Abstract
All mammalian cells display a diverse array of glycan structures that differ from those found on microbial pathogens. Siglecs are a family of sialic acid-binding immunoglobulin-like receptors that participate in the discrimination of ‘self’ and ‘non-self’ and regulate the functions of cells in the innate and adaptive immune systems through recognition of their glycan ligands. In this review, we describe the recent advances in our understanding of the roles of Siglecs in the regulation of immune cell functions in infectious diseases, inflammation, neurodegeneration, autoimmune diseases and cancer.
Siglecs (sialic-acid-binding immunoglobulin-like lectins) were discovered through convergent studies on sialoadhesin (also known as Siglec-1 and CD169) and CD22 (also known as Siglec-2). Sialoadhesin was initially defined as a macrophage adhesion receptor recognizing sialic acids and then shown to be an immunoglobulin superfamily (IgSF) member, and CD22 was characterized as a B cell inhibitory receptor of the IgSF and later shown to recognize sialic acids1, 2. The homology of these proteins with CD33 (also known as Siglec-3) and myelin associated glycoprotein (MAG; also known as Siglec-4) led to the establishment of the Siglec family; to date 14 Siglecs have been identified in humans and 9 in mice3 (Table 1).
Table 1.
Summary of structural and functional properties of the Siglec family. Siglecs are in numerical order based on human Siglecs, with mouse orthologs immediately underneath when established116. Sialoadhesin (Siglec-1), CD22 (Siglec-2), MAG (Siglec-4) and Siglec-15 are conserved Siglecs found in both mouse and man. Other Siglecs are members of the CD33 (Siglec-3) related family, and the mouse orthologs are designated by letter instead of number (e.g. Siglec-E).
| Siglec1 (other names) |
Cell type Expression |
Structure | Sialoside Preference2 | Disease relevance3 |
Ref | ||
|---|---|---|---|---|---|---|---|
| # Ig domains |
Tyrosine motifs |
DAP-12 Binding |
|||||
| Sialoadhesin (Siglec-1, CD169) | Macrophages-tissue subsets Monocytes-activated |
17 | None | No |

|
Autoimmunity HIV-1 infection, PRRSV infection C. jejuni infection Group B Streptococcus infection |
3, 34, 35, 44, 45, 137, 138 |
| CD22 (Siglec-2) | B cells | 7 | ITIM ITIM-like Grb2 |
No | Human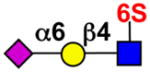
|
Lymphoma, Leukemia, SLE, Rheumatoid arthritis | 3, 93 |
Mouse
| |||||||
| Human CD33 (Siglec-3) | Myeloid progenitors Macrophages Monocytes Microglia Granulocytes |
2 | ITIM ITIM-like |
No |

|
Acute Myeloid Leukemia (AML) Alzheimers disease |
3, 74–76, 79, 80, 82, 137, 138 |
| Mouse CD33 (Siglec-3) | Macrophages Granulocytes |
2 | ITIM-like | unknown | ND | Alzheimers disease | 74 |
| Human/Mouse MAG (Siglec-4) | Oligodendrocytes Schwann cells |
5 | FYN-kinase site | No |
|
Neuro-degeneration | 137, 138 |
| Human Siglec-5 | Neutrophils Monocytes B cells |
4 | ITIM ITIM-like |
No |

|
Group B Streptococcus infection | 3, 5, 137, 139 |
| Human Siglec-6 | Trophoblasts B cells |
3 | ITIM ITIM-like |
No |

|
Pre-eclampsia | 3, 138, 140 |
| Human Siglec-7 | NK cells Monocytes Mast cells |
3 | ITIM ITIM-like |
No |
|
Cancer C. jejuni infection |
3, 42 |
| Human Siglec-8 | Eosinophils Mast cells Basophils |
3 | ITIM ITIM-like |
No |

|
Eosinophilia, Asthma | 3 |
| Mouse Siglec-F (murine paralog of Siglec-8) | Eosinophils Alveolar macrophages Activated microglia |
4 | ITIM ITIM-like |
No |

|
Eosinophilia | 66, 141 |
| Human Siglec-9 | Neutrophils, Monocytes, Dendritic cells NK cells |
3 | ITIM ITIM-like |
No |

|
Chronic lung inflammation | 3 |
| Mouse Siglec-E (murine equivalent of Siglec-9) | Neutrophils, Monocytes, Macrophages Dendritic cells |
3 | ITIM ITIM-like |
No - |

|
Acute lung injury Neuro-inflammation |
3, 70, 78 |
| Human Siglec-10 | B cells Monocytes Eosinophils |
5 | ITIM ITIM-like Grb2 |
No |

|
Lymphoma, leukemia, eosinophilia, allergy | 3, 137, 142, |
| Mouse Siglec-G (murine ortholog of Siglec-10) | B cells Dendritic cells- myeloid |
5 | ITIM ITIM-like Grb2 |
No |
|
Tissue damage, Sepsis GvH disease Autoimmunity |
33, 40, 41, 86, 97, 143 |
| Human Siglec-11 | Macrophages Microglia |
5 | ITIM ITIM-like |
No |

|
Neuro-inflammation | 77, 78 |
| Human Siglec-14 | Neutrophils Monocytes |
3 | None | Yes |

|
Group B Streptococcus infection, COPD | 5, 139 |
| Human and Mouse Siglec-15 | Osteoclasts Macrophages, Dendritic cells |
2 | None 4 | Yes |

|
Osteopetrosis | 131 |
| Human Siglec-16 | Macrophages Microglia |
5 | None | Yes |

|
8 | |
| Mouse Siglec-H | Plasmacytoid dendritic cells Macrophage subsets |
2 | None | Yes | Not yet determined | Viral infection Neuro-inflammation |
3, 78, 144–146 |
Two additional Siglec genes/proteins are found in chimpanzees that are either only expressed in some humans in a form that does not bind sialic acids (Siglec-12), or have been deleted from the human genome (Siglec-13)147, 148.
Specificities are summarized from the cited references or from glycan array data at the http://www.functionalglycomics.org. ND is not determined.
Disease relevance is based on animal models or expression on cells involved in disease processes. Additional references for disease relevance are cited in the text.
Conserved Tyr of unknown function
key:
 NeuAc
NeuAc
 NeuGc
NeuGc
 Gal
Gal
 GalNAc
GalNAc
 GlcNAc
GlcNAc
 Fuc
Fuc
 Sulfate
Sulfate
Siglecs can be divided into 2 groups: those that are conserved across mammals, such as sialoadhesin, CD22, MAG and Siglec-15, and a group of CD33-related Siglecs that are variable across mammals. The CD33-related Siglecs are thought to have expanded from a primordial cluster of Siglec genes that underwent an inverse duplication event over 180 million years ago4. Humans and many other mammals express a much larger set of CD33-related Siglecs than mice and rats, which can be explained by a dramatic loss of Siglec genes in rodents4. As members of the immunoglobulin superfamily, the siglecs are cell surface transmembrane receptors comprised of 2–17 extracellular Ig domains, including a N-terminal V-set domain that contains the sialic acid binding site (Table 1)3. The cytoplasmic domain of most Siglecs have immune receptor tyrosine-based inhibitory motifs (ITIMs) and signal negatively via recruitment of tyrosine phosphatases such as SHP-1 and SHP-2 (also known as tyrosine-protein phosphatase non-receptor type 6 and 11, respectively)3. A few Siglecs, such as Siglec-14, Siglec-15 (Box 1), and Siglec-16 associate with the tyrosine-based activation motif (ITAM) adaptor DAP12 via a positively charged amino acid in their transmembrane region (Table 1) and are predicted to be activating receptors through the recruitment of SYK kinase. Interestingly, most humans express two pairs of Siglecs that share nearly identical ligand binding extracellular regions, but with divergent transmembrane and cytoplasmic regions. The ITIM-containing Siglecs-5 and Siglec-11 are paired with the DAP12-coupled Siglecs-14 and Siglec-16, respectively3. The evolution of these activating receptors from their corresponding inhibitory receptors is thought to have been driven by pathogen exploitation of the inhibitory Siglecs, thereby providing the host with additional activitory pathways by which to combat these pathogens 5–8.
Box 1. Siglec-15 regulates differentiation of Osteoclast.
Osteoclasts play a critical role in bone resorption and as such are a primary target in osteoporosis129. While not considered part of the immune system, they are derived from a monocyte precursor through RANKL stimulation130. Recently, Siglec-15, which is highly conserved in vertebrates131, was shown to be constitutively expressed in osteoclasts132, 133. Mice lacking Siglec-15 develop mild osteopetrosis, a condition that is characterized by dense bone134, 135. In vitro studies have shown that Siglec-15 pairs with DAP12 via a transmembrane domain lysine residue to deliver a signal that positively regulates osteoclast differentiation into their multinucleated state12, 133–135. Importantly, this function requires sialic acid-binding, since a mutant of Siglec-15 that disrupts sialic acid recognition impairs osteoclastogenesis in a manner similar to that seen with Siglec-15−/− cells.
Current treatment strategies for osteoporosis, such as bisphosphates or an antibody targeting RANKL136, ameliorate disease by inhibiting the breakdown of bone through targeting the osteoclasts. Preclinical development is underway for antibodies targeting Siglec-15. These promote Siglec-15 internalization and lysosomal-mediated degradation resulting in reduced expression of Siglec-15 on osteoclast precursor cells, impairing osteoclastogenesis. Targeting Siglec-15 may therefore lead to novel therapies for treatment of osteoporosis.
Most if not all Siglecs are also endocytic receptors that either constitutively cycle between the cell surface and intracellular endosomes, or are induced to undergo endocytosis upon ligation by antibody or multivalent ligands3, 9–15. However, mechanisms of endocytosis vary, with some being clathrin dependent, and others not12, 13, 15. Similarly, while the cytoplasmic Tyr-based motifs are implicated in regulation of endocytosis of some Siglecs9, 13, 14, sialoadhesin has no known regulatory motifs, yet undergoes efficient endocytosis, and can carry ligand bearing cargo into the cell9–11, 14.
Crystal structures of N-terminal regions of sialoadhesin, Siglec-5 and Siglec-7 complexed with various sialic acid ligands have revealed the molecular basis for specificity16–18. Most Siglecs are expressed preferentially in specific cell types, resulting in a complex and partially overlapping expression pattern within the innate and adaptive immune system (Table 1). The role of different Siglecs in disease is therefore determined to a large extent by their expression patterns and the relative importance of different cell populations to the disease in question. Several Siglec polymorphisms linked to human diseases have been described, in particular for CD33, Siglec-8 and Siglec-14 and are discussed further below.
Each Siglec has a distinct preference for binding sialylated glycans, which are found on the surface of all mammalian cells. Each Siglec has preference for specific types of sialylated structures that are expressed on mammalian cells (Table 1) which have been revealed by a variety of methods including glycan array analyses3. Because sialic acids are present on all cells, the glycan ligands of Siglecs are effectively markers of self. Not surprisingly, the interactions of Siglecs with their ligands play a key role in modulating their activity as regulators of immune cell functions. Thus, the Siglecs help immune cells to distinguish between self and non-self, while sialylated pathogens, by cloaking themselves with these self-like ligands can target Siglecs to down-regulate immune cell responses and escape immune surveillance19–23.
Ligand binding can affect the functions of Siglecs in regulation of immune cell functions in different ways, as illustrated in Figure 1 (which will be referred to in the context of specific examples throughout the review). For example, the interactions of Siglecs with cis-binding ligands on the same cell are important for regulating the signaling functions of Siglecs (Fig. 1a, b), as elegantly demonstrated for B cells using ‘knock-in’ mutations of the sialic acid binding sites of CD22 and Siglec-G24, 25 These studies suggest that cis-binding ligands sequester CD22 from association with the BCR, but promote association of Siglec-G with the BCR, exerting opposite effects on setting a threshold for B cell signaling. Trans interactions of Siglecs with soluble glycoconjugates (Fig. 1c, d) or ligands on other cells (Fig. 1e, f) can also modulate signaling functions. For most Siglecs, the precise oligosaccharide ligands, and the protein or lipid carriers that present them to Siglecs for modulation of cellular functions, remain to be elucidated.
Figure 1. Mechanisms by which sialoside ligands of the Siglecs mediate immunomodulatory effects.
Illustrations are generic but are supported by one or more specific examples for individual Siglecs. Modulation of Siglec function by cis (a, b) and trans (c–f) ligands through promoting (a, c) or excluding (b, d) association with an activating receptor. Interactions with trans ligands can also promote apoptosis of the Siglec-expressing cells or drive apoptosis and/or alter migratory properties of the contacted cell (panel e).
In this Review we present recent progress in our understanding of the role of Siglecs in immune cells and in diseases such as infectious disease, lung inflammation, autoimmunity and cancer. In particular, we describe how the interactions of Siglecs and their carbohydrate ligands can modulate cell-cell interactions and the association of the Siglecs with signaling receptors. We also summarize current efforts for the development of therapeutics that target Siglecs and exploit their properties for treatment of human disease. Owing to space constraints, we are unable to provide an in-depth review of other aspects of Siglec structure, evolution and function (for additional information on Siglec structure, evolution and function, see REFS 3, 26–29).
Siglecs and infections
The mammalian immune system has evolved highly sophisticated mechanisms to recognize and respond to a diverse array of pathogens and to eliminate them. Siglecs contribute to this process by helping to regulate both innate and adaptive immune responses via interactions with ‘self’ glycans. However, some human pathogens such as group B Streptococcus (GBS) display sialic acid-based ‘non-self’ ligands that can be recognized by ITIM-bearing inhibitory Siglecs, leading to subversion of host immune responses5, 30, 31. In addition, some pathogens such as Streptococcus pneumoniae secrete enzymes such as sialidases that can destroy Siglec ligands and promote pathogenicity32, 33 (Fig. 2). In contrast, certain Siglecs such as sialoadhesin and the DAP-12-coupled Siglec-14 can interact with pathogen sialic acids and promote host defense functions7, 34, 35. Here we discuss recent progress in understanding how Siglecs modulate immune responses to infections and consider the pathways by which sialylated pathogens can both subvert and promote immune responses through Siglec-dependent interactions.
Figure 2. Siglecs in host-pathogen interactions.
(a) Siglec-10 (Siglec-G in mice) on DCs interacts with CD24 to inhibit DAMP-mediated TLR signaling, which is disrupted by bacterial sialidase to promote infection. (b) Double-stranded (DS) RNA from RNA viruses induces Siglec-G expression in macrophages, which in turn decreases type I interferons through RIG-I degradation and promotes infection. (c) Interactions between Siglec-E and sialoside ligands on Group B Streptococcus (GBS) on macrophages inhibit phagocytosis. (d, e, g) Recognition of various sialoside ligands on GBS, C. jejuni, or HIV by sialoadhesin on macrophages promotes uptake of these pathogens for a variety of outcomes. (f) Siglec-10 recognition of pseudaminic acid on the flagella of Campylobacter jejuni promotes IL-10 release by DCs.
Siglecs modulate cytokine production by DCs and macrophages
Siglecs on dendritic cells (DCs) and macrophages can modulate Toll-like receptor cytokine responses following overexpression or in vitro ligation with anti-siglec antibodies or host-derived glycans 36–39. Another mode of action is based on the proposal that Siglec-G does not directly modulate responses to microbial products but, instead, interacts in cis with a damage-associated molecular pattern (DAMP) receptor, CD24, to suppress DC inflammatory responses following tissue injury40 (Fig. 2a; left panel). Bacterial sialidase from S. pneumoniae can abrogate the sialic acid-dependent Siglec-G–CD24 interaction33, and perhaps other Siglec cis interactions on myeloid cells32, leading to increased pro-inflammatory responses and mortality in models of sepsis (Fig. 2a; right panel).
Siglec-G expression is upregulated on macrophages following infection by RNA viruses such as vesicular stomatitis virus (VSV) or Sendai virus, but not DNA viruses or bacteria41. This upregulation leads to a SHP-2 and Cbl-dependent ubiquitylation and proteosomal degradation of RIG-I and suppression of the interferon-β (IFN-β) response (Fig. 2b). This negative feedback pathway seems to be exploited by RNA viruses since VSV-infected mice lacking Siglec-G show decreased viral titers and reduced mortality accompanied by increased IFN-β production. Interestingly, this effect of Siglec-G on VSV-triggered IFN-β does not require CD24 and is unaffected by sialidase treatment of the macrophages41, suggesting it is sialic acid-independent. The constitutive tyrosine phosphorylation of Siglec-G in macrophages observed following VSV infection may be sufficient to recruit and activate SHP-2-dependent signaling pathways without a requirement for sialic acid ligation41.
Interactions of Siglecs with bacteria
Several human bacterial pathogens can display sialic acid based ligands for Siglecs, including Neisseria meningitidis, Haemophilus influenzae, Campylobacter jejuni, Pseudomonas aeruginosa and GBS31. So far, the best evidence that bacterial sialic acids can subvert immune responses via Siglec interactions has come from studies of GBS. This bacterium displays Siaα2–3Galβ1–4GlcNAc moieties on its capsule that are recognized by Siglec-9 on human neutrophils and its murine equivalent, Siglec-E19, 30 (Fig. 2c). Ligand binding of Siglec-9 suppresses the neutrophil oxidative burst and extracellular trap formation, and thus contributes to pathogen survival19. Following intranasal infection with GBS, SiglecE−/− mice produced higher levels of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-6, but reduced levels of IL-1030. Although this did not affect bacterial numbers, in a sub-lethal systemic challenge with GBS, SiglecE−/− mice showed reduced bacterial dissemination in the brain and kidney, which is consistent with bacterial exploitation of Siglec-dependent inhibitory pathways.
Certain GBS strains can also target the paired receptors Siglec-5 and Siglec-14 in a sialic acid-independent pathway via interactions with cell wall-anchored β protein5, 31. Similar to Siglec-9 ligation, Siglec-5 ligation by β protein suppresses neutrophil killing functions31. In contrast, Siglec-14 ligation by β protein triggers an activation pathway that counterbalances the inhibitory signaling of Siglec-5. Both Siglec-5 and Siglec-14 are expressed on human amniotic epithelium and a SIGLEC-14 null polymorphism may influence responses to GBS infections and preterm births5. Siglec-14 can also interact with H. influenzae in a sialic acid-dependent manner, leading to increased production of IL-8 and TNF-α, again supporting a host protective function of this receptor7.
In contrast to other Siglecs, sialoadhesin is an extended protein with 17 Ig domains that can capture and internalize pathogens in macrophages. Sialoadhesin also lacks intrinsic signaling motifs and has the potential to contribute to host defense via scavenging functions. Direct support for this was provided in an infection model of GBS, where bacteria injected intravenously were taken up by marginal zone macrophages expressing the gene encoding sialoadhesin (Siglec1) 34 (Fig. 2d). This uptake limited bacterial spread to other sites such as kidney, lung and spleen, and in neutropenic mice, this led to increased animal survival compared with Siglec1−/− mice. Additional support for a role of sialoadhesin in host protection to sialylated bacteria was obtained in studies of C. jejuni. C. jejuni is an opportunistic enteric pathogen of humans that displays a variety of ganglioside-like structures on lipo-oligosaccharides that can be recognized by sialoadhesin and other Siglecs35, 42. Injection of mice with C. jejuni expressing GD1a-like ligands for sialoadhesin triggered a MyD88-dependent proinflammatory cytokine and type 1 interferon response that was lost in Siglec1−/− mice35 (Fig. 2e). Since sialoadhesin strongly promotes macrophage phagocytosis of C. jejuni in this system35, cross-talk with TLRs is likely to explain these observations. Besides expressing Siglec ligands on lipo-oligosaccharides, the flagella of C. jejuni display pseudaminic acid, a sugar highly related to sialic acid. A recent study demonstrated that Siglec-10 expressed on human DCs interacted with this sugar and triggered IL-10 production via unknown mechanisms43 (Fig. 2f). This may be important for modulating gut inflammatory responses to this and other enteric pathogens.
Siglecs as receptors for enveloped viruses
Enveloped viruses such as HIV and the porcine reproductive and respiratory syndrome virus (PRRSV) are coated with sialic acids that can be recognized by sialoadhesin on macrophages and promote uptake and infection31. It was recently found that HIV incorporation of ganglioside GM3 into the membrane is required for infection of in vitro matured DCs and two research groups showed independently that sialoadhesin is the major receptor for GM3 that delivers HIV to endosomal compartments to promote trans-infection and dissemination of HIV to T cells44, 45 (Fig. 2g). A key question to be addressed is whether this pathway has an important role in HIV infection and spread in vivo since sialoadhesin is not normally expressed on DCs but is restricted to subsets of inflammatory monocytes and tissue macrophages21. In this regard, HIV can also interact with other Siglecs, including Siglec-3, Siglec-5, Siglec-7 and Siglec-9 via sialylated gp120, also promoting infection of macrophages46
In conclusion, while Siglecs have likely evolved to regulate host immune responses via recognition of ‘self’ glycans, growing evidence indicates that sialylated pathogens target Siglecs to modulate immune cell functions, in some cases contributing to their pathogenicity and ability to survive within the host.
Lung inflammatory diseases
Chronic inflammatory diseases of the lung, such as allergic asthma and chronic obstructive pulmonary disease (COPD), cause progressive damage to lung tissue. Although mechanisms of lung inflammatory diseases differ, innate immune cells such as eosinophils, mast cells, neutrophils and macrophages have established roles in disease pathology47–51 (Table 1). Accumulating evidence suggests that Siglecs have a role in the degree of tissue damage caused by these cells, and therefore may be attractive targets for treatment of lung inflammatory diseases.
Allergic asthma
Eosinophils, mast cells and basophils are granule-containing cells that have a dominant role in allergic asthma52, 53. Siglec-8 is specifically expressed on eosinophils in humans, whereas murine eosinophils and alveolar macrophages express an isofunctional paralog, Siglec-F, that shares a novel specificity for sialylated ligands as described below54–56. Both Siglecs have ITIMs3, and polymorphisms in the gene encoding Siglec-8 are correlated with increased susceptibility to asthma57.
Insights into the regulatory role of Siglec-8 stem from the observation that cross-linking by antibodies or polymeric glycan ligands causes apoptosis of eosinophils58–60. Surprisingly, this Siglec-8-induced apoptosis is enhanced by the cytokine IL-5, which normally promotes eosinophil survival58, 61. These findings suggest that ligation of Siglec-8 by natural ligands may regulate the levels of eosinophils in tissues29, 58 (see Fig. 1e). For murine eosinophils, ligation of Siglec-F with antibodies also causes apoptosis, however, the effect is modest and appears to be mediated by a different mechanism61, which suggests that information gleaned from disease models involving Siglec-F should be interpreted with caution before translating their relevance to Siglec-8 and human disease56, 57.
The specificity of Siglec-8, assessed by glycan microarray analysis, revealed high specificity for a sialylated-sulfated glycan epitope, NeuAcα2–3[6S]Galβ1–4GlcNAc56, 62, 63. Although this specificity is shared with Siglec-F, this mouse paralog also binds with lower affinity to ligands without the sulfate13, 56, 62–65. In mouse models of ovalbumin-induced airway inflammation, Siglec-F ligands are dramatically upregulated on epithelial cells and mucins62, 65–68. In most, but not all, models of lung allergy, mice deficient in either Siglec-F or its ligands (for example ST3Gal3−/− mice) exhibit strongly enhanced eosinophil infiltration62, 67–69. These results support a working model in which trans-activation of Siglec-F by ligands on an opposing cell (see Fig. 1e) or polymeric mucin (see Fig. 1c) is sufficient to dampen eosinophil activation or induce apoptosis to moderate eosinophil accumulation. However, it may not be so simple since in a model of allergic airway inflammation, Siglec-F−/− mice also exhibit increased eosinophils in tissues other than lung66.
Chronic and acute lung inflammation
Neutrophils also have a major role in the pathology of acute and chronic lung inflammatory disease, such as COPD48, 50. Human neutrophils express two inhibitory Siglecs, Siglec-5 and Siglec-9, as well as the activating Siglec-14, whereas murine neutrophils express Siglec-E and low levels of Siglec-F, which are both inhibitory. In a model of lipopolysaccharide-induced lung inflammation, Siglec-E−/− mice showed enhanced neutrophil migration into lung tissue that was blocked by antibody to CD11b β2-integrin70. Neutrophils adherent to the CD11b ligand fibrinogen exhibited enrichment of Siglec-E at the adhesion foci on fibrinogen-coated slides. Moreover, outside-in signalling by CD11b, including phosphorylation of SYK and p38 MAP kinases, was enhanced in Siglec-E−/− neutrophils. Prior treatment of fibrinogen with sialidase enhanced outside-in signalling in WT but not Siglec-E-deficient neutrophils. A follow-up study has shown that, paradoxically, Siglec-E promotes β2 integrin-dependent production of reactive oxygen species by neutrophils in vitro and in vivo and this is required for its suppression of neutrophil recruitment to the lung71. Taken together, the results suggest that CD11b β2-integrin-mediated ‘outside-in’ signaling may be modulated both negatively and positively by Siglec-E through sialic acid-dependent recruitment to the sites of contact with its ligand fibrinogen (see Fig. 1f)70. Although not a Siglec, there are interesting parallels with another neutrophil inhibitory receptor, PILR-α, which mediates high affinity sialic acid-dependent interactions with cis ligands and suppresses neutrophil β2 integrin signaling and chemotactic responses 72.
A recent report has identified a null allele polymorphism of neutrophil activating Siglec-147. Patients with COPD who are homozygous for this null allele had fewer inflammatory exacerbations than patients expressing the WT allele, which suggests that Siglec-14 may promote inflammatory sequelae caused by neutrophils. While the roles of Siglecs continue to emerge, the forgoing examples illustrate that they have an impact on lung inflammatory disease as a consequence of their intrinsic roles in the regulation of the cells that mediate immune responses in the lung.
Siglecs in Neurodegeneration
In the central nervous system (CNS), microglia are specialized resident macrophages that are derived from yolk sac precursors and bone marrow monocytes. They are thought to play a protective role by removal of senile plaques and damaged neurons, but in some conditions they can also exacerbate inflammatory conditions and be neurotoxic73. Microglia express a number of Siglecs including CD33, Siglec-11 and Siglec-16 in humans, and CD33, Siglec-E, Siglec-F, and Siglec-H in mice, several of which have been associated with pathology in neurodegenerative diseases8, 74–78.
Genome-wide association studies have recently identified variants of CD33 as a major risk factor for Alzheimer’s disease79–81. The risk allele of CD33 was associated with increased expression of CD33 on microglial cells, and correlated with decreased in vitro uptake of toxic plaques of amyloid β (Aβ)74–76. These data have led to a model whereby CD33 negatively regulates the ability of microglia to phagocytize toxic Aβ. Furthermore, an additional allele, co-inherited with the aforementioned protective allele, produces CD33 without its V-set domain, which suggests that ligand binding may play a role in suppressing plaque uptake75, 82. Although CD33−/− mice also have a diminished incidence of Aβ plaque pathology74, the mouse may not provide a good model to study the role of CD33 in human disease. In contrast to human CD33, which is an inhibitory Siglec, mouse CD33 lacks a cytoplasmic ITIM and exists in two splice variants. These are predicted to associate with DAP12 due to the presence of a lysine residue within the transmembrane region and thus mediate activatory functions. A better understanding of these processes is needed to define the basis for the association of the CD33 risk allele with Alzheimer’s disease.
In microglia, Siglec-11 and Siglec-E are constitutively expressed and can be neuroprotective by recognising trans sialic acid ligands on neuronal cells (see Fig. 1f), which leads to suppression of an inflammatory response by preventing excessive uptake of apoptotic neuronal debris78. Expression of Siglec-F and Siglec-H under inflammatory conditions has also been proposed to regulate microglial function78. Although, recent studies show that several Siglecs have the potential to play preventative or causative roles in neurodegeneration, further studies are needed to gain a clear understanding of how Siglecs regulate functions of resident and activated microglia and inflammatory monocytes in neurodegenerative diseases.
Siglecs in Autoimmunity
Failure of the immune system to correctly distinguish ‘self’ is one hallmark of autoimmunity. Siglecs are appropriately positioned to aid the immune system in self-recognition because they recognize sialoside ligands present on interacting cells and can dampen cellular activation (Fig. 1f). Insights into the circumstances where Siglec-ligand interactions participate in mechanisms of self-tolerance are beginning to emerge. In particular, recent studies demonstrate an important role for the B cell Siglecs in suppressing immune responses to self-antigens and preventing autoimmunity, and suggest that Siglecs may also directly or indirectly regulate T cell responses relevant to tolerance to self-antigens.
Roles of Siglecs in self-tolerance
Pathogenic autoantibodies arise from the inappropriate stimulation and expansion of self-reactive B cells83. Under healthy conditions, such autoreactive B cells are purged or silenced through mechanisms of B cell tolerance84. The B cell Siglecs contribute to mechanisms of B cell tolerance as inhibitory co-receptors of the B cell receptor (BCR)26. In humans, naïve peripheral B cells express CD22 and Siglec-10, while mouse B cells express CD22 and the Siglec-10 ortholog, Siglec-G. The importance of the combined actions of CD22 and Siglec-G in B cell tolerance have been clearly demonstrated in mice lacking both Siglecs, which develop systemic autoimmunity as they age85, and mice deficient in Siglec-G alone have early onset and increased severity of lupus-like and autoimmune disease models86.
Interactions of B cell Siglecs with their cis ligands87 have a profound, yet complex, influence on the threshold for B cell activation. A recent study showed that B cells from CD22−/− mice and mice bearing a mutant of CD22 that cannot interact with sialic acid, are hyper- and hypo-responsive to BCR stimulation, respectively25, which supports earlier observations that cis CD22 ligands on the B cell limit the association of CD22 with the BCR (see Fig. 1b)3, 88, 89. By contrast, another recent report showed that a point mutation in Siglec-G that ablates sialic acid binding gives rise to decreased association of Siglec-G with the BCR and a decreased threshold for B cell activation24, 25. In humans, mutations in the gene encoding sialic acid 9-O-acetyl esterase (Siae) have been correlated with autoimmunity90. Since sialic acid esterases remove an O-acetyl group from sialic acid, that can otherwise block Siglec binding, sialic acid esterase-deficiency lowers the levels of Siglecs ligands. Furthermore, mice deficient in the enzyme CMP-NeuAc hydroxylase (CMAH) are unable to convert NeuAc to NeuGc the preferred sialic acid for murine Siglecs91, exhibit a hyperactive B cell phenotype91, 92. This phenotype has been attributed to abrogating cis ligand interactions of CD22 with the BCR. This interpretation, however, needs to be re-evaluated in the context of the inhibitory activities of both CD22 and Siglec-G, which have overlapping ligand specificities (Table 1). Given that both CD22 and Siglec-G can independently modulate the activity of the BCR24–26, changes in cis ligands on the B cells, therefore, has the potential to modulate BCR signaling through either Siglec. It is also notable that dramatic remodeling of Siglec ligands of B cells occurs in the germinal center in both mouse91 and man93, yet the functional consequence of this remodeling remains largely unexplored.
Although cis ligands set a threshold for the interaction between CD22 and the BCR, several studies have shown that they do not preclude interactions with trans ligands on other cells94–96. A recent report demonstrates that trans ligands of CD22 on blood vessel endothelial cells are involved in trafficking of mature B cells to Peyer’s patches96. Moreover, recent studies have demonstrated that trans CD22-ligand interactions can have a profound influence on regulation of BCR signaling through CD22. Several studies have used polymers or liposomes displaying both antigen and Siglec ligand and demonstrated that drawing CD22 or Siglec-G together with the BCR has a profound inhibitory effect on BCR signaling97–101 (see Fig. 1c). Co-presentation of antigens with ligands of CD22 or Siglec-G results in strong inhibition of B cell activation, inhibition of tonic BCR signaling through the Akt survival pathway, apoptosis of the antigen-reactive B cell, and consequently induction of B cell tolerance98, 99.
Taken together, these findings suggest that CD22 and Siglec-G may help enforce peripheral B cell tolerance to membrane antigens by a mechanism involving recruitment to the immunological synapse by recognition of sialylated glycans on the cell displaying antigen (Fig. 3). In this regard, since CD22 and Siglec-G/10 recognize a different spectrum of ligands97, and independently suppress B cell activation98, they provide B cells the potential to recognize ‘self’ ligands regardless of the types of sialosides elaborated on the surface of cell.
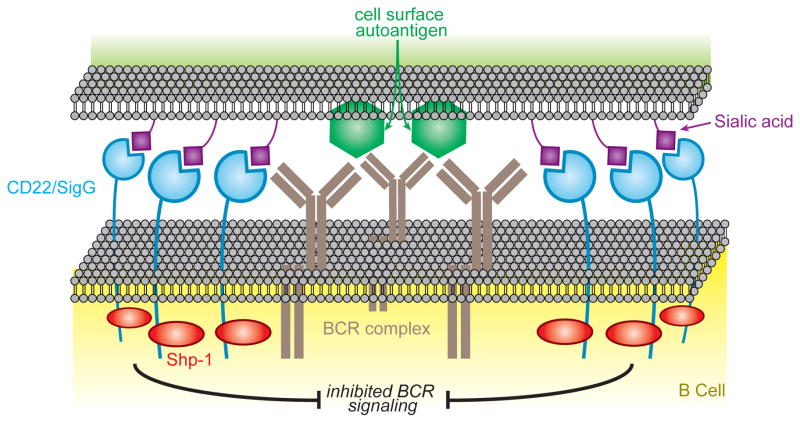
Figure 3. Sialoside ligands on a cell that displays a cell surface antigen draws the B cell. Siglecs into the immunological synapse to inhibit B cell activation.
Mouse B cells express CD22 and Siglec-G and are known to inhibit B cell activation when co-ligated with the BCR by a membrane that displays both antigen and Siglec ligand. Inhibition is initiated by phosphorylation of the Immunoreceptor tyrosine-based inhibitory motifs (ITIMs) on CD22 and Siglec-G, resulting in recruitment of the phosphatase Shp-1 that dampens signaling by dephosphorylation of the BCR signaling complex. This mechanism probably contributes to the role of CD22 and Siglec-G in maintaining peripheral B cell tolerance.
Impact of Siglecs on effector T cells
Several autoimmune disease models have revealed a pro-inflammatory role for sialoadhesin on macrophages in promoting CD4 and CD8 T cell activation. In an experimental autoimmune encephalomyelitis model, Tregs were shown to express high levels of sialoadhesin ligands following activation and their engagement with sialoadhesin on macrophages led to suppression of regulatory T expansion and exacerbation of disease102. Similarly, a subset of T effector cells also upregulates sialoadhesin ligands on activation, leading to T cell apoptosis (see Fig. 1e)103. Hence, the upregulation of sialoadhesin ligands on different T cell subsets appears to play a role in sialoadhesin-dependent immunoregulation.
Although it is generally accepted that the majority of T cells in humans and mice lack Siglec expression, a recent study has proposed that a subset of CD52hi suppressor T cells secrete soluble CD52 that presumably binds to Siglec-10 and suppresses activation of T cells that promote autoimmune diseases such as type 1 diabetes104. Depletion of CD52hi T cells from splenocytes injected into diabetes prone NOD/SCID mice accelerated onset of disease. Although a role of Siglec-10 in this study was inferred from the use of blocking antibodies, details of how Siglec-10 is expressed on T cells and suppresses T cell activation have yet to emerge.
Siglecs In Immune Surveillance of Cancer
Siglecs have long been associated with cancer: CD22 and CD33 were discovered as specific markers of B cell lymphomas and myeloid leukemias long before they were identified as members of the Siglec family105, 106. Several recent reports suggest that siglec deficiencies can increase the potential for generation of lymphomas and leukemias107, 108. Moreover, in keeping with their roles aiding immune cells in distinguishing between self and non-self, Siglecs are emerging as playing a role in regulation of immune surveillance of cancer. This role is particularly relevant in light of the well-documented alterations of glycosylation, including increased sialylation, that occurs on many cancers109.
Cancer cells must evade attack by innate lymphoid NK cells that can recognize transformed cancer cells as altered self, and initiate innate immune responses. NK cells distinguish normal cells from altered cells through an array of activatory and inhibitory receptors that recognize signatures of ‘non-self’ and ‘self’110. Siglec-7 and Siglec-9 are among the inhibitory receptors (Table 1) that are either highly expressed on all NK cells, or expressed on a subset of NK cells, respectively111. It has been shown that recognition of sialic acid containing ligands of Siglec-7 on the surface of an NK target cell draws it into the site of cell contact to dampen NK cell activation112, 113 (see Fig. 1f). Previously, it was speculated that cancer cells up-regulate ligands of Siglec-7 to exploit this mechanism of silencing NK cells113. Indeed, as revealed by two recent studies111, 112, the levels of Siglec-7 and Siglec-9 ligands are greatly elevated on a variety of cancer cells, which decreases their susceptibility to NK cell killing. Intrinsic to these findings is that destroying Siglec ligands by sialidase treatment of the cancer cells abrogates an inhibitory effect through Siglec-7, which also holds true in vivo using mice reconstituted with human NK cells111.
Heavily glycosylated mucins are commonly produced by cancer cells, and can in principle modulate immune surveillance by binding to Siglecs (Fig. 1c, d). Human tumor-produced mucins have been found to bind to Siglec-9 on NK cells, B cells, monocytes, and DCs and down-regulate immune cell responses38, 114. Similarly, when administered to mice, tumor mucins that bind to CD22 deplete marginal zone B cells in a manner similar to that seen in mice bearing tumors115. Thus, soluble mucins produced by tumors may also contribute to escape of cancer cells from immune surveillance.
As seen in these examples, Siglecs effectively reduce innate immune responses against cancer cells by down-regulating immune cells that express them through recognition of sialoside ligands on the cancer cell itself or soluble mucins produced by the cancer cell.
Siglecs as therapeutic targets
Because of the highly restricted expression of Siglecs in the immune system, and the differential expression of Siglecs in various immune cells, they are increasingly recognized as targets for development of immunotherapeutics (Fig. 4)28, 53, 116. Anti-Siglec antibodies that target leukemia and lymphoma blood cancers have been in clinical development for over 20 years, providing a wealth of experience for using antibodies as targeting agents105, 106. More recently, glycan ligand decorated nanoparticles have shown promise as an alternative way to target Siglec-expressing cells11, 117, 118. The majority of the Siglecs are endocytic receptors3, 9, 10, 13, making them candidates for targeted therapeutics that involves delivery of cytotoxic or immuno-modulatory agents into the targeted cell (Fig. 4a)105, 106, 117. This section reviews the current status of agents targeting Siglecs in clinical development, and efforts to develop alternative strategies that exploit Siglecs on immune cells for therapeutic intervention in disease.
Figure 4. Therapeutic potential of targeting Siglecs in disease.
(a) The endocytic property of the Siglecs expressed on lymphoma/leukemia cells (CD22/CD33) or macrophages (sialoadhesin) has been exploited to deliver cargo to the cell type of interest. Toxin or antigens can be delivered via conjugated anti-Siglec antibodies (left) or encapsulated in the lumen of liposomes displaying high affinity Siglec ligands (right). Once endocytosed, toxins are released to cause cell death, while antigens are loaded on MHC or CD1d for presentation to T cells and NKT cells, respectively. It is notable that in the case of targeting via glycan ligands, the modestly reduced endosomal pH is enough to cause dissociation ofSiglecs and their cargo, which allows for recycling of the Siglecs to the cell surface for additional rounds of cargo uptake. (b) Siglec-engaging tolerance-inducing antigenic liposomes (STALs) display both antigen and a high affinity ligand of the B cell Siglecs (CD22 and Siglec-G). STALs enforce association of the Siglecs with the BCR on B cells, inhibiting B cell activation that otherwise occurs when ligands are absent. Through downstream signaling events, a pro-apoptotic signal is delivered that results in apoptosis of the antigen-specific B cells, resulting in antigen-specific B cell tolerance.
Cell depletion therapy for lymphomas and leukemias
An anti-CD33 immunotoxin, Myelotarg™, was approved in the US in 2002 for treatment of acute myeloid leukemia. Although it was later withdrawn for efficacy and safety concerns in 2010, it continues to be actively pursued for treatment of subsets of patients with acute myeloid leukemia119, and there are numerous ongoing clinical trials with anti-CD22 and anti-CD33 antibody-based therapies for B cell lymphomas and myeloid leukemias105, 106, 116, 120. Most of the antibody-based therapeutics are immunotoxins, with anti-CD22 or CD33 antibodies conjugated to calicheamicin (for example gemtuzumab and inotuzumab) or bacterial protein toxin (such as moxetumomab), which rely on the endocytic activity of the Siglec to internalize the toxin into the cell (Fig. 4a, left). Other strategies are radiolabeled antibodies or ‘naked’ antibodies that kill leukemia cells by irradiation or by induction of antibody dependent cellular cytotoxicity or complement induced cytotoxicity, respectively, and are used in combination with standard chemotherapeutics (for example epratuzumab and Y90-epratuzumab)106.
A promising alternative strategy proposed for B cell lymphomas is to use chemotherapeutic loaded nanoparticles decorated glycan ligands that actively target CD22 on lymphoma cells106, 117 (Fig. 4a, right). Doxorubicin-loaded liposomal nanoparticles decorated with glycan ligands of CD22 have shown remarkable efficacy in murine tumor models of B cell lymphoma compared to liposomal formulations without the ligand117. A potential advantage of this approach is that ligands are released from CD22 in the endosomal compartment, allowing ligand-targeted cargo to be pumped into the cell as CD22 recycles from the surface9. The progress in the development of Siglec-targeted therapies for treatment of lymphomas and leukemias has drawn attention to the Siglecs as targets for treatment of immune cell mediated diseases.
Cell depletion therapy for allergic inflammatory disease
The discovery that ligation of Siglec-8 causes apoptosis of eosinophils has stimulated interest in Siglec-8 as a therapeutic target for reducing pathology in inflammatory diseases involving pathology induced by eosinophils53, 58–60, 121. The impact of eosinophil depletion on allergic inflammatory diseases has been explored most directly in mouse models using antibodies to Siglec-F, which effectively deplete eosinophils from blood and tissues122. Using this approach, depletion of eosinophils has been documented to significantly reduce pathology in models of allergic rhinitis123, esophageal eosinophilia124, and allergen induced eosinophil airway remodeling125. A similar approach has been used to demonstrate a beneficial effect of eosinophil depletion on allergen induced intestinal inflammation125.
Antigen targeting to APCs
Siglecs have been used as targets for delivery of antigens to APCs for inducing a more efficient immune response. Proof of principle for this strategy has been documented in vivo for targeting antigens to sialoadhesin positive macrophages using anti-sialoadhesin antibodies coupled to protein antigens (Fig. 4a, left), or liposomes bearing glycan ligands of sialoadhesin carrying protein or glycolipid antigens (Fig. 4a, right), which results in enhanced immune responses for activation of CD4+ T cells and NK T cells, respectively10, 11, 126, 127. Although the efficiency of antigen delivery may not, by itself, be sufficient to warrant a targeted approach, targeted nanoparticles have the potential to incorporate adjuvants and other agents to elicit a desired immune respofnse.
Induction of antigen-specific B cell tolerance
Immunotherapies designed to generally suppress immune responses have been useful in the areas of autoimmunity, allergy and transplantation. However, suppressing the entire adaptive immune system also leads to decreased immunity to pathogens, hence, there is a need for methods that suppress immune responses to the pathogenic antigens and preserve protective immunity. Induction of antigen-specific tolerance has enormous therapeutic potential, but also has major challenges. As described above, co-presenting antigen and ligands of CD22 or Siglec-G on the same surface can have a profound inhibitory effect on B cells (Fig. 3). Liposomal nanoparticles that display both antigen and Siglec ligand were found to induce tolerance through apoptosis of the antigen-reactive B cells98, 99 (Fig. 4b). These Siglec-engaging tolerance-inducing antigenic liposomes (STALs) were investigated for their ability to prevent inhibitory antibodies to the coagulation factor FVIII, which is a well-documented problem for 20–30% of patients with hemophilia A that receive biotherapeutic FVIII as replacement therapy. STALs bearing FVIII and CD22 ligands where shown to successfully tolerize FVIII−/− mice and prevent bleeding in a challenge model following administration of FVIII, while untreated mice with high titer antibodies had no benefit99. The results from this study indicates potential for using such strategies for inducing B cell tolerance in therapeutic settings.
As evident from the this review, roles of Siglecs in regulation of immune cell functions are beginning to emerge, revealing insights into their impact on mechanisms of disease involving the cells that express them. This has led to recognition of Siglecs as targets for cell specific therapies involving delivery of therapeutic cargo or modulation of immune cell functions.
Concluding remarks
Since the discovery of the Siglec family nearly twenty years ago, the roles of Siglec-ligand interactions in regulation of immune cell functions is beginning to emerge. Sialic acid-containing glycans are effectively ‘self’ epitopes, and by contacting them, Siglecs can help immune cells distinguish between ‘self’ and ‘non-self’. These interactions create context specific biology that can either sequester or co-localize the regulatory Siglec with activatory or inhibitory receptors, and thereby regulate the activation status of the cell. As a result of their regulatory activity, Siglecs play an important role in disease processes and have been recognized as therapeutic targets for disease intervention.
Despite this progress, the biological context in which most Siglecs modulate immune cell functions is still poorly understood. In particular, while their roles as inhibitory or activatory co-receptors are well documented, the precise signaling receptors and signaling pathways they modify are still not known for most of them. Moreover, even for well-studied Siglecs like CD22, much is yet to be learned. For example, while the role of CD22 in modulating the activity of the BCR has been extensively studied, CD22 also impacts signaling of B cell TLRs, and has been implicated in trafficking of mature B cells Peyer’s patches85, 96, 128. Applying such considerations to other less studied Siglecs emphasizes that much is yet to be learned about this family of receptors, and major new insights into their biology and roles in immune cell functions will continue to unfold. A deeper understanding of roles of Siglecs in immune cell functions will accelerate the pace of exploiting that knowledge for development of agents for treatment of disease.
Acknowledgments
Work in the authors’ laboratories is supported by grants from the National Institutes of Health (JCP) and the Wellcome Trust (PRC).
Definitions
- Immunoreceptor tyrosine-based activation motif (ITAM)
A short peptide motif containing tyrosine residues found in the cytoplasmic tails of several signaling molecules and in adaptors such as DAP12. The tyrosine is phosphorylated after receptor activation, triggering a cascade of intracellular events that typically results in cellular activation
- Immunoreceptor tyrosine-based inhibitory motif (ITIM)
A short peptide motif containing tyrosine residues found in the cytoplasmic regions of many inhibitory receptors. This is phosphorylated after receptor activation, often by SRC family protein tyrosine kinases, producing a binding site for cytoplasmic phosphatases and other signaling molecules that results in dephosphorylation of activation complexes and inhibition of signaling cascades
- Cis and trans ligands
Siglecs exhibit overlapping specificities for sialic acid containing glycans commonly found on glycoproteins and glycolipids of many different cell types. Siglec ligands can occur on the same cell (cis ligands) or on opposing cells (trans ligands) that are contacted by the Siglec expressing cell
- SHP-1 and SHP-2
Src homology region 2 (SH2) domain-containing phosphatases that bind phosphorylated ITIMs. Recruitment of SHP-1 and -2 by the Siglecs represents a general mechanism for immunomodulation of cellular signaling
- B cell receptor (BCR) complex
On naïve B cells, the BCR complex is composed of membrane form of IgM along with immunoglobulin-α and -β, which initiate BCR signaling through proximal signal components
- Alzheimer’s disease
The most common form of dementia stemming from plaque deposition and neurofibullar tangles in the brain
- Osteopetrosis
A disease characterized by thickening of bones, which stems from an imbalance of bone generation and resorption by osteoblasts and osteoclasts, respectively
- RIG-I
A pattern recognition receptor protein that plays an intricate role in immunity against RNA virus. RIG-I directly recognizes dsRNA and triggers an antiviral response
- gp120
A highly glycosylated protein on the surface of the HIV envelope, which recognizes CD4 on T cells as a portal of entry
- amyloid β
Proteolytically processed peptides of amyloid precursor protein, which can aggregate to form oligomers and give rise to the amyloid plaques found in the brains of Alzheimer patients
- Sialic acids
A family of nine carbon keto sugars that are structurally related to N-acetyl neuraminic acid (NeuAc), the predominant sialic acid in humans. Other common sialic acids are 9-O-acetyl-NeuAc also found in humans, and N-acetyl-gycollyl-neuraminic acid (NeuGc) a sialic acid in most mammalian species, but is not found in humans
- Sialoside
A glycan containing sialic acid linked via its anomeric carbon to the next sugar. In a biological context, sialosides may be complex glycans linked to proteins or lipids
- Hemophilia A
A genetic deficiency in the gene encoding the blood clotting factor VIII. Patients with this disease are treated by intravenous FVIII replacement therapy
References cited
- 1.Crocker PR, et al. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. The EMBO journal. 1994;13:4490–503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sgroi D, Varki A, Braesch-Andersen S, Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J Biol Chem. 1993;268:7011–8. [PubMed] [Google Scholar]
- 3.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, et al. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–17. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 5.Ali SR, et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211:1231–42. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao H, Crocker PR. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology. 2011;132:18–26. doi: 10.1111/j.1365-2567.2010.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angata T, et al. Loss of Siglec-14 reduces the risk of chronic obstructive pulmonary disease exacerbation. Cell Mol Life Sci. 2013;70:3199–210. doi: 10.1007/s00018-013-1311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H, et al. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–15. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly MK, Tian H, Paulson JC. CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells. J Immunol. 2011;186:1554–63. doi: 10.4049/jimmunol.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delputte PL, et al. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS One. 2011;6:e16827. doi: 10.1371/journal.pone.0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki N, et al. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc Natl Acad Sci U S A. 2013;110:7826–31. doi: 10.1073/pnas.1219888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuible M, et al. Mechanism and function of monoclonal antibodies targeting siglec-15 for therapeutic inhibition of osteoclastic bone resorption. J Biol Chem. 2014;289:6498–512. doi: 10.1074/jbc.M113.494542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateno H, et al. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter RB, et al. ITIM-dependent endocytosis of CD33-related Siglecs: role of intracellular domain, tyrosine phosphorylation, and the tyrosine phosphatases, Shp1 and Shp2. J Leukoc Biol. 2008;83:200–11. doi: 10.1189/jlb.0607388. [DOI] [PubMed] [Google Scholar]
- 15.Winterstein C, Trotter J, Kramer-Albers EM. Distinct endocytic recycling of myelin proteins promotes oligodendroglial membrane remodeling. J Cell Sci. 2008;121:834–42. doi: 10.1242/jcs.022731. [DOI] [PubMed] [Google Scholar]
- 16.Attrill H, et al. Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. J Biol Chem. 2006;281:32774–83. doi: 10.1074/jbc.M601714200. [DOI] [PubMed] [Google Scholar]
- 17.May AP, Robinson RC, Vinson M, Crocker PR, Jones EY. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′ sialyllactose at 1.85 A resolution. Molecular cell. 1998;1:719–28. doi: 10.1016/s1097-2765(00)80071-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhuravleva MA, Trandem K, Sun PD. Structural implications of Siglec-5-mediated sialoglycan recognition. J Mol Biol. 2008;375:437–47. doi: 10.1016/j.jmb.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends Immunol. 2009;30:488–93. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaas M, Crocker PR. Sialoadhesin in recognition of self and non-self. Semin Immunopathol. 2012;34:353–64. doi: 10.1007/s00281-012-0310-3. [DOI] [PubMed] [Google Scholar]
- 22.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann N Y Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padler-Karavani V, et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2014;28:1280–93. doi: 10.1096/fj.13-241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutzler S, et al. The ligand-binding domain of Siglec-G is crucial for its selective inhibitory function on B1 cells. J Immunol. 2014;192:5406–14. doi: 10.4049/jimmunol.1302875. [DOI] [PubMed] [Google Scholar]
- 25.Muller J, et al. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci U S A. 2013;110:12402–7. doi: 10.1073/pnas.1304888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller J, Nitschke L. The role of CD22 and Siglec-G in B-cell tolerance and autoimmune disease. Nat Rev Rheumatol. 2014 doi: 10.1038/nrrheum.2014.54. [DOI] [PubMed] [Google Scholar]
- 27.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–92. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012;33:413–20. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiwamoto T, Katoh T, Tiemeyer M, Bochner BS. The role of lung epithelial ligands for Siglec-8 and Siglec-F in eosinophilic inflammation. Curr Opin Allergy Clin Immunol. 2013;13:106–11. doi: 10.1097/ACI.0b013e32835b594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YC, et al. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog. 2014;10:e1003846. doi: 10.1371/journal.ppat.1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YC, Nizet V. The interplay between Siglecs and sialylated pathogens. Glycobiology. 2014 doi: 10.1093/glycob/cwu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang YC, Uchiyama S, Varki A, Nizet V. Leukocyte inflammatory responses provoked by pneumococcal sialidase. mBio. 2012;3 doi: 10.1128/mBio.00220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen GY, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29:428–35. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang YC, et al. Role of macrophage sialoadhesin in host defense against the sialylated pathogen group B Streptococcus. J Mol Med (Berl) 2014 doi: 10.1007/s00109-014-1157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaas M, et al. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J Immunol. 2012;189:2414–22. doi: 10.4049/jimmunol.1200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem Biophys Res Commun. 2008;369:878–83. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 37.Boyd CR, et al. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J Immunol. 2009;183:7703–9. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta M, et al. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem Biophys Res Commun. 2010;402:663–9. doi: 10.1016/j.bbrc.2010.10.079. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki K, et al. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol. 2012;188:4690–700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- 40.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–78. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Avril T, Wagner ER, Willison HJ, Crocker PR. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun. 2006;74:4133–41. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephenson HN, et al. Pseudaminic Acid on Campylobacter jejuni Flagella Modulates Dendritic Cell IL-10 Expression via Siglec-10 Receptor: A Novel Flagellin-Host Interaction. J Infect Dis. 2014 doi: 10.1093/infdis/jiu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo-Useros N, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS biology. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puryear WB, et al. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou Z, et al. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One. 2011;6:e24559. doi: 10.1371/journal.pone.0024559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pappas K, Papaioannou AI, Kostikas K, Tzanakis N. The role of macrophages in obstructive airways disease: chronic obstructive pulmonary disease and asthma. Cytokine. 2013;64:613–25. doi: 10.1016/j.cyto.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Deckers J, Branco Madeira F, Hammad H. Innate immune cells in asthma. Trends Immunol. 2013;34:540–7. doi: 10.1016/j.it.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Caramori G, Pandit A, Papi A. Is there a difference between chronic airway inflammation in chronic severe asthma and chronic obstructive pulmonary disease? Curr Opin Allergy Clin Immunol. 2005;5:77–83. doi: 10.1097/00130832-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–48. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 52.Ilmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic Clin Pharmacol Toxicol. 2014;114:109–17. doi: 10.1111/bcpt.12163. [DOI] [PubMed] [Google Scholar]
- 53.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–36. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Floyd H, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 56.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–35. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 57.Gao PS, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18:713–9. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 2009;330:608–12. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 60.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–4. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao H, et al. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 2013;8:e68143. doi: 10.1371/journal.pone.0068143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiwamoto T, et al. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133:240–7. e1–3. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bochner BS, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 64.Patnode ML, et al. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 2013;288:26533–45. doi: 10.1074/jbc.M113.485409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo JP, et al. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–43. doi: 10.1165/rcmb.2010-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang M, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho JY, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzukawa M, et al. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–48. doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMillan SJ, Richards HE, Crocker PR. Siglec-F-dependent negative regulation of allergen-induced eosinophilia depends critically on the experimental model. Immunol Lett. 2014;160:11–16. doi: 10.1016/j.imlet.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McMillan SJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood. 2013;121:2084–94. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMillan SJ, Sharma RS, Richards HE, Hegde V, Crocker PR. Siglec-E Promotes beta2-Integrin-dependent NADPH Oxidase Activation to Suppress Neutrophil Recruitment to the Lung. J Biol Chem. 2014;289:20370–20376. doi: 10.1074/jbc.M114.574624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Shiratori I, Uehori J, Ikawa M, Arase H. Neutrophil infiltration during inflammation is regulated by PILRalpha via modulation of integrin activation. Nat Immunol. 2013;14:34–40. doi: 10.1038/ni.2456. [DOI] [PubMed] [Google Scholar]
- 73.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 74.Griciuc A, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–43. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malik M, et al. CD33 Alzheimer’s risk-altering polymorphism, CD33 expression, and exon 2 splicing. J Neurosci. 2013;33:13320–5. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bradshaw EM, et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–50. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angata T, et al. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–74. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 78.Linnartz-Gerlach B, Kopatz J, Neumann H. Siglec functions of microglia. Glycobiology. 2014 doi: 10.1093/glycob/cwu044. [DOI] [PubMed] [Google Scholar]
- 79.Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertram L, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–32. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raj T, et al. CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer’s disease susceptibility. Hum Mol Genet. 2014;23:2729–36. doi: 10.1093/hmg/ddt666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–7. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 84.Basten A, Silveira PA. B-cell tolerance: mechanisms and implications. Curr Opin Immunol. 2010;22:566–74. doi: 10.1016/j.coi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–27. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 86.Bokers S, et al. Siglec-G deficiency leads to more severe collagen-induced arthritis and earlier onset of lupus-like symptoms in MRL/lpr mice. J Immunol. 2014;192:2994–3002. doi: 10.4049/jimmunol.1303367. [DOI] [PubMed] [Google Scholar]
- 87.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci U S A. 1998;95:7469–74. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 89.Grewal PK, et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol Cell Biol. 2006;26:4970–81. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Surolia I, et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–7. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naito Y, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–22. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cariappa A, et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med. 2009;206:125–38. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura N, et al. Human B-lymphocytes express alpha2–6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. J Biol Chem. 2007;282:32200–7. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- 94.Collins BE, et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci U S A. 2004;101:6104–9. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lanoue A, Batista FD, Stewart M, Neuberger MS. Interaction of CD22 with alpha2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur J Immunol. 2002;32:348–55. doi: 10.1002/1521-4141(200202)32:2<348::AID-IMMU348>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 96.Lee M, et al. Transcriptional programs define specialization of lymphoid tissue capillary and high endothelium and reveal novel trafficking mechanisms. Nat Immunol. 2014 doi: 10.1038/ni.2983. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–87. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. J Immunol. 2013;191:1724–31. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Macauley MS, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123:3074–83. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A. 2009;106:2500–5. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adachi T, et al. CD22 serves as a receptor for soluble IgM. Eur J Immunol. 2012;42:241–7. doi: 10.1002/eji.201141899. [DOI] [PubMed] [Google Scholar]
- 102.Wu C, et al. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Immunol. 2009;182:6508–16. doi: 10.4049/jimmunol.0804247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kidder D, Richards HE, Ziltener HJ, Garden OA, Crocker PR. Sialoadhesin ligand expression identifies a subset of CD4+Foxp3- T cells with a distinct activation and glycosylation profile. J Immunol. 2013;190:2593–602. doi: 10.4049/jimmunol.1201172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bandala-Sanchez E, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat Immunol. 2013;14:741–8. doi: 10.1038/ni.2610. [DOI] [PubMed] [Google Scholar]
- 105.Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011;17:6417–27. doi: 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- 106.Sullivan-Chang L, O’Donnell RT, Tuscano JM. Targeting CD22 in B-cell malignancies: current status and clinical outlook. BioDrugs. 2013;27:293–304. doi: 10.1007/s40259-013-0016-7. [DOI] [PubMed] [Google Scholar]
- 107.Uckun FM, Goodman P, Ma H, Dibirdik I, Qazi S. CD22 EXON 12 deletion as a pathogenic mechanism of human B-precursor leukemia. Proc Natl Acad Sci U S A. 2010;107:16852–7. doi: 10.1073/pnas.1007896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simonetti G, et al. SIGLEC-G deficiency increases susceptibility to develop B-cell lymphoproliferative disorders. Haematologica. 2014 doi: 10.3324/haematol.2013.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 110.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–74. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 111.Jandus C, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124:1810–20. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicoll G, et al. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur J Immunol. 2003;33:1642–8. doi: 10.1002/eji.200323693. [DOI] [PubMed] [Google Scholar]
- 114.Belisle JA, et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol Cancer. 2010;9:118. doi: 10.1186/1476-4598-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toda M, et al. Ligation of tumour-produced mucins to CD22 dramatically impairs splenic marginal zone B-cells. Biochem J. 2009;417:673–83. doi: 10.1042/BJ20081241. [DOI] [PubMed] [Google Scholar]
- 116.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–8. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen WC, et al. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–86. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen WC, et al. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One. 2012;7:e39039. doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravandi F, et al. Gemtuzumab ozogamicin: time to resurrect? J Clin Oncol. 2012;30:3921–3. doi: 10.1200/JCO.2012.43.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mims A, Stuart RK. Developmental therapeutics in acute myelogenous leukemia: are there any new effective cytotoxic chemotherapeutic agents out there? Curr Hematol Malig Rep. 2013;8:156–62. doi: 10.1007/s11899-013-0158-1. [DOI] [PubMed] [Google Scholar]
- 121.Farid S, Mirshafiey A, Razavi A. Siglec-8 and Siglec-F, the new therapeutic targets in asthma. Immunopharmacol Immunotoxicol. 2012;34:721–6. doi: 10.3109/08923973.2011.589453. [DOI] [PubMed] [Google Scholar]
- 122.Zimmermann N, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–63. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim YH, et al. Antiallergic effect of anti-Siglec-F through reduction of eosinophilic inflammation in murine allergic rhinitis. Am J Rhinol Allergy. 2013;27:187–91. doi: 10.2500/ajra.2013.27.3866. [DOI] [PubMed] [Google Scholar]
- 124.Rubinstein E, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–16. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song DJ, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–41. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen WC, Sigal DS, Saven A, Paulson JC. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22. Leuk Lymphoma. 2012;53:208–10. doi: 10.3109/10428194.2011.604755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ooms K, et al. Evaluation of viral peptide targeting to porcine sialoadhesin using a porcine reproductive and respiratory syndrome virus vaccination-challenge model. Virus Res. 2013;177:147–55. doi: 10.1016/j.virusres.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 128.Kawasaki N, Rademacher C, Paulson JC. CD22 regulates adaptive and innate immune responses of B cells. J Innate Immun. 2011;3:411–9. doi: 10.1159/000322375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 130.Udagawa N, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87:7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–46. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 132.Hiruma Y, Hirai T, Tsuda E. Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem Biophys Res Commun. 2011;409:424–429. doi: 10.1016/j.bbrc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 133.Ishida-Kitagawa N, et al. Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12) J Biol Chem. 2012;287:17493–502. doi: 10.1074/jbc.M111.324194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hiruma Y, et al. Impaired osteoclast differentiation and function and mild osteopetrosis development in Siglec-15-deficient mice. Bone. 2013;53:87–93. doi: 10.1016/j.bone.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 135.Kameda Y, et al. Siglec-15 Regulates Osteoclast Differentiation by Modulating RANKL-Induced Phosphatidylinositol 3-Kinase/Akt and Erk Pathways in Association With Signaling Adaptor DAP12. J Bone Miner Res. 2013;28:2463–75. doi: 10.1002/jbmr.1989. [DOI] [PubMed] [Google Scholar]
- 136.Silva-Fernandez L, Rosario MP, Martinez-Lopez JA, Carmona L, Loza E. Denosumab for the treatment of osteoporosis: a systematic literature review. Reumatol Clin. 2013;9:42–52. doi: 10.1016/j.reuma.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 137.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–19. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 138.Brinkman-Van der Linden EC, Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J Biol Chem. 2000;275:8625–32. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- 139.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–73. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 140.Brinkman-Van der Linden EC, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–31. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 141.Lunnon K, et al. Systemic inflammation modulates Fc receptor expression on microglia during chronic neurodegeneration. J Immunol. 2011;186:7215–24. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- 142.Munday J, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355:489–97. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Toubai T, et al. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014 doi: 10.1182/blood-2013-12-545335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–6. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Puttur F, et al. Absence of Siglec-H in MCMV infection elevates interferon alpha production but does not enhance viral clearance. PLoS Pathog. 2013;9:e1003648. doi: 10.1371/journal.ppat.1003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–8. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 147.Mitra N, et al. SIGLEC12, a human-specific segregating (pseudo)gene, encodes a signaling molecule expressed in prostate carcinomas. J Biol Chem. 2011;286:23003–11. doi: 10.1074/jbc.M111.244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X, et al. Specific inactivation of two immunomodulatory SIGLEC genes during human evolution. Proc Natl Acad Sci U S A. 2012;109:9935–40. doi: 10.1073/pnas.1119459109. [DOI] [PMC free article] [PubMed] [Google Scholar]