other members of the β1 family of integrins.4 The potential usefulness of this novel inhibitor for pre-B-ALL treatment was tested in our established in vitro and in vivo assays.2, 4 We evaluated the effect of TBC3486 on de-adhesion of patient-derived ALL cells (LAX7R) using established adhesion assays. As a control for our studies, a close structural analog was used that lacks activity toward α4β1 integrin (THI0012). After activating LAX7R cells with 1 mM Mn2+, leukemia cells were co-cultured with the murine stromal cell line OP9.6, 7 Subsequently, LAX7R cells were treated with different doses of TBC3486 (5, 10 and 25 μM) and its control, THI0012 (5, 10 and 25 μM), for 4 days. TBC3486 dose-dependently inhibited adhesion of ALL cells (Figure 1a), albeit the adhesion was not completely blocked. The dose of 25 μM was selected for subsequent studies. The concentrations of compound required for inhibition in these assays are higher than previously reported.4 This is due to the fact that TB3486 is highly protein bound in the presence of 20% serum (used in these assays), which significantly reduces the amount of free compound available to bind to the integrin receptor. Next, we determined whether TBC3486 decreases binding of three xenograft cells derived from primary pre-B-ALL cases (LAX7R, ICN3 and SFO3) to the counter-receptor of α4 integrin, human VCAM-1. Adhesion assays were performed as previously described4, 8, 9 by culturing ALL samples treated with TBC3486 (25 μM) or THI0012 (25 μM) on hVCAM-1-coated plates for 2 days. Compared with the control group, TBC3486-treated ALL cells showed significantly less adhesion to hVCAM-1 (Figures 1c, e and g); however, the adhesion was not completely blocked. CD49d (MFI) is expressed with higher intensity in LAX7R compared with the other two samples (ICN3 and SFO3) (data not shown), which may explain why TBC3486 blocked a larger percentage of LAX7R adhesion to VCAM-1. In addition to blocking cell adhesion, TBC3486 treatment also specifically targeted the expression of integrin α4, but not integrin α5 and α6 (Figure 1b). The treatment with TBC3486 did not affect cell viability in all three cases (Figures 1d, f and h) compared with the THI0012 control. Taken together, TBC3486 leads to the partial de-adhesion of pre-B-ALL cells from its counter-receptor VCAM-1 under the conditions described.
Figure 1.
Effects of TBC3486 treatment on adhesion and integrin expression of ALL cells. (a) Percentage of adhesion of LAX7R cells treated with different doses of TBC3486 and THI0012 on OP9 for 4 days. *P<0.05, mean±s.d., performed in triplicates. (b) Expression of α4, α5 and α6 integrins presented as histograms after 4 days of TBC3486 (25 μM) and THI0012 (25 μM) treatment. Three ALL cells (c,d: LAX7R; e,f: ICN3; g,h: SF03) were treated with THI0012 control (white bar) or TBC3486 (red bar) on plates coated with human VCAM-1 or 2% bovine serum albumin as control for 2 days. (c, e, g) Adhesion of ALL cells ( × 400 magnification) and % of adhering cells is shown. (d, f, h) Viability of leukemia cells treated with TBC3486 or THI0012 control was determined by trypan blue exclusion.
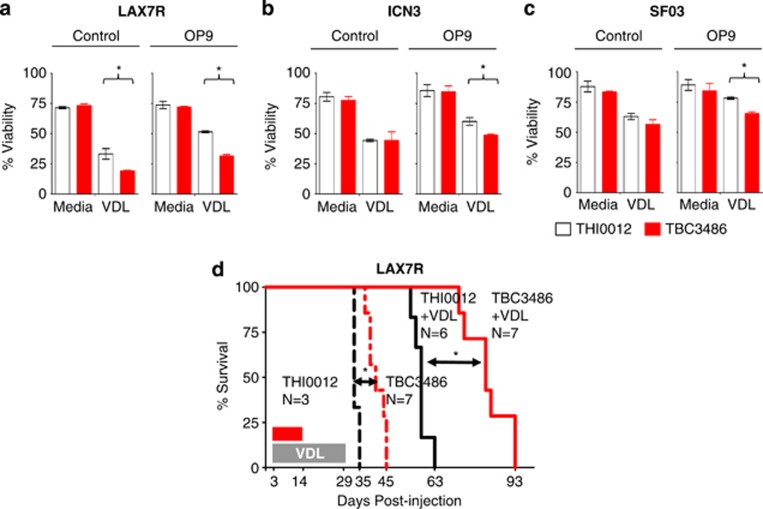
We previously showed that antibody-mediated integrin α4 blockade can sensitize leukemia cells to chemotherapy.2 Therefore, we evaluated whether TBC3486 can not only de-adhere cells from its counter-receptors but also sensitize them to chemotherapy. To study the drug sensitivity of human leukemia, ALL (LAX7R) cells were co-cultured with murine calvaria-derived OP9 stromal cells, which allow in vitro studies beyond 2 days. Then this co-culture was exposed to chemotherapy as previously described.9, 10 ALL cells were treated with chemotherapy (vincristine, dexamethasone, L-asparaginase; VDL) or saline as control for 4 days, with TBC3486 (25 μM) or control THI0012. TBC3486 with VDL treatment reduced the cell viability of human leukemia LAX7R (51±1% vs 31±1% P<0.05) (Figure 2a), ICN3 (60±3% vs 49±1% P<0.05) (Figure 2b) and SFO3 (78±1% vs 65±1% P<0.05) (Figure 2c), indicating that this combination therapy sensitized leukemia cells to chemotherapy in vitro. Nevertheless, all chemoprotection afforded by OP9 cells was removed by TBC3486 in spite of only partial de-adhesion. This observation at least suggests that effects over and above adhesion and physical chemoprotection provided by stromal cells are mediated through α4 integrin.
Figure 2.
TBC3486 treatment sensitizes ALL cells to chemotherapy. (a–c) ALL cells (a) LAX7R; (b) ICN3; (c) SF03) were co-cultured with (right panel) or without OP9 cells (left panel) for 4 days and treated with TBC3486 (25 μM) or THI0012 (25 μM) in combination chemotherapy (VDL: V=0.0005 μM, D=0.005 nM, L=0.0005 IU/ml) in vitro and viability of leukemia cells was determined by trypan blue exclusion *P<0.05; experiments were performed in triplicates. (d) ALL cells (LAX7R) were injected into NSG mice (5 × 104 cells/mouse) and recipient mice were then treated with 10 mg/kg/day TBC3486 or THI0012 control, starting on day 3 post leukemia injection. The daily administrations were split into two intraperitoneal injections per day for 2 weeks. In addition, mice were treated with vincristine (0.5 mg/kg once a week), dexamethasone (8 mg/kg five times a week) and L-asparaginase (800 IU/kg five times a week) for 4 weeks. Kaplan–Meier survival curve was analyzed and MST was calculated for each group: THI0012 (n=3, MST=33 days) (black dashed); TBC3486 (n=7, MST=41 days) (red dashed); THI0012+VDL (n=6, MST=58 days) (black solid); TBC3486+VDL (n=7, MST=82 days) (red solid). *P<0.05, log-rank test.
To determine whether TBC3486 can prolong the survival of xenotolerant mice bearing human leukemia cells in vivo, LAX7R cells were injected into NOD/SCID hosts. Three days after leukemia cell transfer, mice received either TBC3486 or THI0012 (control) (10 mg/kg/d) daily for 2 weeks with two daily intraperitoneal injections to ensure stable plasma levels, with or without VDL chemotherapy. TBC3486-treatment (n=7) resulted in prolonged survival time compared with THI0012 control mice (n=3) (median survival time (MST)=41 vs 33 days, P=0.001). In combination with chemotherapy, the Kaplan–Meier survival curve indicated that TBC3486+VDL-treatment (n=6) resulted in a significantly prolonged survival time (compared with the VDL+THI0012 control (n=7) (MST=82 vs 58 days, P=0.0003; Figure 2d).
TBC3486 preferentially inhibits the high-affinity form of the integrin α4.4 It may thus interfere with the function of lymphocytes entertaining the inflammatory response in multiple sclerosis, but may not interfere with that of lymphocytes causing inflammatory bowel disease. Which β-integrin is the more relevant partner for α4 in the context of drug resistance in ALL is unclear. In addition, TBC3486 mimics VCAM-1 binding, inducing the high-affinity conformation of integrin α4; by contrast, natalizumab recognizes and blocks the ligand-binding moiety of α4 in any conformation and without affecting activation status. Whether, therefore, TBC3486-binding of integrin α4 might elicit outside-in signaling would have to be addressed in further studies. Although unknown off-target effects of the compound cannot be excluded, it should be emphasized that the control compound, THI0012, is structurally identical to TBC3486 except it is the opposite enantiomer. This simple change in stereochemistry eliminates its activity toward α4β1. The fact that this control compound has no activity in any of the in vitro or in vivo studies described here also argues that the efficacy of TBC3486 in these studies is directly related to its activity toward α4β1 integrin.
Like many small molecules, TBC3486 is endowed with a rather shorter half-life, requiring more frequent dosing than natalizumab. A shorter half-life should likely be an advantage in leukemia treatment, but challenges at establishing the optimal timing of anti-integrin treatments (how often, how much and so on) relative to the chemotherapy must not be underestimated. Natalizumab led to the complete eradication of pre-B-ALL in our xenotransplant model in two out of three pre-B-ALL cases. By contrast, adjuvant treatment with TBC3486 was associated only with prolonged survival, not with leukemia cell eradication. Although formal side-by-side comparisons were not performed, this may at least in part be due to the differences in treatment schedules (TBC3486: twice daily for 2 weeks, natalizumab: once weekly for 4 weeks), but additional differences of the two compounds may also contribute. As the half-life of natalizumab is much longer, therapeutic drug levels are likely to be continuously maintained for the entire duration of the 4-week VDL cycle.
Taken together, our data demonstrate that small-molecule inhibition of integrin α4 using TBC3486, currently in preclinical evaluation, is a promising approach for targeting chemotherapy-resistant leukemia. Further studies are warranted to understand and evaluate preclinically adjuvant small-molecule inhibition of integrins to overcome relapse of ALL.
Acknowledgments
The study was supported by the Hyundai Hope on Wheels, V-Foundation, St Baldrick's Scholar Award and NIH R01CA172896. HB acknowledges support from LOEWE OSF TP5a and Deutsche Forschungsgemeinschaft BO3553/1-1.
The authors declare no conflict of interest.
References
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YT, Gang EJ, Geng H, Park E, Huantes S, Chudziak D, et al. Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood. 2013;121:1814–1818. doi: 10.1182/blood-2012-01-406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L, Merrill JT, McInnes IB, Peakman M. Optimization of current and future therapy for autoimmune diseases. Nat Med. 2012;18:59–65. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- Vanderslice P, Woodside DG, Caivano AR, Decker ER, Munsch CL, Sherwood SJ, et al. Potent in vivo suppression of inflammation by selectively targeting the high affinity conformation of integrin alpha4beta1. Biochem Biophys Res Commun. 2010;400:619–624. doi: 10.1016/j.bbrc.2010.08.114. [DOI] [PubMed] [Google Scholar]
- Cannella B, Gaupp S, Tilton RG, Raine CS. Differential efficacy of a synthetic antagonist of VLA-4 during the course of chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 2003;71:407–416. doi: 10.1002/jnr.10487. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Hemler ME. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7987. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. The role of alpha 4-integrin in T lymphocyte migration into the inflamed and noninflamed central nervous system. Curr Top Microbiol Immunol. 1998;231:51–64. doi: 10.1007/978-3-642-71987-5_4. [DOI] [PubMed] [Google Scholar]
- Park E, Gang EJ, Hsieh YT, Schaefer P, Chae S, Klemm L, et al. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011;118:2191–2199. doi: 10.1182/blood-2011-04-351239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, et al. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene. 2013;33:2169–2178. doi: 10.1038/onc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]