Abstract
Background
Litchi is an evergreen woody tree widely cultivated in subtropical and tropical regions. Defective flowering is a major challenge for litchi production in time of climate change and global warming. Previous studies have shown that high temperature conditions encourage the growth of rudimentary leaves in panicles and suppress litchi flowering, while reactive oxygen species (ROS) generated by methyl viologen dichloride hydrate (MV) promote flowering and abortion of rudimentary leaves. To understand the molecular function of the ROS-induced abortion of rudimentary leaves in litchi, we sequenced and de novo assembled the litchi transcriptome.
Results
Our assembly encompassed 82,036 unigenes with a mean size of 710 bp, and over 58% (47,596) of unigenes showed significant similarities to known sequences in GenBank non-redundant (nr) protein database. 5,865 unigenes were found to be differentially expressed between ROS-treated and un-treated rudimentary leaves, and genes encoding signaling components of plant hormones such as ABA and ethylene were significantly enriched.
Conclusion
Our transcriptome data represents the comprehensive collection of expressed sequence tags (ESTs) of litchi leaves, which is a vital resource for future studies on the genomics of litchi and other closely related species. The identified differentially expressed genes also provided potential candidates for functional analysis of genes involved in litchi flowering underlying the control of rudimentary leaves in the panicles.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2164-15-805) contains supplementary material, which is available to authorized users.
Keywords: Rudimentary leaf, Abortion, Flowering, Transcriptome, Reactive oxygen species, Litchi
Background
Litchi is one of the most important subtropical evergreen fruit trees in southern Asia. A major factor determining litchi crop production is the competition between vegetative and reproductive growth during floral differentiation. Floral initiation in litchi could be triggered by low temperatures and enhanced by droughts in autumn and winter [1, 2]. In the following spring, the apical buds will break and elongate as the air temperature and soil moisture rise. Next, the axillary or apical panicle primordia will emerge and become visible as “whitish millets” [3]. At this millet stage, floral buds are considered to be mixed buds containing axillary or apical panicle primordia, leaf primordia and rudimentary leaves. Whether these mixed buds could develop into flowers are largely dependent on the environmental conditions. Under normal climate conditions, the growth of panicle primordia will prevail and the rudimentary leaves will abscise. However, if the buds are exposed to high temperature, the rudimentary leaves could develop into fully expanded leaves and the panicle primordia will cease to develop and shrink [4]. Suppressing the growth of the rudimentary leaves encourages panicle development. Warm winter and hot spring potentially resulted from global warming present a major threat to litchi flowering. In order to develop a counter measure, it is important to understand the genetics behind litchi bud development, and the abortion of the rudimentary leaves.
We have previously shown that ethylene could promote abortion of rudimentary leaves. It is associated with an increase in H2O2 [5], a type of reactive oxygen species (ROS). It is well known that environmental stresses stimulate ROS production in plants [6]. They act as key signals in response to stresses [7]. In Arabidopsis, H2O2 has been shown to be elevated in leaves at the time of floral transition [8]. It has been proposed that H2O2 bursts generate a signal in leaves associated with either the induction of flowering or leaf senescence [9]. The ethylene-induced H2O2 might act as a signal which induces abortion of rudimentary leaves in the panicle and promotes flowering in litchi. Methyl viologen dichloride hydrate (MV) is a ROS producer in plants. It can generate superoxide by accepting an electron from PSI to become a reduced free radical, which is immediately reoxidized by dioxygen, producing superoxide in chloroplast [10]. MV also induces the increase of superoxide production in mitochondria, where complexes I and III are the major electron donors [11]. Superoxide is then transformed to H2O2 by superoxide dismutase [12, 13]. When the litchi leafy panicles were treated with MV, it was found that ROS accumulated, the rudimentary leaves abscised and the numbers of flowers per panicle increased [14]. We have also shown that ROS increased the expression of LcLFY and LcAP1 in panicles [14, 15]. Studies on Arabidopsis and other plants indicated that LFY (LEAFY) is a transcription factor which determines the floral meristem identity and is strongly expressed in the flower buds [16, 17]. Constitutive expression of Arabidopsis AP1 (APETALA1) has also been shown to promote flowering in citrus [18]. AP1 is involved in the transition from floral induction to flower formation and constitutes a hub in the corresponding network of regulatory genes [19, 20]. Beside a few ROS responsive EST clones derived from a suppression subtractive hybridization (SSH) library screen [21], little is known about the transcriptional network controlling litchi flowering.
Without a litchi reference genome, de novo transcriptome assembly using Illumina short RNA-Seq reads is the most cost effective approach for generating a large collection of ESTs suitable for subsequent transcriptome analysis. This method has been successfully applied to Chinese bayberry (Myrica rubra) and watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai var. lanatus) for fruit development and ripening studies [22, 23], pear (Pyrus pyrifolia) for bud dormancy analysis [24, 25], and litchi (Litchi chinesis) and melon (Cucumis melo) for fruit abscission study [26, 27]. Though litchi fruit transcriptome sequencing data were published [27], those of leaves are unknown. In this study, we have constructed a litchi reference transcriptome for rudimentary leaves using Illumina RNA Sequencing (RNA-seq). We also used digital gene expression assay to profile the transcriptome dynamics of ROS treated rudimentary leaves, in order to elucidate genetic network of the ROS-induced abortion.
Results
MV induced abortion of rudimentary leaves
In our previous study, we found that an early sign of abortion of rudimentary leaves was downward growth of the leaves [4]. To confirm the effect of MV treatment, shoot cuttings were treated with water or MV in a growth chamber. The proximal angle (α) and the distal angle (β) of the third and the fourth MV-treated rudimentary leaves were measured (Figure 1A). We used this experimental system for the easy control of light intensity and temperature. Furthermore, in our preliminary study, it was confirmed that detached shoots could survive in water without wilting for at least 2 d. To avoid the immediate effect of the cutting from trees, treatments were carried out after the shoots were placed in water for 2 h. The results showed that proximal angle α had no significant change during the treatments, while the distal angle β significantly increased after the treatment (Table 1). The ROS-treated rudimentary leaves showed epinasty as characterized by downward curvature of leaves (Figure 1B, C, D), presenting an early sign of abortion [4].
Figure 1.

Images of the new flushes. (A) Image of a new flush showing the first to forth rudimentary leaves, (B) Image of a new flush in 0 h of ROS treatment, (C) Image of a new flush in 5 h of ROS treatment, (D) Image of a new flush in 10 h of ROS treatment. α, proximal angle of the rudimentary leaves; β, distal angle of the rudimentary leaves; numbers from 1 to 4 indicate the first, the second, the third and the fourth rudimentary leaves respectively.
Table 1.
Effects of ROS on angles of the rudimentary leaves
| Time of treatment | Proximal angle α (°) | Distal angle β (°) |
|---|---|---|
| 0 h | 51.03 ± 1.09 a | 83.60 ± 1.79 c |
| 5 h | 51.83 ± 1.03 a | 111.36 ± 2.16 b |
| 10 h | 52.88 ± 1.01 a | 126.02 ± 2.16 a |
Values are means ± SE from 30 rudimentary leaves. The differences among all the treatment means were evaluated by Duncan’s multiple range tests at a 0.01 probability level using a SPSS program (SPSS Inc. Chicago, IL, USA). Different lower-case letters indicate significant differences.
Sequence, assembly and annotation of a litchi reference transcriptome
To obtain a reference litchi transcriptome for the rudimentary leaves, a RNA-Seq library has been constructed using RNA from all leaf samples. As shown in Table 2, we have generated 6.13 G of total nucleotides with a Q20 percentage of 97.8%. The Trinity package assembled 82,036 unigenes with a mean size of 710 bp (Table 3). The size distributions of these unigenes are shown in Additional file 1. 58% (47,596/82,036) of unigenes could be annotated by BLASTx (E-value < 1e−5) using the NCBI nr database, while 34,368 were annotated using the Swiss-Prot protein database. In addition, 13,728 and 16,700 unigenes could be annotated according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Cluster of Orthologous Groups of protein (COG) database, respectively. About 10% (8,191/82,036) unigenes could be assigned to a homolog in all four databases (Figure 2A). Based on the NCBI nr database, 23.0% of the unigenes showed homology (1e−20 < E-value < 1e--5), 50.0% of those showed strong homology (1e−100 < E-value < 1e−20) and the remaining 27.0% were very strong homology (E-value < 1e−100) to available plant sequences (Figure 2B). As shown in Figure 2C, 28,556 unigenes were annotated to 4 top-hit species, including Glycine max, Arabidopsis thaliana, Medicago truncatula and Populus trichocarpa. 23,278 unigenes could be classified into 3 gene ontology (GO) categories: cellular component, biological process, and molecular function (Additional file 2). 13,728 unigenes of the rudimentary leaves in litchi were mapped into 274 KEGG pathways (Additional file 3). The maps with the highest unigene representation were ribosome pathway (ko03010) with 629 ungenes counted, followed by protein processing in endoplasmic reticulum (ko04141), starch and sucrose metabolism (ko00500), RNA transport (ko03013), purine metabolism (ko00230), Spliceosome (ko03040) and plant hormone signal transduction pathway (ko04075).
Table 2.
Throughput and quality of RNA-seq of the reference library and the DGE libraries
| Libaries | Total | Total Nucleotides | Q20 percentage | N percentage | GC percentage |
|---|---|---|---|---|---|
| Reads | (nt) | ||||
| Reference library | 61,338,190 | 6,133,819,000 | 97.82% | 0.01% | 46.73% |
| Lc0h-1 | 30,382,747 | 1,488,754,603 | 98.51% | 0.01% | 46.11% |
| Lc0h-2 | 30,181,112 | 1,478,874,488 | 98.53% | 0.01% | 45.85% |
| Lc5h-1 | 28,942,560 | 1,418,185,440 | 98. 45% | 0.01% | 46.11% |
| Lc5h-2 | 32,276,093 | 1,581,528,557 | 98.48% | 0.01% | 46.28% |
| Lc10h-1 | 30,733,719 | 1,505,952,231 | 98.50% | 0.01% | 45.90% |
| Lc10h-2 | 31,382,314 | 1,537,733,386 | 98.51% | 0.01% | 45.83% |
One reference library was constructed by mixing RNA extracted from ROS-treated rudimentary leaves in 0 h, 5 h and 10 h of treatment. 6 DGE libraries were constructed from 0 h, 5 h and 10 h of ROS-treated rudimentary leaves. Each time point of treatment had 2 biological replicates. All libraries were sequenced using HiSeq 2000. Q20 percentage indicates the percentage of sequences with sequencing error rate lower than 1%. N percentage is the percentage of nucleotides which could not be sequenced.
Table 3.
Summary of the transcriptome assembly
| Assembly statistics | |
|---|---|
| Total number of unigenes | 82, 036 |
| Mean length of unigenes (bp) | 710 |
| Sequences with E-value < 1e−5 against nr | 47, 596 |
Figure 2.
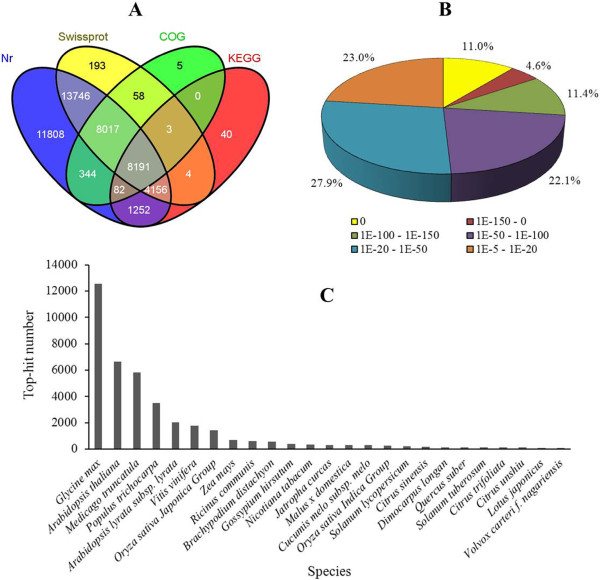
Characteristics of homology search of litchi unigenes. (A) Venn diagram of number of unigenes annotated by BLASTx with a cut-off E-value 1e−05 against protein databases. Numbers in the circles indicate the number of unigenes annotated by single or multiple databases, (B) E-value distribution of the top BLASTx hits against the nr database, (C) Number of unigenes matching the 25 top species using BLASTx in the nr database.
Identify differentially expressed genes using digital gene expression tag (DGE)
To identify differentially expressed genes in response to ROS, 6 DGE libraries have been generated from ROS-treated rudimentary leaves at 0 h, 5 h and 10 h post treatment, with two biological replicates. The libraries produced over 1.41 G of 49 nt single-end read data with a Q20 percentage of about 98%. The percentage of unassigned base “N” is 0.01% and the average GC content is around 45% (Table 2). To assay the normality of the RNA-Seq data in the 6 DGE libraries, we calculated the distribution of unique reads in each DGE libraries. This value is the ratio of the number of bases in a gene covered by unique mapping reads to the total bases in that from our transcriptome reference database. The distribution over different reads abundance categories showed similar patterns among all six libraries. Above 36% of the sequences have coverage more than 80% (Additional file 4). Next we calculated the unigene expression using the uniquely mapped DGE reads and normalized the results to RPKM. Results from the two biological replicates are highly similar, suggesting good reproducibility of the method (Additional file 5). We performed a pairwise comparison using 0 h as the control, and 5 h, or 10 h as the treatments. We also identified differentially expressed unigenes with FDR (false discovery rate) ≤ 0.001 and absolute value of log2 Ratio ≥ 1. As a result, 5,865 unigenes were referred to as differentially expressed genes (DEGs) and used for the subsequent analysis (Additional file 6).
GO-term analysis of differentially expressed genes
To examine the expression profile of the 5,865 DEGs, the expression data υ (from 0 h to 10 h of ROS treatment) were normalized to 0, log2 (υ5h/υ0h), log2 (υ10/υ0h). 5,623 DEGs could be clustered into 8 profiles by Short Time-series Expression Miner software (STEM), in which 5,087 were clustered into 4 profiles (p-value ≤ 0.05), including two down-regulated patterns (Profile 1 and Profile 0) and two up-regulated patterns (Profile 6 and Profile 7) (Figure 3 A-D, Additional file 7). Profile 1 and 0 contained 2,209 and 826 DEGs respectively, while Profile 6 and 7 contained 1,381 and 671 DEGs (Additional file 7). Next, the DEGs within the up- and down-regulated cluster groups were subjected to GO-term analysis. They were classified into 3 main categories including cellular component, biological process, and molecular function. Under the cellular component category, a large number of up-regulation, as well as down-regulation DEGs were categorized as cell part, cell and organelle. Under biological process category, most of those were classified into cellular process and metabolic process. For molecular function category, catalytic activity and binding were the top abundant subcategories (Figure 3 E).
Figure 3.
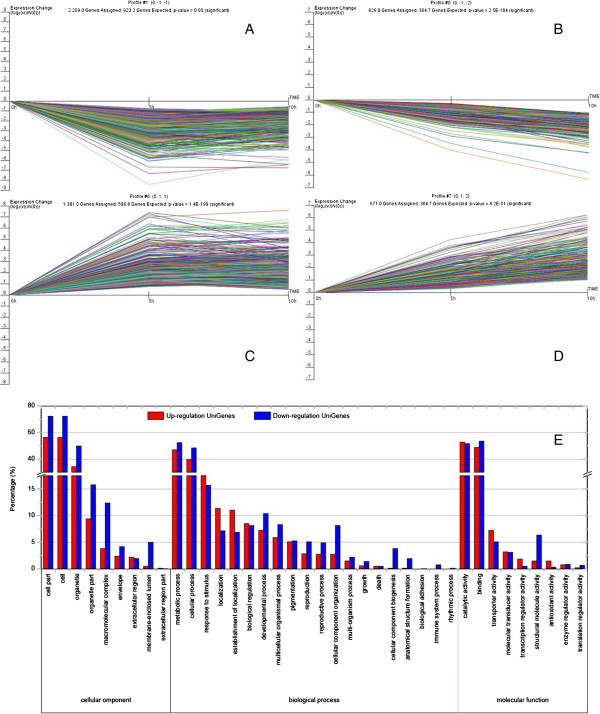
DEGs expression profiles (A-D) and their GO classification (E) in the MV-generated ROS-treated rudimentary leaves. Profile 1 (A) and profile 0 (B) indicating a down-regulated trend, profile 6 (C) and profile 7 (D) indicating an up-regulated trend during 0 to 10 h of ROS treatment. The down-regulation DEGs are union of DEGs in profile 1 and 0. The up-regulation DEGs are union of DEGs in profile 6 and 7.
KEGG pathway enrichment analysis of differentially expressed genes
DEGs were subjected to KEGG pathway enrichment analysis. 27.4% (1,606/5,865) of the DEGs could be annotated. The 10 top KEGG pathways with the highest representation of the DEGs are shown in Table 4. The ribosome (ko03010), plant hormone signal transduction (ko04075), glycolysis/gluconeogenesis (ko00010), starch and sucrose metabolism (ko00500), purine metabolism (ko00230), phenylpropanoid biosynthesis (ko00940), pyrimidine metabolism (ko00240), pyruvate metabolism (ko00620), DNA replication (ko03030) and plant-pathogen interaction (ko04626) pathways are significantly enriched. The 122 unigenes among 837 DEGs (14.58%) in profile 1, and 11 unigenes accounting for 5.16% of 213 in profile 0 were annotated to ribosome pathway, whereas in profile 6 and 7, only 2 unigenes accounting for 0.65% of 307 DEGs, 7 accounting for 7.64% of 144 DEGs were annotated to this pathway.
Table 4.
10 top KEGG pathways with high representation of the DEGs
| Pathways | No. of DEGs with pathway annotation | Pathway ID | ||||
|---|---|---|---|---|---|---|
| All profiles | Profile 1 | Profile 0 | Profile 6 | Profile 7 | ||
| (% of 1606) | (% of 837) | (% of 213) | (% of 307) | (% of 144) | ||
| Ribosome | 147 (9.15%) | 122 (14.58%) | 11 (5.16%) | 2 (0.65%) | 11 (7.64%) | ko03010 |
| Plant hormone signal transduction | 65 (4.05%) | 17 (2.03%) | 9 (4.23%) | 22 (7.12%) | 9 (6.25%) | ko04075 |
| Glycolysis/Gluconeogenesis | 62 (3.86%) | 32 (3.82%) | 7 (3.29%) | 12 (3.90%) | 10 (6.94%) | ko00010 |
| Starch and sucrose metabolism | 57 (3.55%) | 20 (2.39%) | 14 (6.57%) | 11 (3.58%) | 8 (5.56%) | ko00500 |
| Purine metabolism | 47 (2.93%) | 32 (3.82%) | 10 (4.69%) | 4 (1.30%) | 1 (0.69%) | ko00230 |
| Phenylpropanoid biosynthesis | 44 (2.74%) | 12 (1.37%) | 6 (2.82%) | 12 (3.91%) | 6 (4.17%) | ko00940 |
| Pyrimidine metabolism | 44 (2.74%) | 30 (3.58%) | 11 (5.16%) | 2 (0.65%) | 1 (0.69%) | ko00240 |
| Pyruvate metabolism | 43 (2.68%) | 20 (2.39%) | 3 (1.41%) | 12 (3.91%) | 7 (4.86%) | ko00620 |
| DNA replication | 40 (2.49%) | 31 (3.70%) | 9 (4.22%) | 0 (0.00%) | 0 (0.00%) | ko03030 |
| Plant-pathogen interaction | 35(2.18%) | 16 (1.91%) | 7 (3.29%) | 6 (1.95%) | 1 (0.69%) | ko04626 |
DEGs in the signal transduction pathways in response to ROS
Table 5 shows the number of the DEGs involved in the plant hormone signal transduction pathway during 0 to 10 h of ROS-treatment. Numbers of those DEGs in the 4 significantly different expression patterns were calculated. A total of 57 DEGs were annotated in the plant hormone signal transduction pathways, including auxin, cytokinine, gibberellin, abscisic acid, ethylene, brassinosteroid and jasmonic acid.
Table 5.
Number of DEGs involved in the plant hormone signal transduction pathway during 0 to 10 h of ROS treatment
| Components | All profiles | Profile 1 | Profile 0 | Profile 6 | Profile 7 |
|---|---|---|---|---|---|
| Auxin | |||||
| AUX1 | 4 | 0 | 1 | 2 | 1 |
| AUX/IAA | 1 | 1 | 0 | 0 | 0 |
| GH3 | 7 | 4 | 1 | 0 | 0 |
| SAUR | 3 | 0 | 0 | 0 | 0 |
| Cytokinine | |||||
| CRE1 | 2 | 0 | 2 | 0 | 0 |
| AHP | 1 | 0 | 0 | 1 | 0 |
| B-ARR | 1 | 0 | 0 | 1 | 0 |
| A-ARR | 1 | 0 | 1 | 0 | 0 |
| Gibberellin | |||||
| 3GID1 | 2 | 0 | 0 | 1 | 1 |
| DELLA | 2 | 1 | 0 | 1 | 0 |
| Abscisic acid | |||||
| PYR/PYL | 3 | 0 | 1 | 2 | 0 |
| PP2C | 2 | 0 | 0 | 0 | 2 |
| SnRK2 | 4 | 0 | 0 | 1 | 3 |
| ABF | 3 | 0 | 0 | 1 | 2 |
| Ethylene | |||||
| ETR | 3 | 0 | 0 | 3 | 0 |
| CTR1 | 1 | 0 | 0 | 1 | 0 |
| MPK6 | 2 | 2 | 0 | 0 | 0 |
| EBF1/2 | 2 | 0 | 0 | 2 | 0 |
| EIN3 | 3 | 0 | 0 | 3 | 0 |
| ERF1/2 | 2 | 0 | 0 | 2 | 0 |
| Brassinosteroid | |||||
| BKI1 | 1 | 0 | 0 | 0 | 0 |
| BSK | 1 | 0 | 1 | 0 | 0 |
| TCH4 | 4 | 3 | 0 | 1 | 0 |
| CYCD3 | 2 | 0 | 2 | 0 | 0 |
| Jasmonic acid | |||||
| JAR1 | 3 | 2 | 0 | 0 | 0 |
| JAZ | 5 | 4 | 0 | 0 | 0 |
In the auxin signal transduction pathway, 4 unigenes encoding auxin influx transport protein (AUX1) were found to be differentially expressed, among which two were annotated to profile 6, and one to profile 7 showing different pattern of up-regulation. It was found that 7 DEGs encode gretchenhagen-3 (GH3) or GH3 family protein. Four of them were clustered to profile 1, and 1 to profile 0 showing down-regulated trends. Only 1 DEG belonging to profile 1 was annotated to auxin-induced protein AUX/IAA. In the auxin signal transduction pathway, 7 out of the 15 DEGs showed down-regulated trends, and 3 of those showed up-regulated trends, indicating that DEGs of the down-regulated trends in this pathway were more than those of up-regulated trends. Similar results were found in the cytokinine, gibberellin, brassinosteroid and jasmonic acid signal transduction pathway. In the cytokinine signaling pathway, 2 unigenes encoding cytokinin receptor 1B or 1 (CRE1) and 1 encoding type-a response regulator (A-ARR) showed down-regulated trends, while the Unigene0026793 encoding histidine phosphotransfer protein (AHP) showed an up-regulated trend. In the gibberellin signal transduction pathway, 4 unigenes encoding gibberellic acid receptor or DELLA protein were found to be differentially expressed, where 1 was identified as a down-regulated profile, and the other as up-regulated profiles. In the brassinosteroid signaling pathway, 6 out of the 8 DEGs showed down-regulated trends, while 1 showed as an up-regulated trend (Table 5, Figure 4).
Figure 4.
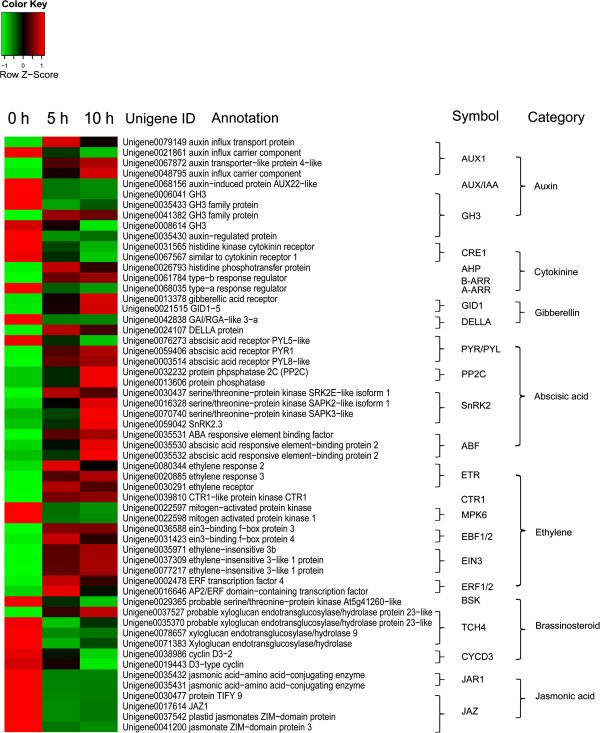
Heat map diagram of expression levels for DEGs annotated in the plant hormone signal transduction pathways analyzed by KEGG. Data for gene expression level were normalized to z-score. The original KEGG map is shown in Additional file 9.
In the abscisic acid signal transduction pathway, 11 out of the 12 DEGs were clustered to profile 6 or 7 showing up-regulated trends. They encode abscisic acid receptors PYR (PYRabactin resistance)/PYL (PYR1-like), type 2C protein phosphatase (PP2C), SNF1 related protein kinase 2 (SnRK2), and ABA responsive element binding factor (ABF). Only Unigene0076273 encoding abscisic acid receptor showed a down-regulated trend. In the ethylene signal pathway, 11 out of 13 DEGs were also clustered to up-regulated profiles, including 3 unigenes encoding ethylene receptor (ETR), 2 encoding ein3-binding F-box protein (EBF1/2), 3 encoding ethylene-insensitive 3b (EIN3) and 2 encoding ERF transcription factor ERF1/2. Only 2 unigenes (Unigene0022597 and Unigene0022598) encoding mitogen activated protein kinase (MPK6) showed down-regulated trends (Table 5, Figure 4). These results showed that DEGs of the up-regulated trend in the abscisic acid and ethylene signaling pathway were much more than those of down-regulated trend, suggesting that most genes involved in these hormone signal transduction pathway were induced by ROS.
Confirm unigenes expression using real-time quantitative reverse transcription PCR
To confirm the accuracy and reproducibility of the transcriptome analysis results, 7 unigenes were selected for real-time quantitative reverse transcription PCR (qRT-PCR) validation. RNA samples from the ROS-treated rudimentary leaves were used as templates. Primers of the candidate unigenes are shown in Additional file 8. The expression profiles of the candidate unigenes revealed by qRT-PCR data were consistent with those derived from sequencing (Figure 5). Linear regression analysis of the fold change of the gene expression ratios between RNA-seq and qRT-PCR showed significantly positive correlation (Figure 6), confirming our transcriptome analysis.
Figure 5.
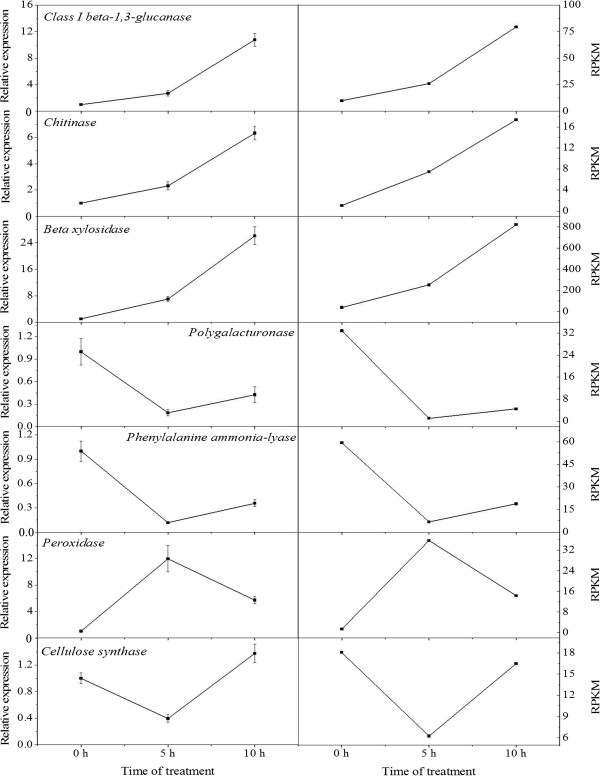
Candidate unigene expression levels revealed by qRT-PCR (left side) and RNA-seq (right side). Data from qRT-PCR are means of three replicates and bars represent SE. RPKM from RNA-seq are means of two replicates.
Figure 6.
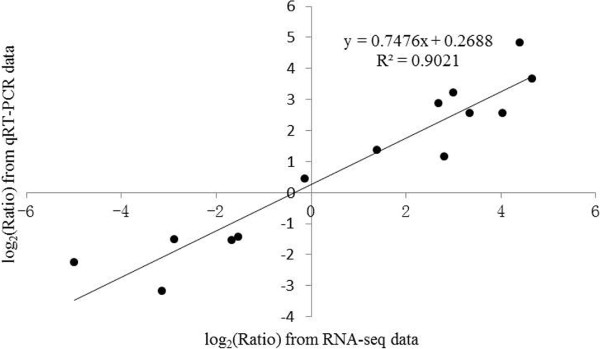
Coefficient analysis of fold change data between qRT-PCR and RNA-seq. Seven unigenes were selected for qRT-PCR. Data indicating relative transcript level from qRT-PCR are means of three replicates, and RPKMs from RNA-seq are means of two replicates. Scatterplots were generated by the log2 expression ratios from RNA-seq (x-axis) and qRT-PCR (y-axis).
Expression levels of the candidate genes in response to ROS
Based on the KEGG pathway enrichment analysis, it was found that most of the unigenes encoding the ABA and ethylene signal transduction components were induced by ROS. We then selected 10 candidate unigenes encoding these components and analyzed their expression levels during 0 to 10 h of ROS treatment. Primers of the candidate unigenes are shown in Additional file 8. The ABA and ethylene signal transduction components are shown in Additional file 9. Analysis of the gene expression revealed by qRT-PCR shows that genes encoding the ABA signal transduction components, including the abscisic acid receptor PYR1, protein phosphatase 2C, ABA responsive element-binding protein 2 and serine/threonine-protein kinase SRK2E-like isoform 1 increased while that of the PYL5-like decreased during 0 to 10 h of treatment. Genes encoding the ethylene signal transduction components, including the ethylene response 3, Ein3-binding f-box protein 3, ethylene-insensive 3b and ERF transcription factor 4 increased while that of the mitogen-activated protein kinase decreased by the treatment (Figure 7).
Figure 7.
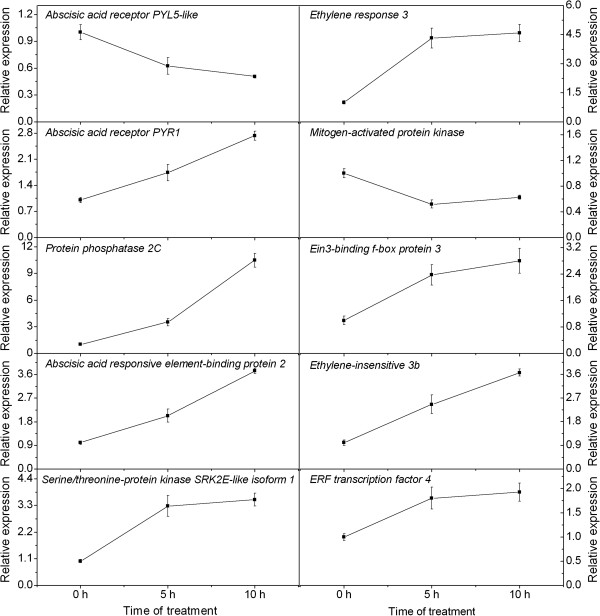
Expression levels of the candidate unigene encoding the ABA (left side) and ethylene (right side) signal transduction components revealed by qRT-PCR. Data from qRT-PCR are means of three replicates and bars represent SE.
Discussion
Stresses induce flowering in evergreen woody fruit trees [28–30]. ROS act as stress signals and are recognized as important signaling components in a wide range of processes. They are generated in chloroplast and peroxisomes in the light, in mitochondria in the dark and in non-green tissues [31, 32]. These signals are perceived specifically by diverse mechanisms, such as the direct redox modification of transcription factors and other proteins, resulting in the regulation of transcriptome [33]. Our previous study showed that ROS, induced by MV, promoted continuative development of panicle primordia and abortion of rudimentary leaves in leafy panicle in litchi [14]. To understand the molecular mechanisms underlying flowering in litchi, identifying ROS responsive genes in rudimentary leaves is needed as well as those in panicle primordia. We had established an SSH library and had identified 93 ROS responsive genes in the panicle primordia [21]. In the present study, we sought to identify the stress-responsive genes in the litchi rudimentary leaves. To overcome the lack of a reference litchi genome, we used RNA-Seq data to de novo assemble a reference transcriptome. In total, our assembly contains 82,036 unigenes with a mean size of 710 bp. 47,596 unigenes were annotated to public protein databases. Using the transcriptome as a reference, we performed DESeq and identified 5,865 differentially expressed genes between un-treated (0 h) and ROS-treated (5 h or 10 h) rudimentary leaves. 2,052 unigenes showed up-regulated trends and 3,035 showed down-regulated trends from 0 to 10 h of treatments. Compared to the 93 ROS responsive genes identified by previous SSH experiment [21], RNA-Seq has identified significantly more DEGs in the rudimentary leaf libraries.
Plant hormones are signal molecules produced within the plant, and occur in extremely low concentrations, but regulate a wide range of processes, including determining the formation of flowers, stems, leaves, the shedding of leaves, the development and ripening of fruit, and in response to biotic and abiotic stresses. The plant hormone signals are perceived and transmitted to the nuclear by series signal transduction components to induce gene expression, resulting in a series of physiological processes. Our KEGG pathway enrichment analysis of the DEGs indicated that unigenes encoding the hormone signaling components were significantly enriched in the differentially expressed groups after MV treatment. These hormones included auxin, cytokinine, gibberellin, abscisic acid, ethylene, brassinosteroid, and jasmonic acid, suggesting that their signal components are responsive to ROS. It is believed that ROS signaling and redox balance is integrated with salicylic acid (SA) signaling [33]. SA-signaling pathway has been proved to have a role in controlling gene expression during senescence [34]. Though the SA-signaling pathway was not found to be significantly enriched in the ROS-treated rudimentary leaves, some unigenes, such as NPR1 and PAD4 encoding SA-signaling components were found to be differentially expressed (Additional file 6, Unigene0034828 and Unigene0014177), suggesting that SA might also be involved in the ROS-induced rudimentary leaf abortion.
Abscisic acid (ABA) is an essential hormone to control plant growth, development and adaptation to environmental stresses [35]. We found that 11 out of the 12 DEGs encoding abscisic acid signal components were up-regulated. The unigenes encoding PYR /PYL, PP2C, SnRK2, and ABF were induced by MV-driven ROS. Our gene expression levels of the components determined by qRT-PCR were consistent with those by RNA-seq, further confirming that the ABA signal transduction components were ROS responsive. ABA is essential for abscission and senescence of aged organs. It is involved in shading-induced abscission of apple fruits [36], and ethylene-associated abscission activation in citrus fruitlets [37]. In the present study, MV treatment induced downward growing of the rudimentary leaves, an early sign of abortion of the rudimentary leaves [4]. The cross-talk between ABA and ROS signaling is well known [38, 39], and ROS is also involved in the ABA-enhanced LcAP1 expression in litchi [40]. Therefore, we hypothesize that the increase in the gene expression of ABA signaling components might play a role in the ROS-induced abortion of litchi rudimentary leaves.
We have also identified 13 differentially expressed genes encoding ethylene signaling components, and 11 of them were up-regulated after MV-treatment. Gene expression levels determined by qRT-PCR indicated that the unigenes encoding ethylene response 3, Ein3-binding f-box protein 3, ethylene-insensive 3b and ERF transcription factor 4 increased while that of the mitogen-activated protein kinase decreased by the ROS treatment, consisting with those revealed by RNA-seq. Our present study suggested that the ethylene signaling transduction components were ROS responsive. Ethylene as a gaseous hormone is involved in a variety of plant developmental adaptations including seed germination, organ senescence, fruit ripening, abscission and stress responses [41]. In agricultural practice, growers often use ethephon as ethylene producer to control rudimentary leaf growth in panicles and promote continual panicle development. We found that ethylene could increase H2O2 levels in the rudimentary leaves [5]. We also showed that the petioles of rudimentary leaf displayed downward growing after MV-ROS treatment (Figure 1), similarly to those of the ethylene-treated leaves, which is a phenomenon of epinasty, suggesting that the MV-ROS could function through ethylene to induce abscission of litchi rudimentary leaves.
In Arabidopsis thaliana, MV was used to study the influence of chloroplastic ROS generation at the transcriptional level [42]. We compared our DEGs in response to MV with those identified by Scarpeci et al. [42]. To our surprise, 84% (267/316) of their differentially expressed homology genes were found in our GEGs data set, including genes encoding some plant hormone signaling transduction components. Our study provided more information on the influence of MV generated-ROS on plant transcriptome.
Conclusions
In summary, we reported a comprehensive litchi leaf EST dataset generated by de novo assembly of next generation sequencing data. It is a valuable resource for future litchi genomic studies and will also benefit researches in other closely related species with significant agricultural importance. The differentially expressed genes dataset will also provide useful candidate genes for functional analysis of litchi flowering underlying the control of rudimentary leaf.
Methods
Plant material and experiment procedures
Commercially cultivated thirteen-year-old litchi trees cv. Nuomici grafted onto ‘Huaizhi’ were selected. The trees were grown at the experimental orchard of South China Agricultural University. About 6 cm length of branches with new flushes were cut off from the trees and immediately placed in water. Two hours later, the cuttings were treated with water or solutions containing 40 μM MV (Sigma) according to the method of Zhou et al. [14]. All the cuttings were placed in a growth chamber at 160 μmol/m2 s1 photosynthetic photon flux density at 20°C. The third and the fourth of the 0 h, 5 h and 10 h MV-treated rudimentary leaves as shown in Figure 1A were sampled, frozen in liquid nitrogen and stored at −80°C for RNA-seq and qRT-PCR. The proximal angle α, and the distal angle β of the third rudimentary leaves as shown in Figure 1A were measured. The angular dimension was measured by degree.
RNA isolation, library construction and EST sequencing
RNA of the rudimentary leaves from the 0 h, 5 h and 10 h ROS-treated rudimentary leaves were extracted using kits from Huayueyang Biotechnology Co., LTD., according to the manufacturer’s protocol. Samples were collected from replicate bundles (6–10 shoot cuttings in one bundle as one replicate). Each treatment had 2 replicates. In order to identify differentially expressed genes (DEGs) in response to ROS, RNA from the 6 samples were used to construct libraries for digital gene expression (DGE) profiling analyses. The libraries were as follows: Lc0h-1 and Lc0h-2 as replicate libraries for 0 h of ROS treatment, Lc5h-1 and Lc5h-2 for 5 h of ROS treatment, Lc10h-1 and Lc10h-2 for 10 h of ROS treatment. The transcriptome assembly library as a reference library was constructed by mixing equal amounts of RNA from the above 6 samples. Briefly, total mRNA was isolated with Oligo (dT) cellulose, fragmented and reverse transcripted with random primers. Second-strand cDNA were synthesized by DNA polymerase I and RNase H. Then the cDNA fragments were purified with QiaQuick PCR extraction kit, under gone end repair, dA-tailing and ligated to Illumina adapters. The ligation products were size fractioned by agarose gel electrophoresis, and fragments were excised for PCR amplification. The amplified fragments were sequenced using Illumina HiSeq™ 2000 by Gene Denovo Co. (Guangzhou, China).
De novo assembly and annotation
For the assembly library, raw reads were filtered to remove those containing adapter and reads with more than 5% unknown nucleotides. Low quality reads were also remove, in which the percentage of low Q-value (≤10) base was more than 20%. Clean reads were de novo assembled by the Trinity Program [43]. To annotate the unigenes, we used BLASTx program (http://www.ncbi.nlm.nih.gov/BLAST/) at NCBI with an E-value threshold of 1e−5 to NCBI nr database (http://www.ncbi.nlm.nih.gov), the Swiss-Prot protein database (http://www.expasy.ch/sprot), the KEGG database (http://www.genome.jp/kegg), and the COG database (http://www.ncbi.nlm.nih.gov/COG). The sequence direction of the unigenes was determined according to the best alignment results. When the results were conflicted among databases, the direction was determined consecutively by nr, Swiss-Prot, KEGG and COG. When a unigene could not be aligned, sequence direction would be confirmed using ESTscan program. GO annotation was analyzed by Blast2GO software. Functional classification of the unigenes was performed using WEGO software. KEGG pathway annotation was undergone by Blastall software against the KEGG database. The dataset is available from the NCBI Short Read Archive (SRA) with an accession number SRA158542 (http://www.ncbi.nlm.nih.gov/sra).
Expression annotation
Raw sequence data of the libraries for DGE profiling analyses were filtered to remove those containing adapter and reads with more than 10% unknown nucleotides, and reads with more than 50% of low quality base (value ≤5). Clean reads were mapped into the transcriptome reference database using SOAPaligner/soap2 software. Not more than 2 mismatch bases were permitted, and unique mapped reads were obtained. The number of unique-match reads was calculated and normalized to RPKM (reads per kb per million reads) for gene expression analysis.
Comparison of unigene expression between treatments was according to DESeq as described by Abders and Huber [44]. P-value corresponds to differential gene expression test. FDR is a method to determine the threshold of P-value. In this experiment, the DEGs between 0 h and 5 h, or between 0 h and 10 h of ROS treatment were restricted with FDR ≤ 0.001 and the absolute value of log2 Ratio ≥ 1.
Gene expression data υ (from 0 h to 10 h of ROS treatment) were normalized to 0, log2 (υ5h/υ0h), log2 (υ10/υ0h). DEGs were clustered by STEM [45]. The clustered profiles with p-value ≤ 0.05 were considered as significantly expressed. Then the DEGs were subjected to GO classifications using WEGO [46], and KEGG pathway annotation undergone by Blastall software against the KEGG database.
Real-time PCR confirmation of the RNA-Seq data
First-strand cDNA was generated from 1 μg total RNA isolated from the rudimentary leaves using the superscript first-strand synthesis system (Invitrogen, USA). Primers for quantitative reverse transcription PCR (qRT-PCR) were designed using Primer Premier 5.0 software (Premier, Canada) and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The litchi homologue Actin (GenBank accession number:HQ588865.1) was selected as reference. All the primers are shown in Additional file 8. qRT-PCR was performed on a Bio-Rad iQ5 Optical System Real Time PCR System (Bio-Rad, USA) using a SYBR Green based PCR assay. Each reaction mixture was 20 μL containing 6 μL of diluted first-strand cDNAs and 250 nM of each primer, SYBR Green PCR Master Mix (TaKaRa, Japan) 10 μL. The qPCRs were run as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s in 96-well optical reaction plates (Bio-rad, USA). Each qRT-PCR analysis was performed in triplicate. Expression levels of the tested reference genes were determined by CT values and calculated by 2-△△Ct.
Electronic supplementary material
Additional file 1: Size distributions of unigenes in the reference library. (PDF 10 KB)
Additional file 2: GO assignment of all unigenes in the reference library. (PDF 123 KB)
Additional file 3: Pathway annotation of unigenes of the rudimentary leaves in litchi. (XLSX 21 KB)
Additional file 4: Distribution of unigenes’ coverage in the 6 DGE libraries. (PDF 576 KB)
Additional file 5: RPKM of the unigenes indicating gene expression levels. A, RPKM of unigenes in two replicate libraries of Lc0h; B, RPKM of unigenes in two replicate libraries of Lc5h; C, RPKM of unigenes in two replicate libraries of Lc10h. (PDF 149 KB)
Additional file 6: Differentially expressed unigenes. This table shows all the DEGs and their RPKM values in the MV-treated leaves. (XLS 608 KB)
Additional file 7: Profiles order based on the P-value significance of number assigned versus expected. Numbers in the backets indicate the number of the DEGs assigned. (PDF 206 KB)
Additional file 8: Primer sequences of the reference gene and candidate unigenes for qRT-PCR. (PDF 48 KB)
Additional file 9: Enriched plant hormone signal transduction pathway. The signal transduction components marked with red rectangles are considered to be differentially expressed. (PDF 98 KB)
Acknowledgements
This work was supported by the National Natural Science Foundation (project no. 31071760) and the Agricultural Industry Project (project no. CARS-33-08).
Abbreviations
- A-ARR
Type-a response regulator
- ABF
ABA responsive element binding protein
- AHP
Histidine phosphotransfer protein
- AP1
APETALA1
- AUX1
Auxin influx transport protein
- AUX/IAA
Auxin-induced protein
- COG
Cluster of Orthologous Groups of protein
- DEG
Differentially expressed gene
- DGE
Digital gene expression tag
- CRE
Cytokinin receptor
- GH3
Gretchenhagen-3
- EBF
Ein3-binding F-box protein
- EIN
Ethylene insensitive
- EST
Expressed sequence tag
- ETR
Ethylene receptor
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LFY
LEAFY
- MPK
Mitogen activated protein kinase
- MV
Methyl viologen dichloride hydrate
- PYL
PYR1-like
- PYR
PYRabactin resistance
- qRT-PCR
Quantitative reverse transcription PCR
- ROS
Reactive oxygen species
- SAPK
Serine/threonine-protein kinase.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BY contributed to the design of the research and wrote the manuscript. LX performed sample collection, RNA isolation and gene expression. ZS contributed to data analysis and manuscript revision. CH and HJ participated in the design of the research. ZQ carried out physiological determination. All authors read and approved the final manuscript.
Contributor Information
Xingyu Lu, Email: luxingyu90@163.com.
Hyeji Kim, Email: heidikim219@hotmail.com.
Silin Zhong, Email: silin.zhong@cuhk.edu.hk.
Houbin Chen, Email: hbchen@scau.edu.cn.
Zhiqun Hu, Email: zhqhu@scau.edu.cn.
Biyan Zhou, Email: zhoubiyan@scau.edu.cn.
References
- 1.Menzel CM, Simpson DX. Effect of temperature on growth and flowering of litchi (Litchi chinensis Sonn.) cultivars. J Hort Sci. 1988;63:349–360. [Google Scholar]
- 2.Chen HB, Huang HB. Low temperature requirements for floral induction in lychee. Acta Hort. 2005;665:195–202. [Google Scholar]
- 3.Huang HB, Chen HB. A phase approach towards floral formation in lychee. Acta Hort. 2005;665:185–194. [Google Scholar]
- 4.Zhou BY, Chen HB, Huang XM, Li N, Hu ZQ, Gao ZG, Lu Y. Rudimentary leaf abortion with the development of panicle in litchi: changes in ultrastructure, antioxidant enzymes and phytohormones. Sci Hort. 2008;117:288–296. doi: 10.1016/j.scienta.2008.04.004. [DOI] [Google Scholar]
- 5.Zhou B, Huang X, Chen H, Shu W, Hu Z, Liu W, Xiang C, Zhang S. Effects of ethylene on rudimentary leaf and panicle primordium: antioxidant enzymes, hydrogen peroxide and nitric oxide. Acta Hort. 2013;975:247–254. [Google Scholar]
- 6.Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–270. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 7.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signaling. J Exp Bot. 2014;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann P, Heinlein C, Orendi G, Zentgraf U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006;29:1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- 9.Bañuelos GR, Argumedo R, Patel K, Ng V, Zhou F, Vellanoweth RL. The developmental transition to flowering in Arabidopsis is associated with an increase in leaf chloroplastic lipoxygenase activity. Plant Sci. 2008;174:366–373. doi: 10.1016/j.plantsci.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodge AD. The mode of action of bipyridylium herbicides, paraquat and diquat. Endeav. 1971;30:30–135. doi: 10.1016/0160-9327(71)90039-1. [DOI] [PubMed] [Google Scholar]
- 11.Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 12.Kraus TE, Fletcher RA. Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant Cell Physiol. 1994;35:45–52. [Google Scholar]
- 13.Sabarinath S, Bharti S, Khanna-Chopra R. Superoxide dismutase and abiotic stress tolerance. Physiol Mol Biol Plants. 2005;11:187–198. [Google Scholar]
- 14.Zhou B, Li N, Zhang Z, Huang X, Chen H, Hu Z, Pang X, Liu W, Lu Y. Hydrogen peroxide and nitric oxide promote reproductive growth in Litchi chinensis. Biol Plant. 2012;56:321–329. doi: 10.1007/s10535-012-0093-3. [DOI] [Google Scholar]
- 15.Cui ZY. Master Thesis. South China Agricultural University, College of Horticulture. 2010. Cloning and expression analysis of AP1 and CDPK homologue gene in Litchi (Litchi chinensis Sonn.) [Google Scholar]
- 16.Ahearn KP, Johnson HA, Weigel D, Wagner DR. NFL1, a Nicotiana tabacum LFY-like gene, controls meristem initiation and floral structure. Plant Cell Physiol. 2001;42:1130–1139. doi: 10.1093/pcp/pce143. [DOI] [PubMed] [Google Scholar]
- 17.Ma YP, Fang XH, Chen F, Dai SL. DFL, a FLORICAULA/LEAFY homologue gene from Dendranthema lavandulifolium is expressed both in the vegetative and reproductive tissues. Plant Cell Rep. 2008;27:647–654. doi: 10.1007/s00299-007-0489-2. [DOI] [PubMed] [Google Scholar]
- 18.Pena L, Martin-Trillo M, Juarez J, Pina JA, Navarro L, Martinez-Zapater JM. Constitutive expression of Arabidopsis LFY or APETALA1 genes in citrus reduces their generation time. Nat Biotechnol. 2001;19:263–267. doi: 10.1038/85719. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann K, Wellmer F, Muiño JM, Ferrier T, Wuest SE, Kumar V, Serrano-Mislata A, Madueño F, Krajewski P, Meyerowitz EM, Angenent GC, Riechmann JL. Orchestration of floral initiation by APETALA1. Science. 2010;328:85–89. doi: 10.1126/science.1185244. [DOI] [PubMed] [Google Scholar]
- 20.Wellmer F, Riechmann JL. Gene networks controlling the initiation of flower development. Trends Genet. 2010;26:519–527. doi: 10.1016/j.tig.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Liu WW, Kim HJ, Chen HB, Lu XY, Zhou BY. Identification of MV-generated ROS responsive EST clones in floral buds of Litchi chinensis Sonn. Plant Cell Rep. 2013;32:1361–1372. doi: 10.1007/s00299-013-1448-8. [DOI] [PubMed] [Google Scholar]
- 22.Feng C, Chen M, Xu CJ, Bai L, Yin XR, Li X, Allan AC, Ferguson IB, Chen K. Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genomics. 2012;13:19. doi: 10.1186/1471-2164-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo SG, Liu JA, Zheng Y, Huang MY, Zhang HY, Gong GY, He HJ, Ren Y, Zhong SL, Fei ZJ, Xu Y. Characterization of transcriptome dynamics during watermelon fruit development: sequencing, assembly, annotation and gene expression profiles. BMC Genomics. 2011;12:454. doi: 10.1186/1471-2164-12-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GQ, Li WS, Zheng PH, Xu T, Chen LJ, Liu DF, Hussain S, Teng YW. Transcriptomic analysis of ‘Suli’ pear (Pyrus pyrifolia white pear group) buds during the dormancy by RNA-Seq. BMC Genomics. 2012;13:700. doi: 10.1186/1471-2164-13-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai S, Saito T, Sakamoto D, Ito A, Fujii H, Takaya M. Transcriptome analysis of Japanese Pear (Pyrus pyrifolia Nakai) flower buds transitioning through endodormancy. Plant Cell Physiol. 2013;54:1132–1151. doi: 10.1093/pcp/pct067. [DOI] [PubMed] [Google Scholar]
- 26.Corbacho J, Romojaro F, Pech JC, Latche A, Gomez-Jimenez MC. Transcriptomic events involved in melon mature-Fruit abscission comprise the sequential induction of Cell-Wall degrading genes coupled to a stimulation of endo and exocytosis. PLoS One. 2013;8:e58363. doi: 10.1371/journal.pone.0058363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Wang Y, Huang X, Li J, Wang H, Li J. De novo assembly and characterization of fruit transcriptome in Litchi chinensis Sonn and analysis of differentially regulated genes in fruit in response to shading. BMC Genomics. 2013;14:552. doi: 10.1186/1471-2164-14-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunez-Elisea R, Davenport TL. Flowering of mango trees in containers as influenced by seasonal temperature and water stress. Sci Hort. 1994;58:57–66. doi: 10.1016/0304-4238(94)90127-9. [DOI] [Google Scholar]
- 29.Manochai P, Sruamsiri P, Wiriya-alongkorn W, Naphrom D, Hegele M, Bangerth F. Year around off season flower induction in longan (Dimocarpus longan Lour.) trees by KClO3 applications: potentials and problems. Sci Hort. 2005;104:379–390. doi: 10.1016/j.scienta.2005.01.004. [DOI] [Google Scholar]
- 30.Zhou BY, Chen HB, Wu CB. An overview on natural triggers and stress signals in relation to flowering in Litchi chinensis and Dimocarpus longan. Acta Hort. 2014;1029:137–144. [Google Scholar]
- 31.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sierla M, Rahikainen M, Salojärvi J, Kangasjärvi J, Kangasjärvi S. Apoplastic and chloroplastic redox signaling networks in plant stress responses. Antioxid Redox Signal. 2013;18:2220–2239. doi: 10.1089/ars.2012.5016. [DOI] [PubMed] [Google Scholar]
- 33.Wrzaczek M, Brosche M, Kangasjarvi J. ROS signaling loops - production, perception, regulation. Curr Opin Plant Biol. 2013;16:575–582. doi: 10.1016/j.pbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Morris K, A.-H.-Mackerness S, Page T, John C, Fred M, Alex M, Carr JP, Buchanan-Wollaston V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000;23:677–685. doi: 10.1046/j.1365-313x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 35.Adie BAT, Perez-Perez J, Perez-Perez MM, Godoy M, Sanchez-Serrano JJ, Schmelz EA, Solano R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell. 2007;19:1665–1681. doi: 10.1105/tpc.106.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Dardick CD, Beers EP, Callanhan AM, Xia R, Yuan R. Transcriptomics of shading-induced and NAA induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol. 2011;11:138. doi: 10.1186/1471-2229-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez-Cadenas A, Mehouachi J, Tadeo FR, Primo-Millo E, Talon M. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta. 2000;210:636–643. doi: 10.1007/s004250050054. [DOI] [PubMed] [Google Scholar]
- 38.Jiang M, Zhang J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell Environ. 2003;26:929–939. doi: 10.1046/j.1365-3040.2003.01025.x. [DOI] [PubMed] [Google Scholar]
- 39.Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 40.Cui Z, Zhou B, Zhang Z, Hu Z. Abscisic acid promotes flowering and enhances LcAP1 expression in Litchi chinensis Sonn. South Afr J Bot. 2013;88:76–79. doi: 10.1016/j.sajb.2013.05.008. [DOI] [Google Scholar]
- 41.Wang FF, Cui XK, Sun Y, Dong CH. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 2013;32:1099–1109. doi: 10.1007/s00299-013-1421-6. [DOI] [PubMed] [Google Scholar]
- 42.Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol. 2008;66:361–378. doi: 10.1007/s11103-007-9274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng QD, Chen ZH, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics. 2006;7:191. doi: 10.1186/1471-2105-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye J, Fang L, Zheng HK, Zhang Y, Chen J, Zhang ZJ, Wang J, Li ST, Li RQ, Bolund L, Wang J. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:W293–W297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Size distributions of unigenes in the reference library. (PDF 10 KB)
Additional file 2: GO assignment of all unigenes in the reference library. (PDF 123 KB)
Additional file 3: Pathway annotation of unigenes of the rudimentary leaves in litchi. (XLSX 21 KB)
Additional file 4: Distribution of unigenes’ coverage in the 6 DGE libraries. (PDF 576 KB)
Additional file 5: RPKM of the unigenes indicating gene expression levels. A, RPKM of unigenes in two replicate libraries of Lc0h; B, RPKM of unigenes in two replicate libraries of Lc5h; C, RPKM of unigenes in two replicate libraries of Lc10h. (PDF 149 KB)
Additional file 6: Differentially expressed unigenes. This table shows all the DEGs and their RPKM values in the MV-treated leaves. (XLS 608 KB)
Additional file 7: Profiles order based on the P-value significance of number assigned versus expected. Numbers in the backets indicate the number of the DEGs assigned. (PDF 206 KB)
Additional file 8: Primer sequences of the reference gene and candidate unigenes for qRT-PCR. (PDF 48 KB)
Additional file 9: Enriched plant hormone signal transduction pathway. The signal transduction components marked with red rectangles are considered to be differentially expressed. (PDF 98 KB)


