Abstract
Background and Purpose
Initial National Institutes of Health Stroke Scale (NIHSS) score is highly predictive of outcome after ischemic stroke. We examined if grouping strokes by presence of individual NIHSS symptoms could provide prognostic information additional or alternative to the NIHSS total score.
Methods
Ischemic strokes from the Greater Cincinnati Northern Kentucky Stroke Study in 2005 were used to develop the model. Latent Class Analysis (LCA) was implemented to form groups of patients with similar retrospective NIHSS (rNIHSS) item responses. Profile group was then used as an independent predictor of discharge modified Rankin and mortality using logistic regression and Cox proportional hazards model.
Results
A total of 2,112 stroke patients were identified in 2005. Six distinct profiles were characterized. Consistent with the profile patterns, the median rNIHSS total score decreased from profile A “most severe” [median(IQR): 20(15,25)] to profile F “mild” [1(1,2)]. Two profiles falling between these extremes, C and D, both had median rNIHSS total score of 5, but different survival rates. Compared with A, C was associated with 59% risk reduction for death, whereas D with 70%. C patients were more likely to have decreased level of consciousness and abnormal language, whereas D patients were more likely to have abnormal right arm and right leg motor function.
Conclusions
Six rNIHSS profiles were identifiable using LCA. In particular, two symptom profiles with identical median rNIHSSS were observed with widely disparate outcomes, which may prove useful both clinically and for research studies as an enhancement to the overall NIHSS score.
Keywords: Ischemic stroke, NIHSS, latent class analysis, mild stroke
Introduction
The NIH Stroke Scale (NIHSS) measures neurological function in patients with signs and symptoms of stroke.1 It has been well validated2 and is commonly used in both the clinical and research settings. The NIHSS neurologic examination includes 15 individual elements that measure motor and sensory function, language and speech production, vision, level of consciousness and attention, and neglect. The elements are summed to provide an overall assessment of stroke severity, with the score ranging from 0 to 42. Stroke severity at onset has been associated with mortality, functional disability, length of hospital stay, and recovery.3,4 Initial NIHSS total score is highly predictive of outcome after ischemic stroke (IS). However, even among patients with mild strokes, defined by NIHSS scores between 0 and 5, approximately one third have significant disability following their stroke.5,6
Using an overall score, the individual components that contribute to that score are, for the most part, considered equal and interpretation is the same for a total score of 10 whether it indicates severe loss of consciousness or indicates motor weakness. Latent class analysis (LCA) is a technique that can be used to identify groups of individuals with similar response patterns on measured variables, optimizing the use of the individual components and their clustering to form identifiable phenotypes. The study objective was to examine if grouping strokes by presence of individual NIHSS components could provide additional or alternative prognostic information to the total NIHSS score.
Methods
The Greater Cincinnati and Northern Kentucky Stroke Study (GCNKSS) is an epidemiology study with major goals of determining temporal trends in the incidence rate, causes, treatment, and outcome of stroke in a biracial metropolitan population of approximately 1.3 million. Data for one-year study periods has been collected approximately every 5 years beginning in 1993. This present analysis is restricted to the 2005 study period and uses the 1999 study period data for validation. Ischemic strokes in patients 20 years and older where included. The GCNKSS methods have been described in detail elsewhere.7,8 Briefly, research nurses extracted information from the medical charts of all area residents who were either inpatients or discharged from an emergency department with primary or secondary stroke-related International Classification of Disease, 9th Revision (ICD-9) discharge codes 430-436 at the 16 hospitals active in this area in 2005. All stroke-related visits to public health clinics, hospital-based outpatient clinics, and family practice centers were reviewed. In addition, potential stroke cases in a random sample of 51 of the 832 primary care physicians’ offices and 25 of the 126 nursing homes in the area were assessed. A similar sampling strategy was employed during the 1999 study period. Sampling weights were not included in this analysis for both simplification of the methods and because the interest was on behavioral relationships and not necessarily population relationships.9 All potential stroke events were reviewed by a study physician to determine whether a stroke had occurred. If the potential case met the clinical criteria adapted from the Classification of Neurological Disorders III and epidemiological studies of stroke for cerebral ischemia, then it was included in this study as an ischemic case. Only one IS event per subject was included, recurrent IS events within the study period were excluded from the current analysis. Death following stroke was tracked using study records, the Ohio and Kentucky death registers, and the Social Security Death Index. To ensure completeness of overall survival data, survival status was truncated at 12/31/2009 for 2005 and at 12/31/2003 for 1999 (i.e., patients alive after these dates were censored at that time). Institutional review boards of all involved research institutions and hospitals approved the study protocol.
Retrospective NIHSS
The original intent of the NIHSS was to be scored at the time of patient evaluation with symptoms of stroke, but this is not always possible. Williams et al. developed an algorithm for computing the NIHSS score from the patient's medical record of physical examination and history.10 The retrospective NIHSS (rNIHSS) has been shown to be adequately scored from the initial history and physical examination documented by a stroke team physician, and that the magnitude of the NIHSS score does not influence the validity of the retrospective scoring method.11,12 The rNIHSS validated scoring method was used in this study.
Data Analyses
Due to the majority of scores on the rNIHSS being 0 or 1, items were dichotomized as normal (0) versus abnormal (≥1). LCA was used to combine the 15 dichotomized rNIHSS items to classify stroke patients into discrete profiles. Stroke patients with similar patterns of presence or absence of rNIHSS elements are grouped together within a latent profile. The latent class model differs from traditional models, such as ordinary least squares regression analysis that describe only those relationships between observed variables, by including one or more discrete unobserved (latent) variables. The resulting model parameters are: (1) the proportion of subjects with in a given profile membership and (2) the probability of having a score ≥1 for an item given that profile membership. In this study, an example would be the probability that a member of profile A has an abnormal score for rNIHSS item best gaze. The latent class model requires that the number of latent classes be specified, and the ideal number to specify is commonly determined by increasing the number of classes and assessing model fit in a stepwise fashion. Initially, two classes were specified in the model. The number of classes was deemed optimal at the point when no statistically significant drop in model fit was noted with an increase in the number of classes. The Lo-Mendell-Rubin adjusted likelihood ratio test, average posterior probability, and group membership probabilities were also used to assess model fit.13 The analysis was implemented using finite mixture modeling in Mplus 5 using maximum likelihood.14
Once the appropriate number of classes was determined, class membership was used as an independent predictor of clinical patient outcomes: 30- and 90-day mortality, time to death, and modified Rankin (mRS) at discharge ≤1 or back to baseline. Kaplan-Meier life table analysis, Cox proportional hazards and logistic regression models adjusted for age, race, gender, and pre stroke mRS were used for analysis of the outcomes. In addition, the analysis was repeated among those patients with mild strokes defined by rNIHSS scores ≤5 and included of rNIHSS total score in the models.
The 2005 profile groupings were validated by applying the 2005 profile pattern to the IS events collected during the 1999 study period for patients 20 years and older.
Results
A total of 2,244 clinically defined IS cases in patients 20 years and older were identified in 2005, of which, 15 cases were excluded due to missing rNIHSS assessment. For patients with more than one IS occurrence during the year, the rNIHSS from the earliest case was used. A total of 2,112 IS patients were included in this analysis. Characteristics of this study group are shown in Table 1.
Table 1.
Demographics and stroke risk factors of the ischemic strokes from 2005, overall and by profile
| 2005 | Profile |
|||||||
|---|---|---|---|---|---|---|---|---|
| IS Cases | A | B | C | D | E | F | p-value | |
| Characteristic | (N=2112) | (n=222) | (n=150) | (n=208) | (n=319) | (n=448) | (n=765) | |
| Age, median (IQR) | 72 (60, 81) | 77.0 (67, 83) | 78.0 (68, 84) | 79 (68, 86) | 71 (59, 81) | 71 (58, 80) | 69 (56, 79) | <0.01 |
| Race (black) | 470 (22%) | 49 (22%) | 30 (20%) | 41 (20%) | 88 (28%) | 103 (23%) | 159 (21%) | 0.18 |
| Gender (female) | 1178 (56%) | 137 (62%) | 82 (55%) | 132 (63%) | 189 (59%) | 253 (56%) | 385 (50%) | <0.01 |
| Pre-stroke Rankin, median (IQR) | 2 (0, 3) | 2 (1,3) | 2 (1,3) | 2 (1,3) | 2 (0,3) | 1 (0,3) | 1 (0,2) | <0.01 |
| Hypertension | 1658 (79%) | 164 (74%) | 132 (89%) | 153 (74%) | 261 (82%) | 348 (78%) | 600 (78%) | <0.01 |
| Diabetes | 710 (34%) | 66 (30%) | 53 (35%) | 75 (36%) | 122 (38%) | 134 (30%) | 260 (34%) | 0.15 |
| Current smoking | 533 (26%) | 38 (19%) | 21 (15%) | 33 (17%) | 94 (30%) | 135 (31%) | 212 (28%) | <0.01 |
| Atrial fibrillation | 457 (22%) | 80 (36%) | 61 (41%) | 65 (31%) | 60 (19%) | 86 (19%) | 105 (14%) | <0.01 |
| CAD | 759 (36%) | 73 (33%) | 74 (49%) | 84 (41%) | 109 (34%) | 160 (36 %) | 259 (34%) | <0.01 |
| CHF | 413 (20%) | 71 (32%) | 43 (29%) | 56 (27%) | 56 (18%) | 77 (17%) | 110 (14%) | <0.01 |
| Prior Stroke | 557 (26%) | 66 (30%) | 47 (31%) | 65 (31%) | 97 (30%) | 123 (27%) | 159 (21%) | <0.01 |
| rNIHSS total score, median (IQR) | 4 (2, 7) | 20 (15, 25) | 12 (9, 16) | 5 (3, 7) | 5 (4, 8) | 4 (3, 6) | 1 (1, 2) | <0.01 |
IQR indicates interquartile range; CAD, coronary artery disease; CHF, congestive heart failure; rNIHSS, retrospective National Institutes of Health Stroke Scale.
In determining the optimal number of latent profiles, a total of six latent class models were evaluated with the number of latent profiles specified as two to seven. Based on model fit criteria, a six profile solution was deemed adequate.
The six distinct profiles identified within the IS cases from 2005 are shown in Figure 1. Based on the profile patterns, we labeled the profiles as severe (profiles A and B), moderate to mild (profiles C, D, and E), and mild (profile F). Profile A (222 patients; 11% of the total) represents severe stroke with decreased level of consciousness, facial palsy, abnormal motor function on the right side, language deficit and slurred speech. Profile B (150 patients; 7%) also represents severe stroke but with some decreased level of consciousness, facial palsy, abnormal motor function on left side, and slurred speech. Of note, all IS patients in Profile B had abnormal left arm function (probability = 1.00). Profile C (208 patients; 10%) captured strokes with language deficit and signs of slurred speech. Profile D (319 patients; 15%) included strokes with facial palsy, abnormal motor function on the right side, and slurred speech. Profile E (448 patients; 21%) included strokes with facial palsy and abnormal motor function on the left side. Profile F (765 patients; 36%) represents mild stroke with low probabilities of abnormal findings on all 15 items.
Figure 1.
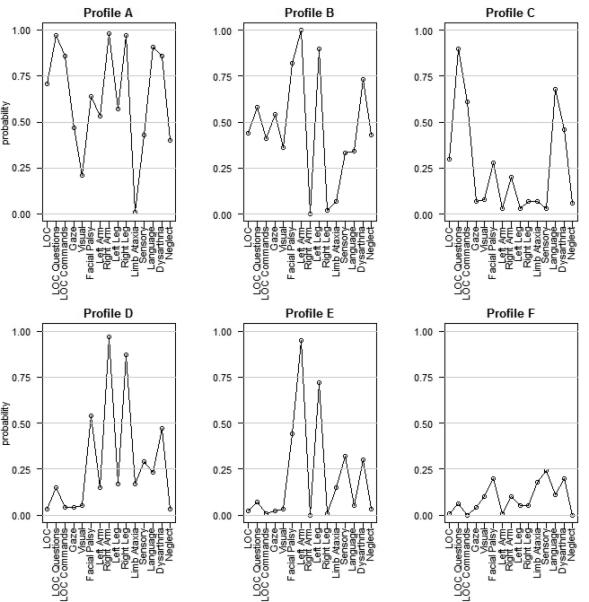
rNIHSS profile patterns: probability of observing an abnormal response for each rNIHSS element by profile
Footnote:
LOC = Level of consciousness.
The 15 rNIHSS items are noted on the x-axis.
The probability of an abnormal score (≥1) for each of the 15 rNIHSS items is shown on the y-axis.
To evaluate the profile classifications, the six profiles were compared for demographic and disease risk characteristics (Table 1). No significant differences across profiles were observed for race and history of diabetes. However, significant differences in age, gender, pre-stroke mRS, smoking status, history of hypertension, atrial fibrillation, coronary artery disease (CAD), congestive heart failure (CHF), prior stroke, and rNIHSS total score were observed. The profiles representing the more severe strokes, as indicated by higher rNIHSS total scores, included patients that tended to be older, not current smokers, had a history of atrial fibrillation, CHF, and prior stroke.
Consistent with the profile patterns, the median rNIHSS total score decreased from the most severe profile A [median (Interquartile range): 20 (15, 25)] to the mild profile F [1 (1, 2)]. Of note, two profiles falling between these extremes, C and D, both had median rNIHSS total score of 5, but different survival rates (Figures 2 and 3). After adjusting for age, gender, race, and pre-stroke mRS, profiles C and D had statistically significant different 90-day mortality and time to event survival rates (Table 2).
Figure 2.
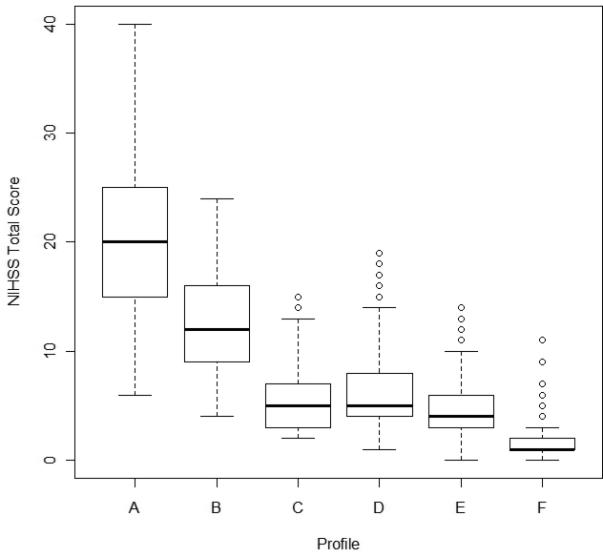
Boxplots of rNIHSS total score by profile
Figure 3.
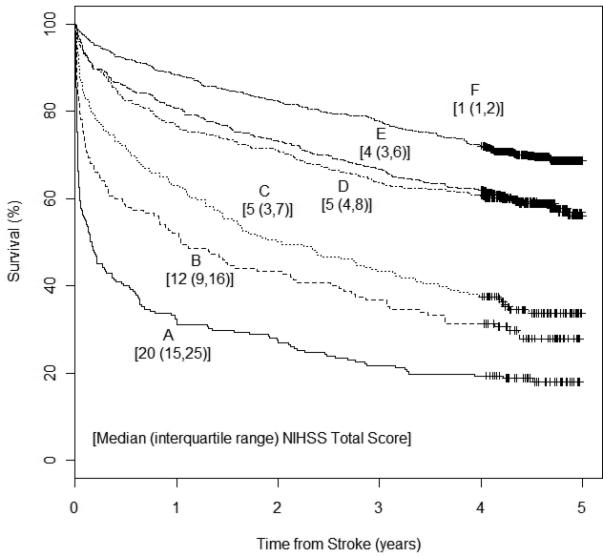
Kaplan-Meier curves by profile
Table 2.
Outcome measures by rNIHSS item profile (n=2112)
| Discharge Rankin ≤1 or back to baseline |
30 day mortality |
90 day mortality |
Survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Profile | # of events | OR | 95% CI | # of events | OR | 95% CI | # of events | OR | 95% CI | # of events | HR | 95% CI |
| A (n=222) | 17 | Ref | - | 97 | Ref | - | 122 | Ref | - | 181 | Ref | - |
| B (n=150) | 12 | 0.97 | (0.44, 2.13) | 38 | 0.43 | (0.27, 0.69) | 53 | 0.44 | (0.28, 0.69) | 107 | 0.60 | (0.47, 0.76) |
| C (n=208) | 47 | 2.90 | (1.58, 5.33) | 33 | 0.20 | (0.13, 0.33) | 48 | 0.20* | (0.13, 0.32) | 136 | 0.41* | (0.33, 0.51) |
| D (n=319) | 84 | 3.66 | (2.07, 6.50) | 22 | 0.11 | (0.07, 0.19) | 35 | 0.12* | (0.07, 0.18) | 132 | 0.30* | (0.24, 0.37) |
| E (n=448) | 156 | 5.54 | (3.19, 9.62) | 28 | 0.11 | (0.07, 0.17) | 46 | 0.11 | (0.08, 0.17) | 184 | 0.31 | (0.25, 0.39) |
| F (n=765) | 385 | 12.10 | (7.02, 20.86) | 20 | 0.05 | (0.03, 0.09) | 40 | 0.06 | (0.04, 0.10) | 231 | 0.23 | (0.19, 0.29) |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
Adjusted for age, gender, race, and pre stroke modified Rankin.
OR indicates odds ratio; CI, confidence interval; HR, hazard ratio.
Profiles C and D significantly different with p-value < 0.05.
Compared with the most severe profile A, profile C was associated with 80% reduction in risk of 90-day mortality, whereas profile D was associated with 88% reduction. Similarly for overall survival, compared to the most severe profile A, profile C was associated with 59% reduction in risk of death, whereas profile D was associated with 70% reduction in risk of death. Recall that C profile patients were more likely to have decreased level of consciousness and abnormal language, whereas D profile patients were more likely to have abnormal right arm and right leg motor function.
To compare the performance of the profiles among patients with mild stroke, a secondary analysis was performed including only those patients with rNIHSS total scores that were less than or equal to five. A total of 1362 IS patients were included in this secondary analysis. There were 115 (8%) patients in profile C, 161 (12%) in profile D, 330 (24%) in profile E and 756 (56%) in profile F. There were no patients in profile A and only 8 patients in profile B with rNIHSS total scores ≤5; thus patients in profiles A and B were not included in the mild stroke analysis.
The median rNIHSS total score was highest in profile D [4 (3, 5)] and lowest in profile F [1 (1, 2)]. Even though, all patients had rNIHSS total scores ≤ 5, profile C continued to show worse survival compared with the other three profiles (supplemental Figures S1 and S2, please see http://stroke.ahajournals.org). After adjusting for age, gender, race, pre-stroke mRS, and rNIHSS total score statistically significant differences between profiles were observed for discharge mRS ≤1 or back to baseline and overall survival (Table S1).
To validate these results, the 2005 profiles were applied to the data collected during the 1999 study period. Characterization of 1999 cases using the profiles yielded similar associations with outcome. A total of 2,459 clinically defined IS cases among patients 20 years and older were identified in 1999, and again the first IS event for each patient within the period was used, of which, 40 cases were excluded due to missing rNIHSS assessment; 2,277 cases were available for analysis. The median rNIHSS total score was the same both study periods, 4 (IQR 2, 7). The demographic characteristics of the 1999 IS cases were similar to that of the 2005 cases; median age 74 years, 18% black, and 55% female (Table S2). Again, no significant differences across profiles were observed for race and history of diabetes. For cases from 1999, there was no difference for hypertension and CAD (Table S2). Significant differences in age, gender, pre-stroke mRS, smoking status, history of atrial fibrillation, CHF, prior stroke, and rNIHSS total score were observed. Again, profiles C and D, both had similar median rNIHSS total scores of 5 and 4.5, respectively, but different survival rates (Figures S3 and S4). After adjusting for age, gender, race, and pre-stroke mRS, profiles C and D had statistically significant different 30-day and 90-day mortality, and time to event survival rates (Table S3).
Discussion
This is the first study to apply, and validate, Latent Class Analysis (LCA) to the rNIHSS, resulting in the identification of six discrete profile patterns. This study showed that the LCA method of analyzing rNIHSS items in IS patients provides an alternative approach for summarizing prognostic information for forecasting functional outcome and death compared to using the raw rNIHSS total score. In particular, two symptom profiles with identical median rNIHSS total scores but with widely disparate outcomes were identified. This observed difference in outcome may in part be due to more patients in profile C having aphasia compared to patients in profile D which further emphasizes the issue with considering individual components equal, such that a single point on the NIHSS for aphasia is equivalent to a slight sensory loss, when the two likely have dramatically different impacts on outcome. As shown in Figure 1, the profile patterns reflect the rNIHSS total score as well as the individual components. Patients with a high rNIHSS total score are more likely to fall into profiles A and B as these profiles had high probabilities of abnormal score on several rNIHSS items. Consistent with previous studies, the IS patients in these two profiles, due to high total rNIHSS, are more likely to experience poor outcomes compared with the other profiles.3,4 When the rNIHSS total score is indicative of a less severe stroke (profiles C-E), the profile membership can aid in patient prognosis, such that an IS patient with a rNIHSS total score of 5 is more likely to have a better outcome if a member of profile D compared to an IS patient with rNIHSS total score of 5 but a member of profile C. Differences in outcomes by profile membership remained among IS patients when examining only mild stroke (defined by rNIHSS total score ≤ 5). The validation of the profile patterns on 1999 IS cases revealed similar findings, which suggest that we may be capturing true response patterns that are associated with functional and mortality outcomes. In addition, when comparing the six profiles for differences in demographic and disease risk characteristics, we found that the profiles representing the more severe strokes included patients that tended to be older, not current smokers, with history of atrial fibrillation, CHF, prior stroke and higher rNIHSS total scores; similar to the findings noted by Kleindorfer et al.15
Our findings among mild IS differ from those reported by Leira et al. Their work indicated that increased prospectively collected baseline NIHSS total scores were associated with poor 3-month outcome, but that no individual NIHSS items or syndromic combination of NIHSS scores were found to be associated with outcome.16 This is not unexpected as there are substantial differences between our study and that of Leira et al. First, the sample size for the Leira et al. study was much smaller, with a total of 194 mild IS patients. The NIHSS combinations explored in the Leira et al. study were based on clinical syndromes used in clinical practice, not combinations generated in a modeling approach as was done in this study. Additionally, the functional outcome measure differed by both scale and timing. The Leira et al. study measured 3-month functional outcome based on both the Glasgow Outcome Scale and the modified Barthel Index, while this current study evaluated hospital discharge mRS scores. Lastly, the NIHSS scores were collected prospectively in the Leira et al. study, whereas this current study used retrospective NIHSS scores.
One limitation to this study is that the data were collected retrospectively. Although the retrospective NIHSS has been validated among the entire range of possible scores, it is possible that some discrepancies between the retrospective and prospective methods of scoring remain, thus limiting our generalizability to retrospectively scored NIHSS. For this analysis, rNIHSS items were dichotomized, which may limit the sensitivity due to loss of information. However, with this analytic method, there is a gain in interpretation of the profile patterns as probabilities reflected as the probability of an abnormal response for each of the rNIHSS items. The limited range of rNIHSS scores in our study may be due to the retrospective data collection and epidemiology study setting, and it further highlights the need for validation of the study findings in a prospective trial setting. Previous studies have also opted to dichotomize items even with prospective NIHSS assessments.16 The LCA method requires a specification of the number of true classes in the population of interest. Although the adequacy of the number of classes selected was checked, it is possible that we over- or under-estimated the number. Nevertheless, we were able to validate our findings on a similar but independent sample, which offer reassurance that our model is correct.
In conclusion, the NIHSS is a well validated and highly useful tool for capturing stroke severity with uses in both the clinical and research setting. The rNIHSS profiles identified in this study might be clinically useful for prognosis, and could conceivably be used in future clinical trial design.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke Division, grant R01NS30678.
Footnotes
Disclosures
H.Sucharew, J.C.Khoury, C.J.Moomaw, K.Alwell, B.M.Kissela, O.Adeoye, P.Khatri, and D.Kleindorfer receive research support from the National Institutes of Health. S.Belagaje, D.Woo, M.Flaherty, S.Ferioli, L.Heitsch, and J.Broderick have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, et al. Measurement of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH Stroke Scale. Arch Neurol. 1989;46:660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS TPA stroke trial. Stroke. 2000;31:2335–2341. doi: 10.1161/01.str.31.10.2335. [DOI] [PubMed] [Google Scholar]
- 4.Young FB, Weir CJ, Lees KR. Comparison of the National Institutes of Health stroke scale with disability outcome measures in acute stroke trials. Stroke. 2005;36:2187–2192. doi: 10.1161/01.STR.0000181089.41324.70. [DOI] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–31. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 6.Khatri P, Conaway MK, Johnston KC. ASAP Investigators. Ninety-day outcome rates of prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43:560–562. doi: 10.1161/STROKEAHA.110.593897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The greater Cincinnati/Northern Kentucky stroke study: Preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, et al. The unchanging incidence and case-fatality of stroke in the 1990s: A population-based study. Stroke. 2006;37:2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 9.Winship C, Radbill L. Sampling weights and regression analysis. Sociological Methods & Research. 1994;23:230–257. [Google Scholar]
- 10.Williams LS, Engin YY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 11.Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, et al. Reliability and validity of estimating the NIH Stroke Scale score from medical records. Stroke. 1999;30:1534–1537. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- 12.Lindsell CJ, Alwell K, Moomaw CJ, Kleindorfer DO, Woo D, Flaherty ML, et al. Validity of a retrospective National Institutes of Health Stroke Scale scoring methodology in Patients with severe stroke. Journal of Stroke and Cerebrovascular Diseases. 2005;14:281–283. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–78. [Google Scholar]
- 14.Muthen LK, Muthen BO. Mplus Users’ Guide. 5th ed. Muthen & Muthen; Los Angeles, CA: 1998-2007. pp. 131–184. [Google Scholar]
- 15.Kleindorfer D, Lindsell C, Alwell K, Moomaw CJ, Woo D, Flaherty ML, et al. Patients lining in impoverished areas have more severe ischemic strokes. Stroke. 2012;43:2055–2059. doi: 10.1161/STROKEAHA.111.649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leira EC, Ludwig BR, Gurol ME, Torner JC, Adams HP., Jr The types of neurological deficits might not justify withholding treatment in patients with low total National Institutes of Health Stroke Scale scores. Stroke. 2012;43:782–786. doi: 10.1161/STROKEAHA.111.620674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


