Abstract
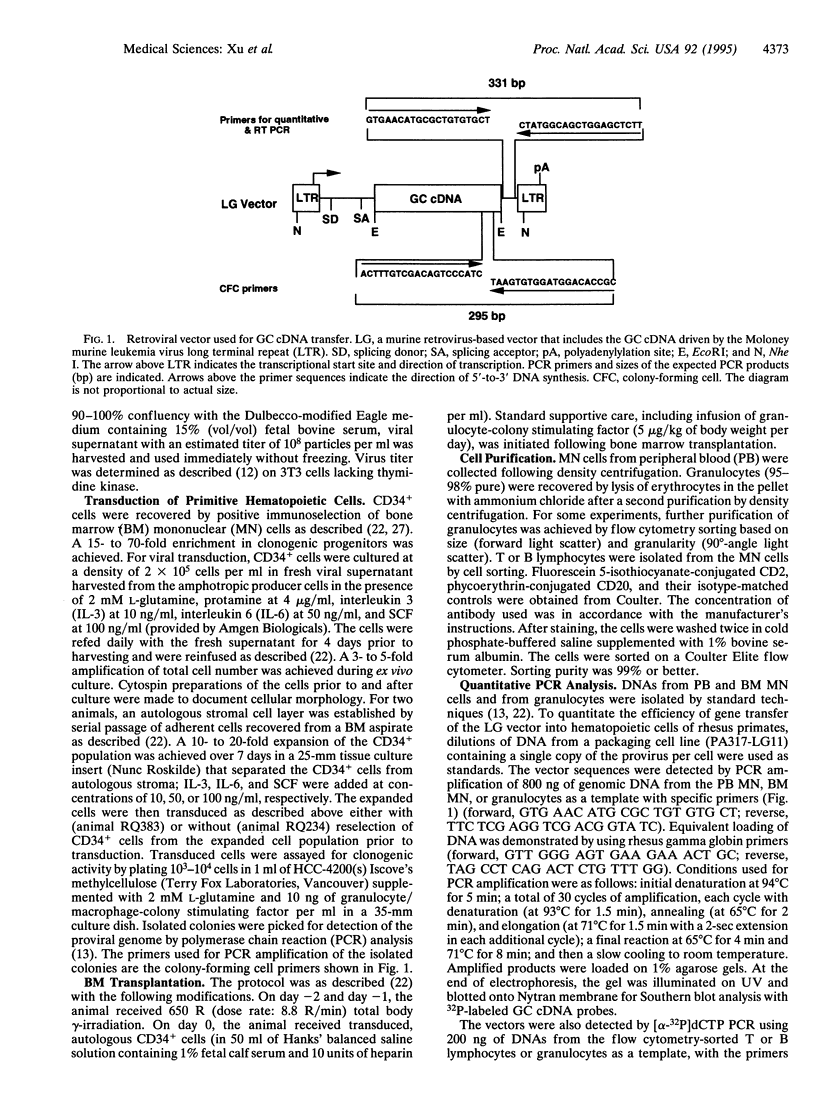
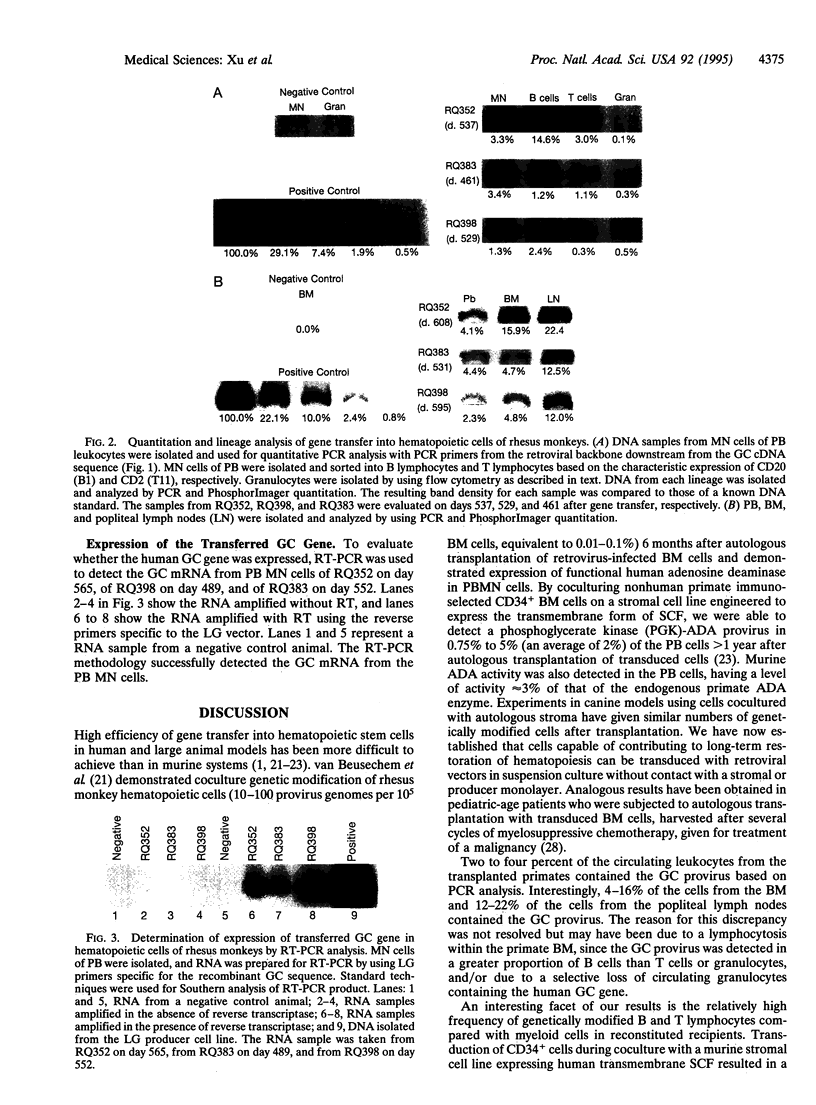
Successful gene transfer into stem cells would provide a potentially useful therapeutic modality for treatment of inherited and acquired disorders affecting hematopoietic tissues. Coculture of primate bone marrow cells with retroviral producer cells, autologous stroma, or an engineered stromal cell line expressing human stem cell factor has resulted in a low efficiency of gene transfer as reflected by the presence of 0.1-5% of genetically modified cells in the blood of reconstituted animals. Our experiments in a nonhuman primate model were designed to explore various transduction protocols that did not involve coculture in an effort to define clinically useful conditions and to enhance transduction efficiency of repopulating cells. We report the presence of genetically modified cells at levels ranging from 0.1% (granulocytes) to 14% (B lymphocytes) more than 1 year following reconstitution of myeloablated animals with CD34+ immunoselected cells transduced in suspension culture with cytokines for 4 days with a retrovirus containing the glucocerebrosidase gene. A period of prestimulation for 7 days in the presence of autologous stroma separated from the CD34+ cells by a porous membrane did not appear to enhance transduction efficiency. Infusion of transduced CD34+ cells into animals without myeloablation resulted in only transient appearance of genetically modified cells in peripheral blood. Our results document that retroviral transduction of primate repopulating cells can be achieved without coculture with stroma or producer cells and that the proportion of genetically modified cells may be highest in the B-lymphoid lineage under the given transduction conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- BRADY R. O., KANFER J. N., SHAPIRO D. METABOLISM OF GLUCOCEREBROSIDES. II. EVIDENCE OF AN ENZYMATIC DEFICIENCY IN GAUCHER'S DISEASE. Biochem Biophys Res Commun. 1965 Jan 18;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991 May 23;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Barton N. W., Furbish F. S., Murray G. J., Garfield M., Brady R. O. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1913–1916. doi: 10.1073/pnas.87.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science. 1992 May 8;256(5058):794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kay A., Saven A., Garver P., Thurston D., Dawson A., Rosenbloom B. Enzyme replacement therapy for Gaucher disease. Blood. 1991 Sep 1;78(5):1183–1189. [PubMed] [Google Scholar]
- Bienzle D., Abrams-Ogg A. C., Kruth S. A., Ackland-Snow J., Carter R. F., Dick J. E., Jacobs R. M., Kamel-Reid S., Dubé I. D. Gene transfer into hematopoietic stem cells: long-term maintenance of in vitro activated progenitors without marrow ablation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):350–354. doi: 10.1073/pnas.91.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Moritz T., Donahue R. E., Luskey B. D., Kessler S. W., Martin D. I., Orkin S. H., Nienhuis A. W., Williams D. A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993 Oct 1;82(7):1975–1980. [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Moen R. C., Krance R. A., Mirro J., Jr, Anderson W. F., Ihle J. N. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993 Jan 9;341(8837):85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- Correll P. H., Colilla S., Dave H. P., Karlsson S. High levels of human glucocerebrosidase activity in macrophages of long-term reconstituted mice after retroviral infection of hematopoietic stem cells. Blood. 1992 Jul 15;80(2):331–336. [PubMed] [Google Scholar]
- Correll P. H., Colilla S., Karlsson S. Retroviral vector design for long-term expression in murine hematopoietic cells in vivo. Blood. 1994 Sep 15;84(6):1812–1822. [PubMed] [Google Scholar]
- Correll P. H., Fink J. K., Brady R. O., Perry L. K., Karlsson S. Production of human glucocerebrosidase in mice after retroviral gene transfer into multipotential hematopoietic progenitor cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8912–8916. doi: 10.1073/pnas.86.22.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll P. H., Kew Y., Perry L. K., Brady R. O., Fink J. K., Karlsson S. Expression of human glucocerebrosidase in long-term reconstituted mice following retroviral-mediated gene transfer into hematopoietic stem cells. Hum Gene Ther. 1990 Fall;1(3):277–287. doi: 10.1089/hum.1990.1.3-277. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Kessler S. W., Bodine D., McDonagh K., Dunbar C., Goodman S., Agricola B., Byrne E., Raffeld M., Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992 Oct 1;176(4):1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. K., Correll P. H., Perry L. K., Brady R. O., Karlsson S. Correction of glucocerebrosidase deficiency after retroviral-mediated gene transfer into hematopoietic progenitor cells from patients with Gaucher disease. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2334–2338. doi: 10.1073/pnas.87.6.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Kessler S. W., Orenstein J. M., Justement J. S., Jaffe E. S., Fauci A. S. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988 Nov 11;242(4880):919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- Hobbs J. R., Jones K. H., Shaw P. J., Lindsay I., Hancock M. Beneficial effect of pre-transplant splenectomy on displacement bone marrow transplantation for Gaucher's syndrome. Lancet. 1987 May 16;1(8542):1111–1115. doi: 10.1016/s0140-6736(87)91673-4. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S. Treatment of genetic defects in hematopoietic cell function by gene transfer. Blood. 1991 Nov 15;78(10):2481–2492. [PubMed] [Google Scholar]
- Luskey B. D., Rosenblatt M., Zsebo K., Williams D. A. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells. Blood. 1992 Jul 15;80(2):396–402. [PubMed] [Google Scholar]
- Lynch C. M., Miller A. D. Production of high-titer helper virus-free retroviral vectors by cocultivation of packaging cells with different host ranges. J Virol. 1991 Jul;65(7):3887–3890. doi: 10.1128/jvi.65.7.3887-3890.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Muench M. O., Firpo M. T., Moore M. A. Bone marrow transplantation with interleukin-1 plus kit-ligand ex vivo expanded bone marrow accelerates hematopoietic reconstitution in mice without the loss of stem cell lineage and proliferative potential. Blood. 1993 Jun 15;81(12):3463–3473. [PubMed] [Google Scholar]
- Muench M. O., Moore M. A. Accelerated recovery of peripheral blood cell counts in mice transplanted with in vitro cytokine-expanded hematopoietic progenitors. Exp Hematol. 1992 Jun;20(5):611–618. [PubMed] [Google Scholar]
- Nolta J. A., Hanley M. B., Kohn D. B. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood. 1994 May 15;83(10):3041–3051. [PubMed] [Google Scholar]
- Nolta J. A., Yu X. J., Bahner I., Kohn D. B. Retroviral-mediated transfer of the human glucocerebrosidase gene into cultured Gaucher bone marrow. J Clin Invest. 1992 Aug;90(2):342–348. doi: 10.1172/JCI115868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T., Boggs S., Robbins P., Bahnson A., Patrene K., Wei F. S., Wei J. F., Li J., Lucht L., Fei Y. Efficient transfer and sustained high expression of the human glucocerebrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdén O., Groth C. G., Erikson A., Bäckman L., Granqvist S., Månsson J. E., Svennerholm L. Long-term follow-up of the first successful bone marrow transplantation in Gaucher disease. Transplantation. 1988 Jul;46(1):66–70. doi: 10.1097/00007890-198807000-00011. [DOI] [PubMed] [Google Scholar]
- Sato N., Sawada K., Koizumi K., Tarumi T., Ieko M., Yasukouchi T., Yamaguchi M., Takahashi T. A., Sekiguchi S., Koike T. In vitro expansion of human peripheral blood CD34+ cells. Blood. 1993 Dec 15;82(12):3600–3609. [PubMed] [Google Scholar]
- Weinthal J., Nolta J. A., Yu X. J., Lilley J., Uribe L., Kohn D. B. Expression of human glucocerebrosidase following retroviral vector-mediated transduction of murine hematopoietic stem cells. Bone Marrow Transplant. 1991 Nov;8(5):403–412. [PubMed] [Google Scholar]
- Xu L., Stahl S. K., Dave H. P., Schiffmann R., Correll P. H., Kessler S., Karlsson S. Correction of the enzyme deficiency in hematopoietic cells of Gaucher patients using a clinically acceptable retroviral supernatant transduction protocol. Exp Hematol. 1994 Feb;22(2):223–230. [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]




