Abstract
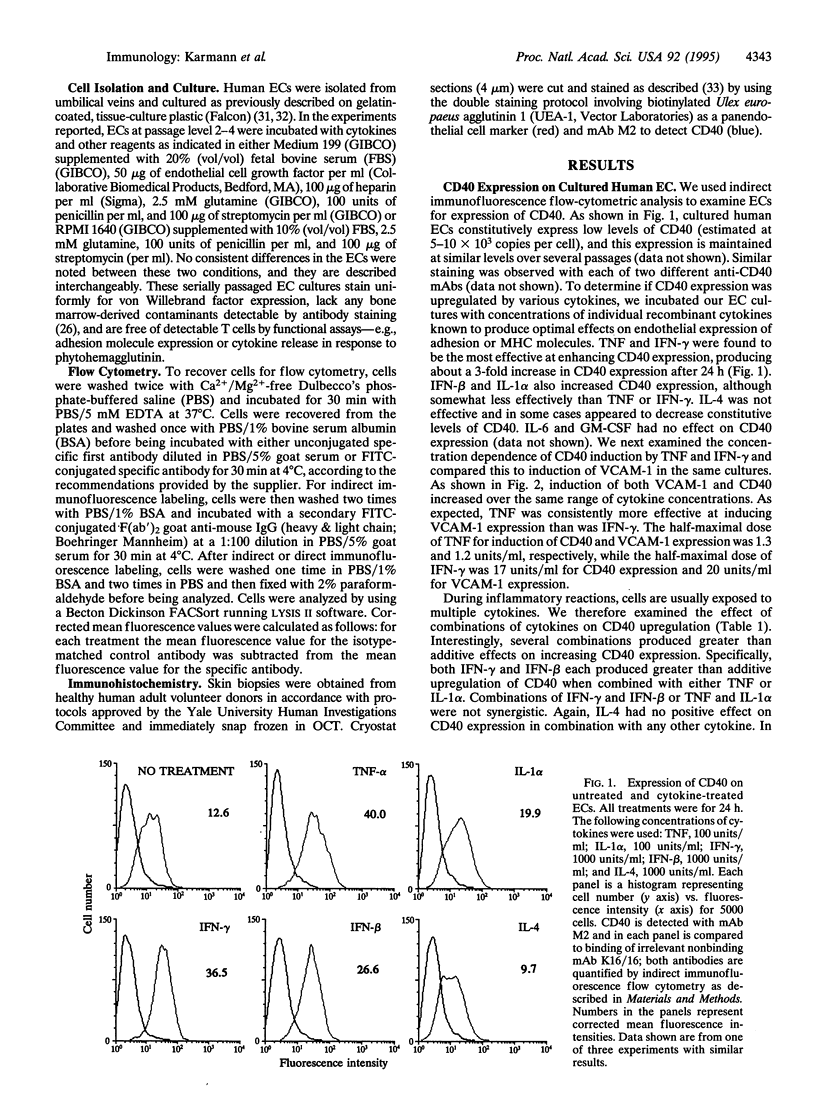
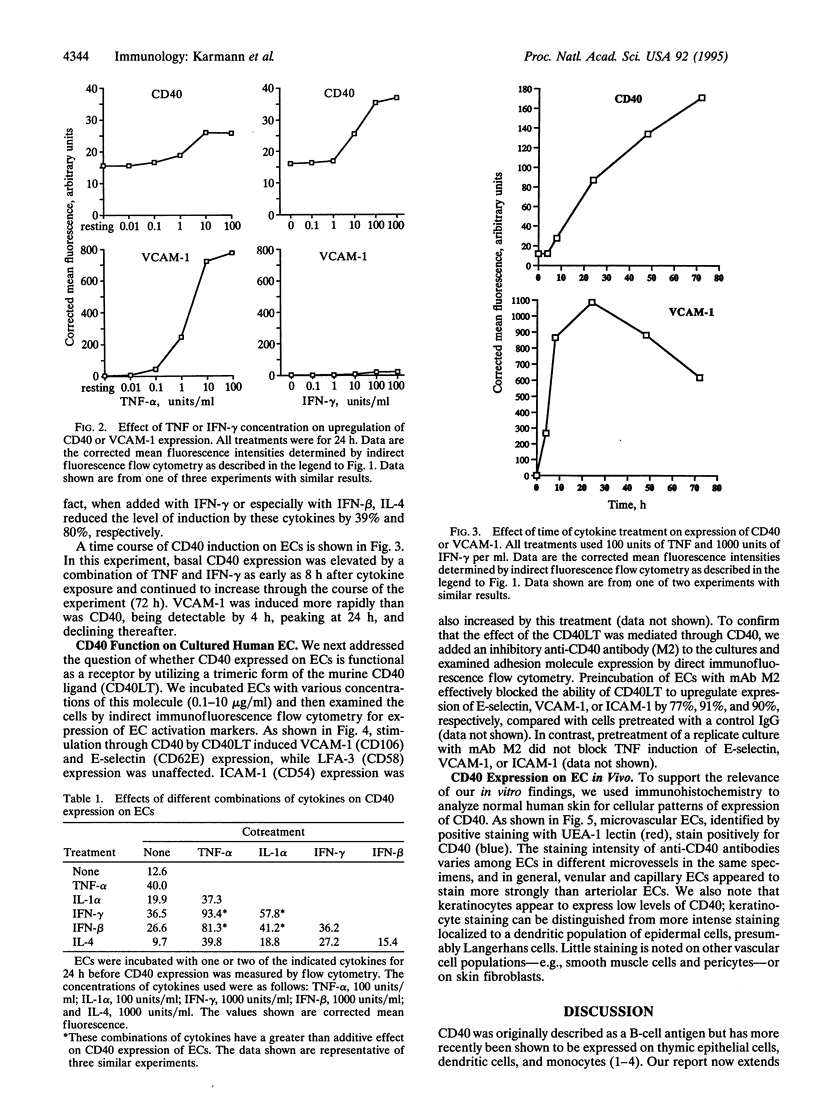
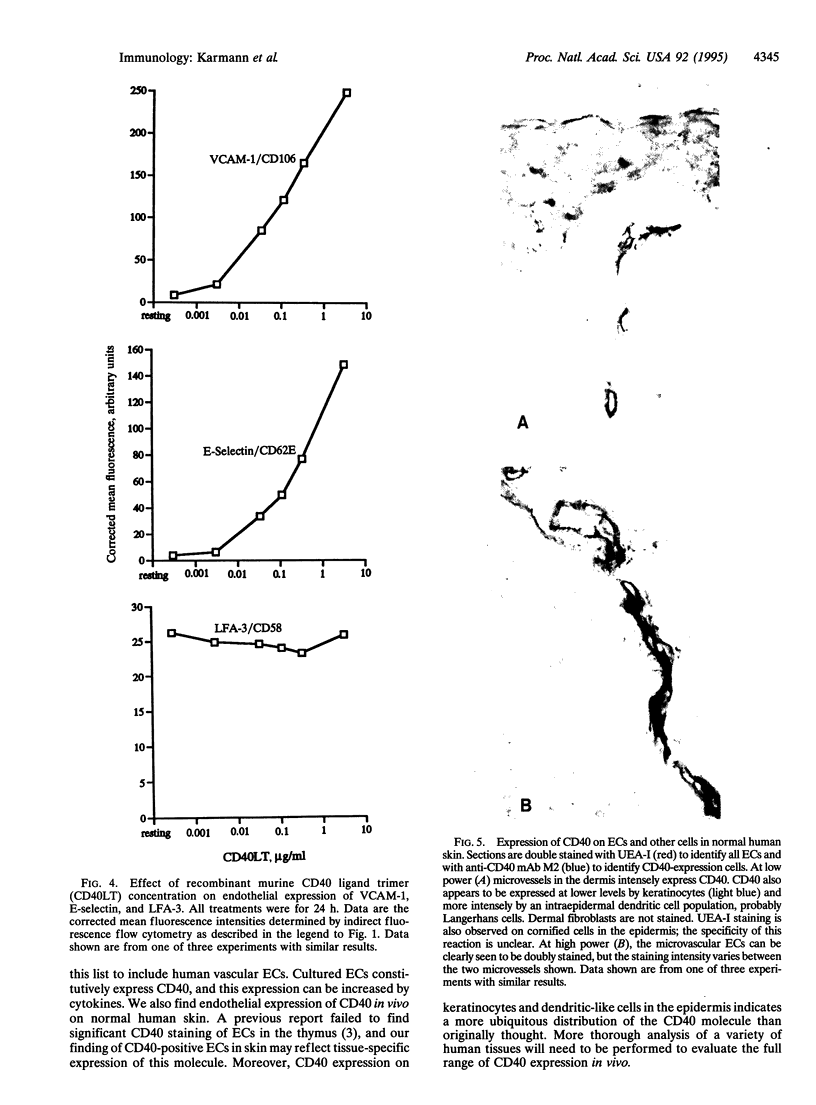
Cultured human umbilical vein endothelial cells (EC) constitutively express a low level of CD40 antigen as detected by monoclonal antibody binding and fluorescence flow cytometric quantitation. The level of expression on EC is increased about 3-fold following 24 h treatment with optimal concentrations of tumor necrosis factor, interleukin 1, interferon beta, or interferon gamma; both interferons show greater than additive induction of CD40 when combined with tumor necrosis factor or interleukin 1. Expression of CD40 increases within 8 h of cytokine treatment and continues to increase through 72 h. A trimeric form of recombinant murine CD40 ligand acts on human EC to increase expression of leukocyte adhesion molecules, including E-selectin, vascular cell adhesion molecule 1, and intercellular adhesion molecule 1. CD40 may be detected immunocytochemically on human microvascular EC in normal skin. We conclude that endothelial CD40 may play a role as a signaling receptor in the development of T-cell-mediated inflammatory reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. W., Lee H. S., Waldman W. J., Sedmak D. D., Morgan C. J., Ward J. S., Orosz C. G. Alloantigenicity of human endothelial cells. 1. Frequency and phenotype of human T helper lymphocytes that can react to allogeneic endothelial cells. J Immunol. 1992 Jun 15;148(12):3753–3760. [PubMed] [Google Scholar]
- Alderson M. R., Armitage R. J., Tough T. W., Strockbine L., Fanslow W. C., Spriggs M. K. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993 Aug 1;178(2):669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Banchereau J., de Paoli P., Vallé A., Garcia E., Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan 4;251(4989):70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Shu G. Association between IL-6 and CD40 signaling. IL-6 induces phosphorylation of CD40 receptors. J Immunol. 1990 Sep 1;145(5):1400–1406. [PubMed] [Google Scholar]
- DiSanto J. P., Bonnefoy J. Y., Gauchat J. F., Fischer A., de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- Durie F. H., Aruffo A., Ledbetter J., Crassi K. M., Green W. R., Fast L. D., Noelle R. J. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chronic forms of graft-vs-host disease. J Clin Invest. 1994 Sep;94(3):1333–1338. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie F. H., Foy T. M., Masters S. R., Laman J. D., Noelle R. J. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994 Sep;15(9):406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Fanslow W. C., Srinivasan S., Paxton R., Gibson M. G., Spriggs M. K., Armitage R. J. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994 Oct;6(5):267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- Foy T. M., Laman J. D., Ledbetter J. A., Aruffo A., Claassen E., Noelle R. J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994 Jul 1;180(1):157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Loh R., Jabara H., Rosen R. S., Chatila T., Fu S. M., Stamenkovic I., Geha R. S. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy A. H., Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992 Aug 1;149(3):775–782. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Hollenbaugh D., Grosmaire L. S., Kullas C. D., Chalupny N. J., Braesch-Andersen S., Noelle R. J., Stamenkovic I., Ledbetter J. A., Aruffo A. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992 Dec;11(12):4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. C., Savage C. O., Pober J. S. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990 May 1;171(5):1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994 Jun;1(3):167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Korpelainen E. I., Gamble J. R., Smith W. B., Goodall G. J., Qiyu S., Woodcock J. M., Dottore M., Vadas M. A., Lopez A. F. The receptor for interleukin 3 is selectively induced in human endothelial cells by tumor necrosis factor alpha and potentiates interleukin 8 secretion and neutrophil transmigration. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11137–11141. doi: 10.1073/pnas.90.23.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
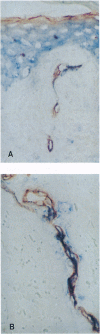
- Petzelbauer P., Bender J. R., Wilson J., Pober J. S. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993 Nov 1;151(9):5062–5072. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. The role of endothelial cells in inflammation. Transplantation. 1990 Oct;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw B. R., Fanslow W. C., 3rd, Armitage R. J., Campbell K. A., Liggitt D., Wright B., Davison B. L., Maliszewski C. R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994 Nov 1;180(5):1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Savage C. O., Hughes C. C., McIntyre B. W., Picard J. K., Pober J. S. Human CD4+ T cells proliferate to HLA-DR+ allogeneic vascular endothelium. Identification of accessory interactions. Transplantation. 1993 Jul;56(1):128–134. doi: 10.1097/00007890-199307000-00024. [DOI] [PubMed] [Google Scholar]
- Savage C. O., Hughes C. C., Pepinsky R. B., Wallner B. P., Freedman A. S., Pober J. S. Endothelial cell lymphocyte function-associated antigen-3 and an unidentified ligand act in concert to provide costimulation to human peripheral blood CD4+ T cells. Cell Immunol. 1991 Oct 1;137(1):150–163. doi: 10.1016/0008-8749(91)90065-j. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Armitage R. J., Strockbine L., Clifford K. N., Macduff B. M., Sato T. A., Maliszewski C. R., Fanslow W. C. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992 Dec 1;176(6):1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Clark E. A., Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989 May;8(5):1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Torres R. M., Clark E. A. Differential increase of an alternatively polyadenylated mRNA species of murine CD40 upon B lymphocyte activation. J Immunol. 1992 Jan 15;148(2):620–626. [PubMed] [Google Scholar]
- Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. Mice deficient for the CD40 ligand. Immunity. 1994 Aug;1(5):423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]