Abstract
This study investigated the impact of Alzheimer's disease (AD) on conjunctive and relational binding in episodic memory. Mild AD patients and controls had to remember item-color associations by imagining color either as a contextual association (relational memory) or as a feature of the item to be encoded (conjunctive memory). Patients' performance in each condition was correlated with cerebral metabolism measured by FDG-PET. The results showed that AD patients had an impaired capacity to remember item-color associations, with deficits in both relational and conjunctive memory. However, performance in the two kinds of associative memory varied independently across patients. Partial least square analyses revealed that poor conjunctive memory was related to hypometabolism in an anterior temporal-posterior fusiform brain network, whereas relational memory correlated with metabolism in regions of the default mode network. These findings support the hypothesis of distinct neural systems specialized in different types of associative memory and point to heterogeneous profiles of memory alteration in Alzheimer's disease as a function of damage to the respective neural networks.
Keywords: associative memory, binding, Alzheimer's disease, FDG-PET
1. Introduction
Binding processes in memory can create an associative link between independent items or between items and context into episodic memories (relational memory) (Cohen et al., 1999). An alternative process, conjunctive binding, allows associations to be encoded as a united representation of features or a single entity (Mayes, Montaldi, & Migo, 2007; O'Reilly & Rudy, 2001). Whereas memory for novel relational associations relies on the hippocampus, parahippocampal cortex and regions of the default mode network (posterior cingulate cortex/precuneus, lateral parietal and medial prefrontal cortex) (Diana, Yonelinas, & Ranganath, 2007; Mayes et al., 2007; Ranganath & Ritchey, 2012), conjunctive memory involves the perirhinal cortex (Diana, Yonelinas, & Ranganath, 2010; Haskins, Yonelinas, Quamme, & Ranganath, 2008; Staresina & Davachi, 2008).
Probable Alzheimer's disease (AD) is characterized by gradually progressive cognitive deficits typically starting with severe impairment of episodic memory (McKhann et al., 2011). Given that binding of information is a key feature of episodic memory, characterization of the memory deficit in AD should consider whether this crucial mechanism is compromised. Current evidence indicates that patients with Alzheimer's disease (AD) show impaired long-term relational memory, as evidenced by decreased ability to remember novel associations between words or pictures (Algarabel et al., 2012; Duchek et al., 1991; Gallo et al., 2004; Lindeboom et al., 2002; Lowndes et al., 2008; Wolk et al., 2011), between objects and locations (Bucks and Willison, 1997; Fowler et al., 2002; Hanaki et al., 2011; Huijbers et al., 2011; Kessels et al., 2005; Lee et al., 2003; Swainson et al., 2001), between verbal information and their source (Dalla Barba et al., 1999; Multhaup and Balota, 1997) and between faces and names (Pariente et al., 2005; Sperling et al., 2003). In contrast, little is known with regard to the effect of AD on conjunctive memory (i.e., object-feature associations). Studies of short term memory have indicated that AD patients are impaired at remembering conjunctions of visual features (i.e., shape and color) (Della Sala et al., 2012; Parra et al., 2009, 2010a, 2010b). In long-term memory, two studies indicated that AD patients make more errors than controls when recalling the color of studied objects (Della Sala et al., 2000; Lloyd-Jones, 2005).
However, as no study directly contrasted long-term conjunctive and relational memory in AD, it is not known whether patients are differentially impaired on these forms of associative memory. Based on known patterns of cerebral changes in the course of AD, particularly early atrophy and dysfunction of the entorhinal and perirhinal cortices (Gour et al., 2011; Juottonen et al., 1998), we predict impaired conjunctive memory in AD (Didic et al., 2011; Wolk, Mancuso, Kliot, Arnold, & Dickerson, 2013). Additionally, altered functional connectivity within the default mode network in AD (Pievani, de Haan, Wu, Seeley, & Frisoni, 2011) may be associated with the disturbance of relational processes in episodic memory. Interestingly, healthy older adults retain the capacity to learn new conjunctions, but demonstrate impairment on relational memory tasks (Bastin, Diana, et al., 2013). If AD affects both kinds of associative memory, behavioral performance profiles might differentiate AD from normal aging. In particular, if conjunctive binding is disproportionately impaired in patients, this may represent a signature of AD.
We assessed the impact of mild stage AD on long-term relational and conjunctive memory by testing memory for novel word-color associations encoded under two conditions. The context detail condition was designed to create an arbitrary, relational, association between separate entities such that color becomes a contextual detail related to the item. In contrast, the item detail condition was designed to create a unified, conjunctive, representation such that color is integrated as a feature of the item (Diana et al., 2010). Patients' brain metabolic activity at rest (FDG-PET) was analysed with spatio-temporal Partial Least Squares (McIntosh, Bookstein, Haxby, & Grady, 1996) in order to assess the relation of behavioral performance and activity in functional cerebral networks. We expect that the neural correlates of conjunctive and relational memory will dissociate. Given heterogeneity in cognitive and metabolic profiles in AD (Davidson et al., 2010; Salmon et al., 2009), we hypothesized that both relational and conjunctive memory may be variably compromised across patients due to variable amount of hypometabolism in distinct networks even if, as a group, the patients may present with deficits in both kinds of associative memory. Impaired performance in the item detail condition may be associated with hypometabolism in a temporal network encompassing the perirhinal cortex, whereas deficits in the context detail condition would be related to dysfunction within the default mode network.
2. Materials and methods
2.1 Participants
The participants in this study were 30 patients with a diagnosis of probable Alzheimer's disease (19 women) (McKhann et al., 2011) and 24 healthy older adults (14 women). All participants were community-dwelling, were native French speakers and had normal or corrected-to-normal vision. Patients with probable AD were recruited in memory clinics in the Liège area and were selected on the basis of general examination, neurological and neuropsychological assessments, and neuroimaging. Temporoparietal hypometabolism (FDG-PET) and cortical and/or hippocampal atrophy on structural magnetic resonance image (MRI) were taken as biomarkers. Structural MRI showed mild leukoaraiosis consistent with aging and degenerative processes. Patients were included if their symptoms corresponded to a mild stage of AD, as indicated by a MMSE score above 20 (Folstein, Folstein, & McHugh, 1975; Hugonot-Diener, 2001). Healthy older participants had no global cognitive decline (as confirmed by their score superior to 132/144 on the Mattis dementia rating scale (Mattis, 1973)) or psychiatric problems, were free of medication that could affect cognitive functioning, and reported being in good health.
Table 1 presents the demographic and clinical characteristics of both groups. Patients and healthy controls were comparable in terms of age, years of education and gender distribution. Patients showed a significant cognitive decline as assessed by the Mattis Dementia Rating Scale (Mattis, 1973) when compared to controls. There was no group difference on the Geriatric Depression Scale (Yesavage et al., 1983).
Table 1.
Demographic and clinical characteristics of the participants.
| AD (n = 30) | Controls (n = 24) | p-value | |
|---|---|---|---|
| Age (years) | 77.1 (6.4) | 75.9 (8.1) | .56 |
| Education (years) | 12.1 (3.6) | 11.6 (2.7) | .62 |
| Gender F/M* | 19/11 | 14/10 | .70 |
| Mattis DRS | 125.9 (8.1) | 140.5 (3.1) | < .001 |
| MMSE | 23.9 (2.0) | - | |
| GDS | 2.9 (2.3) | 2.6 (1.5) | .58 |
Standard deviations in brackets. DRS Dementia Rating Scale. GDS Geriatric Depression Scale.
Chi-square test; other analyses used t-tests.
Written informed consent was obtained from all participants according to the declaration of Helsinki. The study was approved by the Ethics Committee of the University Hospital of Liège.
2.2 Materials and procedure
Mild AD patients and healthy older adults performed a source memory task where word items were associated with one of two background colors (red or green) under two conditions. In the context detail condition, participants were asked to imagine the item in a situation with a green 100-euro bill if the background was green or with a red stop signal if the background was red. In the item detail condition, participants were asked to imagine the item as though it were the same color as the background.
A list of 40 concrete nouns, as well as the associated descriptive sentences were selected from the materials used by Diana and colleagues (Diana, Yonelinas, & Ranganath, 2008; Diana et al., 2010) and translated into French. Each sentence provided an explanation as to why the word item might be associated with a stop sign or a 100-euro bill (context detail condition) or why the item might be green or red (item detail condition). The words were randomly divided in two sets of 20 items. Each word had a sentence for both the item detail and context detail conditions such that assignment of the words to the two conditions could be counterbalanced across participants. The descriptive sentences were selected based on a pilot study in young adults that matched performance between conditions (Diana et al., 2010). Examples of sentences in the item detail condition are “The turtle is red because kids at the beach painted the shell so it would stand out amongst the other turtles” for the association “turtle-red”, and “The cloth is green because the waiter used it to clean up spilled pea soup” for the association “cloth-green”. Examples of sentences in the context detail condition are “The monkey is on the stop sign to show people that they should turn right to get to the zoo” for the association “monkey-red”, and “The sock has a 100-euro bill in it because the traveler put the bill in his sock to keep it safe” for the association “sock-green”.
Participants were tested individually in two sessions about one week apart. Each participant performed both conditions, which were administered in distinct sessions in order to minimize the contamination of one encoding condition on the other. Half of the participants started with the item detail condition, while the other half were first given the context detail condition. Stimuli were presented on a laptop computer. Each trial consisted of the presentation of a word against a background color (either green or red), with a sentence at the bottom of the screen. Before each task, participants were informed that their memory for the association between each word and the background color would be subsequently tested. In the item detail condition, they were asked to imagine the item as if it were the same color as the background, to read the sentence explaining why the item is that color, and to report whether this explanation was easy or difficult to imagine. In the context detail condition, participants were asked to imagine the item interacting with a stop sign (red background) or with a 100-euro bill (green background), to read the sentence explaining why the item is associated with the stop sign or the 100-euro bill, and to report whether that explanation was easy or difficult to imagine. Pictures of a stop sign and a 100-euro bill were shown before the task. The stimulus remained on the screen until a response was made. After a 20s interval filled with conversation, the test phase began in which the participants were presented with a randomized list of the studied words shown one at a time. For each word, they were asked to indicate whether the associated background was red or green. The test phase was also self-paced.
2.3 Neuroimaging data acquisition
2.3.1 Cerebral metabolism
At the end of the first session, AD patients' brain metabolic activity was measured during quiet wakefulness with eyes closed and ears unplugged after intravenous injection of an average of 180 MBq of 18F-2-fluoro-2-deoxy-d-glucose (FDG). PET images were acquired on a Siemens/CTI (Knoxville, TN) ECAT HR+ scanner (3D mode; 63 image planes; 15.2 cm axial field of view; 4.4 mm axial resolution; and 2.4mm slice interval). Images of the tracer distribution in the brain were used for analysis; the scan starting time was 30 min after tracer injection. Scan duration was 20 min. Images were reconstructed using the filtered backprojection method including corrections for measured attenuation, random and scatter effects using the standard software supplied by the scanner manufacturer.
2.3.2 Magnetic resonance imaging
MRI was performed at the end of the second session in AD patients. Subjects were equipped with earplugs and their heads were stabilized with foam pads to minimize head motion. In all AD patients, a high-resolution T1-weighted anatomical image was acquired on a 3T headonly scanner (Magnetom Allegra, Siemens Medical Solutions, Erlangen, Germany) operated with the standard transmit-receive quadrature head coil [TR 7.92 ms, TE 2.4 ms, FA 15°, 176 sagittal slices, FoV = 256 × 224 mm2, slice thickness 1 mm, matrix size 256 × 224 (Deichmann, Schwarzbauer, & Turner, 2004)].
2.4 Cerebral image preprocessing and analyses
All imaging data were preprocessed with SPM8 (Wellcome Department of Cognitive Neurology, London, UK). For each participant, the structural MRI image was segmented and normalized to the MNI space using unified segmentation (Ashburner & Friston, 2005). A mean image of all patients' normalized segmented grey matter image was computed and used as mask in the statistical analyses. Each subject's PET image was coregistered to the corresponding MRI image and normalized by applying parameters from the spatial normalization of the anatomical MRI data. The normalized PET images were smoothed with an isotropic 12 mm full-width half-maximum (FWHM) Gaussian kernel. All anatomical images were also submitted to VBM8 toolbox in SPM8 (http://dbm.neuro.unijena.de/vbm.html). Modulated images of regional grey matter density were extracted with default parameters and smoothed (8 mm FWHM).
In order to determine the set of brain regions where metabolism positively correlated with performance in the item detail and the context detail conditions, AD patients' PET data were analyzed using spatiotemporal Partial Least Squares (PLS) (McIntosh et al., 1996), a multivariate method that operates on the covariance between the metabolic values in the voxels and the behavioral scores to identify one component (latent variable, LV) that optimally relates the two. For this analysis, we used non-rotated behavioral PLS where a design matrix consisting of a contrast representing condition comparison (item detail performance - context detail raw performance, contrast [1 -1]) and the image data matrix (one mean-centered PET image per subject) were submitted to singular value decomposition. The resulting LV has a singular value which represents the amount of covariance between the design matrix and the image matrix accounted by the LV. Each brain voxel has a weight on the LV, known as a salience, that indicates how that voxel is related to the LV. The salience was positive for voxels correlating with item detail performance but not with context detail performance (item detail > context detail condition) or negative when the correlation was specifically with the context detail performance (context detail > item detail condition). Another analysis was run looking for pattern of regions whose metabolism covaried with performance in both conditions (conjunction of item and context detail conditions, contrast [1 1]). The significance for the LV was determined by a permutation test, which involves a random reordering of the data matrix and calculation of a new LV for each reordering. The singular value of each newly permuted LV is compared to the singular value of the original LV, yielding a probability of the number of occurrences that the permuted values exceed the original value. Five hundred permutations were conducted and the statistical significance level was set at p < .05. Finally, the reliability of the saliences for the brain voxels characterizing each LV was assessed by a bootstrap analysis of the standard errors using 100 bootstrap samples (Efron & Tibshirani, 1986). A reliable contribution for a given voxel was defined as a ratio of salience to standard error superior or equal to 3 (cluster size > 5, p < .005).
Finally, a PLS analysis was performed in order to assess the correlation between performance in the two conditions and grey matter density. As for the analysis of PET data, the significance of the latent variable was assessed with 500 permutations and a statistical threshold set at p < .05.
3. Results
3.1 Source memory performance
Table 2A presents the proportions of correct source judgments (correctly recalled background color) for the item detail and the context detail conditions in each group. The proportions were submitted to a 2 (group) by 2 (condition) repeated measure ANOVA. There was a significant main effect of group, F(1, 52) = 46.10, p < .001, indicating that AD patients produced less accurate source judgments than healthy controls. Performance was also better in the item detail than the context detail condition, F(1, 52) = 14.6, p < .001, as also observed in Bastin, Diana et al. (2013). The group by condition interaction was not significant, F(1, 52) = 0.52, p = .47.
Table 2.
Proportions of correct source judgments as a function of group and condition. A. Global performance. B. Performance assessed separately for easy-to-imagine and difficult-to-imagine associations.
| AD | Controls | ||
|---|---|---|---|
| A. | Item detail | .63 (.13) | .87 (.13) |
| Context detail | .56 (.13) | .77 (.18) | |
| B. | Item detail | ||
| Easy to imagine | .66 (.17) | .94 (.11) | |
| Difficult to imagine | .56 (.21) | .77 (.19) | |
| Context detail | |||
| Easy to imagine | .58 (.20) | .78 (.18) | |
| Difficult to imagine | .58 (.17) | .74 (.25) | |
Standard deviations in parentheses.
In order to more directly compare the degree of impairment of patients across both conditions and account for the global difference in level of performance (i.e., globally better performance in the item detail condition), z-scores were calculated for the AD group referenced to the control mean and standard deviation (Wolk et al., 2013; Wolk, Signoff, & DeKosky, 2008). Control-referenced z-scores for the item detail and context detail conditions were -1.83 and -1.12, respectively. In AD patients, a paired-samples t-test revealed a significantly lower z-score in the item detail than the context detail condition [t(29) = -3.69, p < 0.001]. This differential impairment may however be due to the fact that performance was closer to chance in the context detail than in the item detail condition. Indeed, as age-related differences exist only in the condition where color was encoded as a contextual detail (Bastin, Diana, et al., 2013), this would leave less room for the deficit to manifest itself in AD patients in the relational memory condition. When comparing only high-performing patients and controls (i.e., scoring above the median, so well-above floor), we observed a main effect of group, F(1, 24) = 34.74, p < .001, but no main effect of task (p = .25) and no interaction (p = .19). The comparison of control-referenced z-scores confirmed greater impairment in the item detail than in the context detail conditions [t(11) = -4.46, p < .001].
At encoding, participants reported whether the association with the color was easy versus difficult to imagine on the basis of the provided explanation. A group by condition ANOVA on the proportions of easy-to-imagine judgments showed that AD patients judged the associations to be easy to imagine less frequently than did controls (M = .50 in AD and .58 in controls, F(1, 52) = 5.07, p < .05). There was no effect of condition, F(1, 52) = 0.45, p = .50, and no interaction, F(1, 52) = 1.36, p = .24.
The proportions of correct source responses were further analyzed by considering the ratings that the participants provided during the encoding phase (see Table 2B). Thus, proportions of correct source judgments were computed separately for associations judged as easy to imagine and for associations judged as difficult to imagine. A 2 (group) by 2 (condition) by 2 (encoding judgment: easy versus difficult) ANOVA on the proportions of correct responses showed a main effect of group, F(1, 51) = 36.54, p < .001, and a main effect of condition, F(1, 51) = 7.89, p < .01, as in the previous analysis. There was also a significant main effect of encoding judgment, F(1, 51) = 12.46, p < .01. This was qualified by the significant condition by encoding judgment interaction, F(1, 51) = 7.48, p < .01. This showed that, for associations that were easy to imagine, there was a greater proportion of correct source responses in the item detail than the context detail condition (HSD Tukey test, p < .001). In contrast, for associations that were difficult to imagine, there was no difference in the proportion of correct source responses between the two conditions (p = .92). No other interaction was significant (ps > .19). Thus, the efficiency at mentally integrating the color into the item seems more beneficial to subsequent memory for the color than the efficiency at mentally imagining an interaction between the item and a colored object. This was observed in controls as well as in AD patients.
AD patients' performance in both conditions varied largely (variation coefficient of 20.8% in the item detail condition and 23.1% in the context detail condition) and patients' scores in the two conditions did not correlate significantly (r = .29, p = .11). One cannot exclude however that the lack of correlation is partly driven by the fact that patients' performance in the context detail condition was close to chance. The following analysis of PET data explored the metabolic correlates of performance variability that are specific to each condition and investigated whether common metabolic correlates can also be found.
3.2 Metabolic correlates of source memory performance
In patients with mild Alzheimer's disease, PLS analyses revealed a significant pattern of metabolic activity that correlated specifically with each condition (accounting for 76.48 % of the covariance in the data; p < .05, Tables 3 and 4). AD patients' proportion of correct responses in the item detail condition was specifically and positively correlated with the metabolic activity of a network encompassing regions extending from the ventral temporopolar cortex to the posterior fusiform cortex on the left hemisphere, as well as the uncus, the amygdala, the perirhinal and parahippocampal gyri on the right hemisphere (Figure 1 A). There was also a correlation with the anterior and middle cingulate cortex and right superior prefrontal cortex (BA 6/9). With regard to performance in the context detail condition (context detail > item detail condition), there was a significant positive correlation with the precuneus, the anterior medial prefrontal cortex and the right temporoparietal junction (Figure 1 B), and with the right middle prefrontal cortex (BA46).
Table 3.
Peak MNI coordinates of brain regions specifically associated with performance in the item detail condition in AD patients.
| Laterality | Region | BA | x | y | z | k | BR |
|---|---|---|---|---|---|---|---|
| Item detail > Context detail | |||||||
| L | Fusiform cortex | 20 | -42 | -16 | -24 | 1488 | 5.77 |
| R | Fusiform cortex | 20 | 56 | -28 | -30 | 283 | 3.48 |
| R | Uncus | 36 | 26 | 2 | -38 | 231 | 3.70 |
| R | Parahippocampal cortex | 30 | 20 | -40 | -8 | 212 | 3.60 |
| R | Amygdala | 24 | -4 | -22 | 18 | 3.14 | |
| R | Lingual cortex | 19 | 26 | -72 | 4 | 75 | 4.78 |
| L | Superior frontal cortex | 6 | -18 | -2 | 54 | 105 | 5.53 |
| R | Superior frontal cortex | 9 | 18 | 40 | 34 | 62 | 4.36 |
| R | Superior frontal cortex | 6 | 16 | -6 | 58 | 49 | 3.74 |
| R | Cingulate cortex | 24 | 4 | 8 | 30 | 73 | 3.58 |
| R | Anterior cingulate cortex | 24 | 14 | 28 | 24 | 30 | 4.17 |
| L | Precentral gyrus | 4 | -28 | -22 | 52 | 53 | 3.86 |
| L | Parieto-occipital sulcus | 7 | -16 | -62 | 34 | 32 | 3.59 |
BA. Brodman area; k. Cluster size; BR. Bootstrap ratio (salience/standard error).
Table 4.
Peak MNI coordinates of brain regions specifically associated with performance in the context detail condition in AD patients.
| Laterality | Region | BA | x | y | z | k | BR |
|---|---|---|---|---|---|---|---|
| Context detail > Item detail | |||||||
| R | Precuneus | 7 | 8 | -62 | 60 | 490 | -4.26 |
| L | Precuneus | 7 | -4 | -60 | 60 | 202 | -4.76 |
| L | Anterior medial frontal cortex | 10 | -4 | 60 | 2 | 206 | -4.99 |
| R | Middle frontal cortex | 10 | 40 | 52 | 10 | 151 | -4.11 |
| R | Middle frontal cortex | 46 | 44 | 34 | 30 | 80 | -4.04 |
| R | Temporoparietal junction | 39 | 62 | -54 | 16 | 35 | -3.33 |
| L | Paracentral gyrus | 6 | -2 | -34 | 68 | 32 | -3.62 |
BA. Brodman area; k. Cluster size; BR. Bootstrap ratio (salience/standard error).
Figure 1.
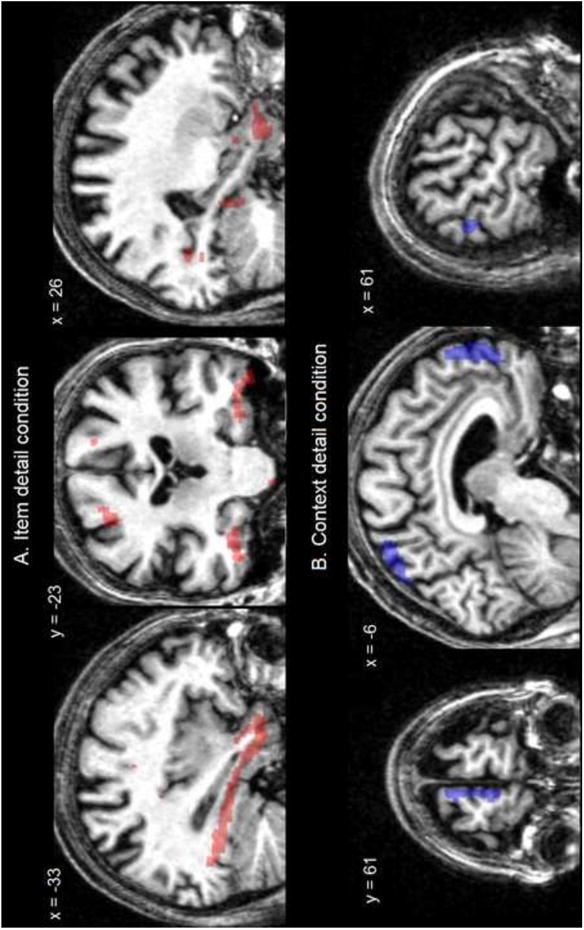
Results of the PLS analyses showing metabolic regional activity significantly correlated with the proportion of correct source judgments (A) when color was integrated as an item feature (conjunctive memory) and (B) when color was associated to the item as a contextual detail (relational memory) in patients with Alzheimer's disease. Results rendered on a patient anatomical image.
An analysis looking for a set of regions commonly associated with performance in both conditions did not reveal any significant latent variable (p > .12). When the neural correlates of each condition were evaluated individually (data not shown), the PLS analyses revealed broadly the same networks as the analysis contrasting the two conditions. Notably, the correlation with performance in the item detail condition again showed the involvement of the fusiform cortex, medial temporal cortex around the collateral sulcus and the temporal pole (bootstrap ratio > 3, p < .005). The only difference was that the occipito-temporal network was less extended in the left hemisphere, but more extended in the right-hemisphere, including also the right anterior hippocampus. As for the network correlating with performance in the context detail condition, it involved the anterior medial prefrontal cortex, the temporoparietal cortex, and also the dorsomedial prefrontal cortex and the retrosplenial cortex, but not the precuneus (bootstrap ratio > 3, p < .005).
3.3 Voxel-based morphometry analyses
The PLS analysis looking for correlation between grey matter density and performance in the item detail or context detail conditions did not reveal any significant latent variable (p > .81). No site showed correlation with performance in both conditions (conjunction, LV, p > .57). This lack of significant correlation suggests that the above-mentioned metabolic correlates were not driven by atrophy.
4. Discussion
The current study investigated relational and conjunctive encoding in early Alzheimer's disease by comparing long-term memory for information that was associated with an item either as a contextual detail (context detail condition, measuring relational memory) or as a feature of that item (item detail condition, measuring conjunctive memory). Mild AD patients demonstrated a deficit in both kinds of associative memory. Importantly, the patients' performance varied widely in both tasks and this variation was related to the functional integrity of distinct networks of cerebral regions. Indeed, deficits in the item detail condition were associated with hypometabolism in the left parahippocampal gyrus and fusiform gyrus from the ventral temporopolar cortex to the occipitotemporal region, as well as in the right anterior extrahippocampal medial temporal cortex and amygdala. In contrast, poor memory performance in the context detail condition was related to decreased activity in the anterior medial prefrontal cortex, precuneus and right temporoparietal junction.
This study provides novel evidence for impaired conjunctive memory in AD. The finding of impaired relational memory in mild AD also adds to previous evidence for deficits in memory for inter-item associations and item-context associations (Algarabel et al., 2012; Dalla Barba, Nedjam, & Dubois, 1999; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Kessels, Feijen, & Postma, 2005; Sperling et al., 2003; Swainson et al., 2001; Wolk, Dunfee, Dickerson, Aizenstein, & DeKosky, 2011). More critically, by directly comparing both kinds of associative memory in a single study, we show that conjunctive binding in long-term memory is as much, if not more, disrupted as relational memory in AD. This pattern of impairment contrasts with decline due to healthy aging which affects relational memory more than conjunctive memory (Bastin, Diana, et al., 2013). Vulnerability of conjunctive memory may thus be a cognitive marker of Alzheimer's disease, as suggested by Didic et al. (2011) in their theoretical review.
Furthermore, Didic et al. (2011) predicted that AD is associated with an early deficit of long-term item/conjunctive memory due to initial deterioration of extrahippocampal medial temporal lobe regions. The results of our cognitive-metabolic correlation analysis support this hypothesis and point to a relation between AD patients' performance in the item detail condition and the functional integrity of parahippocampal and fusiform regions. The finding of significant clusters in the fundus of the collateral sulcus in the rostral portion of the fusiform and parahippocampal gyri, corresponding to the perirhinal cortex (Ding & Van Hoesen, 2010; Insausti et al., 1998) is consistent with fMRI studies of conjunctive memory in healthy young adults. These fMRI studies showed that the perirhinal cortex is activated when participants learn new associations where two words are integrated to form a new concept (Haskins et al., 2008) or when color is encoded as an item feature (Diana et al., 2010; Staresina & Davachi, 2006, 2008).
However, the regions where metabolism correlated with performance in the item detail condition also included more posterior regions such as the occipitotemporal cortex. In line with our findings, earlier work in Mild Cognitive Impairment (MCI) has found that impaired visual object recognition memory was associated with hypoperfusion in the medial temporal lobe extending to the occipitotemporal junctions (Guedj et al., 2006). The network associated with conjunctive binding in AD thus combines the ventral visual stream and anterior medial temporal lobe. These regions also belong to the perceptual-mnemonic/feature-conjunction neural network (Baxter, 2009; Bussey & Saksida, 2005, 2007; Graham, Barense, & Lee, 2010) which is assumed to support the representation of feature conjunctions of increasing complexity. According to this view, the perirhinal cortex subserves memory and perception of complex conjunctive representations. A refinement of the view was proposed by Staresina and Davachi (2010), who pointed to a functional distinction along the MTL-ventral visual pathway with regard to perceptual integration and memory formation of unitized representations. More specifically, in that fMRI study, participants were presented with pictures of objects visually intact or separated into two or four fragments, surrounded by a color background. The encoding instruction was to imagine the object in the background color, so that participants had to mentally unitize the object fragments into a single representation. Memory for these objects and the associated color was subsequently tested. The results showed that successful encoding of object and object-color associations activated the perirhinal cortex independently of the level of fragmentation. In contrast, the anterior fusiform gyrus and inferior temporal cortex were sensitive to both the level of fragmentation and subsequent memory effect, and occipitotemporal cortices showed only effects of fragmentation level. This suggests that the components of a visual image are fused into an integrated entity in the ventral visual processing stages before reaching the perirhinal cortex where experience with this integrated item is encoded into long-term memory.
Recent evidence showed that MCI and AD patients have an impaired capacity to perceptually discriminate between objects with overlapping features, reflecting compromised representation of complex conjunctions (Kivisaari, Monsch, & Taylor, 2013; Newsome, Duarte, & Barense, 2012). Consequently, the impaired ability of AD patients to remember the color associated with the word in the item detail condition may stem from a deficiency of the integration process itself. That is, our finding of decreased metabolism in the occipitotemporal cortices may indicate that the patients failed to create a fused image of the item in the designated color. During the encoding phase, AD patients found it more difficult to imagine the new item-color associations than healthy controls, which may suggest a decreased capacity to mentally create a perceptual representation of the stimulus. This was however not specific to the item detail condition, as patients judged new associations as equally difficult to imagine in the item detail and the context detail condition. Alternatively, their memory deficit may be driven by failures in encoding the integrated association into long-term memory, as suggested by hypometabolism in the perirhinal cortex. Finally, the involvement of the ventral temporal pole may indicate an impairment in interpreting the item-color associations on the basis of the provided explanation (e.g., ‘the cloth is green because the waiter used it to clean up spilled pea soup’) due to either semantic conceptual unitization processing or integration with emotion (Patterson, Nestor, & Rogers, 2007; Wong & Gallate, 2012). Any of these problems may result in impaired conjunctive memory in the current task. Future studies should explore which of these processing deficits best explain AD patients' difficulty integrating color as an item feature. Another possibility, to be tested, is that AD patients' deficit originates from altered connectivity between structures along the ventral visual-medial temporal lobe-temporal pole pathway, so that the products of perceptual unitization, conceptual integration and memory processing do not converge into a coherent memory representation.
With respect to relational memory, AD patients' performance in the context detail condition correlated with metabolic activity in the anterior medial prefrontal cortex (BA 10), the precuneus, and the right temporoparietal junction. These regions have been involved in the default mode network (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Fox et al., 2005; Kahn, Andrews-Hanna, Vincent, Snyder, & Buckner, 2008; Vincent et al., 2006) and belong to an episodic memory neural system (Rugg & Vilberg, 2013) whose general role may consist in building a mental representation of the relationships between entities, contextual details, actions and outcomes (Ranganath & Ritchey, 2012). Moreover, the association of metabolism in the anterior medial prefrontal cortex, precuneus and temporoparietal junction with item-context associative memory is consistent with the results from numerous fMRI studies indicating that source memory judgments and the retrieval of qualitative details encoded with the studied items activate these regions (Cansino, Maquet, Dolan, & Rugg, 2002; Diana et al., 2010; Hayama, Vilberg, & Rugg, 2012; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Lundstrom, Ingvar, & Petersson, 2005; Rugg, Fletcher, Chua, & Dolan, 1999; Simons, Gilbert, Owen, Fletcher, & Burgess, 2005; Yonelinas, Otten, Shaw, & Rugg, 2005).
The present findings have some relevance to the current debate about the impact of MCI and Alzheimer's disease on recollection and familiarity memory functions. Research characterizing patients' memory deficits in terms of recall of qualitative details about an item (recollection) and retrieval based on feeling of oldness devoid of any recall of contextual information (familiarity) has consistently shown that MCI and AD patients have impaired recollection. However, the results regarding familiarity are divergent. On the one hand, several reports of impaired familiarity in MCI and AD patients (Algarabel et al., 2009; Ally, Gold, & Budson, 2009; Didic et al., 2013; Embree, Budson, & Ally, 2012; Wolk et al., 2013; Wolk et al., 2008) are consistent with the prediction that item familiarity should be disrupted very early in the course of Alzheimer's disease due to initial deterioration of the extrahippocampal medial temporal lobe regions on which it depends (Didic et al., 2011). On the other hand, there is contrasting evidence of spared familiarity in MCI and AD, notably in memory tasks for pictures (Embree et al., 2012; O'Connor & Ally, 2010; Westerberg et al., 2013; Westerberg et al., 2006), but also in verbal memory tasks (Anderson et al., 2008; Belleville, Menard, & Lepage, 2011; Genon et al., 2013, 2014; Serra et al., 2010).
Although the contribution of recollection and familiarity has not been evaluated in the current tasks, previous work with this procedure demonstrated that performance in the item detail condition is more dependent on familiarity than in the context detail condition (Bastin, Diana, et al., 2013; Diana, Van den Boom, Yonelinas, & Ranganath, 2011; Diana et al., 2008, 2010). The deficit that AD patients manifested in the item detail condition may thus indicate disrupted familiarity for integrated items in relation to hypometabolism of extrahippocampal MTL regions. Nevertheless, as discussed above, this conclusion cannot be verified here because patients may fail to create an integrated perceptual and conceptual representation of the item-color conjunction before it is available for familiarity-related memory processes. More generally, it is likely that contradictory evidence regarding the integrity of familiarity in the course of Alzheimer's disease arises because of the influence of various task-related or patient-related factors, such as the intrinsic properties of the methods used to estimate recollection and familiarity (Wolk et al., 2008), the extent to which memory decisions benefit from the use of processing fluency (Ally, 2012; Bastin, Willems, Genon, & Salmon, 2013), or the severity and nature of patients' cognitive deficits (e.g., Bastin, Willems, et al., 2013; Hoppstadter et al., 2013).
In conclusion, it appears that associative memory is not globally and indiscriminately affected by Alzheimer's disease. Even though the task setting was identical in the item detail and context detail conditions, the different encoding instructions promoted distinct binding processes which are dissociable in terms of their cerebral metabolic correlates and variably affected among patients with mild Alzheimer's disease. Consistent with the theoretical views proposing that memory for item-context associations and memory for intra-item details rely on distinct neural networks (Diana et al., 2007; Mayes et al., 2007; Ranganath & Ritchey, 2012), this variability relates to the amount of hypometabolism affecting a network centered on the parahippocampal and fusiform gyri, and an anterior-posterior midline network respectively. Several questions remain to be clarified in future studies. One question is whether the relative dysfunction of the two types of associative memory also varies in the prodromal stages of Alzheimer's disease and whether they decline similarly in the course of the pathology. The precise nature of the impairment of conjunctive memory should also be investigated to determine whether it stems from the integration process itself or subsequent memory processes. Finally, one fundamental question is whether the neural underpinnings of these associative memory disorders in Alzheimer's disease reflect regional alteration or altered network connectivity.
Highlights.
- Alzheimer's disease disrupts relational and conjunctive binding in episodic memory
- Relational binding scores related to default mode network resting-state metabolism
- Conjunctive binding scores are associated with integrity of anterior temporal network
- Variable alteration of specialized memory systems occurs in Alzheimer's disease
Acknowledgments
This work was supported by SAO-FRA and the King Baudouin Foundation (grant 2011-R12860-003), the Inter-University Attraction Pole P7/11, F.R.S.-FNRS (FRSM grant 3.4511.11 and travel funding 2012/V 3/5/110-IB/JN- 758), the University of Liège, and the National Institute of Mental Health (grant nos. MH83734 and MH59352). C.B. is a research associate and F.C. is a research director at the F.R.S.-FNRS.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algarabel S, Escudero J, Mazon JF, Pitarque A, Fuentes M, Peset V, Lacruz L. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer's patients. Neuropsychologia. 2009;47:2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Fuentes M, Escudero J, Pitarque A, Peset V, Mazon JF, Meléndez JC. Recognition memory deficits in mild cognitive impairment. Aging, Neuropsychology, and Cognition. 2012;19(5):608–619. doi: 10.1080/13825585.2011.640657. [DOI] [PubMed] [Google Scholar]
- Ally BA. Using pictures and words to understand recognition memory deterioration in amnestic mild cognitive impairment and Alzheimer's disease: a review. Current Neurology and Neuroscience Reports. 2012;12(6):687–694. doi: 10.1007/s11910-012-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69(3):504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22(2):177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bastin C, Diana RA, Simon J, Collette F, Yonelinas AP, Salmon E. Associative memory in aging: The effect of unitization on source memory. Psychology and Aging. 2013;28(1):275–283. doi: 10.1037/a0031566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Willems S, Genon S, Salmon E. Enhancing the salience of fluency improves recognition memory performance in mild Alzheimer's disease. Journal of Alzheimer's Disease. 2013;33:1033–1039. doi: 10.3233/JAD-2012-121678. [DOI] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61:667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Belleville S, Menard MC, Lepage E. Impact of novelty and type of material on recognition in healthy older adults and persons with mild cognitive impairment. Neuropsychologia. 2011;49(10):2856–2865. doi: 10.1016/j.neuropsychologia.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Willison JR. Development and validation of the Location Learning Test (LLT) : A test of visuo-spatial learning designed for use with older adults and in dementia. The Clinical Neuropsychologist. 1997;11:273–286. [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temproal lobe: An alternative approach. Current Opinion in Neurobiology. 2005;15:730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G, Nedjam Z, Dubois B. Confabulation, executive functions, and source memory in Alzheimer's disease. Cognitive Neuropsychology. 1999;16(3-5):385–398. [Google Scholar]
- Davidson JE, Irizarry MC, Bray BC, Wetten S, Galwey N, Gibson R, Borrie M, Delisle R, Feldman HH, Hsiung GY, Fornazzari L, Gauthier S, Guzman D, Loy-English I, Keren R, Kertesz A, George-Hyslop PS, Wherrett J, Monsch AU. An exploration of cognitive subgroups in Alzheimer's disease. Journal of the International Neuropsychological Society. 2010;16(2):233–243. doi: 10.1017/S1355617709991160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Kinnear P, Spinnler H, Stangalino C. Color-to-figure matching in Alzheimer’s disease. Archives of Clinical Neuropsychology. 2000;15:571–585. doi: 10.1016/s0887-6177(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Parra MA, Fabi K, Luzzi S, Abraham S. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. 2012;50:833–840. doi: 10.1016/j.neuropsychologia.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Diana RA, Van den Boom W, Yonelinas AP, Ranganath C. ERP correlates of source memory: unitized source information increases familiarity-based retrieval. Brain Research. 2011;1367:278–286. doi: 10.1016/j.brainres.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. The effects of unitization on familiarity-based source memory: testing a behavioral prediction derived from neuroimaging data. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(4):730–740. doi: 10.1037/0278-7393.34.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Journal of Cognitive Neuroscience. 2010;22(8):1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, Ceccaldi M. Which memory system is impaired first in Alzheimer's disease? Journal of Alzheimer's Disease. 2011;27(1):11–22. doi: 10.3233/JAD-2011-110557. [DOI] [PubMed] [Google Scholar]
- Didic M, Felician O, Barbeau EJ, Mancini J, Latger-Florence C, Tramoni E, Ceccaldi M. Impaired visual recognition memory predicts Alzheimer's disease in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2013;35(5-6):291–299. doi: 10.1159/000347203. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW. Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Human Brain Mapping. 2010;31:1359–1379. doi: 10.1002/hbm.20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Cheney M, Ferraro FR, Storandt M. Paired associate learning in senile dementia of the Alzheimer type. Archives of Neurology. 1991;48:1038–1040. doi: 10.1001/archneur.1991.00530220054019. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Statistical Sciences. 1986;1:54–77. [Google Scholar]
- Embree LM, Budson AE, Ally BA. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50(9):2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. Journal of the International Neuropsychological Society. 2002;8:58–71. [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is instrincisally organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer's disease: Evidence for impaired recall-to-reject. Neuropsychology. 2004;18(3):556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Genon S, Bahri MA, Collette F, Angel L, d'Argembeau A, Clarys D, Kalenzaga S, Salmon E, Bastin C. Cognitive and neuroimaging evidence of impaired interaction between self and memory in Alzheimer's disease. Cortex. 2014;51:11–24. doi: 10.1016/j.cortex.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Genon S, Collette F, Feyers D, Phillips C, Salmon E, Bastin C. Item familiarity and controlled associative retrieval in Alzheimer's disease: An fMRI study. Cortex. 2013;49:1566–1584. doi: 10.1016/j.cortex.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Gour N, Ranjeva JP, Ceccaldi M, Confort-Gouny S, Barbeau E, Soulier E, Guye M, Didic M, Felician O. Basal functional connectivity within the anterior temporal network is associated with performance on declarative memory tasks. Neuroimage. 2011;58:687–697. doi: 10.1016/j.neuroimage.2011.05.090. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Guedj E, Barbeau E, Didic M, Felician O, de Laforte C, Ceccaldi M, Mundler O, Poncet M. Identification of subgroups in amnestic mild cognitive impairment. Neurology. 2006;67:356–358. doi: 10.1212/01.wnl.0000225076.73312.d4. [DOI] [PubMed] [Google Scholar]
- Hanaki R, Abe N, Fujii T, Ueno A, Nishio Y, Hiraoka K, Shimomura T, Iizuka O, Shinohara M, Hirayama K, Mori E. The effects of aging and Alzheimer’s disease on associative recognition memory. Neurological Sciences. 2011;32:1115–1122. doi: 10.1007/s10072-011-0748-4. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? Journal of Cognitive Neuroscience. 2012;24(5):1127–1137. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory : An event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppstadter M, King AV, Frolich L, Wessa M, Flor H, Meyer P. A combined electrophysiological and morphological examination of episodic memory decline in amnestic mild cognitive impairment. Frontiers in Aging Neuroscience. 2013;5:51. doi: 10.3389/fnagi.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugonot-Diener L. MMS version consensuelle GRECO. In: Hugonot-Diener L, editor. La consultation en gériatrie. Paris: Masson; 2001. pp. 13–20. [Google Scholar]
- Huijbers MJ, Bergmann HC, Olde Rikkert MGM, Kessels RPC. Memory for emotional pictures in patients with Alzheimer’s dementia: Comparing picture-location binding and subsequent recognition. Journal of Aging Research. 2011;2011:1–9. doi: 10.4061/2011/409364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkänen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporpolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkänen A, Partanen K, Soininen H. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiology of Aging. 1998;19(1):15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(1):129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RPC, Feijen J, Postma A. Implicit and explicit memory for spatial information in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2005;20:184–191. doi: 10.1159/000087233. [DOI] [PubMed] [Google Scholar]
- Kivisaari SL, Monsch AU, Taylor KI. False positives to confusable objects predict medial temporal lobe atrophy. Hippocampus. 2013;23(9):832–841. doi: 10.1002/hipo.22137. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Rahman S, Hodges JR, Sahakian BJ, Graham KS. Associative and recognition memory for novel objects in dementia: Implications for diagnosis. European Journal of Neuroscience. 2003;18:1660–1670. doi: 10.1046/j.1460-9568.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. Journal of Neurology, Neurosurgery and Psychiatry. 2002;73:126–133. doi: 10.1136/jnnp.73.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones TJ. The role of color in the implicit memory performance of healthy older adults and individuals with Alzheimer’s disease. Neuropsychology. 2005;19:44–53. doi: 10.1037/0894-4105.19.1.44. [DOI] [PubMed] [Google Scholar]
- Lowndes GJ, Saling MM, Ames D, Chiu E, Gonzalez LM, Savage GR. Recall and recognition of verbal paired associates in early Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14:591–600. doi: 10.1017/S1355617708080806. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Windsor England: NFER-Nelson; 1973. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein F, Haxby J, Grady C. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup KS, Balota DA. Generation effects and source memory in healhty older adults and in adults with dementia of the Alzheimer type. Neuropsychology. 1997;11:382–391. doi: 10.1037//0894-4105.11.3.382. [DOI] [PubMed] [Google Scholar]
- Newsome RN, Duarte A, Barense MD. Reducing perceptual interference improves visual discrimination in mild cognitive impairment: implications for a model of perirhinal cortex function. Hippocampus. 2012;22(10):1990–1999. doi: 10.1002/hipo.22071. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory : Principles of cortical and hippocampal function. Psychological Review. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- O'Connor MK, Ally BA. Using stimulus form change to understand memorial familiarity for pictures and words in patients with mild cognitive impairment and Alzheimer's disease. Neuropsychologia. 2010;48(7):2068–2074. doi: 10.1016/j.neuropsychologia.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente J, Cole S, Henson R, Clare L, Kennedy A, Rossor M, Cipolotti L, Puel M, Demonet JF, Chollet F, Frackowiak RS. Alzheimer’s patients engage an alternative network during a memory task. Annals of Neurology. 2005;58:870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala S. Short-term memory binding deficits in Alzheimer’s disease. Brain. 2009;132:1057–1066. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Della Sala S. Visual short-term memory binding in Alzheimer’s disease and depression. Journal of Neurology. 2010;257:1160–1169. doi: 10.1007/s00415-010-5484-9. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews: Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurology. 2011;10(9):829–843. doi: 10.1016/S1474-4422(11)70158-2. doi:10.1016/S 1474-4422(11)70158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nature Reviews: Neuroscience. 2012;13:1–15. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PML, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology. 2013;23:255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E, Kerrouche N, Perani D, Lekeu F, Holthoff V, Beuthien-Baumann B, Sorbi S, Lemaire C, Collette F, Herholz K. On the multivariate nature of brain metabolism impairment in Alzheimer's disease. Neurobiology of Aging. 2009;30:186–197. doi: 10.1016/j.neurobiolaging.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Serra L, Bozzali M, Cercignani M, Perri R, Fadda L, Caltagirone C, Carlesimo GA. Recollection and familiarity in amnesic mild cognitive impairment. Neuropsychology. 2010;24(3):316–326. doi: 10.1037/a0017654. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. Journal of Neurophysiology. 2005;94:813–820. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. The Journal of Neuroscience. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20(8):1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. The Journal of Neuroscience. 2010;30(29):9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn DB, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AP, Fox MD, Shannon BJ, Andrews JR, Raichle KA, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Mayes AR, Florczak SM, Chen Y, Creery J, Parrish T, Weintraub S, Mesulam MM, Reber PJ, Paller KA. Distinct medial temporal contributions to different forms of recognition in amnestic mild cognitive impairment and Alzheimer's disease. Neuropsychologia. 2013;51(12):2450–2461. doi: 10.1016/j.neuropsychologia.2013.06.025. http://dx.doi.org/10.1016/j.neuropsychologia.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. A medial temporal lobe division of labor: Insights from memory in aging and early Alzheimer's disease. Hippocampus. 2011;21:461–466. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Mancuso L, Kliot D, Arnold SE, Dickerson BC. Familiarity-based memory as an early cognitive marker of preclinical and prodromal AD. Neuropsychologia. 2013;51:1094–1102. doi: 10.1016/j.neuropsychologia.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, DeKosky ST. Recollection and familiarity in amnestic mild cognitive impairment: A global decline in recognition memory. Neuropsychologia. 2008;46:1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: A review of the empirical evidence. Brain Research. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum V, Huang V, Adey M, Leier O. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


