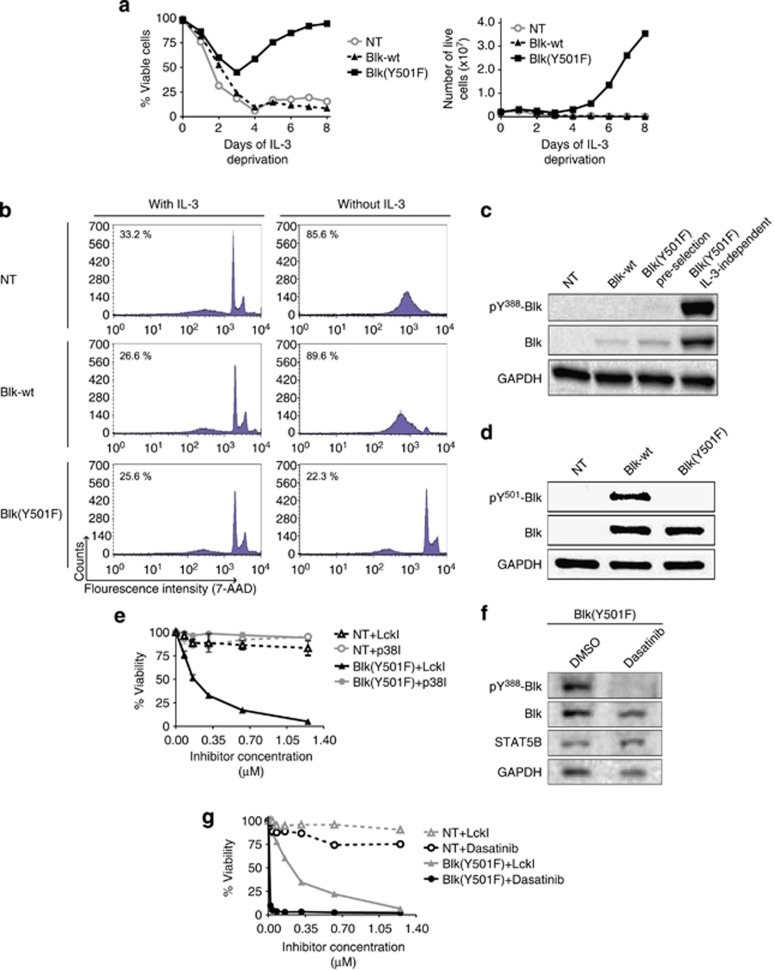
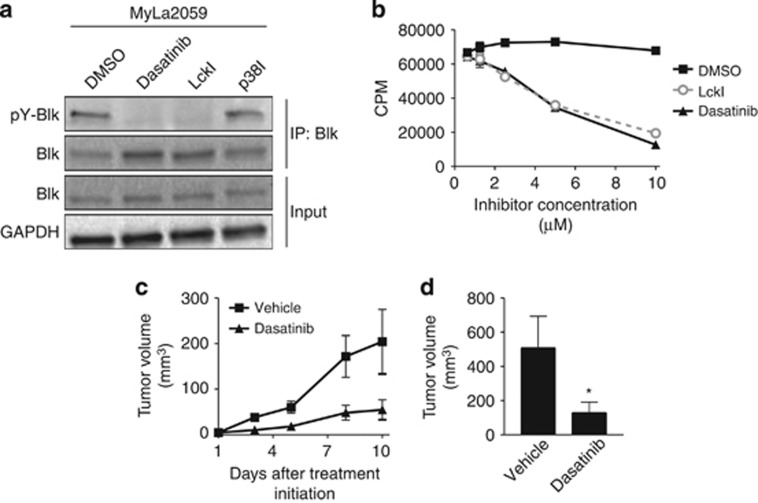
Cutaneous T-cell lymphoma (CTCL) is the most frequent primary lymphoma of the skin. Patients diagnosed in early stages often experience an indolent disease course and have a favorable prognosis. Yet, the disease follows an aggressive course in a substantial fraction (15–20%) of patients and despite recent progress in novel therapies, advanced disease remains a major challenge as relapses are common and cure is rare.1 Recently, it was discovered,2 and independently confirmed in a meta-analysis study,3 that malignant T cells in the majority of patients display ectopic expression of the B-lymphoid tyrosine kinase (Blk), a member of the Src kinase family. Importantly, gene knockdown experiments showed that Blk promoted the proliferation of malignant T cells from CTCL patients,2 suggesting that Blk—in analogy with other Src family members—may function as an oncogene. In support, Montero-Ruiz et al.4 provided evidence that Blk is implicated in childhood acute lymphoblastic leukemia. However, studies in mice suggested that murine Blk also has tumor-suppressive functions depending on the specific cellular context.5 To study the oncogenic potential of human Blk, we therefore transfected a cytokine (IL-3)-dependent lymphoid cell line (Ba/F3) with plasmids expressing either wild-type (wt) Blk or a constitutively active form of Blk lacking the kinase-inhibitory site due to a tyrosine-to-phenylalanine substitution at amino-acid position 501 (Y501F). Stable transfectants were established by selecting for the plasmid-encoded blasticidin resistance gene, and before experimentation, transformed cells were maintained in blasticidin- and IL-3-supplemented growth media. As shown in Figure 1a, the constitutively active form of Blk (Y501F) was fully able to transform growth factor (IL-3)-dependent Ba/F3 cells into IL-3-independent cells, whereas non-transfected and Blk-wt-transfected Ba/F3 cells remained dependent on exogenous IL-3 to survive and proliferate. In accordance, IL-3 deprivation induced massive apoptosis in non-transfected and Blk-wt-transfected Ba/F3 cells, whereas no increase in apoptosis was observed in Blk(Y501F)-transfected Ba/F3 cells following IL-3 withdrawal (Figure 1b). As expected, Blk(Y501F) was phosphorylated on the activating tyrosine (Y388) and not on the inhibitory tyrosine phosphorylation site (Y501), whereas Blk-wt was heavily phosphorylated on the kinase-inhibitory site (Y501) (Figures 1c and d). The well-characterized Src family kinase inhibitor, Lck inhibitor (LckI, Calbiochem, San Diego, CA, USA), selectively inhibited the proliferation of Blk(Y501F)-transfected Ba/F3 cells, whereas an inhibitor of mitogen-activated protein kinase p38 (SB203580, Selleck Chemicals, Houston, TX, USA) did not (Figure 1e). Likewise, the dual-specificity inhibitor of Bcr-Abl and the Src family kinases, dasatinib (Sprycel, Selleck Chemicals), inhibited Y388 phosphorylation and proliferation of the Blk(Y501F)-transfected Ba/F3 cells (Figures 1f and g). Taken together, these results indicate that the active form of human Blk is able and sufficient to transform cytokine-dependent lymphoid cells into cytokine-independent cells. These findings support the previous observation made by others6 that murine Blk(Y495F) is lymphomagenic in mice. In keeping, enzymatic inhibition by LckI and other Src family kinase inhibitors, as well as siRNA-mediated knockdown of Blk, inhibits proliferation of Blk-positive malignant T cells including MyLa2059 and MyLa2000 (ref. 2 and data not shown). As dasatinib profoundly inhibited Blk(Y501F)-transformed Ba/F3 cells and tyrosine phosphorylation of Blk in the MyLa2059 cells (Figure 2a), we hypothesized that dasatinib—which is used for treatment of chronic myelogenous leukemia and other malignancies7—has a potential for treatment of CTCL. To address this hypothesis, we initially studied the effect of dasatinib on malignant proliferation in vitro. As shown in Figure 2b, dasatinib inhibited the spontaneous proliferation of the malignant CTCL T-cell line MyLa2059 in a concentration-dependent manner. Likewise, the Blk-positive CTCL cell lines MyLa2000 and PB2B2 were also inhibited by dasatinib, whereas the Blk-negative Sezary Syndrome cell line (SeAx) was resistant (Supplementary Figure S1). As malignant T cells, including some cell lines obtained from CTCL2 and peripheral T-cell lymphoma patients,8 often express Fyn (another Src kinase), we cannot exclude the possibility that the effect of dasatinib was partially mediated through an inhibition of both Blk and Fyn. However, Fyn is not tyrosine phosphorylated in the malignant MyLa cells suggesting that Fyn may not be functionally active in these cells (data not shown). The observation that dasatinib inhibited Blk-positive tumor cells prompted us to examine the effect of dasatinib on tumor growth in a xenograft transplantation model of CTCL.9, 10 In a preliminary experiment, mice were inoculated subcutaneously (s.c.) with MyLa2059 cells and treated orally with different concentrations of dasatinib (or vehicle as a control) to evaluate the effect on tumor formation in vivo. The average time of tumor onset was significantly (P<0.05) delayed from day 13 in the control group (N=5) to day 20 in animals (N=6) treated with dasatinib (data not shown). Next, we addressed whether dasatinib inhibited growth of already established tumors. Accordingly, eight mice were inoculated s.c. with MyLa2059 cells and following detection of palpable tumors (day 1) the mice were treated with either dasatinib (Sprycel) (40 mg/kg) or vehicle as control. Tumor dimensions were measured in each group on days 3, 5, 8 and 10. As shown in Figure 2c, dasatinib significantly inhibited tumor growth. Likewise, volume of the resected tumors harvested on day 10 was significantly lower in the dasatinib-treated mice when compared with the control mice, confirming that dasatinib does inhibit CTCL tumor growth in vivo (Figure 2d). As the malignant T cells do not express the Bcr-Abl oncogene (data not shown), the present finding suggests that Blk functions as an oncogene in the CTCL cells. As NF-κB is active in CTCL2 and supports growth of dasatinib/imatinib-resistant cells,11, 12 we tested dasatinib in combination with NF-κB inhibitors. Interestingly, dasatinib and NF-κB inhibitors had an additive effect on malignant proliferation in vitro (data not shown), which might explain why Blk(Y501F)-transformed Ba/F3 cells were more sensitive to dasatinib than the malignant MyLa cells. In summary, (i) Blk is enzymatically active in malignant CTCL cells and expressed in situ,2 (ii) its constitutively active form confers cytokine independence (Figure 1) and (iii) it promotes tumor growth in vivo as indicated by the effect of dasatinib on tumor growth in mice (Figure 2). Taken together, these findings strongly suggest that Blk is a potential therapeutic target in CTCL for dasatinib and other clinical-grade dual-specificity Bcr-Abl and Src family kinase inhibitors. As dasatinib and other dual-specific inhibitors are already used in treatment of other hematological malignancies with a high efficacy, tolerability and compliance,7 these drugs are attractive novel candidates for the treatment of CTCL expressing Blk.
Figure 1.
Blk(Y501F) drives resistance to apoptosis and cytokine-independent proliferation. (a) Ba/F3 Blk-wt and Ba/F3 Blk(Y501F), as well as non-transfected Ba/F3 cells (NT), were cultured in the absence of IL-3. Graph to the left depicts the percentage of viable cells at different time points, where each data point represents the mean of a duplicate-sample count. The graph to the right depicts the total number living cells. The data are representative of two independent experiments. (b) Ba/F3 NT, Ba/F3 Blk-wt and Ba/F3 Blk(Y501F) were cultured with or without IL-3 for 6 days before apoptosis was determined by 7-aminoactinomycin D (7-AAD) staining using flow cytometry. Percentages in upper left corner of individual graphs indicate mean percentages of apoptotic cells from three independent experiments, of which the data shown are representative. (c) Western blot (WB) analysis using antibodies specific for Blk-activating phosphotyrosine-388, and antibodies recognizing total Blk, of NT, Blk-wt, and Blk(Y501F) (pre-selection) Ba/F3 cells after 16 h of IL-3 deprivation, as well as of IL-3-independent Ba/F3 Blk(Y501F) cells post-selection established in the experiment depicted in (a). GAPDH was used as loading control. (d) WB analysis using antibodies targeting the Blk-deactivating phoshotyrosine-501 in Ba/F3 NT cells and stable Ba/F3 transfectants. GAPDH was used as loading control. (e) Ba/F3 NT and IL-3-independent selected Ba/F3 Blk(Y501F) cells were treated with different concentrations of either LckI, p38 inhibitor (p38I) or vehicle (DMSO (dimethyl sulfoxide)) for 48 h before cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data points represent mean percentage viability of LckI- and p38I-treated cells, relative to vehicle-treated controls, from median values of three independent experiments performed in triplicates, with error bars representing ±s.e.m. Only the IL-3-dependent Ba/F3 NT cells were grown in IL-3-supplemented media. (f) IL-3-independent selected Ba/F3 Blk(Y501F) cells were treated with 10 μM of dasatinib or vehicle (DMSO) for 1 h before the cells were lysed and WB analysis using anti-pY388-Blk and anti-Blk antibodies was performed. STAT5B and GAPDH served as loading controls. (g) Ba/F3 NT and IL-3-independent selected Ba/F3 Blk(Y501F) cells were treated with different concentrations of either dasatinib, LckI or vehicle (DMSO) for 48 h before cell viability was determined by MTT assay. Data points represent mean percentage viability of dasatinib- and LckI-treated cells, relative to vehicle. Only the IL-3-dependent Ba/F3 NT cells were grown in IL-3-supplemented media.
Figure 2.
Dasatinib inhibits proliferation of malignant CTCL cells and tumor growth in a xenograft model of CTCL. (a) Malignant cells obtained from a lesional biopsy of a patient with CTCL (MyLa2059) were treated with 10 μM of either dasatinib, LckI, p38I or vehicle (DMSO (dimethyl sulfoxide)) for 1 h before the cells were lysed and Blk immuprecipitated (IP: Blk) followed by western blot (WB) analysis of the level of Blk phosphorylation using anti-phosphotyrosine and anti-Blk antibodies. WB analysis of the input lysates (Input) using anti-Blk and anti-GAPDH antibodies were included for reference. (b) Malignant cells (MyLa2059; 4 × 103) were cultured for 72 h with dasatinib, LckI or vehicle (DMSO) and [3H]-thymidine were added for 20 h before measurement of [3H]-thymidine incorporation. Results are shown as mean counts per minute of triplicate cultures with error bars representing ±s.d. (c, d) Four NOD.Cg-Prkdcscid B2mtm1Unc/J mice per group were inoculated s.c. with 1 × 106 MyLa2059 cells into both flanks as described elsewhere.10 When a mouse developed a palpable tumor (day 1) treatment with either vehicle (10% DMSO, 90% propylene glycol) or 40 mg/kg dasatinib orally 5 days a week was initiated. Mice with palpable tumors were allocated alternately to the group receiving vehicle or the group receiving dasatinib. The length and width of the tumors were measured continuously until the experiment was terminated on day 10 post treatment initiation where the tumors were excised and measured. (c) Graph showing the average tumor volume per mouse ±s.e.m. at different time points after treatment initiation. The tumor volume was calculated using the formula V=(a × b2)/2, where a defines the length (mm) and b the width (mm) of the tumor. (d) Bar graph showing the average tumor volume per mouse ±s.e.m. of the excised tumors at day 10 post treatment initiation. The ellipsoid tumor volume was calculated using the formula (a × b × c) × π/6, where a, b and c designate tumor diameters (mm) for length, width and depth, respectively. *P<0.05 denotes a significant difference using a one-tailed two-sample t-test.
In conclusion, our study provides novel evidence that human Blk—in its active form—is an oncogene with the potential to support growth of lymphoid cells in vitro and to promote tumor growth in vivo. Thus, Blk is a potential novel therapeutic target in CTCL.
Acknowledgments
This project was funded by the Calsberg Foundation, the Novo Nordic Research Foundation, the Lundbeck Foundation, the Danish Research Council (FSS under Det Frie Forskningsråd), the Danish Cancer Society (Kræftens Bekæmpelse) and Dansk Kræftforsknings Fond. JB was funded from a grant from the Danish Strategic Research Council rewarded to M Givskov. We thank K Kaltoft for the gift of the MyLa2059 cell line.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. 2013;54:752–759. doi: 10.3109/10428194.2012.729831. [DOI] [PubMed] [Google Scholar]
- Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Kneitz H, Eriksen KW, Lovato P, et al. Ectopic expression of B-lymphoid kinase in cutaneous T-cell lymphoma. Blood. 2009;113:5896–5904. doi: 10.1182/blood-2008-09-181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kester MS, Borg MK, Zoutman WH, Out-Luiting JJ, Jansen PM, Dreef EJ, et al. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J Invest Dermatol. 2012;132:2050–2059. doi: 10.1038/jid.2012.117. [DOI] [PubMed] [Google Scholar]
- Montero-Ruíz O, Alcántara-Ortigoza MA, Betancourt M, Juárez-Velázquez R, González-Márquez H, Pérez-Vera P. Expression of RUNX1 isoforms and its target gene BLK in childhood acute lymphoblastic leukemia. Leuk Res. 2012;36:1105–1111. doi: 10.1016/j.leukres.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Zhang H, Peng C, Hu Y, Li H, Sheng Z, Chen Y, et al. The Blk pathway functions as a tumor suppressor in chronic myeloid leukemia stem cells. Nat Genet. 2012;44:861–871. doi: 10.1038/ng.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek SN, Dordai DI, Reim J, Dintzis H, Desiderio S. Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7351–7356. doi: 10.1073/pnas.95.13.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17:5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- Palomero T, Couronné L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46:166–170. doi: 10.1038/ng.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejsgaard T, Kopp K, Ralfkiaer E, Willumsgaard AE, Eriksen KW, Labuda T, et al. A novel xenograft model of cutaneous T-cell lymphoma. Exp Dermatol. 2010;19:1096–1102. doi: 10.1111/j.1600-0625.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- Kopp KL, Dabelsteen S, Krejsgaard T, Eriksen KW, Geisler C, Becker JC, et al. COX-2 is a novel target in therapy of mycosis fungoides. Leukemia. 2010;24:2127–2129. doi: 10.1038/leu.2010.221. [DOI] [PubMed] [Google Scholar]
- Lounnas N, Frelin C, Gonthier N, Colosetti P, Sirvent A, Cassuto JP, et al. NF-kappaB inhibition triggers death of imatinib-sensitive and imatinib-resistant chronic myeloid leukemia cells including T315I Bcr-Abl mutants. Int J Cancer. 2009;125:308–317. doi: 10.1002/ijc.24294. [DOI] [PubMed] [Google Scholar]
- Shao W, Growney JD, Feng Y, O'Connor G, Pu M, Zhu W, et al. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int J Cancer. 2010;127:2199–2208. doi: 10.1002/ijc.25218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.