Abstract
Purpose:
Estrogen (E2)-stimulated growth re-emerges after a c-Src inhibitor blocking E2-induced apoptosis. A resulting cell line, MCF-7:PF, is selected with features of functional estrogen receptor (ER) and over-expression of insulin-like growth factor-1 receptor beta (IGF-1Rβ). We addressed the question of whether the selective ER modulator (SERM), 4-hydroxytamoxifen (4-OHT) or other SERMs could target ER to prevent E2-stimulated growth in MCF-7:PF cells.
Methods:
Protein levels of receptors and signaling pathways were examined by immunoblotting. Expression of mRNA was measured through real-time RT-PCR. Recruitment of ER or nuclear receptor coactivator 3 (SRC3) to the promoter of ER-target gene was detected by chromatin-immunoprecipitation (ChIP).
Results:
4-OHT and other SERMs stimulated cell growth in an ER-dependent manner. However, unlike E2, 4-OHT suppressed classical ER-target genes as does the pure antiestrogen ICI 182,780 (ICI). ChIP assay indicated that 4-OHT did not recruit ER or SRC3 to the promoter of ER-target gene, pS2. Paradoxically, 4-OHT reduced total IGF-1Rβ but increased phosphorylation of IGF-1Rβ. Mechanistic studies revealed that 4-OHT functioned as an agonist to enhance the non-genomic activity of ER and activate focal adhesion molecules to further increase phosphorylation of IGF-1Rβ. Disruption of membrane-associated signaling, IGF-1R and focal adhesion kinase (FAK), completely abolished 4-OHT-stimulated cell growth.
Conclusions:
This study is the first to recapitulate a cellular model in vitro of acquired tamoxifen resistance developed in athymic mice in vivo. Importantly, it provides a rationale that membrane-associated pathways may be valuable therapeutic targets for tamoxifen resistant patients in clinic.
Keywords: selective estrogen receptor modulator (SERM), resistance, insulin-like growth factor-1 receptor beta (IGF-1Rβ), non-genomic pathway, focal adhesion molecules
1. Introduction
The clinical application of the laboratory strategy of long-term adjuvant antihormone therapy for the treatment of breast cancer (1) has significantly improved breast cancer survival. Selection of patients whose tumors express the estrogen receptor (ER) are more likely to respond to long-term adjuvant tamoxifen (TAM) (2, 3) or aromatase inhibitors (AIs) (4) than those without ER. However, acquired resistance to antihormone therapy remains a challenge as adjuvant therapy is extended (3, 5).
The evolution of acquired resistance to TAM treatment was discovered using MCF-7 tumors transplanted in athymic mice to mimic years of adjuvant treatment in patients (6-8). Long-term therapy generates selection pressure for cell populations that evolve from acquired TAM resistance, ubiquitously observed in metastatic breast cancer, to eventually expose a vulnerability that is expressed as E2-induced apoptosis (8-10). Acquired resistance to TAM or other selective ER modulators (SERMs) is unique in that the growth of resistant tumors is dependent on SERMs (6-8). Acquired TAM resistance during the treatment of metastatic breast cancer occurs within one or two years (11), consistent with the model of SERM resistance in athymic mice (6, 8). An AI (depleting E2) or fulvestrant (ICI 182,780; a pure antiestrogen that destroys the ER) is effective as second-line therapy after TAM failure (12, 13). Thus, it appears that acquired resistance to SERMs is initially able to utilize either E2 or a SERM as the growth stimulus in ER-positive TAM-resistant breast tumors. However, no mechanism has been established to explain this paradox.
We describe a new model of antihormone-resistant breast cancer in vitro that exhibits the characteristics of acquired TAM resistance in vivo. The MCF-7:5C cell line emerged unexpectedly from an established MCF-7 cell line after long-term E2 deprivation, i.e. simulated AI resistance (14). The E2-deprived cell lines (14, 15) created from MCF-7 cells have the unique ability to undergo E2-induced apoptosis that has clinical significance for the treatment (16) and prevention of breast cancer (17). We discovered that if a c-Src inhibitor is applied, E2-induced apoptosis is initially blocked in MCF-7:5C cells (18), but with extended treatment, E2-stimulated growth re-emerges (19). Unexpectedly, the derived cell line MCF-7:PF was found to mimic the SERM/E2-stimulated models in vivo (6), thereby providing the opportunity to decipher the mechanism of SERM-stimulated growth. Here, we provide evidence that 4-hydroxytamoxifen (4-OHT)-stimulated growth of MCF-7:PF is ER-dependent despite suppression of classical ER-target genes. However, 4-OHT functions as an agonist to enhance the non-genomic activity of ER and activates focal adhesion molecules to further increase phosphorylation of IGF-1Rβ. All of these events promote 4-OHT-stimulated cell growth. Overall, the constant nuclear pressure causes broad activation of membrane-associated signaling to aid breast cancer cell survival during the selection process required for acquired TAM resistance.
2. Materials and Methods
2.1 Materials
Estradiol and FAK inhibitor (PF573228) were purchased from Sigma-Aldrich (St. Louis, MO); ICI 182,780 (ICI) was purchased from Tocris (Park Ellisville, MO). SERMs: 4-hydroxytamoxifen (4-OHT) was purchased from Sigma-Aldrich (St. Louis, MO), raloxifene was a kind gift from Eli Lilly (Indianapolis, IN), endoxifen was gifted from Dr. James Ingle (Mayo Clinic, Rochester, MN), bazedoxifene (BZA) was gifted from Dr. Ronald Grigg (University of Leeds, UK), EM652 was gifted from AstraZeneca (UK). c-Src inhibitor, PP2, and IGF-1Rβ inhibitor, AG1024, were purchased from CalBiochem (San Diego, CA). Sources of antibodies for Western blot were as follows: ERα (sc-544), mouse IGF-1Rβ (sc-462), and rabbit IGF-1Rβ (sc-713) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Total MAPK (#9102), phosphorylated MAPK (#9101), total Akt (#9272), phosphorylated Akt (#9271), total STAT3 (#4903), phosphorylated STAT3 (#9131), total FAK (#3285), phosphorylated FAK (Y397, #3283), phosphorylated FAK (Y576/577, #3281), phosphorylated p130CAS (#4014), phosphorylated IGF-1Rβ (#3024), and phosphorylated c-Src (#2101) antibodies were from Cell Signaling Technology (Beverly, MA). Total c-Src (GD11) and anti-phosphotyrosine 4G10 antibodies were from Millipore (Temecula, CA). Total p130CAS (Cat: 610272) was purchased from BD Biosciences (San Jose, CA).
2.2 Cell culture conditions and cell proliferation assays
The ER-positive wild-type human breast cancer MCF-7 cells, estrogen-deprived MCF-7:5C cells, and MCF-7:PF cells were cultured as previously described (19). The DNA fingerprinting pattern of all cell lines are consistent with the report by the ATCC (19). The DNA content of the cells, a measure of proliferation, was determined as previously described (19) using a DNA fluorescence Quantitation kit (Bio-Rad Laboratories, Hercules, CA).
2.3 Immunoblotting
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with Protease Inhibitor Cocktail Set I and Phosphatase Inhibitor Cocktail Set II (Calbiochem, San Diego, CA). Immunoblotting was performed as previously described (19).
2.4 Immunoprecipitation
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with Protease Inhibitor Cocktail Set I and Phosphatase Inhibitor Cocktail Set II (Calbiochem, San Diego, CA). Supernatants containing 1 mg total protein were incubated with antibody against the target protein at 4°C for 4 h before addition of 40 μL Protein G beads (Millipore, Temecula, CA), and incubation was continued at 4°C overnight. The protein G beads with immunocomplex were centrifuged at 14,000 rpm for 20 s. The supernatant was carefully removed. The beads were washed twice with cell lysis buffer (Cell Signaling Technology, Beverly, MA) and then boiled in 20 μL of 2× Laemmli buffer (Bio-Rad Laboratories, Hercules, CA). Immunoblotting was conducted as described above.
2.5 Subcellular protein fractionation
Plasma membrane protein was extracted using Mem-PER Eukaryotic Membrane Protein Extraction Reagent kit, and nuclear and cytoplasmic proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents kit following the manufacturer's instructions. The kits were purchased from Pierce Biotechnology (Rockford, IL). CD73 antibody (sc-25603, used as the marker of plasma membrane protein), LDH-A antibody (sc-27230, used as the marker of cytosol protein), and Lamin-A/C (sc-20681, used as the marker of nuclear protein) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.6 Quantitative real-time RT-PCR
Quantitative real-time RT-PCR assays were conducted as previously described (18) using the SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA) and a 7900HT Fast Real-time PCR System (Applied Biosystems). All primers were synthesized by Integrated DNA Technologies (San Diego, CA).
2.7 Transient transfection reporter gene assays
Transient transfection assays were performed using a dual-luciferase system (Promega, Madison, WI) as previously described (18). Briefly, MCF-7:PF cells were transfected for 24 hours. Then, transfected MCF-7:PF cells were treated with vehicle control (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), ICI (10−6 mol/L), E2 (10−9 mol/L) plus ICI (10−6 mol/L), and 4-OHT (10−6 mol/L) plus ICI (10−6 mol/L) for 24 hours. Cells were harvested to measure luciferase activity following manufacturer’s instruction (Promega, Madison, WI).
2.8 Chromatin-immunoprecipitation (ChIP) assay
The standard ChIP assay was conducted as previously described (20). Briefly, MCF-7:5C and MCF-7:PF cells were treated with different compounds as indicated for 45 minutes. Immunoprecipitation with antibodies against ERα and steroid receptor coactivator-3 (SRC3) were purchased from Santa Cruz (Santa Cruz, CA). RT-PCR was performed using primers specific for the pS2 promoter (20).
2.9 Statistical Analysis
All reported values are the means ± SE. Statistical comparisons were determined with two-tailed Student's t tests. Results were considered statistically significant if the p value was <0.05.
3. Results
3.1 The ER agonist activity of SERMs is significantly elevated in MCF-7:PF cells
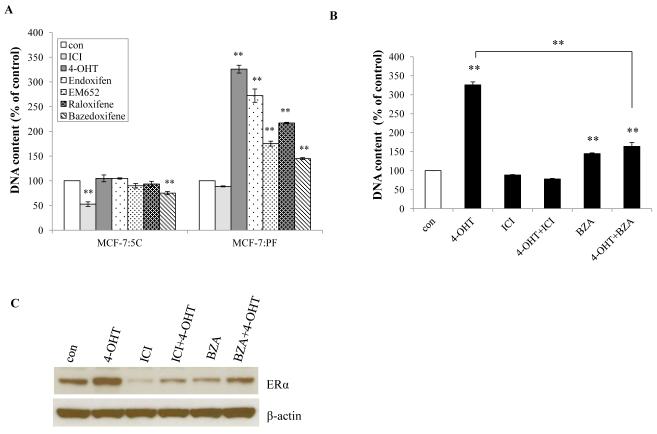
Our recent publication shows the proliferative response to E2 in the selected and reprogrammed cell line, MCF-7:PF, occurs in an ER-dependent manner (19). Here, we addressed the question of whether SERMs could block E2-stimulated growth. In long-term E2-deprived MCF-7:5C cells (simulating AI resistance), no SERM had an inhibitory effect except for bazedoxifene (BZA) (21), whereas all SERMs significantly stimulated cell growth in MCF-7:PF cells (Fig. 1A). Raloxifene did not stimulate MCF-7:PF cell growth as effectively as tamoxifen metabolites (22), 4-OHT and endoxifen, nor did the structurally related EM652 (23). Endoxifen and 4-OHT had similar actions in MCF-7:PF cells (Fig. 1A). The stimulation by 4-OHT could be completely blocked by ICI and partially blocked by BZA (Fig. 1B). Both ICI and BZA reduced levels of ERα protein in MCF-7:PF cells (Fig. 1C). It suggests that SERM-stimulated growth is in an ER-dependent manner.
Figure 1. Cell response to various SERMs.
(A) Growth response to various antiestrogens. MCF-7:5C and MCF-7:PF cells were treated with vehicle (0.1% EtOH; con) and antiestrogens: ICI, 4-OHT, Endoxifen, EM652, Raloxifene, and Bazedoxifene (BZA) at a final concentration of 1μM (10−6 mol/L). Cells were harvested after 7 days treatment and cell viability was quantitated by determination of total DNA. p<0.001, ** compared with control. (B) ICI and BZA blocked the proliferation activated by 4-OHT. MCF-7:PF cells were treated with vehicle (0.1% EtOH) and different compounds or combination at the same concentration as above. Cells were harvested after 7 days treatment and total DNA was determined as above. p<0.001, ** compared with control. (C) ICI and BZA reduced levels of ERα. ERα of MCF-7:PF cells was examined by Western blot after 24 hours treatment with different compounds at the same concentration as in (A). All the data shown are representative of at least three separate experiments with similar results.
3.2 4-OHT consistently suppresses classical estrogen-responsive element (ERE)-regulated transcriptional pathways in MCF-7:PF cells
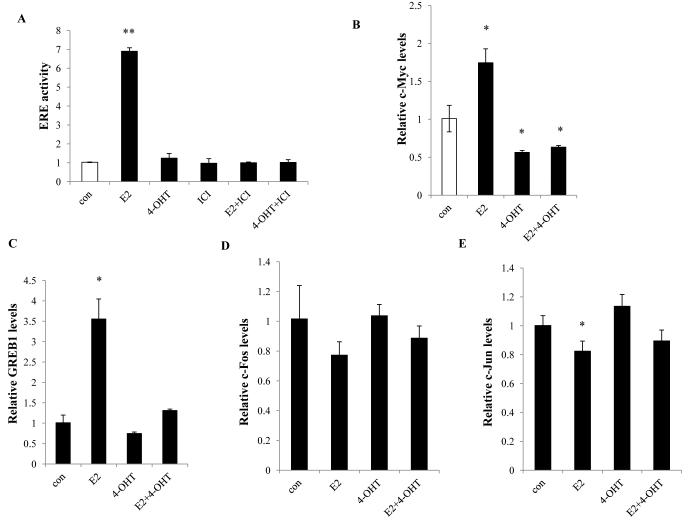
To test whether 4-OHT could also activate classical ERE-regulated endogenous ER-target genes as well as stimulating cell growth, MCF-7:PF cells were treated with 4-OHT in the absence or presence of E2 for 24 hours. Unlike E2, 4-OHT was unable to activate ERE activity in MCF-7:PF cells (Fig. 2A). Interestingly, 4-OHT and ICI both inhibited ER-target genes encoding c-Myc, GREB1, pS2, and PR (Fig. 2B, 2C, and S1), all of which were ER-target genes induced by E2 in MCF-7:PF cells (Fig. 2B, 2C, and S1). Inhibition of E2-stimulated pS2 by 4-OHT occurred in MCF-7, MCF-7:5C, and MCF-7:PF cell lines (Fig. S2) regardless of their different biological effects on inhibiting E2-stimulated growth (MCF-7), having no inhibitory action on cell growth (MCF-7:5C), or stimulating full growth (MCF-7:PF). Although non-classical ER-regulated transcriptional factors, AP-1 family members have been reported to be involved in the TAM resistance (24), we did not observe the up-regulation of c-Fos/c-Jun mRNA levels by 4-OHT (Fig. 2D and 2E), whereas E2 had a potential to reduce c-Fos/c-Jun mRNA levels in MCF-7:PF cells (Fig. 2D and 2E). These findings imply that 4-OHT is able to consistently suppress the classical ERE-regulated transcriptional pathways, even at time of growth stimulation.
Figure 2. 4-OHT regulated ER transcriptional pathways in MCF-7:PF cells.
(A) ERE-reporter gene activity. MCF-7:PF cells were transfected with ERE firefly luciferase and renilla luciferase plasmids as in Materials and Methods. (B), (C) Expression of classical ER-target genes. MCF-7:PF cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and E2 (10−9 mol/L) plus 4-OHT (10−6 mol/L) for 24 hours. Expression of c-Myc (B) and GREB1(C) was quantitated by real-time RT-PCR. p<0.05, * compared with control, p<0.001, ** compared with control. (D), (E) Expression of non-classical ER-regulated genes. RNA samples were the same as in (B) and (C). Expression of c-Fos (D) and c-Jun (E) was quantitated by real-time RT-PCR. p<0.05, * compared with control.
3.3 4-OHT does not recruit ERα and SRC3 to the promoter of pS2 in MCF-7:PF cells
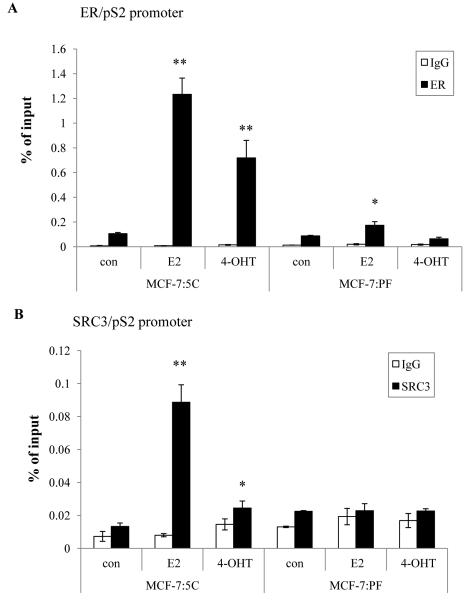
Since coactivators may be involved in promoting the agonistic properties of TAM (25), we tested the recruitment of ERα and the coactivator SRC3 to the promoter region of pS2 gene by chromatin immunoprecipitation (ChIP). MCF-7:5C and MCF-7:PF cells were treated in parallel with vehicle (0.1% EtOH), E2, or 4-OHT for 45 min, and thereafter harvested for ChIP. As noted in wild-type MCF-7 cells (20), we found that both E2 and 4-OHT recruited ERα to the pS2 promoter in MCF-7:5C cells (Fig. 3A). In contrast, E2 weakly recruited ERα to the pS2 promoter in MCF-7:PF cells, and 4-OHT showed no ERα recruitment (Fig. 3A). Recruitment of SRC3, which plays an important role in transcriptional activation of many ER-regulated genes (26), followed a similar pattern to ERα in MCF-7:5C cells (Fig. 3B). However, neither E2 nor 4-OHT recruited SRC3 to the pS2 promoter in MCF-7:PF cells (Fig. 3B). These results provide further evidence to suggest that 4-OHT-stimulated growth of MCF-7:PF does not involve recruitment of ERα and SRC3 to the promoters of ER target genes.
Figure 3. 4-OHT did not recruit ERα or SRC3 to the promoter of pS2 in MCF-7:PF cells.
(A) Recruitment of ERα at the pS2 proximal promoter. MCF-7:5C and MCF-PF cells were treated with vehicle (0.1% EtOH; con), E2 (10−9 mol/L), and 4-OHT (10−6 mol/L) for 45 min. ChIP assay was performed as in Materials and Methods. p<0.05, * compared with control, p<0.001, ** compared with control. (B) Recruitment of SRC3 at the pS2 proximal promoter. MCF-7:5C and MCF-PF cells were treated the same as above. ChIP assay was performed as described in Materials and Methods. p<0.05, * compared with control, p<0.001, ** compared with control. All the data shown are representative of at least three separate experiments with similar results.
3.4 4-OHT stimulates membrane-associated signaling pathways to promote cell growth in MCF-7:PF cells
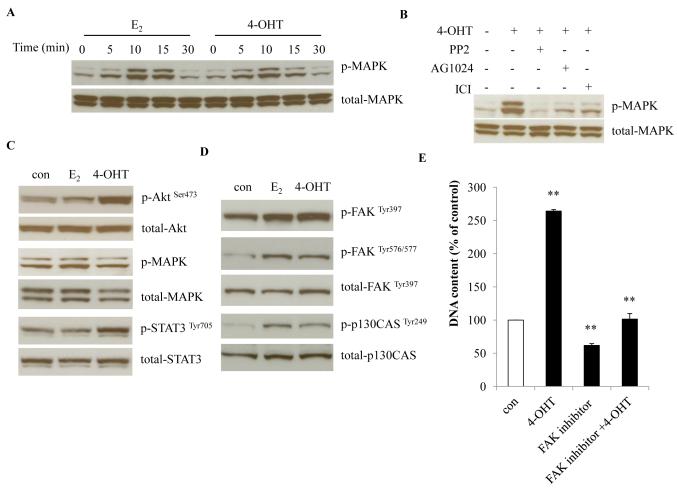
We observed that 4-OHT rapidly activated MAPK signaling, in a similar pattern as E2 (Fig. 4A, 27), although without clear redistribution of ERα in the plasma membrane (Fig. S3A). Inhibition of ER (ICI), c-Src (PP2), and IGF-1R (AG1024) were each sufficient to block the 4-OHT-mediated MAPK activation (Fig. 4B), which demonstrates that ER, c-Src and IGF-1R participate in the rapid non-genomic action of 4-OHT in MCF-7:PF cells. With prolonged treatment, 4-OHT was more potent than E2 in the activation of Akt and STAT3, whereas MAPK signaling was not significantly different among the treatment groups (Fig. 4C). Both 4-OHT and E2 could activate focal adhesion molecules FAK and p130CAS (Fig. 4D). The activation of FAK and STAT3 could be blocked by the c-Src inhibitor, PP2 (Fig. S3B). Importantly, inhibition of c-Src and FAK completely blocked 4-OHT-stimulated cell growth, but not E2-induced proliferation (Fig. 4E, S3C, S3D, and S3E). Together, these results suggest that focal adhesion molecules contribute to the development of resistance in this model.
Figure 4. 4-OHT activated membrane-associated signaling in MCF-7:PF cells.
(A) Rapid activation of MAPK. MCF-7:PF cells were treated with E2 (10−9 mol/L) or 4-OHT (10−6 mol/L) for the time points indicated. p-MAPK was examined by Western blot. Total MAPK was measured as loading control. (B) Selective inhibition of 4-OHT-induced MAPK activation. MCF-7:PF cells were treated with 4-OHT (10−6 mol/L) alone or in the presence of specific inhibitors, PP2 (5×10−6 mol/L), AG1024 (10−5 mol/L), and ICI (10−6 mol/L) for 10 minutes. p-MAPK was examined by Western blot. (C) Activation of growth pathways by 4-OHT or E2. MCF-7:PF cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), or 4-OHT (10−6 mol/L) for 72 hours. p-Akt, p-MAPK, and p-STAT3 were determined by Western blot. Respective total proteins were examined as loading controls. (D) Activation of focal adhesion molecules by 4-OHT and E2. Cell lysates were the same as in (C). p-FAK and p-p130CAS were determined by Western blot. Respective total proteins were examined as loading controls. (E) The FAK inhibitor, PF573228, blocked the proliferation induced by 4-OHT. MCF-7:PF cells were seeded in 24-well plates as above. One day later, they were treated with vehicle (0.1% DMSO), 4-OHT (10−6 mol/L), PF573228 (10−6 mol/L), and PF573228 (10−6 mol/L) plus 4-OHT (10−6 mol/L) for 7 days. Total DNA was determined as above as a measure for cell proliferation. p<0.001, ** compared with control.
3.5 4-OHT increases phosphorylation of IGF-1R to promote cell growth
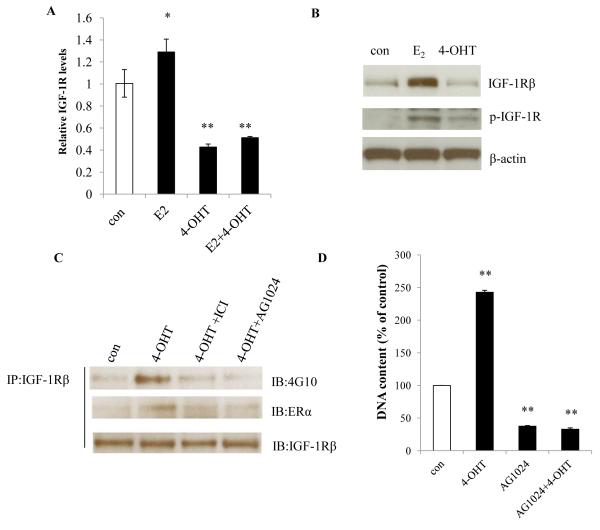
Membrane-associated IGF-1Rβ has been found to be responsible for the activation of the phosphatidylinositol-3 kinases (PI3K)/Akt signaling pathway in MCF-7:PF cells (19). Here, E2 increased the expression of IGF-1Rβ transcript and protein (Fig. 5A and 5B), however, levels of IGF-1Rβ transcript and protein were markedly reduced by 4-OHT, even in the presence of E2 (Fig. 5A and 5B). While 4-OHT did not increase total IGF-1Rβ, it did induce phosphorylation of IGF-1Rβ through ER, which was inhibited by ICI (Fig. 5C). Interaction between ER and activated IGF-1Rβ was increased by 4-OHT in MCF-7:PF cells (Fig. 5C). Furthermore, a specific inhibitor of IGF-1Rβ (AG1024) alone prevented cell growth (Fig. 5D) and completely abolished 4-OHT-stimulated cell growth in MCF-7:PF cells (Fig. 5D). All together, our results indicate that 4-OHT exerts distinct functions on nuclear ER and membrane-associated ER in resistant model (Fig. 6). A variety of membrane-associated molecules are activated by 4-OHT to form a complicated signaling network (Fig. 6). ER and IGF-1Rβ are two critical molecules to integrally modulate signaling between nucleus and plasma membrane (Fig. 6).
Figure 5. 4-OHT increased phosphorylation of IGF-1R in MCF-7:PF cells.
(A) Expression of IGF-1Rβ mRNA. MCF-7:PF cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), or E2 (10−9 mol/L) plus 4-OHT (10−6 mol/L) for 72 hours. IGF-1Rβ mRNA was quantitated through real-time RT-PCR. p<0.05, * compared with control, p<0.001, ** compared with control. (B) Protein levels of p-IGF-1Rβ and total IGF-1Rβ. MCF-7:PF cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), or 4-OHT (10−6 mol/L) for 72 hours. Total IGF-1Rβ and p-IGF-1Rβ were determined by Western blot. (C) Interaction between ERα and p-IGF-1Rβ. MCF-7:PF cells were treated with vehicle (0.1% EtOH), 4-OHT (10−6 mol/L), 4-OHT (10−6 mol/L) plus ICI (10−6 mol/L), and 4-OHT (10−6 mol/L) plus AG1024 (10−5 mol/L) for 24 hours. Protein-protein interaction was determined by immunoprecipitation. (D) Growth response to the IGF-1R inhibitor. MCF-7:PF cells were treated with vehicle control (0.1% DMSO), 4-OHT (10−6 mol/L), AG1024 (10−5 mol/L), and AG1024 (10−5 mol/L) plus 4-OHT (10−6 mol/L) for 7 days. Total DNA was determined as above. p<0.001, ** compared with control.
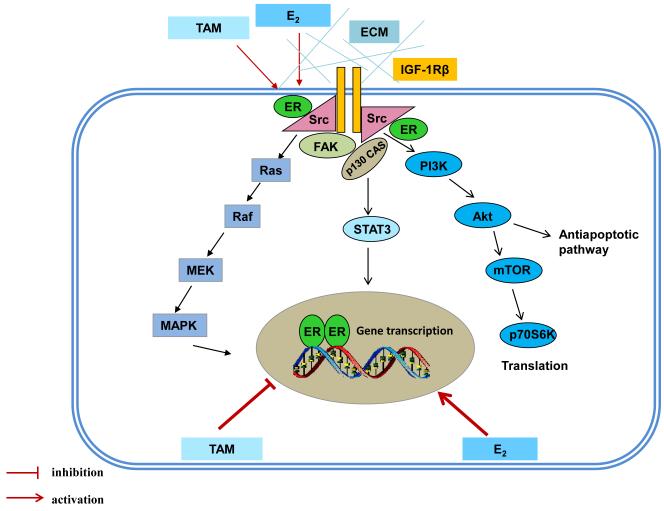
Figure 6. Genomic and nongenomic signal transduction pathways in tamoxifen resistant model.
E2 and TAM exert differential functions on nuclear ER. E2 activates classical ER-target genes but TAM acts to block gene activation. Both E2 and TAM increases the non-genomic activity of ER through membrane-associated molecules such as c-Src, IGF-1Rβ, and FAK to enhance downstream signaling cascades.
4. Discussion
For the first time, we established a TAM-resistant model entirely in vitro which faithfully replicates SERM/E2-stimulated growth in vivo (28, 29). We document the mechanistic changes that membrane-associated pathways play as an essential requirement in the mediation of TAM resistance despite the constant inhibition of nuclear ER-target genes.
Our results demonstrate that ER is a major driver of growth utilized by both E2 and TAM in resistant models in vivo and in vitro (Fig. 1B, 6, 28). As a result, ER remains a therapeutic target in breast cancers that are resistant to both first- and second-line endocrine interventions (21, 30). This is illustrated recently by the fact that BZA inhibits the growth of both TAM-sensitive and -resistant ER-positive breast cancer xenografts (30). Indeed, the SERM used by Wardell et al (30) in vivo are recapitulated here in vitro (Fig. 1A and 1B). In contrast to E2 that activated classical ER-target genes, 4-OHT continued to act as an effective antiestrogen to inhibit classical ER-target genes (Fig. 2B, 2C, and S1), even at the time of growth stimulation. The question, therefore, arises as to the function of classical ER-target genes in the process of selecting a new population to acquire TAM resistance. The results presented here are consistent with our previous finding in vivo that growth of tumors by TAM or a fulvestrant/E2 combination is potentially independent of ER transcriptional activity, as evidenced by lack of induction of E2-responsive genes (31). Other groups have reported similar observations with TAM suppressing classical ERE-regulated genes despite acquired resistance in vitro (32, 33) or in vivo (34). However, the aforementioned model in vitro (32, 33) does not demonstrate SERM-stimulated growth. Moreover, we observed that 4-OHT effectively inhibited the ER-target gene, pS2, induced by E2 in three cell lines with differential biological responsiveness to 4-OHT and E2 (Fig. S2). Together, these observations highlight that TAM may continue to function as an antiestrogen for classical ER-target genes even when acquired resistance occurs. It also illustrates that the function of ER is modified in the process of acquired SERM resistance.
A significant alteration of ER function observed in TAM-resistant cells is the enhancement of the non-genomic activity (Fig. 4A) involving a multi-molecular complex containing IGF-1Rβ, c-Src, Shc, and ER (27, Fig. 4B). Inhibition of c-Src and FAK completely blocked 4-OHT-stimulated growth but not E2-stimulated growth (Fig. 4E, S3C, S3D, and S3E), confirming the important role of membrane-associated signaling in 4-OHT-stimulated growth. It needs to be noted here how c-Src integrally regulates the non-genomic function of ER and interactions among functional membrane-associated molecules. Our observations demonstrate that blocking E2-induced stress is the major contribution of the c-Src inhibitor, PP2, to establish a reprogrammed cell line, MCF-7:PF (18, 19). However, c-Src tyrosine kinase is not permanently suppressed if the c-Src inhibitor is removed from the medium (19). PP2 is a reversible blocker even after months of treatment (19). The recovery c-Src tyrosine kinase has multifaceted functions to regulate 4-OHT-promoted cell growth. In addition to the mediation of the non-genomic pathway of ER (Fig. 4B), c-Src was involved in the activation of FAK and STAT3 by 4-OHT (Fig. S3B). Tyrosine phosphorylated focal adhesion molecules, such as FAK and p130CAS (Fig. 4D) provide docking sites for the redistribution of a small fraction of ER that associates with the cell membrane (33, 35), and mediate the cross-talk between the ER and growth factor receptors (27, 35). All of these results illustrate that 4-OHT modulates a variety of membrane-associated molecules to enhance the non-genomic pathways in TAM-resistant cells (Fig. 6).
Membrane-associated growth factor receptors, such as IGF-1R are another permissive components resulting in TAM resistance (34-37). IGF-1R is regulated by E2 in an ER-dependent manner (19, 38). Cross-talk between IGF-1R and ER signaling pathways results in synergistic growth (39). Here, we provide evidence that 4-OHT functions as an antiestrogen to suppress the gene expression of IGF-1Rβ, even in the presence of E2 (Fig. 5A). Interestingly, 4-OHT had the capacity to simultaneously activate IGF-1Rβ through ER (Fig. 5B and 5C), facilitating its interaction with ER (Fig. 5C) to activate non-genomic pathways (Fig. 4B). Our observations precisely define the mechanisms underlying the paradoxical finding in vivo that acquired resistance to TAM is associated with loss of total IGF-1R (34, 37, 40) but keep (40) or gain of phosphorylated IGF-1R (34, 37). In contrast to the result that the non-genomic action of ER can rapidly (within minutes) activate IGF-1Rβ (27), we detected increased phosphorylation of IGF-1Rβ by 4-OHT after 24 hours (Fig. 5B and 5C). Currently, it is unclear how 4-OHT activates IGF-1Rβ through ER. Deposition of fibrinogen in the plasma membrane (19) may facilitate the interactions between IGF-1R and focal adhesion molecules, which may participate in the activation of IGF-1Rβ (41). c-Src is a critical molecule to mediate the functions of focal adhesion molecules (33). However, inhibition of c-Src did not suppress phosphorylation of IGF-1Rβ, which was increased to a higher level in MCF-7:PF cells (Fig. S4). This result implies that other molecules may be involved in the activation of IGF-1Rβ. All together, distinct functions on nuclear ER and membrane-associated ER by 4-OHT differentially affect the levels of total and phosphorylated IGF-1Rβ. Cross-talk between ER and IGF-1Rβ appears to reinforce one another to stimulate breast cancer cell growth (Fig. 6).
Acquired resistance to SERMs is not one-dimensional with a simple solution. Resistant cell populations are in constant evolution depending upon selection pressure and the availability of growth stimuli that enhances population plasticity and survival of new clones. This plasticity is well illustrated by the MCF-7 cell line. There are few ER-positive cell lines (42), but based on results using MCF-7 cells, progress in breast cancer research and patient care has been profound (43). For the first time, we have recapitulated an entirely new MCF-7:PF cell line with both E2 and SERM-stimulated growth dependent on ER signaling. 4-OHT exerts distinct functions on nuclear and extra-nuclear ER (Fig. 6). The non-genomic activity of ER is diversely modulated via multiple membrane-associated molecules to subvert long-term nuclear suppression by TAM. The targeting of these membrane-associated pathways and seeking new unanticipated combination therapies with the MCF-7:PF cells may have further clinical potential to decipher and treat endocrine-resistant patients.
Supplementary Material
MCF-7:PF cells are derived from MCF-7:5C cells after long-term treated with the c-Src inhibitor and E2.
Both E2 and SERMs stimulate cells to grow in an ER-dependent manner.
4-OHT exerts distinct functions on nuclear ER and membrane-associated ER in resistant model. 4-OHT constantly suppresses classical nuclear ER-target genes as does the pure antiestrogen fulvestrant. However, 4-OHT functions as an agonist to enhance the non-genomic activity of ER.
4-OHT acts as an antagonist to reduce total IGF-1Rβ but increases phosphorylation of IGF-1Rβ. Additionally, 4-OHT activates focal adhesion molecules to further increase phosphorylation of IGF-1Rβ, which further enhances the cross-talk between ER and IGF-1Rβ to promote cell growth.
Disruption of membrane-associated signaling, IGF-1R and FAK, completely abolishes 4-OHT-stimulated cell growth.
Acknowledgements
VCJ is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. We are particularly grateful to the Avon Foundation for supporting this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared.
References
- 1.Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr relat Cancer. 2014;21:R235–46. doi: 10.1530/ERC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Martino S, et al. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol. 2007;25:2006–11. doi: 10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 6.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–87. [PubMed] [Google Scholar]
- 7.Gottardis MM, Wagner RJ, Borden EC, Jordan VC. Differential ability of antiestrogens to stimulate breast cancer cell (MCF-7) growth in vivo and in vitro. Cancer Res. 1989;49:4765–69. [PubMed] [Google Scholar]
- 8.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 9.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 10.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108:18879–86. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingle JN, Ahmann DJ, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 12.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 15.Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 16.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan P, Griffith OL, Agboke FA, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510–20. doi: 10.1158/0008-5472.CAN-12-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan P, Agboke FA, McDaniel RE, et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2014;50:457–68. doi: 10.1016/j.ejca.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–78. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis-Wambi JS, Kim H, Curpan R, Grigg R, Sarker MA, Jordan VC. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor α and cyclin D1. Mol Pharmacol. 2011;80:610–20. doi: 10.1124/mol.111.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–9. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 23.Schafer JI, Liu H, Tonetti DA, Jordan VC. The interaction of raloxifene and the active metabolite of the antiestrogen EM-800 (SC 5705) with the human estrogen receptor. Cancer Res. 1999;59:4308–13. [PubMed] [Google Scholar]
- 24.Schiff R, Reddy P, Ahotupa M, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst. 2000;92:1926–34. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 25.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 26.Labhart P, Karmakar S, Salicru EM, et al. Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci USA. 2005;102:1339–44. doi: 10.1073/pnas.0409578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–81. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–3. [PubMed] [Google Scholar]
- 29.Lee ES, Schafer JM, Yao K, et al. Cross-resistance of triphenylethylene-type antiestrogens but not ICI 182,780 in tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:4893–9. [PubMed] [Google Scholar]
- 30.Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19:2420–31. doi: 10.1158/1078-0432.CCR-12-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osipo C, Meeke K, Cheng D, et al. Role for HER2/neu and HER3 in fulvestrant-resistant breast cancer. Int J Oncol. 2007;30:509–20. [PubMed] [Google Scholar]
- 32.Hutcheson IR, Knowlden JM, Madden TA, et al. Oestrogen receptor-mediated modulation of the EGFR/MAPK pathway in tamoxifen-resistant MCF-7 cells. Breast Cancer Res Treat. 2003;81:81–93. doi: 10.1023/A:1025484908380. [DOI] [PubMed] [Google Scholar]
- 33.Riggins RB, Thomas KS, Ta HQ, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–15. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- 34.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–33. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 35.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–60. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 36.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 37.Fagan DH, Uselman RR, Sachdev D, Yee D. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72:3372–80. doi: 10.1158/0008-5472.CAN-12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee AV, Jackson JG, Gooch JL, et al. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13:787–96. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 39.Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF Pathway Regulates ERα through a S6K1-Dependent Mechanism in Breast Cancer Cells. Mol Endocrinol. 2011;25:516–28. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drury SC, Detre S, Leary A, et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer. 2011;18:565–77. doi: 10.1530/ERC-10-0046. [DOI] [PubMed] [Google Scholar]
- 41.Shin DH, Lee HJ, Min HY, et al. Combating resistance to anti-IGFR antibody by targeting the integrin β3-Src pathway. J Natl Cancer Inst. 2013;105:1558–70. doi: 10.1093/jnci/djt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweeney EE, McDaniel RE, Maximov PY, Fan P, Jordan VC. Models and mechanisms of acquired antihormone resistance in breast cancer: significant clinical progress despite limitations. Horm Mol Biol Clin Investig. 2012;9:143–63. doi: 10.1515/hmbci-2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.