Abstract
Background
Ceratopteris richardii is a useful experimental system for studying gametophyte development and sexual reproduction in plants. However, few tools for cloning mutant genes or disrupting gene function exist for this species. The feasibility of systemic gene silencing as a reverse genetics tool was examined in this study.
Results
Several DNA constructs targeting a Ceratopteris protoporphyrin IX magnesium chelatase (CrChlI) gene that is required for chlorophyll biosynthesis were each introduced into young gametophytes by biolistic delivery. Their transient expression in individual cells resulted in a colorless cell phenotype that affected most cells of the mature gametophyte, including the meristem and gametangia. The colorless phenotype was associated with a 7-fold decrease in the abundance of the endogenous transcript. While a construct designed to promote the transient expression of a CrChlI double stranded, potentially hairpin-forming RNA was found to be the most efficient in systemically silencing the endogenous gene, a plasmid containing the CrChlI cDNA insert alone was sufficient to induce silencing. Bombarded, colorless hermaphroditic gametophytes produced colorless embryos following self-fertilization, demonstrating that the silencing signal could be transmitted through gametogenesis and fertilization. Bombardment of young gametophytes with constructs targeting the Ceratopteris filamentous temperature sensitive (CrFtsZ) and uroporphyrin dehydrogenase (CrUrod) genes also produced the expected mutant phenotypes.
Conclusion
A method that induces the systemic silencing of target genes in the Ceratopteris gametophyte is described. It provides a simple, inexpensive and rapid means to test the functions of genes involved in gametophyte development, especially those involved in cellular processes common to all plants.
Background
Plants differ from animals by incorporating into their life cycle a multicellular, haploid phase, the gametophyte, which alternates with a diploid, sporophyte phase. Although the gametophyte is extremely reduced and inconspicuous in flowering plants, it is essential for sexual reproduction in all land plants as it produces gametes, facilitates fertilization, and, for at least a brief time, nurtures the young embryo. We have used the homosporous fern Ceratopteris richardii as a model system for studying gametophyte development because Ceratopteris gametophytes are autotrophic, small (~1 mm) and develop rapidly [1]. They can also be manipulated to develop as males or hermaphrodites by the pheromone antheridiogen [2] and are easily crossed. Because all gametophytes are haploid, mutations affecting the gametophyte development are easily selected within days of spore mutagenesis and growth on selective medium. While Ceratopteris has proven to be a useful genetic system for dissecting its sex determination pathway [3-5], it has yet to be stably transformed, which makes it difficult to clone genes known only for their mutant phenotype or to test the functions of gametophytically expressed genes.
Recent advances in epigenetic gene silencing have led to its use as a reverse genetics tool for examining gene function in plants and animals [6-13]. Referred to as post-transcriptional gene silencing (PTGS), co- or sense-suppression, antisense suppression, quelling or RNA interference (RNAi) depending on the organism or the method employed, these processes result in post-transcriptional and sequence-specific gene silencing upon introduction of a transgene or double-stranded RNA (dsRNA) (reviewed in [14-16]). What links these processes together is the presence of small 21 – 23 nt RNA molecules that mediate the degradation of complementary homologous RNA. Genetic screens in Arabidopsis thaliana, Caenorhabditis elegans and Neurospora crassa have identified homologous genes required for gene silencing [17-20], indicating that they share a common and evolutionarily conserved mechanism that is likely to be present in all plants, including ferns. One striking feature of gene silencing is that the silencing effects are non-cell autonomous and spread to neighboring cells. For this reason, transient expression rather than stable integration of a transgene is sufficient to induce phenotypes resulting from gene silencing.
This study investigates the feasibility of DNA vector based gene silencing as a reverse genetics technique for studying gene function in gametophytes. Our selection of Ceratopteris genes to target was based upon three criteria: they result in visible phenotypes when mutated in other flowering plants; they are expressed in the gametophyte; and they are present in a Ceratopteris EST library generated from germinating spores [21]. The three genes selected included protoporphyrin IX magnesium chelatase (CrChlI), filamentous temperature sensitive Z (CrFtsZ), and uroporphyrin dehydrogenase (CrUrod), which are necessary for chlorophyll biosynthesis or chloroplast development. Here we show that systemic gene silencing occurs in Ceratopteris gametophytes when appropriate transgene constructs are introduced into young gametophytes by particle bombardment, that the silencing effects are non-cell autonomous, and that the phenotype resulting from gene silencing can be transmitted from the gametophyte to the sporophyte generation, although at low frequencies. By comparing the efficiency of various gene-silencing constructs, we also show that cDNA constructs without any recognizable promoter sequence are sufficient to induce silencing in gametophytes at high frequencies.
Results and discussion
Biolistic introduction of a CrChlI potential hairpin-forming construct suppresses the endogenous CrChlI gene
A Ceratopteris EST that encodes a putative protein that is >75% identical in amino acid sequence to the barley ChlI protein was initially selected to test silencing of endogenous genes in Ceratopteris. This gene, which is required for chlorophyll biosynthesis, was chosen because its inactivation results in an easily scorable phenotype. In barley, plants homozygous for ChlI mutations are yellow-seedling lethal, whereas heterozygotes are yellow-green [22]. Because the introduction of silencing transgenes by biolistic bombardment of tissues has been shown to trigger systemic gene silencing in angiosperms [23-26], the same method was applied here. Hermaphroditic Ceratopteris gametophytes were initially bombarded with the 35S:irintCrChlI plasmid (Fig. 1), which potentially drives the expression of a dsRNA hairpin with an intron loop that, if spliced, forms a 393 bp double-stranded CrChlI RNA molecule. Such an inverted repeat construct design is known to be very efficient in inducing gene silencing in flowering plants [13]. At the time of bombardment, 6d-old hermaphrodites are very small (~0.3 mm) and beginning to initiate a lateral, multicellular meristem (Fig. 2A). The simple morphology of the hermaphroditic gametophyte, which consists of a single layer of cells dotted with egg-forming archegonia and sperm-forming antheridia, makes it simple to detect gene silencing in all cells of the gametophyte while its gender makes it possible to self-fertilize gametophytes and assess the transmission of a silenced phenotype to the sporophyte generation following fertilization. If silencing of the endogenous CrChlI gene spreads from the bombarded to neighboring cells, gametophytes co-bombarded with 35S:irintCrChlI and the GUS reporter pFF19G plasmid (Fig. 1) were expected to develop a sectored or completely yellow-to-colorless prothallus but express GUS in only a single cell of the gametophyte that was present at the time of bombardment. As shown in Figure 2, hermaphrodites co-bombarded with both plasmids generated colorless cells throughout most of their prothalli, including the meristem, antheridia and archegonia, yet displayed GUS activity only in older cells of the gametophyte. While GUS activity could be detected in several adjacent cells (Fig. 2C), this is most likely due to diffusion of either the GUS protein or reaction product from an individual cell transiently expressing the GUS gene. Only one GUS positive sector was observed in >99% of all GUS positive gametophytes and more than two GUS sectors never observed. Of the gametophytes co-bombarded with the 35S:irintCrChlI and pFF19G and having a colorless or GUS positive phenotype, ~89% were both colorless and GUS positive seven days after bombardment (Table 1), indicating a high efficiency of introduction of both plasmids into the same cell. These results demonstrate that the presence of this CrChlI expression construct in one or a small number of cells is sufficient to inactivate the endogenous CrChlI gene in almost all cells of the growing prothallus leading to a colorless phenotype, especially in cells formed after bombardment.
Figure 1.
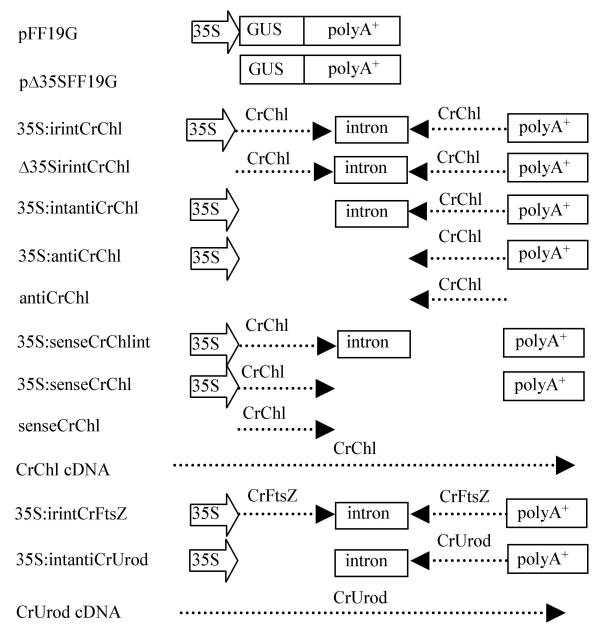
DNA constructs used to bombard gametophytes. Plasmid sequences are not shown.
Figure 2.
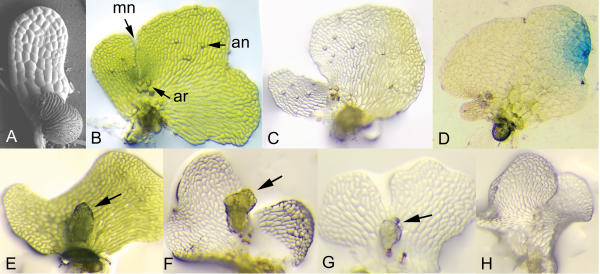
Gametophyte phenotypes after bombardment with 35S:irintCrChlI. (A) Scanning electron microscopy of 6 day old hermaphrodite initiating a lateral meristem. (B) Non-bombarded 15 day-old hermaphrodite showing meristem notch (mn), archegonia (ar) and antheridia (an). (C) A 15d-old hermaphrodite 8d after bombardment with a large colorless sector including the meristem and gametangia. (D) Bombarded colorless gametophyte stained for GUS activity. (E)-(H) Phenotypes of self-fertilized colorless gametophytes ~3 weeks after bombardment. Embryos are indicated by arrows.
Table 1.
Frequencies of gametophyte phenotypes after bombardment with various plasmids.
| Plasmids introduced | % colorless & GUS positivea,b | % colorless & GUS negativea | % green & GUS positivea | nc |
| 35S:irintCrChl+pFF19G | 88.8 (+/-1.36)d | 6.8 (+/-0.46)d | 4 (+/- 1.41)d | 1263 |
| irintCrChl+pFF19G | 81.7 (+/-0.87) | 8.3 (+/-1.20) | 10 (+/-1.53) | 775 |
| 35S:intantiCrChl+pFF19G | 61.0 (+/-1.73) | 11.0 (+/-1.73) | 28.0 (+/-3.21) | 564 |
| 35S:antiCrChl+pFF19G | 12.0 (+/-1.53) | 0 | 88.0 (+/-1.53) | 724 |
| antiCrChl+pFF19G | 0.7 (+/-0.33) | 0 | 99.3 (+/-0.33) | 479 |
| 35S:senseCrChlint+pFF19G | 37.7 (+/-1.12) | 6.3 (+/-0.19) | 56 (+/-1.00) | 559 |
| 35S:senseCrChl+pFF19G | 2.3 (+/-0.88) | 0 | 97.7 (+/-0.88) | 761 |
| senseCrChl+pFF19G | 1.7 (+/-0.33) | 0 | 98.7 (+/-0.42) | 677 |
| CrChl cDNA+pFF19G | 13.7 (+/-0.88) | 0 | 86.0 (+/-1.16) | 563 |
| Δ35SpFF19G+ | 48.5 (+/-6.36) | 51.0 (+/-5.66) | 0.5 (+/-0.71) | 470 |
| 35SinintCrChl | ||||
| pFF19G | 0 | 0 | 100 | 600 |
| % with lesions & GUS positivea,b | % with lesions & GUS negativea | % without lesions & GUS positive | nc | |
| CrUrod cDNA+pFF19G | 61.0 (+/-0.71) | 20.2 (+/-0.69) | 18.9 (+/-0.43) | 774 |
| 35S:intantiCrUrod+pFF19G | 55.7 (+/-1.40) | 13.5 (+/-1.61) | 31.1 (+/- 2.25) | 472 |
aAll gametophytes were stained 7 days after shooting; each percent represents the average of three replicates; standard error is given in parentheses. bthe differences between treatments for all pairwise comparisons are statistically significant (z>3.4, P < 0.0003) with the exception of 35S:senseCrChl+pFF19G and senseCrChl+pFF19G. ctotal number of gametophytes displaying a phenotype and scored. dthe average of 5 replicates.
The relative abundance of endogenous CrChlI transcripts in silenced gametophytes was assessed by quantitative real-time RT-PCR using cDNA generated from green and colorless gametophytes 6d after bombardment with the 35S:irintCrChlI plasmid. The abundance of CrChII was normalized to the abundance of the Ceratopteris EF1α gene in both gametophyte populations; the latter did not vary between the two populations (Fig. 3A). A ~7-fold decrease in the abundance of CrChIl transcripts was observed in colorless gametophytes compared to green gametophytes (Fig. 3B) indicating that the silencing mechanism interferes either with CrChlI transcription or the stability of the endogenous CrChlI transcripts.
Figure 3.
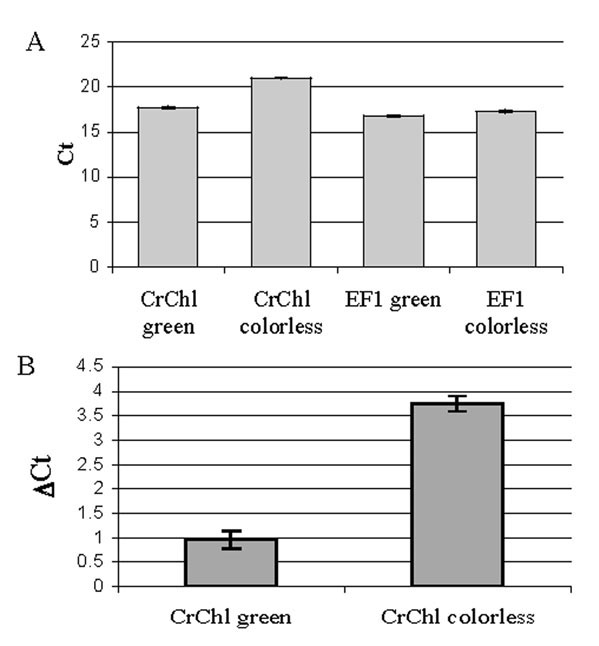
Results of quantitative PCR comparing CrChlI message abundance in green and colorless gametophytes. (A) Ct values using PCR templates derived from green and colorless gametophytes and primers specific for either the CrChlI or the Ceratopteris EF1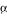 gene. (B) ΔCt values of CrChlI abundance, normalized to EF1α, in green and colorless gametophytes.
gene. (B) ΔCt values of CrChlI abundance, normalized to EF1α, in green and colorless gametophytes.
Endogenous gene silencing in the gametophyte is both reversible and heritable
The heritability of the colorless phenotype of gametophytes bombarded with 35S:irintCrChlI was assessed by placing 195 colorless hermaphrodites individually into microtiter wells and adding water, allowing sperm to swim to and fertilize the egg. After three weeks, four classes of gametophytes, illustrated in Figure 2E,2F,2G,2H, were observed, including those that turned green and produced a green embryo (32%; Fig. 2E); those that remained colorless and produced a green embryo (26%; Fig. 2F); those that remained colorless and produced a colorless embryo (7%) that did not develop beyond the stage illustrated in Figure 2G; and those that remained colorless and produced no embryo (35%; Fig. 2H). The inability of many colorless gametophytes to form sporophytes was not due to the lack of antheridia, motile sperm or archegonia as these structures appeared to develop normally, but may be due to the lack of sufficient photosynthate to support embryonic growth after fertilization. The relative proportions of each gametophyte class indicate that the silenced CrChlI gene is almost equally likely to reactivate or remain silenced in the gametophyte, but tends to reactivate upon or after fertilization. Reactivation of the endogenous CrChlI gene, reflected by the greening of colorless gametophytes, also indicates that CrChlI silencing is reversible and, therefore, epigenetic. Although the percentage of colorless embryos that developed from colorless gametophytes was low, their presence demonstrates that the silenced state can be maintained through gametogenesis and fertilization and can be transmitted to the next sporophyte generation. Because colorless sporophytes did not develop beyond the embryonic stage, it was not possible to assess whether the colorless phenotype could be maintained through meiosis and the subsequent gametophyte generation.
Defining transgene elements required for gene silencing
While a potential hair-pin forming construct was found to be effective in promoting the systemic silencing of the endogenous CrChlI gene, the assembly of this construct involves a two-step cloning procedure, which is a limitation for high throughput screening of gene function. For this reason, a variety of elements were removed from the inverted repeat-intron construct (illustrated in Fig. 1) and their ability to induce gene silencing assessed.
To determine if silencing of the endogenous CrChlI gene requires a promoter, the 35S promoter was deleted to give Δ35S:irintCrChlI; gametophytes were co-bombarded with this plasmid plus pFF19G. Surprisingly, the absence of the 35S promoter resulted in a high percentage of colorless gametophytes, with ~82% of the colorless and/or GUS-positive gametophytes displaying both phenotypes (Table 1), indicating that either the CrChlI DNA sequences alone are sufficient for gene silencing, or that transcription of irintCrChlI can occur and promote gene silencing in the absence of a plant promoter. To test the latter possibility, gametophytes were co-bombarded with two constructs: the 35S:irintCrChlI plasmid as a marker for transformation; and the pFF19G construct from which the 35S promoter had been removed (to give Δ35S:GUS). The latter construct was used as a marker for in vivo gene expression of the GUS gene in the absence of a plant promoter. About one-half of the colorless gametophytes that developed after bombardment were also GUS positive (Table 1), although the intensity of GUS staining was less than that observed in gametophytes bombarded with pFF19G (data not shown). No gametophytes bombarded with 35S:irint, a plasmid lacking the GUS reading frame (see materials and methods) stained positive for GUS activity (data not shown). Since a promoterless GUS construct was found to promote GUS activity in the gametophyte, transcription of the CrChl1I silencing transgene is also likely to occur in the absence of a plant promoter. The systemic silencing of genes using promoterless silencer constructs similar to that described here has also been observed in angiosperms [23].
To address the contribution of the intron, the 35S promoter and a second copy of the transgene to CrChlI silencing in the gametophyte, each was deleted from 35S:irintCrChlI then co-bombarded with pFF19G into gametophytes. In comparing constructs with the CrChlI fragment cloned in an antisense orientation, the proportion of colorless, GUS positive gametophytes decreased ~28% with the deletion of the sense copy of CrChlI from 35S:irintCrChlI (giving 35S:intantiCrChlI) and decreased a further 49% when the intron was also deleted (giving 35S:antiCrChlI) (Table 1). Almost no colorless gametophytes were observed when bombarded with the antiCrChlI plasmid, which lacks the 35S promoter, intron, poly(A)+ signal and the second (sense) copy of CrChlI. The same trend was observed when gametophytes were bombarded with the CrChlI fragment cloned in the sense orientation (Table 1). These results indicate that the intron plays an important but unknown role in gene silencing and that the intron and the 35S promoter together enhance the efficiency of endogenous gene silencing. In angiosperms, an intron used to separate the two copies of a gene in an inverted orientation (as in the 35S:irintCrChlI construct) also increases the efficiency of gene silencing, although how this occurs is unclear [13].
To determine the efficiency of CrChlI silencing by the unmodified CrChlI cDNA from which the CrChlI EST was originally derived, gametophytes were co-bombarded with pFF19G plus the CrChlI cDNA plasmid (Fig. 1). About 14% of the GUS positive gametophytes were colorless (Table 1), which is significantly greater than the ~1–2% observed following bombardment with the antiCrChlI or senseCrChlI plasmids that also lack a 35S promoter and intron sequences (Table 1). The differences in frequency observed are likely due to differences in the size of the CrChlI insert, as the senseCrChlI and antiCrChlI plasmids harbor a 393bp cDNA insert while the cDNA plasmid harbors a 1.2kb cDNA insert. A similar correlation between the length of the silencer transgene and silencing efficiency has been observed in angiosperms [26].
CrFtsZ and CrUrod gene silencing in the gametophyte
Gametophytes were bombarded with two additional genes selected from a Ceratopteris EST library to further test the application of gene silencing as a reverse genetics tool in Ceratopteris gametophytes. The two chosen, CrFtsZ and CrUrod, encode putative proteins that are at least 70% identical in amino acid sequence to the FtsZ and Urod proteins in angiosperms. The FtsZ gene encodes a tubulin-like component of the filamentous plastoskeleton and the plastid division ring, which is essential for chloroplast division in plants [27-29]. While Arabidopsis has four FtsZ genes representing two gene families that differ in the cellular targeting of the FtsZ protein [28,30], inactivation of a single member of either family results in cells with fewer and larger chloroplasts, a phenotype that should be easy to visualize in Ceratopteris gametophytes. Following bombardment with the inverted repeat-intron-forming CrFtsZ construct 35S:irintCrFtsZ (Fig. 1), hermaphrodites developed a prothallus with cells having larger and as few as three chloroplasts per cell compared to non-bombarded gametophytes with >50 chloroplasts per cell (Fig. 4A,4B). The larger-and-fewer chloroplast phenotype was not evident until three weeks after bombardment, indicating that the phenotype requires new cells divisions to occur. Because GUS activity fades in time and is usually undetectable three weeks after bombardment, the frequencies of GUS positive and CrFtsZ silenced phenotypes were impossible to determine for this gene. Gametophytes with altered chloroplast morphology also formed shallow meristems and no functional archegonia or antheridia, preventing self-fertilization of the affected hermaphrodites (Fig. 4C). Whether a functional CrFtsZ gene product is directly or indirectly involved in the development of meristem and gametangia is unknown, but this result suggests that CrFtsZ inactivation also can affect the differentiation of the gametangia and the organization of the multicellular meristem.
Figure 4.
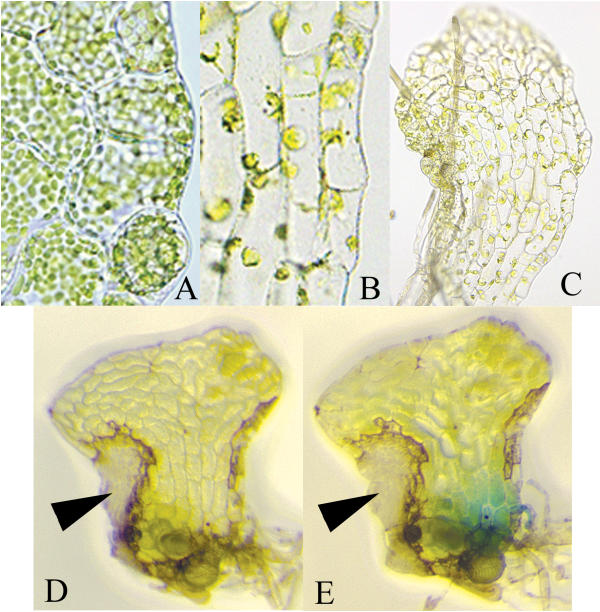
Phenotypes of sporophytes and gametophytes bombarded with various plasmids. (A) Chloroplasts of non-bombarded gametophyte. (B) Chloroplastsof gametophyte ~3 weeks after bombardment with the 35S:irintCrFtsZ plasmid. (C) Hermaphrodite prothallus ~3 weeks after bombardment with 35S:irintCrFtsZ. (D), (E) Gametophyte 7d after bombardment with 35S:irtantiCrUrod before (D) and after (E) staining for GUS activity. The lesion associated with the inactivation of the CrUrod gene is indicated by an arrow.
The Urod gene encodes an enzyme that catalyzes the decarboxylation of uroporphyrinogen III to coproporphyrinogen III, a precursor of chlorophyll and heme production in plants [31,32]. Maize plants heterozygous for Les22, a mutation of a Urod gene, develop discrete, tiny, colorless or necrotic spots on their leaf blades, while homozygous plants are seedling lethal [33]. The lesions in Les22 heterozygotes result from the accumulation of uroporphyrin, which is toxic to cells upon exposure to light. Inactivation of the endogenous Ceratopteris CrUrod gene was expected either to inhibit growth of the gametophyte or lead to gametophytes that developed necrotic lesions of unknown size. About 60% of the hermaphrodites co-bombarded with the construct 35S:intantiCrUrod (Fig. 1) and pFF19G developed one or two discrete necrotic lesions within one week of bombardment (Figs. 4D,4E) and were also GUS positive (Table 1). Each lesion consisted of several adjacent colorless cells that eventually died. GUS staining could be detected only in cells of the mature gametophyte that were likely present at the time of bombardment. The GUS-positive cells may have survived following bombardment with the 35S:intantiCrUrod construct because each had produced sufficient CrUROD protein prior to bombardment and were thus able to catalyze the decarboxylation of uroporphyrin. Of the gametophytes that developed lesions or were GUS positive following bombardment with the original CrUrod cDNA plasmid plus pFF19G, 61% were both GUS positive and developed lesions (Table 1), a percentage similar to that obtained when gametophytes were bombarded with the intron-antisense construct 35S:intantiCrUrod plus pFF19G.
Application of gene silencing for high throughput analyses of gene function
Our results show that DNA vector-based gene silencing in the fern gametophyte is effective in systemically inactivating targeted genes and results in a gene-specific mutant phenotype that is apparent throughout most of the gametophyte. While PTGS and RNAi have been shown to silence genes in flowering plants, the fern gametophyte offers many technical advantages as a system for studying gene function. One is that the method for generating a silenced phenotype is rapid and robust, requiring only a cDNA plasmid clone, a biolistic apparatus and a plate of six-day-old Ceratopteris gametophytes. Since each gametophyte matures quickly and is not much larger than a yeast colony, large numbers (>100) of transformed and gene-silenced gametophytes can be generated with each bombardment and identified one to two weeks following bombardment. For this reason, it is feasible to bombard and test a minimum of 500 different cDNA clones within a two-week period, making this method suitable for high throughput testing of gene function. Like an onion epidermal peel, each hermaphroditic gametophyte is a single cell layer thick, making phenotypes easy to observe without interference from adjacent cell layers. Yet another technical advantage of the fern gametophyte is that lethal phenotypes associated with the inactivation of essential genes, such as the CrUROD gene, can be easily recognized because the gametophyte is not bombarded until after it has germinated from the spore. By varying the age of the gametophyte at the time of bombardment, one can control when during development gene silencing occurs.
The method described will be useful for identifying the biological functions of genes that are involved in post-germination processes in the gametophyte, including meristem development, archegonia, antheridia, sperm and egg differentiation, sperm chemotaxis, fertilization and early embryo development. Most of these processes are hallmarks of the plant gametophyte that are difficult to study in flowering plants because their gametophytes develop embedded within sporophytic tissues of the flower. Because the gold particles cannot penetrate the spore coat, genes that control processes that only occur prior to the emergence of the prothallus cannot be silenced using the method described in this study. Germination, the initial cell division, establishment of polarity as well as sex determination are among the processes that occur during these early days of growth [5]. Recently, an RNAi method first described in Marsilea vestita [34-36] was applied to Ceratopteris spores [21]. In this study, spores were incubated in the presence of in vitro synthesized dsRNA corresponding to each of five genes known to be expressed in germinating spores. Although phenotypes associated with each treatment were not reported, mixing spores with dsRNA was shown to reduce steady state mRNA levels after a 24 hr incubation period. Should this method prove fruitful in generating informative phenotypes, the combination of the two techniques will allow the examination of gene function throughout all stages of gametophyte development.
Conclusions
1) The expression of endogenous genes in the fern gametophyte can be systemically suppressed by introducing a transgene construct into single cells of the gametophyte by particle bombardment.
2) DNA constructs that promote the expression of potential hairpin-intron loop or antisense transcripts are the most effective, although constructs having a promoterless gene are sufficient in inducing systemic gene silencing in the gametophyte.
3) The silencing of the three genes tested (CrChlI, CrFtsZ and CrUrod) resulted in phenotypes that mimic mutant phenotypes in other plants.
4) The method developed is a useful reverse genetic tool to quickly and efficiently screen the functions of gametophytically expressed genes.
Methods
Gametophyte culture and imaging
The hermaphroditic her19 mutant used in this study is described in Eberle and Banks [37]. Hermaphrodite cultures were generated from sterilized spores plated on fern medium, or FM, consisting of 0.5× MS salts, pH 6.5 as previously described [37]. In preparation for bombardment, spores suspended in water were plated on 60 mm petri dishes at a density of 2500–3000 spores per dish. Cultures were then placed in plastic bags to maintain high humidity and incubated at 30°C. To self-fertilize gametophytes, individual virgin hermaphrodites were placed into the wells of 96-well microtiter plates containing FM plus agar; enough water to submerge each gametophyte was then added to each well. Electron microscopy was done as previously described [2]. All light photography was done using a Spot II camera attached to a Leica dissecting microscope; images were processed with Adobe Photoshop.
Gametophyte bombardment
Several parameters were optimized before using the following conditions for bombardment, including the age of the gametophyte at time of bombardment, the amount of DNA delivered, gold size, rupture disc type, and shelf placement in the biolistic apparatus. 1.6 μm gold was prepared and coated with unmodified plasmid DNA purified using a Qiagen kit (Qiagen, CA) prior to bombardment using a PDS 1000 Helium System, all according to manufacturer's instructions (BioRad, CA). Gametophyte cultures were placed on a shelf 9 cm below the stopping screen of the apparatus; 1100 psi rupture discs were used. Gametophytes were bombarded 6 days after spore inoculation, and the amount of DNA delivered was 0.7 μg per shot. Unless indicated otherwise, gametophytes were scored for phenotype and histologically stained for beta-glucuronidase (GUS) activity [38] 6 days after bombardment. In comparing the efficiencies of different plasmid constructs in inducing gene silencing, the null hypothesis (i.e., the efficiency of gene inactivation between two different constructs is the same) was tested by applying the z test for two proportions.
DNA constructs
A skeleton silencing DNA vector, named 35S:irint, into which a targeted gene could be easily cloned in opposing orientations separated by an intron was made by removing the GUS coding region from pFF19G [39] by digestion with Nru1 and Pst1 and replacing the GUS fragment with another containing the castor bean catalase intron 1 [40]. This intron was PCR amplified using pIGI121Hm [41] as template and the two adapter/primer sequences: 5' CGACGGACCGATCTAGAACATGGATCCCTACAGGGTA and 5' TCAGCTGCAGACTAGTTACAGGACGGACGAGTCGACGGTTC. The PCR product was digested with Pst1 and NruI then ligated to the pFF19G-minus GUS vector.
The genes targeted for silencing were chosen from a Ceratopteris EST database of ~3700 cDNAs generated from germinating, 20 hour-old spores [21]. The cloning vector for the cDNA library from which ESTs were derived was pCMVSPORT6 (Invitrogen, CA). The inverted repeat-intron CrChlI (Cr referring to Ceratoperis richardii) gene silencing construct (35S:irintCrChlI) was made by PCR amplifying two 393 bp fragments from a Ceratopteris cDNA clone (GenBank accession number BE642494) using the primer pairs 5'GATACGGACCGGTTCTGGCAATCCAGAGGAA and 5'ATGCGGATCCAAGGCAATTGGGAATCACTG for cloning CrChlI in the sense orientation, and 5' GACGGTCGACAAGGCAATTGGGAATCACTG and 5'CGTAACTAGTGTTCTGGCAATCCAGAGGAA for cloning CrChlI in the antisense orientation. Constructs for targeting the CrFtsZ gene (35S:irintCrFtsZ) were made by PCR amplifying two 435 bp CrFtsZ fragments from a Ceratopteris cDNA clone (GenBank accession number BE64351) using the primer pairs 5'CATACGGACCGGCTCTTGAGGCCATTGAAAG and 5'ATGCGGATCCGGATCAGCCAAGCTGGTAAC for cloning in the sense orientation, and 5'CGTAACTAGTGCTCTTGAGGCCATTGAAAG and 5'GACGGTCGACGGATCAGCCAAGCTGGTAAC for cloning in the antisense orientation. All sense orientation PCR fragments were digested with RsrII plus BamHI and cloned into the same sites of the skeleton silencing vector. All antisense orientation PCR fragments were digested with SalI and SpeI and cloned into the same sites of silencing vector. The promoterless construct irintCrChlI was made by removing a 791 bp HindIII- RsrII fragment that contains the enhanced 35S promoter. Additional CrChlI constructs were generated by deleting various sequences from 35S:irintCrChlI by digestion with appropriate restriction enzymes followed by religation of the plasmid, or were generated as intermediates during the cloning of the final 35S:irintCrChlI construct. The antisense construct for silencing the endogenous CrUrod gene (GenBank accession number BE642240) was made by PCR amplifying a 413 bp CrUrod fragment from the appropriate Ceratopteris cDNA clone using the primers 5'CGTAACTAGTGCTGAGAAGCACCCCAGTTTC3' and 5'GACGGTCGACAAAGACTTTGGGTGCCTGATG3'. The SalI and SpeI digested PCR fragments were then cloned into the SalI and SpeI sites of 35S:irint.
Quantitative PCR
RNA was isolated from pools of 80 green and 80 colorless gametophytes one week after bombardment with the 35S:irintCrChlI plasmid using an RNeasy Plant Mini kit (Qiagen, CA). Total RNA was reverse transcribed using 200 U SuperscriptII reverse transcriptase (Invitrogen, CA) in the presence of d(T)15. Single-stranded cDNA was then used as a template in a real time PCR reaction using SYBR green PCR Master Mix from Applied Biosystems. Approximately 2 ng of cDNA, corresponding to the amount of RNA isolated from 3 gametophytes, was used as a template. The reactions were performed in an ABI Prism 7700 machine with real-time SYBR Green I detection using default parameters and the primers 5'AACGAGCAGGATGTGAAATG3' and 5'AACGAGCAGGATGTGAAATCG3'. Reactions were performed in quadruplicate for each template to assess standard deviation of threshold cycle (Ct) measurements of the amount of CrChlI transcripts in the green and colorless samples. Each measurement was normalized to the amount of CrEF1α (•Ct) (GenBank accession number BE642078) transcript using the same amplification conditions and the primers 5' CAGACCAGTCGGAGCAAAAGT3' and 5'TCCTGTGGGAAGGGTGGAA3'. The fold decrease in abundance of CrChlI in green and colorless gametophytes is equal to 2•(•Ct).
Authors' contributions
GR made the constructs. MT carried out the plant transformations and molecular analysis of the transgenic plants. GR, MT and JB participated in the design of this study. JB conceived the study, participated in its design and drafted the manuscript together with MH and MT. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Luke Gumaelius, Drew Schultz, Amber Hopf, and Erlinda Embuscado for technical assistance, insightful discussions and help with harvesting plants and spores, and thank Kenzo Nakamura for graciously providing the pIG121Hm plasmid. We also thank Dr. Stan Roux for providing the cDNA library from which ESTs were generated at the Purdue University Genomics Facility. This work was supported by the National Science Foundation and the Purdue Agricultural Research Programs.
Contributor Information
George Rutherford, Email: gruther@bilbo.bio.purdue.edu.
Milos Tanurdzic, Email: milos@purdue.edu.
Mitsuyasu Hasebe, Email: mhasebe@nibb.ac.jp.
Jo Ann Banks, Email: banksj@purdue.edu.
References
- Chatterjee A, Roux SJ. Ceratopteris richardii: a productive model for revealing secrets of signaling and development. J Plant Growth Regul. 2000;19:284–289. doi: 10.1007/s003440000032. [DOI] [PubMed] [Google Scholar]
- Banks JA, Hickok L, Webb MA. The Programming of Sexual Phenotype in the Homosporous Fern ceratopteris-richardii. Int J Plant Sci. 1993;154:522–534. doi: 10.1086/297135. [DOI] [Google Scholar]
- Banks JA. The TRANSFORMER genes of the fern Ceratopteris simultaneously promote meristem and archegonia development and repress antheridia development in the developing gametophyte. Genetics. 1997;147:1885–1897. doi: 10.1093/genetics/147.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain E, Hass B, Banks JA. Characterization of mutations that feminize gametophytes of the fern Ceratopteris. Genetics. 2001;159:1271–1281. doi: 10.1093/genetics/159.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JA. Gametophyte development in ferns. Annu Rev Plant Phys. 1999;50:163–+. doi: 10.1146/annurev.arplant.50.1.163. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Chuang CF, Meyerowitz EM. Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. P Natl Acad Sci USA. 2000;97:4985–4990. doi: 10.1073/pnas.060034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Echeverri C, Oegema K, Coulson A, Jones SJ, Copley RR, Duperon J, Oegema J, Brehm M, Cassin E, Hannak E, Kirkham M, Pichler S, Flohrs K, Goessen A, Leidel S, Alleaume AM, Martin C, Ozlu N, Bork P, Hyman AA. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–176. doi: 10.1016/S0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Misquitta L, Paterson BM. Targeted disruption of gene function in Drosophila by RNA interference (RNA-i): a role for nautilus in embryonic somatic muscle formation. Proc Natl Acad Sci U S A. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutjesdijk PA, Singh SP, Liu Q, Hurlstone CJ, Waterhouse PA, Green AG. hpRNA-mediated targeting of the Arabidopsis FAD2 gene gives highly efficient and stable silencing. Plant Physiol. 2002;129:1723–1731. doi: 10.1104/pp.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 2001;27:581–590. doi: 10.1046/j.1365-313X.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Pickford AS, Catalanotto C, Cogoni C, Macino G. Quelling in Neurospora crassa. Adv Genet. 2002;46:277–303. doi: 10.1016/S0065-2660(02)46010-5. [DOI] [PubMed] [Google Scholar]
- Vaucheret H, Beclin C, Fagard M. Post-transcriptional gene silencing in plants. J Cell Sci. 2001;114:3083–3091. doi: 10.1242/jcs.114.17.3083. [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Isolation of quelling-defective (qde) mutants impaired in posttranscriptional transgene-induced gene silencing in Neurospora crassa. Proc Natl Acad Sci U S A. 1997;94:10233–10238. doi: 10.1073/pnas.94.19.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogoni C, Macino G. Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature. 1999;399:166–169. doi: 10.1038/20215. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Plasterk RH. A genetic link between co-suppression and RNA interference in C. elegans. Nature. 2000;404:296–298. doi: 10.1038/35005113. [DOI] [PubMed] [Google Scholar]
- Mourrain P, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Jouette D, Lacombe AM, Nikic S, Picault N, Remoue K, Sanial M, Vo TA, Vaucheret H. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell. 2000;101:533–542. doi: 10.1016/S0092-8674(00)80863-6. [DOI] [PubMed] [Google Scholar]
- Stout SC, Clark GB, Archer-Evans S, Roux SJ. Rapid and efficient suppression of gene expression in a single-cell model system, Ceratopteris richardii. Plant Physiol. 2003;131:1165–1168. doi: 10.1104/pp.016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A, Kannangara CG, von Wettstein D, Hansson M. Molecular basis for semidominance of missense mutations in the XANTHA-H (42-kDa) subunit of magnesium chelatase. Proc Natl Acad Sci U S A. 1999;96:1744–1749. doi: 10.1073/pnas.96.4.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Vain P, Angell S, Baulcombe DC. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell. 1998;95:177–187. doi: 10.1016/s0092-8674(00)81749-3. [DOI] [PubMed] [Google Scholar]
- Palauqui JC, Balzergue S. Activation of systemic acquired silencing by localised introduction of DNA. Curr Biol. 1999;9:59–66. doi: 10.1016/S0960-9822(99)80016-5. [DOI] [PubMed] [Google Scholar]
- Crete P, Leuenberger S, Iglesias VA, Suarez V, Schob H, Holtorf H, van Eeden S, Meins F. Graft transmission of induced and spontaneous post-transcriptional silencing of chitinase genes. Plant J. 2001;28:493–501. doi: 10.1046/j.1365-313X.2001.01171.x. [DOI] [PubMed] [Google Scholar]
- Klahre U, Crete P, Leuenberger SA, Iglesias VA, Meins F., Jr. High molecular weight RNAs and small interfering RNAs induce systemic posttranscriptional gene silencing in plants. Proc Natl Acad Sci U S A. 2002;99:11981–11986. doi: 10.1073/pnas.182204199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Stokes KD, Rutherford SM, Percival AL, Lee WY. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell. 1998;10:1991–2004. doi: 10.1105/tpc.10.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reski R. Rings and networks: the amazing complexity of FtsZ in chloroplasts. Trends Plant Sci. 2002;7:103–105. doi: 10.1016/S1360-1385(02)02232-X. [DOI] [PubMed] [Google Scholar]
- Strepp R, Scholz S, Kruse S, Speth V, Reski R. Plant nuclear gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. Proc Natl Acad Sci U S A. 1998;95:4368–4373. doi: 10.1073/pnas.95.8.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, McAndrew RS. The Plastid Division Machine. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:315–333. doi: 10.1146/annurev.arplant.52.1.315. [DOI] [PubMed] [Google Scholar]
- Elder GH, Roberts AG. Uroporphyrinogen decarboxylase. J Bioenerg Biomembr. 1995;27:207–214. doi: 10.1007/BF02110035. [DOI] [PubMed] [Google Scholar]
- von Wettstein D., Gough, S., and Kannangara, C.G. Chlorophyll Biosynthesis. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GS, Yalpani N, Briggs SP, Johal GS. A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell. 1998;10:1095–1105. doi: 10.1105/tpc.10.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink VP, Wolniak SM. Centrin is necessary for the formation of the motile apparatus in spermatids of Marsilea. Mol Biol Cell. 2001;12:761–776. doi: 10.1091/mbc.12.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink VP, Wolniak SM. The efficacy of RNAi in the study of the plant cytoskeleton. J Plant Growth Regul. 2000;19:371–384. doi: 10.1007/s003440000043. [DOI] [PubMed] [Google Scholar]
- Tsai CW, Wolniak SM. Cell cycle arrest allows centrin translation but not basal body formation during spermiogenesis in Marsilea. J Cell Sci. 2001;114:4265–4272. doi: 10.1242/jcs.114.23.4265. [DOI] [PubMed] [Google Scholar]
- Eberle JR, Banks JA. Genetic interactions among sex-determining genes in the fern Ceratopteris richardii. Genetics. 1996;142:973–985. doi: 10.1093/genetics/142.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans MC, Maliga P, Vieira J, Messing J. The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants. J Biotechnol. 1990;14:333–344. doi: 10.1016/0168-1656(90)90117-T. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ario T, Hattori T, Nakamura K, Asahi T. Isolation and characterization of two tightly linked catalase genes from castor bean that are differentially regulated. Plant Mol Biol. 1994;25:507–516. doi: 10.1007/BF00043878. [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313X.1994.6020271.x. [DOI] [PubMed] [Google Scholar]


