Background: The role of NFIC and KLF4 and their interrelationship during dentinogenesis remain unclear.
Results: Our study establishes the Nfic-Klf4 dentin matrix protein 1 (Dmp1)-Dspp pathway in odontoblasts.
Conclusion: NFIC regulates KLF4 during dentinogenesis.
Significance: These data demonstrate the regulation of DSPP via NFIC-KLF4 axis.
Keywords: Dentin, Differentiation, Gene Regulation, Signaling, Tooth Development, DSPP, KLF5, NFIC, Dentinogenesis, Odontoblast
Abstract
Odontoblasts are a type of terminally differentiated matrix-secreting cells. A number of molecular mechanisms are involved in the differentiation of odontoblasts. Several studies demonstrated that Krüppel-like factor 4 (KLF4) promotes odontoblast differentiation via control of dentin sialophosphoprotein (DSPP). Because nuclear factor I-C (NFIC) is also known to control DSPP, we investigated the relationship between NFIC and KLF4 during odontoblast differentiation. Klf4 mRNA expression was significantly decreased in Nfic−/− pulp cells compared with wild type cells. In immunohistochemistry assays, dentin matrix protein 1 (Dmp1), and DSP protein expression was barely observed in Nfic−/− odontoblasts and dentin matrix. Nfic bound directly to the Klf4 promoter and stimulated Klf4 transcriptional activity, thereby regulating Dmp1 and DSPP expression during odontoblast differentiation. Nfic or Klf4 overexpression promoted mineralized nodule formation in MDPC-23 cells. In addition, Nfic overexpression also decreased Slug luciferase activity but augmented E-cadherin promoter activity via up-regulation of Klf4 in odontoblasts. Our study reveals important signaling pathways during dentinogenesis: the Nfic-Klf4-Dmp1-Dspp and the Nfic-Klf4-E-cadherin pathways in odontoblasts. Our results indicate the important role of NFIC in regulating KLF4 during dentinogenesis.
Introduction
Odontoblasts are a type of neural crest-derived matrix-secreting cells that are vital for dentin formation and mineralization (1). During odontoblast differentiation, regulation of dentin sialophosphoprotein (DSPP)2 expression is critical because DSPP is a representative odontoblast marker gene (2).
The nuclear factor I (NFI) family of site-specific transcription factors, encoded by four genes in vertebrates (termed Nfia, Nfib, Nfic, and Nfix), plays essential developmental roles in the transcriptional modulation of various cell types (3). NFI proteins contain a highly conserved NH2-terminal domain that mediates DNA binding and a dimerization domain is gene-specific (4). Disruption of the Nfic gene in mice leads to abnormal tooth roots that are predominantly caused by abnormal odontoblast differentiation and dentin formation during root formation (5–7). Previously, we suggested that Nfic transcriptionally activates DSPP expression (8).
The roles of Krüppel-like factor 4 (KLF4) have been extensively studied in many different physiological processes, including development, cytodifferentiation, and maintenance of normal tissue homeostasis (9). KLF4 is specifically expressed in the polarizing and elongating odontoblasts of the mouse incisor at embryonic day 16.5, and in the molar from embryonic day 18.5 to postnatal day 3 (10). Recently, KLF4 overexpression in human dental pulp cells led to down-regulate the cell proliferation rate and up-regulate odontoblast-related genes, such as dentin matrix protein 1 (DMP1), DSPP, and alkaline phosphatase (ALP) (11, 12). Therefore, NFIC and KLF4 are important regulators of odontoblast-related genes, but the interrelationship between NFIC and KLF4 during odontoblast differentiation remains unclear. We investigated the roles of NFIC and KLF4 and their relationship during odontoblast differentiation and dentin formation.
EXPERIMENTAL PROCEDURES
DNA Microarray
Total RNA from pulp cells of wild type (Nfic+/+) and Nfic−/− mice were sent to Origen Labs for DNA microarray analysis on the GeneChip Sample Cleanup Module (Qiagen, Valencia, CA). Expression data were analyzed using Affymetrix MicroArray Suite (version 5.0). Signal intensities of all probe sets were scaled to the target value of 500.
Primary Cell Culture and Cell Lines
The mandibles were removed from 17-day-old (P17) wild type and Nfic−/− mice, and primary pulp cell culture was conducted as described previously (6, 13). Briefly, after the incisors were dissected out, they were cracked longitudinally using a 27-gauge needle on a 1-ml syringe. Pulp tissues were then minced to explants and placed in 60-mm culture dishes (Nunc, Rochester, NY). Explants were weighed down with a sterile cover glass and cultured in DMEM Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and antibiotics (100 units/ml, penicillin-G, 100 mg/ml streptomycin, and 2.5 mg/ml fungizone, Invitrogen). The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2, and cells at passage 2 were used in the experiments. All experiments using mice were approved by the Seoul National University Institutional Animal Care and Use Committee (SNU-111013-2).
MDPC-23 (a generous gift from Dr. J. E. Nör, School of Dental Medicine, University of Michigan, Ann Arbor, MI) and HEK293 cells (ATCC, Rockville, MD) were grown and maintained in DMEM supplemented with 10% FBS and antibiotics in a 5% CO2 atmosphere at 37 °C. Ameloblast lineage cells (ALC; a generous gift from Dr. T. Sugiyama, Akita University School of Medicine, Akita, Japan) were cultured in minimum essential medium (Invitrogen) supplemented with 5% FBS, 10 ng/ml recombinant human epithelial growth factor (EGF; Sigma-Aldrich), and antibiotics. To induce differentiation of MDPC-23 and ALC cells, 80–90% confluent cells were cultured in DMEM or minimum essential medium supplemented with 5% FBS, ascorbic acid (50 mg/ml), and β-glycerophosphate (10 mm) for up to 1 or 2 weeks.
Plasmid Constructs
The pCH-Nfic expression plasmid was provided by Dr. R. M. Gronostajski (State University of New York at Buffalo, Buffalo, NY) (14). siRNAs were synthesized (Integrated DNA Technologies, San Diego, CA) based on the chosen 19 nucleotides of Nfic (5′-CCG GTG AAG AAG ACA GAG A-3′), and these siRNA plasmids were prepared using the pSUPER-retro-neo-GFP retro virus siRNA expression vector (OligoEngine, Seattle, WA) according to the manufacturer's instructions. Klf4 cDNAs were amplified by PCR and subcloned into FLAG-tagged pcDNA3 vector (Invitrogen). The pcDNA3-FLAG-Slug, pGL2-Klf4, -Slug, and -E-cadherin plasmids were purchased from Origene Company (Rockville, MD). Deletion mutants of the Klf4 promoter regions were generated by PCR amplification and subcloned into pGL3 (Promega, Madison, WI).
Real-time PCR Analyses
Total RNA was extracted from MDPC-23 cells as well as pulp tissue using TRIzol® reagent according to the manufacturer's instructions (Invitrogen). Total RNA (2 μg) was reverse transcribed for 1 h at 50 °C with 0.5 mg oligo(dT) and 1 μl (50 international units) Superscript III enzyme (Invitrogen) in a 20-μl reaction. One microliter of the reverse transcription product was PCR-amplified using the primer pairs. For real-time PCR, the specific primers for Nfic, Snail, Slug, Klf4, vimentin, E-cadherin, N-cadherin, Dmp1, and Dspp were synthesized as listed in Table 1. Real-time PCR was performed on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Carlsbad, CA) using SYBR GREEN PCR Master Mix (Takara Bio Inc., Otsu, Shiga, Japan) according to the manufacturer's instructions. PCR conditions were 95 °C for 1 min, 94 °C for 15 s, and 60 °C for 34 s for 40 cycles. All reactions were run in triplicate and were normalized to the housekeeping gene GAPDH. Relative differences in PCR results were calculated using the comparative cycle threshold (CT) method.
TABLE 1.
Real-time PCR primer sequences
| Gene name | Primer |
|---|---|
| mNfic | |
| Forward | 5′-GAC CTG TAC CTG GCC TAC TTT G-3′ |
| Reverse | 5′-CAC ACC TGA CGT GAC AAA GCT C-3′ |
| mKlf4 | |
| Forward | 5′-CTG AAC AGC AGG GAC TGT-3′ |
| Reverse | 5′-GTG TGG GTG GCT GTT CTT TT-3′ |
| mSnail | |
| Forward | 5′-TCT GAA GAT GCA CAT CCG AAG C-3′ |
| Reverse | 5′-TTG CAG TGG GAG CAG GAG AAT-3′ |
| mSlug | |
| Forward | 5′-GGC TGC TTC AAG GAC ACA TTA GAA C-3′ |
| Reverse | 5′-GGT CTG CAG ATG TGC CCT CA-3′ |
| mE-cadherin | |
| Forward | 5′-CGT CCT GCC AAT CCT GAT GA-3′ |
| Reverse | 5′-ACC ACT GCC CTC GTA ATC GAA C-3′ |
| mVimentin | |
| Forward | 5′-AAA GCG TGG CTG CCA AGA AC-3′ |
| Reverse | 5′-GTG ACT GCA CCT GTC TCC GGT A-3′ |
| mN-cadherin | |
| Forward | 5′-CGC CAA TCA ACT TGC CAG AA-3′ |
| Reverse | 5′-TGG CCC AGT GAC GCT GTA TC-3′ |
| mDmp1 | |
| Forward | 5′-AGT GAAG TCA TCA GAA GAA AGT CA-3′ |
| Reverse | 5′-TAC TGG CCT CTG TCG TAG CC-3′ |
| mDspp | |
| Forward | 5′-ATT CCG GTT CCC CAG TTA GTA-3′ |
| Reverse | 5′-CTG TTG CTA GTG GTG CTG TT-3′ |
| mGapdh | |
| Forward | 5′-AGG TCG GTG TGA ACG GAT TTG-3′ |
| Reverse | 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′ |
Western Blot Analyses
To prepare whole cell extracts, cells were washed three times with PBS, scraped into 1.5-ml tubes, and pelleted by centrifugation at 12,000 rpm for 2 min at 4 °C. After removal of the supernatant, pellets were suspended in lysis buffer (50 mm Tris-Cl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, and 2 mm EDTA, pH 7.4) and incubated for 15 min on ice. Cell debris was removed by centrifugation. Proteins (30 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell BioScience, Dassel, Germany). Membranes were blocked for 1 h with 5% nonfat dry milk in PBS containing 0.1% Tween 20 (PBS-T) and incubated overnight at 4 °C with the primary antibody diluted in PBS-T buffer (1:1000). Rabbit polyclonal anti-Nfic and anti-DSP antibodies were produced as described previously (6). The mouse monoclonal anti-HA (MMS-101P) antibody was purchased from Covance (Emeryville, CA). Other antibodies, including E-cadherin (sc-7870), N-cadherin (sc-7939), TGFβ-RI (sc-398), Slug (sc-1539), and GAPDH (sc-25778), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal anti-E-cadherin (3195) antibody was purchased from Cell Signaling Technology (Danvers, MA). Antibodies against N-cadherin (ab12221) and Dmp1 (ab103203) were purchased from Abcam (Cambridge, MA). After washing, membranes were incubated for 1 h with anti-mouse (sc-2031), anti-rabbit (sc-2004), or anti-goat (sc-2768) IgG secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology). Labeled protein bands were detected using an enhanced chemiluminescence system (Dogen, Cambridge, MA). The quantification analyses were performed using ImageJ (http://imagej.nih.gov/ij/).
Transient Transfection and Luciferase Assays
HEK293 and MDPC-23 cells were seeded in 12-well culture plates at a density of 1.5 × 105 cells per well. Cells were transiently transfected with reporter constructs using Metafectene PRO reagent (Biontex, Planegg, Martinsried, Germany). pGL2-Klf4, -Slug, -E-cadherin, or pGL3-DSPP were transfected into cells with Nfia, Nfib, Nfic, Nfix, Klf4, or Slug vectors, or the Nfic siRNA vector. Also, cells were transiently transfected with Klf4 siRNA (Santa Cruz Biotechnology) using Lipofectamine RNAiMAX reagent (Invitrogen). Following the addition of luciferin (50 μl) to the cell lysate (50 μl), luciferase activity was determined using an analytical luminescence luminometer according to the manufacturer's instructions (Promega, Madison, WI).
Cells were transiently transfected with microRNA (miR) control and miR145 (Invitrogen) using Lipofectamine RNAiMAX reagent (Invitrogen). Following the addition of lysis buffer (100 μl) to the cell lysate, Western blot assay was performed.
Chromatin Immunoprecipitation (ChIP) Assays
After transfection with the indicated plasmid DNA using the metafectene Pro reagent (Biontex), MDPC-23 cells were treated with formaldehyde (1% final concentration) for 10 min at 37 °C, rinsed twice with cold PBS, and swollen on ice in lysis buffer (1% SDS, 10 mm EDTA, and 50 mm Tris-HCl (pH 8.1)) for 10 min. Nuclei were collected and sonicated on ice. Supernatants were obtained by centrifugation for 10 min and were diluted 10-fold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.1), and 167 mm NaCl). The fragmented chromatin mixture was incubated for 4 h with anti-Nfic, anti-Klf4, or anti-FLAG antibodies (F3165, Sigma-Aldrich, 1:250) on a rotator at 4 °C. Protein A/G PLUS-agarose (30 μl; sc-2003, Santa Cruz Biotechnology) was added and incubated at 4 °C for 1 h with rotation to collect the antibody/chromatin complex. The precipitated chromatin complexes were recovered and reversed according to the manufacturer's protocol (Upstate Biotechnology). The final DNA pellets were recovered and analyzed by PCR using the specific primers for Slug (300 bp), Klf4 (650 bp), or the E-cadherin promoter region (750 bp) listed in Table 2. The following PCR conditions were used: 94 °C for 30 s; 55 °C for 30 s; and 72 °C for 1 min for 35 cycles. PCR products were electrophoresed in 1% agarose gels, stained with ethidium bromide, and visualized under ultraviolet light.
TABLE 2.
The list for ChIP assay primer
| Promoter name | Primer |
|---|---|
| mSlug pro | |
| Forward | 5′-GAA GCC AAG TCG CCG TAG-3′ |
| Reverse | 5′-GGC GCC TCT GAA GTC ACC-3′ |
| mKlf4 pro | |
| Forward | 5′-CAT TGC AGC GCT CCT CTT C-3′ |
| Reverse | 5′-GGC TCG AAA GTC CTG CCA-3′ |
| mE-cad pro | |
| Forward | 5′-AAA AGG GAG CCG GTC AGG T-3′ |
| Reverse | 5′-AGG AGT CTA GCA GAA GTT CTT-3′ |
DNA Affinity Precipitation Assays
Transfected MDPC-23 cells were washed with ice-cold PBS, collected by centrifugation, and resuspended in RIPA buffer (50 mm Tris-Cl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, 1 mm PMSF, 1 mm Na3VO4, and 1 mm NaF) supplemented with protease inhibitors (Roche Molecular Biochemicals, Mannheim, Germany). Lysates were rotated on a rotating platform for 30 min at 4 °C and purified by centrifugation at 13,000 rpm for 5 min at 4 °C. Binding assays were performed by mixing nuclear extract proteins (600 μg) and biotinylated specific wild type or mutated Nfic binding site oligonucleotides (6 μg) (Cosmogenetech, Seoul, Korea) of Klf4 promoter in binding buffer (12% glycerol, 12 mm HEPES-NaOH (pH 7.9), 4 mm Tris-Cl (pH 7.9), 60 mm KCl, 1 mm EDTA, and 1 mm DTT). Mutated positions in the sequence are underlined. Lysates were incubated at room temperature for 30 min. Next, 60 μl of streptavidin-agarose beads (Thermo Scientific, Rockford, IL) were added. The mixture was incubated for 2 h at 4 °C with rotating. Beads were pelleted and washed three times with PBS. Nfic (wild type or mutant forms) bound to the oligonucleotides was detected by SDS-PAGE and immunoblotting using the mouse monoclonal anti-Nfic antibody.
Tissue Preparation and Immunohistochemistry
All experiments involving animals were performed according to the Dental Research Institute guidelines of Seoul National University. Mice tooth were decalcified in 10% EDTA (pH 7.4), embedded in paraffin, and processed for immunohistochemistry. Sections were incubated overnight at 4 °C with rabbit polyclonal Nfic, Klf4, E-cadherin, N-cadherin, Dmp1, and DSP as the primary antibodies (dilutions of 1:100–1:200). Secondary anti-rabbit or -mouse IgG antibodies were added to the sections for 30 min at room temperature and then reacted with the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA). Signals were converted using a diaminobenzidine kit (Vector Laboratories). Nuclei were stained with hematoxylin.
Alizarin Red S Staining
Cells were fixed for 20 min with 4% paraformaldehyde in PBS. Cell were stained with 1% alizarin red S (Sigma-Aldrich) solution in 0.1% NH4OH (pH 4.2) for 20 min at room temperature. Stained cells were collected by centrifugation at 13,000 rpm for 10 min at 4 °C. Cell lysates were solubilized by vortexing or pipetting with 0.5 ml of 5% SDS in 0.5 N HCl for 30 min at room temperature. Solubilized stain (0.1 ml) was transferred to the wells of a 96-well plate, and absorbance was measured at 405 nm.
Fluorescence Microscopy
Cells in Laboratory-Tek chamber slides (Nunc) were washed with PBS, fixed for 10 min at room temperature with 4% paraformaldehyde in PBS, and permeabilized for 5 min in PBS containing 0.5% Triton X-100. After washing and blocking, cells were incubated for 1 h with anti-Nfic (1:200) and Alexa Fluor® 488 Phalloidin (A12379, Invitrogen) antibodies in blocking buffer (PBS and 1% bovine serum albumin), followed by the addition of a fluorescein isothiocyanate (FITC) or Cy3-conjugated anti-rabbit IgG antibody (1:200, Amersham Biosciences). After washing, cells were visualized using fluorescence microscopy (AX70; Olympus Optical Co., Tokyo, Japan). Chromosomal DNA in the nucleus was stained using DAPI.
Statistical Analyses
Statistical analyses were performed using Student's t tests. Statistical significance is denoted (*, p < 0.01). All statistical analyses were performed using SPSS software (version 19.0; IBM Corp., Armonk, NY).
RESULTS
Differential Gene Expression Profiling in Nfic−/− Pulp Cells
Nfic−/− mice exhibited a lack of dentin formation by abnormal odontoblasts, which failed to express Dspp (6). To determine which genes cause abnormal differentiation of odontoblasts in Nfic−/− mice, we performed microarray analyses with pulp cells isolated from Nfic−/− and wild type mice. Among 190,230 probes on the BeadChip, 2,338 genes differed by factors of greater than 2-fold in pulp cells of Nfic−/− mice compared with wild type. Of these, 1,343 genes were up-regulated, whereas 995 genes were down-regulated. Among these genes, some are related to morphologic changes of the cells and are reported to impact odontoblast differentiation, including Snail, Fox, KLF, and TGF-β family genes (Table 3).
TABLE 3.
Summary of microarray analyses between WT and Nfic−/− primary pulp cells
| Gene symbol | GenBankTM accession no. | Gene function | Fold change |
|---|---|---|---|
| Snail1 | NM_011427 | Snail homolog 1 | 0.649 |
| Snail2 (Slug) | NM_011415 | Snail homolog 2 | 2.414 |
| Klf3 | NM_008453 | Kruppel-like factor 3 | 0.557 |
| Klf5 | NM_009769 | Kruppel-like factor 5 | 0.371 |
| Klf8 | NM_173780 | Kruppel-like factor 8 | 1.582 |
| Klf12 | AK012473 | Kruppel-like factor 12 | 4.845 |
| Tgfβ RI | NM_009370 | Transforming growth factor, β receptor I | 2.807 |
| Dmp1 | NM_016779 | Dentin matrix protein 1 | 0.115 |
Klf4 Is Down-regulated in Nfic−/− Odontoblasts
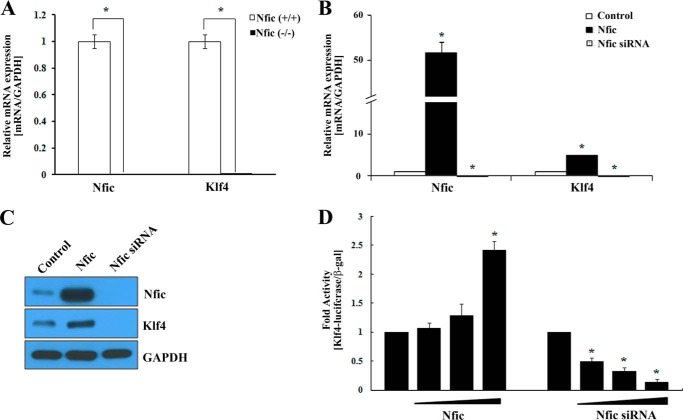
KLF5 belongs to the KLF family, which has several members that regulate epithelial-mesenchymal transition, including KLF4 (15), KLF8 (16), and KLF17 (17). In the microarray results, Klf5 showed a greater than 2-fold decrease in expression in Nfic−/− pulp cells compared with wild type. However, Klf4 was not identified (Table 3). Nevertheless, KLF5 and KLF4 have similarities and differences in regulating dental cell differentiation (10). In addition, KLF4 function during tooth development has been reported, whereas KLF5 has not (12). Therefore, we examined Klf4 mRNA expression in pulp cells from Nfic−/− and wild type mice using real-time PCR. Notably, Klf4 mRNA expression was significantly decreased in Nfic−/− pulp cells compared with wild type (Fig. 1A).
FIGURE 1.
The effect of Nfic on Klf4 expression in human dental pulp and MDPC-23 odontoblastic cells. A, Nfic and Klf4 expression was analyzed using real-time PCR in Nfic+/+ (wild type) and Nfic−/− primary pulp cells. An asterisk denotes values significantly different from the wild type (p < 0.01). B and C, expression of Nfic and Klf4 mRNA, and protein was analyzed by real-time PCR (B) and Western blotting (C) after transfection with Nfic-expressing or Nfic siRNA constructs in MDPC-23 cells. GAPDH was used as a whole cell loading control. D, Klf4 promoter activity was evaluated by luciferase assays in HEK293 cells transfected with Nfic-expressing or Nfic siRNA constructs. Data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01).
To investigate the functional consequences of Nfic-induced Klf4 expression, we determined the effect of Nfic on Klf4-mediated transcriptional activation. Nfic overexpression accelerated Klf4 mRNA and protein levels, whereas siRNA-mediated silencing of Nfic decreased Klf4 expression in odontoblastic MDPC-23 cells (Fig. 1, B and C). In addition, increasing concentrations of Nfic significantly enhanced expression of luciferase reporter genes under the control of the mouse Klf4 promoter. In contrast, depletion of Nfic using a specific siRNA suppressed promoter activity of the Klf4 reporter construct in 293 cells (Fig. 1D).
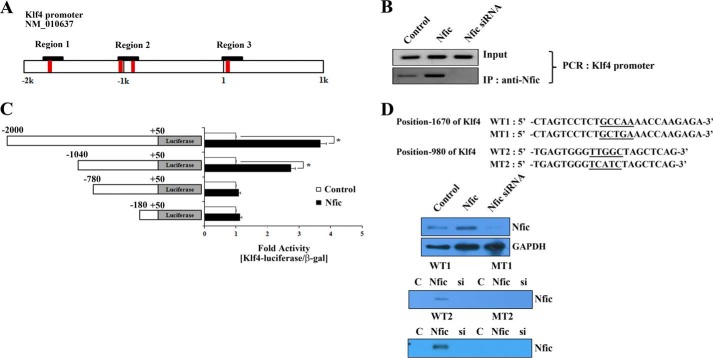
Nfic Is Recruited to the Klf4 Promoter in Odontoblasts
A computer search using the TESS program revealed four Nfic-binding sites, TTGGCXXXXXGCCAA regions, in the Klf4 promoter (Fig. 2A). Results were similar to those predicted by other programs. To investigate whether Nfic-mediated activation of the Klf4 promoter occurs through recruitment of Nfic to the endogenous Klf4 promoter, we performed ChIP assays with the primer containing region 2 of Klf4 promoter. The Klf4 promoter could be precipitated using an Nfic-specific antibody in Nfic-expressing MDPC-23 cells but not in Nfic-silenced MDPC-23 cells (Fig. 2B). These results suggest that Nfic has an important role in activating Klf4 transcriptional activity and expression in odontoblasts.
FIGURE 2.
Nfic binding to the Klf4 promoter and transcriptional activity of Klf4 in odontoblasts. A, a schematic illustration of the Nfic putative binding elements in the 2-kb mouse Klf4 promoter. B, Nfic binding to the Klf4 promoter was investigated using ChIP assays after transfection with Nfic-expressing or Nfic siRNA constructs in MDPC-23 cells. C, an Nfic-binding motif in the Klf4 promoter was identified by luciferase reporter assays in MDPC-23 cells co-transfected with Nfic-expressing constructs and truncated Klf4 promoter reporter constructs. Luciferase activity was normalized to β-gal. Data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01). D, the Nfic binding motif in the Klf4 promoter was confirmed by DNA affinity precipitation (DNAP) assays using extracts from MDPC-23 cells that had been transfected with Nfic-expressing or Nfic siRNA constructs. Biotinylated oligonucleotides corresponding to the Klf4 promoter (WT or mutated) or the −1670/−980 region of the Klf4 promoter were used as probes. A schematic illustration shows the sequence of the proximal mouse Klf4 promoter at the nucleotide −1670 and −980 regions. C, control; MT, mutant type.
To identify the responsive Nfic sites among the four putative sites, we isolated the Klf4 promoter, which is a region spanning to −2,000, using genomic DNA PCR. We created a series of deletion constructs in pGL3 and transiently transfected them into MDPC-23 cells in the presence or absence of Nfic-expressing constructs. Subsequent luciferase assays indicated that Nfic stimulated Klf4 promoter activity. Luciferase assays of the truncation mutants revealed that the region near −1,670 and −980 of Klf4 promoter were required for promoter activation. Therefore, the region near −1,670 and −980 appears to be involved in Nfic-mediated activation of the Klf4 promoter. However, the −980 region of Klf4 promoter was more important than the −1,670 region because the reporter construct without the −980 region exhibited severely diminished transcriptional activity compared with the reporter construct without the −1,670 region (Fig. 2C).
To confirm Nfic recruitment to the Klf4 promoter in vitro, we performed DNA affinity precipitation experiments using wild type- or mutant-biotinylated oligonucleotides corresponding to the −1,670/−980 region of the Klf4 promoter containing the Nfic-binding sites (Fig. 2D) and extracts from MDPC-23 cells that had been transfected with Nfic-expressing or Nfic siRNA constructs. In agreement with the luciferase assay data, the wild type −1,670/−980 region of the Klf4 promoter bound Nfic protein, but not Nfic proteins with mutations in these regions. In addition, the −980 region of Klf4 promoter recruited Nfic more strongly than the −1,670 region of the Klf4 promoter (Fig. 2D). These results suggest that these regions are important for Nfic recruitment to the Klf4 promoter in odontoblasts.
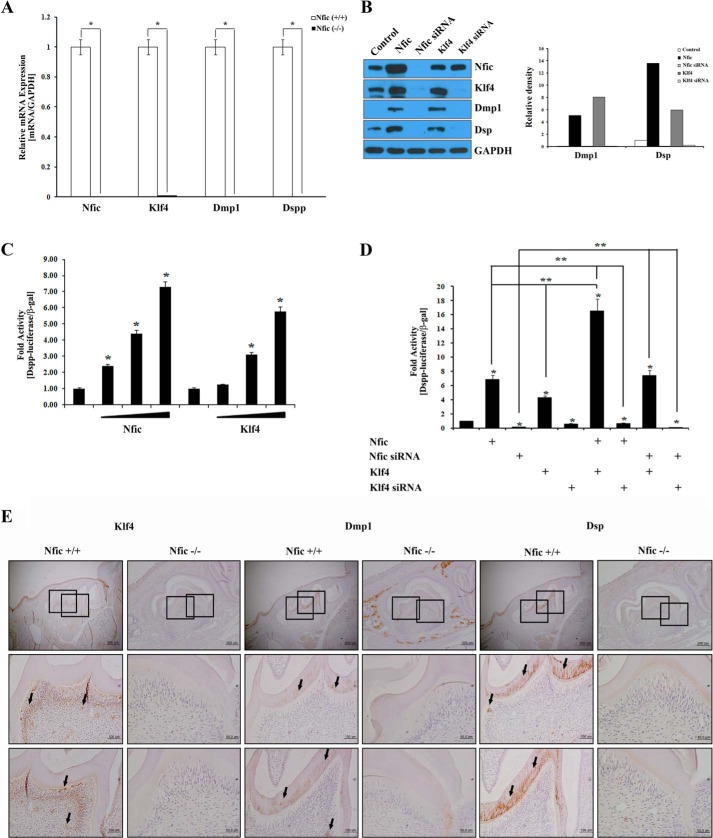
Nfic Cooperates with Klf4 to Regulate Dmp1 and Dspp in Odontoblasts
Mesenchymal cells express Dmp1 and Dspp in mouse incisors, which are important for odontoblast differentiation (18). We observed regulation of Klf4 mRNA and protein by Nfic in odontoblasts (Fig. 1). Therefore, we next examined Dmp1 and Dspp expression using real-time PCR in primary pulp cells from wild type and Nfic−/− mice. The expression levels of Klf4, Dmp1, and Dspp mRNAs were significantly decreased in the Nfic−/− primary pulp cells compared with wild type pulp cells (Fig. 3A). To confirm these findings, we analyzed the expression of their respective proteins using Western blotting in MDPC-23 cells that were transfected with Nfic-expressing, Nfic siRNA, or Klf4-expressing constructs or Klf4 siRNA. The data showed that Dmp1 and Dsp proteins were significantly increased in Nfic- or Klf4-expressing cells compared with control. However, Nfic or Klf4 inactivation by siRNA decreased Klf4, Dmp1, and Dsp protein expression (Fig. 3B).
FIGURE 3.
Enhanced Dmp1 and Dspp expression by the Nfic-Klf4 axis in human dental pulp cells and odontoblasts. A, Nfic, Klf4, Dmp1, and Dspp expression was analyzed by real-time PCR in Nfic+/+ (wild type) and Nfic−/− primary pulp cells. B, Nfic, Klf4, Dmp1, and DSP protein expression was analyzed by Western blotting (left panel) and its quantification (right panel) in MDPC-23 cells transfected with Nfic-expressing, Nfic siRNA, or Klf4-expressing constructs or Klf4 siRNA. GAPDH was used as a whole cell loading control. C and D, transcriptional activity of the Dspp promoter was evaluated by luciferase assays using Nfic-expressing, Nfic siRNA, or Klf4-expressing constructs, or Klf4 siRNA in MDPC-23 cells. Control samples were transfected with only Dspp promoter constructs. Nfic-expressing and Klf4-expressing constructs were transfected in dose-dependent manner (B; 0.1, 0.5, or 1 ug). Data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01); double asterisks denote values significantly different among the groups (p < 0.01). E, Klf4, Dmp1, and DSP protein expression (arrows) was detected by immunohistochemistry of Nfic+/+ (wild type) and Nfic−/− mice on postnatal day 14. Scale bars, 100 μm.
To investigate whether Nfic and Klf4 influence odontoblast differentiation by up-regulating Dspp, we measured Dspp promoter activity after transfection with Nfic- and Klf4-expressing constructs in MDPC-23 cells. Interestingly, Nfic and Klf4 increased Dspp promoter activity approximately ∼6–7-fold compared with control in a dose-dependent manner (Fig. 3C). Next, we investigated whether Nfic, acting as an upstream regulator of Klf4, is required for Klf4-mediated Dmp1-Dspp transcriptional regulation using a mouse Dspp-luciferase construct in MDPC-23 cells, which express quantifiable levels of Klf4. As expected, overexpression of Nfic or Klf4 significantly induced Dspp transcriptional activity in MDPC-23 cells (∼5–7-fold; lanes 2 and 4). Overexpression of Nfic and Klf4 showed a synergistic effect on Dspp transcriptional activity (16.5-fold; lanes 2 and 4 compared with lane 6). In contrast, when endogenous Nfic or Klf4 was suppressed by Nfic siRNA or Klf4 siRNA, activity of the Dspp promoter was disrupted (2–9-fold; lanes 3 and 5). Moreover, Klf4 facilitated Dspp promoter activity in Nfic-silenced cells (7.4-fold; lane 3 compared with lane 8), but not in Klf4-silenced cells (0.5-fold; lane 8 compared with lane 9, Fig. 3D). However, Nfic did not enhance Dspp promoter activity in Klf4-silenced cells (0.4-fold; lane 2 compared with lane 7), but in Klf4-overexpressed cells (16-fold; lane 2 compared with lane 6, Fig. 3D). These results suggest the Nfic-Klf4-Dmp1-Dspp cascade has an important role during odontoblast differentiation.
To confirm regulation of the Klf4-Dmp1-Dspp axis by Nfic in vivo, immunohistochemical staining was performed on tooth sections of 14-day-old Nfic−/− and wild type mice using polyclonal anti-Klf4, -Dmp1, and -Dsp antibodies. In postnatal day 14 molars, Klf4 protein was noticeably reduced in Nfic−/− odontoblasts and predentin compared with wild type. In wild type mice, Dmp1 and Dsp protein expression was observed predominantly in the dentin matrix and pulp cells, whereas their expression was barely detectable in Nfic−/− odontoblasts and the dentin matrix (Fig. 3E).
To gain insight into the expression pattern of Nfic and Dsp during odontoblast differentiation in vitro, MDPC-23 cells were grown in differentiation medium for 14 days. Nfic protein was expressed at the beginning of the culture, decreased from days 3 to 5, and increased from days 7 to 14. Dsp expression was detected on day 5 and continued to increase through day 14 during odontoblast differentiation (Fig. 4A). We also determined the effect of altered Klf4 expression on dentin mineralization. Alizarin red S staining was performed to evaluate mineralization nodules by culturing MDPC-23 cells in odontoblastic induction medium for 7 days. Overexpression of Nfic or Klf4 induced mineralized nodule formation on day 7 in MDPC-23 cells. Remarkable nodules were noted in the negative control group, whereas scarce nodules were observed in the Nfic siRNA transfected group (Fig. 4B). These results demonstrate that enforced expression of Nfic or Klf4 significantly promotes odontoblast differentiation and mineralization.
FIGURE 4.
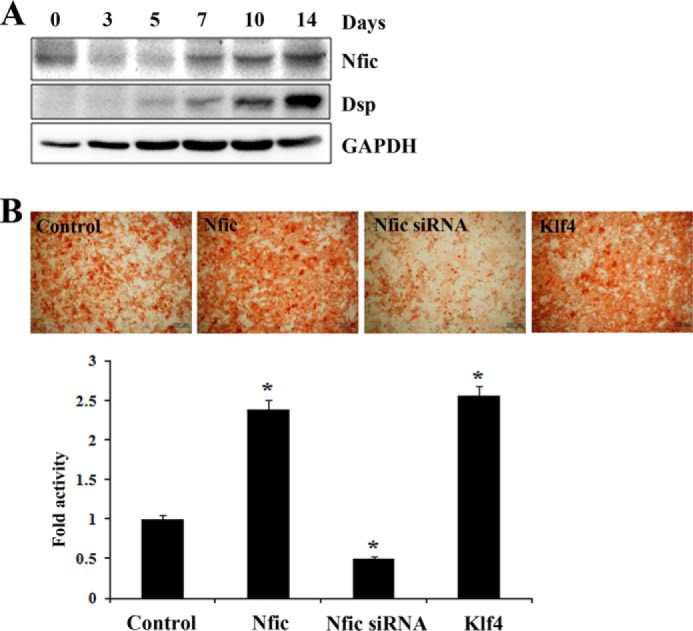
The effect of Nfic and Klf4 on odontoblast differentiation and dentin mineralization in vitro. A, Nfic and Dsp protein expression after MDPC-23 odontoblastic cell culture for 14 days in differentiation medium analyzed by Western blotting. B, the effect of Nfic and Klf4 on mineralized nodules formation in MDPC-23 cells 7 days after transfection of Nfic-expressing, Nfic siRNA, or Klf4-expressing constructs analyzed by alizarin red S staining. Quantification of mineralization was measured by colorimetric spectrophotometry. Data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01). Scale bars, 200 μm.
Nfic Is a Target for miR-145 in Odontoblasts
miR-145 decreased Dmp1 and Dspp expression by binding to the 3′-UTRs of Klf4 genes in mouse primary dental pulp cells and controlling odontoblast differentiation and dentin formation (19). We examined the effect of miR-145 on Nfic regulation. In silico assays revealed binding sites for miR-145 within the 3′-UTR of Nfic (Fig. 5A). In Western blot analyses, Nfic, Klf4, and DSP expression was decreased when miR-145 was enforced (Fig. 5B). These data demonstrate that miR-145 may bind to the 3′-UTR of the Nfic gene and decrease both Klf4 and Dsp expression. This suggests that miR-145 regulates cell differentiation via Nfic-Klf4 pathways.
FIGURE 5.
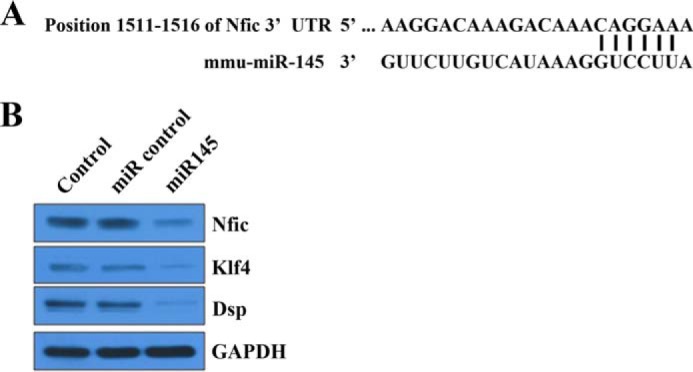
The regulation Nfic-Klf4 axis by miR-145 in odontoblasts. A, in silico assays reveal binding sites for miR-145 within the 3′-UTR of Nfic. B, NFIC, Klf4, and DSP protein expression was analyzed by Western blotting in MDPC-23 cells transfected with miR control or miR-145. mmu, M. musculus.
Nfic Maintains Epithelial Differentiation Status in Odontoblasts
The previous reports have shown the expression change of E-cadherin and N-cadherin in ameloblasts and odontoblasts during tooth development. In addition, these cells undergo actin rearrangement and cell shape change during amelogenesis and odontogenesis (20, 21). KLF4 regulated epithelial cell marker genes such as E-cadherin as a transcription factor, and then it induced epithelial cellular characters (22). To investigate the expression patterns of E-cadherin, N-cadherin, TGFβ-RI, TGF-β, and Nfic during ameloblast and odontoblast differentiation in vitro, we cultured ameloblastic ALC and odontoblastic MDPC-23 cells for 14 days and evaluated protein expression using Western blot analyses. Ameloblastic ALC and odontoblastic MDPC-23 cells showed opposite patterns in the expression of E-cadherin, N-cadherin, TGFβ-RI, TGF-β, and Nfic during their differentiation. During odontoblast differentiation, Nfic and E-cadherin expression gradually increased over time, whereas the expression of N-cadherin and TGF-β gradually decreased (Fig. 6A). We also investigated the correlation between cellular morphology and actin arrangement in odontoblasts in the presence or absence of Nfic. Parental MDPC-23 cells grow as dispersed cells and rarely formed clusters. In contrast, Nfic-expressing MDPC-23 cells formed tightly packed patches of epithelial sheets, were smaller than control cells, and had a nearly complete disappearance of central actin stress fibers with a transition to circumferential actin cables. The altered morphology and cytoskeletal modifications were reversible by siRNA-mediated inactivation of Nfic in MDPC-23 cells (Fig. 6B).
FIGURE 6.
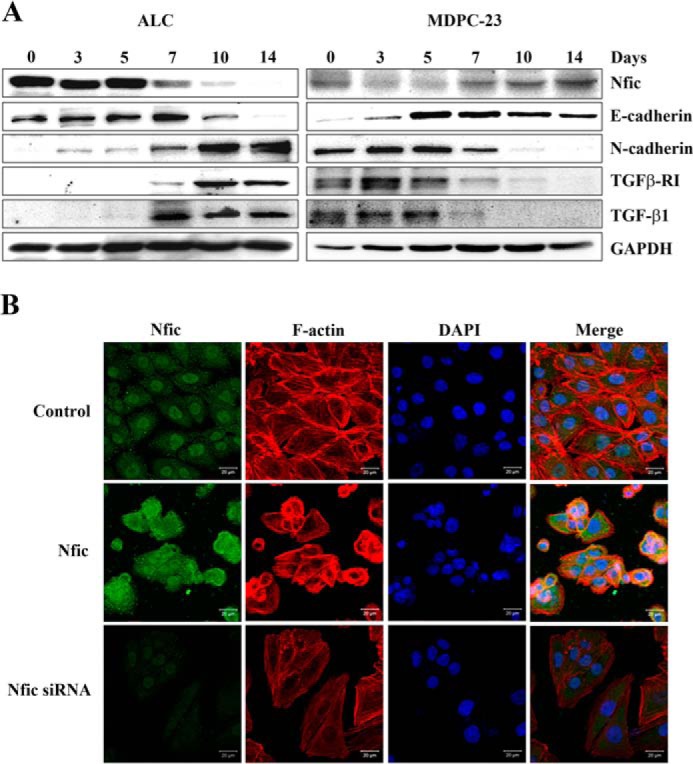
Expression pattern of Nfic, cadherins, and TGF-β signaling factors during ameloblast and odontoblast differentiation in vitro. A, protein levels of Nfic, E-cadherin, N-cadherin, TGFβ-RI, and TGF-β were determined by Western blotting in ALC (left panel) or odontoblastic MDPC-23 cell (right panel) cultures after 14 days in differentiation medium. GAPDH was used as a loading control. B, Nfic expression (green) and phalloidin staining (F-actin, red) with nuclei counterstained (blue) was detected by fluorescence microscopy in MDPC-23 cells transfected with Nfic-expressing or Nfic siRNA constructs. Scale bars, 50 μm.
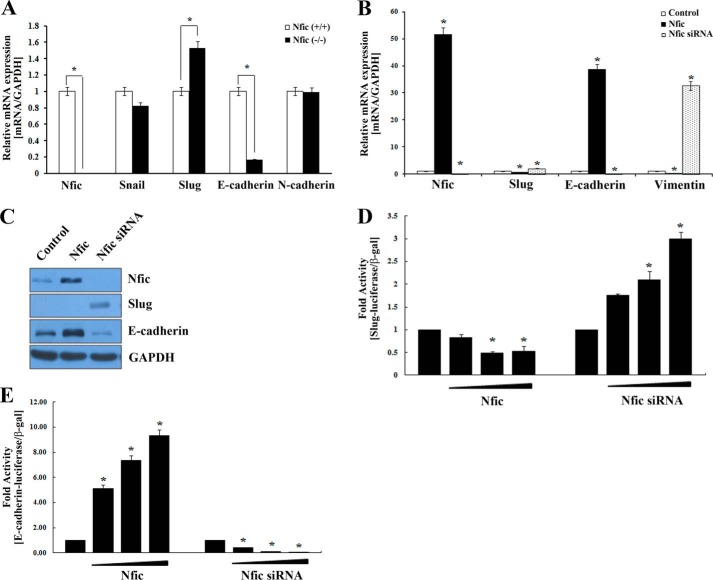
The Snail family members, Snail 1 and Slug, which encode zinc finger-type transcription factors, have been shown to be involved in neural crest formation and subsequent epithelial status (23, 24). Consistent with microarray data, Slug expression was significantly increased in Nfic−/− pulp cells compared with wild type, whereas E-cadherin expression was decreased (Fig. 7A).
FIGURE 7.
Effects of Nfic on Snail, Slug, E-cadherin, N-cadherin, vimentin, and F-actin in human dental pulp cells and odontoblastic MDPC-23 cells. A, Nfic, Snail, Slug, E-cadherin, and N-cadherin mRNA expression in Nfic+/+ (wild type) and Nfic−/− primary pulp cells analyzed by real-time PCR. B and C, expression of Nfic, Slug, E-cadherin, and vimentin mRNA (B) and protein (C) in MDPC-23 cells transfected with Nfic-expressing or Nfic siRNA constructs analyzed by real-time PCR and Western blotting, respectively. D, the transcriptional activity of Slug promoter was evaluated by luciferase assay in HEK293 cells transfected with Nfic-expressing or Nfic siRNA constructs. E, the transcriptional activity of E-cadherin promoter was evaluated by luciferase assay in HEK293 cells transfected with Nfic-expressing or Nfic siRNA constructs. Data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01).
A distribution of vimentin accompanies odontoblast polarization (25). Nfic overexpression decreased expression of Slug and vimentin, which play important roles in the polarization of odontoblasts, but enhanced E-cadherin expression, which plays an important role in cellular adhesion. However, E-cadherin expression was decreased after Nfic inactivation by siRNA (Fig. 7, B and C). Additionally, Nfic overexpression decreased Slug luciferase activity but augmented E-cadherin promoter activity, whereas siRNA-mediated Nfic inactivation increased Slug promoter activity but decreased E-cadherin promoter activity (Fig. 7, D and E).
Klf4 Is Recruited to the E-cadherin Promoter
A computer search using the TESS program revealed two Nfic-binding sites in the Slug promoter and four Nfic-binding sites in the E-cadherin promoter (Fig. 8A). To investigate whether Nfic could bind to the Slug or E-cadherin promoter, we performed ChIP assays. As shown in Fig. 8B, Nfic did not associate with the Slug or E-cadherin promoters. However, ChIP assays using a primer set for E-cadherin indicated that Klf4 proteins were recruited to the E-cadherin promoter after expression of either Klf4 or Nfic (Fig. 8C). Klf4 activated E-cadherin expression by binding the GC-rich/E-box region of the E-cadherin promoter, resulting in alterations in epithelial cell morphology and migration (26). Thus, Nfic is critical for recruitment to the Klf4 promoter, but not to the Slug and E-cadherin promoters, in vivo.
FIGURE 8.
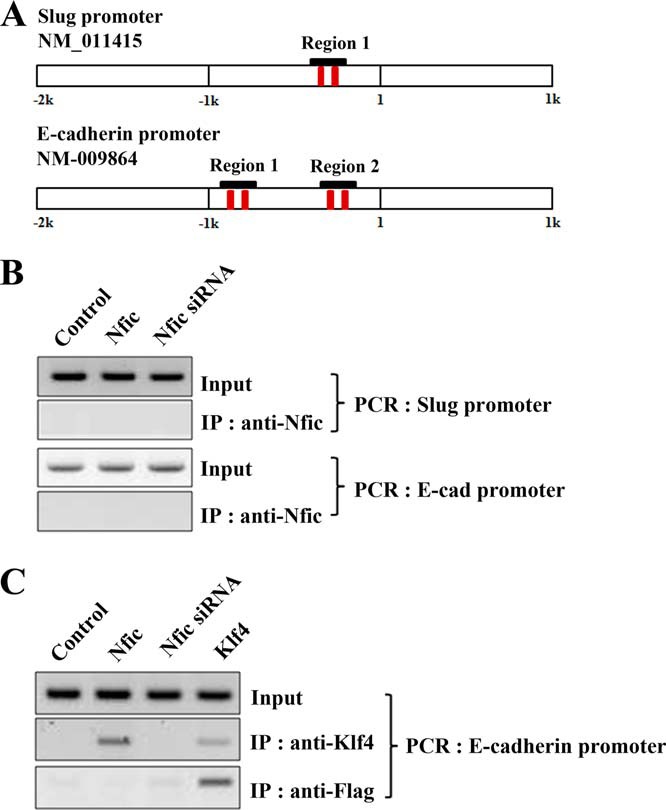
Klf4 binds the E-cadherin promoter. A, a schematic illustration of the putative Nfic binding elements in the 2-kb mouse Slug and E-cadherin promoters. B, Nfic binding to the Slug and E-cadherin promoter was investigated using ChIP analyses after transfection with Nfic-expressing or Nfic siRNA constructs in MDPC-23 cells. C, Klf4 binding to the E-cadherin promoter was investigated using ChIP analyses after transfection with the Nfic-expressing or Nfic siRNA, or Klf4-expressing constructs, in MDPC-23 cells.
Nfic and Nfix, but Not Nfia and Nfib, Influence Klf4 Transcription
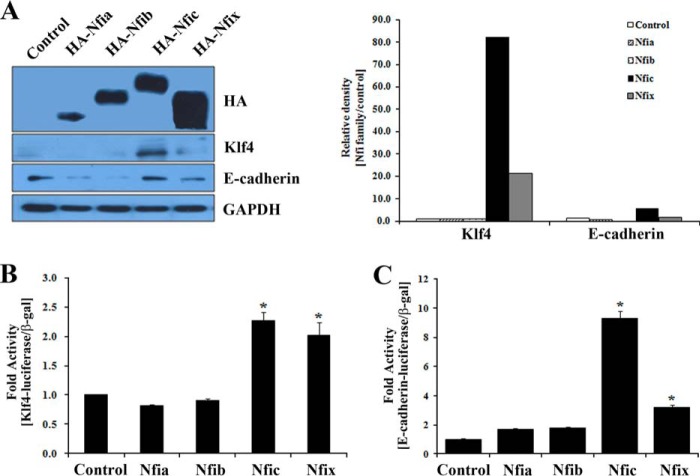
To investigate the function of the four NFI factors on the regulation of KLF4 and E-cadherin, we examined the effects of NFI family member overexpression on Klf4 and E-cadherin promoter activity. Expression of each of the NFI isoforms was confirmed by Western blotting with the anti-HA antibody after transfection. Nfic and Nfix overexpression significantly induced expression of Klf4 and E-cadherin in odontoblasts (Fig. 9A).
FIGURE 9.
Effects of NFI family members on the expression of Klf4 and E-cadherin. A, expression of HA, Klf4, and E-cadherin protein was analyzed by Western blotting (left panel) and its quantification (right panel) in MDPC-23 cells transfected with HA-tagged Nfia-, Nfib-, Nfic-, or Nfix-expressing constructs. B and C, the transcriptional activity of the Klf4 or E-cadherin promoters was evaluated by luciferase assay in HEK293 cells transfected with Nfia-, Nfib-, Nfic-, or Nfix-expressing constructs. The data are presented as the mean ± S.D. of triplicate experiments. An asterisk denotes values significantly different from the control (p < 0.01).
Next, expression vectors for Nfia, Nfib, Nfib, or Nfix were co-transfected with Klf4 or E-cadherin reporter constructs into MDPC-23 cells. Transfection of Nfic or Nfix constructs significantly enhanced the Klf4 and E-cadherin promoter activity, suggesting that the two isoforms function as transcriptional activators for the expression of Klf4 and E-cadherin. In contrast, transfection with Nfia or Nfib-expressing constructs did not noticeably activate Klf4 or E-cadherin (Fig. 9, B and C).
DISCUSSION
Tooth development involves a complex sequence of reciprocal epithelial-mesenchymal interactions. Dental epithelial cells from the dental organ differentiate into ameloblasts, whereas ectomesenchymal cells from the dental papilla differentiate into odontoblasts (27). Differentiating odontoblasts elongate, polarize, and produce dentin by synthesizing and secreting Dmp1 and DSPP, important markers of odontoblast differentiation (2, 18). Klf4 promotes odontoblast differentiation via up-regulation of Dmp1 by binding its promoter and Dmp1 subsequently induces Dspp expression during odontoblast differentiation (12, 19, 28, 29). These studies imply that Klf4-Dmp1-Dspp signaling controls odontoblast differentiation and dentin formation. In our study, we report that Nfic directly binds to the Klf4 promoter and transactivates its expression. Therefore, we suggest that the Nfic-Klf4-Dmp1-Dspp cascade controls odontoblast differentiation and dentin formation. This view is also supported by two other findings. First, Klf4, Dmp1, and Dsp protein expression was significantly decreased in Nfic−/− odontoblasts compared with wild type odontoblasts. In addition, Nfic or Klf4 overexpression promoted mineralized nodule formation in odontoblastic MDPC-23 cells. Dmp1 protein was expressed in the abnormal dentin matrix of Nfic−/− mice but was not expressed in the normal dentin matrix of wild type mice. This result is in agreement with our previous investigations that abnormal dentin is formed by aberrant odontoblasts in Nfic−/− mice that exhibit a bone-like phenotype, as observed the expression of Dmp1 in surrounding alveolar bone (6).
In our previous studies, Nfic−/− mice developed normal molar crowns but short molar roots that contain aberrant odontoblasts and abnormal dentin formation. Gross observations of the teeth of Nfic−/− mice revealed normal molar crowns, short molar roots, and thin incisors (14). However, in microscopic observations, the pulp side of a root forming molar of an Nfic−/− mouse was covered with polygonal, disorganized, and abnormal odontoblasts because ectomesenchymal pulp cells failed to differentiate into normal odontoblasts (5). An incisor from Nfic−/− mice also contained abnormal odontoblasts in the pulp, and some of the cells were trapped in osteodentin-like mineralized tissues (6). In this study, we have used pulp cells of Nfic−/− mice incisors for the experiments because we can easily obtain the more pulp cells form incisors than molars.
It was also reported that miR-143 and miR-145 control odontoblast differentiation via regulation of Klf4 and Osx transcription factors. miR-143 and miR-145 expression decreases during odontoblast differentiation in vitro and thus releases the expression of Klf4 and Osx regulating Dspp (19). Consistent with these results, in the present study, miR-145 decreased Nfic, Klf4, and Dsp expression in odontoblasts. It implies that miR-143 and miR-145 regulated Klf4 and Dsp via the control of Nfic, an upstream effector molecule of Klf4. Furthermore, we have shown that Nfic directly regulates Osx expression but acts downstream of the BMP2-Runx2 pathway in osteoblasts (30). These findings suggest that miR-143 and miR-145 also regulates odontoblast differentiation via the miR-143/145-Nfic-Klf4/Osx-Dspp pathway.
E-cadherin and N-cadherin, which are expressed during odontogenesis, are involved in the regulation of various biological processes such as cell recognition, intercellular communication, cell fate, cell polarity, boundary formation, and morphogenesis (31). Although not observed in vivo, E-cadherin is expressed in cultured pulp cells (32). In this study, odontoblastic MDPC-23 cells showed opposite patterns in the expression of Nfic/E-cadherin and TGF-β/N-cadherin. This result is in agreement with our previous investigations that demonstrate cross-talk between Nfic and TGF-β1 signaling regulates cell differentiation in odontoblasts (33).
During the cytodifferentiation stage, odontoblasts become highly complex, polarized secretory cells based on the appearance of a complex cytoskeleton (34). Differentiating odontoblasts establish palisade-like layers due to the development of junctional complexes containing tight junctions and adherens junctions (35, 36). In our previous study, Nfic expression strikingly alters the morphology and intercellular adhesion of odontoblasts (37). siRNA-mediated inactivation of Nfic also caused altered morphology and cytoskeletal modifications in MDPC-23 cells. These results suggest that Nfic induces the transition of cellular morphology by regulating the expression of Klf4, Slug, and E-cadherin in odontoblasts.
Nfi genes (Nfia, Nfib, Nfic, and Nfix) exhibit promoter-specific differences in their maximal transcriptional activation potentials due to differences in their carboxyl-terminal regions (38). NFI proteins exhibit cell type- and promoter-specific differences in their repression properties, with NFIC and NFIX, but not NFIA and NFIB, repressing the mouse mammary tumor virus promoter in HeLa cells. NFIC-mediated repression occurs by interference with coactivator (e.g. p300/CBP or SRC-1A) function at the mouse mammary tumor virus promoter (39). Although it is widely accepted that NFI proteins differ in their repression and activation potentials, their ability to regulate transcription is poorly understood. In our study, Nfic and Nfix influenced Klf4 transcription but Nfia and Nfib did not. Nfic and Nfix are thought to regulate Klf4 because the DNA-binding affinities of NFIC and NFIX were higher than those of NFIA and NFIB; however, no direct evidence yet supports this notion.
To our knowledge, this work is the first to investigate the Nfic-Klf4-Dmp1-Dspp and the Nfic-Klf4-E-cadherin signaling pathways in odontoblasts, as well as their functional implications during dentin formation. This information will lead to a comprehensive understanding of the role of NFIC in dentinogenesis.
This work was supported by National Research Foundation of Korea Grants NRF-2013R1A2A2A01010911, NRF-2013037491, and NRF-2013M3A9B2076480.
- DSPP
- dentin sialophosphoprotein
- NFIC
- nuclear factor I-C
- KLF4
- Krüppel-like factor 4
- DMP1
- dentin matrix protein 1
- ALC
- ameloblast lineage cells.
REFERENCES
- 1. Arana-Chavez V. E., Massa L. F. (2004) Odontoblasts: the cells forming and maintaining dentine. Int. J. Biochem. Cell Biol. 36, 1367–1373 [DOI] [PubMed] [Google Scholar]
- 2. D'Souza R. N., Cavender A., Sunavala G., Alvarez J., Ohshima T., Kulkarni A. B., MacDougall M. (1997) Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J. Bone Miner Res. 12, 2040–2049 [DOI] [PubMed] [Google Scholar]
- 3. Gronostajski R. M. (2000) Roles of the NFI/CTF gene family in transcription and development. Gene 249, 31–45 [DOI] [PubMed] [Google Scholar]
- 4. Bandyopadhyay S., Starke D. W., Mieyal J. J., Gronostajski R. M. (1998) Thioltransferase (glutaredoxin) reactivates the DNA-binding activity of oxidation-inactivated nuclear factor I. J. Biol. Chem. 273, 392–397 [DOI] [PubMed] [Google Scholar]
- 5. Park J. C., Herr Y., Kim H. J., Gronostajski R. M., Cho M. I. (2007) Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J. Periodontol. 78, 1795–1802 [DOI] [PubMed] [Google Scholar]
- 6. Lee D. S., Park J. T., Kim H. M., Ko J. S., Son H. H., Gronostajski R. M., Cho M. I., Choung P. H., Park J. C. (2009) Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J. Biol. Chem. 284, 17293–17303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oh H. J., Lee H. K., Park S. J., Cho Y. S., Bae H. S., Cho M. I., Park J. C. (2012) Zinc balance is critical for NFI-C mediated regulation of odontoblast differentiation. J. Cell. Biochem. 113, 877–887 [DOI] [PubMed] [Google Scholar]
- 8. Kwon A., Park H. J., Baek K., Lee H. L., Park J. C., Woo K. M., Ryoo H. M., Baek J. H. (2012) Suberoylanilide hydroxamic acid enhances odontoblast differentiation. J. Dent. Res. 91, 506–512 [DOI] [PubMed] [Google Scholar]
- 9. Evans P. M., Liu C. (2008) Roles of Krupel-like factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim. Biophys. Sin. 40, 554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z., Couble M. L., Mouterfi N., Magloire H., Chen Z., Bleicher F. (2009) Spatial and temporal expression of KLF4 and KLF5 during murine tooth development. Arch. Oral Biol. 54, 403–411 [DOI] [PubMed] [Google Scholar]
- 11. Lin H., Xu L., Liu H., Sun Q., Chen Z., Yuan G., Chen Z. (2011) KLF4 promotes the odontoblastic differentiation of human dental pulp cells. J. Endod. 37, 948–954 [DOI] [PubMed] [Google Scholar]
- 12. Lin H., Liu H., Sun Q., Yuan G., Zhang L., Chen Z. (2013) KLF4 promoted odontoblastic differentiation of mouse dental papilla cells via regulation of DMP1. J. Cell. Physiol. 228, 2076–2085 [DOI] [PubMed] [Google Scholar]
- 13. Couble M. L., Farges J. C., Bleicher F., Perrat-Mabillon B., Boudeulle M., Magloire H. (2000) Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif. Tissue Int. 66, 129–138 [DOI] [PubMed] [Google Scholar]
- 14. Steele-Perkins G., Butz K. G., Lyons G. E., Zeichner-David M., Kim H. J., Cho M. I., Gronostajski R. M. (2003) Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol. Cell. Biol. 23, 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y. N., Abou-Kheir W., Yin J. J., Fang L., Hynes P., Casey O., Hu D., Wan Y., Seng V., Sheppard-Tillman H., Martin P., Kelly K. (2012) Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor β-initiated prostate cancer epithelial-mesenchymal transition. Mol. Cell. Biol. 32, 941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X., Lu H., Urvalek A. M., Li T., Yu L., Lamar J., DiPersio C. M., Feustel P. J., Zhao J. (2011) KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene 30, 1901–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gumireddy K., Li A., Gimotty P. A., Klein-Szanto A. J., Showe L. C., Katsaros D., Coukos G., Zhang L., Huang Q. (2009) KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat. Cell Biol. 11, 1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin C., D'Souza R., Feng J. Q. (2007) Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J. Dent Res. 86, 1134–1141 [DOI] [PubMed] [Google Scholar]
- 19. Liu H., Lin H., Zhang L., Sun Q., Yuan G., Zhang L., Chen S., Chen Z. (2013) miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback loop. J. Biol. Chem. 288, 9261–9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diekwisch T. (1989) Localization of microfilaments and microtubules during dental development in the rat. Acta Histochem. Suppl. 37, 209–212 [PubMed] [Google Scholar]
- 21. Hosoya A., Nakamura H., Ninomiya T., Yoshiba K., Yoshiba N., Nakaya H., Wakitani S., Yamada H., Kasahara E., Ozawa H. (2006) Immunohistochemical localization of α-Smooth muscle actin during rat molar tooth development. J. Histochem. Cytochem. 54, 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiwari N., Meyer-Schaller N., Arnold P., Antoniadis H., Pachkov M., van Nimwegen E., Christofori G. (2013) Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8). PLoS One 8, e57329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abe S., Hamada K., Miura M., Yamaguchi S. (2012) Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol. Int. 36, 927–936 [DOI] [PubMed] [Google Scholar]
- 24. del Barrio M. G., Nieto M. A. (2002) Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129, 1583–1593 [DOI] [PubMed] [Google Scholar]
- 25. Lesot H., Meyer J. M., Ruch J. V., Weber K., Osborn M. (1982) Immunofluorescent localization of vimentin, prekeratin and actin during odontoblast and ameloblast differentiation. Differentiation 21, 133–137 [DOI] [PubMed] [Google Scholar]
- 26. Yori J. L., Johnson E., Zhou G., Jain M. K., Keri R. A. (2010) Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J. Biol. Chem. 285, 16854–16863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mina M., Kollar E. J. (1987) The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 32, 123–127 [DOI] [PubMed] [Google Scholar]
- 28. Narayanan K., Srinivas R., Ramachandran A., Hao J., Quinn B., George A. (2001) Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. U.S.A. 98, 4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson M. P., Zhu Q., Wang S., Liu Q., Liu Y., Wang X., Yuan B., Ruest L. B., Feng J. Q., D'Souza R. N., Qin C., Lu Y. (2013) The rescue of dentin matrix protein 1 (DMP1)-deficient tooth defects by the transgenic expression of dentin sialophosphoprotein (DSPP) indicates that DSPP is a downstream effector molecule of DMP1 in dentinogenesis. J. Biol. Chem. 288, 7204–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee D. S., Choung H. W., Kim H. J., Gronostajski R. M., Yang Y. I., Ryoo H. M., Lee Z. H., Kim H. H., Cho E. S., Park J. C. (2014) NFI-C regulates osteoblast differentiation via control of Osterix expression. Stem Cells 32, 2467–2479 [DOI] [PubMed] [Google Scholar]
- 31. Heymann R., About I., Lendahl U., Franquin J. C., Obrink B., Mitsiadis T. A. (2002) E- and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am. J. Pathol. 160, 2123–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iida K., Takeda-Kawaguchi T., Hada M., Yuriguchi M., Aoki H., Tamaoki N., Hatakeyama D., Kunisada T., Shibata T., Tezuka K. (2013) Hypoxia-enhanced derivation of iPSCs from human dental pulp cells. J. Dent Res. 92, 905–910 [DOI] [PubMed] [Google Scholar]
- 33. Lee D. S., Yoon W. J., Cho E. S., Kim H. J., Gronostajski R. M., Cho M. I., Park J. C. (2011) Crosstalk between nuclear factor I-C and transforming growth factor-β1 signaling regulates odontoblast differentiation and homeostasis. PLoS One 6, e29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slavkin H. C., Bringas P., Jr. (1976) Epithelial-mesenchyme interactions during odontogenesis. IV. Morphological evidence for direct heterotypic cell-cell contacts. Dev. Biol. 50, 428–442 [DOI] [PubMed] [Google Scholar]
- 35. Arana-Chavez V. E., Katchburian E. (1997) Development of tight junctions between odontoblasts in early dentinogenesis as revealed by freeze-fracture. Anat. Rec. 248, 332–338 [DOI] [PubMed] [Google Scholar]
- 36. ten Cate J. M., Damen J. J., Buijs M. J. (1998) Inhibition of dentin demineralization by fluoride in vitro. Caries Res. 32, 141–147 [DOI] [PubMed] [Google Scholar]
- 37. Nilsson J., Helou K., Kovács A., Bendahl P. O., Bjursell G., Fernö M., Carlsson P., Kannius-Janson M. (2010) Nuclear Janus-activated kinase 2/nuclear factor 1-C2 suppresses tumorigenesis and epithelial-to-mesenchymal transition by repressing Forkhead box F1. Cancer Res. 70, 2020–2029 [DOI] [PubMed] [Google Scholar]
- 38. Chaudhry A. Z., Vitullo A. D., Gronostajski R. M. (1998) Nuclear factor I (NFI) isoforms differentially activate simple versus complex NFI-responsive promoters. J. Biol. Chem. 273, 18538–18546 [DOI] [PubMed] [Google Scholar]
- 39. Chaudhry A. Z., Vitullo A. D., Gronostajski R. M. (1999) Nuclear factor I-mediated repression of the mouse mammary tumor virus promoter is abrogated by the coactivators p300/CBP and SRC-1. J. Biol. Chem. 274, 7072–7081 [DOI] [PubMed] [Google Scholar]