Significance
Angiosperm range expansion and diversification have been major biotic upheavals in the Earth history. Mechanisms involved in their successful diversification have mainly called upon intrinsic processes at the plant level, leaving the influence of the global tectonics poorly explored. We investigate evolution of paleogeography and climate and correlate it with the diversification of angiosperms by using a general circulation model. We show that Pangea breakup induced an important expansion of temperate zones during the late Cretaceous which was concomitant to the rise of angiosperms. We suggest that the breakup of Pangea led to the onset of new humid bioclimatic continents, which in turn may have provided new external conditions for ecological expansion of the angiosperms and their diversification.
Keywords: climate modeling, paleogeography
Abstract
In 1879, Charles Darwin characterized the sudden and unexplained rise of angiosperms during the Cretaceous as an “abominable mystery.” The diversification of this clade marked the beginning of a rapid transition among Mesozoic ecosystems and floras formerly dominated by ferns, conifers, and cycads. Although the role of environmental factors has been suggested [Coiffard C, Gómez B (2012) Geol Acta 10(2):181–188], Cretaceous global climate change has barely been considered as a contributor to angiosperm radiation, and focus was put on biotic factors to explain this transition. Here we use a fully coupled climate model driven by Mesozoic paleogeographic maps to quantify and discuss the impact of continental drift on angiosperm expansion and diversification. We show that the decrease of desertic belts between the Triassic and the Cretaceous and the subsequent onset of long-lasting humid conditions during the Late Cretaceous were driven by the breakup of Pangea and were contemporaneous with the first rise of angiosperm diversification. Positioning angiosperm-bearing fossil sites on our paleobioclimatic maps shows a strong match between the location of fossil-rich outcrops and temperate humid zones, indicating that climate change from arid to temperate dominance may have set the stage for the ecological expansion of flowering plants.
Angiosperms have gradually dominated terrestrial environments after their appearance during the Early Cretaceous (1, 2). Their radiation was characterized by high and rapid diversification (3, 4), high rates of speciation throughout the Cretaceous (5), and unprecedented ecological dominance. Most hypotheses to explain angiosperm radiation invoke biotic (instrinsic) factors, such as pollinating insects (6), coevolution with herbivorous insects (7), morphological novelties (8), or ecophysiological innovations (9–11) as well as macroevolutionary patterns (1). However, recent studies have shown that extrinsic influences combined with biotic factors may drive species diversity at the multimillion-year time scale (6, 12), reviving the potential role of global climate change (13, 14) on angiosperm radiation. Such a combination is supported by fossil data, as illustrated by the latest studies based on the European megafossil plant record that provided a scenario in which angiosperm radiation was concomitant, in space and time, with the evolution of the physical environment (15).
Although the Cretaceous climate is described as warm and equable, onset of such climatic conditions is gradual (16) and results from long-term processes that occurred throughout the Mesozoic. Climate simulations were conducted using a fully coupled ocean–atmosphere general circulation model (FOAM) for five continental configurations, from the Middle Triassic [225 million years ago (Ma)] to the Late Cretaceous (70 Ma). The coupling of the Lund–Potsdam–Jena dynamic global vegetation model (LPJ) within FOAM experiments helped to account for vegetation feedbacks on the climate system and to build the most accurate paleoclimatic maps for each of the five periods. Three atmospheric pCO2 levels have been tested for each paleogeography (560 ppm, 1,120 ppm, and 2,240 ppm). This range covers the large uncertainties of pCO2 estimates for these geological periods.
To validate our paleoclimatic experiments, the geographical distribution of climate-sensitive sediments such as evaporites (dry or seasonally dry climate indicators) and coals (humid climate indicators) have been compared with our maps of simulated biomes for each time period (Fig. S1). Overall, for every time period, the spatial fit between coals and humid biomes is higher for 1,120-ppm and 2,240-ppm pCO2 scenarios than for 560 ppm (Table S1 and Fig. S1). Still, relative distribution of arid and humid zones does not show major changes between 1,120 and 2,240 ppm (Fig. S1), and coals cannot discriminate between these two scenarios. For the Carnian and Toarcian, we select the 1,120-ppm scenario, which is consistent with most studies that agree on a background pCO2 of ca. 1,000 ppm for these periods. For the Cretaceous, comparisons of our simulations with oceanic latitudinal thermal gradients reconstructed from paleoceanographic data (17–25) show that pCO2 of 2,240 ppm is required to produce sea surface temperatures comparable with data for the Aptian and Cenomanian, whereas for the Maastrichian, data-derived sea surface temperature gradient is better reproduced at 1,120 ppm (Fig. S2). Based on these comparisons, the best-fit model–data scenario corresponds to the simulations at 1,120 ppm, except for the Aptian and Cenomanian simulations, for which CO2 concentrations of 2,240 ppm are used.
Climatic Evolution
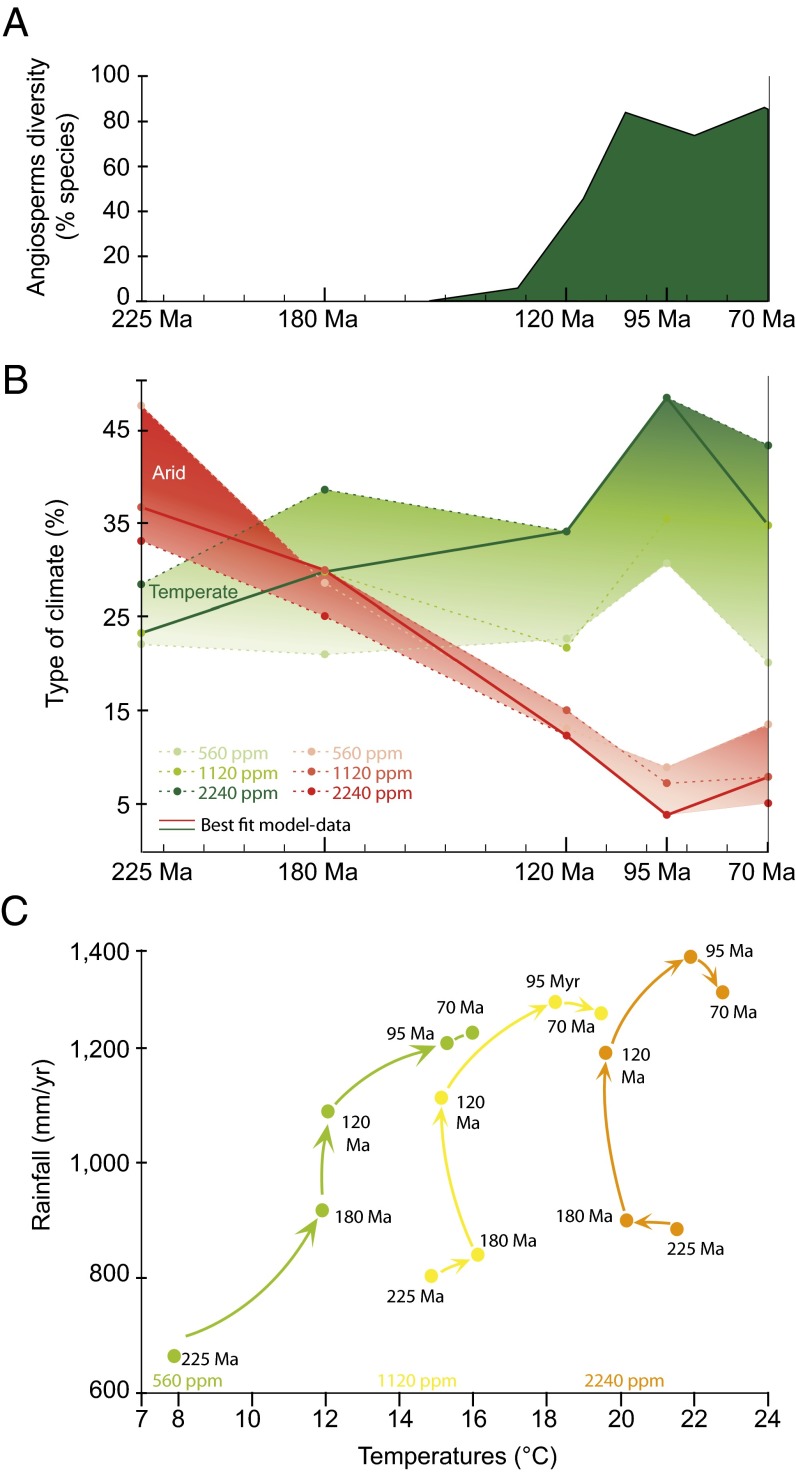
Our simulations show that the evolution of paleogeography triggers strong changes in rainfall patterns (Fig. 1C). Continental rainfall increases (ca. +60%) from the Middle Triassic (225 Ma) to reach maximal values at the Cenomanian (95 Ma) for 1,120 ppm and 2,240 ppm. Despite a slight decrease between 95 Ma and 70 Ma, high precipitation rates are maintained for the Late Cretaceous. Changes in atmospheric CO2 do not affect this global pattern of evolution of temperatures and precipitation for the different continental configuration (Fig. 1C). The Middle Triassic (225 Ma) and the Early Jurassic (180 Ma) remain the driest conditions. The Early Cretaceous configuration (120 Ma) appears as pivotal, with the global onset of new climatic conditions (higher rainfall and temperatures) which can favor macroevolutionary changes in the flora, such as angiosperm diversification.
Fig. 1.
Relationship between rainfall, temperatures, simulated biomes, and angiosperm diversification during the Mesozoic. (A) Evolution of angiosperm diversity during the Mesozoic [from de Boer et al. (10)] showing the rapid rise of diversity in the Early Cretaceous and the high values for the Cenomanian (95 Ma). (B) Evolution of desert surfaces and temperate biome fractions during the Mesozoic for the three pCO2 scenarios (560, 1,120, and 2,240 ppm) and five paleogeographies. Biomes were defined from the PFTs defined in the LPJ scheme (Table S2). The temperate biome fraction is considered as the sum of the three LPJ temperate woody PFTs and the herbaceous temperate PFT. Solid lines indicate the scenarios retained as the best-fitting with paleoclimatic data (Supporting Information). (C) Global continental mean air temperature (°C) and rainfall (mm/y) simulated by FOAM-LPJ with a pCO2 of 560 ppm (green), 1,120 ppm (yellow), and 2,240 ppm (orange). From the Triassic (225 Ma) to the mid and Late Cretaceous, precipitations highly increase. For all values of pCO2 the Triassic (225 Ma) and Jurassic (180 Ma) are the driest simulations, and the Cenomanian (95 Ma) and Maastrichtian (70 Ma) are the wettest. The Early Cretaceous (120 Ma) is pivotal with the onset of humid and hot conditions.
Changes in continental configuration affect the global climate (Fig. 1B) through three mechanisms. First, the land mass dispersion due to the breakup of Pangaea modifies the sources of moisture advection to the continents. Smaller land masses involve a more widespread distribution of precipitation over the continents due to multiple moisture sources. Conversely, with a vast supercontinent, rainout of air masses from coastal to inland regions results in very dry continental interiors. The latitudinal position of land masses is also crucial because the presence of large continental areas underneath the dry descending branch of Hadley cells induces arid conditions. From the Middle Triassic (225 Ma) to the Cenomanian (95 Ma), continental areas located in this arid belt (e.g., 20°S–40°S) drop from 38.5% to only 23% of the total continental surface (TCS), favoring the global rainfall increase. Last, fragmentation of Pangea and the subsequent increase in latent heat release leads to warmer mid–high latitudes, through higher atmospheric near-infrared absorption and associated positive cloud radiative feedbacks (26).
Simulated biomes highlight climate changes induced by paleogeography. The modern-like climatic zonation, with tropical, temperate, and boreal biomes and desert areas, is found in each simulation although with large changes in their relative fraction (Fig. 1B). For instance, extensive desert areas characterize the Middle Triassic and Early Jurassic, indicating rather dry conditions at these time periods (Fig. 1). Conversely, the Cretaceous simulations show an important decrease in these arid regions from 37% to 4% of the TCS between 225 Ma and 95 Ma for the best-fit model–data scenario (Fig. 1B). Temperate biomes show an opposite trend with a continuous increase from the Triassic to the Cenomanian with the largest change occurring between the Aptian and the Cenomanian (34–48%).
Global Climate and Angiosperm Expansion and Diversification
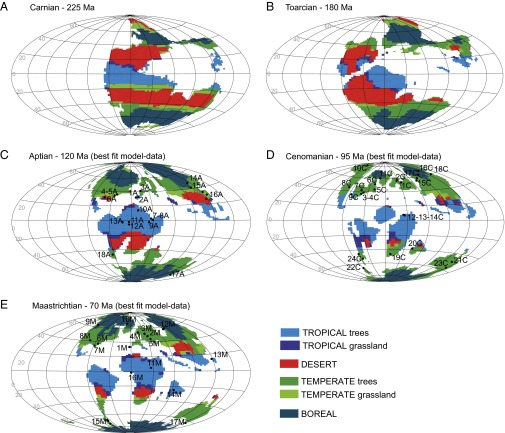
Earliest angiosperms are dated from the Early Cretaceous but were neither diverse nor abundant at this time (1). According to recent studies they were essentially limited to small shrubby, herbaceous, or aquatic forms (1, 15). Within a few tens of millions of years, their diversity strongly increased, as they represent 40% of the macrofossil flora from the latest Cretaceous (27). In Europe, the megafossil record from the Barremian (ca. 130 Ma) to the Campanian (ca. 84 Ma) shows that this increase in diversity happened in a sequential scenario involving migration and radiation of angiosperms into new environments (15). During the Aptian (120 Ma), a nonnegligible part of the global angiosperm fossil record is composed of pollen grains found close to the equator according to the prevailing angiosperm fossils sites (i.e., Gabon, Congo, Tunisia, Egypt, and Brazil in the Gondwana continent) published by Friis et al. (2). According to our simulations, 45% of angiosperm localities correspond to tropical biomes, whereas 40% are found under temperate biomes (Table 1, Fig. 2, and Table S3). From the Aptian to the Cenomanian, the number of angiosperm localities fitting with simulated temperate biomes almost doubles (Table 1). The sites are more diverse, numerous, and scattered over the globe and include higher-latitude sites which became temperate only during the mid-Cretaceous (e.g., Alaska, Siberia, and Greenland localized around 60° N; Fig. 2 D and E, sites 6, 10, 11, and 18). Although some fossil localities correspond to simulated temperate climates that have replaced cold climates at high latitudes when the supercontinent gets fragmented, other angiosperms colonize sites that were already temperate. In the southern hemisphere, this might be explained by the persistence of desertic areas that would have prevented full ecological expansion of angiosperms during the Aptian. This hypothesis will need to be confirmed by future studies because the discontinuous and scattered nature of the continental sedimentary record is a strong limitation to establish an accurate point-by-point correlation between the angiosperms and the simulated biomes. In any case, our modeling experiments demonstrate that the climate simulated during the mid-Cretaceous favors the expansion of temperate biomes (Fig. 1B) and, compared with the angiosperm record, results in a 76% match between fossil sites and temperate area. In addition, around 8% of the fossil sites correspond to simulated boreal biomes for the three Cretaceous paleogeographies. A closer look at the geographical localities reveals that most of these sites are localized at the border between boreal and temperate biomes (Fig. 2, sites 14 and 15 for the Aptian; sites 16 and 17 for the Cenomanian; and sites 9, 10, and 12 for the Maastrichtian). The main region of disagreement is continental Siberia, a region for which a long-running discrepancy between models and data does exist (28). Some mechanisms, not explicitly represented in our climate model, may help to decrease this discrepancy. The climate biological feedback (29) and the chemistry climate feedback (30) are among those mechanisms but are still not well understood, hampering our capacity to move forward in this area. In any case, it is not the main purpose of this paper to solve this long standing issue, but we note that those processes (among others) may induce warmer and wetter conditions, which in turn may amplify the latitudinal extension of temperate biomes at the expense of boreal biomes and may potentially improve the match between angiosperm sites and temperate biomes.
Table 1.
Percentage of angiosperm-rich fossil sites found in the tropical, desert, temperate, and cold biomes for the Cretaceous period and LPJ methods
| Biomes | Aptian (120 Ma) LPJ, % | Cenomanian (95 Ma) LPJ, % | Maastrichtian (70 Ma) LPJ, % |
| Tropical | 45 | 16 | 26.3 |
| Desert | 10 | 0 | 0 |
| Temperate | 40 | 76 | 63.2 |
| Cold | 5 | 8 | 10.5 |
Fig. 2.
Biomes simulated with the FOAM-LPJ model during the Mesozoic and angiosperm diversification. The five maps correspond to simulated biomes with the best-fit pCO2 scenario. Black circles represent the major angiosperm fossil sites listed by Friis et al. (1). See Table S3 for more details. (A) Middle Triassic, Carnian (225 Ma). (B) Early Jurassic, Toarcian (180 Ma). (C) Early Cretaceous, Aptian (120 Ma); 1A, Portugal; 2A, Spain; 3A, Great Britain; 4A, Virginia: 5A, Maryland and Washington, DC; 6A, Texas; 7A, Israel; 8A, Jordan; 9A, Egypt; 10A, Tunisia; 11A, Gabon; 12A, Congo; 13A, Brazil; 14A, Transbaikalia; 15A, Mongolia; 16A, China; 17A, Australia; 18A, South America. (D) Early–Late Cretaceous, Cenomanian (95 Ma); 1C, Czech Republic; 2C, Germany; 3C, Virginia; 4C, Maryland and Washington, DC; 5C, New Jersey; 6C, Massachusetts; 7C, Alabama; 8C, Kansas; 9C, Texas; 10C, Alaska; 11C, Greenland; 12C, Israel; 13C, Jordan; 14C, Lebanon; 15C, Kazakhstan; 16C, Siberia: 17C, Northeastern Russia; 18C, East of Russia; 19C, Southern Africa; 20C, Madagascar; 21C, Australia; 22C, Antarctica; 23C, New Zealand; 24C, South America. (E) Climate and angiosperm fossils sites for the Late Cretaceous, Maastrichtian (70 Ma); 1M, Portugal; 2M, Austria; 3M, Germany; 4M, Sweden; 5M, Romania; 6M, North Carolina; 7M, Georgia; 8M, Colorado; 9M, Canada; 10M, Greenland; 11M, Sudan; 12M, Kazakhstan; 13M, Japan; 14M, India; 15M, Antarctica; 16M, Nigeria; 17M, New Zealand.
Among fundamental questions regarding the angiosperm diversification, the timing of major lineages divergences remains debated because of discrepancies between the earliest record of fossil taxa and molecular-based phylogenetic inferences. The latter suggest that the origin and the initial diversification of angiosperms have occurred between 180 Ma and 140 Ma (31), predating the oldest known angiosperm fossil record, dated ca. 125 Ma (32). A similar discrepancy occurs between the first appearance of some current clades such as the diversification of Rosids (33) in the fossil outcrops and the divergence time estimated with molecular phylogenies. Here the dramatic ecological radiation of angiosperms acknowledged in the second half of the Cretaceous by numerous fossil outcrops (1) coincides with the Cenomanian fragmented world and the development of temperate continental islands. This observation supports the hypothesis of a major diversification at that time (Fig. 1 A and B, and Fig. 2D) and suggests a slight overestimation of divergence time by current molecular phylogeny dating.
For angiosperm evolution, the mid-Cretaceous is a key period for both diversification and geographical settings. Global paleogeographic changes throughout the Mesozoic involved major climatic and paleoenvironmental evolution with the expansion of temperate biomes that culminated during the Cenomanian at the expense of desertic areas (Fig. 1B). The location of the fossil-rich regions at this time under temperate biomes suggests that the development of temperate humid conditions set the stage for a major range expansion of angiosperm clades (Fig. 2D). The Aptian–Cenomanian is also considered a key period for diversification (ref. 5 and Fig. 1A). In addition, a closer inspection of the biome maps shows that continuous latitudinal desert belts largely limited potential migration pathways of flowering plants between the tropics and the temperate regions during Triassic and Jurassic times. Breakup of these large latitudinal desert belts begins during the Early Cretaceous but is not achieved until the Cenomanian. These barriers might explain the shift of the angiosperms from low latitudes to midlatitudes between the beginning and the middle of the Cretaceous, although testing such a hypothesis may requires more appropriate models and data and is beyond the scope of this study. The progressive decrease of arid zones building connectivity between humid tropical and warm temperate zones ever since the Cenomanian (as presently in southern China or between Florida and the Appalachians) may add a facilitating factor for diversification and migration.
Allopatric speciation and radiation, usually enhanced by orogeny processes and relief creation, rather involves here the fragmentation of the supercontinent into many small land masses. Additional overall long-term rise of global sea level, reaching a maximum during the mid-Cretaceous (34), has enhanced isolation phenomena leading to numerous archipelagos. By the emergence of new bioclimatic continents we suggest that this mechanism has set the stage for ecological expansion of angiosperms and could have ultimately led to the successful ecological radiation and diversification of angiosperms (Fig. 1C). This may involve the scenario inferred by Coiffard et al. (15), with a migration in most of the continental surfaces in three phases, being globally relevant and not restricted to Europe. We favor an abiotic first-order context allowing angiosperm radiation by a global climate change driven by both a breakup of Pangea in Gondwana and Laurasia and the drift of the smaller land masses. It is in this new Cretaceous global context that novel functional traits, such as biological innovation with interaction with pollinating insects (7), ecological adaptation (15), morphological novelties with the symmetry of the flower (8), and ecophysiological innovations with evolution of densely veined leaves (10, 35), have combined and collectively explain the evolutionary success of the angiosperms. Still, none of the local or global scale is exclusive, and both act in a pluralistic way because these biotic interactions may have driven a local-scale success of several radiations. In addition, angiosperm phenotypic innovation occurred with repeated bursts (4), which is consistent with the development of several new bioclimatic continents during the Upper Cretaceous, as depicted here.
Materials and Methods
The model experiments were performed with the Fast Ocean-Atmosphere model (FOAM) developed by Jacob (36). FOAM successfully simulates many aspects of the present-day climate and compares well with other contemporary medium-resolution climate models; it has also been used previously to investigate Cretaceous and Neoproterozoic climates (17, 37–39). This model is a fully coupled ocean–atmosphere general circulation model. The atmosphere component has a horizontal resolution of R15 (4.5° latitude × 7.5° longitude, ∼499 km × 817 km) and 18 levels in the vertical. The ocean component has 24 vertical levels and a horizontal resolution of 1.4° latitude × 2.8° longitude, ∼155 km × 305 km. A coupler links the ocean and atmospheric models. The experiments were integrated for 2,000 y without any flux corrections or deep ocean acceleration. During the last 50 y of model integration, there is no apparent drift in the upper ocean (between the surface and 300 m depth). The results discussed above correspond to the mean climate averaged over the last 50 y. The climate–vegetation interaction was accounted for in FOAM using the LPJ dynamic global vegetation model (40). The coupling procedure has been previously developed by Donnadieu et al. (41). The thermal and evaporative characteristics of the simulated vegetation are implemented back in FOAM boundary conditions in terms of evaporative capacity; roughness length; and shortwave, longwave, and visible albedos and is updated yearly [see Donnadieu et al. (41) for more details on the coupling between FOAM and LPJ].
Numerical climate modeling is subject to uncertainties because simulations are highly dependent on the prescribed boundary conditions. We ran FOAM for five distinct time periods ranging from the Late Triassic (225 Ma) to the Late Cretaceous (70 Ma). The land–ocean distributions for the model experiments come from the global paleogeography of Sewall et al. (42) for the Cretaceous, Dera et al. (43) for the Jurassic, and Donnadieu et al. (26) for the Triassic. Each paleogeography includes a reconstruction of the topography as well. The Earth’s orbit is fixed for modern time values. Solar intensity is fixed to 1,367 W⋅m−2. All these parameters were kept constant to isolate the influence of the evolving land–ocean configurations.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for improving the manuscript with thoughtful and constructive remarks. This study was supported by the Agence Nationale pour la Recherche (ANR) as part of PhyloSpace Project ANR 2009 PEXT 002 and has also received the support of Labex Centre d'Etudes de la Biodiversité Amazonienne (ANR-10-LABX-2501). This work was granted access to the High Performance Computing resources of Très Grand Centre de Calcul du CEA under the allocation 2014-012212 made by Grand Equipement National de Calcul Intensif.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324002111/-/DCSupplemental.
References
- 1.Friis EM, Crane PR, Pedersen KR. Early Flowers and Angiosperm Evolution. Cambridge, UK: Cambridge Univ Press; 2011. [Google Scholar]
- 2.Magallón S, Castillo A. Angiosperm diversification through time. Am J Bot. 2009;96(1):349–365. doi: 10.3732/ajb.0800060. [DOI] [PubMed] [Google Scholar]
- 3.Darwin F, Seward AC. More Letters of Charles Darwin. A Record of His Work in a Series of Hitherto Unpublished Letters. London: John Murray; 1903. Vols 1 and 2. [Google Scholar]
- 4.Lupia R, Crane PR, Lidgard S. In: Biotic Response to Global Change: The Last 145 Million Years. Culver SJ, Rawson PF, editors. Cambridge, UK: Cambridge Univ Press; 2000. pp. 223–243. [Google Scholar]
- 5.Crepet WL, Niklas KJ. Darwin’s second ‘abominable mystery’: Why are there so many angiosperm species? Am J Bot. 2009;96(1):366–381. doi: 10.3732/ajb.0800126. [DOI] [PubMed] [Google Scholar]
- 6.Grimaldi D. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Ann Mo Bot Gard. 1999;86(2):373–406. [Google Scholar]
- 7.Farrell BD. “Inordinate fondness” explained: Why are there so many beetles? Science. 1998;281(5376):555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- 8.Busch A, Zachgo S. Flower symmetry evolution: Towards understanding the abominable mystery of angiosperm radiation. BioEssays. 2009;31(11):1181–1190. doi: 10.1002/bies.200900081. [DOI] [PubMed] [Google Scholar]
- 9.Boyce CK, Lee JE. An exceptional role for flowering plant physiology in the expansion of tropical rainforests and biodiversity. Proc Biol Sci. 2010;277(1699):3437–3443. doi: 10.1098/rspb.2010.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer HJ, Eppinga MB, Wassen MJ, Dekker SC. A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat Commun. 2012;3(1221):1221. doi: 10.1038/ncomms2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feild TS, et al. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc Natl Acad Sci USA. 2011;108(20):8363–8366. doi: 10.1073/pnas.1014456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies TJ, et al. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc Natl Acad Sci USA. 2004;101(7):1904–1909. doi: 10.1073/pnas.0308127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElwain JC, Willis K, Lupia R. In: A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Baldwin IT, et al., editors. New York: Springer; 2005. pp. 133–165. [Google Scholar]
- 14.Coiffard C, Gómez B. Influence of latitude and climate on spread, radiation and rise to dominance of early angiosperms during the Cretaceous in the Northern Hemisphere. Geol Acta. 2012;10(2):181–188. [Google Scholar]
- 15.Coiffard C, Gomez B, Daviero-Gomez V, Dilcher DL. Rise to dominance of angiosperm pioneers in European Cretaceous environments. Proc Natl Acad Sci USA. 2012;109(51):20955–20959. doi: 10.1073/pnas.1218633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pucéat E, Lécuyer C, Sheppard SMF, Dromart G, Reboulet S, Grandjean P. Thermal evolution of Cretaceous Tethyan marine waters inferred from oxygen isotope composition of fish tooth enamels. Paleoceanography. 2003;18(2):7-1–7-12. [Google Scholar]
- 17.Price GD, Passey BH. Dynamic polar climates in a greenhouse world: Evidence from clumped isotope thermometry of early Cretaceous belemnites. Geology. 2013;41(8):923–926. [Google Scholar]
- 18.Littler K, Robinson SA, Bown PR, Nederbragt AJ, Pancost RD. High sea-surface temperatures during the Early Cretaceous Epoch. Nat Geosci. 2011;4(3):169–172. [Google Scholar]
- 19.Sinninghe Damsté JS, van Bentum ZC, Reichart G-J, Pross J, Schouten S. A CO2 decrease-driven cooling and increased latitudinal temperature gradient during the mid-Cretaceous Oceanic Anoxic Event 2. Earth Planet Sci Lett. 2010;248(1-2):426–437. [Google Scholar]
- 20.Mutterlose J, Malkoc M, Schouten S, Sinninghe Damsté JS, Forste A. TEX86 and stable δ18O paleothermometry of early Cretaceous sediments: Implications for belemnite ecology and paleotemperature proxy application. Earth Planet Sci Lett. 2010;298(3-4):286–298. [Google Scholar]
- 21.Pucéat E, et al. Revised phosphate-water fractionation equation reassessing paleotemperatures derived from biogenic apatite. Earth Planet Sci Lett. 2010;298(1-2):135–142. [Google Scholar]
- 22.Pucéat E, et al. Fish tooth δ18 O revising Late Cretaceous meridional upper ocean water temperature gradients. Geology. 2007;35(2):107–110. [Google Scholar]
- 23.Steuber T, Rauch M, Masse J-P, Graaf J, Malkoc M. Low-latitude seasonality of Cretaceous temperatures in warm and cold episodes. Nature. 2005;437(7063):1341–1344. doi: 10.1038/nature04096. [DOI] [PubMed] [Google Scholar]
- 24.Bice KL, Norris RD. Possible atmospheric CO2 extremes of the Middle Cretaceous (late Albian-Turonian) Paleoceanography. 2002;17(4):22-1–22-17. [Google Scholar]
- 25.Pearson PN, et al. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature. 2001;413(6855):481–487. doi: 10.1038/35097000. [DOI] [PubMed] [Google Scholar]
- 26.Donnadieu Y, Goddéris Y, Pierrehumbert R, Fluteau F, Dromart G. Modelling the primary control of paleogeography on Cretaceous climate. Geochem Geophys Geosyst. 2006;7(11):Q11019. [Google Scholar]
- 27.Lidgard S, Crane PR. Angiosperm diversification and Cretaceous floristic trends: a comparison of palynofloras and leaf macrofloras. Paleobiology. 1990;16(1):77–93. [Google Scholar]
- 28.Spicer RA, et al. The Late Cretaceous continental interior of Siberia: A challenge for climate models. Earth Planet Sci Lett. 2008;267(1-2):228–235. [Google Scholar]
- 29.Kump LR, Pollard D. Amplification of Cretaceous warmth by biological cloud feedbacks. Science. 2008;320(5873):195. doi: 10.1126/science.1153883. [DOI] [PubMed] [Google Scholar]
- 30.Beerling DJ, Fox A, Stevenson DS, Valdes PJ. Enhanced chemistry-climate feedbacks in past greenhouse worlds. Proc Natl Acad Sci USA. 2011;108(24):9770–9775. doi: 10.1073/pnas.1102409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikström N, Savolainen V, Chase MW. Evolution of the angiosperms: calibrating the family tree. Proc Biol Sci. 2001;268(1482):2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421(6925):807–814. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, et al. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proc Natl Acad Sci USA. 2009;106(10):3853–3858. doi: 10.1073/pnas.0813376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller KG, Mountain GS, Wright JD, Browning JVA. 180-million-year record of sea level and ice volume variations from continental margin and deep-sea isotopic records. Oceanography. 2011;24(2):40–53. [Google Scholar]
- 35.Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc Biol Sci. 2009;276(1663):1771–1776. doi: 10.1098/rspb.2008.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob RL. 1997. Low frequency variability in a simulated atmosphere ocean system. Thesis (Univ of Wisconsin, Madison)
- 37.Poulsen CJ, Pierrehumbert RT, Jacob RL. Impact of ocean dynamics on the simulation of the Neoproterozoic “snowball Earth”. Geophys Res Lett. 2001;28(8):1575–1578. [Google Scholar]
- 38.Poulsen CJ, Jacob RL, Pierrehumbert RT, Huynh TT. Testing paleogeographic controls on a Neoproterozoic snowball Earth. Geophys Res Lett. 2002;29(11):10-1–10-4. [Google Scholar]
- 39.Poulsen CJ. Absence of a runaway ice-albedo feedback in the Neoproterozoic. Geology. 2003;31(6):473–476. [Google Scholar]
- 40.Sitch S, et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biol. 2003;9(2):161–185. [Google Scholar]
- 41.Donnadieu Y, Goddéris Y, Bouttes N. Exploring the climatic impact of the continental vegetation on the Mezosoic atmospheric CO2 and climate history. Clim Past. 2009;5(1):85–96. [Google Scholar]
- 42.Sewall JO, Wal RSW, van de Zwan CJ, van der Oosterhout C, Dijkstra HA, Scotese CR. Climate model boundary conditions for four Cretaceous time slices. Clim Past. 2007;3(4):647–657. [Google Scholar]
- 43.Dera G, Donnadieu Y. Modeling evidences for global warming, Arctic seawater freshening, and sluggish oceanic circulation during the Early Toarcian anoxic event. Paleoceanography. 2012;27(2):PA2211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.