Abstract
Background
Past research has linked patient-physician communication with improved emotional, physical, and social health. One component of communication, patient-clinician information engagement (PCIE) predicts improved short-term patient-reported outcomes such as treatment satisfaction through perceptions of feeling informed. However, the relationship between PCIE and longer term cancer-related problems has not previously been examined.
Objective
This study examines the influence of PCIE on self-reported problems associated with cancer diagnosis and treatment based on a longitudinal survey among a randomly selected sample from the 2005 Pennsylvania Cancer Registry.
Methods
We surveyed 1,293 respondents diagnosed with colorectal, breast, or prostate cancers in 2006 and 2007 (baseline response rate was 64%, retention rate was 65%). We predicted an index of cancer-related problems at one-year follow-up with the baseline cancer-related problem index and PCIE, controlling for demographic and clinical factors using regression analyses. The mean age of participants was 65 years, about half were female, and 86% were White.
Results
Having more cancer-related problems and PCIE at baseline significantly predicted more cancer-related problems at follow-up. Additionally, baseline cancer-related problems and PCIE interacted significantly (p=0.01) – PCIE was associated with more cancer-related problems at follow-up among participants who reported more rather than fewer symptoms at baseline.
Conclusion
If respondents reported engaging more with their physicians at baseline, they reported experiencing more cancer-related issues at follow-up; this pattern was stronger among those reporting more baseline problems. Increased discussion of cancer information with physicians may maintain the salience of these problems in cancer survivors’ minds over time.
Keywords: Patient-clinician communication, self-reported outcomes, cancer
INTRODUCTION
Effective communication between patients and their physicians plays an integral role in shaping cancer patients’ experiences and interactions with their health provider.1 As the U.S. health care system continues to encourage engaging patients as active participants and decision-makers in their care,2–4 the information that patients receive becomes increasingly vital to the their physical and psychological well-being. Cancer patients are known to seek information about their cancer, treatment, and quality of life issues from many sources,5, 6 including both medical and non-medical sources.7 This study focuses on how patients’ engagement with a fundamental source of information, their doctor, affects their self-reported experience of problems associated with their cancer diagnosis among a representative sample of cancer patients in Pennsylvania.
Communication of information is important in the cancer care setting, as it may impact how patients receive bad news about their diagnosis, understand new and complex material, navigate a multifaceted health care system, manage uncertainty, and make important treatment and lifestyle decisions.1 However, promoting patient-clinician communication does not necessarily influence patients’ outcomes in positive ways. Of 21 studies included in a review of patient-clinician communication on health outcomes,8, 9 sixteen reported positive results, four reported non-significant results, and one was inconclusive.
On one hand, evidence suggests that patient-physician communication has positive effects not just on perceptions of the relationship, but on patients’ subsequent psychological and physical health outcomes as well. For example, Martinez and colleagues found that patient-clinician information engagement led to improved treatment decision satisfaction, and that this relationship was mediated through feeling informed.10 An earlier study by Stewart and colleagues found that patients who felt that common ground was achieved with their physicians were more likely to have improved health status and more efficient hospital care.8 In an experiment of a communication training program for health professionals, the training led to improved ratings by patients, fewer surgery complications, faster transfer to less intensive care levels, as well as shorter hospital stays.11
In contrast, other studies have reported null or negative outcomes. This meant that increased patient-clinician communication did not always lead to better health consequences. In a study among breast and testicular cancer patients, reassurance by doctors that symptoms were not sinister yielded mixed results with regard to anxiety.12 A randomized controlled trial of a patient-centered care training program for general practice teams that treated diabetes found that although patients attending trained practiced teams reported better communication and treatment satisfaction, they did not experience improved metabolic control or diabetes-specific quality of life compared to the control group.13 Unexpectedly, the study also reported that diabetes patients in the intervention group gained more weight at follow-up, potentially contributing to higher cardiovascular risk. In a qualitative study among cancer patients, Leydon and colleagues reported that additional information may sometimes exacerbate fear, undermine patients’ hopes, and lead to more worry.14 Clearly, more research must be conducted in order to better understand the relationship between patient-clinician communication and disease-related outcomes.
One important effect of patient-clinician communication that requires further exploration is patient-reported outcomes related to cancer diagnosis and treatment. Patient-reported outcomes broadly encompass a diversity of measures that examine the impact of disease on patients’ lives, including health-related quality-of-life, functional status, symptom status, overall well-being, satisfaction with care, and treatment adherence.15Patient-reported outcomes can be defined as “any aspect of an individual’s health status that comes directly from the individual, without modification or interpretation by another observer”.16There are three possible relationships between patient-clinician engagement and self-reported outcomes in cancer patients. One possible effect of enhanced patient-clinician engagement is an improvement in patients’ experience of cancer-related problems, perhaps because clinicians could help cancer patients and their caregivers anticipate, identify, and better manage issues such as physical symptoms and anxiety. For instance, one experiment found that routine collection and use of health-related quality-of-life data from patients led to better subsequent quality-of-life and emotional functioning.17 Another possible effect is that more engagement with clinicians could lead patients to be more likely to report having symptoms. An example of this was a study that reported that the use of patient-reported quality-of-life assessments in visits led to a greater percentage of patients identifying with moderate to severe problems in various health domains.18 This could be because increased engagement exacerbates patients’ underlying concerns about experiencing post-diagnosis problems, as illustrated by a study that found that anxiety can be exacerbated through medical discussions.12 The third possibility is that there may be no relationship between patient-clinician engagement and patients’ experience of cancer-related problems at all. The current study examines the relationship between patient-clinician information engagement (PCIE) and self-reported problems related to cancer diagnosis and treatment in a population-based sample of breast, prostate, and colorectal cancer patients.
MATERIALS AND METHODS
Study population and procedure
We randomly sampled breast, prostate, and colorectal cancer patients stratified by cancer type from the 2005 Pennsylvania Cancer Registry (PCR), which included patients diagnosed with these cancers between January 2005 and December 2005. We obtained permission to access patient data from the PCR through the Pennsylvania state health department. The sampling frame included all patients with breast, prostate, or colorectal cancer who were reported to the PCR in time to have their data compiled by the commencement of the study in September 2006 (approximately 95% of all incident cases in 2005). We over-sampled cancer patients who were diagnosed with Stage IV disease and also African-American patients in order to improve the statistical power for subgroup analyses.
The overall study design was a three-round longitudinal survey of the sampled population. The analyses in this study utilized data from only the first two rounds. The baseline of the survey was conducted in the fall of 2006 (Round 1), and a follow-up survey in the fall of 2007 (Round 2) was conducted among participants who consented to being contacted after the first round. Round 1 survey included 2,013 patients, and 1,293 (64.2%1) patients participated in the Round 2 survey. We mailed surveys to participants based on Dillman’s method for mail surveys.19 Potential participants were first contacted with a notice letter explaining the study objectives and included opt-out instructions. The survey (tailored according to the type of cancer), a small monetary incentive2 , and a stamped addressed envelope to return the survey were sent to participants. Participants who had not return the survey or opted out of the study were mailed an additional letter and survey two weeks later. The baseline questionnaire was developed following literature review, expert consultation, a pilot study with 29 cancer patients, and appropriate revisions following the pilot testing. All study participants provided informed consent prior to participation and the University of Pennsylvania Institutional Review Board approved this study.
Survey measures
Dependent variable – Cancer-related problem index at Round 2
The outcome variable in this analysis was an index comprising patients’ self-reported experience of the following nine cancer-related issues in the preceding 12 months of the follow-up survey: 1) physical symptoms (e.g. pain, fatigue, sleep problems, bowel problems…), 2) memory or concentration problems, 3) fertility or menstrual problems,3 4) sexual problems, 5) changes in appearance, 6) anxiety or depression, 7) financial problems, 8) work-related problems, and 9) social or family-related problems. A higher score indicated that respondents experienced more of these problems within the past year. While Cronbach’s alpha is not an appropriate measure of reliability for this index, since the individual experience items that make it up are not expected to correlate with one another, strong evidence for reliability comes from the correlation between this measure and the same index measured one year later (r=.67).20, 21
Independent variable – Cancer-related problem index at Round 1
We postulated that participants’ baseline experience of problems would be predictive of their subsequent reporting of cancer-related issues. The baseline problem index was based on a question that asked participants if they experienced any of the same nine issues (described in the previous section) after their initial cancer diagnosis and treatment. The coding and summation of this measure was identical to the Round 2 problem index.
Independent variable – Patient-clinician information engagement (PCIE) scale at Round 1
We measured PCIE from eight items in the Round 1 survey as described by Martinez et al.10 Essentially, participants were instructed to think back to the first few months of their cancer diagnosis and recall if they: 1) sought information about treatments from their treating doctor, 2) sought treatment information from other doctors or health professionals, 3) actively looked for information about their cancer from their treating doctor, 4) looked for cancer information from other doctors or health professionals, 5) discussed information from other sources with their treating doctor, 6) received suggestions from their treating doctor to get information from other sources, 7) actively looked for information about quality-of-life issues from their treating doctor, and 8) looked for quality-of-life information from other doctors or health professionals. These items demonstrated reasonable internal consistency (Cronbach’s alpha=0.78). Each of the eight items was transformed to Z-scores and the average of the eight Z-scores formed the PCIE scale.
Control variables
Demographic variables (age in years, gender, education level, and ethnicity), psychological variables (worry about cancer at diagnosis), non-clinical sources of cancer information (media or interpersonal sources), and cancer-related variables (cancer type, stage of disease at diagnosis, type of treatment received, health status, frequency of physician visits, overall cancer experience, and being told about the presence of metastatic disease) were measured in the questionnaire.
Psychological variables
We postulated that worry about one’s cancer condition at baseline may potentially confound the relationship between PCIE and participants’ experience of cancer-related problems. For instance, higher worry at baseline may lead one to engage in more information-seeking from physician sources and may also be associated with more self-reported symptoms of anxiety. Therefore, worry at diagnosis was included as a control variable in the analyses. Respondents were asked to describe how worried they were about what might happen to them when they were first diagnosed with their cancer (5-point scale ranging from 1 ‘Not at all worried’ to 5 ‘Extremely worried’).
Non-clinical sources of medical information
Other non-medical sources of cancer information may also confound the relationship between PCIE and reported experience of cancer-related problems. For instance, seeking for cancer information from the media may motivate patients to engage with their physician for cancer information-seeking. Portrayals of cancer information in the media could also prime patients to be more aware of their cancer-related problems. Parallel to the PCIE scale, we measured patients’ non-medical information-seeking from responses indicating whether patients sought information about three domains: 1) treatment, 2) cancer information, and 3) quality-of-life issues information from non-medical sources. The different non-medical sources included: 1) television or radio, 2) books, brochures or pamphlets, 3) newspapers or magazines, 4) the internet other than personal e-mail, 5) family members, friends, or co-workers, 6) other cancer patients, 7) support groups, and 8) telephone hotlines from the American Cancer Society. The responses from these items were summed within each domain and the summed scores were standardized and averaged to form the non-medical seeking scale (Cronbach’s alpha=0.83).
Cancer-related characteristics
Individual clinical characteristics of participants may similarly influence PCIE and experiences of cancer-related problems. We controlled for important factors including respondents’ cancer type (breast, prostate or colorectal cancer), AJCC/UICC TNM stage (derived from the PCR data), type of treatment received, health status at baseline, frequency of physician visits, overall subjective cancer experience, and being told about the presence of metastatic disease by their physician. We recoded respondents’ receipt of various treatments for their cancers into three binary variables indicating whether respondents received surgery, radiation therapy, or systemic treatment (chemotherapy or hormonal therapy) respectively.4 As each cancer type may have disease-specific staging and treatment protocols, we further controlled for the interaction between cancer type and stage as well as the interaction between cancer type and treatment received.
Analytic procedure
Analyses were conducted using the Stata Statistical Software (Release 10). We performed multiple imputation to address missing data according to the procedure prescribed by Allison22 using the ICE program.23 Essentially, the imputation model comprised of the dependent variable, all independent variables, and the additional interactions described in the preceding section on measures. Using this procedure, we generated 15 datasets with imputed values of independent variables. Missing data on the dependent variable was not imputed. To reflect the distribution of cases in the PCR by cancer type, date of diagnosis, cancer stage, and demographic variables, post-stratification weights were applied to the data for analyses using the Survey program. This enabled inferences about patients with colon, breast, and prostate cancer in Pennsylvania to be made based on the results. We next used the MIM program to estimate the regression coefficients across the imputed datasets.
We first performed a preliminary assessment to test the assumption of linearity for the relationship between PCIE (grouped into ten levels) and Round 2 cancer-related problem index using analysis-of-variance (ANOVA) based on the unimputed dataset. The test of linearity was strongly significant (F=74.3, 1 df, p<.0005), the deviation from linearity test was marginally significant (F=2.0, 8 df, p=.048), and eta-squared (.06) was similar to the R-squared (.07). These findings indicated that linear regression will be appropriate for analyzing the hypothesized relationship between PCIE and the Round 2 cancer-related problem index.
Weighted point-estimates of zero-order correlations of PCIE and both rounds of the problem index were computed by averaging correlation estimates from each of the imputed datasets. To test the level of significance of these correlations, we performed bivariate regressions using these variables with the procedures suggested by Sribney.24
We estimated the effects of PCIE and Round 1 cancer-related problems with a series of linear regression models.5 In Model 1, PCIE and the index of Round 1 cancer-related problems were entered as predictor variables to estimate their unadjusted main effects. In Model 2, we estimated the main effects of PCIE and Round 1 cancer-related problems controlling for individual characteristics (demographics,6 worry at diagnosis, and clinical variables). Model 3 included an interaction term between PCIE and Round 1 cancer-related problems (centered at its mean), controlling for individual characteristics. We performed additional post-estimation analyses to assess the nature of the interaction effect by predicting the number of cancer-related problems at Round 2 based on the regression coefficients in the Model 3, varying the levels of PCIE and the number of problems at Round 1 (one standard deviation above and below their respective means), and keeping all other control variables at their mean values.
RESULTS
The demographic profile of the sample population approximately matched the profile of the general population of cancer patients in Pennsylvania with the above three cancers. Mean age of the sample population was about 65 years, 51% was female, almost half had some college education and higher, and the majority of respondents were White. The mean number of cancer-related problems at baseline 2.5 out of a maximum possible score of 9 in comparison to the mean number of problems at Round 2 which was slightly lower at 2.1. Table 1 describes other important characteristics of the sample.
Table 1.
Sample characteristics (N=1,293)
| Sample characteristic | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|
| Mean | S.E. | % | Mean | S.E. | % | |
| PCIE | 0.1 | 0.0 | 0.0 | 0.0 | ||
| Baseline cancer-related problem index | 2.5 | 0.1 | 2.3 | 0.1 | ||
| Follow-up cancer-related problem index | 2.1 | 0.6 | 1.9 | 0.6 | ||
| Age | 65.5 | 0.3 | 67.8 | 0.5 | ||
| Female | 51.4 | 51.2 | ||||
| Education | ||||||
| High school and below | 52.4 | 51.7 | ||||
| Some college and above | 47.6 | 48.3 | ||||
| Race/ ethnicity | ||||||
| White | 86.2 | 88.1 | ||||
| Black | 10.4 | 8.4 | ||||
| Hispanic or other | 3.4 | 3.4 | ||||
| Worry at diagnosis | 3.73 | 0.05 | 3.60 | 0.05 | ||
| Non-medical seeking | 0.1 | 0.0 | 0.0 | 0.0 | ||
| Cancer type | ||||||
| Colon | 31.9 | 31.5 | ||||
| Breast | 34.8 | 35.0 | ||||
| Prostate | 33.3 | 33.5 | ||||
| Cancer stage | ||||||
| Stage 0 | 9.0 | 12.2 | ||||
| Stage 1 | 20.2 | 19.6 | ||||
| Stage 2 | 44.7 | 46.6 | ||||
| Stage 3 | 13.0 | 12.1 | ||||
| Stage 4 | 13.2 | 9.5 | ||||
| Treatment received | ||||||
| Surgery | 73.3 | 72.7 | ||||
| Radiation therapy | 48.4 | 49.3 | ||||
| Systemic therapy | 56.5 | 51.7 | ||||
| Health status | 3.2 | 0.0 | 3.2 | 0.0 | ||
| Frequency of doctor visits | 3.6 | 0.0 | 3.5 | 0.0 | ||
| Cancer experience | 3.6 | 0.0 | 3.7 | 0.0 | ||
| Informed of metastatic disease | 14.3 | 11.7 | ||||
The correlations between PCIE and cancer-related problems at Round 1 and Round 2 are displayed in Table 1. These correlations were highly significant for all bivariate associations between these variables. The correlation between the problem indices in Round 1 and Round 2 were strong while the correlations between these variables and PCIE were medium based on Cohen’s criteria.25
Table 3 shows the results of the regression models predicting cancer-related problems at Round 2. Model 1 shows that higher PCIE and problems at Round 1 were both associated with more problems at Round 2. Controlling for potential confounders in Model 2, the coefficients of PCIE and cancer-related problems at Round 1 were slightly diminished but still statistically significant. This indicates that increased PCIE was associated with higher levels of cancer-related problems at Round 2, over and above the predicted effect of the presence of problems at baseline. Model 3 indicates a significant positive interaction between PCIE and baseline cancer-related problems. This suggests that the magnitude of effects of PCIE on patients’ experience of cancer-related problems at Round 2 was contingent on the level of problems experienced in Round 1.
Table 3.
Weighted ordinary-least-squares (OLS) regression analyses predicting Round 2 cancer-related problem index
| Predictors | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | |
| PCIE | 0.22** | 0.07 | 0.19* | 0.09 | 0.24** | 0.09 |
| Round 1 cancer-related problem index | 0.63*** | 0.02 | 0.56*** | 0.03 | 0.54*** | 0.03 |
| Interaction of PCIE and Round 1 cancer-related problem index | 0.11** | 0.04 | ||||
p<.05
p<.01
p<.001
Notes: Model 1 presents the main effects of PCIE and Round 1 cancer-related problem index without the control variables. Models 2 and 3 controlled for demographic characteristics (age, education level, and ethnicity), psychological variables (worry about cancer at diagnosis), non-clinical sources of cancer information (media or interpersonal sources), and cancer-related variables (cancer type, stage of disease at diagnosis, type of treatment received, health status, frequency of physician visits, overall cancer experience, and being told about the presence of metastatic disease).
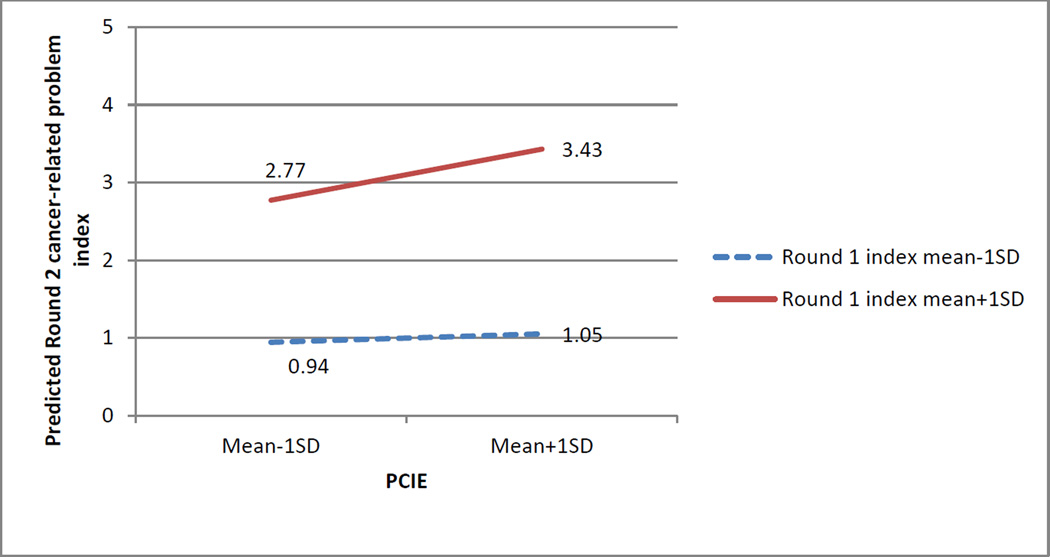
Figure 2 displays the effects of the interaction between PCIE and cancer-related problems in Round 1 based on the coefficients from the final regression model, at varying levels of these variables (one standard deviation above and below their respective means). Among respondents who have low levels of cancer-related problems at baseline, increasing levels of PCIE was not associated with appreciable change in Round 2 problem index. In comparison, for respondents who had more problems at baseline, increased PCIE was associated with more cancer-related problems at follow-up.
DISCUSSION
This study found that as patients discussed with and sought information at baseline from their physicians and other health professionals about their cancer treatment, quality of life and other cancer-related issues, there was a tendency for patients to report experiencing more cancer-related issues the following year. This relationship was stronger among patients who had reported more cancer-related issues at baseline. On the surface, these are discouraging results—why would talking to one’s doctor lead to worse patient-reported outcomes? There are three possible reasons proposed here: increased salience, the quality or content of the discussion, and a false sense of security.
One plausible reason is that conversation with doctors leads to higher awareness of the various possible cancer-related issues a patient may experience, which may lead to higher reporting of issues, but not actual experience of them. Discussing cancer-related symptoms with the doctor may establish it as a medical concern worthy of attention and reporting, and the heightened awareness could lead to a lower threshold for labeling a particular issue as a problem. Although the survey items attempted to capture actual experience rather than awareness of or susceptibility to issues related to their cancer diagnoses and treatment, more objective measures of cancer-related problems and refined measures of patients’ subjective perceptions of these problems would help to answer whether salience is responsible for this study’s findings.
Second, the measure used in this study was a measure of quantity rather than quality or content of the information engagement. Unclear or unsatisfactory communication can lead to worse patient-reported outcomes.26, 27 It is possible that patients who had more trouble communicating with their doctors engaged in more of it. Although quality-of-life information was included in the PCIE measure, the nature of discussions between patients and oncologists tend to focus on symptoms and treatment and less on psychological, social, and spiritual concerns,28 which could also affect patients’ ability to cope with the latter concerns. However, this explanation seems at odds with another set of findings from the same dataset, which demonstrated that PCIE increased the feeling of being informed as well as treatment decision satisfaction.10 Without a clearer picture of the nature of the patient-clinician discussions, levels of health literacy, and patients’ perceptions of the discussion within this population, it is not possible to conclude that more PCIE, regardless of its content, leads to increased experiences of cancer-related problems.
Third, more PCIE may have led to greater experience of problems via a false sense of security. Patients who talk more with their doctors may feel less concerned about the problems they will face, and therefore act less vigilantly in their self-care. This explanation may hold especially true for those patients who had more cancer-related issues at baseline, and it is consistent with the results of the interaction term tested here. These potential causal mechanisms should be tested in future studies.
The present study offers several improvements to the existing literature regarding patient-clinician communication and patient-reported outcomes, specifically physical and psychosocial issues related to one’s cancer diagnosis and treatment. First, it utilizes a representative population-based sample of patients diagnosed with three of the most prevalent cancers in Pennsylvania, as opposed to studies that typically involve convenience samples of patients in individual oncology clinics. Second, as this study was based on a longitudinal analysis, we were able to make more confident inferences about the causal direction between PCIE and cancer-related problems while controlling for potential confounders in contrast with studies that show cross-sectional associations. Finally, the probability sample weighting allowed for the extrapolation of findings at least to the sampling frame of cancer patients in Pennsylvania.
There are several limitations with the present study. First, the sample was composed of Pennsylvanian breast, prostate, and colorectal patients, and the results may not be generalizable to other patient populations in terms of disease or geographic location. Research among other patients should be conducted to examine if these findings could be replicated. Second, the PCIE measure used here has only captured the extent to which patient information engagement has taken place for various cancer-related topics with their doctor. It is possible that other measures of patient-clinician communication, such as levels of understanding and rapport, the content discussed, and the clinician’s perceptions could yield different results. Future research should incorporate the nature of the patient-physician communication from such measures. Third, the index of cancer-related problem in this present study assessed the self-reported presence or absence of a limited set of health and lifestyle issues. The severity and the extent to these symptoms impacted patients’ well-being and lifestyles were not measured. Other dimensions of quality-of-life issues were also not measured. Therefore, additional research using patient-reported outcome measures that include other important dimensions or measures of impact on patients’ well-being is recommended. Fourth, there may be other potential unmeasured confounders related to the clinical status of patients at baseline. For instance, patients with baseline problems that were severe, difficult to treat or reverse, or that progress over time, might be more likely to report increased PCIE and experiencing of one of the nine cancer-related problems at follow-up, over and above their tendency to experience them in the first year after diagnosis. While this analysis did control for certain measures of patients’ clinical status at baseline (for example, stage, health status, frequency of physician visits, and subjective cancer experience), the presence and severity of problems that were difficult to resolve might not have been captured in these measured variables.
However, despite these limitations, this study does call into question the seemingly intuitive positive effects of promoting greater patient-clinician communication in the care of cancer patients. It demonstrates that the conventional wisdom that more patient engagement with their physicians will lead to better long term health outcomes cannot be assumed, and highlights the need to further examine the underlying causal pathways and the role of moderating factors.
Figure 1.
Predicted Round 2 cancer-related problem index with varying levels of PCIE and Round 1 cancer-related problem index
Note: Predicted values based on weighted regression coefficients from Model 3, adjusting for all control variables at their respective means.
Table 2.
Weighted Pearson’s correlations between PCIE, Round 1 and Round 2 cancer-related problem indices
| Round 1 cancer-related problem index |
Round 2 cancer-related problem index |
|
|---|---|---|
| PCIE | 0.27*** | 0.25*** |
| Round 1 cancer-related problem index | - | 0.67*** |
p<.05
p<.01
p<.001
Footnotes
The non-respondents in Round 2 were due to refusal to be re-contacted after Round 1 (255 cases, 12.7%) and no response after the second mailed survey (465 cases, 23.0%).
The incentive amounted to either $3 or $5 in Round 1 and was $3 in Round 2.
“Menstrual problems” were omitted for the survey questionnaire meant for respondents with prostate cancer.
These treatment types are not mutually exclusive. Respondents may report having received one or more treatments for their cancer.
The regression analyses were conducted with and without applying sampling weights. The findings were substantively identical and hence, only the results with the weighted analyses are reported here.
Gender was omitted from the list of confounder variables due to gender-specific cancer types in our dataset. This did not alter the findings or conclusions from the analyses.
REFERENCES
- 1.Epstein RM, Street RL., Jr . Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 2.Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335:24–27. doi: 10.1136/bmj.39246.581169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibbard JH. Engaging health care consumers to improve the quality of care. Med Care. 2003;41:I61–I70. doi: 10.1097/00005650-200301001-00007. [DOI] [PubMed] [Google Scholar]
- 4.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: What is its role in cancer screening? Cancer. 2004;101:1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi C, Czaja R, Buis M, Derk D. Patient use of treatment-related information received from the cancer information service. Cancer. 1993;71:1326–1337. doi: 10.1002/1097-0142(19930215)71:4<1326::aid-cncr2820710426>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Rutten LJ, Arora NK, Bakos AD, Aziz N, Rowland J. Information needs and sources of information among cancer patients: A systematic review of research (1980–2003) Patient Educ Couns. 2005;57:250–261. doi: 10.1016/j.pec.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Hesse BW, Moser RP, Rutten LJ, Kreps GL. The health information national trends survey: Research from the baseline. J Health Commun. 2006;11(Suppl 1):vii–xvi. doi: 10.1080/10810730600692553. [DOI] [PubMed] [Google Scholar]
- 8.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 9.Stewart MA. Effective physician-patient communication and health outcomes: A review. CMAJ. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez LS, Schwartz JS, Freres D, Fraze T, Hornik RC. Patient–clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Educ Couns. 2009;77:384–390. doi: 10.1016/j.pec.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trummer UF, Mueller UO, Nowak P, Stidl T, Pelikan JM. Does physician-patient communication that aims at empowering patients improve clinical outcome? A case study. Patient Educ Couns. 2006;61:299–306. doi: 10.1016/j.pec.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Stark D, Kiely M, Smith A, Morley S, Selby P, House A. Reassurance and the anxious cancer patient. Br J Cancer. 2004;91:893–899. doi: 10.1038/sj.bjc.6602077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: Impact on current wellbeing and future disease risk. the diabetes care from diagnosis research team. BMJ. 1998;317:1202–1208. doi: 10.1136/bmj.317.7167.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leydon GM, Boulton M, Moynihan C, et al. Cancer patients' information needs and information seeking behaviour: In depth interview study. BMJ. 2000;320:909–913. doi: 10.1136/bmj.320.7239.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: A review of recent research and policy initiatives. CA Cancer J Clin. 2007;57:278–300. doi: 10.3322/CA.57.5.278. [DOI] [PubMed] [Google Scholar]
- 16.Lipscomb J, Reeve BB, Clauser SB, et al. Patient-reported outcomes assessment in cancer trials: Taking stock, moving forward. J Clin Oncol. 2007;25:5133–5140. doi: 10.1200/JCO.2007.12.4644. [DOI] [PubMed] [Google Scholar]
- 17.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 18.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 19.Dillman DA, Dillman DA. Mail and Internet Surveys : The Tailored Design Method. 2nd ed. New York: J. Wiley; 2000. [Google Scholar]
- 20.Bollen K, Lennox R. Conventional wisdom on measurement: A structural equation perspective. Psychol Bull. 1991;110:305. [Google Scholar]
- 21.Streiner DL. Being inconsistent about consistency: When coefficient alpha does and doesn't matter. J Pers Assess. 2003;80:217–222. doi: 10.1207/S15327752JPA8003_01. [DOI] [PubMed] [Google Scholar]
- 22.Allison PD. Missing Data. 07-136. Thousand Oaks, Calif. ; London: Sage Publications; 2002. [Google Scholar]
- 23.Royston P. Multiple imputation of missing values: Further update of ice, with an emphasis on interval censoring. Stata J. 2007;7:445–464. [Google Scholar]
- 24.Sribney B. Estimating correlations with survey data. [accessed March 18, 2010]; Available from URL: http://www.stata.com/support/faqs/stat/survey.html. [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 26.Kerr J, Engel J, Schlesinger-Raab A, Sauer H, Holzel D. Communication, quality of life and age: Results of a 5-year prospective study in breast cancer patients. Ann Oncol. 2003;14:421–427. doi: 10.1093/annonc/mdg098. [DOI] [PubMed] [Google Scholar]
- 27.Kerr J, Engel J, Schlesinger-Raab A, Sauer H, Holzel D. Doctor-patient communication: Results of a four-year prospective study in rectal cancer patients. Dis Colon Rectum. 2003;46:1038–1046. doi: 10.1097/01.DCR.0000074690.73575.99. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez KL, Bayliss N, Alexander SC, et al. How oncologists and their patients with advanced cancer communicate about health-related quality of life. Psychooncology. 2009 doi: 10.1002/pon.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]