Abstract
SidJ is a Dot/Icm effector involved in the trafficking or retention of ER-derived vesicles to Legionella pneumophila vacuoles whose mutation causes an observable growth defect, both in macrophage and amoeba hosts. Given the crucial role of this effector in L. pneumophila virulence we investigated the mechanisms shaping its molecular evolution. The alignment of SidJ sequences revealed several alleles with amino acid variations that may influence the protein properties. The identification of HGT events and the detection of balancing selection operating on sidJ evolution emerge as a clear result. Evidence suggests that intragenic recombination is an important strategy in the evolutionary adaptive process playing an active role on sidJ genetic plasticity. This pattern of evolution is in accordance with the life style of L. pneumophila as a broad host-range pathogen by preventing host-specialization and contributing to the resilience of the species.
Introduction
Legionella pneumophila is a ubiquitous bacterium in freshwater environments as well as in many man-made water systems worldwide known for its ability to cause pneumonia in humans [1]. L. pneumophila are subject to predation by eukaryotic phagocytes, such as amoeba and ciliates, so the bacterium's survival and spread depends on the ability to hijack the phagocytic vacuole, to create a replicative niche, to prevent phagosome-lysosome fusion and evade host immune system. In humans, L. pneumophila reaches the lungs after inhalation of contaminated aerosol droplets where the similar mechanisms allow L. pneumophila to hijack another phagocyte, lung-based macrophages, leading to infection [2]–[10]. Since human-to-human transmission of L. pneumophila has not been observed the human infection is an evolutive dead end for Legionella. Consequently, protozoan hosts are believed to provide the primary evolutionary pressure for the acquisition and maintenance of virulence factors, resulting largely from the organism's need to replicate in an intracellular niche and also avoid predation by environmental protozoa [4], [5], [8], [10].
The long-term co-evolution of L. pneumophila with free-living amoebae has influenced the genomic structure of this organism since amoeba may act as a gene melting pot, allowing diverse microorganisms to evolve by gene acquisition and loss, and then either adapt to the intra-amoebal lifestyle or evolve into new pathogenic forms [8], [10]–[12]. This lifestyle, namely the interaction with different protozoan in different environments, may have prevented host-specialization and be responsible for the evolutionary story of L. pneumophila [13]. Several studies showed that L. pneumophila clinical isolates showed less genetic diversity than man-made and natural environmental isolates [14]–[19]. This evidence supports the hypothesis proposed by Coscollá and González-Candelas [16] that isolates of L. pneumophila recovered from clinical cases are a limited, non-random subset of all genotypes existing in nature, perhaps representing an especially adapted group of clones.
The virulence of L. pneumophila is dependent on the Dot/Icm type IVB protein secretion system responsible for the translocation of at least 290 effectors into the host cell where they act on diverse host cell pathways [20]–[22]. Functional redundancy among groups of substrates that target similar host processes has been commonly reported since elimination of a single substrate gene rarely leads to detectable defects in intracellular growth under standard laboratory conditions [3]–[5], [23]. Indeed, its particular large repertoire of effectors seems to be the basis for the broad host range of L. pneumophila, since replication within different hosts requires specific sets of substrates [23], [24]. Inter-domain horizontal gene transfer from eukaryotes and subsequent evolution of eukaryotic-like translocated effectors has enabled L. pneumophila to adapt to the intracellular lifestyle through exploitation of evolutionarily conserved eukaryotic cell mechanisms [3], [12] Indeed, many of the dot/icm effectors harbor eukaryotic-like motifs that mediate the interaction with host proteins and organelles to modulate host cell functions, establishing molecular mimicry as a major virulence strategy in L. pneumophila pathogenesis [5], [21], [24]. Although the vast majority of individual Dot/Icm-secreted substrates are genetically dispensable for the intracellular replication of L. pneumophila, critical components for both intracellular growth and disease within animals have been identified. Indeed, only SdhA, SidJ and AnkB have been described as essential for maximal intracellular replication, suggesting that certain proteins in L. pneumophila selectively provide an advantage to the pathogen in certain hosts [3], [4], [20], [25]–[28]. Furthermore, both sdhA and sidJ are conserved among strains of Legionella pneumophila and Legionella longbeachae of known genome sequence [29]–[32].
SidJ modulates host cellular pathways through the membrane remodeling of the L. pneumophila containing vacuoles by the efficient acquisition of ER specific proteins [4], [27]. The SidJ locus is presented in an operon-like structure with three other members of the SidE family, namely, sdeC, sdeB and sdeA [29]–[32]. Nevertheless, SidJ clearly is the sole protein responsible for the growth defect observed in the sidJ mutant since neither of those genes is required for intracellular growth in macrophages [33], [34]. Moreover, sidJ expression is not coregulated by the same mechanisms that rule the expression of sdeC, sdeA, and sdeB [27], which are significantly induced when L. pneumophila enters the postexponential growth phase [33]. Compared to wild-type strains, the sidJ deletion mutant did not display any detectable growth defect in AYE broth, but resulted in ∼15-fold reduction in intracellular growth within macrophages, and causes a significant growth defect in amoeba [27]. Given the role of SidJ in establishing successful infections and the diversity of host cells encountered by L. pneumophila in nature, it is possible that this gene product is a target for host specialization and adaptive evolution, and that variation in sidJ may reflect an increase in the fitness of L. pneumophila in certain environments. Our goal was to determine the genetic structure and allelic diversity of L. pneumophila populations inferred from sidJ gene and to identify the molecular mechanisms operating in the evolution of this virulence-related gene.
The identification of HGT events within L. pneumophila and the detection of balancing selection operating on sidJ evolution emerge from the present work. Our results indicate that intragenic recombination is favored as a strategy in the evolutionary adaptive process playing an active role in sidJ genetic plasticity.
Materials and Methods
L. pneumophila strains
Thirty two unrelated strains of L. pneumophila were selected for complete sequencing of the sidJ gene to determine the genetic structure and molecular evolution (Table 1). Strains were selected from several others in order to capture the maximum genetic variability, since they represented the allelic diversity determined in early studies from the complete sequence of dotA and type II protein secretion system (T2S) related genes [18], [19]. These also included twelve isolates from 9 sites comprising natural and man-made environments, and seventeen clinical-related L. pneumophila type and reference strains, eleven from L. pneumophila subsp. pneumophila, three L. pneumophila subsp. fraseri strains and three L. pneumophila subsp. pascullei. The sequences from eleven L. pneumophila subsp. pneumophila genome sequenced strains [31], [32], [35]–[39] were also included in this work. Previously published sequences of partial rpoB gene from the studied strains were also used for comparison purposes (Table S1).
Table 1. L. pneumophila unrelated strains, isolated from distinct environments, type and reference strains included in this study and distribution of L. pneumophila strains into clusters according with rpoB and sidJ gene sequences.
| Strain designation | Environmental type | Subspecies | Reference of the source | Clusters | |
| rpoB | sidJ | ||||
| Aço13 | Natural | L. pneumophila subsp. pneumophila | [93] | A | B |
| Aço20 | Natural | L. pneumophila subsp. pneumophila | [93] | A | A |
| Agn2 | Natural | L. pneumophila subsp. pneumophila | [18] | A | C |
| Alf 18 | Natural | L. pneumophila subsp. pneumophila | [94] | A | C |
| Felg244 | Natural | L. pneumophila subsp. pneumophila | [40] | A | C |
| Ice27 | Natural | L. pneumophila subsp. pneumophila | [18] | A | B |
| Ice30 | Natural | L. pneumophila subsp. pneumophila | [18] | A | C |
| NMex1 | Natural | L. pneumophila subsp. pneumophila | [94] | A | C |
| NMex49 | Natural | L. pneumophila subsp. pneumophila | [94] | A | A |
| HL06041035 | Man-made | L. pneumophila subsp. pneumophila | [12] | A | B |
| IMC23 | Man-made | L. pneumophila subsp. pneumophila | [95] | A | C |
| LPE059 | Man-made | L. pneumophila subsp. pneumophila | [31] | A | A |
| Ma36 | Man-made | L. pneumophila subsp. pneumophila | [18] | A | C |
| Por3 | Man-made | L. pneumophila subsp. pneumophila | [18] | A | B |
| 130b | Clinical-related | L. pneumophila subsp. pneumophila | [39] | A | D |
| 797-PA-H (ATCC 43130) | Clinical-related | L. pneumophila subsp. pneumophila | [96] | A | D |
| Alcoy | Clinical-related | L. pneumophila subsp. pneumophila | [38] | A | C |
| ATCC43290 | Clinical-related | L. pneumophila subsp. pneumophila | [30] | A | A |
| Chicago 2 (ATCC 33215) | Clinical-related | L. pneumophila subsp. pneumophila | [97] | A | A |
| Concord 3 (ATCC 35096) | Clinical-related | L. pneumophila subsp. pneumophila | [98] | A | A |
| Corby | Clinical-related | L. pneumophila subsp. pneumophila | [37] | A | C |
| Lens | Clinical-related | L. pneumophila subsp. pneumophila | [36] | A | D |
| Lorraine | Clinical-related | L. pneumophila subsp. pneumophila | [12] | A | A |
| Paris | Clinical-related | L. pneumophila subsp. pneumophila | [36] | A | B |
| Philadelphia 1 (ATCC 33152T) | Clinical-related | L. pneumophila subsp. pneumophila | [35] | A | A |
| Thunder Bay | Clinical-related | L. pneumophila subsp. pneumophila | [32] | A | A |
| Los Angeles 1(ATCC 33156T) | Clinical-related | L. pneumophila subsp. fraseri | [99] | B | E |
| Dallas 1E (ATCC 33216) | Clinical-related | L. pneumophila subsp. fraseri | [98] | B | E |
| Lansing 3 (ATCC 35251) | Clinical-related | L. pneumophila subsp. fraseri | [84] | B | A |
| U8W (ATCC 33737T) | Clinical-related | L. pneumophila subsp. pascullei | [84] | C | E |
| U7W (ATCC 33736) | Clinical-related | L. pneumophila subsp. pascullei | [84] | C | E |
| MICU B (ATCC 33735) | Clinical-related | L. pneumophila subsp. pascullei | [84] | C | E |
DNA extraction, polymerase chain reaction (PCR), cloning and DNA sequencing
The extraction of genomic DNA from the previously selected L. pneumophila strains was carried out as previously described by Costa and colleagues [40]. PCRs were performed to amplify the sidJ locus (2625 bp) using the primer sets described in Table S2. In general, PCR was carried out using 150–200 ng DNA, 2.0 mM MgCl2, 1X reaction buffer, 0.2 µM each dNTP, 5 pmol each primer, and 1 U Taq polymerase (Invitrogen) in 50 µl reaction volumes with the following PCR profile: 5 min a 95°C; 30 cycles of 95°C, 45 s; 50°C, 45 s; a 72°C, 3 min; 7 min at 72°C. Moreover, in some cases it was necessary to adjust the annealing temperatures for individual strains. The amplified PCR products were detected on 1.0% agarose gels stained with ethidium bromide and were purified for sequencing by using an NZYGelpure extraction kit (NZYTech, Lda., Portugal). To obtain the full–length genes the PCR products were cloned using NZY-A PCR cloning kit (NZYTech, Lda., Portugal) according to the manufacturer instructions. Positive clones were selected on Luria-Bertani agar plates containing 20 µg ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), 0.5 mM IPTG (isopropyl-β-D-1-thiogalactopyranoside), and 100 µg ml−1 ampicillin. Plates were incubated overnight at 37°C in selective media. Positive clones were confirmed by PCR with the same primers used for amplification, and plasmid DNA was extracted using Zyppy Plasmid Miniprep Kit (Zymo Research, USA) according to the manufacturer instructions. Gene sequences were determined by Macrogen Corporation (Netherlands).
For PCR amplification of the sdeC, laiE, sdeB and sedA genes, primers were designed based on the corresponding genes from L. pneumophila strain Philadelphia 1, namely, lpg2153, lpg2154, lpg2156 and lpg2157, respectively (Fig. S1 and Table S2). PCR amplifications were performed as previously described. Several annealing temperatures between 40 and 55°C were tested for 1 min. The amplified PCR products were detected and purified as abovementioned. For confirmation purposes, all PCR products were sequenced with the primers used for amplification by Macrogen Corporation (Netherlands).
Sequence analysis
The quality of the sequences was manually checked using the Sequence Scanner software (https://products.appliedbiosystems.com). Phylogenetic analyses were performed using MEGA5 package [41]. Alignment against the corresponding genes found in eleven genome sequenced L. pneumophila strains obtained from the public databases (Table S1), was performed using the multiple alignment CLUSTAL software [42], included on MEGA5 package. For coding loci alignments were performed with the amino acid sequences and gaps were later introduced in the corresponding nucleotide alignments, thus keeping the correct frame for translation. A multiple alignment of amino acid sequences was obtained using ClustalΩ [43] manually corrected where necessary. The MEGA5 package was used to derive the multiple alignments of nucleotide and positions of doubtful homology were removed using Gblocks [44].
Maximum likelihood (ML) phylogenetic trees were obtained for sidJ and rpoB loci with PhyML 3.0 [45] with HKY +G [46] and TrN +G+I models [47], respectively. The most appropriate model of nucleotide substitution and likelihood scores assessed by TOPALi V2.5 [48] and by jModeltest [49]. The best model was determined by using the Akaike Information Criterion (AIC) [50], [51]. ML phylogenetic analysis was performed for the amino acid alignment by PhyML 3.0 [45] using the JTT +G+F model [52]. The most appropriate model of amino acid substitution and likelihood scores were assessed by ProtTest 2.4 [53]. Supports for the nodes were evaluated by bootstrapping with 1000 pseudoreplicates.
Genetic variability analyses were performed with DnaSP software [54]. Mean non-synonymous mutations among the three groups were compared through one-way analysis of variance (ANOVA) after arcsine square root data transformation to fulfill ANOVA assumptions.
The locations of the variable nucleotide positions were displayed graphically using the programs PSFIND and HAPPLOT written by Dr Thomas S. Whittam and available at the STEC Center website (http://www.shigatox.net/stec/cgi-bin/programs).
Molecular Evolution
Neighbour-net analysis [55] was performed and converted to a splits graph using the drawing algorithms implemented in SplitsTree4 software – version 4.6 [56]. The neighbour-net method was based on the pairwise distance matrices calculated with the Jukes–Cantor correction [57] of the sidJ sequences alignment performed on the MEGA5 package [41].
Intragenic recombination was screened within the aligned sequences using the program RDP3 [58]. This program identifies recombinant sequences and recombination breaking points using several methods. We choose six of them: RDP [59], GENECONV [60], BootScan [61], Maximum Chisquared Test (MaxChi; [62]), CHIMAERA [63] and Sister Scan (SiScan; [64]). The analysis was performed with default settings for the detection methods, a Bonferroni corrected P-value cut-off of 0.05, and a requirement that each potential event had to be detected simultaneously by four or more methods. The breakpoint positions and recombinant sequence(s) inferred for every detected potential recombination event were manually checked and adjusted where necessary using the extensive phylogenetic and recombination signal analysis features available in RDP3.
The GARD method [65] implemented in datamonkey server [66] was also used to search for evidence of phylogenetic incongruence, and to identify the number and location of breakpoints corresponding to recombination events.
Neutrality tests and positive selection analysis
Tajima's D [67], Fu and Li's D* and F* [68] and Fu's Fs [69] statistics were calculated for testing the mutation neutrality hypothesis [70], as previously described by Coscollá and colleagues [71] and Costa and colleagues [19]. These statistics were calculated with the program DNASP4.0 [54] using a statistical significance level α = 0.025 and applying the false discovery rate [72], [73] to correct for multiple comparisons and 1000 replicates in a coalescent simulation.
Estimates of the number of non-synonymous and synonymous substitutions at each locus (dN/dS) were calculated using the modified Nei–Gojobori method [74] with Jukes-Cantor correction [57] implemented in MEGA5 package [41].
In order to investigate the presence of positively selected codons in sidJ locus, the estimates of both positive and purifying selection at each amino acid site were calculated from the ratio of non-synonymous to synonymous substitutions, known as ω, as previously described [18]. Nucleotide sequences alignment from L. pneumophila strains were constructed using the MEGA5 package [41] and analyses were conducted using the Selecton version 2.1 software [75], [76]. The significance of the ω scores was obtained by using a Likelihood Ratio Test that compares two nested models: a null model that assumes no selection (M8a) [77] and an alternative model that does (M8) [78].
Four physicochemical properties (volume, polarity, charge and hydrophobicity) were used to characterize the results of amino acid substitutions in comparisons of translated homologous sequences [79], [80]. Corresponding dG values were obtained using Miyata's matrix [81] and were calculated per one amino acid substitution so that they would not depend on the rates of nucleotide substitutions per se [82].
Nucleotide sequence accession numbers
The complete sidJ sequences from L. pneumophila strains determined in this study were deposited in the EMBL Nucleotide Sequence Database with Accession No. HG531934–HG531954.
Results and Discussion
Sequence analysis and genetic structure inferred from sidJ
The complete sequence of sidJ (2625 bp) was determined from 32 L. pneumophila strains (Table 1) to determine the mechanisms shaping this fundamental virulence-related gene evolution. All L. pneumophila studied strains yielded the analyzed gene with the expected size.
Sequences from an internal fragment of the rpoB gene, previously obtained from the same L. pneumophila strains [17], [18], [83], were included in the analysis (Table S1) because the inferred rpoB tree agrees with phylogenetic and phenotypic analyses [84]–[86], that allow the separation of the three L. pneumophila subspecies.
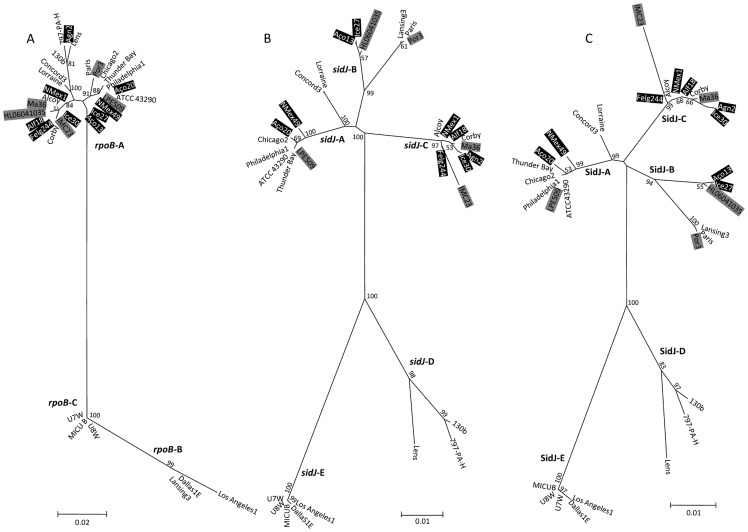
A comparative analysis between the phylogeny obtained with an internal fragment of rpoB gene, used as a marker of vertical inheritance in L. pneumophila, and the corresponding phylogeny of sidJ was performed to study congruence between this inheritance and the phylogeny of sidJ. Maximum likelihood (ML) phylogenetic trees were obtained for sidJ and rpoB gene sequences (Fig. 1A and B). The topology of the two inferred trees was not congruent since, depending on the gene, most strains had different relationships with each other and with L. pneumophila type and reference strains (Fig. 1A and B). The analysis of the rpoB gene from the 32 strains matched the three different L. pneumophila subspecies, namely, L. pneumophila subsp. pneumophila (cluster rpoB-A), L. pneumophila subsp. fraseri (cluster rpoB-B) and L. pneumophila subsp. pascullei (cluster rpoB-C), comprising 81.2%, 9.4% and 9.4% of all strains, respectively (Fig. 1A and Table 1). While the inferred rpoB tree agrees with phylogenetic and taxonomic analyses [80]–[82] with three clusters matching L. pneumophila subsp., in the inferred sidJ tree five major clusters were identified supported by very high bootstrap values (cluster A to E) (Fig. 1B). One important observation from this study is that the strains previously grouped in the rpoB-A cluster (Fig. 1A) (L. pneumophila subsp. pneumophila) were split into four discrete groups in the sidJ sequence-based analysis (cluster A to D) (Fig. 1B). Equally relevant is the fact that the majority of the strains previously clustered in the rpoB-B and rpoB-C clusters (Fig. 1A) (L. pneumophila subsp. fraseri and L. pneumophila subsp. pascullei, respectively) were merged into a single group in the sidJ inferred dendrogram (cluster sidJ-E) (Fig. 1B). A similar significant evolutionary drift was observed for the strain Lansing 3, that belonged to cluster rpoB-B with all other L. pneumophila subsp. fraseri strains, since it was grouped in a distinct cluster in the ML tree inferred from the sidJ gene (sidJ-A) along with other L. pneumophila subsp. pneumophila strains (Table 1). These incongruencies are discussed below in the context of intragenic recombination. Moreover, the strains were not evenly distributed in these clusters. Natural and man-made environmental isolates were only found in clusters sidJ-A to C, while clusters sidJ-D and sidJ-E were composed exclusively by clinical-related strains (Table 1).
Figure 1. Maximum likelihood phylogenetic trees of L. pneumophila isolates, type and references strains ( Table 1 ) from DNA sequences of rpoB (A), sidJ (B) and from deduced amino acid sequences of SidJ (C).
Bootstrap support values (1,000 replicates) for nodes higher than 50% are indicated next to the corresponding node.
Additionally, a phylogenetic comparison between the previously obtained clusters from rpoB and sidJ genes and the corresponding deduced amino acid sequences was also performed. The ML phylogenetic tree was obtained for SidJ (Fig. 1C). The deduced amino acid sequences from the partial rpoB gene sequences of all isolates and reference strains were the same, despite the nucleotide differences detected (results not shown). On the other hand, the clusters inferred from the partial deduced amino acid sequences of sidJ (Fig. 1C) were consistent with the previously obtained nucleotide-based subgroups. These findings indicate that most sidJ nucleotide polymorphisms result in amino acid changes, in contrast to what was observed for rpoB [18]. Moreover, incongruence between lineage relationships was observed for sidJ clusters A to C when compared to the nucleotide-based tree (Fig. 1B and C).
Genetic variability of sidJ gene
The overall nucleotide sequence diversity of rpoB varied from 0 to 0.032 with an average of 0.043±0.006. (Table S3). The diversity of sidJ nucleotide sequences from the five defined clusters was higher than that observed for rpoB sequences, varying between 0 and 0.070 with an average of 0.033±0.003. The sidJ-B subgroup was the most polymorphic with genetic pairwise differences varying from 0 to 0.026 with an average of 0.015±0.003 (Table S3). On the other hand, the diversity within sidJ-D and sidJ-E clusters was rather lower. The diversity within the two most representative clusters, sidJ-A and sidJ-C, varied between 0 and 0.022 with an average of 0.010±0.004 and between 0 and 0.013 with an average of 0.004±0.001, respectively.
Genetic variability of 32 L. pneumophila unrelated strains was estimated based on the sidJ sequences using genetic diversity parameters, not directly dependent on sample size. Moreover, the genetic variability of L. pneumophila populations based on strain origin was also estimated from sidJ from natural environmental strains, man-made environmental strains and clinical-related strains (Table 2). The highest haplotype (h) was found in clinical-related strains presenting 13 distinct alleles. On the contrary, the haplotype diversity (Hd) was higher in natural and man-made environmental isolates since all strains were different from each other. The nucleotide diversities (π), number of polymorphic nucleotide sites (S), population mutation ration (θ), average number of pairwise nucleotide differences (k), and total number of mutations (η) were higher in clinical-related strains. Non-synonymous mutations were more frequent in man-made environmental strains (39.26%, 68 of 184), while in clinical-related strains and natural populations, mutations accounting for differences among alleles accounted for 32.20% (123 of 382) and 35.33% (53 of 153), respectively. Nevertheless, these differences were not significant among the three populations (F2,29 = 3.11; p = 0.06). The overall degree of variability detected within sidJ is similar to that previously observed for the pilD gene, a structural component of the T2S involved in virulence-related phenotypes found to be under neutral evolution [19], [87].
Table 2. Summary of genetic diversity parameters for sidJ from L. pneumophila strains.
| sidJ | ||||
| Overall | Natural environment | Man-made environment | Disease-related | |
| Sequence, n | 32 | 9 | 5 | 18 |
| Sequence length, L | 2628 | 2628 | 2628 | 2628 |
| Haplotypes, h | 23 | 9 | 5 | 13 |
| Haplotype diversity, Hd | 0.974 | 1.0 | 1.0 | 0.954 |
| (standard deviation) | (0.015) | (0.057) | (0.126) | (0.034) |
| Nucleotide diversity, π | 0.04778 | 0.02459 | 0.03356 | 0.05616 |
| (standard deviation) | (0.00475) | (0.00408) | (0.00521) | (0.00393) |
| Polymorphic sites, S (%) | 432 (16.43) | 151 (17.24) | 181 (20.66) | 385 (43.95) |
| θ (φρομ Σ) | 0.04096 | 0.02117 | 0.03317 | 0.04274 |
| (standard deviation) | (0.01246) | (0.00172) | (0.01662) | (0.01484) |
| Pairwise differences, k | 125.145 | 64.556 | 87.900 | 147.092 |
| Total number of mutations, η | 424 | 153 | 184 | 382 |
| Synonymous mutations (%) | 275 (64.86) | 97 (64.67) | 109 (59.24) | 259 (67.80) |
| Non-synonymous mutations (%) | 149 (35.14) | 53 (35.33) | 68 (39.26) | 123 (32.20) |
| dN/dS | 0.125 | 0.142 | 0.170 | 0.120 |
| dG per one amino acid change | 1.35 | 1.41 | 1.37 | 0.98 |
The rates of non-synonymous substitutions per non-synonymous site (dN) in the coding loci were very low, despite the relatively large values of polymorphic sites, most of which corresponded to synonymous substitutions (dS), ranged between 0.081 in natural isolates to 0.2257 in clinical-related strains. The low dN/dS ratios obtained for sidJ and for the sidJ-related populations indicated that these alleles were under purifying selection (Table 2). In this case, variation occurs only if it does not confer a significant disadvantage on any surviving variant. Because nucleotide substitutions may exert their influence on the function of the final protein product at any of several levels (e.g. DNA, mRNA or protein), dN/dS ratios reflect general restrictions on gene and protein variability. On the other hand, dG values reflect variation purely in protein structural and functional features, indicating some restrictions on the amino acid substitutions at the level of the final functioning product [82]. Based on this analysis we can conclude that the high calculated dG values for the sidJ and for all sidJ-related populations indicates that some of the amino acid substitutions may influence protein properties (Table 2). In fact, despite displaying relatively low dN/dS values, not all amino acid substitutions are conservative, as assessed by changes in amino acid physicochemical properties.
L. pneumophila phylogeny inferred from sidJ sequences
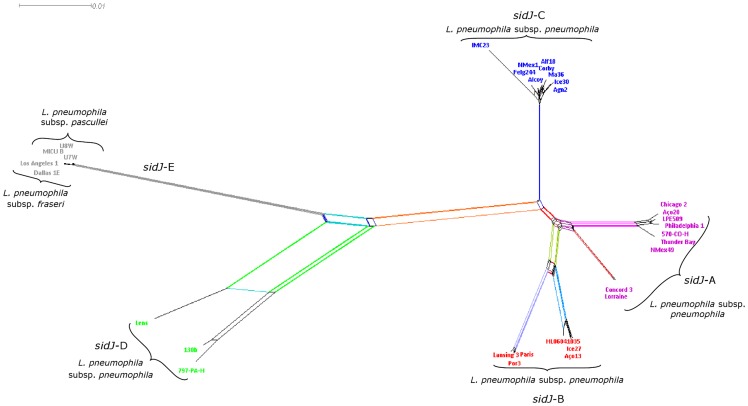
Neighbor-Net analysis [55] has been performed to determine how recombination and horizontal gene transfer events affected the phylogenetic relationships among L. pneumophila strains isolated from distinct environments and locations inferred from sidJ sequences (Fig. 2). The obtained splits graph showed evidence of a network-like evolution, indicating the lack of tree-like relationship between the sidJ sequences (Fig. 2), although it was still possible to reconstruct the previously defined clusters by the ML phylogenetic analysis (Fig. 1). The center of the neighbor net was slightly netted, implying that the data supports many deep conflicting splits. Nonetheless, the clusters previously identified were quite robust (as indicated by the colors in Figure 2) and the divergence of clusters sidJ-A, sidJ-B and sidJ-C from clusters sidJ-D and sidJ-E was noticeable. Moreover, it is obvious the existence of several reticulated events that shaped the evolution of sidJ within L. pneumophila.
Figure 2. Neighbor-net phylogenetic network showing the relationships among L. pneumophila strains (see Table 1 ).
The split graph was estimated with SplitsTree4 from p-distances of the sidJ sequence alignment based on the Jukes–Cantor method. Color code: sidJ-A subgroup is shown in purple, sidJ-B red, sidJ-C blue, sidJ-D green and sidJ-E grey. The relations between and within strains are illustrated by weighted splits with different colors representing simultaneously both grouping in the data and evolutionary distances between taxa, highlighting conflicting signals or alternative phylogenetic histories (recombination or gene transfer) in sidJ molecular evolution.
Determining the influence of recombination on sidJ molecular evolution
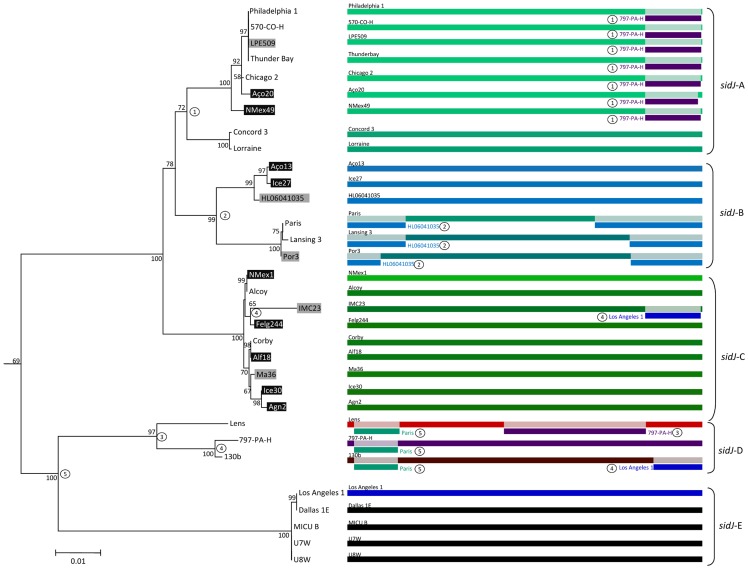
The aforementioned results strongly suggest the existence of recombination events between and within distinct sidJ subgroups. To clarify this hypothesis, evidence for individual recombination events were sought by using two approaches, RDP3 [58] and GARD [65], with only minor differences. Indeed, five putative recombinant regions were identified in this analysis and mapped onto the corresponding ML phylogenetic tree (Fig. 3 and Table S4). From it we were able to identify Potential Recombination Events (PREs) that were compatible with numerous conflicting phylogenetic signals previously observed both in the ML and Neighbor-Net analysis (Fig. 1B and 2).
Figure 3. Maximum likelihood tree from sidJ alignment of L. pneumophila strains.
Bootstrap support values (1,000 replicates) for nodes higher than 50% are indicated. Unique recombination events detected by six recombination detection tests implemented under the RDP3 and GARD based on sidJ amino acid alignment are mapped onto the corresponding breaking point positions in the alignment. Only recombination events that were identified, simultaneously, by four or more methods were selected and numbered according to the RDP analysis (see Table S3).
The identified PREs were limited to strains belonging to the L. pneumophila subsp. pneumophila and aided to explain the previously observed complex evolutionary history of sidJ within this subspecies. Namely, PRE1 involving some of the strains clustered in sidJ-A and the ancestor L. pneumophila subsp. pneumophila strain 797-PA-H as minor parent (Fig. 3), responsible for the bifurcation denoted in the ML and Neighbor-Net analysis (Fig. 1B and 2). PRE2 involving only some strains of sidJ-B cluster and the ancestor L. pneumophila subsp. pneumophila strain HL06041035 as minor parent, reconstructs a previously assigned conflicting signal in the network that originated the split of the cluster into two branches (Fig. 3). Moreover, it was possible to identify PREs that helped to explain the complex evolutionary history observed within strains IMC23, Lens, 130b and 797-PA-H (PRE number 3, 4 and 5 in Fig. 3 and Table S4).
The detection of intragenic recombination events, within a gene, in opposition to intergenic recombination events, between genes, in L. pneumophila has been rarely reported although it is worth noticing that we have found that this form of recombination has a fundamental role on the molecular evolution of L. pneumophila genes critical for virulence, namely in the dotA gene [18] and in sidJ (current study). We anticipate that the reason why the impact of intragenic recombination events on the population structure and genetic diversity of L. pneumophila is underestimated relates with the fact that, despite the ubiquitous character of Legionella sp. in water environments, most studies on genetic variation in L. pneumophila focus on strains isolated from man-made environments, including air conditioning-systems, potable water distribution systems, public fountains, and plumbing fixtures and on clinical-related strains [14]–[16], [36], [88]–[90]. In fact those studies showed clear differences between the populations of clinical-related and man-made environmental isolates, with clinical-related isolates showing less diversity than man-made environmental isolates [14]–[16]. Recently, the first complete genome sequence of a man-made environmental L. pneumophila isolate was determined [31]. It was further demonstrated that this man-made environmental strain was unable to overcome the defense conferred by primary macrophages from mice known to be permissive for clinical-related L. pneumophila strains. Those results also suggested the existence of a host immune surveillance mechanism differing from those currently known in responding to L. pneumophila infection [91].
sidJ gene polymorphism
Multiple alignments of the sidJ sequences revealed numerous substitutions, between and within the defined clusters. We further analyzed the number of polymorphic sites by using DnaSP software [54]. As a whole, the aligned sequences had 16.4% polymorphic nucleotide sites (432 of 2.628 nucleotides), 149 of which predicted amino acid replacements. SidJ length varied between 876 amino acids within cluster sidJ-C and 875 amino acids within the remaining clusters.
The number of polymorphic nucleotide sites detected was somewhat distinct between the defined subgroups (Table 2). The cluster sidJ-D was the most variable subgroup with 3.3% polymorphic sites (86/2.2625 nucleotides), 31 of which predicted amino acid replacements (36%). In contrast, the cluster sidJ-E was the most conserved, with only 0.1% variable sites (3/2625 nucleotides), all predicting amino acid replacements. Clusters sidJ-A and sidJ-B had 2.9% (69/2625 nucleotides) polymorphic sites, 30% and 25% of which predicted amino acid replacements, respectively. An important observation was that although only 43 of 2.2628 nucleotides were polymorphic sites (1.6%) in cluster sidJ-C, 70% corresponded to replacement substitutions.
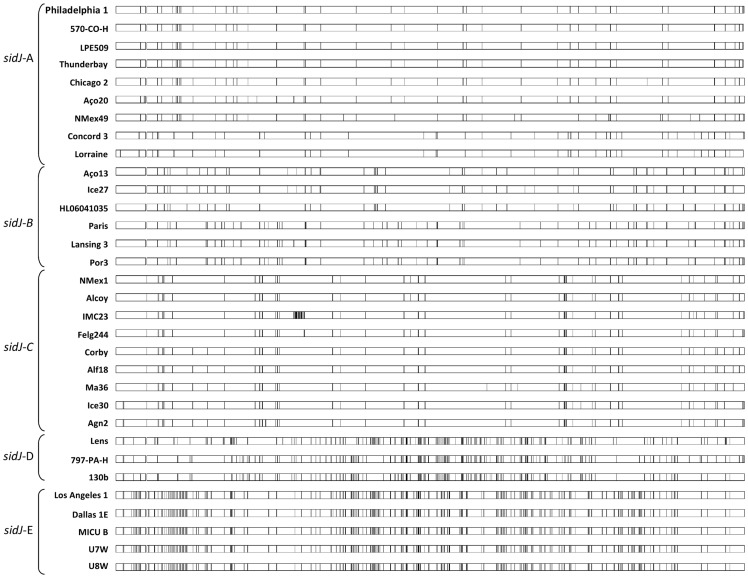
In order to search for mosaic patterns, a hallmark of recombination, sidJ genes were aligned and the positions of sequence differences relative to a guiding sequence were visualized using the Happlot program. Numerous clusters of polymorphic sites that matched the previously identified potential recombination events in sidJ were readily identified by visual inspection, as shown in Fig. 4. This is a remarkable observation since obvious mosaics have only rarely been described, presumably because recombination is so effective that mosaics rapidly become too fragmented for facile recognition.
Figure 4. Graphical display of the location of polymorphic sites (SPNs and INDELs) of sidJ from L. pneumophila strains (see Table 1 ) using the program HAPPLOT when aligned with L. pneumophila strain Philadelphia 1.
Polymorphic nucleotide sites based upon pairwise comparisons are represented by vertical lines. SNPs and INDELS are important drivers of bacterial evolution, by modifying how or whether gene are transcribed and translated.
It is worth notice the degree of nucleotide polymorphisms between sidJ clusters A, B and C when compared with clusters sidJ-D and E, clearly indicating that there are several sidJ alleles. Additionally, sid-D and sid-E clusters were exclusively composed of clinical-related strains. Interestingly, the amino acid variations within cluster sidJ-E, comprising strains belonging to L. pneumophila subsp. pascullei and fraseri, were widely distributed throughout the gene. A similar pattern was also observed for cluster sidJ-D, although a cluster of polymorphic region was detected in the middle region of the gene.
Determining the forces shaping sidJ sequence evolution
In order to discard any influence of positive selection in the detection of recombination events [92], we performed neutrality tests on sidJ gene (Table S5) and complemented them with the analysis of positively selected codons in the coding region. These tests revealed that most variation in this locus was not significantly different from the neutral hypothesis of evolution [67]–[69]. Additionally, the sidJ alignment was analyzed by using a codon based ML method implemented in Selecton package [76]. The server was run with the M8 model [78] and compared with the M8a null model [77]. Likelihood ratio tests between both models were not significant (cut-off value at 0.05) for sidJ. Therefore, the existence of positively selected codons was discarded, reinforcing the existence of recombination events.
sidJ genetic context
Since sidJ is organized in a operon-like structure with members of the sidE family in several clinical-related strains [29]–[32] we considered if the same genetic structure was present in the natural and man-made environmental analyzed strains. Different primer combinations ensured that the associations between sidJ and the sidE-family members could be determined (Fig. S1 and Table S2). We have found that sedC, laiE, sidJ, sedB and sedA genes are structurally linked in all L. pneumophila examined strains, with only one likely exception, since no amplicon was obtained for the man-made environmental strain IMC23. These findings suggest that this operon-like structure has been preserved through evolution, reinforcing the relationship between sidJ and other members of the sidE family.
Conclusions
In sum, the detection of balancing selection operating on sidJ evolution emerges as a clear result from various analyses performed in the present study. Furthermore, sidJ genetic plasticity acquired by frequent recombination events and nonsynonymous mutations is favored as a strategy in the L. pneumophila evolutionary adaptive process. These events are important for increasing L. pneumophila genetic pool by allowing the selection of new allelic forms with increase fitness or, in a more neutral perspective, as merely genetic modifications with no obvious selective advantages. Nevertheless, the detected intragenic recombination events are crucial for the increase of sidJ allelic diversity, contributing for the resilience of L. pneumophila. Further studies focusing the pathogenicity of L. pneumophila natural environmental strains, including the identification of virulent determinants to exploit host functions, will certainly clarify the importance of the reported polymorphism in sidJ.
Supporting Information
Schematic representation of the operon-like structure comprising some members of the sidE family, namely sedC , laiE , sidJ , sedB and sedA in L. pneumophila Philadelphia 1 (lpg2153, lpg2154, lpg2155, lpg2156 and lpg2157, respectively). Primers used for PCR amplifications are also represented (Table S2).
(DOCX)
Locus tag and accession numbers from the L. pneumophila unrelated strains, isolated from distinct environments, type and reference strains included in this study.
(DOCX)
Primers and their sequences designed in this study.
(DOCX)
Genetic pairwise differences, average and standard deviation (SD) for (A) and between (B) sidJ and rpoB clusters. The highest population pairwise differences, average and standard deviation for each gene are marked in bold.
(DOCX)
Potential recombinant events (PRE) identified with RDP3 from the alignment of sidJ obtained from 32 L. pneumophila strains. The minimum number of independent recombination events (IREs) within each identified PRE was inferred by a minimum of four methods and were mapped on the phylogenetic tree (Fig. 3).
(DOCX)
D (Tajima), D* and F* (Fu and Li) and Fs (Fu) statistics obtained from sidJ .
(DOCX)
Acknowledgments
We wish to thank Matilde Moreira-Santos (IMAR, University of Coimbra, Portugal) for advice on the statistics analyses.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All sidJ sequences are available from EMBL Nucleotide Sequence Database with Accession No. HG531934–HG531954.
Funding Statement
The research was funded by Fundação para a Ciência e a Tecnologia (FCT), Portugal and EU-FEDER through grants PEst-C/SAU/LA0001/2011 and PTDC/AGR-TEC/3789/2012. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fields BS (2008) Legionella in the environment. In: Hoffman P, Friedman H, Bendinelli M, editors. Legionella pneumophila: pathogenesis and immunity. New York: Springer Science and Business Media. pp 85–91. [Google Scholar]
- 2. Allombert J, Fuche F, Michard C, Doublet P (2013) Molecular mimicry and original biochemical strategies for the biogenesis of a Legionella pneumophila replicative niche in phagocytic cells. Microbes Infect 15(14–15): 981–988. [DOI] [PubMed] [Google Scholar]
- 3. Richards AM, Von Dwingelo JE, Price CT, Abu Kwaik Y (2013) Cellular microbiology and molecular ecology of Legionella-amoeba interaction. Virulence 15(4): 307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo ZQ (2012) Legionella secreted effectors and innate immune responses. Cell Microbiol 14(1): 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton HJ, Ang DK, van Driel IR, Hartland EL (2010) Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 23: 274–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchrieser C (2011) Legionella: from protozoa to humans. Front Microbiol 12(2): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Escoll P, Rolando M, Gomez-Valero L, Buchrieser C (2013) From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr Top Microbiol Immunol 376: 1–34. [DOI] [PubMed] [Google Scholar]
- 8. Al-Quadan T, Price CT, Abu Kwaik Y (2012) Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella . Trends Microbiol 20(6): 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hubber A, Roy CR (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26: 261–83. [DOI] [PubMed] [Google Scholar]
- 10. Moliner C, Fournier PE, Raoult D (2010) Genome analysis of microorganisms living in amoebae reveals a melting pot of evolution. FEMS Microbiol Rev 34: 281–294. [DOI] [PubMed] [Google Scholar]
- 11. Thomas V, Greub G (2010) Amoeba/amoebal symbiont genetic transfers: lessons from giant virus neighbours. Intervirology 53(5): 254–267. [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Valero L, Rusniok C, Jarraud S, Vacherie B, Rouy Z, et al. (2011) Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genomics 12: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ensminger AW, Yassin Y, Miron A, Isberg RR (2012) Experimental evolution of Legionella pneumophila in mouse macrophages leads to strains with altered determinants of environmental survival. PLoS Pathog 8(5): e1002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison TG, Doshi N, Fry NK, Joseph CA (2007) Comparison of clinical and environmental isolates of Legionella pneumophila obtained in the UK over 19 years. Clin Microbiol Infect 213: 78–85. [DOI] [PubMed] [Google Scholar]
- 15. Harrison TG, Afshar B, Doshi N, Fry NK, Lee JV (2009) Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000–2008). Eur J Clin Microbiol Infect Dis 28: 781–791. [DOI] [PubMed] [Google Scholar]
- 16. Coscollá M, González-Candelas F (2009) Comparison of clinical and environmental samples of Legionella pneumophila at the nucleotide sequence level. Infect Genet Evol 9: 882–888. [DOI] [PubMed] [Google Scholar]
- 17. Costa J, Tiago I, da Costa MS, Veríssimo A (2005) Presence and persistence of Legionella spp. in groundwater. Appl Environ Microbiol 71: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costa J, Tiago I, da Costa MS, Veríssimo A (2010) Molecular evolution of Legionella pneumophila dotA gene, the contribution of natural environmental strains. Environ Microbiol 12: 2711–2729. [DOI] [PubMed] [Google Scholar]
- 19. Costa J, d'Avó AF, da Costa MS, Veríssimo A (2012) A Molecular evolution of key genes for type II secretion in Legionella pneumophila . Environ Microbiol 14(8): 2017–33. [DOI] [PubMed] [Google Scholar]
- 20. Ensminger AW, Isberg RR (2009) Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr Opin Microbiol 12: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hubber A, Roy CR (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26: 261–83. [DOI] [PubMed] [Google Scholar]
- 22. Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, et al. (2013) Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc Natl Acad Sci USA 19 110(8): E707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Connor TJ, Adepoju Y, Boyd D, Isberg RR (2011) Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc Natl Acad Sci USA 108: 14733–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nora T, Lomma M, Gomez-Valero L, Buchrieser C (2009) Molecular mimicry: an important virulence strategy employed by Legionella pneumophila to subvert host functions. Future Microbiol 4(6): 691–701. [DOI] [PubMed] [Google Scholar]
- 25. Molmeret M, Bitar DM, Han L, Abu Kwaik Y (2004) Cell biology of the intracellular infection by Legionella pneumophila . Microbes Infect 6(1): 129–139. [DOI] [PubMed] [Google Scholar]
- 26. Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR (2006) A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA 103: 18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Luo ZQ (2007) The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect Immun 75(2): 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Khodor S, Price CT, Habyarimana F, Kalia A, Abu Kwaik Y (2008) A Dot/Icm translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol Microbiol 70(4): 908–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gomez-Valero L, Rusniok C, Buchrieser C (2011) Comparative and functional genomics of legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front Microbiol. 28 2: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amaro F, Gilbert JA, Owens S, Trimble W, Shuman HA (2012) Whole-genome sequence of the human pathogen Legionella pneumophila serogroup 12 strain 570-CO-H. J Bacteriol 194(6): 1613–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma J, He Y, Hu B, Luo ZQ (2013) Genome sequence of an environmental isolate of the bacterial pathogen Legionella pneumophila . Genome Announc 1(3): e00320–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan MA, Knox N, Prashar A, Alexander D, Abdel-Nour M, et al. (2013) Comparative genomics reveal that host-innate immune responses influence the clinical prevalence of Legionella pneumophila serogroups. PLoS One 8(6): e67298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bardill JP, Miller JL, Vogel JP (2005) IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol 56: 90–103. [DOI] [PubMed] [Google Scholar]
- 34. Luo ZQ, Isberg RR (2004) Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci USA 101: 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chien M, Morozova I, Shi S, Sheng H, Chen J, et al. (2004) The genomic sequence of the accidental pathogen Legionella pneumophila . Science 305: 1966–1968. [DOI] [PubMed] [Google Scholar]
- 36. Cazalet C, Rusniok C, Brüggemann H, Zidane N, Magnier A, et al. (2004) Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet 36: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 37. Glöckner G, Albert-Weissenberger C, Weinmann E, Jacobi S, Schunder E, et al. (2008) Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int J Med Microbiol 298: 411–428. [DOI] [PubMed] [Google Scholar]
- 38. D'Auria G, Jiménez-Hernández N, Peris-Bondia F, Moya A, Latorre A (2010) Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 17: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder GN, Petty NK, Mousnier A, Harding CR, Vogrin AJ, et al. (2010) Legionella pneumophila strain 130b possesses a unique combination of type IV secretion systems and novel Dot/Icm secretion system effector proteins. J Bacteriol 192: 6001–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costa J, da Costa MS, Veríssimo A (2010) Colonization of a therapeutic spa with Legionella spp.: a public health issue. Res Microbiol 161: 18–25. [DOI] [PubMed] [Google Scholar]
- 41. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10): 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higgins DG (1994) CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol. 25: 307–18. [DOI] [PubMed] [Google Scholar]
- 43. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552. [DOI] [PubMed] [Google Scholar]
- 45. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 46. Hasegawa M, Kishino H, Yano T (1985) Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22(2): 160–74. [DOI] [PubMed] [Google Scholar]
- 47. Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512–526. [DOI] [PubMed] [Google Scholar]
- 48. Milne I, Wright F, Rowe G, Marshal DF, Husmeier D, et al. (2004) TOPALi: Software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20: 1806–1807. [DOI] [PubMed] [Google Scholar]
- 49. Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 50. Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19: 716–723. [Google Scholar]
- 51. Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Syst Biol 53: 793–808. [DOI] [PubMed] [Google Scholar]
- 52. Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comp Apps Biosc 8: 275–282. [DOI] [PubMed] [Google Scholar]
- 53. Abascal F, Zardoya R, Posada D (2005) ProtTest: Selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- 54. Librado P, Rozas J (2009) DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 55. Bryant D, Moulton V (2004) Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol 21(2): 255–265. [DOI] [PubMed] [Google Scholar]
- 56. Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Bio Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 57.Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN, editor. Mammalian protein metabolism. New York: Academic Press. pp 21–132. [Google Scholar]
- 58. Martin DP, Lemey P, Lott M, Moulton V, Posada D, et al. (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26: 2462–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin DP, Rybicki E (2000) RDP: detection of recombination amongst aligned sequences. Bioinformatics 16: 562–563. [DOI] [PubMed] [Google Scholar]
- 60. Padidam M, Sawyer S, Fauquet CM (1999) Possible emergence of new geminiviruses by frequent recombination. Virology 265: 218–225. [DOI] [PubMed] [Google Scholar]
- 61. Martin DP, Posada D, Crandall KA, Williamson C (2005) A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res Hum Retrovir 21: 98–102. [DOI] [PubMed] [Google Scholar]
- 62. Maynard Smith J (1992) Analysing the mosaic structure of genes. J Mol Evol 34: 126–129. [DOI] [PubMed] [Google Scholar]
- 63. Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc Natl Acad Sci USA 98: 13757–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gibbs MJ, Armstrong JS, Gibbs AJ (2000) Sister-scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16: 573–582. [DOI] [PubMed] [Google Scholar]
- 65. Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SD (2006) GARD: a genetic algorithm for recombination detection. Bioinformatics 22: 3096–3108. [DOI] [PubMed] [Google Scholar]
- 66. Delport W, Poon AF, Frost SD, Kosakovsky Pond SL (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26: 2455–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fu YX, Li WH (1993) Maximum likelihood estimation of population parameters. Genetics 134: 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura M (1983) The neutral theory of molecular evolution. Cambridge: Cambridge University Press. 384 p. [Google Scholar]
- 71. Coscollá M, Gosalbes MJ, Catalán V, González-Candelas F (2006) Genetic variation in environmental samples of Legionella pneumophila from the Comunidad Valenciana (Spain). Environ Microbiol 4: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 72. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statis Soc B 57: 289–300. [Google Scholar]
- 73. Benjamini Y, Yekutieli D (2005) False discovery rate – adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc 100: 71–93. [Google Scholar]
- 74. Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3: 418–426. [DOI] [PubMed] [Google Scholar]
- 75. Doron-Faigenboim A, Stern A, Mayrose I, Bacharach E, Pupko T (2005) Selecton: a server for detecting evolutionary forces at a single amino-acid site. Bioinformatics 21: 2101–2113. [DOI] [PubMed] [Google Scholar]
- 76.Stern A, Doron-Faigenboim A, Erez E, Martz E, Bacharach E, et al. (2007) Selecton 2007: advanced models for detecting positive and purifying selection using a Bayesian inference approach. Nucleic Acids Res 35(Web Server issue): W506–W511. [DOI] [PMC free article] [PubMed]
- 77. Swanson WJ, Nielsen R, Yang Q (2003) Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol 20: 18–20. [DOI] [PubMed] [Google Scholar]
- 78. Yang Z, Nielsen R, Goldman N, Pedersen AM (2000) Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155: 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bogardt RA, Jones BN, Dwulet FE, Garner WH, Lehman LD, et al. (1980) Evolution of the amino acid substitution in the mammalian myoglobin gene. J Mol Evol 15: 197–218. [DOI] [PubMed] [Google Scholar]
- 80. Kawashima S, Kanehisa M (2000) AAIndex: amino acid index database. Nucleic Acids Res 28: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miyata T, Miyazawa S, Yasunaga T (1979) Two types of amino acid substitutions in protein evolution. J Mol Evol 12: 219–236. [DOI] [PubMed] [Google Scholar]
- 82. Morozova I, Qu X, Shi S, Asamani G, Greenberg JE, et al. (2004) Comparative sequence analysis of the icm/dot genes in Legionella . Plasmid 51: 127–147. [DOI] [PubMed] [Google Scholar]
- 83. Ko KS, Lee HK, Park MY, Park MS, Lee KH, et al. (2002) Population genetic structure of Legionella pneumophila inferred from RNA polymerase gene (rpoB) and DotA gene (dotA) sequences. J Bacteriol 184: 2123–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brenner DJ, Staigerwalt AG, Epple P, Bibb WF, McKinney RM, et al. (1988) Legionella pneumophila serogroup Lansing 3 isolated from a patient with fatal pneumonia, and descriptions of L. pneumophila subsp. pneumophila subsp. nov., L. pneumophila subsp. fraseri subsp. nov., and L. pneumophila subsp. pascullei subsp. nov . J Clin Microbiol 26: 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hookey JV, Saunders NA, Fry NK, Birtles RJ, Harrison TG (1996) Phylogeny of Legionellaceae based on small-subunit ribosomal DNA sequences and proposal of Legionella lytica comb. nov. for Legionella-like amoebal pathogens. Int J Syst Bacteriol 46: 526–531. [Google Scholar]
- 86. Veríssimo A, Morais PV, Diogo A, Gomes C, da Costa MS (1996) Characterization of Legionella species by numerical analysis of whole-cell protein electrophoresis. Int J Syst Bacteriol 46: 41–49. [DOI] [PubMed] [Google Scholar]
- 87. Cianciotto NP (2013) Type II Secretion and Legionella Virulence. Curr Top Microbiol Immunol 376: 81–102. [DOI] [PubMed] [Google Scholar]
- 88. Coscollá M, González-Candelas F (2007) Population structure and recombination in environmental isolates of Legionella pneumophila . Environ Microbiol 9: 643–656. [DOI] [PubMed] [Google Scholar]
- 89. Edwards MT, Fry NK, Harrison TG (2008) Clonal population structure of Legionella pneumophila inferred from allelic profiling. Microbiol 154: 852–864. [DOI] [PubMed] [Google Scholar]
- 90. Kozak NA, Benson RF, Brown E, Alexander NT, Taylor TH, et al. (2009) Distribution of lag-1 alleles and sequence-based types among Legionella pneumophila serogroup 1 clinical and environmental isolates in the United States. J Clin Microbiol 47: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tao L, Zhu W, Hu BJ, Qu JM, Luo ZQ (2013) Induction of rapid cell death by an environmental isolate of Legionella pneumophila in mouse macrophages. Infect Immun 81(9): 3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reed FA, Tishkoff SA (2006) Positive selection can create false hotspots of recombination. Genetics 172: 2011–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Veríssimo A, Marrão G, da Silva FG, da Costa MS (1991) Distribution of Legionella spp. in hydrothermal areas in continental Portugal and the island of São Miguel, Azores. Appl Environ Microbiol 57: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marrão G, Veríssimo A, Bowker RG, da Costa MS (1993) Biofilms as major sources of Legionella spp. in hydrothermal areas and their dispersion into stream water. FEMS Microbiol Ecol 12: 25–33. [Google Scholar]
- 95. Veríssimo A, Vesey G, Rocha GM, Marrão G, Colbourne J, et al. (1990) A hot water supply as the source of Legionella pneumophila in incubators of a neonatology unit. J Hosp Infect 15: 255–263. [DOI] [PubMed] [Google Scholar]
- 96. Thacker WL, Benson RF, Wilkinson HW, Ampel NM, Wing EJ, et al. (1986) 11th serogroup of Legionella pneumophila isolated from a patient with fatal pneumonia. J Clin Microbiol 23: 1146–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. McKinney RM, Wilkinson HW, Sommers HM, Fikes BJ, Sasseville KR, et al. (1980) Legionella pneumophila serogroup six: isolated from cases of Legionellosis, identification by immunofluirescence staining, and immunological response to infection. J Clin Microbiol 12: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bissett ML, Lee JO, Lindquist DS (1983) New serogroup of Legionella pneumophila, serogroup 8. J Clin Microbiol 17: 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McKinney RM, Thacker L, Harris PP, Lewallen KR, Herbert GA, et al. (1979) Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann Inter Med 90: 621–624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the operon-like structure comprising some members of the sidE family, namely sedC , laiE , sidJ , sedB and sedA in L. pneumophila Philadelphia 1 (lpg2153, lpg2154, lpg2155, lpg2156 and lpg2157, respectively). Primers used for PCR amplifications are also represented (Table S2).
(DOCX)
Locus tag and accession numbers from the L. pneumophila unrelated strains, isolated from distinct environments, type and reference strains included in this study.
(DOCX)
Primers and their sequences designed in this study.
(DOCX)
Genetic pairwise differences, average and standard deviation (SD) for (A) and between (B) sidJ and rpoB clusters. The highest population pairwise differences, average and standard deviation for each gene are marked in bold.
(DOCX)
Potential recombinant events (PRE) identified with RDP3 from the alignment of sidJ obtained from 32 L. pneumophila strains. The minimum number of independent recombination events (IREs) within each identified PRE was inferred by a minimum of four methods and were mapped on the phylogenetic tree (Fig. 3).
(DOCX)
D (Tajima), D* and F* (Fu and Li) and Fs (Fu) statistics obtained from sidJ .
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All sidJ sequences are available from EMBL Nucleotide Sequence Database with Accession No. HG531934–HG531954.