Abstract
Cold temperatures are associated with increased mortality and morbidity of cardiovascular and pulmonary disease. Cold exposure causes lung inflammation, pulmonary hypertension (PH) and right ventricle (RV) hypertrophy, but there is no effective therapy due to unknown mechanism. Here we investigated if RNAi silencing of TNF-α decreases cold-induced macrophage infiltration, PH, and pulmonary arterial (PA) remodeling. We found for the first time that continuous cold exposure (5.0°C) increased TNF-α expression and macrophage infiltration in the lungs and pulmonary arteries (PAs) right before elevation of RV systolic pressure. The in vivo RNAi silencing of TNF-α was achieved by IV delivery of recombinant AAV-2 carrying TNFα short hairpin siRNA (TNFshRNA) 24 hours prior to cold exposure. Cold exposure for eight weeks significantly increased RV pressure compared to the warm controls (40.19±4.9 vs. 22.9±1.1 mmHg), indicating that cold exposure caused PH. Cold exposure increased TNF-α, IL-6 and phosphodierterase-1C (PDE-1C) protein expression in the lungs and PAs and increased lung macrophage infiltration. Notably, TNFshRNA prevented the cold-induced increases in TNF-α, IL-6 and PDE-1C protein expression, abolished lung macrophage infiltration, and attenuated PH (26.28±1.6 mmHg), PA remodeling, and RV hypertrophy. PA SMCs isolated from cold-exposed animals showed increased intracellular superoxide levels and cell proliferation along with decreased intracellular cGMP. These cold-induced changes were prevented by TNFshRNA.
Conclusions
Upregulation of TNF-α played a critical role in the pathogenesis of cold-induced PH by promoting pulmonary macrophage infiltration and inflammation. AAV delivery of TNFshRNA may be an effective therapeutic approach for cold-induced PH and PA remodeling.
Keywords: macrophage infiltration, inflammation, pulmonary hypertension, remodeling, smooth muscle cell proliferation
Introduction
The adverse effects of cold temperatures on the systemic circulation are well documented.1–4 However, whether cold exposure affects the pulmonary circulation is largely unexplored. Lungs are open to the environment and are susceptible to cold air stimulation. Clinical studies suggest that inhaled cold air causes pathophysiological responses such as vasoconstriction in the respiratory tract mucosa which may contribute to cold-related respiratory diseases.5 Exposure to cold temperatures is an important public health concern, particularly for those dying from cardiorespiratory diseases.6 In the US, cold weather is associated with increased mortality from pulmonary and cardiovascular diseases.2, 7–8 Cold exposure causes pulmonary vasospasm in patients with Raynaud’s phenomenon.9 Cold weather provokes respiratory systems.10–12 Cold air causes pulmonary vascular responses.13 We recently showed that exposure to cold increased pulmonary arterial (PA) pressure in rats, namely cold-induced pulmonary hypertension (CIPH).14 Prolonged elevation of PA pressure is detrimental to the pulmonary vascular system and the right heart. However, the negative effect of cold temperature on the pulmonary circulation is poorly studied and the underlying mechanism is largely unknown. The study of CIPH has important implications for people who live in cold regions or in winter.
Tumor necrosis factor (TNF)-α is a pro-inflammatory cytokine that plays diverse roles in many pathological processes. TNF-α is produced largely by monocytes and macrophages in response to inflammatory stimuli.15–16 Upon release, TNF-α binds to its receptors which are present on nearly all cells including macrophages and can regulate immune function, apoptosis, autoimmunity, and fever induction.17 The receptors and ligands in the TNF-α family couple directly to signaling pathways that involve cell proliferation, survival and differentiation, where their rapid response is critical to coordinating the proliferation and protective functions of various cells.17 As such, TNF-α and its receptors have been the focus of intense biological research in order to understand their role in a variety of conditions including atherosclerosis, rheumatoid arthritis and autoimmune disease.
Preliminary studies in our lab indicate that cold exposure increases pulmonary TNF-α expression, lung macrophage infiltration, and PA blood pressure and causes PA remodeling. We hypothesized that inhibition of TNF-α would decrease cold-induced pulmonary macrophage infiltration and inflammation, PA remodeling, and PH.
Methods
For details, see the expanded methods section in the Online Supplemental Methods and Data.
Results
Exposure to cold increased RV pressure and upregulated TNF-α protein expression in PAs
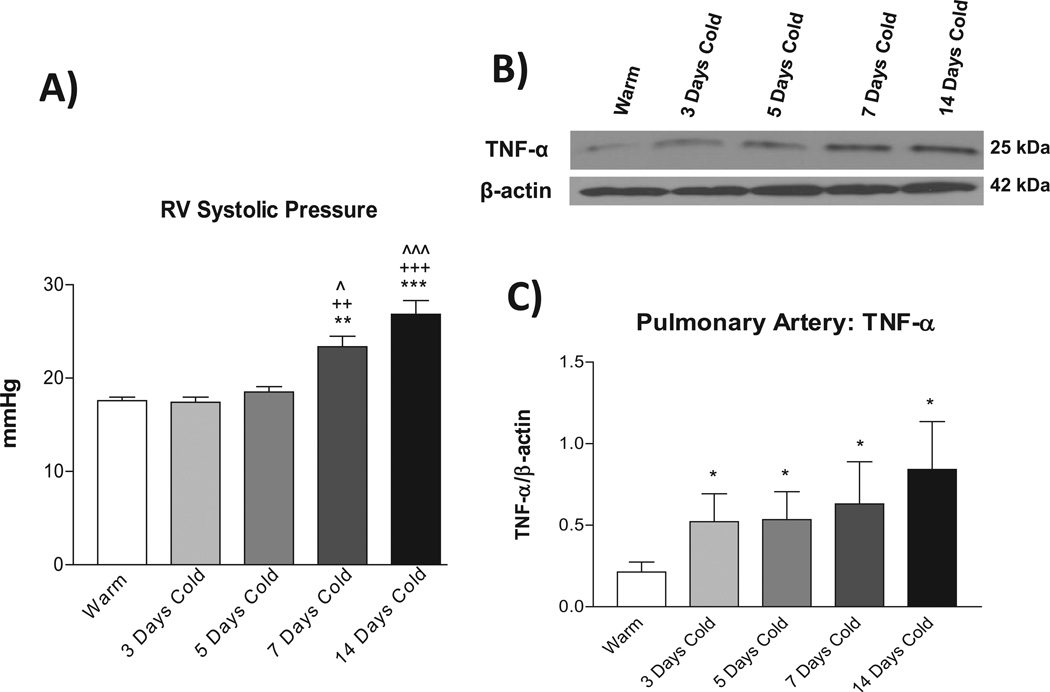
Exposure to moderate cold (5±0.1°C) significantly increased RV systolic pressure, a reliable indicator of PA pressure, 18–22 in a time-dependent manner (Fig. 1A). RV pressure started to elevate slightly at 5 days and reached a significant high level by 7 days after exposure to cold (Fig. 1A), indicating that cold exposure caused PH. In contrast, TNF-α protein expression in PAs was increased as early as 3 days after exposure to cold (Fig. 1B&C). Macrophage infiltration was also increased in the lungs and PAs from 3 days of exposure to cold (Supplemental Fig. S1). IL-6 protein expression in PAs was not increased until 14 days after exposure to cold (Fig. S2). Thus, cold exposure caused pulmonary inflammatory responses. Notably, TNF-α expression was upregulated right before elevation of PA pressure.
Figure 1. Time course of changes in right ventricle (RV) systolic pressure and TNF-α expression in PAs.
A) RV systolic pressure. B) Western blot bands of TNF-α protein expression in PAs. C) Quantification of TNFα protein expression. Data=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs. Warm; ++p<0.01, +++p<0.001 vs. 3 Days Cold; ^p<0.05, ^^^p<0.001 vs. 5 Days Cold. N=6–8.
TNFshRNA attenuated the cold-induced increase in RV pressure and RV hypertrophy
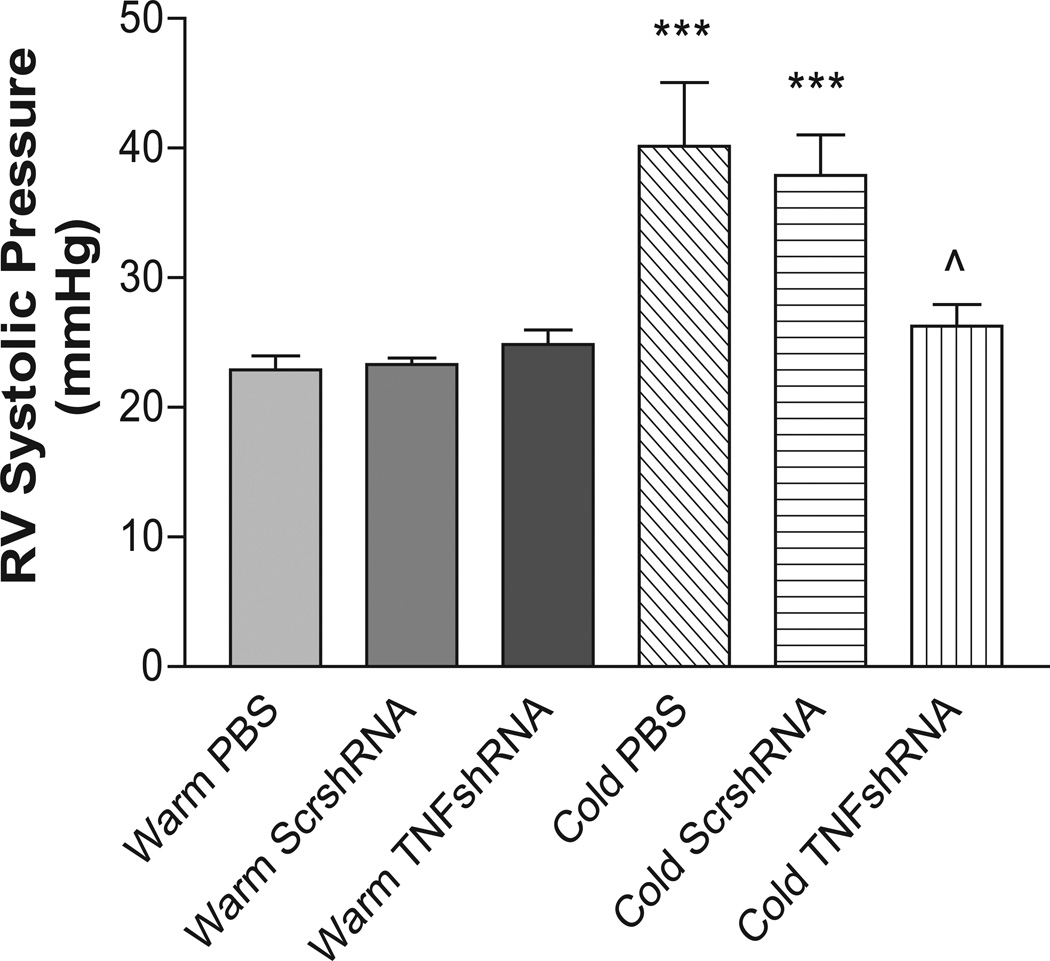
The recombinant AAV-2 carrying the rat TNFshRNA (AAV.TNFshRNA) and scrambled (AAV.ScrshRNA) were constructed as we described previously.23–24 RV systolic pressures in the Cold PBS (40.14±4.9 mmHg) and Cold ScrshRNA (37.91±3.1 mmHg) groups were elevated significantly vs. the control groups (Fig. 2, Fig. S3). Cold exposed animals treated with TNFshRNA, however, had a significantly decreased RV systolic pressure (26.28±1.6 mmHg) that was similar to the three warm groups. It is noted that one single injection of TNFshRNA prevented the cold-induced increase in RV pressure for up to 8 weeks (length of the study) (Fig. 2).
Figure 2. TNFshRNA attenuated the cold-induced increase in RV systolic pressure.
Eight weeks after i.v. delivery of AAV.TNFshRNA, AAV.ScrshRNA or PBS, the animals were anesthetized and RV pressure was recorded using telemetry. Data=means±SEM. ***p<0.001 vs. Warm PBS; ^p<0.05 vs. Cold PBS. N=6.
It seems the AAV constructs were mainly trapped in the pulmonary tissues following delivery via jugular veins because AAV.GFP was expressed in lungs but not in systemic tissues (aortas and kidneys) (Fig. S4). Gene delivery did not affect body weight gain in rats kept in either temperature environment (Fig. S5A), indicating that the AAV construct did not affect animals’ growth.
RV hypertrophy (RVH) is a hallmark of PH. Cold-exposed rats developed RVH as evidenced by a significant increase in the RV weight compared to the warm controls while TNFshRNA prevented RVH (Fig. S5B). Trichrome staining indicated that collagen was not detectable in the RV (Fig. S6). No obvious fibrosis was found.
TNFshRNA attenuated cold-induced PA remodeling
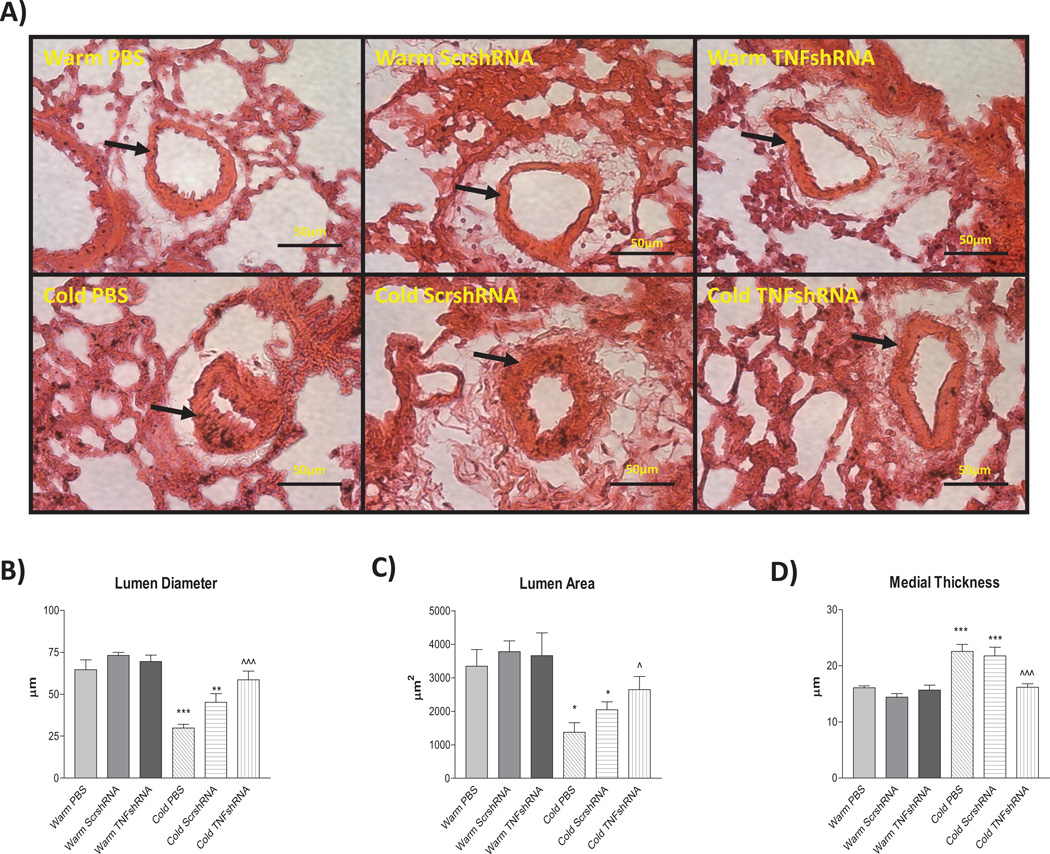
PA remodeling is common in almost all forms of PH. We examined small PAs in the lungs with a diameter of 50–80 µm, which is in line with the third order of PA branching (resistance PAs). The lumen diameter was significantly decreased in the Cold PBS (40.3±4.9µm) and Cold ScrshRNA (44.7±4.7µm) groups compared to Warm PBS (63.2±4.2µm), Warm ScrshRNA (72.4±6.1µm), and Warm TNFshRNA (70.4±4.6µm) (Fig. 3A&B). TNFshRNA prevented the cold-induced decreases in lumen diameter (57.4±3.6µm). Cold exposure also decreased lumen area, which can be partially rescued by TNFshRNA (Fig. 3A&C). We also measured the medial layer thickness which is primarily determined by proliferation of the smooth muscle cells. Cold PBS and Cold ScrshRNA groups had significant increases in medial layer thickness (23.3±0.8µm and 21.3±1.2µm, respectively) vs the Warm PBS (16.1±0.48µm), Warm ScrshRNA (14.8±0.44µm), and Warm TNFshRNA (15.8±0.7µm) groups (Fig. 3A&D). TNFshRNA prevented the cold-induced increase in medial layer thickness (15.93±0.7µm).
Figure 3. TNFshRNA attenuated cold-induced pulmonary artery remodeling.
A) Hematoxylin and eosin (H&E) staining was performed in paraffin embedded lung sections (5 µm). Morphometric analysis of third order pulmonary arteries (50–80 µm) was performed to evaluate the B) lumen diameter, C) lumen area and D) medial layer thickness. Data=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs. Warm PBS; ^p<0.05, ^^^p<0.001 vs. Cold PBS. Photos are shown at 400x. Arrows indicate pulmonary artery and scale bars are 50 µm. N=6.
TNFshRNA prevented the cold-induced increase in lung TNF-α expression
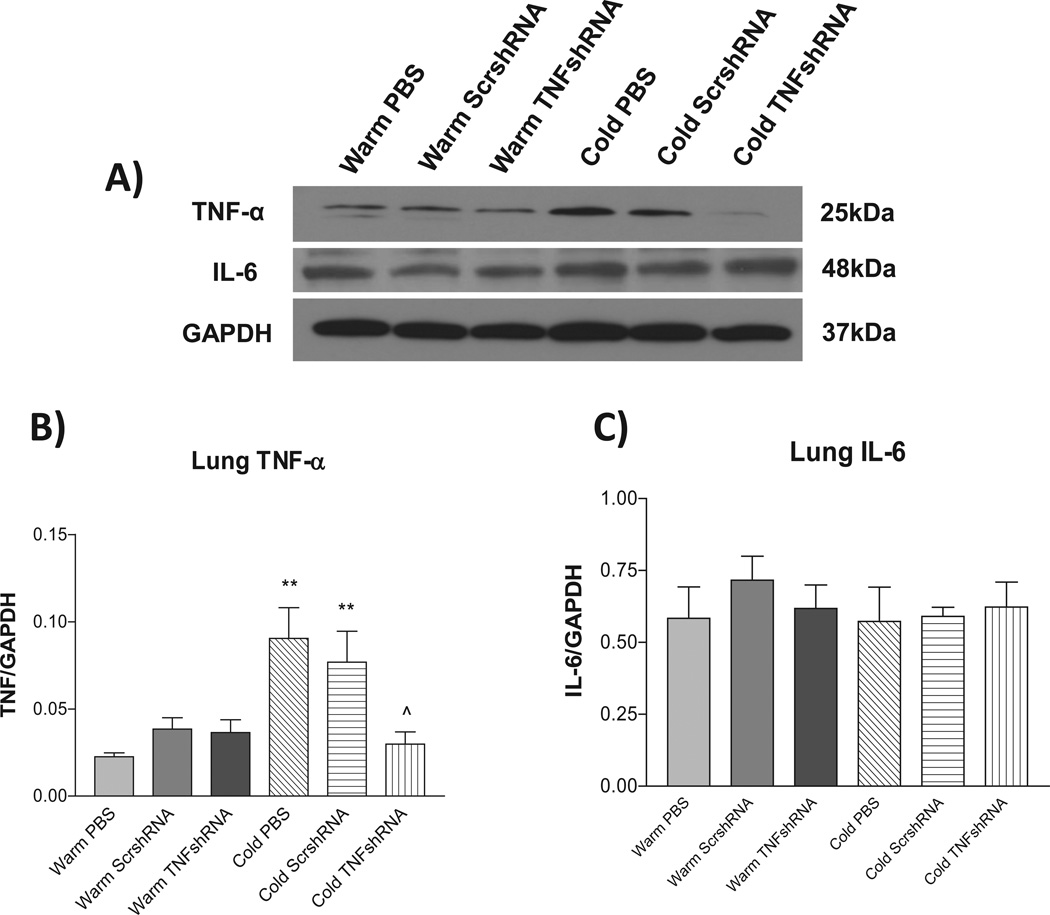
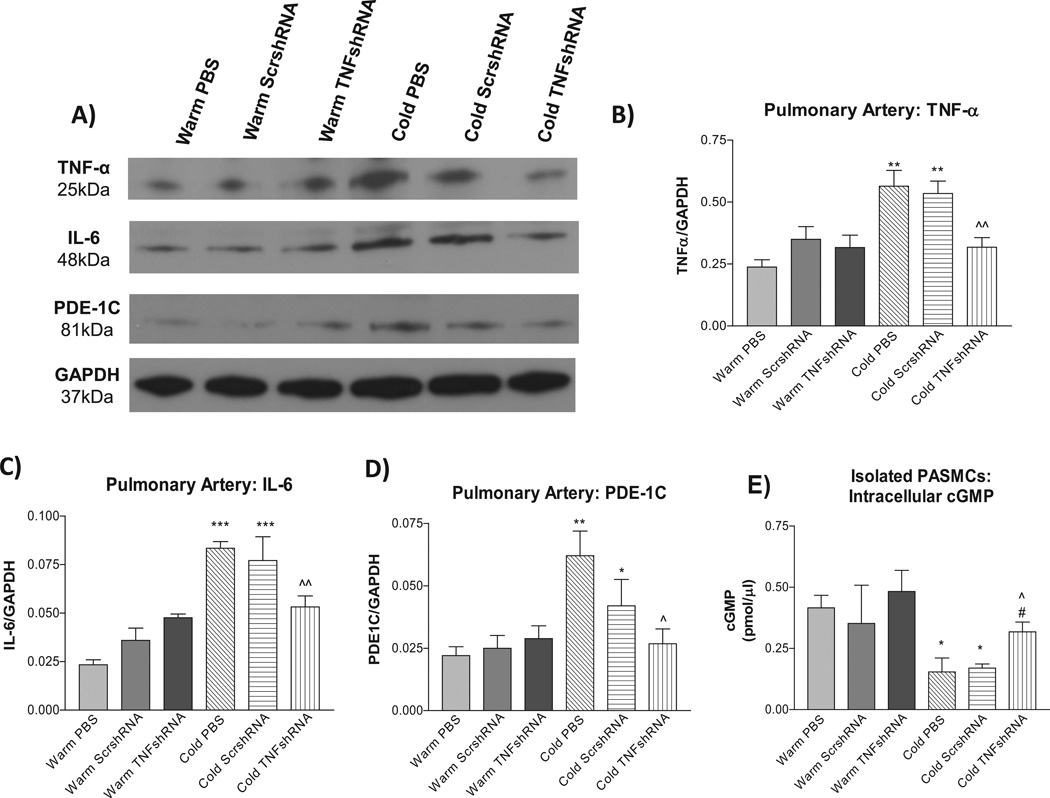
To evaluate the effectiveness of the AAV.TNFshRNA construct, we measured TNF-α protein expression in the lung. Cold PBS and Cold ScrshRNA showed significantly increased lung TNF-α protein expression compared to the three warm control groups (Figs. 4A&B) demonstrating that cold exposure upregulates TNF-α protein expression. TNFshRNA prevented the cold-induced increase in TNF-α mRNA and protein expression in the lung (Fig. 4A&B, Fig. S7), indicating effective in silencing of lung TNF-α. In addition, TNFαshRNA also prevented the cold-induced upregulation of plasma TNFα (Fig. S8).
Figure 4. TNFshRNA prevented the cold-induced increase in lung TNF-α expression.
Western blot was used to measure the lung protein expression of TNF-α and IL-6. A) Representative Western blot of TNF-α and IL-6 protein expression. B) Quantitative analysis of lung TNF-α expression and C) quantitative analysis of lung IL-6 expression. Data=means±SEM. **p<0.01 vs. Warm PBS; ^p<0.05 vs. Cold PBS. N=6.
Unexpectedly, cold exposure did not increase protein expression of lung IL-6 (a pro-inflammatory cytokine) significantly, and TNFshRNA did not have obvious effect on lung IL-6 expression (Fig. 4A and C).
TNFshRNA prevented the cold-induced increases in TNF-α, IL-6, and PDE-1C expression in PAs
TNF-α protein expression was increased in PAs of the Cold PBS and Cold ScrshRNA groups compared to the warm control groups (Fig. 5A and B). TNFshRNA prevented the cold-induced increase in TNF-α in PAs, indicating effective silencing of TNF-α. Contrary to IL-6 expression in the lung (Fig. 4A and C), IL-6 expression in the PAs was increased by cold exposure which can be prevented by TNFshRNA (Fig. 5A and C).
Figure 5. TNFshRNA prevented cold-induced increases in TNF-α, IL-6, and PDE-1C expression in pulmonary arteries (PAs).
Western blot was used to measure protein expression of pulmonary artery TNF-α, IL-6 and PDE-1C. A) Representative Western blots of TNF-α, IL-6 and PDE-1C protein expression. Quantitative analysis of B) TNF-α expression, C) IL-6 expression, and D) PDE-1C expression in PAs. E) TNFshRNA delivery increased intracellular cGMP in isolated PASMCs. A cGMP ELISA kit was used to measure intracellular cGMP in isolated PASMCs. Data=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs. Warm PBS; ^p<0.05, ^^p<0.01 vs. Cold PBS. #p<0.05 vs. Cold ScrshRNA. N=6.
It was reported that phosphodiesterase-1 (PDE-1) may be involved in the development of PH.25 We examined PDE-1 expression in PAs. Cold exposure increased PDE-1C protein expression (Fig. 5A and D), but did not affect PDE1-A or PDE1-B expression (not shown), in PAs compared to the warm controls. Interestingly, TNFshRNA prevented the cold-induced increase in PDE-1C expression in PAs.
PDE-1C hydrolyzes the second messenger cGMP, a vasodilator in the pulmonary circulation. We measured the intracellular level of cGMP in isolated PASMCs. PASMCs isolated from Cold PBS and Cold ScrshRNA groups had a lower cGMP level compared to cells from the warm controls (Fig 5E). TNFshRNA almost abolished the cold-induced decrease in intracellular cGMP in PASMCs (Fig. 5E).
TNFshRNA prevented cold-induced infiltration of macrophages in the lungs
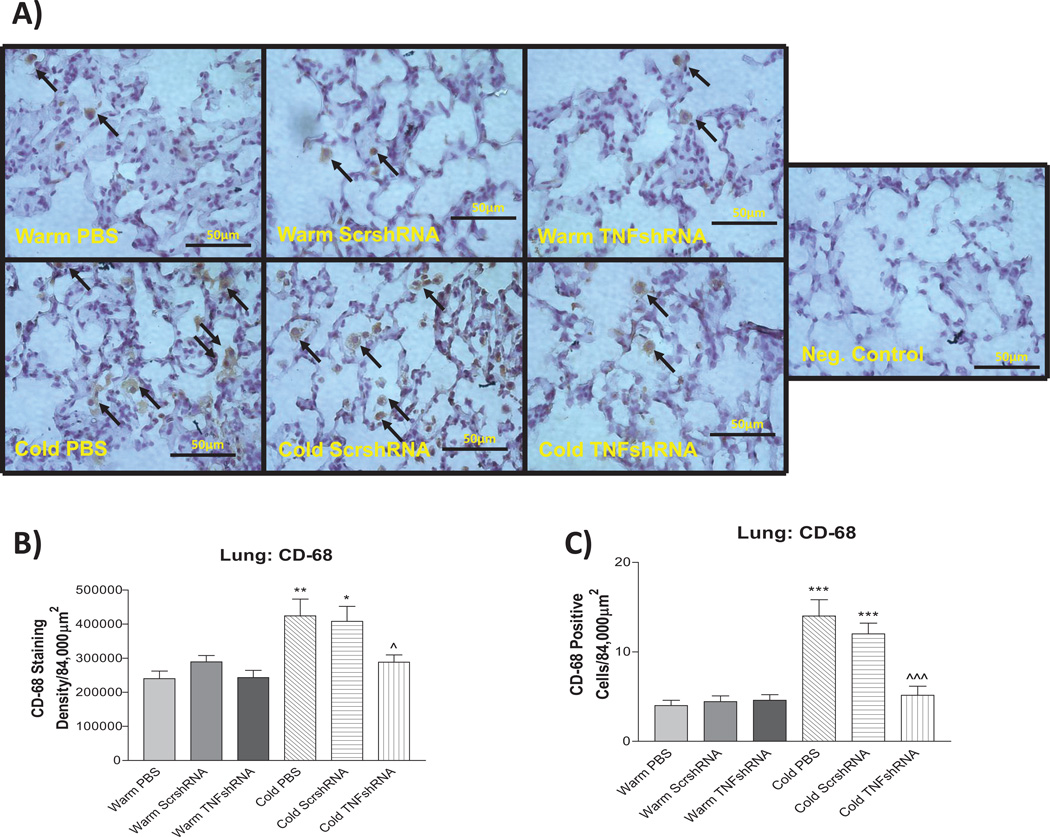
To further evaluate the relationship between cold exposure and inflammation, we examined the macrophage infiltration in the lungs using a CD-68 marker. Cold exposure clearly increased both the density of CD-68 staining and the actual count of CD-68+ cells in the Cold PBS and Cold ScrshRNA groups compared to the warm controls (Fig. 6A–C), indicating that cold exposure increased macrophage infiltration in the lungs. TNFshRNA prevented the cold-induced increases in pulmonary macrophage infiltration (Fig. 6A–C).
Figure 6. TNFshRNA prevented cold-induced infiltration of macrophages in the lungs.
A) IHC analysis of infiltration of macrophages in paraffin embedded lung sections (5µm) using anti-CD-68 primary antibody. B) Densitometry analysis of the CD-68 staining and C) the actual count of CD-68 positive cells. Photos are shown at 400x. Arrows indicate positive staining (brown). Scale bars indicate 50 µm. Data=means±SEM. *p<0.05, **p<0.01, ***p<0.001 vs. Warm PBS; ^p<0.05, ^^^p<0.001 vs. Cold PBS. N=3.
TNFshRNA prevented the cold-induced increase in superoxide in isolated PASMCs
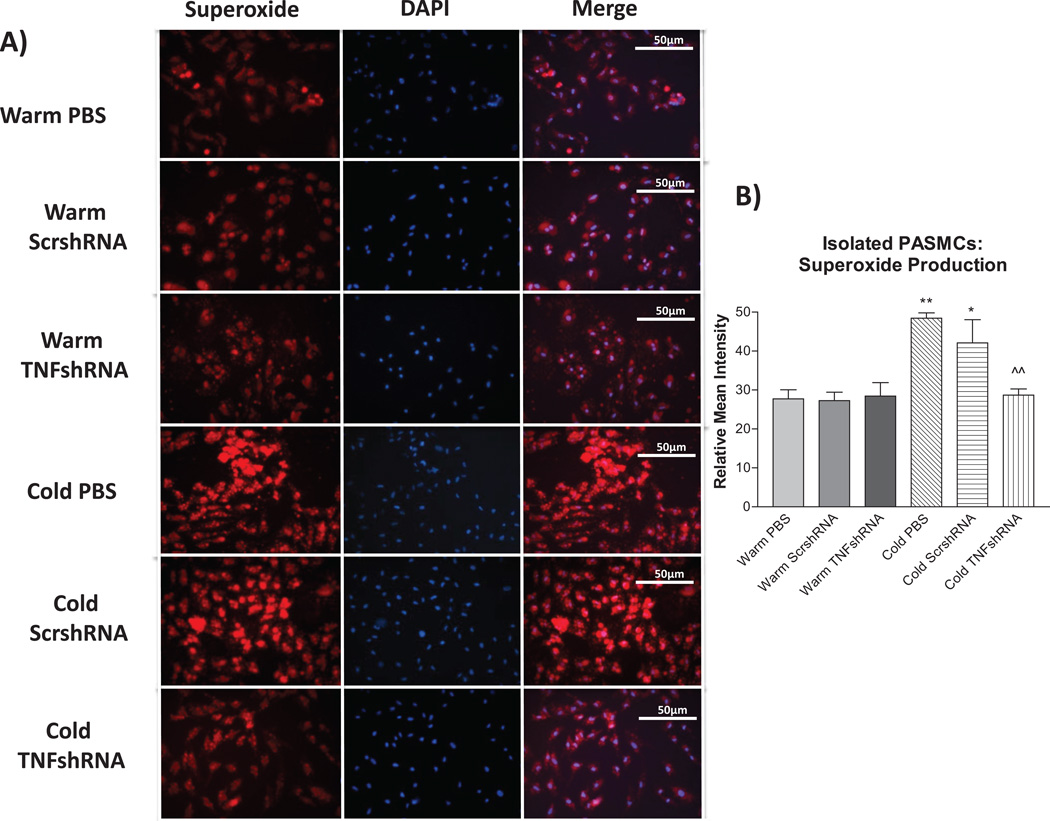
The relationship between TNF-α, superoxide and cold exposure is unclear. We measured superoxide levels in freshly-isolated PA smooth muscle cells (SMCs) as described recently.26–27 Cold exposure significantly increased superoxide levels in PASMCs compared to the three warm control groups (Fig. 7 and B). TNFshRNA prevented the cold-induced increase in superoxide in PASMCs (Fig. 7A and B).
Figure 7. TNFshRNA prevented the cold-induced increase in superoxide in isolated PASMCs.
PASMCs were incubated with dihydroethidium (DHE) followed by DAPI. A) Photos showing representative superoxide production (red), nuclear staining (blue) and the merged images and B) quantification of superoxide production in PASMCs. Cells were viewed at 400×. Data=means±SEM. *p<0.05, **p<0.01 vs. Warm PBS; ^p<0.05, ^^p<0.01 vs. Cold PBS.
TNFshRNA prevented the cold-induced increase in PCNA expression
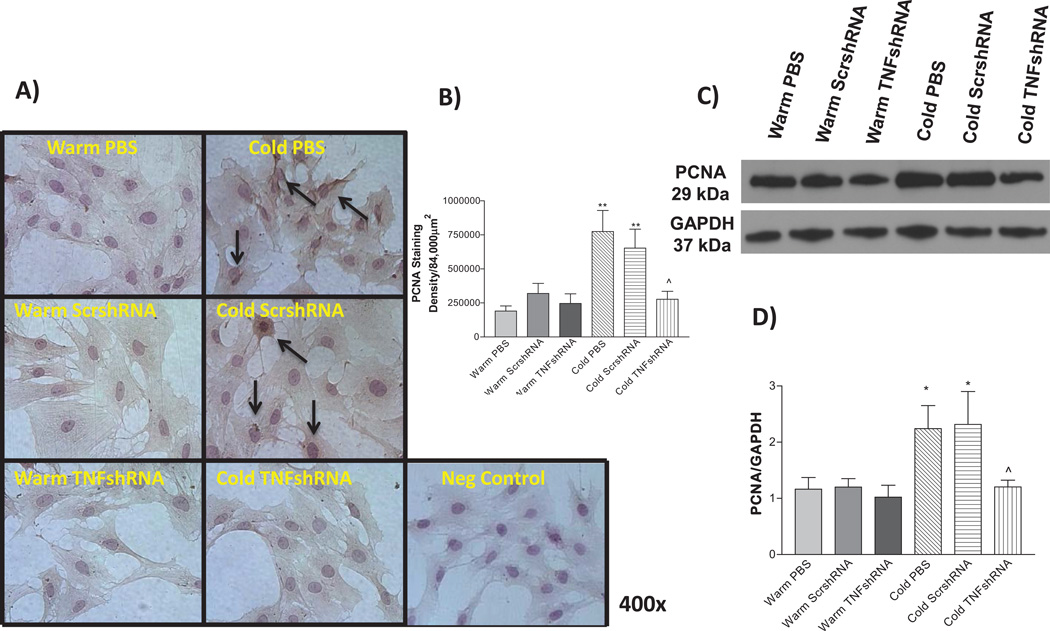
Proliferating cell nuclear antigen (PCNA) is expressed in the cell nuclei during DNA synthesis and replication and is thus considered as a marker of cell proliferation. We evaluated the expression of PCNA in isolated PASMCs using both ICC staining (Fig. 8 A and B) and Western blot (Fig 8C and D) analysis. PCNA expression was increased in the Cold PBS and Cold ScrshRNA groups compared to warm controls (Fig. 8A–D), suggesting increased PASMC proliferation. TNFshRNA prevented the cold-induced increase in PASMC proliferation (Fig. 8A–D).
Figure 8. TNFshRNA prevented the cold-induced increase in PCNA expression.
A) IHC analysis using isolated PASMCs. Cells were stained with an anti-PCNA primary antibody. B) Densitometry analysis of the IHC PCNA staining in PASMCs. C) Representative Western blot of PCNA staining and D) Quantitative analysis of PCNA expression, as measured by Western blot. Photos are shown at 400×. Arrows indicate positive PCNA staining (brown). Data=means±SEM. *p<0.05, **p<0.01 vs. Warm PBS; ^p<0.05 vs. Cold PBS. N=6.
Discussion
This study demonstrated, for the first time, that chronic exposure to cold upregulated TNFα expression and increased macrophage infiltration in PAs and lungs (Figs. 4–6, Fig. S1), indicating that cold exposure caused pulmonary inflammation. Cold-induced pulmonary inflammation was not the consequence of PH because the upregulation of TNF-α and macrophage infiltration preceded the elevation of PA pressure (Figs. 1 & S1). Interestingly, RNAi inhibition of TNF-α protein expression abolished cold-induced inflammation, PH, PA remodeling, and RV hypertrophy (Figs. 2–6, S5). Thus, these findings revealed a previously unidentified role of TNF-α in the pathogenesis of cold-induced PH (CIPH).
One single injection of TNFshRNA not only prevented the PA remodeling but also abolished the increase in PA blood pressure due to exposure to cold. The AAV2 vector we used to deliver TNFshRNA is an effective and non-pathogenic vector that has been used repeatedly to deliver therapeutic genes to the cardiovascular system for long-term control of cardiovascular disease.24, 27–28 Other investigators have shown that AAV2 is an efficient vector for delivering genes to pulmonary vessels (SMCs) and lungs for the treatment of PH29–31 AAV delivery of TNFshRNA prevented the cold-induced upregulation of TNFα mRNA and protein expression in lungs and PAs, indicating effective silencing TNFα (Fig. 4&5, Fig. S7). Indeed, TNFshRNA attenuated the cold-induced increase in TNFα levels in plasma (Fig. S8). Therefore, the results suggest that AAV-based RNAi silencing of TNF-α may be new preventive and therapeutic strategy for cold-related impairment of the pulmonary circulation.
The current experiment clearly showed that cold exposure caused PA remodeling. The major PA remodeling appeared to be the increase in medial layer thickness that is primarily composed of SMCs. PASMCs isolated from cold exposed animals had elevated protein expression of PCNA (Fig. 8), suggesting that cold exposure increased PASMC proliferation. The cold-induced PASMC proliferation may be due to upregulation of TNF-α and inflammation because RNAi silencing of TNF-α prevented the cold-induced increase in PCNA (Fig. 8). Proliferation of PASMCs results in PA hypertrophy and is a feature common to almost all forms of PH.32–34 PA remodeling can lead to narrowing or obliteration of the PAs and increase the PA resistance and the PA pressure.
The unique finding of this study is that cold exposure results in a robust inflammatory response characterized by increased levels of the pro-inflammatory cytokines TNF-α (lungs and PA), IL-6 (PA only), and lung macrophage infiltration. RNAi silencing of TNF-α effectively prevented the cold-induced increases in IL-6 expression and macrophage infiltration, suggesting that the upregulation of TNF-α plays a critical role in cold-induced inflammation. Tuder et al first identified inflammatory infiltrates in the plexiform lesions of PH patients in 1994 and other studies since have shown an increase in additional inflammatory mediators including intracellular adhesion molecules, vascular adhesion molecules, selectins and integrins.35–38 The monocrotaline-induced PH model also shows massive mononuclear infiltration.37 Therefore, inflammation is an important pathological mechanism of PH.39
Cold exposure significantly increased the lung macrophage infiltration (Fig. 6, S1). Macrophages play a vital role in regulation of the immune response through their ability to release inflammatory mediators, including growth factors and complement proteins, and are also vital for antigen presentation to lymphocytes.40–42 Macrophages are also a major source for the production of TNF-α in the lungs.43–44 The increase in lung macrophage infiltration due to cold exposure and the subsequent production of inflammatory mediators including TNF-α may further activate downstream inflammatory pathways in the lung contributing to the pathogenesis of PH. While this mechanism is not fully understood, inhibition of TNF-α may be preventing downstream activation of NF-κB, which is well accepted to induce both the innate and adaptive immune responses.45–47 Interestingly, macrophages are also the targets of TNF-α because TNFshRNA successfully prevented the cold-induced infiltration of macrophages in the lungs (Fig. 6).
Cold exposure increased superoxide levels in isolated PASMCs (Fig. 7). RNAi silencing of TNF-α abolished the cold-induced increase in superoxide production in PASMCs. Therefore, cold-induced increases in TNF-α in the pulmonary vasculature are likely contributing to the overproduction of superoxide in PASMCs. It is well established that increased superoxide production causes SMC proliferation and migration, two components that are vital in vascular remodeling pathologies. Furthermore, TNF-α is known to activate NADPH oxidase48, a major source of vascular superoxide. While there are several ways to block superoxide production in cells (including superoxide dismutase, diphenyl iodonium), this is the first report of viral-mediated TNF-α inhibition to suppress superoxide production in PASMCs.
The current study clearly demonstrated that phosphodiesterase-1C (PDE-1C) expression was increased in the PAs due to cold exposure and that TNF-α inhibition, an anti-inflammatory therapy, prevented the cold-induced PA PDE-1C expression (Fig. 5). While this study did not thoroughly investigate the relationship between TNF-α and PDE-1C, these findings suggest that inflammatory mediators, including TNF-α, may up-regulate PDE-1C expression in response to cold exposure. PDE-1C belongs to a family of enzymes that hydrolyze second messengers, including cGMP, a vital vasodilator and inhibitor of VSMC proliferation in the pulmonary circulation.49–50 The cold-induced decrease in cGMP may be contributing to PH in two ways; 1) decreased cGMP would decrease the vasodilatory response in PASMCs and 2) decreased cGMP would relieve its inhibitory effect on PASMC proliferation. The intracellular cGMP was decreased in PASMCs isolated from cold exposed animals which can be partially rescued by TNFshRNA. Therefore, the beneficial effects of TNF-α inhibition on CIPH, PASMC proliferation and PA remodeling may be mediated, at least in part, by attenuation of cold-induced upregulation of PDE-1C and downregulation of intracellular cGMP in pulmonary vascular cells.
Perspectives
The prevalence of pulmonary and cardiovascular diseases is increased in cold regions or in winter. Cold weather is associated with increased mortality and morbidity from pulmonary and cardiovascular diseases. Cold air causes pulmonary vasoconstriction and provokes cardiovascular complications. The current study yields two significant findings in the pathogenesis of cold-induced pulmonary hypertension. First, cold exposure significantly increased the expression of the pro-inflammatory cytokine TNF-α in the lungs and PAs. Second, inhibition of TNF-α attenuated the cold-induced increase in pulmonary arterial blood pressure, RV hypertrophy, PA remodeling, and pulmonary inflammation. These findings suggest that TNF-α may play a critical role in the pathogenesis of cold-induced pulmonary hypertension and that inhibition of TNF-α may be a potential therapeutic strategy for cold-induced pulmonary vascular disorders.
Supplementary Material
Novelty and Significance.
What is new?
It is new and interesting that prolonged exposure to cold temperatures increased TNF-α protein expression in the lungs and pulmonary arteries (PAs) causing inflammation and leading to pulmonary remodeling and hypertension.
This study demonstrates, for the first time, that in vivo AAV-based RNAi inhibition of TNF-α attenuated cold-induced right ventricle hypertrophy (RVH) as well as cold-induced increases in lung macrophage infiltration. These findings reveal a previously unidentified role of TNF-α in cold-induced lung inflammation and PH.
What is relevant?
-
It is significant that inhibition of TNF-α attenuated cold-induced pulmonary hypertension, RVH, and PA remodeling which provides a new therapeutic approach for the management of cold-induced pulmonary hypertension (CIPH).
This study addresses an important role of TNF-α in CIPH which is a public health concern but remains poorly understood.
Summary
Inhibition of TNF-α attenuated CIPH and reversed cold-induced PA remodeling by suppression of inflammation in the pulmonary system and by decreasing superoxide production in the PA smooth muscle cells, which suggests that upregulation of TNF-α protein expression may be involved in the pathogenesis of CIPH.
Acknowledgments
Source of Funding
This work was supported by NIH R01 HL116863, HL105302, and HL102074 and AHA Predoctoral Fellowship 11PRE7830040.
This publication was made possible by NIH Grant Number 9P20GM104934-06 from the COBRE Program of the National Institute of General Medical Sciences.
Footnotes
Author Contributions
Conception and design: ZS; Performing experiments & Collecting data: PC, KC; Analysis and interpretation: ZS, PC, KC; Drafting the manuscript for important intellectual content: ZS, PC.
Disclosure
Nothing to disclose. The authors confirm that there are no conflicts of interest.
References
- 1.Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health. 1982;72:261–265. doi: 10.2105/ajph.72.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ. Health Perspect. 2002;110:859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata T, Ogihara T, Maruyama A, Mikami H, Nakamaru M, Naka T, Kumahara Y, Nugent CA. The seasonal variation of blood pressure in patients with essential hypertension. Clin Exp Hypertens. A. 1982;4:341–354. doi: 10.3109/10641968209060747. [DOI] [PubMed] [Google Scholar]
- 4.Gyllerup S. Cold climate and coronary mortality in Sweden. Int J Circumpolar Health. 2000;59:160–163. [PubMed] [Google Scholar]
- 5.Mourtzoukou EG, Falagas ME. Exposure to cold and respiratory tract infections. Int J Tuberc Lung Dis. 2007;11:938–943. [PubMed] [Google Scholar]
- 6.Carder M, McNamee R, Beverland I, Elton R, Cohen GR, Boyd J, Agius RM. The lagged effect of cold temperature and wind chill on cardiorespiratory mortality in Scotland. Occup Environ Med. 2005;62:702–710. doi: 10.1136/oem.2004.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keatinge WR, Donaldson GC, Bucher K, Jendritzky G, Cordioli E, Martinelli M, Katsouyanni K, Kunst AE, McDonald C, Nayha S, Vuori I. Winter mortality in relation to climate. Int J Circumpolar Health. 2000;59:154–159. [PubMed] [Google Scholar]
- 8.Keatinge WR. Winter mortality and its causes. Int J Circumpolar Health. 2002;61:292–299. doi: 10.3402/ijch.v61i4.17477. [DOI] [PubMed] [Google Scholar]
- 9.Barr WG, Fahey PJ. Reduction of pulmonary capillary blood volume following cold exposure in patients with Raynaud's phenomenon. Chest. 1988;94:1195–1199. doi: 10.1378/chest.94.6.1195. [DOI] [PubMed] [Google Scholar]
- 10.Davis MS, Malayer JR, Vandeventer L, Royer CM, McKenzie EC, Williamson KK. Cold weather exercise and airway cytokine expression. J Appl Physiol. 2005;98:2132–2136. doi: 10.1152/japplphysiol.01218.2004. [DOI] [PubMed] [Google Scholar]
- 11.Giesbrecht GG. The respiratory system in a cold environment. Aviat Space Environ Med. 1995;66:890–902. [PubMed] [Google Scholar]
- 12.Koskela HO. Cold air-provoked respiratory symptoms: the mechanisms and management. Int J Circumpolar Health. 2007;66:91–100. doi: 10.3402/ijch.v66i2.18237. [DOI] [PubMed] [Google Scholar]
- 13.Greenlees KJ, Tucker A, Robertshaw D, Vader CR. Pulmonary vascular responsiveness in cold-exposed calves. Can J Physiol Pharmacol. 1985;63:131–135. doi: 10.1139/y85-023. [DOI] [PubMed] [Google Scholar]
- 14.Crosswhite P, Sun Z. Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension. 2013;61:585–592. doi: 10.1161/HYPERTENSIONAHA.111.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung cell Mol Physiol. 2001;280:L39–L49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, Potter BJ, Zhang C. Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242–H2249. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 18.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Va c Biol. 2005;25:2335–2342. doi: 10.1161/01.ATV.0000186184.33537.48. [DOI] [PubMed] [Google Scholar]
- 19.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahara N, Kai H, Niiyama H, Mori T, Sugi Y, Takayama N, Yasukawa H, Numaguchi Y, Matsui H, Okumura K, Imaizumi T. Repeated gene transfer of naked prostacyclin synthase plasmid into skeletal muscles attenuates monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Hum Gene Ther. 2004;15:1270–1278. doi: 10.1089/hum.2004.15.1270. [DOI] [PubMed] [Google Scholar]
- 21.van Albada ME, Schoemaker RG, Kemna MS, Cromme-Dijkhuis AH, van Veghel R, Berger RM. The role of increased pulmonary blood flow in pulmonary arterial hypertension. Eur Respir J. 2005;26:487–493. doi: 10.1183/09031936.05.00015405. [DOI] [PubMed] [Google Scholar]
- 22.Zhang TT, Cui B, Dai DZ, Tang XY. Pharmacological efficacy of CPU 86017 on hypoxic pulmonary hypertension in rats: mediated by direct inhibition of calcium channels and antioxidant action, but indirect effects on the ET-1 pathway. J Cardiovasc Pharmacol. 2005;46:727–734. doi: 10.1097/01.fjc.0000184470.58047.79. [DOI] [PubMed] [Google Scholar]
- 23.Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension. 2010;55:1484–1491. doi: 10.1161/HYPERTENSIONAHA.109.146902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Skelley L, Cade R, Sun Z. AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther. 2006;13:1097–1103. doi: 10.1038/sj.gt.3302768. [DOI] [PubMed] [Google Scholar]
- 25.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Wang Q, Sun Z. Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension. 2012;60:818–826. doi: 10.1161/HYPERTENSIONAHA.112.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Skelley L, Wang B, Mejia A, Sapozhnikov V, Sun Z. AAV-Based RNAi Silencing of NADPH Oxidase gp91(phox) Attenuates Cold-Induced Cardiovascular Dysfunction. Hum Gene Ther. 2012;23:1016–1026. doi: 10.1089/hum.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito T, Okada T, Mimuro J, Miyashita H, Uchibori R, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Sakata Y, Shimada K, Ozawa K. Adenoassociated virus-mediated prostacyclin synthase expression prevents pulmonary arterial hypertension in rats. Hypertension. 2007;50:531–536. doi: 10.1161/HYPERTENSIONAHA.107.091348. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101:734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami T, Kanazawa H, Satoh T, Ieda M, Ieda Y, Kimura K, Mochizuki H, Shimada T, Yokoyama C, Ogawa S, Tanabe T, Fukuda K. AAV-PGIS gene transfer improves hypoxia-induced pulmonary hypertension in mice. Biochem Biophys Res Commun. 2007;363:656–661. doi: 10.1016/j.bbrc.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B, Shen M, Xu M, Liu LL, Luo Y, Xu DQ, Wang YX, Liu ML, Liu Y, Dong HY, Zhao PT, Li ZC. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators of inflammation. 2012;2012:840737. doi: 10.1155/2012/840737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Okawa-Takatsuji M, Aotsuka S, Fujinami M, Uwatoko S, Kinoshita M, Sumiya M. Up-regulation of intercellular adhesion molecule-1 (ICAM-1), endothelial leucocyte adhesion molecule-1 (ELAM-1) and class II MHC molecules on pulmonary artery endothelial cells by antibodies against U1-ribonucleoprotein. Clin Exp Immunol. 1999;116:174–180. doi: 10.1046/j.1365-2249.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 37.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 38.Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S, Humbert M. Inflammation in pulmonary arterial hypertension. Chest. 2012;141:210–221. doi: 10.1378/chest.11-0793. [DOI] [PubMed] [Google Scholar]
- 39.Sun Z. Platelet TLR4: a critical link in pulmonary arterial hypertension. Circ. Res. 2014;114:1551–1553. doi: 10.1161/CIRCRESAHA.114.303945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara N, Kobayashi K. Macrophages in inflammation. Current drug targets. Inflammation and allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 41.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews. Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Chen K, Lei H, Sun Z. Klotho Gene Deficiency Causes Salt-Sensitive Hypertension via Monocyte Chemotactic Protein-1/CC Chemokine Receptor 2-Mediated Inflammation. J. Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013101033. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sewing AC, Kantores C, Ivanovska J, Lee AH, Masood A, Jain A, McNamara PJ, Tanswell AK, Jankov RP. Therapeutic hypercapnia prevents bleomycin-induced pulmonary hypertension in neonatal rats by limiting macrophage-derived tumor necrosis factor-alpha. Am J Physiol. Lung cell Mol Physiol. 2012;303:L75–L87. doi: 10.1152/ajplung.00072.2012. [DOI] [PubMed] [Google Scholar]
- 45.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes & Develop. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013;24:433–442. doi: 10.1016/j.cytogfr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermohlen O, Bruning JC, Schutze S, Kronke M. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 49.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. J Am Soc Nephrol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 50.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.