Abstract
The fidelity of protein synthesis depends on the capacity of aminoacyl-tRNA synthetases (AARSs) to couple only cognate amino acid-tRNA pairs. If amino acid selectivity is compromised, fidelity can be ensured by an inherent AARS editing activity that hydrolyses mischarged tRNAs. Here, we show that the editing activity of Escherichia coli leucyl-tRNA synthetase (EcLeuRS) is not required to prevent incorrect isoleucine incorporation. Rather, as shown by kinetic, structural and in vivo approaches, the prime biological function of LeuRS editing is to prevent mis-incorporation of the non-standard amino acid norvaline. This conclusion follows from a reassessment of the discriminatory power of LeuRS against isoleucine and the demonstration that a LeuRS editing-deficient E. coli strain grows normally in high concentrations of isoleucine but not under oxygen deprivation conditions when norvaline accumulates to substantial levels. Thus, AARS-based translational quality control is a key feature for bacterial adaptive response to oxygen deprivation. The non-essential role for editing under normal bacterial growth has important implications for the development of resistance to antimicrobial agents targeting the LeuRS editing site.
Keywords: editing, isoleucine, leucyl-tRNA synthetase, micro-aerobic growth, norvaline
See also: L Ribas de Pouplana (August 2014)
Introduction
Aminoacyl-tRNA synthetases (AARSs) define the genetic code through the covalent pairing of amino acids with their cognate tRNAs to provide the activated aminoacyl moiety for ribosomal protein synthesis. Aminoacylation is a two-step reaction localized within the synthetic active site of the AARS catalytic domain (reviewed in First, 2005; Perona & Gruic-Sovulj, 2013). Firstly, ATP-dependent amino acid activation yields an aminoacyl-adenylate intermediate (AA-AMP) that remains non-covalently bound in the synthetic site. In the second step, either the 2′- or 3′-OH group of the terminal adenosine of the tRNA attacks the carbonyl carbon of AA-AMP, leading to transfer of the aminoacyl moiety onto the tRNA (Fig1). Based on the differences in the architecture and topology of the catalytic domains, AARSs are divided into two classes, class I consisting of 11 members and class II of 13 members (Cusack, 1997; de Pouplana & Schimmel, 2001; Eriani et al, 1990; Perona & Hadd, 2012).
Figure 1. Schematic presentation of editing pathways.
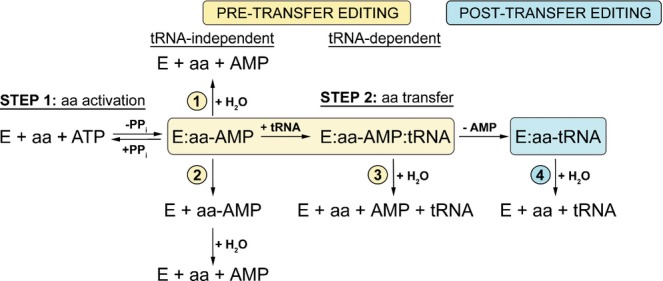
Pre-transfer editing can occur through enhanced dissociation of non-cognate aminoacyl-adenylate (2) or its enzymatic hydrolysis (1, 3), which may be tRNA-independent (1) or tRNA-dependent (3). After transfer, mischarged tRNA can be deacylated through post-transfer editing (4). The colors in the scheme match the color of the domain where the reaction occurs (yellow, catalytic domain; cyan, editing domain; see Fig4).
Many AARSs are incapable of distinguishing between cognate and near-cognate amino acids with high specificity in the synthetic reaction alone. These enzymes instead use intrinsic hydrolytic proof-reading to minimize the error to the level tolerated in protein synthesis (1 in 103–104). Editing may operate through the several pathways localized in the two distinct active sites: the synthetic site at the catalytic core and the editing site in a distinct editing domain (Fig1; reviewed in Perona & Gruic-Sovulj, 2013). Hydrolysis of non-cognate AA-AMP (pre-transfer editing), both tRNA-independent and tRNA-dependent, takes place within the confines of the synthetic site (Fig1, paths 1 and 3) (Cvetesic et al, 2012; Dulic et al, 2010; Gruic-Sovulj et al, 2007; Minajigi & Francklyn, 2010; Splan et al, 2008). The relevance of pre-transfer editing is defined by the kinetic partitioning of AA-AMP within the synthetic site (Dulic et al, 2010; Minajigi & Francklyn, 2010). If the rate of aminoacyl transfer to the tRNA is significantly faster than the rate of AA-AMP hydrolysis, the non-cognate amino acid may evade pre-transfer editing. Misacylated tRNA is then cleared by post-transfer editing at the specialized editing domain. Non-cognate amino acid is delivered to the distant hydrolytic site by translocation of the 3′-end of the misacylated tRNA.
Leucyl-tRNA synthetase (LeuRS) provides a cellular pool of Leu-tRNALeu, thus allowing the programmed insertion of leucine into proteins. Besides leucine, Escherichia coli LeuRS (EcLeuRS) activates structurally similar but non-cognate isoleucine, norvaline, norleucine, and methionine with a frequency that generally exceeds the tolerated translational error (Chen et al, 2000; Cvetesic et al, 2012; Martinis & Fox, 1997; Tang & Tirrell, 2002). To clear errors of aminoacylation, EcLeuRS exhibits efficient post-transfer editing (Cvetesic et al, 2012; Lincecum et al, 2003; Mursinna et al, 2004) localized at a discrete 189 amino acids long peptide, called connective peptide 1 (CP1) that is inserted into the Rossmann fold of the canonical class I AARS catalytic domain (Cusack et al, 2000; Palencia et al, 2012). This feature is shared with homologous isoleucyl- and valyl-tRNA synthetase (IleRS and ValRS) (Dulic et al, 2010; Fukai et al, 2000; Silvian et al, 1999). Isoleucine is generally thought to be a significant threat to the accuracy of leucylation (Boniecki et al, 2008; Lincecum et al, 2003; Lue & Kelley, 2005; Martinis & Fox, 1997; Mursinna et al, 2004). However, some doubt about the promiscuity of LeuRS towards isoleucine comes from the observation that reported discrimination factors for isoleucine (defined as (kcat/Km)Leu/(kcat/Km)Ile) substantially differ [from 630 (Chen et al, 2000) to 5,400 (Tang & Tirrell, 2002)]. So far, the significance of isoleucine editing has not been substantiated by detailed kinetic analyses of the synthetic and editing isoleucine pathways.
Norvaline, a side product of the leucine biosynthetic pathway (Umbarger, 1978), may pose a significant threat to fidelity of protein synthesis under limited-oxygen growth conditions (Soini et al, 2008) where it accumulates to a concentration capable of potentially jeopardizing the accuracy of Leu-tRNALeu synthesis by E. coli. Our in-depth kinetic analysis established that norvaline at millimolar amounts is rapidly activated and transferred to tRNALeu by EcLeuRS. Yet, availability of Nva-tRNALeu for protein synthesis is effectually prevented by rapid hydrolysis of the misacylated tRNA at the LeuRS CP1 editing site (Cvetesic et al, 2012). Single-turnover kinetic analysis revealed the LeuRS CP1 editing site exhibits enhanced substrate specificity for norvaline. This might indicate that the LeuRS editing has been evolutionary optimized to eliminate non-proteinogenic norvaline rather than isoleucine.
To explore whether isoleucine discrimination by LeuRS differs from the mechanisms that assure specificity against norvaline, we performed an extensive kinetic and thermodynamic analyses of LeuRS synthetic and editing pathways using isoleucine as the non-cognate substrate. Unexpectedly, we find that LeuRS discriminates against isoleucine at the activation step with 104-fold specificity that arises from weak ground state binding and decreased rate of the chemical step. Our data show that the prevailing opinion that LeuRS frequently misactivates isoleucine is mistaken because it is based on measurements with impure isoleucine samples that contain traces of leucine. The crystal structure of the ternary complex formed by EcLeuRS, tRNALeu, and the non-cognate isoleucyl-adenylate analogue (Ile-AMS) unveils a novel mechanism of ground state discrimination, whereby the active site geometry ensures selectivity against the most abundant amino acid conformer. The growth of an E. coli strain deprived of LeuRS post-transfer editing displays a high tolerance towards a surplus of isoleucine, but not norvaline, in the media. We thus propose that the prime biological importance of the editing domain of LeuRS is to exclude the non-canonical norvaline from protein synthesis and thus preserve the canonical genetic code under norvaline-rich (micro-aerobic) growth conditions. Our data show that AARS editing is an essential part of the cellular mechanisms that ensure bacterial adaptability to changing environment.
Results
Isoleucine is effectively eliminated in the synthetic reaction of LeuRS
Characterization of the several commercial isoleucine lots, by kinetics and NMR spectroscopy, revealed contamination with leucine in the 0.0019–0.38% range (see Supplementary Materials and Methods, and Supplementary Fig S1 and S2). Although the percentage of leucine appears low, two major kinetic artifacts nonetheless emerged as a consequence of leucine impurities in the isoleucine samples: (i) inhibition of LeuRS editing at high concentrations of isoleucine (Supplementary Fig S3) and (ii) substantially lower discrimination factor for isoleucine at the activation step (100 versus 8,000 for the samples with 0.38% and 0.0019% of leucine, respectively; Supplementary Table S1). The latter may explain the observed inconsistency in the published discrimination factors (from 630 to 5,400) for activation of isoleucine by EcLeuRS (Boniecki et al, 2008; Chen et al, 2000; Lue & Kelley, 2005; Tang & Tirrell, 2002). Our data re-emphasize the difficulties associated with the presence of trace levels of cognate amino acid in a non-cognate amino acid sample (Fersht & Dingwall, 1979b) and thus highlight the importance of a careful analysis of the amino acid substrates.
The problem was addressed by further purification of the commercial isoleucine samples prior to their use in kinetic assays (see Supplementary Materials and Methods). Ultra-pure isoleucine (around 0.00035% of leucine) was produced and used for determination of the kinetic parameters for activation of isoleucine by EcLeuRS. Remarkably, we observed that LeuRS discriminates robustly against isoleucine in the activation step, with a discrimination factor as high as 31,000 (Table1). Thus, utilization of ultra-pure isoleucine revealed that LeuRS discriminates substantially better against isoleucine than was expected based on data with non-purified isoleucine samples (Supplementary Table S1 and Boniecki et al, 2008; Chen et al, 2000; Lue & Kelley, 2005). It thus appears that isoleucine will be mistakenly activated by LeuRS only with frequency 1 in 31,000: significantly lower than the observed error in protein synthesis (1 in 3,300, Loftfield & Vanderjagt, 1972). This suggests that editing of isoleucine is not essential and thus argues against the view that editing of isoleucine by LeuRS is a major defense against mistranslation of leucine codons as isoleucine in E. coli (Boniecki et al, 2008; Lue & Kelley, 2005).
Table 1.
Steady-state parameters for amino acid activation by wild-type (WT) EcLeuRSa
| AA | AA structure | Km (AA) | kcat | kcat/Km | Discrimination factorb |
|---|---|---|---|---|---|
| mM | s−1 | mM−1s−1 | |||
| WT LeuRS | |||||
| Leuc | 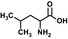 |
0.05 ± 0.01 | 66 ± 2 | 1,320 | |
| Nvac | 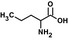 |
4.9 ± 0.4 | 56 ± 1 | 11.4 | 116 |
| Abad | 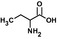 |
85 ± 9 | 20 ± 1 | 0.24 | 5,500 |
| Ilee,g | 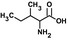 |
26 ± 7 | 1.1 ± 0.1 | 0.042 | 31,429 |
| M40G LeuRS | |||||
| Leuf | 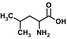 |
0.34 ± 0.03 | 6.9 ± 0.2 | 20 | |
| Nvaf | 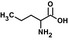 |
17 ± 3 | 4.2 ± 0.3 | 0.247 | 81 |
| Ilee, g |  |
10 ± 2 | 1.14 ± 0.06 | 0.114 | 175 |
The values represent the best fit value ± s.e.m. of at least two independent experiments.
Measured by ATP-PPi exchange assay.
Discrimination factor is defined as (kcat/Km)cognate/(kcat/Km)non-cognate.
Parameters reported in (Cvetesic et al, 2012).
WT or M40G LeuRS were used at d10 nM, e150 nM, or f50 nM concentration, and Leu, Nva, and Ile concentrations were varied over the range 0.1–10 times the Km.
Isoleucine preparation used to determine activation parameters was purified as described in the Supplementary Materials and Methods.
Comparison of isoleucine and norvaline kinetic parameters in amino acid activation by LeuRS (Table1) shows that both non-cognate substrates are discriminated at the level of ground state binding with the more prominent effect in the case of isoleucine. In sharp contrast, isoleucine, but not norvaline, is also significantly discriminated at the catalytic step (kcat is decreased 55-fold). This is unusual because neither ValRS nor IleRS discriminates against non-cognate threonine and valine, respectively, at the level of kcat in activation (Dulic et al, 2010; Fersht, 1977; Fersht & Kaethner, 1976). We further tested activation of the substantially smaller non-cognate α-aminobutyric acid (Aba) by LeuRS and find that kcat is not highly compromised. The major effect (1,700 fold) was again observed at the level of Km (Table1). It thus appears that considerably slower activation is a distinctive feature of isoleucine rejection by LeuRS.
Next, we explored LeuRS discrimination against isoleucine in aminoacylation. Although aminoacyl transfer is generally taken as non-discriminative (Cvetesic et al, 2012; Dulic et al, 2010; Lin et al, 1984; Minajigi & Francklyn, 2010), it is known that tRNA may modulate the activation step (Bovee et al, 2003; Dibbelt et al, 1980; Gruic-Sovulj et al, 2002; Guth et al, 2005; Minajigi & Francklyn, 2010). The two-step aminoacyl-tRNALeu formation was followed under single-turnover conditions using 4-fold excess of the enzyme over tRNA (Fig2A and B). Hydrolysis of Ile-tRNALeu was disabled by use of the LeuRS mutant (D345A LeuRS) inactivated in the CP1 editing site (Cvetesic et al, 2012; Lincecum et al, 2003). Thus, D345A LeuRS pre-incubated with ATP, and a limiting amount of [32P]-tRNA under the saturating conditions was mixed with various concentrations of amino acid (isoleucine or leucine) using a rapid chemical quench instrument. Several modes of mixing were tested, and no difference in reaction kinetics was observed. Time-course of AA-[32P]-tRNA formation fit best to a single exponential equation at each concentration of the amino acid (either isoleucine or leucine). The curves were carefully inspected for a lag in product formation at the moderate amino acid concentrations, and no lag was observed (Fig2C), indicating that the binding step is a rapid equilibrium. The observed first-order rate constants (kobs) extracted from the exponential curves were plotted versus amino acid concentration, and in each case, the resulting curve was fit to a hyperbola from which the apparent binding constant (Kd) and the maximal composite rate of the activation and transfer chemical steps (kchem) were directly obtained (Fig2A and B). Hyperbolic dependency is consistent with a minimal (simplified) reaction scheme (Figure3), whereby the initial binding of amino acid is a rapid equilibrium reaction, and the subsequent steps in aminoacyl-tRNA formation, amino acid activation and/or transfer, define the maximal single-turnover rate (Johnson, 1992).
Figure 2. Kinetic analyses of leucine or isoleucine-dependent synthetic pathway.
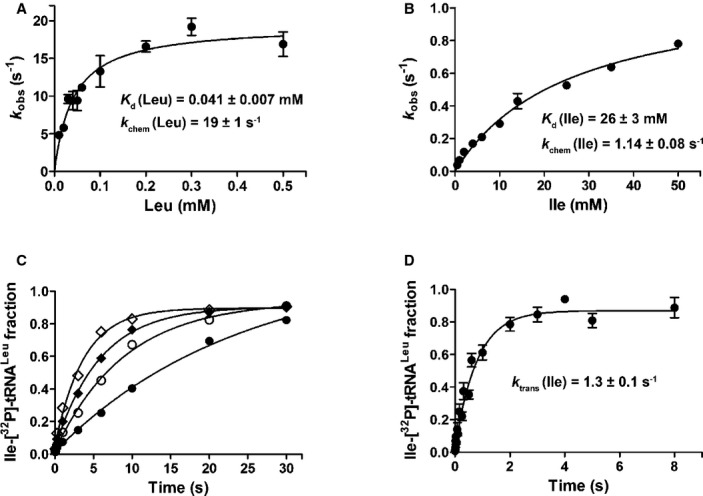
- Plot of single-turnover leucylation rate constant by D345A LeuRS versus leucine concentration.
- Plot of single-turnover isoleucylation rate constant by D345A LeuRS vs. isoleucine concentration.
- Representative isoleucylation time-courses by D345A LeuRS performed at 2 (•), 4 (○), 6 (♦) or 10 (◊) mM isoleucine concentration.
- Single-turnover isoleucine transfer by D345A LeuRS. Errors bars correspond to the s.e.m. from three independent experiments.
Figure 3. Aminoacetylation reaction mechanism.
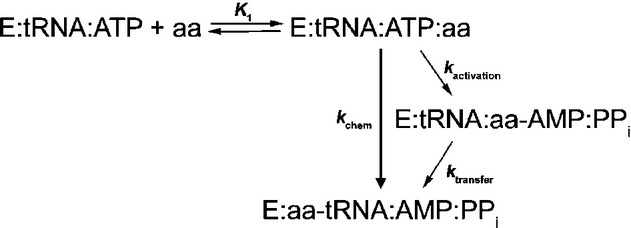
A minimal reaction scheme for the mechanism whereby the initial binding of amino acid is in rapid equilibrium and the subsequent reaction steps define the maximal single-turnover rate of aminoacylation.
The observed 500-fold difference in Kd (Kd (Leu) = 41 μM and Kd (Ile) = 26 mM) is consistent with the 500-fold observed difference in Km in the ATP-PPi exchange reaction. A significant portion of the selectivity also arises at the chemical steps of aminoacyl-tRNA formation (kchem(Ile) = 1.14 s−1 and kchem(Leu) = 19 s−1). Discrimination against isoleucine in the overall two-step aminoacylation ([kchem/Kd]Leu/[kchem/Kd]Ile = 11,000) is thus consistent with discrimination exerted in the first step, showing that tRNALeu does not modulate LeuRS specificity.
Idiosyncratic discrimination against isoleucine at the aminoacyl transfer step
To investigate whether LeuRS also discriminates against isoleucine at the transfer step, the LeuRS:AA-AMP complex preformed in situ was mixed with a limiting amount of [32P]-tRNA and the AA-[32P]-tRNA formation was followed in time. Interestingly, a 50-fold slower transfer of isoleucine (1.3 s−1; Fig2D) as compared with leucine (58 s−1; Cvetesic et al, 2012) was observed. Several controls were undertaken that proved that the transfer step, not slow activation, has been indeed followed (see Materials and Methods). Finally, LeuRS:Ile-AMP was isolated by size-exclusion chromatography prior to use in the single-turnover transfer assay. The derived kobs was 0.5 s−1, confirming unequivocally the slow transfer of the isoleucyl moiety. To provide further insight into the specificity of the LeuRS transfer step, transfer of Aba to tRNALeu was also followed. Interestingly, Aba is rapidly transferred (26 ± 3 s−1) comparable with the reported rapid transfer of norvaline moiety (Cvetesic et al, 2012). It thus appears that slow transfer is an idiosyncratic feature of isoleucine as a substrate of LeuRS. Apparently, the aminoacyl transfer in some cases can embody specificity against amino acid. It has been recently shown that Mycoplasma mobile PheRS also transfers tyrosine 5-fold slower than the cognate phenylalanine to the tRNAPhe (Yadavalli & Ibba, 2013).
Structural basis of isoleucine discrimination by LeuRS
To understand the structural basis for the high discrimination against isoleucine by LeuRS, we determined the crystal structure of EcLeuRS bound to the cognate tRNALeu and a non-hydrolysable analogue of isoleucyl-adenylate (Ile-AMS) at 2.4 Å resolution (Table2). The complex adopts an aminoacylation-like conformation where the 3′ end of the tRNA and Ile-AMS are bound in the kinetically productive position for the transfer of isoleucyl moiety to the tRNA (Fig4A).
Table 2.
Data collection and refinement statistics
| E. coli LeuRS:tRNALeu (UAA):IleAMS | |
|---|---|
| Data collection | |
| Space group | C2 |
| Cell dimensions | |
| a, b, c (Å) | 155.62, 67.58, 224.59 |
| α, β, γ (°) | 90.00, 105.0, 90.00 |
| Resolution (Å) | 46.5–2.5 (2.500–2.505) |
| Rsym | 17.0 (68.5) |
| I/σI | 7.0 (1.8) |
| Completeness (%) | 97.1 (98.7) |
| Redundancy | 3.17 (3.18) |
| Refinement | |
| Resolution (Å) | 2.5 |
| No. reflections work/free | 72414/3828 |
| Rwork/Rfree | 0.213/0.290 |
| No. atoms | |
| Protein | 6907[A]b/6897[D] |
| tRNA | 1756[B]/1692[E] |
| Ligand | 124 (4 × IleAMS) |
| Mg2+ | 1[B]/1[E] |
| Zn2+ | 1[A]/1[D] |
| Water/other | 306 |
| B-factors | |
| Protein | 22.5[A]/74.4[D] |
| tRNA | 30.13[B]/85.7[E] |
| Ligand | 9.6[A1]c/28.7[A2]c 49.7[D1]/144.5[D2] |
| Mg2+ | 25.2[B]/52.1[E] |
| Zn2+ | 65.7[A]/100.4[D] |
| Water | 19.8[Z] |
| R.M.S. deviations | |
| Bond lengths (Å) | 0.010 |
| Bond angles (°) | 1.502 |
Values in parentheses are for highest-resolution shell.
Values are for each molecule in the asymmetric unit with chain indicator given in square brackets.
Number 1 denotes Ile-AMS ligands bound into the synthetic site, and number 2 bound into the editing site.
Figure 4. Structure of the E. coli LeuRS:tRNALeu complex with the non-cognate Ile-AMP analogue.
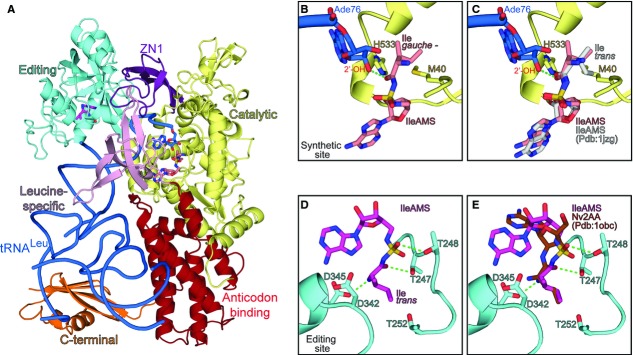
- Cartoon representation of the EcLeuRS:tRNALeu:Ile-AMS ternary complex showing the different domains colored as follows: yellow, catalytic domain; purple, ZN1 domain; cyan, editing domain; pink, leucine-specific domain; red, anticodon-binding domain; orange, C-terminal domain. The tRNALeu is represented in blue ribbon with the last base Ade76 in sticks; the Ile-AMS analogues bound in the synthetic, and editing sites are shown as salmon and magenta sticks, respectively (carbon atoms).
- Ade76 of tRNALeu and Ile-AMS bound in the synthetic site of E. coli LeuRS. The isoleucine part of Ile-AMS shows a gauche conformation. The residues involved in isoleucine discrimination (see text) are represented as sticks.
- Same as (B) but with Ile-AMS from the IleRS:Ile-AMS complex (Pdb:1jzq) docked into the LeuRS synthetic site. Isoleucine adopts trans conformation in IleRS, which is not compatible with the LeuRS synthetic site.
- Ile-AMS bound in the CP1-editing site of LeuRS, where isoleucine adopts trans conformation. Some of the key residues involved in post-transfer editing of non-cognate amino acids and T252 (involved in leucine rejection) at the editing site of LeuRS are shown in sticks.
- Same as (D) but with norvaline post-transfer editing analogue (Nv2AA) docked into the editing site of LeuRS. The position of the aminoacyl part of isoleucine and norvaline is equivalent.
At a protein level, there are no major structural differences at the synthetic site when the cognate (Leu-AMS) or non-cognate (Ile-AMS) AA-AMP analogues are bound. Some minor changes (about 0.8 Å movements) are observed in the positions of the terminal ribose of the tRNA and the residue His537, but their significance is not clear. Interestingly, in spite of the slight movement of the 2′-OH of Ade76, its relative distance to the carbonyl carbon atom of isoleucine remains compatible with the transfer step (2.8 Å) (Fig4B). This is intriguing as isoleucyl transfer to the tRNA proceeds with 50-fold decreased rate as compared with the leucyl transfer (see Discussion). Discrimination against isoleucine is ensured by the lack of van der Waals contacts between its β-branched side chain and the synthetic site pocket. Indeed, inspection of the LeuRS:Leu-AMS structure bound to tRNA revealed that both δ-C atoms of the leucine side chain are well accommodated within the hydrophobic pocket which contributes favorably to the binding energy. The striking feature of the LeuRS:tRNALeu:Ile-AMS ternary complex is the particular conformation of the bound isoleucine substrate, which appears to be imposed by the geometry of the synthetic site. L-isoleucine presents four different conformations in solution: trans, with a dihedral angle X2 (formed by carbons α, β, γ and δ1) of about 170 degrees, gauche – (X2 ˜ 300 degrees), gauche + (X2 ˜ 66 degrees), and gauche 100 (X2 ˜ 100 degrees). The trans conformation is the most favorable energetically and hence the most abundant in solution (˜ 81%), followed by gauche – (˜15%), while the other two conformations account for less than 4% (Hansen et al, 2010; Lovell et al, 2000). Remarkably, our structure reveals that the conformation of isoleucine bound in the synthetic site corresponds to a ‘gauche – like’ conformation (X2 = 243 degrees) and that binding of the trans conformation is sterically prevented by the geometry of the LeuRS synthetic site, in particular by Met40 (Fig4B and C). In contrast, isoleucine adopts the more favored trans conformation when bound to the cognate IleRS synthetic site (Nakama et al, 2001). This suggests that isoleucine recognition by LeuRS exhibits a novel mechanism of discrimination at the ground state level that results in a very weak amino acid binding affinity. To provide more quantitative insight, binding of isoleucine and leucine to EcLeuRS was followed using isothermal titration calorimetry (ITC). Thermodynamic analysis confirmed very weak binding of isoleucine (Kd > 10 mM, Fig5A), consistent with Km (26 mM) and kinetic Kd (26 mM) obtained in the activation and two-step aminoacylation reactions, respectively (Table1 and Fig2). Indeed, LeuRS displays three orders of magnitude higher affinity for cognate leucine (Kd = 60 μM, Fig5B) than for isoleucine. The Kd values extracted from ITC measurements of Ile-AMS or Leu-AMS binding to LeuRS are substantially lower compared with the values for the free amino acids, confirming the significant contribution of the AMP moiety to the energetics of binding (˜103), while retaining a significant difference of affinity between the cognate and non-cognate AA-AMP analogues (Fig5C and D).
Figure 5. Isothermal titration calorimetry experiments showing the binding of cognate and non-cognate amino acids or AA-AMP analogues to E. coli LeuRS.
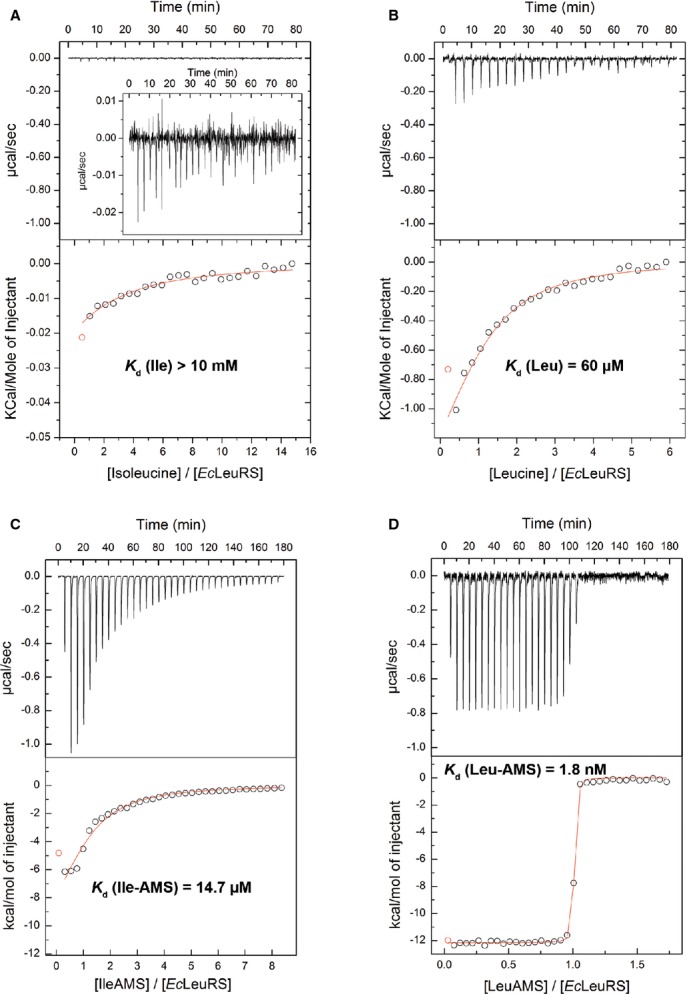
A–D EcLeuRS at 70 μM in the sample cell titrated by (A) isoleucine at 10 mM and (B) leucine at 2 mM, and titration of EcLeuRS at 20–25 μM by (C) Ile-AMS at 0.6 mM and (D) Leu-AMS at 0.3 mM. The upper graphs show the raw data (in same scale to show the affinity differences), and the bottom graphs show the ligand concentration dependence of the heat released upon binding after normalization. Kd values represent the average from at least two independent experiments (the associated errors are about 5%). Inset panel show amplification of the binding profile.
Most likely as a consequence of the high concentration of the analogue used in crystallization, we observed a second Ile-AMS molecule bound in the LeuRS CP1 editing site, which adopts a different conformation compared with that bound in the synthetic site (Fig4D). The AMP moiety is also bound differently from the configuration observed in the complexes of the pre- and post-transfer editing analogue of norvaline bound in the LeuRS CP1 editing site (Lincecum et al, 2003). The reason for this is uncertain although it is consistent with kinetic data showing that hydrolysis of Ile-AMP does not occur within the CP1 editing site (see below). In contrast, the aminoacyl part, including the amino and carbonyl groups, occupies equivalent positions to those of norvaline in the CP1 editing site (Fig4E) (Lincecum et al, 2003). Interestingly, the conformation of isoleucine at the editing site of LeuRS adopts the most stable conformation in solution (trans). This indicates that interconversion of isoleucine between different rotamers is not restricted by the structure of the adenylate and suggests that the ‘gauche – like’ conformation of isoleucine at the LeuRS synthetic site is selected from the pool of different conformations existing in solution rather than due to an artificial effect of the adenylate.
Methionine 40 is essential for LeuRS discrimination against isoleucine
The LeuRS:tRNA:Ile-AMS structure indicates that the positions of Met40 and possibly His533 impose steric constraints which are not compatible with the binding of the trans conformation of isoleucine (Fig4B and C; Supplementary Fig S4). These residues are observed invariably in the same conformation in various LeuRS structures. We substituted Met40 with glycine and observed a 2.6-fold drop in the Km for isoleucine in the activation step as compared to WT (Table1). This likely originates from loosening of the steric constraints of the amino acid binding site. Surprisingly, however, the M40G substitution induced a 200-fold loss in the capacity of the synthetic site to discriminate against isoleucine (Table1). The effect originates predominantly from the cognate reaction; a 7-fold increase in Km and 10-fold decrease in kcat yield 70-fold less efficient leucine activation relative to the WT reaction. The structure of LeuRS in complex with Leu-AMS and tRNA (Palencia et al, 2012) shows that the beta carbon of Met40 side chain is in van der Waals contact with the beta carbon of the leucine side chain of Leu-AMS and thus contributes to binding and productive positioning of leucine for the chemical reaction. Thus, it appears that Met40 plays a dual role in the synthetic site: (i) it enhances cognate leucylation and (ii) it sterically discriminates against binding of the isoleucine in the favorable trans conformation. It must be emphasized that even in the M40G substituted LeuRS active site, isoleucine is kinetically discriminated, which indicates that slow activation is primarily not a consequence of the selected gauche – conformation. Interestingly, the M40G substitution impairs kcat and Km values for norvaline similarly as to leucine, leaving the discrimination factor in amino acid activation unchanged.
Overall editing of isoleucine is limited by the rate of Ile-tRNALeu formation
In the presence of isoleucine and absence of tRNA, EcLeuRS accumulates AMP as a consequence of tRNA-independent editing (Fig6A). The observed rate is comparable to hydrolysis of cognate Leu-AMP (kobs = 0.0100 ± 0.0003 s−1) and is 20-fold slower than editing of norvaline (Cvetesic et al, 2012). Interestingly, Ile-AMP accumulates above the enzyme concentration (Fig6B), which shows that a portion of Ile-AMP is released prior to hydrolysis (Fig1, pathway 2). In contrast, Leu-AMP does not significantly dissociate into solution, presumably as a consequence of its lower dissociation constant (Fig5D). The distinction between AA-[32P]-AMP and [32P]-AMP formation is possible because of utilization of [α-32P]-ATP (Gruic-Sovulj et al, 2005). The released AA-AMPs are prone to slow non-enzymatic hydrolysis, which usually does not substantially contribute to AMP formation. To confirm this, non-enzymatic Ile-AMP hydrolysis was followed by the cold-chase assay (Gruic-Sovulj et al, 2005). The derived catalytic constant (kobs = (2.2 ± 0.2) × 10−4 s−1) was 45-fold smaller than the rate constant for AMP formation in the presence of LeuRS (0.0100 ± 0.0003 s−1, Fig6A), showing that the observed editing is predominately an enzyme-based activity. Inactivation of the CP1 editing site by D345A substitution did not influence the rate of tRNA-independent editing of isoleucine (kobs = 0.013 s−1), thus strongly indicating that pre-transfer editing does not reside in the CP1 editing site. This is consistent with the recently established model, whereby AARS synthetic sites host pre-transfer editing activity (Cvetesic et al, 2012; Dulic et al, 2010; Gruic-Sovulj et al, 2007; Minajigi & Francklyn, 2010; Splan et al, 2008).
Figure 6. Editing of isoleucine by WT LeuRS.
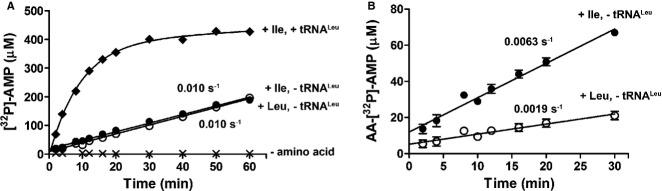
- AMP formation by 5 μM WT LeuRS in the absence of amino acid (×), in the presence of 50 mM Leu and absence of tRNALeu (○), and in the presence of 50 mM Ile and presence (♦) or absence of 20 μM tRNALeu (•).
- AA-AMP formation by 5 μM WT LeuRS in the absence of tRNALeu and in the presence of 50 mM Leu (○), or 50 mM Ile (•). Errors bars correspond to the s.e.m. from three independent experiments.
In the presence of tRNA, isoleucine is edited more rapidly (Fig6A) due to tRNA misacylation and subsequent post-transfer editing at the LeuRS CP1 domain. To quantitatively describe tRNA-dependent (overall) editing of isoleucine, kcat and Km kinetic constants were extracted (Km = 31 mM and kcat = 0.104 s−1, Supplementary Fig S3B). The high Km is consistent with the high Km and Kd for isoleucine in activation and aminoacylation, respectively (Table1, Fig2B). The kcat is significantly slower than the previously determined steady-state rate of Ile-tRNALeu deacylation (kobs = 2 s−1; Cvetesic et al, 2012), indicating that kcat reflects Ile-tRNALeu formation. To demonstrate this by an alternative approach, activation of isoleucine was followed under the editing reaction conditions. The catalytic constant (0.16 s−1) was highly similar to the aforementioned kcat, thus confirming that the overall editing of isoleucine is limited by the rate of the synthetic pathway.
Isoleucine is edited only by the post-transfer LeuRS pathway
A significantly slower isoleucyl transfer step (1.3 s−1) opens the possibility that tRNA-dependent pre-transfer editing (if it exists in the synthetic site of LeuRS) competes effectively with the transfer step. To address this issue, we followed the initial steady-state formation of AA-[32P]-tRNA and [32P]-AMP by the WT and deacylation-defective LeuRS enzymes in the parallel aminoacylation assays, one relying on [32P]-tRNA another on [α-32P]-ATP (Cvetesic et al, 2012). Time-points were sampled to ensure conditions where the tRNA is predominantly non-aminoacylated.
Enhanced AMP consumption during aminoacylation is diagnostic of hydrolytic editing. Hence, an AMP/AA-tRNA ratio above 1 in the presence of the post-transfer editing-deficient enzyme indicates the existence of tRNA-dependent pre-transfer editing. Here, we show that deacylation-defective LeuRS forms AMP and Ile-tRNALeu at identical rates (Fig7A), indicating that Ile-AMP formed in the synthetic site is stoichiometrically used in Ile-tRNALeu synthesis. As expected, WT LeuRS accumulates AMP substantially faster than Ile-tRNALeu because of active post-transfer editing (Fig7B). Thus, despite the inherently slower isoleucyl transfer step, tRNA-dependent pre-transfer editing of isoleucine does not operate in the LeuRS synthetic site. Instead, isoleucine editing relies almost exclusively on the post-transfer pathway.
Figure 7. AMP and Ile-tRNALeu production in parallel reaction assays.
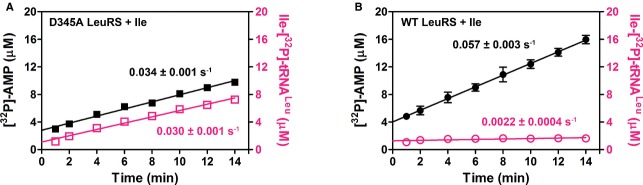
A, B Time-courses following AMP production are illustrated with filled symbols, while time-courses following Ile-tRNALeu production are illustrated with empty magenta-colored symbols. (A) 250 nM D345A LeuRS. (B) 250 nM WT LeuRS. Because of the low Ile-tRNALeu accumulation by WT LeuRS, the formation rate of 0.0022 s−1 (B) should be taken as an approximate value. The rate constants represent the best fit value ± s.e.m. of three independent experiments.
E. coli strain incapable of LeuRS editing tolerates substantial concentration of isoleucine
To study in vivo requirements for editing, an E. coli MG1655 strain that relies on the editing-defective D345A LeuRS was constructed by replacement of the WT leuS gene with the gene for D345A LeuRS using genetic recombination. Error-prone conditions were introduced by supplementing the minimal media with either norvaline or isoleucine. Inactivation of the LeuRS CP1 editing site did not induce any growth defect in the absence of non-cognate amino acid supplements, arguing against universal requirements for AARS editing under non-error-prone conditions (Fig8A). In sharp contrast, a clear distinction between deacylation-defective and WT LeuRS strains was observed in the presence of norvaline. Norvaline (in concentrations as low as 1.5 mM) completely inhibits the growth of the editing-defective strain, yet exhibits no impact on the growth of the WT strain (Fig8A). This is consistent with the recently demonstrated high capacity of LeuRS to edit norvaline (Cvetesic et al, 2012). Replot of the growth rates versus norvaline concentrations yields IC50 of about 0.1 mM (Fig8B). Conversely, up to 20 mM isoleucine did not induce a substantial effect on the growth of either the deacylation-defective or the WT strain (Fig8C). This is consistent with some previous work (Karkhanis et al, 2007). An unspecific inhibitory effect on both strains was observed with isoleucine above 30 mM (IC50 of 51 mM; Fig8D), presumably as a consequence of the artificially high amino acid concentration. We sought to confirm the non-specificity of the effect by performing the same experiment with leucine (Fig8E and F). The same behavior was observed, however at lower leucine concentration in agreement with its known signaling role in E. coli (Newman & Lin, 1995). Since the high surplus of isoleucine is well tolerated by the cells deprived of LeuRS editing, it is unlikely the CP1 editing domain subsists to ensure isoleucine editing.
Figure 8. Norvaline, isoleucine, and leucine toxicity.
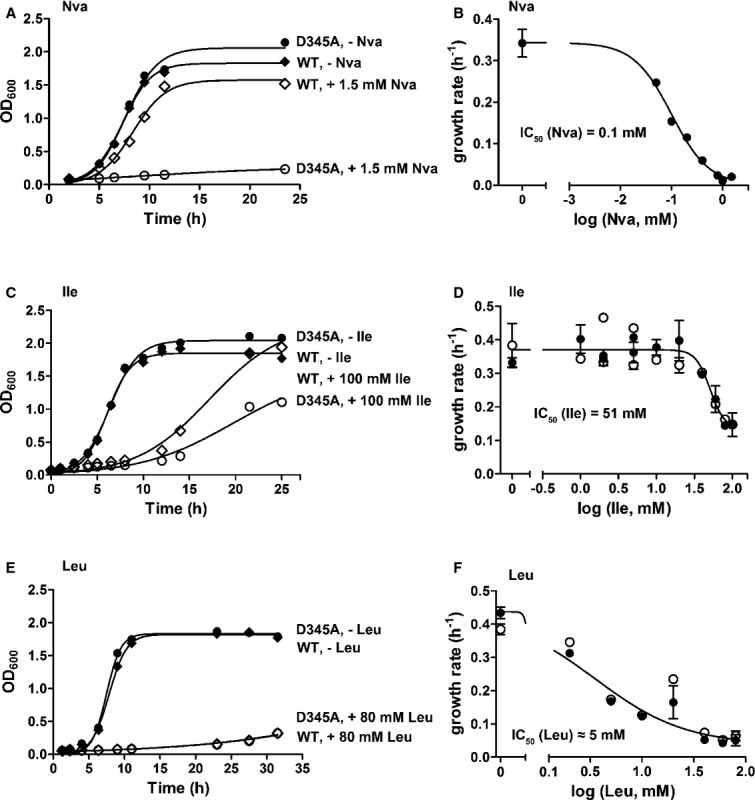
- Growth curves of LeuRS WT (♦) or editing-deficient (D345A LeuRS) (•) E. coli strains in the presence (◊, ○) or absence (♦, •) of 1.5 mM Nva.
- Concentration-dependent toxicity of norvaline. Errors bars correspond to the s.e.m. from four independent experiments.
- Growth curves of LeuRS WT (♦) or editing-deficient (D345A LeuRS) (•) E. coli strains in the presence (◊, ○) or absence (♦, •) of 100 mM Ile.
- Concentration-dependent toxicity of isoleucine. Errors bars correspond to the s.e.m. from four independent experiments.
- Growth curves of LeuRS WT (♦) or editing-deficient (D345A LeuRS) (•) E. coli strains in the presence (◊, ○) or absence (♦, •) of 80 mM Leu.
- Concentration-dependent toxicity of leucine. Errors bars correspond to the s.e.m. from four independent experiments.
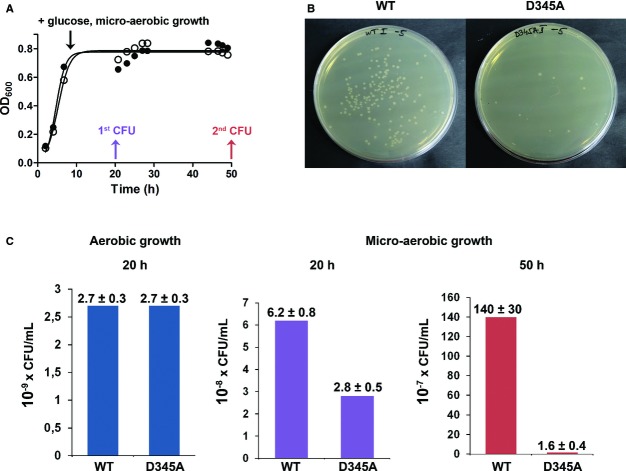
LeuRS editing is crucial for E. coli viability under micro-aerobic conditions
To explore whether LeuRS editing is a prerequisite for accurate translation under growth conditions where norvaline accumulates, we tested the viability of the WT and LeuRS editing-deficient strains (D345A) under micro-aerobic conditions. Inactivation of editing had no impact on the growth curves (Fig9A), presumably because the cultures still contained a substantial level of oxygen or did not accumulate norvaline to a substantial concentration. However, as a consequence of impaired oxygen transfer, the cells reached a stationary phase at a significantly lower OD600 than observed at the aerobic growth (Fig8A). Rigorous micro-aerobic conditions were then promoted at the stationary phase by a decrease in agitation. Norvaline production was further stimulated by adding glucose to the culture at regular time intervals (see Materials and Methods). Cell viability was sampled via the colony forming unit assay 10 and 40 h after promotion of micro-aerobic growth. The strain incapable of LeuRS editing exhibited a two-fold drop relative to the WT strain in the number of viable cells after 10 h of micro-aerobic growth, whereas the difference increased to 87-fold after 40 h (Fig9B and C). The 18-fold drop in viability (2.8 × 108 versus 1.6 × 107; Fig9C) during prolonged growth under micro-aerobic conditions, which is observed only with the D345A strain, clearly links LeuRS editing with E. coli survival under oxygen-limited, norvaline-rich growth conditions.
Figure 9. Cell viability under micro-aerobic conditions.
- Growth curves of LeuRS WT (•) or editing-deficient (D345A LeuRS) (○) E. coli strains. The black arrow indicates the start of the glucose feed and promotion of the micro-aerobic conditions by reduced agitation. The purple and red arrows indicate the time-points when the cultures were sampled for the CFU assay.
- Representative plate dilutions of both WT and D345A strains from the CFU assay performed after 50 h of growth under micro-aerobic conditions.
- Cell viability of WT and D345A strains grown in aerobic conditions (blue column chart, 20 h of growth) and micro-aerobic conditions (purple column chart, 20 h of growth, red column chart, 50 h of growth). Note that labels of the y-axis for each of the column charts are in different orders of magnitude. Errors correspond to the s.e.m. from four independent experiments.
Discussion
Here, we find that EcLeuRS discriminates robustly against isoleucine at the amino acid activation and aminoacylation steps, with specificity of better than 104-fold (Table1, Fig2). This is substantially higher than the minimal discrimination that is generally accepted to be necessary for sufficient translational fidelity (1 in 3,300, Loftfield & Vanderjagt, 1972) and thus obviates the need for post-transfer editing of isoleucine. Our data correct the misconception that isoleucine mimics leucine in the LeuRS synthetic reactions. The essential difference between this work and previous studies that reported weak isoleucine discrimination by EcLeuRS (Boniecki et al, 2008; Chen et al, 2000; Lue & Kelley, 2005; Martinis & Fox, 1997) is the purification step we introduced to eliminate traces of leucine from isoleucine samples. Remarkably, all tested commercial isoleucine samples were contaminated by leucine to various extents (0.0019–0.38%, Supplementary Materials and Methods). Contamination of isoleucine by leucine, a problem which has not previously been highlighted, leads to substantial underestimation of LeuRS specificity for isoleucine (Supplementary Table S1). Indeed, production and utilization of ultra-pure isoleucine (around 0.00035% of leucine) were necessary for a clear demonstration of the inability of isoleucine to challenge the accuracy of leucylation. Several lines of evidence now indicate that the major threat for error-free leucylation is instead posed by the non-proteinogenic amino acid norvaline (see below).
Two idiosyncratic features of EcLeuRS discrimination against isoleucine are: (i) specificity is also exercised at the chemical steps and (ii) ground state discrimination operates in part via rejection of the most abundant isoleucine conformer in solution (trans). This differs from so far described specificity mechanisms in class Ia editing AARSs (Cvetesic et al, 2012; Dulic et al, 2010; Fersht & Dingwall, 1979b). An origin of specificity established at the chemical steps is not clear yet. It has been recently argued for DNA polymerases, another family of editing enzymes, that the non-cognate substrate binding energy may be used to misalign the catalytic residues (Johnson, 2010). Fluorescence analyses indeed indicated that T7 DNA-polymerase bound to near-cognate nucleotide adopts a mismatch conformation that impedes catalysis and promotes substrate release (Tsai & Johnson, 2006). The crystal structure of the LeuRS:tRNALeu complex bound to an Ile-AMP analogue, however, does not provide evidence for a distinct, long-lived non-cognate conformation that could be responsible for the slow chemical step, although this possibility cannot be fully excluded. Thus, it seems plausible to assume that no active site determinants participate in unproductive Ile-AMP binding. Instead, assembly of the catalytic residues for the isoleucyl transfer step likely occurs through a slower isoleucine-dependent conformational change, which may limit the measured ktrans. One may also argue that isoleucine is specifically distinguished at the transition state. Interestingly, α-aminobutyrate, which lacks side chain binding capacity, is activated and transferred at rates comparable to the cognate reaction. This may indicate that the amino acid side chain does not significantly contribute to transition state stabilization in LeuRS. However, steric constraints imposed by the different branching of the isoleucine side chain may influence the functional contacts at the transition states.
A lack of sufficient binding energy for the β-branched isoleucine side chain in combination with preferential binding of the less abundant isoleucine conformation (gauche –) results in very weak affinity for isoleucine (Table1, Figs2 and 5). The established low affinity, however, might not suffice to provide the high specificity observed against isoleucine (˜31,000). Instead, a significant portion of the selectivity also arises at the chemistry steps. Our data open a new perspective about AARS selectivity mechanisms, showing that specificity against non-cognate amino acids may arise in part from preferential binding of higher-energy conformers. This complements the double-sieve model (Fersht, 1998), which proposes that a synthetic site acts as a coarse-sieve that rejects larger amino acids solely by steric exclusion. It remains to be seen whether this mechanism is unique for isoleucine rejection by EcLeuRS or is widespread among different LeuRSs or even other AARSs. Indeed, structural and kinetic analyses demonstrate that Met40 is a key determinant of EcLeuRS specificity against isoleucine (Table1, Fig4C). However, whereas Met40 is conserved in bacteria, it is not conserved among LeuRSs from other domains of life (Supplementary Fig S5). Some archaeal LeuRSs have alanine instead of methionine, while the cytosolic eukaryotic LeuRS mostly have proline. We produced M40A LeuRS and tested its activation of isoleucine. Minimal effects on the Km and kcat values were observed (Km = 20 ± 4 mM, kcat = 1.05 ± 0.08 s−1), consistent with a model whereby the β-carbon atom of Met40 plays the most prominent role in steric exclusion of the trans isoleucine. Additional support for the model comes from the observation that homologous positions in IleRSs are occupied by the absolutely conserved glycine, presumably to preclude steric clash with the isoleucine in trans conformation (Supplementary Fig S5).
Specificity in non-editing AARSs generally originates from the lack of synthetic site binding determinants for the non-cognate amino acids, providing insufficient binding energy for substantial ground and transition state stabilization (Perona & Hadd, 2012). Alternatively, the synthetic site may drive unproductive binding of the non-cognate amino acid (Bullock et al, 2003) or may impose steric hindrance to ensure selectivity (Arnez et al, 1999). Consequently, a prominent increase in Km and decrease in kcat jointly establish 105–107-fold specificity (Fersht & Dingwall, 1979a; Uter et al, 2005). If an AARS is unable to discriminate with at least 10-3 frequency against non-cognate amino acid in the synthetic reaction, editing becomes critical. This generally holds for AARSs which are incapable of exerting specificity at the catalytic steps (Fersht, 1977; Fersht & Kaethner, 1976; Beebe et al, 2003; Guo et al, 2009; Ling et al, 2012; Sankaranarayanan et al, 2000; Yadavalli & Ibba, 2013). Intriguingly, LeuRS resembles editing tRNA synthetases in its specificity against norvaline, while it discriminates against isoleucine in a manner more closely related to non-editing AARSs. Consistent with this, in vivo studies show that loss of LeuRS post-transfer editing had dramatic consequences on cell growth only in the presence of norvaline. The strain lacking LeuRS post-transfer editing tolerates isoleucine at a concentration substantially higher than its physiological concentration in E. coli (20 mM (Fig8C and D) versus 0.3 mM (Bennett et al, 2009)), thus underpinning the irrelevance of isoleucine editing. This finding has important implications to the development of antimicrobial agents. Recently, boron-based benzoxaborole compounds have been developed that inhibit fungal (Rock et al, 2007) and bacterial (Hernandez et al, 2013) LeuRSs, by covalently trapping enzyme-bound tRNALeu in the CP1 editing site. However, in clinical trials, benzoxaborole-resistant bacterial mutants emerged, showing that the cell can establish antibiotic resistance at the expense of LeuRS post-transfer editing (Hernandez et al, 2013). This is consistent with the reported irrelevance of isoleucine editing in vivo. To decrease the frequency with which resistant mutants emerge, norvaline-rich conditions could be artificially induced to promote growth conditions where LeuRS post-transfer editing is essential for cell survival (Fig8A and B).
The combination of both high amino acid specificity and an active post-transfer editing mechanism against non-cognate tyrosine and alanine was previously recognized in E. coli PheRS (Reynolds et al, 2010) and ProRS (Beuning & Musier-Forsyth, 2000), respectively. Therefore, the appearance of dual checkpoints in at least PheRS, LeuRS, and ProRS indicates that E. coli relies on several quality control mechanisms to ensure adaptability under highly different physiological conditions. It has recently been suggested that requirements for quality control mechanisms may be species-specific and condition-dependent (Beuning & Musier-Forsyth, 2001; Reynolds et al, 2010; SternJohn et al, 2007). In accordance, we show here that AARS editing may operate to preserve canonical translation only under particular conditions of growth. Under normal aerobic conditions, E. coli easily tolerates editing-deficient LeuRS, implying that LeuRS post-transfer editing is irrelevant for normal growth as already demonstrated for E. coli PheRS editing (Reynolds et al, 2010). Conversely, mistranslation of leucine codons under micro-aerobic conditions significantly decreases cell viability (Fig9B and C). Its toxicity is likely a consequence of misfolding and aggregation (Drummond & Wilke, 2009) of the norvaline enriched proteins (Weber & Miller, 1981). Indeed, growth in a norvaline-rich environment is rescued by the powerful LeuRS post-transfer editing activity which eliminates norvaline from the genetic code (Fig8A). This capacity provides E. coli with flexibility to encounter both oxygen-rich and oxygen-deprived ecological niches. Our results show that AARS-mediated translational quality control is interconnected with the E. coli adaptive response mechanisms to provide survival under quickly changing oxygen environments.
Materials and Methods
EcLeuRS kinetic assays
EcLeuRS enzymes and tRNALeuUAA were produced and purified by standard procedures (Cvetesic et al, 2012). The enzymes used in single-turnover assays were subjected to a second purification step designed to remove Leu-AMP bound in the enzyme's active site, as described (Cvetesic et al, 2012).
[32P]-tRNALeu used in kinetic assays was prepared using tRNA nucleotidyltransferase (Wolfson & Uhlenbeck, 2002). All rate constants obtained through [32P]-tRNALeu measurement were corrected by the factor that reflects the proportion of functional [32P]-tRNALeu (Cvetesic et al, 2012).
All assays were performed at 37 °C in the standard LeuRS reaction buffer that contains 100 mM Hepes-KOH, pH 7.5, 10 mM MgCl2, 150 mM KCl, and 5 mM DTT with appropriate amounts of enzyme, amino acid, or ATP. Steady-state reactions were supplemented with 100 μg/ml BSA for enzyme stabilization. All experiments were routinely repeated at least three times.
ATP-PPi exchange was performed using 4 mM ATP and 1 mM [32P]-PPi (Cvetesic et al, 2012). The parameters for isoleucine activation were determined using the ultra-pure sample (around 0.00035% leucine).
Aminoacyl-adenylate synthesis assay was performed as described (Cvetesic et al, 2012). The reactions included 0.5 mM [α-32P]-ATP (0.01–0.1 mCi/ml), 0.004 U/μl inorganic pyrophosphatase (IPPase), with or without 20 μM active tRNALeu.
The Ile-AMP stability test was performed with ultra-pure isoleucine, as previously published (Cvetesic et al, 2012).
[32P]-AMP and Ile-[32P]-tRNALeu formations were followed in parallel steady-state assays as described (Cvetesic et al, 2012). Briefly, the reactions were supplemented with 0.004 U/μl IPPase, using either 0.25 μM WT or D345A LeuRS in the presence of 200 μM ATP, 20 μM active tRNALeu, and 50 mM Ile (ultra-pure).
The transfer step was measured using D345A LeuRS:Ile-AMP or D345A LeuRS:Aba-AMP complexes pre-formed by incubation of 60 μM D345A LeuRS with 8 mM ATP, 0.008 U/μl IPPase, and 10 mM Ile or 100 mM Aba, respectively, for 15 min at 37°C. Equal volumes of 60 μM LeuRS:AA-AMP were mixed with 12 μM active [32P]-tRNALeu in the KinTek RQF-3 instrument, as described (Cvetesic et al, 2012; Dulic et al, 2010). Several controls were performed to confirm that the measured rate constant represents the true transfer rate constant and is not limited by slow amino acid activation. Both, the incubation time for in situ formation of LeuRS:AA-AMP and the concentration of Ile or Aba were varied, and no influence on the observed rate constant was revealed. Furthermore, transfer of isoleucine was measured using the isolated D345A LeuRS:Ile-AMP non-covalent complex. The complex was formed as described above, cooled by placing on ice, and isolated by size-exclusion chromatography on Sephadex G-25. Due to its instability, the isolated complex was constantly kept at low temperature, the syringe used for loading the complex was cooled in ice prior to use, and a construction that enables ice cooling of the syringe mounted on the sample port was used throughout data collection. The complex was preheated to 37°C by 1–2 min incubation in the RQF-3 instrument, prior to mixing with tRNA.
Single-turnover isoleucylation was performed using the KinTek RQF-3 instrument by mixing equal volumes of 60 μM D345A LeuRS, 12 μM [32P]-tRNALeu, 8 mM ATP, and 0.008 U/μl IPPase incubated in one syringe, with various concentrations of isoleucine incubated in the second syringe. Single-turnover leucylations were performed in the same manner, albeit 20 μM D345A LeuRS and 2 μM tRNALeu were used. Time-courses were fit to a first-order exponential equation, and the extracted rate constants (kobs) were fit to the hyperbolic binding equation (kobs = S0 × kchem/S0 + Kd). Various mixing modes showed no influence on the extracted rate constants.
Construction of the editing-deficient D345A-LeuRS E. coli strain
E. coli strain MG1655 was obtained from the E. coli Genetic Stock Center and used as the parent strain to create the editing-deficient D345A LeuRS strain. Replacement of the chromosomal WT leuS with the gene for D345A LeuRS was performed using the pKOV vector, according to the published procedures (Link et al, 1997). The positives were selected by their sensitivity to norvaline and confirmed by sequencing (for details see Supplementary Materials and Methods).
Amino acid toxicity experiments
The WT and editing-deficient D345A-LeuRS strains were inoculated into LB media from the colonies grown on LB plates and were grown overnight at 37°C, 200 rpm. The cultures were diluted 1:100 into minimal M9 medium with 0.2% glucose and the various concentrations of either norvaline, isoleucine, or leucine. The cultures were grown at 37°C, 200 rpm, and the growth curves were generated by following OD600. For both, the WT and deacylation-deficient strains, two independently obtained strains were used as biological replicates. To obtain growth rates, the growth curves were fitted to the logistic model y = A/(1 + e(4 × μ/A × (λ−t) + 2)), where A represents the maximum cell growth, λ represents the length of the lag phase, and μ represents the growth rate. All experiments were performed in duplicates; the obtained growth rates were averaged, plotted against amino acid concentration, and fitted to the sigmoidal dose-response curve to acquire IC50.
Cell viability under micro-aerobic conditions
The WT and editing-deficient D345A-LeuRS MG1655 strains were inoculated into LB media from single colonies and grown overnight in aerobic conditions at 37°C, 200 rpm, until saturation was reached. Two independently obtained WT and D345A strains were used as biological replicates. The starter cultures were diluted 1:100 into minimal M9 medium supplemented with 2% glucose. To achieve micro-aerobic growth conditions, the M9 medium was degassed by sonication prior to inoculation, and the experiment was performed in 50 ml of media incubated in 50 ml conical tubes. Growth was followed at OD600, and the cultures were agitated at 150 rpm at 37°C, until they reached a stationary growth phase. Agitation was then lowered to 100 rpm to reduce the oxygen transfer, and the cultures were supplemented with glucose at regular time intervals (approximately every 2 h during the first 12 h, and then after 14 h, 1 ml of 50% glucose was added). The colony forming unit assay was performed after 20 and 50 h of growth. Decimal and serial dilutions were made in phosphate buffered saline, and 100 μl of each dilution was evenly spread on LB plates and incubated overnight. Colony count was performed only with plate dilutions that had more than 30 and less than 300 colonies. CFU/ml for WT and D345A strain was expressed as an average of two biological replicas, each with at least two dilution replicas.
Control experiment was performed under aerobic conditions. 10 ml of M9 medium was inoculated 1:100 with starter cultures (WT or D345A strain) and incubated in 50-ml conical tubes. Growth was followed at OD600, and the cultures were agitated at 37°C, 200 rpm to maximize oxygen transfer. CFU assay was performed as described above.
Crystallization
Production and purification of LeuRS and tRNALeu in vitro transcript for crystallization were followed as described (Palencia et al, 2012). Crystallization was performed at 20°C by the hanging drop vapor diffusion method. A solution containing 51 μM LeuRS, 58 μM tRNALeu, and 2.5 mM Ile-AMS (purchased from RNA-TEC, Leuven, Belgium) was prepared prior to the crystallization experiments. Crystals were obtained by mixing 2 μl of this solution with 2 μl of the reservoir solution containing 0.1 M Bis-Tris (pH 5.5), 23–25% PEG 3350, and 0–200 mM ammonium acetate. The crystals were frozen in liquid nitrogen prior to X-ray exposure without added cryoprotectant. Structure determination and refinement were performed by standard procedures (for details see Supplementary Materials and Methods).
Isothermal titration calorimetry (ITC) measurements
ITC measurements were taken at 25°C using an ITC200 Micro-calorimeter (MicroCal Inc). Experiments included 26 stepwise, automated injections (Vinjection = 1.5 μl) of amino acid (2–10 mM), or AA-AMP analogue (0.3–0.6 mM) solutions into the sample cell containing the protein (20–70 μM) in 25 mM Tris–HCl pH 7.4, 150 mM NaCl, 5 mM MgCl2, and 5 mM 2-β-mercaptoethanol buffer. Amino acids (purchased from Sigma) and AA-AMP analogues (acquired from RNA-TEC) were dissolved in the same buffer as the protein, and when necessary, the pH was readjusted. For the experiments with isoleucine, ultra-pure isoleucine sample was used (around 0.00035% of leucine contamination). Control experiments were performed under identical conditions by injecting the ligands into the buffer to correct the dilution heats into the buffer. Binding isotherms were fit by nonlinear regression using Origin Software version 7.0 (MicroCal Inc). The initial data point was routinely not used in the fitting. The data were fit to a one-site binding model using the software provided by MicroCal (Turnbull & Daranas, 2003; Wiseman et al, 1989).
Accession codes
Protein data bank: Coordinates have been deposited with accession code 4cqn.
Acknowledgments
We are indebt to Ivana Weygand-Durasevic for access to the research facilities at the University of Zagreb, Croatia, Mario Cindric, and Marko Mocibob for fruitful discussions about the contamination problem, Mirna Bilus for assistance with the CFU assay, and John J. Perona and Morana Dulic for careful reading of the manuscript. This work was supported by the Unity through Knowledge Fund (Grant No 8/13 to IGS) and in part by the grant from Croatian Science Foundation (09.01/293). SC and AP acknowledge the access to the EMBL-ESRF-ILL-IBS Partnership for Structural Biology facilities.
Author contributions
NC designed and executed all kinetic and in vivo experiments under the guidance of IGS. AP performed structural and ITC analyses with input from SC. IH purified isoleucine. IGS directed the project and wrote the manuscript with input from all coauthors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information for this article is available online: http://emboj.embopress.org
References
- Arnez JG, Dock-Bregeon AC, Moras D. Glycyl-tRNA synthetase uses a negatively charged pit for specific recognition and activation of glycine. J Mol Biol. 1999;286:1449–1459. doi: 10.1006/jmbi.1999.2562. [DOI] [PubMed] [Google Scholar]
- Beebe K, Ribas de Pouplana L, Schimmel P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 2003;22:668–675. doi: 10.1093/emboj/cdg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuning PJ, Musier-Forsyth K. Hydrolytic editing by a class II aminoacyl-tRNA synthetase. Proc Natl Acad Sci USA. 2000;97:8916–8920. doi: 10.1073/pnas.97.16.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuning PJ, Musier-Forsyth K. Species-specific differences in amino acid editing by class II prolyl-tRNA synthetase. J Biol Chem. 2001;276:30779–30785. doi: 10.1074/jbc.M104761200. [DOI] [PubMed] [Google Scholar]
- Boniecki MT, Vu MT, Betha AK, Martinis SA. CP1-dependent partitioning of pretransfer and posttransfer editing in leucyl-tRNA synthetase. Proc Natl Acad Sci USA. 2008;105:19223–19228. doi: 10.1073/pnas.0809336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee ML, Pierce MA, Francklyn CS. Induced fit and kinetic mechanism of adenylation catalyzed by Escherichia coli threonyl-tRNA synthetase. Biochemistry. 2003;42:15102–15113. doi: 10.1021/bi0355701. [DOI] [PubMed] [Google Scholar]
- Bullock TL, Uter N, Nissan TA, Perona JJ. Amino acid discrimination by a class I aminoacyl-tRNA synthetase specified by negative determinants. J Mol Biol. 2003;328:395–408. doi: 10.1016/s0022-2836(03)00305-x. [DOI] [PubMed] [Google Scholar]
- Chen JF, Guo NN, Li T, Wang ED, Wang YL. CP1 domain in Escherichia coli leucyl-tRNA synthetase is crucial for its editing function. Biochemistry. 2000;39:6726–6731. doi: 10.1021/bi000108r. [DOI] [PubMed] [Google Scholar]
- Cusack S. Aminoacyl-tRNA synthetases. Curr Opin Struct Biol. 1997;7:881–889. doi: 10.1016/s0959-440x(97)80161-3. [DOI] [PubMed] [Google Scholar]
- Cusack S, Yaremchuk A, Tukalo M. The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue. EMBO J. 2000;19:2351–2361. doi: 10.1093/emboj/19.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetesic N, Perona JJ, Gruic-Sovulj I. Kinetic partitioning between synthetic and editing pathways in class I aminoacyl-tRNA synthetases occurs at both pre-transfer and post-transfer hydrolytic steps. J Biol Chem. 2012;287:25381–25394. doi: 10.1074/jbc.M112.372151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbelt L, Pachmann U, Zachau HG. Serine activation is the rate limiting step of tRNASer aminoacylation by yeast seryl tRNA synthetase. Nucleic Acids Res. 1980;8:4021–4039. doi: 10.1093/nar/8.17.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DA, Wilke CO. The evolutionary consequences of erroneous protein synthesis. Nat Rev Genet. 2009;10:715–724. doi: 10.1038/nrg2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic M, Cvetesic N, Perona JJ, Gruic-Sovulj I. Partitioning of tRNA-dependent editing between pre- and post-transfer pathways in class I aminoacyl-tRNA synthetases. J Biol Chem. 2010;285:23799–23809. doi: 10.1074/jbc.M110.133553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Fersht A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. 1st edn. New York: W. H. Freeman; 1998. [Google Scholar]
- Fersht AR, Kaethner MM. Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry. 1976;15:3342–3346. doi: 10.1021/bi00660a026. [DOI] [PubMed] [Google Scholar]
- Fersht AR. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry. 1977;16:1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- Fersht AR, Dingwall C. Cysteinyl-tRNA synthetase from Escherichia coli does not need an editing mechanism to reject serine and alanine. High binding energy of small groups in specific molecular interactions. Biochemistry. 1979a;18:1245–1249. doi: 10.1021/bi00574a020. [DOI] [PubMed] [Google Scholar]
- Fersht AR, Dingwall C. Evidence for the double-sieve editing mechanism in protein synthesis. Steric exclusion of isoleucine by valyl-tRNA synthetases. Biochemistry. 1979b;18:2627–2631. doi: 10.1021/bi00579a030. [DOI] [PubMed] [Google Scholar]
- First E. Catalysis of the tRNA aminoacylation reaction. In: Ibba M, Francklyn CS, Cusack S, editors. The Aminoacyl-tRNA Synthetases. Landes Bioscience/Eurekah.com: Georgetown, Texas; 2005. pp. 328–347. (eds) [Google Scholar]
- Fukai S, Nureki O, Sekine S, Shimada A, Tao J, Vassylyev DG, Yokoyama S. Structural basis for double-sieve discrimination of L-valine from L-isoleucine and L-threonine by the complex of tRNA(Val) and valyl-tRNA synthetase. Cell. 2000;103:793–803. doi: 10.1016/s0092-8674(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Gruic-Sovulj I, Landeka I, Soll D, Weygand-Durasevic I. tRNA-dependent amino acid discrimination by yeast seryl-tRNA synthetase. Eur J Biochem. 2002;269:5271–5279. doi: 10.1046/j.1432-1033.2002.03241.x. [DOI] [PubMed] [Google Scholar]
- Gruic-Sovulj I, Uter N, Bullock T, Perona JJ. tRNA-dependent aminoacyl-adenylate hydrolysis by a nonediting class I aminoacyl-tRNA synthetase. J Biol Chem. 2005;280:23978–23986. doi: 10.1074/jbc.M414260200. [DOI] [PubMed] [Google Scholar]
- Gruic-Sovulj I, Rokov-Plavec J, Weygand-Durasevic I. Hydrolysis of non-cognate aminoacyl-adenylates by a class II aminoacyl-tRNA synthetase lacking an editing domain. FEBS Lett. 2007;581:5110–5114. doi: 10.1016/j.febslet.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Guo M, Chong YE, Shapiro R, Beebe K, Yang XL, Schimmel P. Paradox of mistranslation of serine for alanine caused by AlaRS recognition dilemma. Nature. 2009;462:808–812. doi: 10.1038/nature08612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth E, Connolly SH, Bovee M, Francklyn CS. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry. 2005;44:3785–3794. doi: 10.1021/bi047923h. [DOI] [PubMed] [Google Scholar]
- Hansen DF, Neudecker P, Kay LE. Determination of isoleucine side-chain conformations in ground and excited states of proteins from chemical shifts. J Am Chem Soc. 2010;132:7589–7591. doi: 10.1021/ja102090z. [DOI] [PubMed] [Google Scholar]
- Hernandez V, Crepin T, Palencia A, Cusack S, Akama T, Baker SJ, Bu W, Feng L, Freund YR, Liu L, Meewan M, Mohan M, Mao W, Rock FL, Sexton H, Sheoran A, Zhang Y, Zhang YK, Zhou Y, Nieman JA, et al. Discovery of a novel class of boron-based antibacterials with activity against gram-negative bacteria. Antimicrob Agents Chemother. 2013;57:1394–1403. doi: 10.1128/AAC.02058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA. Transient-State Kinetic Analysis of Enzyme Reaction Pathways. Vol. 20. San Diego: Academic Press Inc; 1992. Vol. [Google Scholar]
- Johnson KA. The kinetic and chemical mechanism of high-fidelity DNA polymerases. Biochim Biophys Acta. 2010;1804:1041–1048. doi: 10.1016/j.bbapap.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis VA, Mascarenhas AP, Martinis SA. Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J Bacteriol. 2007;189:8765–8768. doi: 10.1128/JB.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SX, Baltzinger M, Remy P. Fast kinetic study of yeast phenylalanyl-tRNA synthetase: role of tRNAPhe in the discrimination between tyrosine and phenylalanine. Biochemistry. 1984;23:4109–4116. doi: 10.1021/bi00313a015. [DOI] [PubMed] [Google Scholar]
- Lincecum TL, Jr, Tukalo M, Yaremchuk A, Mursinna RS, Williams AM, Sproat BS, Van Den Eynde W, Link A, Van Calenbergh S, Grotli M, Martinis SA, Cusack S. Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol Cell. 2003;11:951–963. doi: 10.1016/s1097-2765(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Ling J, Peterson KM, Simonovic I, Soll D, Simonovic M. The mechanism of pre-transfer editing in yeast mitochondrial threonyl-tRNA synthetase. J Biol Chem. 2012;287:28518–28525. doi: 10.1074/jbc.M112.372920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AJ, Phillips D, Church GM. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftfield RB, Vanderjagt D. The frequency of errors in protein biosynthesis. Biochem J. 1972;128:1353–1356. doi: 10.1042/bj1281353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell SC, Word JM, Richardson JS, Richardson DC. The penultimate rotamer library. Proteins. 2000;40:389–408. [PubMed] [Google Scholar]
- Lue SW, Kelley SO. An aminoacyl-tRNA synthetase with a defunct editing site. Biochemistry. 2005;44:3010–3016. doi: 10.1021/bi047901v. [DOI] [PubMed] [Google Scholar]
- Martinis SA, Fox GE. Non-standard amino acid recognition by Escherichia coli leucyl-tRNA synthetase. Nucleic Acids Symp Ser. 1997;36:125–128. [PubMed] [Google Scholar]
- Minajigi A, Francklyn CS. Aminoacyl transfer rate dictates choice of editing pathway in threonyl-tRNA synthetase. J Biol Chem. 2010;285:23810–23817. doi: 10.1074/jbc.M110.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursinna RS, Lee KW, Briggs JM, Martinis SA. Molecular dissection of a critical specificity determinant within the amino acid editing domain of leucyl-tRNA synthetase. Biochemistry. 2004;43:155–165. doi: 10.1021/bi034919h. [DOI] [PubMed] [Google Scholar]
- Nakama T, Nureki O, Yokoyama S. Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-tRNA synthetase. J Biol Chem. 2001;276:47387–47393. doi: 10.1074/jbc.M109089200. [DOI] [PubMed] [Google Scholar]
- Newman EB, Lin R. Leucine-responsive regulatory protein: a global regulator of gene expression in E. coli. Annu Rev Microbiol. 1995;49:747–775. doi: 10.1146/annurev.mi.49.100195.003531. [DOI] [PubMed] [Google Scholar]
- Palencia A, Crepin T, Vu MT, Lincecum TL, Jr, Martinis SA, Cusack S. Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat Struct Mol Biol. 2012;19:677–684. doi: 10.1038/nsmb.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona JJ, Hadd A. Structural diversity and protein engineering of the aminoacyl-tRNA synthetases. Biochemistry. 2012;51:8705–8729. doi: 10.1021/bi301180x. [DOI] [PubMed] [Google Scholar]
- Perona JJ, Gruic-Sovulj I. Synthetic and Editing Mechanisms of Aminoacyl-tRNA Synthetases. Top Curr Chem. 2013;344:1–41. doi: 10.1007/128_2013_456. [DOI] [PubMed] [Google Scholar]
- de Pouplana LR, Schimmel P. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell. 2001;104:191–193. doi: 10.1016/s0092-8674(01)00204-5. [DOI] [PubMed] [Google Scholar]
- Reynolds NM, Ling J, Roy H, Banerjee R, Repasky SE, Hamel P, Ibba M. Cell-specific differences in the requirements for translation quality control. Proc Natl Acad Sci USA. 2010;107:4063–4068. doi: 10.1073/pnas.0909640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock FL, Mao W, Yaremchuk A, Tukalo M, Crepin T, Zhou H, Zhang YK, Hernandez V, Akama T, Baker SJ, Plattner JJ, Shapiro L, Martinis SA, Benkovic SJ, Cusack S, Alley MR. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Dock-Bregeon AC, Rees B, Bovee M, Caillet J, Romby P, Francklyn CS, Moras D. Zinc ion mediated amino acid discrimination by threonyl-tRNA synthetase. Nat Struct Biol. 2000;7:461–465. doi: 10.1038/75856. [DOI] [PubMed] [Google Scholar]
- Silvian LF, Wang J, Steitz TA. Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- Soini J, Falschlehner C, Liedert C, Bernhardt J, Vuoristo J, Neubauer P. Norvaline is accumulated after a down-shift of oxygen in Escherichia coli W3110. Microb Cell Fact. 2008;7:30. doi: 10.1186/1475-2859-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splan KE, Ignatov ME, Musier-Forsyth K. Transfer RNA modulates the editing mechanism used by class II prolyl-tRNA synthetase. J Biol Chem. 2008;283:7128–7134. doi: 10.1074/jbc.M709902200. [DOI] [PubMed] [Google Scholar]
- SternJohn J, Hati S, Siliciano PG, Musier-Forsyth K. Restoring species-specific posttransfer editing activity to a synthetase with a defunct editing domain. Proc Natl Acad Sci USA. 2007;104:2127–2132. doi: 10.1073/pnas.0611110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Tirrell DA. Attenuation of the editing activity of the E. coli leucyl-tRNA synthetase allows incorporation of novel amino acids into proteins in vivo. Biochemistry. 2002;41:10635–10645. doi: 10.1021/bi026130x. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Johnson KA. A new paradigm for DNA polymerase specificity. Biochemistry. 2006;45:9675–9687. doi: 10.1021/bi060993z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull WB, Daranas AH. On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J Am Chem Soc. 2003;125:14859–14866. doi: 10.1021/ja036166s. [DOI] [PubMed] [Google Scholar]
- Umbarger HE. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Uter NT, Gruic-Sovulj I, Perona JJ. Amino acid-dependent transfer RNA affinity in a class I aminoacyl-tRNA synthetase. J Biol Chem. 2005;280:23966–23977. doi: 10.1074/jbc.M414259200. [DOI] [PubMed] [Google Scholar]
- Weber AL, Miller SL. Reasons for the occurrence of the twenty coded protein amino acids. J Mol Evol. 1981;17:273–284. doi: 10.1007/BF01795749. [DOI] [PubMed] [Google Scholar]
- Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Wolfson AD, Uhlenbeck OC. Modulation of tRNAAla identity by inorganic pyrophosphatase. Proc Natl Acad Sci USA. 2002;99:5965–5970. doi: 10.1073/pnas.092152799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavalli SS, Ibba M. Selection of tRNA charging quality control mechanisms that increase mistranslation of the genetic code. Nucleic Acids Res. 2013;41:1104–1112. doi: 10.1093/nar/gks1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.