Abstract
High doses of sodium salicylate (SS) have long been known to induce temporary hearing loss and tinnitus, effects attributed to cochlear dysfunction. However, our recent publications reviewed here show that SS can induce profound, permanent, and unexpected changes in the cochlea and central nervous system. Prolonged treatment with SS permanently decreased the cochlear compound action potential (CAP) amplitude in vivo. In vitro, high dose SS resulted in a permanent loss of spiral ganglion neurons and nerve fibers, but did not damage hair cells. Acute treatment with high-dose SS produced a frequency-dependent decrease in the amplitude of distortion product otoacoustic emissions and CAP. Losses were greatest at low and high frequencies, but least at the mid-frequencies (10-20 kHz), the mid-frequency band that corresponds to the tinnitus pitch measured behaviorally. In the auditory cortex, medial geniculate body and amygdala, high-dose SS enhanced sound-evoked neural responses at high stimulus levels, but it suppressed activity at low intensities and elevated response threshold. When SS was applied directly to the auditory cortex or amygdala, it only enhanced sound evoked activity, but did not elevate response threshold. Current source density analysis revealed enhanced current flow into the supragranular layer of auditory cortex following systemic SS treatment. Systemic SS treatment also altered tuning in auditory cortex and amygdala; low frequency and high frequency multiunit clusters up-shifted or down-shifted their characteristic frequency into the 10-20 kHz range thereby altering auditory cortex tonotopy and enhancing neural activity at mid-frequencies corresponding to the tinnitus pitch. These results suggest that SS-induced hyperactivity in auditory cortex originates in the central nervous system, that the amygdala potentiates these effects and that the SS-induced tonotopic shifts in auditory cortex, the putative neural correlate of tinnitus, arises from the interaction between the frequency-dependent losses in the cochlea and hyperactivity in the central nervous system.
Keywords: sodium salicylate, auditory cortex, amygdala, medial geniculate body, auditory nerve, distortion product otoacoustic emission, tinnitus, startle reflex
Introduction
Salicylate, the active ingredient in aspirin, is one of the most effective, inexpensive and widely used antipyretic, analgesic and anti-inflammatory drugs. However, when consumed in large quantities (6-8 g/day) (Myers and Bernstein, 1965) it induces temporary hearing loss and tinnitus (Cazals, 2000; Day et al., 1989; Lobarinas et al., 2004; McFadden and Wightman, 1983; McFadden et al., 1984a). Although, salicylate-induced cochlear dysfunction is considered reversible in humans and in animals, recent reports suggest that prolonged, high-dose treatments with sodium salicylate (SS) can permanently decrease the neural output of the cochlea (Chen et al., 2010) and lead to neural degeneration in vitro (Wei et al., 2010; Zheng and Gao, 1996). The severity of SS-induced hearing loss has been closely linked to plasma salicylate levels (Cazals, 2000; Day et al., 1989); however, the relationship between tinnitus and serum salicylate concentration is somewhat less predictable, possibly due to the all or none nature of the phenomenon (McFadden et al., 1984b). Because high doses of the drug reliably induce tinnitus, SS has been widely used to study the perceptual, anatomical, behavioral and neurophysiological aspects of tinnitus in animal models (Jastreboff et al., 1988b; McFadden et al., 1984a; Ochi and Eggermont, 1996; Wallhausser-Franke et al., 2003). While salicylate and aspirin have long been known to impair cochlear function (Stypulkowski, 1990), more recent studies indicate that it affects many different parts of the central nervous system (CNS) (Bauer et al., 2000; Gong et al., 2008; Lu et al., 2011; Mahlke and Wallhausser-Franke, 2004; Panford-Walsh et al., 2008; Sun et al., 2009). Paradoxically, the functional impairments seen in the CNS differ in several important ways from those seen in the cochlea. In the auditory periphery, SS depresses cochlear sensitivity by reducing outer hair cell (OHC) electromotility (Kakehata and Santos-Sacchi, 1996; Tunstall et al., 1995) and decreases the neural output of the cochlea. In contrast, systemic treatment with a high-dose SS causes the auditory cortex (AC) to become hyperactive in response to high-level sound stimulation (Lu et al., 2011; Sun et al., 2009; Yang et al., 2007). These changes may be related to altered γ-aminobutyric acid (GABA) and serotonin mediated neurotransmission in the CNS (Bauer et al., 2000; Caperton and Thompson, 2011; Gong et al., 2008; Liu et al., 2003; Lu et al., 2011; Xu et al., 2005). In addition, high-dose SS increased expression of c-fos, an activity-dependent protein, in the AC, as well as several non-classical auditory regions (e.g., amygdala) associated with stress, anxiety and emotion (Wallhausser-Franke et al., 2003). Over the past few years, we have attempted to systematically evaluate the behavioral and physiological manifestations of SS-induced tinnitus and ototoxicity and to integrate the physiological changes seen in the periphery with those observed in the CNS.
Results
Behavioral Measure of Tinnitus
At high doses, SS has long been known to induce temporary tinnitus in humans (Cazals, 2000; McFadden, 1982); therefore, researchers have used SS to validate their behavioral models, establish drug dose-response relationships and test compounds that might suppress tinnitus (Bauer et al., 1999; Jastreboff et al., 1997; Lobarinas et al., 2011; Lobarinas et al., 2006; Panford-Walsh et al., 2008; Ruttiger et al., 2003). Some of the early behavioral paradigms designed to assess tinnitus did so by comparing the performance of a control group with a SS-treated group and were not suitable for assessing tinnitus in individual animals (Heffner and Harrington, 2002; Jastreboff et al., 1988a). To overcome this limitation, we developed a behavioral paradigm described previously that combined two methods: schedule induced polydipsia and shock avoidance conditioning (SIPAC) (Lobarinas et al., 2004; Lobarinas et al., 2011). Food restricted rats with free access to water spontaneously begin to lick for water (polydipsia) by delivering a small food pellet once per minute. Even though the rats were not thirsty, they lick for water while waiting for the next pellet to arrive. Once a rat licked for water at a high rate (polydipsic), the licking behavior was put under stimulus control by presenting 30 sec Sound intervals (noise or tones, 40 or 60 dB SPL, random order) followed by 30 sec Quiet intervals. Since licks occurring during Sound intervals were paired with foot shock, polydipsic rats learned to lick for water during Quiet intervals and to refrain from licking during Sound intervals. Data from a typical SIPAC trained rat are presented in Figure 1A. During the first 8 days of baseline testing, the rat licked at a high rate during Quiet intervals, but rarely licked during Sound intervals, resulting indicating that the rat could discriminate between Sound trials and Quiet trials. Treatment with saline on day 9 did not affect licking behavior during days 9-12 of baseline testing. However, when the rat was treated with 350 mg/kg SS (i.p.), the lick rate during Quiet intervals decreased dramatically and was comparable to that during sound trials. This salicylate effect was measured twice (day 13 and14); each day, salicylate was injected 2 hours before the behavioral measurement. These results suggest that the rat was experiencing a phantom sound during the Quiet intervals and therefore refrained from licking. During the drug washout period, the lick rate during Quiet intervals recovered to the prior baseline rate indicating an absence of tinnitus during the washout period.
Figure 1.
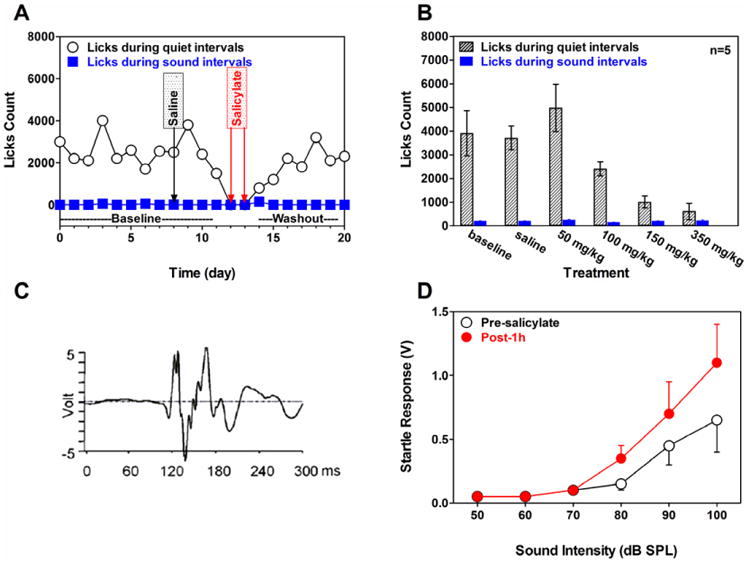
(A) Typical behavior of a rat trained on the SIPAC paradigm. Lick counts during Quiet intervals and Sound intervals were obtained during each day of baseline testing, treatment with saline, treatment with 350 mg/kg/d SS and the washout period. (B) SS dose-response function obtained with SIPAC paradigm. Mean lick counts (±SEM, n=5) measured during Quiet intervals (gray) and Sound intervals (blue). During baseline and saline sessions, lick counts were high during Quiet intervals and low during sound interval; licking behavior remained largely unchanged after treatment with 50 mg/kg SS. Lick counts in Quiet systematically declined as SS dose increased from 100 to 350 mg/kg/d; lick count at 150 mg/kg/d and 350 mg/kg/d significantly less (p<0.05) than baseline, behavior consistent with tinnitus. Lick counts during Sound intervals were unaffected by SS dose. (C) Typical startle response of a rat to a 50-ms broadband noise presented at 115 dB SPL. Voltage-time output from piezo transducer following startle stimulus. (D) Mean startle amplitude (±SEM, n=12) plotted as a function of startle stimulation level pre-salicylate and 1 h after treatment with 250 mg/kg of salicylate.
SS Dose-Response Function
To determine the dose of SS that would reliably induce tinnitus, we monitored SIPAC behavior in a group (n = 5) of Sprague-Dawley rats treated with various doses of SS or saline as previously reported (Lobarinas et al., 2004). Figure 1B shows the mean (±SEM) lick count during Sound intervals and Quiet intervals during daily 2 h test sessions. During baseline testing, the lick count during Quiet intervals was high while the lick count during Sound intervals was low, consistent with the absence of tinnitus. Lick counts in Quiet and Sound intervals were unaffected by saline or 50 mg/kg SS. The 100 mg/kg dose of SS caused a moderate, but non-significant decrease of licks in Quiet intervals compared to the baseline. In contrast, the 150 mg/kg and 350 mg/kg dose caused a statistically significant decrease in licks in Quiet intervals. Lick counts during Sound intervals remained largely unchanged across all conditions. These results as well as those of others (Ruttiger et al., 2003) indicate that SS doses of 150 mg/kg or higher induce tinnitus-like behavior.
SS-Induced Hyperactivity
Tinnitus and sensorineural hearing loss (SNHL) (McFadden et al., 1984a) are often accompanied by a rapid growth of loudness and hyperacusis (loudness intolerance) (Dauman and Bouscau-Faure, 2005; Gu et al., 2010; Jastreboff, 2007). Among patients whose primary complaint is tinnitus, roughly 50-75% has hyperacusis (Andersson et al., 2002; Baguley, 2003; Dauman et al., 2005). Conversely, among patients whose main complaint was hyperacusis, 86% experienced tinnitus (Anari et al., 1999). While there is no widely accepted procedure for assessing hyperacusis or loudness intolerance in animals, one metric that may provide insights is the acoustic startle reflex, a sudden motor response to intense acoustic stimuli (Blaszczyk, 2003; Davis et al., 1982; Ison et al., 2007). Figure 1C is an example of acoustic startle reflex waveform elicited in a rat using a 115 dB SPL, 50 ms noise burst as previously reported (Sun et al., 2009). The acoustic startle motor response was assessed by placing the rat on a platform mounted on a piezoelectric transducer and measuring the RMS voltage output during the acoustic startle reflex response (Sun et al., 2009). Figure 1D shows the mean (±SEM, n = 12) amplitude of the acoustic startle reflex as a function of intensity before and 1 h after treatment with 250 mg/kg of SS as reported previously (Sun et al., 2009). The startle amplitude to broadband noise was elicited around 80 dB SPL and the amplitude increased with intensity. SS treatment resulted in a 5-10 dB leftward shift of the input/output (I/O) function and an increase in response amplitude. At 100 dB SPL the startle amplitude 1 h post-treatment was nearly twice as large as pre-treatment values. Saline treatment did not cause an increase in startle reflex (data not shown) (Sun et al., 2009).
Acute Effects of SS on distortion product otoacoustic emissions (DPOAE)
DPOAE are believed to arise from the prestin-mediated electromotile response of OHCs in combination with the +80 mV endocochlear potential (Liberman et al., 2002; Schmiedt et al., 2002). High doses of SS inhibit the binding of chloride at the anion-binding site on prestin thereby suppressing OHC electromotility (Santos-Sacchi et al., 2006); this leads to a loss of cochlear amplification and a decrease in DPOAE amplitude (Liberman et al., 2002). We have found that high doses of SS caused a frequency-dependent reduction of DPOAE amplitude (Stolzberg et al., 2011). Figure 2 shows the acute reductions in DPOAE I/O functions in 5 Sprague-Dawley rats treated with 300 mg/kg of SS (i.p.). Robust DPOAEs were present at all 6 test frequencies (F2=6, 8, 12, 16, 24, and 30 kHz respectively) before SS treatment. However, at 2 h post-treatment, DPOAE amplitudes had decreased significantly at low frequencies (2F1-F2 ≤ 10.7 kHz) and high frequencies (2F1-F2 ≥ 20 kHz), whereas little change occurred at the mid-frequencies (2F1-F2 =16 kHz), a frequency region previously associated with the pitch of salicylate-induced tinnitus (Ralli et al., 2010; Yang et al., 2007). The mild reduction in mid-frequency DPOAEs surrounded by large losses at low and high frequencies could alter the tonotopic organization in the CNS as discussed below.
Figure 2.
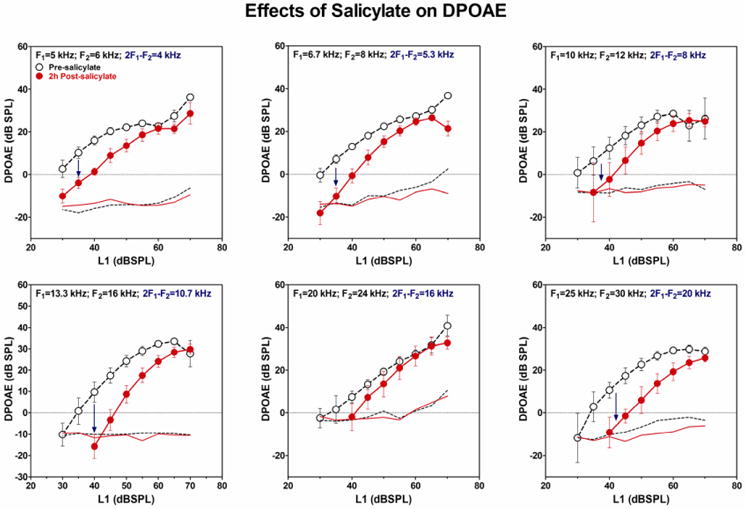
Mean (±SEM, n=5) DPOAE amplitudes plotted as a function of L1 intensity pre-salicylate and 2 h post-salicylate (300 mg/kg). F2/F1 =1.2, L1 = +10 dB re L2, F1, F2, 2F1-F2 indicated in each panel. DPOAE amplitudes decreased significantly (dark blue arrows) at all frequencies following salicylate treatment except at 2F1-F2 = 16 kHz. Dashed line shows noise floor at frequencies surrounding 2F1-F2.
Chronic Effects of SS on DPOAE
The peripheral auditory system, like other parts of the nervous system, can adapt and compensate to long-term acoustic stimulation (Boettcher et al., 1992; Canlon, 1997). This also appears to be true for chronic salicylate treatment (Chen et al., 2010; Yu et al., 2008) as illustrated in Figure 3 which shows the effects of repeated salicylate treatments on DPOAE amplitudes at three frequencies (F2 = 8, 12 and 16 kHz) with L1 = 60 dB SPL. Rats were treated with 300 mg/kg/day SS for 4 days in the first period and for 4 days in the second period with a 2 day break in between. Changes in DPOAE amplitude were normalized to the DPOAE amplitude measured prior to starting the salicylate treatments (blue square). During period 1 and 2, DPOAEs were measured 2 h post-SS; note the reduction of DPOAE during period 1 and period 2 (open squares). Each salicylate treatment period was followed by a rebound enhancement in DPOAE amplitude (red triangles); the DPOAE amplitude enhancement was still observed at 70 post-treatment days (red circle), presumably reflecting a sustained over expression of prestin (Yu et al., 2008).
Figure 3.
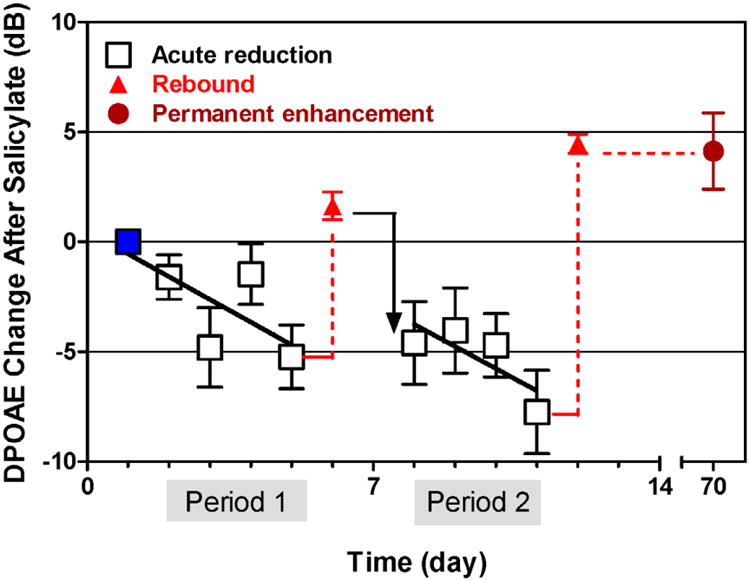
Mean change (±SEM) in DPOAE amplitude after repeated salicylate injections. Sprague-Dawley rats (n=8) were injected with 300 mg/kg/d of salicylate for 4 days in period 1 and for 4 days in period 2. During period 1 and period 2, DPOAE were measured on each day of injection (open squares) and again after 2 days of rest (red filled triangles) and finally 70 day later. DPOAE measured at 3 frequencies (F2 = 8, 12, and 16 kHz) and at L1 = 60 dB SPL). Measurement made 2 h after each injection period (squares), 24 h after each rebound/rest period (red triangle) and at 70 days (dark red circle). Note decline in DPOAE amplitude during daily salicylate treatment and rebound in DPOAE amplitude after 2-day rest intervals and at 70 days.
Acute Effects of SS on Compound Action Potential (CAP)
The N1 amplitude of the CAP to tone bursts reflects neural responses from type I auditory nerve fibers that contact inner hair cells (IHCs). Since high doses of SS can disrupt neural function, we investigated the acute effects of a high systemic dose of SS on the CAP (Chen et al., 2010; Stolzberg et al., 2011). Figure 4 compares the mean (n=5) CAP I/O functions evoked by tone bursts (8, 16 and 40 kHz) pre- and post-SS (300 mg/kg, i.p.). The dashed lines in Figure 4, A-D show a linear relationship between the logarithm of CAP amplitude and sound level in dB SPL. The normal pre-treatment CAP-I/O functions (black open circles) are nonlinear due to the cochlear amplification. After SS treatment, the I/O functions were shifted to the right at low intensities by approximately 30 dB at 8 and 40 kHz (Figure 4, panel A, C) and by 20 dB at 16 kHz (Figure 4B). In addition, the CAP-I/O function became linear (slope close to 1) at high-frequencies (slope=0.97, Figure 4C, 40 kHz, red circles) and low frequencies (slope=0.75, Figure 4A, 8 kHz) indicating a nearly complete loss of cochlear amplification. However, the 16 kHz I/O function remained nonlinear (slope=0.58, Figure 4B) indicating that the cochlear amplifier was still functional at the mid-frequencies. Thus, SS caused less impairment at mid-frequencies compared to low or high frequencies consistent with the DPOAE results. These frequency-dependent changes may be related to mid-frequency tinnitus reported previously (Yang et al., 2007).
Figure 4.
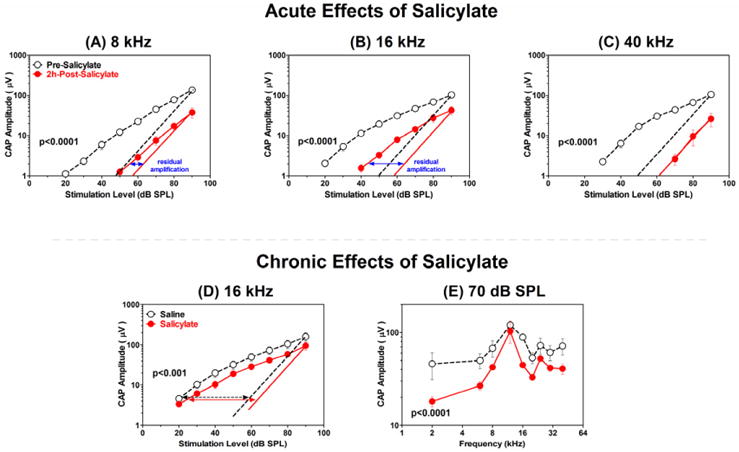
(A-C) Mean (±SEM, n=5) CAP input/output function obtained pre- and 2 h post-SS (300 mg/kg) at 8, 16 and 40 kHz. Dashed line represents a linear relationship between log CAP amplitude and stimulus level in dB SPL. Post-SS CAP input/output functions shifted approximately 30 dB to the right at low intensities at 8 and 40 kHz and about 20 dB to the right at 16 kHz. Post-SS input/output functions linear or nearly linear at 40 and 8 kHz respectively; 16 kHz input/output function retains its nonlinearity. Residual nonlinear amplification shown by the horizontal blue arrows; cochlear amplification retained at 16 kHz, but lost or greatly reduced at 40 and 8 kHz respectively. (D-E) The permanent effects of repeated salicylate treatments (200 mg/kg/day, 5 days/week for 3 weeks) on mean (±SEM, n=6) CAP amplitude. Note parallel shift to the right of CAP-I/O function (D) and a frequency-dependent CAP amplitude reduction at 70 dB SPL (E) with less impairment in the middle frequency region.
Chronic Effects of SS on CAP
Chronic SS treatment does not damage sensory hair cells in vivo or in vitro; however, its effects on auditory nerve function are unclear (Wei et al., 2010; Zheng et al., 1996). To determine if chronic SS treatment could permanently reduce the neural output of the cochlea, we recorded CAP from rats (n = 6) treated 5 days per week for 3 consecutive weeks either with SS (200 mg/kg/d) or saline as described in detail previously (Chen et al., 2010). The CAP-I/O functions were obtained four weeks after the last treatment. Figure 4D compares the 16 kHz CAP I/O functions in a SS group versus a saline control group. Even though the CAP-I/O function in the SS-treated group (red circles) was slightly smaller than in the control group (black circles), nonlinear cochlear amplification was similar in both groups suggesting that the OHC and cochlear amplifier were still largely intact. Figure 4E compares the CAP amplitudes as a function of frequency at 70 dB SPL. CAP amplitudes were smaller in the SS-treated group than in the control group at low and high frequencies, but were not much different in the mid-frequency region, consistent with the acute effects of SS (Figure 4A-C). The reduced CAP amplitudes in the chronic SS-treated group are indicative of long-term functional or structural damage to spiral ganglion neurons (SGN) consistent with the reduced auditory brainstem response amplitudes observed after chronic SS treatment (Chen et al., 2010).
Salicylate-Induced SGN damage
To determine if chronic SS treatment could damage SGN, we treated postnatal day 3 rat cochlear organotypic cultures with SS for 48 h as described in detail in our earlier publication (Wei et al., 2010). Hair cells were labeled with Alexa-488 conjugated phalloidin (Invitrogen A12379, diluted by 1:200) and auditory nerve fibers and SGN were immunolabeled with a monoclonal antibody against class III beta-tubulin and Cy3-conjugated secondary antibody. The hair cells in the cultures treated with 1, 5 or 10 mM of SS for 48 h appeared normal and comparable to those in the control group (Figure 5, A-D). Mean (n= 5/group) cochleograms showing hair cell loss as a function of cochlear place were obtained for the SS treated group and control group. There was little hair cell loss in either group and no significant difference between groups as reported in detail in our earlier paper (Wei et al., 2010). However, the number of auditory nerve fibers radiating out to the hair cells decreased with increasing SS dose and many blebs and breaks were present on the nerve fibers treated with SS (Figure 5A-D). The number of nerve fiber fascicles per 100 μm width was significantly reduced from 22.7 in controls (n=4) to 14.6 in the 1 mM SS treatment group (n=4) as reported previously (Wei et al., 2010). The cell bodies of the SGN in control cultures were large and round whereas those treated with SS were smaller and fewer were present. These results indicate that high doses of SS damage SGN, but not hair cells, consistent with earlier findings (Zheng et al., 1996). Gene expression analysis and caspase labeling indicated that SGN and nerve fiber degeneration occurred by apoptosis (Wei et al., 2010). More recent in vivo results also indicate that SGN in adult guinea pigs degenerate via caspase-mediated apoptosis after long term treatment with high doses of SS (Feng et al., 2011; Feng et al., 2010). Taken together, these results indicate that SS treatment not only causes damage to SGNs in the developmental stage but also in adult animals.
Fig. 5.
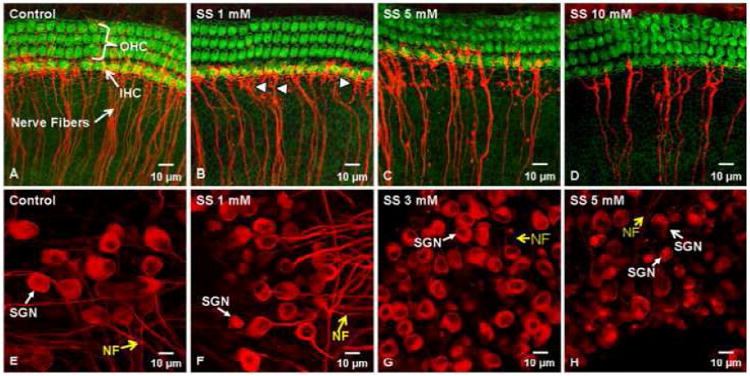
Cochlear organotypic cultures from postnatal day 3 rats labeled with Alexa-488 conjugated phalloidin to identify F-actin that is heavily expressed in the stereocilia and cuticular plate of outer hair cells (OHC) and inner hair cells (IHC). Nerve fibers (NF) and spiral ganglion neurons (SGN) labeled with a monoclonal antibody against class III –tubulin and Cy3-conjugated secondary antibody (red). (A-D) Control culture and cultures treated with escalating doses of SS for 48 h. Note loss of NF as SS dose increases; arrowhead points to NF. (E-H) Photomicrographs show SGN and NF from postnatal day 3 cochlear cultures. Note large round SGN and nerve fibers (NF) emanating from the soma of control cultures. Treatment with escalating doses of SS for 48 h results in loss of NF and shrinkage of SGN. (From Wei et al., 2010 with permission).
AC Hyperactivity
The preceding results show that high doses of SS decrease the neural output of the cochlea and elevate CAP thresholds in a frequency dependent manner (Figure 4A-C). SS, however, also readily crosses the blood brain barrier where it can reach concentrations as high as 1.4 mM (Jastreboff et al., 1986) and exert powerful effects on CNS metabolism (Liu et al., 2003; Thurston et al., 1970; Wallhausser-Franke et al., 1996) and neurotransmitter systems such as serotonin and γ-aminobutyric acid (GABA) (Akada et al., 2003; Bauer et al., 2000; Wang et al., 2008). Interestingly, high doses of SS significantly increased c-fos expression in AC suggesting that this region is one of several preferentially affected by SS (Wallhausser-Franke et al., 2003). To evaluate the electrophysiological consequences of SS, we recorded local field potentials (LFP), which largely reflect the pre-synaptic inputs near the recording site and the post-synaptic spike discharges of multiunit clusters from the AC before and after administering 300 mg/kg (i.p.) of SS. SS significantly enhanced sound evoked activity in the AC despite the fact that SS reduced the neural output of the cochlea. The AC amplitude enhancement can be clearly seen by examining the LFP in response to noise bursts (Figure 6A). Consistent with our previous results (Lobarinas et al., 2006; Sun et al., 2009; Yang et al., 2007), SS induced a threshold shift of approximately 20 dB SPL in agreement with the CAP data in Figure 4. Paradoxically, the amplitude of the LFP increased at a faster than normal rate so that at 80 dB SPL or greater the AC response was larger than normal. SS treatment causes the AC to become hyperactive at high intensities in awake animals or animals that are anesthetized with ketamine and xylazine (Lobarinas et al., 2006; Sun et al., 2009; Yang et al., 2007). Some mechanistic insights have been gleaned from pharmacologic interventions. Systemic treatment with baclofen, which increases GABAB-mediated inhibition, vigabatrin, which increases GABA neurotransmitter concentrations, and isoflurane anesthesia, which increases GABA-mediated inhibition, suppressed the SS-induced enhancement of the sound-evoked AC amplitude enhancement (Lu et al., 2011). Taken together, these results suggest that the sound-evoked hyperactivity in the AC after SS treatment may be due to a reduction in GABA-mediated inhibition.
Figure 6.
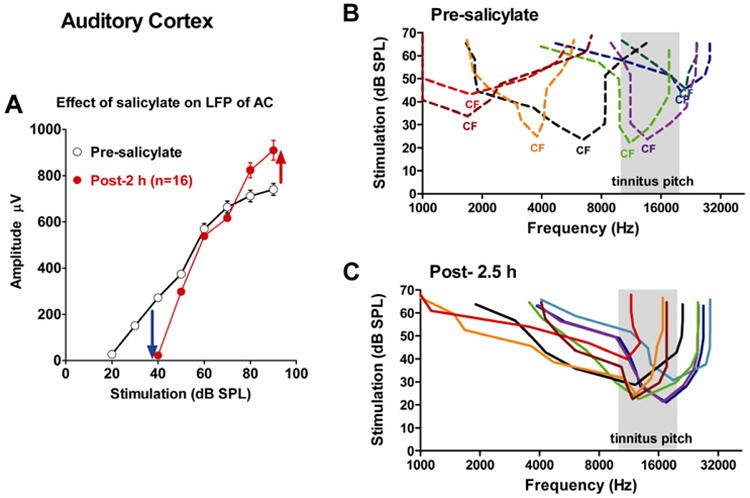
(A) Mean (±SEM, n=16) AC LFP I/O functions measured with noise bursts pre- and 2 h post treatment with SS (300 mg/kg, i.p.). SS induced a threshold shift of approximately 20 dB (blue arrow), but increased LFP amplitudes at high levels (red arrow). (A) FRF of AC neurons measured pre-SS (color coded, dashed lines) and (B) 2 h post-SS (color-code in panel C same as in panel B; but dashed lines converted to solid lines).
AC Tonotopy
In earlier pharmacologic studies, we showed that iontophoresis of bicuculline, a GABAA receptor antagonist, into AC decreased single neuron thresholds and expanded the excitatory response area (Wang et al., 2002). These observations and others suggest that considerable spectral integration occurs in the AC from horizontal interconnections that bring together acoustic information from remote frequencies (Eggermont and Roberts, 2004; Kaur et al., 2005; Metherate et al., 2005). Under normal conditions, potent GABAergic circuits suppress information from remote frequencies in order to maintain sharp tuning at the neuron's characteristic frequency (CF). When we administered SS systemically to rats under ketamine/xylazine anesthesia, we observed striking changes in tuning and threshold primarily in low-CF and high-CF AC neurons; much smaller changes were seen in mid-frequency (10-24 kHz) neurons as previously reported (Stolzberg et al., 2011). Figure 6B-C shows the tuning curves of 8 AC multiunit clusters before and 2.5 h after SS treatment (300 mg/kg). The CFs of neurons below 10 kHz and above 20 kHz shifted their CFs into the 10-20 kHz range (shaded area, predicted tinnitus pitch). In contrast, neurons with CFs between 10 and 20 kHz showed little change in CF; however, their tuning curves became wider at suprathreshold levels. The migration of low and high CF units towards the 10-20 kHz regions presumably results from an interaction between certain features of the peripheral losses (Figures 3-4) and central factors: (1) AC neurons receive excitatory inputs from a broad range of frequencies, but inhibition in the AC normally narrows the excitatory response area. When SS is applied to brain slices from AC, it reduces inhibitory post-synaptic currents (Wang et al., 2006) and selectively reduces current-evoked firing in fast-spiking GABAergic AC neurons (Su et al., 2009). Therefore, we speculate that when SS reaches the AC in vivo, it reduces GABAergic inhibition thereby permitting some AC neurons to respond to a broader range of frequencies. (2) Because SS produces a frequency-dependent loss of cochlear sensitivity that is greatest at low and high frequencies but least at the mid-frequencies (Figure 2 and 4), we hypothesize that disinhibited low-CF and high-CF neurons shift their CFs into the mid-frequency range where there is less hearing loss (Stolzberg et al., 2011). The net result is that there are now more AC neurons tuned to the mid-frequencies where the tinnitus pitch from SS has been measured with several different behavioral paradigms (Brennan and Jastreboff, 1991; Kizawa et al., 2010; Yang et al., 2007).
AC Circuitry
Recent in vitro studies indicate that SS produces dissimilar effects on the current-evoked firing of neurons located in different layers of AC (Su et al., 2009). These results suggest that high doses of SS may induce layer specific electrophysiological changes within the microcircuitry of primary AC (A1). A1, like most neocortical tissue, contains a heterogeneous population of neurons arranged in roughly six horizontal, interconnected layers (Prieto et al., 1994; Winguth and Winer, 1986). One method used to detect changes in the microcircuitry activation patterns across different cortical layers is to apply current source density (CSD) analysis to sound-evoked LFPs recorded by electrodes positioned at different cortical layers (Happel et al., 2010; Kaur et al., 2005). CSD analysis significantly improves the spatial localization of neural activity by taking into account field potentials on neighboring electrodes. CSD maps (Figure 7, left) reveal sinks (gSk and sSk), representing the depolarization of neurons, and sources (dark blue), reflecting passive repolarization and possibly the active inhibition of neurons.
Figure 7.
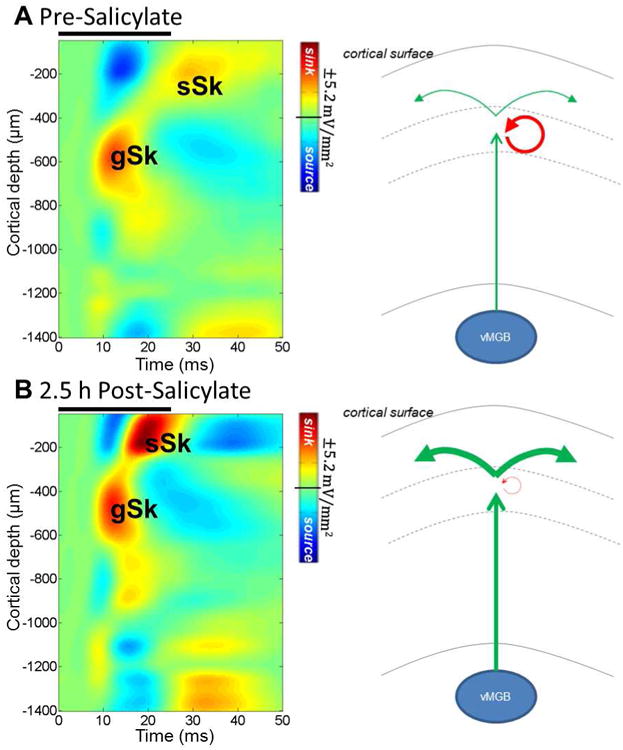
Effects of SS on sound processing in the microcircuitry of A1. Left panels: Heat maps showing mean sound-evoked current source density (CSD) responses with amplitude (mV/mm2) of current sink indicated in red and amplitude of current source indicated in blue. Location of CSD sinks and sources relative to cortical surface indicated on ordinate; approximate depth of cortical layers 1-6 shown adjacent to ordinate. Change in CSD sources and sinks plotted as a function of time (abscissa) after onset of noise burst (25 ms). CSD heat maps are shown for the same rat before (A) and following (B) systemic 250 mg/kg of SS (i.p.). Note increase in gSk and even greater increase in sSk after salicylate treatment (panel B vs. A). Black bars above maps indicate 25 ms broadband noise burst at 90 dB SPL. Right panels: Highly simplified schematics of activation patterns in response to sound. Normally, strong thalamic drive is initially received by granular layers) from the ventral portion of the medial geniculate body (vMGB), followed by recurrent inhibition (red arrow) and activation of local and long range excitatory intracortical projections (green arrows) which play a role in spectral integration. Following a high systemic dose of SS, thalamic activation of the granular layer (gSk) is enhanced (indicated by increased thickness of vertical arrow in panel B), but reduced recurrent inhibition by SS treatment results in abnormally large activation of excitatory intracortical projections (sSk, indicated by extra large increase in thickness of horizontal arrows in panel B). Increased activation of these intracortical fibers manifests as broadened multiunit frequency receptive fields.
To determine if high doses of SS would significantly alter the temporal and spatial properties of the CSD generated in A1, we used a 32 channel, linear microelectrode array to record the LFPs generated in different layers of the AC pre- and post-SS treatment; the methods and the results of this study are described in detail in a recent publication (Stolzberg et al., 2012). The primary effects of systemic SS treatment (250 mg/kg, i.p.) were significantly enhanced activation (indicated by increased thickness of the vertical green arrow in Figure 7B vs. 7A) of the granular layer (granular sink, gSk) and even greater activation (indicated by increased thickness of left and right horizontal green arrows in Figure 7B vs. 7A) of supragranular layers (supragranular sink, sSk) of A1. The amplitude of gSk primarily reflects activation of dendrites within granular layers of A1 arising from thalamocortical projections originating in the ventral medial geniculate body (MGB) of the thalamus. The sSk primarily reflects activation of dendritic arbors of infragranular pyramidal cells that receive input from intracortical horizontal fibers that play a major role in spectral and temporal integration occurring within the cortex (Happel et al., 2010; Ojima et al., 1991; Ojima et al., 1992). Following systemic SS treatment, we found that the supragranular response to broadband noise bursts exhibited a significantly shorter latency to its peak amplitude and the supragranular response amplitude (sSk) was significantly enhanced relative to baseline recordings. The supragranular response was enhanced to such an extent following SS that it was often greater than the granular sink response at high sound stimulus levels (80 and 90 dB SPL) (Stolzberg et al., 2012). The results from CSD analysis indicate that much of the enhanced sound-evoked response recorded from AC following systemic SS treatment has primarily intracortical origins. These results support the hypothesis that inhibition within A1 is compromised during SS-induced tinnitus (Eggermont et al., 2004). We further speculate that the intracortical gain enhancement may result in the recruitment of higher cortical areas and/or extralemniscal structures during tinnitus and/or hyperacusis (Stolzberg et al., 2012).
Medial Geniculate Body
Although SS readily enters the brain, its effects vary substantially across regions of the CNS. High doses of SS produced little c-fos labeling in most auditory nuclei in the brainstem and midbrain, but sometimes increased c-fos in the MGB (Wallhausser-Franke et al., 2003) suggesting that this region might also be affected by SS. To test this hypothesis, we made preliminary measurement of the LFP from the MGB before and after administering SS (300 mg/kg, i.p.). Figure 8A shows LFP amplitude as a function of noise burst intensity pre- and 2 h post-SS. The threshold of the LFP was approximately 30 dB SPL prior to SS treatment. Two hours post-SS, the threshold had increased to 50 dB SPL, a threshold shift of roughly 20 dB, similar to the threshold shift of the CAP (Figure 4) and AC LFP (Figure 6). Although SS increased threshold and decreased LFP amplitudes at low intensities, the response increased rapidly with sound level such that LFP amplitude was roughly 30% greater than normal at 100 dB SPL. Preliminary recordings were also obtained from multiunit clusters in the MGB. Figure 8B shows the discharge rate of a multiunit cluster plotted as a function of frequency using tone bursts (50 ms) presented at 60 dB SPL. The discharge rate vs. frequency plot at 60 dB SPL (Figure 8B) shows that the post-SS firing rate was higher than Pre-SS; i.e., the multiunit cluster was hyperactive over a broad range of frequencies after SS treatment. Figure 8C shows the threshold vs. frequency plots for this multiunit cluster before and 2 h post-SS. The CF of this multiunit cluster was originally near 12 kHz and the CF-threshold was near 0 dB SPL. However, 2 h after SS treatment, thresholds mainly increased in the tip of the tuning curve, but not at low frequencies resulting in much broader frequency tuning. These preliminary results indicate that systemic SS not only leads to sound-evoked hyperactivity in AC, but also the MGB, which provides excitatory inputs to A1.
Figure 8.
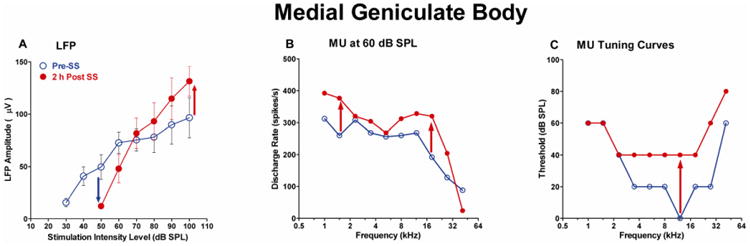
Effects of SS (300 mg/kg, i.p.) on MGB. (A) Mean (±SEM, n=5) LFP input/output function to noise bursts recorded pre- and 2 h post-SS. LFP threshold increased approximately 20 dB after SS treatment. LFP amplitude was below normal at stimulation level below 70 dB SPL (blue arrow), but was approximately 30% greater than normal at 100 dB SPL (red arrow). (B) Discharge rate vs. frequency plots recorded at 60 dB SPL from a representative multiunit cluster pre- and 2 h post-SS. Note increase in firing rate Post-SS (red, up arrows). (C) Tuning curve showing threshold vs. frequency pre- and 2 h post-SS. The tuning curve is broader post-SS due to selective threshold elevation around the original characteristic frequency (CF) near 12 kHz.
Lateral amygdala (LA)
The amygdala, which assigns emotional significance to sensory events (e.g., fear conditioned learning), contains several nuclei with distinct functions. (Davis, 1992; Fanselow and LeDoux, 1999; Maren and Quirk, 2004; McKernan and Shinnick-Gallagher, 1997; Rogan et al., 1997; Tsvetkov et al., 2002). The LA, part of the basolateral complex of the amygdala, receives information from the auditory system and other senses, and its major outputs project to the basolateral and centromedial nuclei of the amygdala. The amygdala, which connects the classical auditory pathway with the limbic system, forms reciprocal connections with the auditory thalamus and AC (Bordi and LeDoux, 1992; Budinger et al., 2008; LeDoux, 2007; LeDoux, 2000; Romanski et al., 1993). High doses of SS, that can induce tinnitus, increase c-fos expression in the amygdala (Wallhausser-Franke et al., 2003) whereas low doses of SS that do not induce tinnitus fail to up regulate c-fos expression. Based on these results, we hypothesized that high doses of SS would strongly activate the LA. We tested this hypothesis by recording LFP I/O functions and multiunit discharge patterns in the LA before and after SS treatment (300 mg/kg, i.p.) as recently reported (Chen et al., 2012). Two hours after systemic SS treatment, the LFPs in the LA elicited by noise bursts were depressed at low intensities as recently noted (Figure 9A) (Chen et al., 2012). SS treatment elevated LA response thresholds approximately 20 dB similar to the shifts seen in the cochlea, MGB and AC. However, at stimulation intensities of 60 dB SPL or higher, post-SS LFP amplitudes were almost twice as large as pre-treatment values.
Figure 9.
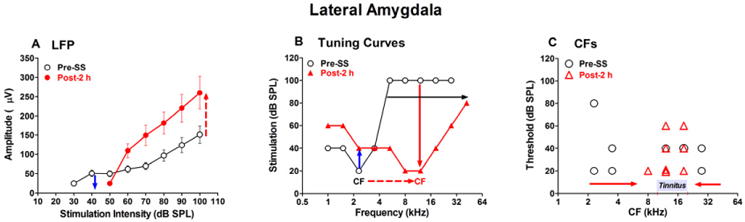
Effects of SS (300 mg/kg, i.p.) on the lateral amygdala (LA). (A) Mean (±SEM, n=19) LFP input/output functions elicited with noise bursts pre- and 2 h post-SS. Salicylate induced a 20 dB threshold shift and decreased the amplitude at low intensities (blue, down arrow). Post-SS amplitudes were much higher than normal at stimulation intensities of 60 dB SPL or higher (red, up arrow). (B) Tuning curve (threshold vs. frequency plot) of a representative LA multiunit cluster. Original CF threshold was 20 dB near 2 kHz. CF shifted to approximately 12 kHz 2 h post-SS, CF threshold remained at 20 dB SPL, but the tuning curve was much broader. (C) CFs of 8 multiunit clusters in the LA before (black circles) and 2 h post-SS (red triangles). Most low-CF and high-CF neurons up-shift or down-shift (arrows) their CFs to the mid-frequencies where the tinnitus pitch was detected in our previous behavioral studies (Yang et al., 2007). CFs of mid-frequency neurons largely unchanged.
Neurons in the LA respond to acoustic stimuli and are sharply tuned (Figure 9B) as we and others have noted (Bordi et al., 1992; Chen et al., 2012). The frequency receptive field (FRF) of the LA multiunit cluster shown in Figure 9B originally had a CF near 2 kHz, a CF-threshold around 20 dB SPL and responded well to stimuli below 6 kHz. The FRF changed dramatically 2 h after SS treatment; the CF shifted up from approximately 2 kHz to 12 kHz; the high-frequency edge of the FRF expanded from roughly 6 kHz to more than 32 kHz and the tuning curve became much broader. These results suggest that neurons in the LA receive excitatory inputs from a broad range of frequencies (i.e., spectral integration), but the broad excitatory response is narrowed by potent inhibitory circuits in the CNS. SS appears to unmask these latent excitatory inputs permitting the neurons to respond to a much broader range of frequencies similar to what we reported in the AC (Figure 6B-C) (Stolzberg et al., 2011). The CF-shifts in LA were accompanied by large, frequency-dependent changes in threshold. The threshold at the original CF, 2 kHz, increased from approximately 20 dB SPL to 40 dB SPL. In contrast, a dramatic reduction in threshold occurred at the new CF, ∼12 kHz; the threshold at this frequency decreased from approximately 100 dB SPL to 20 dB SPL. The frequency-dependent nature of these threshold shifts may be due to a combination of factors; the SS-induced peripheral changes in sensitivity which were greater at low frequencies than mid-frequencies (Figure 4) plus the SS-induced reduction in inhibition mainly located at frequencies above this units original low CF, ∼2 kHz. Similar to what was observed in the AC, SS induced a frequency-dependent shift in CF such that low CF neurons (<10 kHz) and high CF neurons (>20 kHz) generally shifted their CFs toward the mid-frequencies while those at the mid-frequencies maintained nearly the same CF. The CF-shifts in the amygdala are illustrated in Figure 9C which plots the CF and CF-threshold of each neuron before SS and the new CF and CF-threshold 2 h post-SS. Of the 8 multiunit clusters evaluated, 4 low-frequency multi-unit clusters and 2 high-frequency multi-unit clusters shifted their CFs to become sensitive to mid-frequencies. The CF-shifts were minimal among units originally tuned to the mid-frequencies. The CF shifts in the LA, which resemble those seen in the AC (Figure 6), result in an overrepresentation of mid-frequency neurons that reside in a frequency range that corresponds to the tinnitus pitch measured in behavioral studies (Brennan et al., 1991; Kizawa et al., 2010; Yang et al., 2007).
Round Window Application of SS
The neural hyperactivity seen in the AC, MGB and LA after systemic drug treatment reflects the combined effects that SS exerts on the cochlea and CNS. To determine if the hyperactivity observed in the AC originated in the cochlea or CNS, we applied SS to the round window membrane and evaluated its effects on the CAP and AC LFP in the same animal as described previously (Sun et al., 2009). SS-solution (∼50 μl, 25 mg/ml) was delivered to the round window through a fine plastic tubing (∼ 100 μm in diameter). The solution was allowed to sit on the round window for 5-10 minutes then was removed by absorbent paper before recording the CAP. The round window administration of SS reduced the amplitude of both CAP (Figure 10A) and AC LFP (Figure 10B) by approximately 50% and elevated the threshold of the CAP and AC by roughly 25 dB. These results together with those presented above (Figure 4A-C) and by others indicate that SS, whether given locally or systemically, reduces the neural output of the cochlea at suprathreshold intensities (Fitzgerald et al., 1993). In contrast, if SS is given systemically, it reduces the neural output of the cochlea, but paradoxically increases the neural activity in the AC, MGB and LA at suprathreshold intensities. Moreover, in previous studies we found that systemic SS (250 mg/kg, i.p.) treatment had little effect on the mean (±SEM) amplitude of the LFP recorded from the inferior colliculus (IC) (Figure 10C) (Sun et al., 2009). One interpretation of these results is that the reduced neural output of the cochlea is already partially amplified by the time it reaches the IC. Further amplification occurs as the neural signal propagates from the IC to the AC (Sun et al., 2009) and within AC itself (Stolzberg et al, 2012). Taken together, these results indicate the systemic SS application increases the gain of the central auditory pathway to compensate for a reduced cochlear response (Lu et al., 2011).
Figure 10.
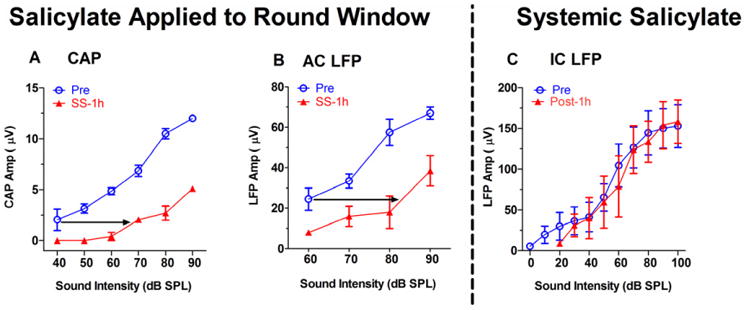
Effect of SS application (∼50 μl, 25 mg/ml) to the cochlear round window membrane on the mean (±SEM) CAP (A) and AC LFP (B) input/output functions. The cochlear application of SS elevated threshold approximately 20 dB and reduced the amplitude of the CAP and AC LFP at all intensity levels. (C) Mean (±SEM, n=4) LFP input/output function from IC pre- and 1 h post systemic SS treatment (i.p. 250 mg/kg).
SS Applied to AC Leads to Hyperactivity
The preceding results suggest that the sound-evoked hyperactivity observed in the AC, MGB and LA following systemic treatment results from the direct effects of SS on the CNS. To test this hypothesis, we recorded the LFP from the AC before and after applying a 100 μl of SS (2 mM) directly on the AC as recently reported (Lu et al., 2011). The amplitude of the AC LFP increased substantially after local SS treatment (Figure 11A). However, the threshold of the LFP was unaffected (Figure 11B) as reported in our recent publication (Chen et al., 2012). These results suggest that the threshold elevation associated with systemic SS treatment originates in the cochlea whereas the suprathreshold hyperactivity arises in the CNS.
Figure 11.
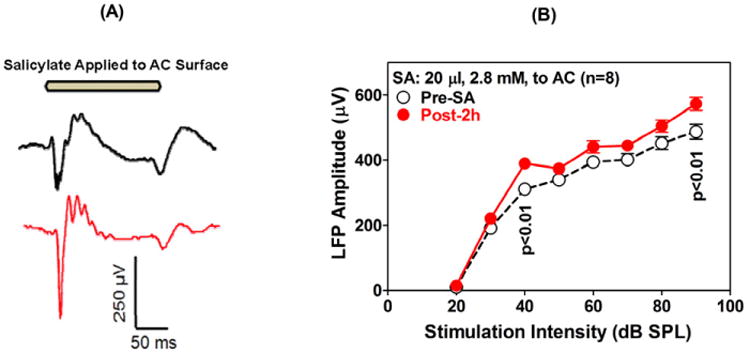
(A) LFP recorded from AC in response to a 200 ms tone burst (bar on the top) presented at 90 dB SPL. The LFP was recorded pre (top) and approximately 1 minute after applying SS (100 μl, 2 mM) on the AC (bottom). The LFP amplitude increased roughly two-fold after SS treatment. (B) Mean LFP amplitudes after applying SS on the AC (20 μL, 2.8 mM). Note increase of LFP amplitude without a threshold shift.
Infusion of SS into LA Induces AC Hyperactivity
Since neurons in the amygdala project to the AC (Bordi et al., 1992; Budinger et al., 2008; Romanski et al., 1993), the SS-induced changes observed in the LA are likely to influence activity in the AC. To test this hypothesis, recordings were obtained from the AC before and after infusing SS (2.8 mM, 20 μl) into the ipsilateral LA as recently noted (Chen et al., 2012). Two hours after infusing SS into the LA, the amplitude of the AC LFP was greatly enhanced to noise bursts presented at suprathreshold intensities, but the response threshold was not shifted as illustrated in Figure 12A. These results clearly demonstrate that SS has a direct effect on the LA and that the LA in turn can enhance sound-evoked activity in the AC.
Figure 12.

Infusion of SS (20 μl, 2.8 mM) into LA while recording from AC pre- and post-treatment. (A) Mean (±SEM, n=18) AC LFP input/output function to noise bursts obtained pre- and 2 h post-SS. Note large increase in LFP amplitude without threshold shift. (B-C) Matrix of peristimulus time histograms (PSTHs) obtained with tone bursts presented at 10 different frequencies (x-axis) at 6 intensities ranging from 0 to 100 dB SPL (y-axis). PSTH in each square shows the firing rate as a function of time (5 ms bin width, 500 ms total duration) to the 50 ms tone burst. Dashed blue line in panel B outlines FRF of the AC multiunit cluster prior to SS treatment; dashed red line in panel C outlines FRF of the multiunit cluster 2 h after infusing SS into the LA (blue dashed line from panel B also shown in panel C). Red horizontal arrow in panel C shows expansion of FRF towards the high frequencies. (D) Mean (±SEM) tuning curve of 3 (out of 16) multiunit clusters showing expansion of response area of low-frequency neurons towards the middle frequencies after SS infusion; response area shift towards the tinnitus pitch.
To determine if infusion of SS into the LA would alter AC tuning, we recorded from 16 multiunit clusters in the AC pre- and post-treatment as recently reported (Chen et al., 2012). While all of the multiunit clusters showed an increase in firing rate at suprathreshold intensities, only 3 of 16 multiunit clusters in AC showed a significant FRF expansion (Figure 12D). Figure 12B shows FRF of one of the unit with a CF near 3.5 kHz. After perfusion of SS into the LA (20 μl, 2.8 mM), the suprathreshold AC responses were typically enhanced (compare height of PSTHs in Figure 12C to those in Figure 12B) and the FRF expanded towards the high frequencies (compare red dashed line to blue dashed line in Figure 12C). In this case, the high-frequency edge of the FRF expanded from 5.3 kHz to 12.1 kHz, while the low-frequency edge remained unchanged. These results suggest that the SS-induced CF shifts seen in the AC during systemic SS treatment (Figure 6B-C) may be partially mediated by the LA. Since only one dose of SS was applied to the LA in this study, it is not clear if higher doses of the drug would increase the magnitude or likelihood of inducing retuning of AC neurons.
Discussion
Salicylate Ototoxicity
High-dose SS is a reliable inducer of hearing loss and tinnitus, but the mechanisms that underlie the chronic and acute effects of the drug are not fully understood. In the cochlea, high-dose SS reduces OHC electromotility (Kakehata et al., 1996; Tunstall et al., 1995) by displacing chloride from its anion-binding site on prestin thereby interfering with OHC electromotility (Oliver et al., 2001; Santos-Sacchi et al., 2006) and reducing cochlear amplification and DPOAE. The OHC functional loss following a single high dose of SS recovers after 1-2 days; however, repeated SS treatments can induce long lasting increases in DPOAE amplitude and OHC electromotility due to prestin up-regulation (Huang et al., 2005; Yang et al., 2009; Yu et al., 2008). Although high dose SS has been reported to cause hair cell damage (Feng et al., 2010), we and others have not observed hair cell degeneration in vitro or in vivo (Chen et al., 2010; Huang et al., 2005; Wei et al., 2010; Yu et al., 2008; Zheng et al., 1996). On the other hand, prolonged, high-dose SS treatment can permanently reduce the neural output of the cochlea (i.e., CAP) by either inducing apoptosis in SGN or impairing neural function (Chen et al., 2010; Feng et al., 2011; Wei et al., 2010).
Our results show that both the temporary and permanent changes in DPOAE and CAP are frequency-dependent with the greatest loss at low and high frequencies and the least in the mid-frequencies. The mechanisms that lead to this frequency-dependent change are currently unknown, but the functional implications of this may play an important role in the tonotopic remodeling observed in the AC and LA following systemic SS treatment as discussed below (Chen et al., 2012; Stolzberg et al., 2011).
Both systemic administration of SS and direct application of SS on to the AC induced hyperactivity in the AC. A major difference between the two modes of drug delivery is that the former induced a large, frequency dependent threshold shift whereas the latter did not induce a threshold shift when applied to the AC or LA, but did induce hyperactivity in the AC. One interpretation of these results is that the hearing loss induced by systemic SS treatment is cochlear in origin whereas the sound evoked hyperactivity observed in the AC, MGB and LA originates in the CNS.
Cortical Retuning
Systemic SS treatment induced large CF shifts in the AC and LA resulting in tonotopic shifts of low and high CF multiunit clusters into the mid-frequency region that has been linked to the pitch of SS induced tinnitus (Brennan et al., 1991; Chen et al., 2012; Stolzberg et al., 2011; Yang et al., 2007). The increased numbers of neurons, some originally tuned to the mid-frequencies and others re-tuned to the mid-frequencies (10-20 kHz), would result in an increase in the total number of spontaneous discharges associated with the mid-frequencies possibly leading to a phantom sound sensation as we previously hypothesized (Chen et al., 2012). The mechanisms responsible for these large CF shifts are not fully understood, but are likely to involve at least two factors. One factor is the frequency dependent loss in cochlear sensitivity that was greatest at low and high frequencies (“-” inside arrows indicates decreased output, length of arrow indicates degree of loss) and least in the mid-frequencies. In addition to reducing the overall output of the cochlea, SS changed the tonotopic profile of excitation resulting in relatively stronger excitation at the mid-frequencies than at lower and higher frequencies as schematized in Figure 13 (compare normal profile in top row to profile in arrow 1 below). Importantly, when SS was applied to the round window, it reduced the neural output of the cochlea and reduced the sound-driven response in AC. In contrast, local application of SS to the AC primarily increased activity in the AC without elevating threshold. These results indicate that SS can directly increase the gain of the central auditory system (Figure 13, arrow 3; “X” inside of arrows indicate increased gain) thereby amplifying the frequency-dependent losses emanating from the SS-impaired cochlea which in turn leads to alterations in AC tonotopy.
Figure 13.
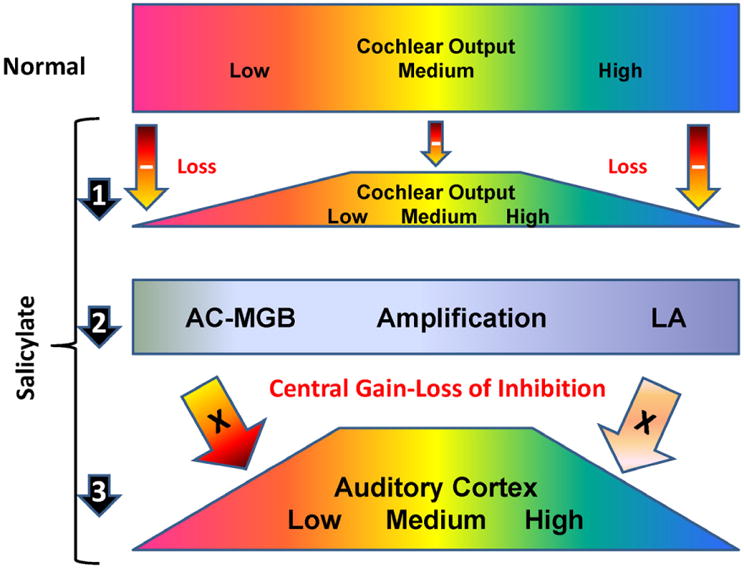
Schematic illustrating the effects of SS and the cochlea and CNS. (1) SS-induced a frequency-dependent reduction in cochlear output with greater loss at low and high frequencies compared to the mid-frequencies. Compare normal profile in top row to profile in arrow 1; note frequency dependent loss; “-” inside arrows indicates decreased output, length of the arrow indicates degree of loss). (2) SS-induced increase in central gain resulting in part from a loss of inhibition within the AC and MGB. In addition, the LA acts to further amplify (arrow 2) the frequency-dependent changes in the cochlear output and (3) the enhancement in AC activity and CF shifts that focuses AC tonotopy to the mid-frequencies. The increased number of mid-frequency neurons leads to an increase in the total number of spontaneous discharges associated with the mid-frequencies; this over-representation may lead to a mid-frequency, phantom sound sensation. X's within arrow indicate amplification of incoming neural signals from the MGB and LA. The frequency-dependent changes in the cochlea amplified by the LA and AC results in an enhanced mid-frequency response and altered AC tonotopy (arrow 3).
A more detailed CSD analysis of the current sources and sinks revealed that the supragranular layers of the AC, which receives parallel fibers from adjacent regions of A1, was depolarized earlier and more robustly in response to sound stimulation after systemic SS treatment (Stolzberg et al., 2012). The supragranular layer receives horizontal fibers that, under the appropriate conditions, allow for long-range spectral integration of acoustic energy (Happel et al., 2010; Moeller et al., 2010; Wang et al., 2002). Our results suggest that SS directly disinhibits intracortical circuits, which results in an enhanced intracortical excitation delivering unusually strong and broad excitatory signals along the tonotopy of A1resulting in broadly tuned frequency receptive fields. The migration of CFs to the mid-frequencies is likely a consequence of the frequency-dependent losses in the cochlea that are greatest at low and high frequencies and least at mid-frequencies paired with the cortical disinhibition.
Amygdala
Although the amygdala lies outside the classical auditory pathway, it has reciprocal connections with AC and MGB (Bordi et al., 1992; Budinger et al., 2008; Romanski et al., 1993). Because the amygdala can assign emotional significance to sensory stimuli, regulate attention and influence memory, it has the potential to modulate sound-evoked activity within the auditory pathway (LeDoux, 2007; LeDoux, 2000; McGaugh et al., 1996). Immunolabeling studies have revealed strong c-fos expression in the amygdala as well as the AC after a high dose of SS (Wallhausser-Franke et al., 2003). We found that high doses of systemic SS increased the amplitude of sound evoked activity in both the LA and AC. The AC amplitude enhancement could result from an increase in spike rate, enhanced neural synchrony or the combination of both effects. Indeed, a large decrease in the latency to the peak sSk response in the CSD was observed after salicylate treatment and the width of the peaks in the sSk and gSk waveform became narrower (Stolzberg et al., 2012). These results imply that the LA by the mechanisms discussed above plays an important role in enhancing sound evoked activity in the AC as schematized in Figure 13 (arrows 2-3). In addition, infusion of SS into the LA also increased sound evoked activity in the AC and sometimes resulted in the expansion of FRF of neurons in the AC whereas direct application of SS on the AC did not induce such large FRF expansion. These results suggest that the SS-induced CF shift of the AC may be partially mediated by the direct effects of SS on the LA.
Startle Reflex and CNS Hyperactivity
Although SS elevated CAP thresholds and reduced the neural output of the cochlea, sound-evoked activity in the AC, MGB, and LA was paradoxically increased at suprathreshold stimulation intensities. We have suggested that the SS-induced hyperactivity in the AC might be related to the enhancement of the acoustic startle reflex due to a decrease in GABAergic inhibition (Lu et al., 2011; Sun et al., 2009). SS has been shown to alter GABAergic inhibition in the IC, AC and hippocampus (Bauer et al., 2000; Gong et al., 2008; Wang et al., 2006; Wang et al., 2008). Since neural activity in these regions can modulate the acoustic startle reflex response (Bowen et al., 2003; Caine et al., 1992; Campeau and Davis, 1995) it is conceivable that the SS-induced hyperactivity in these regions contributes to the enhancement of the acoustic startle response. One way to test this hypothesis would be to infuse SS into the LA and/or AC and determine if this leads to an increase in the acoustic startle reflex.
Synopsis
The effects of high doses of SS have generally been thought to be transient in nature, mostly confined to the cochlea, and frequency-independent. However, high doses of SS selectively damage spiral ganglion neurons, but not the hair cells. Moreover, prolonged treatment with high-doses of SS permanently reduced the CAP from the auditory nerve, but not DPOAE that arise from OHC. SS can penetrate the blood brain barrier and directly affect neural activity at sites within the central auditory pathway as well as regions outside the classical auditory pathway such as the LA. The interaction between SS-induced peripheral deficits and SS-induced central hyperactivity may contribute to the tonotopic reorganization in the AC and the amygdala which in turn may give rise to a phantom sound sensation in quiet. Previous immunolabeling studies and the results presented here suggest that the functional consequences of high dose salicylate may be more profound, diverse and widespread than previously believed (Wallhausser-Franke et al., 1996; Wallhausser-Franke et al., 2003). While the present series of studies provides new insights on potential mechanisms of SS-induced tinnitus and hyperacusis, the fact that SS can exert direct effects on the CNS suggests that the mechanisms that give rise to SS-induced tinnitus are likely to be different from those that give rise to tinnitus induced by noise exposure or age-related hearing loss.
Highlights.
Salicylate suppresses cochlear function selectively with less effect at mid-frequency
Salicylate enhances neural activity in auditory thalamus, cortex, and amygdala
Systemic salicylate application results in frequency map reorganization in the brain
More neurons turn to represent tinnitus frequency after systemic salicylate injection
Acknowledgments
This research was supported in part by grants from NIH (R01DC009091-05; R01DC009219-05; F31DC010931-03), the Tinnitus Research Initiative and the Tinnitus Research Consortium.
Abbreviations
- AC
auditory cortex
- CAP
compound action potential
- CF
characteristic frequency
- CNS
central nervous system
- CSD
current source density
- DPOAE
distortion product otoacoustic emission
- gSk
granular sink
- IC
inferior colliculus
- IHC
inner hair cell
- I/O
input/output
- LA
lateral amygdala
- LFP
local field potential
- MGB
medial geniculate body
- OHC
outer hair cell
- SGN
spiral ganglion neuron
- SIPAC
schedule induced polydipsia and shock avoidance conditioning
- SNHL
sensorineural hearing loss
- SS
sodium salicylate
- sSk
supragranular sink
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akada S, Takeda S, Ogawa R. Salicylate action on medullary inspiratory neuron activity in a brainstem-spinal cord preparation from newborn rats. Anesth Analg. 2003;96:407–11. doi: 10.1097/00000539-200302000-00020. table of contents. [DOI] [PubMed] [Google Scholar]
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand Audiol. 1999;28:219–30. doi: 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- Andersson G, Lindvall N, Hursti T, Carlbring P. Hypersensitivity to sound (hyperacusis): a prevalence study conducted via the Internet and post. Int J Audiol. 2002;41:545–54. doi: 10.3109/14992020209056075. [DOI] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. J R Soc Med. 2003;96:582–5. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–82. doi: 10.1016/s0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121:457–62. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW. Startle response to short acoustic stimuli in rats. Acta Neurobiol Exp (Wars) 2003;63:25–30. doi: 10.55782/ane-2003-1451. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Spongr VP, Salvi RJ. Physiological and histological changes associated with the reduction in threshold shift during interrupted noise exposure. Hear Res. 1992;62:217–36. doi: 10.1016/0378-5955(92)90189-t. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–22. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Brennan JF, Jastreboff PJ. Generalization of conditioned suppression during salicylate-induced phantom auditory perception in rats. Acta Neurobiol Exp. 1991;51:15–27. [PubMed] [Google Scholar]
- Budinger E, Laszcza A, Lisona H, Scheich H, Ohlc FW. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: Bottom-up and top-down processing of neuronal information via field AI. Brain Res. 2008;1220:2–32. doi: 10.1016/j.brainres.2007.07.084. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and prepulse inhibition in the rat. Pharmacol Biochem Behav. 1992;43:1201–8. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–27. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B. Protection against noise trauma by sound conditioning. Ear Nose Throat J. 1997;76:248–50. 253–5. [PubMed] [Google Scholar]
- Caperton KK, Thompson AM. Activation of serotonergic neurons during salicylate-induced tinnitus. Otol Neurotol. 2011;32:301–7. doi: 10.1097/MAO.0b013e3182009d46. [DOI] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Chen GD, Manohar S, Salvi R. Amygdala hyperactivity and tonotopic shift after salicylate exposure. Brain Res. 2012;1485:63–76. doi: 10.1016/j.brainres.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–9. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol. 2005;125:503–9. doi: 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RO, Graham GG, Bieri D, Brown M, Cairns D, Harris G, Hounsell J, Platt-Hepworth S, Reeve R, Sambrook PN, et al. Concentration-response relationships for salicylate-induced ototoxicity in normal volunteers. Br J Clin Pharmacol. 1989;28:695–702. doi: 10.1111/j.1365-2125.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Feng H, Yin SH, Tang AZ, Tan SH. Salicylate Initiates Apoptosis in the Spiral Ganglion Neuron of Guinea Pig Cochlea by Activating Caspase-3. Neurochem Res. 2011;36:1108–15. doi: 10.1007/s11064-011-0455-9. [DOI] [PubMed] [Google Scholar]
- Feng H, Yin SH, Tang AZ, Cai HW, Chen P, Tan SH, Xie LH. Caspase-3 activation in the guinea pig cochlea exposed to salicylate. Neurosci Lett. 2010;479:34–9. doi: 10.1016/j.neulet.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JJ, Robertson D, Johnstone BM. Effects of intra-cochlear perfusion of salicylates on cochlear microphonic and other auditory responses in the guinea pig. Hear Res. 1993;67:147–56. doi: 10.1016/0378-5955(93)90242-s. [DOI] [PubMed] [Google Scholar]
- Gong N, Zhang M, Zhang XB, Chen L, Sun GC, Xu TL. The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology. 2008;54:454–63. doi: 10.1016/j.neuropharm.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104:3361–70. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel MF, Jeschke M, Ohl FW. Spectral integration in primary auditory cortex attributable to temporally precise convergence of thalamocortical and intracortical input. J Neurosci. 2010;30:11114–27. doi: 10.1523/JNEUROSCI.0689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/s0378-5955(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Huang ZW, Luo Y, Wu Z, Tao Z, Jones RO, Zhao HB. Paradoxical enhancement of active cochlear mechanics in long-term administration of salicylate. J Neurophysiol. 2005;93:2053–61. doi: 10.1152/jn.00959.2004. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE. Age-Related Hearing Loss in C57BL/6J Mice has both Frequency-Specific and Non-Frequency-Specific Components that Produce a Hyperacusis-Like Exaggeration of the Acoustic Startle Reflex. J Assoc Res Otolaryngol. 2007;8:539–50. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ. Tinnitus retraining therapy. Prog Brain Res. 2007;166:415–23. doi: 10.1016/S0079-6123(07)66040-3. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Sasaki CT. An animal model for tinnitus. Laryngoscope. 1988a;98:280–6. doi: 10.1288/00005537-198803000-00008. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hansen R, Sasaki PG, Sasaki CT. Differential uptake of salicylate in serum, cerebrospinal fluid, and perilymph. Arch Otolaryngol Head Neck Surg. 1986;112:1050–3. doi: 10.1001/archotol.1986.03780100038004. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988b;102:811–22. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Zhou S, Jastreboff MM, Kwapisz U, Gryczynska U. Attenuation of salicylate-induced tinnitus by Ginkgo biloba extract in rats. Audiol Neurootol. 1997;2:197–212. doi: 10.1159/000259244. [DOI] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J Neurosci. 1996;16:4881–9. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–45. doi: 10.1016/j.neuroscience.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Kizawa K, Kitahara T, Horii A, Maekawa C, Kuramasu T, Kawashima T, Nishiike S, Doi K, Inohara H. Behavioral assessment and identification of a molecular marker in a salicylate-induced tinnitus in rats. Neuroscience. 2010;165:1323–32. doi: 10.1016/j.neuroscience.2009.11.048. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–4. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Liu J, Li X, Wang L, Dong Y, Han H, Liu G. Effects of salicylate on serotoninergic activities in rat inferior colliculus and auditory cortex. Hear Res. 2003;175:45–53. doi: 10.1016/s0378-5955(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–14. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Dalby-Brown W, Stolzberg D, Mirza NR, Allman BL, Salvi R. Effects of the potassium ion channel modulators BMS-204352 Maxipost and its R-enantiomer on salicylate-induced tinnitus in rats. Physiol Behav. 2011;104:873–9. doi: 10.1016/j.physbeh.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol. 2006;(Suppl):13–9. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, Sun W. GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience. 2011;189:187–98. doi: 10.1016/j.neuroscience.2011.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlke C, Wallhausser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hear Res. 2004;195:17–34. doi: 10.1016/j.heares.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature reviews Neuroscience. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McFadden D. Tinnitus: Facts, Theories, and Treatments. National Academy Press; Washington, D.C: 1982. [PubMed] [Google Scholar]
- McFadden D, Wightman FL. Audition: some relations between normal and pathological hearing. Annu Rev Psychol. 1983;34:95–128. doi: 10.1146/annurev.ps.34.020183.000523. [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS, Pasanen EG. Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear Res. 1984a;16:251–60. doi: 10.1016/0378-5955(84)90114-x. [DOI] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS, Pasanen EG. Temporary hearing loss induced by combinations of intense sounds and nonsteroidal anti-inflammatory drugs. Am J Otolaryngol. 1984b;5:235–41. doi: 10.1016/s0196-0709(84)80033-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A. 1996;93:13508–14. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–11. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hear Res. 2005;206:146–58. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Moeller CK, Kurt S, Happel MF, Schulze H. Long-range effects of GABAergic inhibition in gerbil primary auditory cortex. Eur J Neurosci. 2010;31:49–59. doi: 10.1111/j.1460-9568.2009.07039.x. [DOI] [PubMed] [Google Scholar]
- Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol. 1965;82:483–93. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- Ochi K, Eggermont JJ. Effects of salicylate on neural activity in cat primary auditory cortex. Hear Res. 1996;95:63–76. doi: 10.1016/0378-5955(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Ojima H, Honda CN, Jones EG. Patterns of axon collateralization of identified supragranular pyramidal neurons in the cat auditory cortex. Cereb Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- Ojima H, Honda CN, Jones EG. Characteristics of intracellularly injected infragranular pyramidal neurons in cat primary auditory cortex. Cereb Cortex. 1992;2:197–216. doi: 10.1093/cercor/2.3.197. [DOI] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–3. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Panford-Walsh R, Singer W, Ruttiger L, Hadjab S, Tan J, Geisler HS, Zimmermann U, Kopschall I, Rohbock K, Vieljans A, Oestreicher E, Knipper M. Midazolam reverses salicylate-induced changes in brain-derived neurotrophic factor and arg3.1 expression: implications for tinnitus perception and auditory plasticity. Mol Pharmacol. 2008;74:595–604. doi: 10.1124/mol.108.046375. [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Peterson BA, Winer JA. Laminar distribution and neuronal targets of GABAergic axon terminals in cat primary auditory cortex (AI) Mechan Dev. 1994;344:383–402. doi: 10.1002/cne.903440305. [DOI] [PubMed] [Google Scholar]
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. Otol Neurotol. 2010;31:823–31. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–50. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Ruttiger L, Ciuffani J, Zenner HP, Knipper M. A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res. 2003;180:39–50. doi: 10.1016/s0378-5955(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Song L, Zheng J, Nuttall AL. Control of mammalian cochlear amplification by chloride anions. J Neurosci. 2006;26:3992–8. doi: 10.1523/JNEUROSCI.4548-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Lang H, Okamura HO, Schulte BA. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J Neurosci. 2002;22:9643–50. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chen GD, Allman BL, Salvi RJ. Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience. 2011;180:157–64. doi: 10.1016/j.neuroscience.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chrostowski M, Salvi RJ, Allman BL. Intracortical Circuits Amplify Sound-Evoked Activity in Primary Auditory Cortex Following Systemic Injection of Salicylate in the Rat. J Neurophysiol. 2012 doi: 10.1152/jn.00946.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stypulkowski PH. Mechanisms of salicylate ototoxicity. Hear Res. 1990;46:113–45. doi: 10.1016/0378-5955(90)90144-e. [DOI] [PubMed] [Google Scholar]
- Su YY, Luo B, Wang HT, Chen L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear Res. 2009;253:60–6. doi: 10.1016/j.heares.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–34. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston JH, Pollock PG, Warren SK, Jones EM. Reduced brain glucose with normal plasma glucose in salicylate poisoning. J Clin Invest. 1970;49:2139–45. doi: 10.1172/JCI106431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Gale JE, Ashmore JF. Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. J Physiol. 1995;485(Pt 3):739–52. doi: 10.1113/jphysiol.1995.sp020765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Braun S, Langner G. Salicylate alters 2-DG uptake in the auditory system: a model for tinnitus? Neuroreport. 1996;7:1585–8. doi: 10.1097/00001756-199607080-00010. [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–54. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–31. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience. 2010;168:288–99. doi: 10.1016/j.neuroscience.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winguth SD, Winer JA. Corticocortical connections of cat primary auditory cortex (AI): laminar organization and identification of supragranular neurons projecting to area AII. J Comp Neurol. 1986;248:36–56. doi: 10.1002/cne.902480104. [DOI] [PubMed] [Google Scholar]
- Xu H, Gong N, Chen L, Xu TL. Sodium salicylate reduces gamma aminobutyric acid-induced current in rat spinal dorsal horn neurons. Neuroreport. 2005;16:813–6. doi: 10.1097/00001756-200505310-00007. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–53. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Yang K, Huang ZW, Liu ZQ, Xiao BK, Peng JH. Long-term administration of salicylate enhances prestin expression in rat cochlea. Int J Audiol. 2009;48:18–23. doi: 10.1080/14992020802327998. [DOI] [PubMed] [Google Scholar]
- Yu N, Zhu ML, Johnson B, Liu YP, Jones RO, Zhao HB. Prestin up-regulation in chronic salicylate (aspirin) administration: an implication of functional dependence of prestin expression. Cell Mol Life Sci. 2008;65:2407–18. doi: 10.1007/s00018-008-8195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur J Neurosci. 1996;8:1897–905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]


