Abstract
The human γ-globin gene and its orthologous galago γ-globin gene evolved from an ancestral ε-globin gene. In galago, expression of the γ-gene remained restricted to the embryonic stage of development, whereas in humans, expression of the γ-gene was recruited to the fetal stage. To localize the cis-elements responsible for this developmentally distinct regulation, we studied the expression patterns of the human γ-gene driven by either the human or the galago γ-promoters in transgenic mice. γ-gene transcription driven by either promoter reached similar levels in embryonic erythropoiesis. In adult erythropoiesis the γ-gene was silenced when controlled by the galago γ-promoter, but it was expressed at a high level when it was linked to the human γ-promoter. By a series of γ-promoter truncations the sequences required for the down-regulation of the galago γ-globin gene were localized to the minimal promoter. Furthermore, by interchanging the TATA, CCAAT, and CACCC elements between the human and galago minimal promoters we found that whereas each box made a developmentally distinctive contribution to γ-globin gene expression, the CACCC box was largely responsible for the down-regulation of the γ-gene in adult erythropoiesis.
The human β-globin locus provides a model to study the regulation of gene expression during development. Expression of the five productive globin genes of this locus is confined to erythrocytes and it is developmentally regulated during ontogeny. The ε-globin gene is expressed in the first 8 weeks of gestation and silenced afterward; then the γ-genes are active predominately during the fetal period; and, finally, globin expression is switched to the β- and δ-genes after birth (1). Early studies in transgenic mice suggested that the gene or the immediate vicinity, presumably the promoter, is responsible for the developmental regulation of globin genes (2–5). Thus, the human β-globin transgene, like the endogenous mouse β-globin genes, is active only during fetal liver and adult bone marrow erythropoiesis, whereas the developmental pattern of the human γ-globin transgene follows that of the orthologous βh1 gene, i.e., the expression is restricted to the yolk sac embryonic erythropoiesis. Although in these studies the developmental regulation of the human genes could be replicated in transgenic mice, the level of expression was extremely low and was subjected to effects of the chromatin neighboring the integration sites. Physiological levels of globin gene expression are achieved by linking the genes to the locus control region (LCR) (6). However, this linkage results in alteration of the developmental profile of the transgenes. For example, when coupled with the LCR, the proper temporal regulation of the β-globin gene is abolished unless a γ-globin gene is also present in the construct (7, 8). The effect of the LCR on the developmental regulation of the γ-gene was shown to be context-dependent, whereas proper regulation of the γ-globin gene was observed in transgenic mice carrying a cosmid- or YAC-based γ-globin construct (9, 10), the γ-gene is deregulated in plasmid-based constructs (11).
The human γ-globin genes originate from an ancestral embryonic gene. The fetal pattern of expression of the human γ-gene is a unique feature for simian primates; all the orthologous γ-genes in other mammals are expressed exclusively in the embryonic tissues. Sequence comparisons show that the proximal promoters of the human and galago (Galago crassicaudatus, a prosimian primate) γ-genes are highly conserved (12). However, whereas the human γ-gene is expressed during the fetal stage of development, the expression of the galago γ-gene is restricted to the embryonic stage (12). This developmental regulation of the galago γ-globin gene has been reproduced in transgenic mice. Expression of a galago γ-transgene linked to the human HS2 or HS3 is restricted to the yolk sac (13, 14); in contrast, expression of the human γ-globin gene linked to μLCR persists in the fetal liver and adult stages of erythropoiesis of transgenic mice (11, 15). This difference provided us an opportunity to localize the elements responsible for developmental specificity of the γ-globin genes. By exchanging individual elements of the human and galago γ-gene promoters we show that the minimal promoter, in particular the γCACCC box is largely responsible for the down-regulation of the γ-globin gene in adult erythropoiesis.
Materials and Methods
Constructs. The constructs used in this study were assembled by conventional molecular cloning techniques. To facilitate production of the human/galago chimeric minimal promoters a SalI site was introduced between the CACCC and the distal CCAAT boxes of the galago minimal promoter (equivalent to the BalI site in the human γ-promoter) by oligonucleotide direct-site mutagenesis; and a NaeI site was introduced to the minimal galago γ-promoter between the proximal CCAAT and the TATA boxes (equivalent to the NaeI site in the human γ-promoter). In the galago/human chimeric promoters the CACCC region was the AvaII/BalI fragment (–159 to –130) in the human γ-gene promoter or the galago counterpart; the BalI/NaeI fragment (–130 to –50 in human) or the galago counterpart contained the duplicate CCAAT boxes; and the NaeI/NcoI fragment (–50 to +52 in human) or corresponding galago fragment contained the TATA box. A 17-bp oligonucleotide containing the CACCC box of the human ε-globin gene (GGACCTGACTCCACCCC) was used for replacing the corresponding region of the human γCACCC box in the context of the μLCRAγ construct. A superscript h (human) or g (galago) was used in the designation of the constructs to facilitate discrimination between the two promoters. All mutations were confirmed by DNA sequencing.
Production of Transgenic Mice, DNA Analysis, and Globin Expression Analysis. All experimental procedures were as described (15, 16).
Results
Experimental Model. Fetal hemoglobin can be abundantly produced in the red cells of human adult individuals, as demonstrated by mutations causing hereditary persistence of fetal hemoglobin (1) or δβ-thalassemia (1). Individuals homozygous for deletional hereditary persistence of fetal hemoglobin (HPFH) have 100% hemoglobin F and they are hematologically normal. Also, hemoglobin F can be elevated in human adults in a variety of acquired conditions, and it is typically elevated in the majority of patients with thalassemia and sickle cell disease. It is likely that specific elements of the γ-globin gene promoter are involved in the control of γ-gene expression in adult erythropoiesis. It is difficult, if not impossible, to investigate the role of these elements in the adult stage of development by using βYAC transgenic mice because of autonomous silencing of γ-gene expression. Therefore, to investigate γ-gene promoter elements contributing to γ-gene expression in the adult we used a transgenic mouse model whose phenotype mimics the phenotype of HPFHs and it is characterized by abundant γ-gene expression in the adult. The transgene contains a γ-globin gene with a promoter extended to position –382 linked to a 2.5-kb μLCR. This transgene is characterized by γ-gene expression in yolk sac and fetal liver erythropoiesis, but also by continuation of high levels of γ-gene expression in the cells of adult erythropoiesis (15), indicating that all the cis-elements necessary for activation of the γ-globin in all developmental stages are present in the construct. This model provided us an opportunity to ask which elements in the γ-globin gene promoter are involved in the control of γ-gene expression in the adult erythropoiesis.
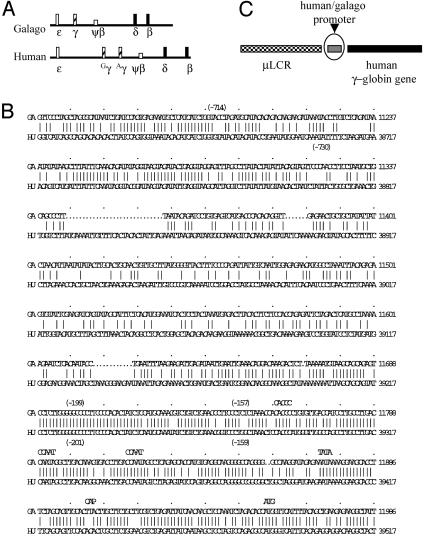
The genomic arrangements of the β-globin locus of galago and human are very similar (Fig. 1A). Sequence comparisons of the γ-gene promoters of the two species are depicted in Fig. 1B. The highly conserved region between the two promoters extends ≈250 bp 5′ to the cap site (12).
Fig. 1.
(A) Diagram of the galago and human β-globin loci. Rectangle boxes are the expressed genes, and the square boxes are pseudogenes. (B) Alignment of the human and galago promoters. Numbers in parentheses indicate the promoter truncations used in the constructs. (C) The basic configuration of the constructs. Each construct contains the μLCR and the human Aγ-globin gene from the start codon (ATG) to +1951 (HindIII site). The promoters in each construct are specified in the text.
The basic configuration of the constructs is outlined in Fig. 1C, and the detailed promoter design is described in Materials and Methods. All constructs contain the same μLCR cassette and the same Aγ-gene-coding sequence, but they differ in the structure of their promoters.
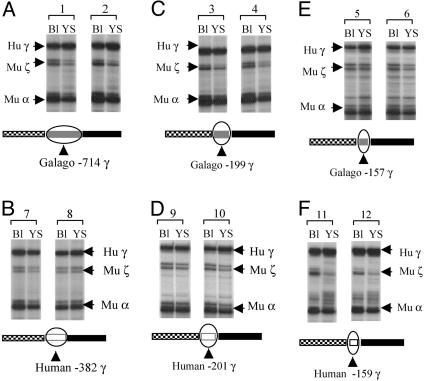
The Galago γ-Globin Gene Promoter Is Competent for Driving Expression of the Human Aγ-Globin Gene. Transgenic mice carrying galago γ-globin gene constructs linked to HS2 or HS3 of the LCR express the γ-globin gene in the embryonic stage of erythropoiesis; γ-gene expression occurs in the early fetal liver stage of definitive erythropoiesis of transgenic mice, but no γ-gene expression occurs in the adult stage (13, 14). In contrast, such expression is characteristic of transgenic mice carrying various human γ-globin gene constructs (15). If the galago γ-promoter is the major determinant of γ-gene silencing in adult erythropoiesis, it is expected that replacement of the human γ-promoter with the galago promoter will silence the human γ-gene in the adult stage of erythropoiesis. This expectation assumes that the galago γ-promoter is able to drive the human γ-globin gene expression to a high level during embryonic erythropoiesis. To test this we replaced the human γ-promoter with a 769-bp galago promoter spanning from –714g to the start codon of the gene. The μLCR was placed upstream of the chimeric galago γ-promoter/human γ-gene, forming μLCR(–714g)Aγ. Two transgenic mouse lines carrying intact copies of the construct were established. γ-gene expression in embryonic erythropoiesis was determined in 12-days-postcoitum yolk sac and fetal blood samples by RNase protection assay. In Fig. 2A we present representative RNase protection assays showing the level of γ-gene expression and the expression of endogenous murine α- and ζ-mRNAs. The level of human γ-mRNA (corrected per copy) of the Aγ(–714g) line A was 15.4 ± 1.4 in day 12 blood and 36.0 ± 1.8 in day 12 yolk sac. In line B these values were 9.9 ± 1.6 and 16.9 ± 3.2, respectively. These results are similar to those of the μLCR(–201h)Aγ control mice (10.4 ± 9.5 in day 12 blood and 21.0 ± 7.4 in day 12 yolk sac; Table 1) and indicate that the galago γ-gene promoter is able to drive the human γ-gene to high levels of expression in embryonic cells.
Fig. 2.
The galago γ-globin gene promoter has the capability to drive high-level expression of the human γ-globin gene in transgenic mice. For each construct two images of RNase protection assay are shown, each one representing the results from an individual fetus. The line diagrams appearing below each image indicate the tested constructs. Hu γ, 170-bp protected fragment from exon 2 of the human γ-globin gene; Mu ζ, 151-bp protected fragment from the murine ζ-globin gene; Mu α, 128-bp protected fragment from the murine α-globin gene; Bl, blood; YS, yolk sac.
Table 1. Expression of the human γ-gene in transgenic mice carrying a μLCRAγ in which the human γ-promoter was substituted by a -199 galago promoter.
| Human γ, % of murine α-mRNA per copy
|
|||||
|---|---|---|---|---|---|
| Embryonic erythropoiesis
|
Definitive erythropoiesis
|
||||
| Line | Copy no. | Day 12 blood | Day 12 yolk sac | Day 12 fetal liver | Adult blood |
| μLCR(-199g)Aγ | |||||
| C | 24 | 5.8 ± 0.6 | 20.4 ± 5.1 | 5.5 ± 0.8 | 0.8 ± 0.2 |
| D | 18 | 21.1 ± 6.8 | 31.7 ± 10.7 | 8.5 ± 2.0 | 1.2 |
| E | 7 | 22.1 ± 1.0 | 41.7 ± 7.0 | 10.4 ± 2.4 | 1.3 ± 0.4 |
| Mean | 16.3 ± 9.1 | 31.3 ± 10.7 | 8.1 ± 2.5 | 1.1 ± 0.3 | |
| μLCR(-201h)Aγ | |||||
| Mean* | 10.4 ± 9.5 | 21.0 ± 7.4 | 11.8 ± 2.8 | 16.5 ± 4.5 | |
The Galago γ-Gene Promoter Down-Regulates Human γ-Gene Expression in Adult Erythropoiesis. To determine which sequences of the galago γ-promoter are responsible for the down-regulation of γ-gene expression we produced a construct carrying the human γ-gene driven by a galago γ-gene promoter truncated to –199g. The position of galago –199g γ corresponds to the human –201hγ.We have previously shown that the –201h γ transgenic mice display high levels of γ-globin gene expression in the embryonic and fetal stages of erythropoiesis and similarly high levels of γ-gene expression in the adult stage, i.e., they display a phenotype that mimics HPFH. Analysis of γ-mRNA levels and copy numbers of the transgene was performed in the three lines carrying intact copies of the μLCR (–199g)Aγ construct (Table 1). As shown in the table and Fig. 2 C and D high levels of γ-gene expression were observed in the embryonic cells of these transgenes, providing further evidence that the galago promoter is able to drive the human γ-gene to a high level of expression. The γ-level of mRNA in the adult blood in the three galago –199g γ lines was ≈1% of murine α mRNA per copy (Table 1), about 15 times lower than in the control –201h Aγ mice. These results suggest that the down-regulation of human γ-gene expression in the galago promoter/human γ transgenic mice is controlled by cis-regulatory elements located in the galago γ-gene promoter.
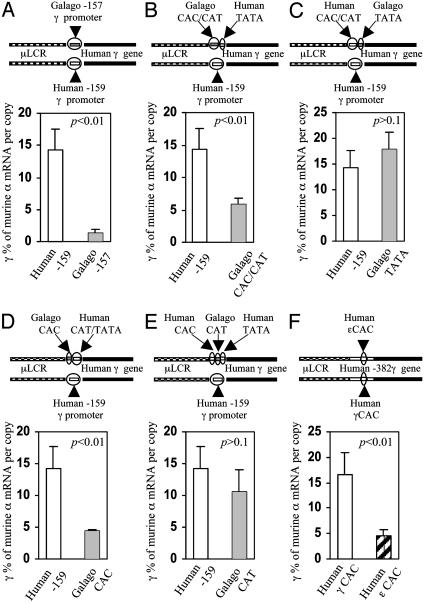
A Minimal Promoter of the Galago γ-Gene Is Sufficient to Down-Regulate γ-Globin Gene Expression in Adult Erythropoiesis. To further locate the elements responsible for down-regulation of γ-gene expression, we truncated the galago γ-promoter to position –157g and produced a construct μLCR(–157g)Aγ (Fig. 2E). This minimal galago γ-promoter contains three conventional motifs, CACCC, CCAAT, and TATA. Six transgenic mouse lines carrying intact copies of this construct were established. An analogous construct containing the minimal human γ-promoter [μLCR(–159h)Aγ] was produced to serve as control, and four transgenic mouse lines carrying this control construct were established. During embryonic erythropoiesis the γ-mRNA level in the human –159hγ mice (Fig. 2F) was similar to that in the μLCR(–382h) Aγ mice (26.6 ± 8.8% vs. 21.0 ± 7.4% in day 12 yolk sac, Fig. 2B), and the μLCR(–201h)Aγ mice (Table 1 and Fig. 2D), indicating that the minimal human γ-promoter is sufficient to drive the human γ-gene to high levels of expression. Similarly, as shown in Fig. 2E, in the embryonic stage, the galago –157g γ promoter is able to drive the human γ-gene to a level indistinguishable from that of the human –159h γ promoter (25.4 ± 4.2% and 26.6 ± 8.8% of murine α-mRNA per copy,). However, whereas in the human –159h γ mice γ-gene expression continues in the definitive erythropoiesis, γ-gene expression is progressively down-regulated in the definitive erythropoiesis of the galago –157g γ mice (Fig. 3A and Table 2). Thus, the amount of γ-mRNA in the day 12 fetal liver of the galago –157g γ mice was reduced to about one-half of that in the human –159h γ mice (8.3% vs. 14.0%). The largest decrease was observed in the adult blood: γ-gene expression in the galago –157g γ mice decreased to 1.4 ± 0.5% of murine α-globin mRNA, i.e., it was 10-fold lower compared to the human –159h γ mice. Because the truncated galago promoter actually maintains full promoter strength in the embryonic cells, we conclude that the minimal galago promoter is sufficient to down-regulate human γ-gene expression in the definitive erythropoiesis.
Fig. 3.
The galago minimal promoter silences expression of the γ-globin gene in adult erythropoiesis in transgenic mice. Effects of the galago γ-promoter or its promoter elements on humanγ-gene expression were assessed in the adult blood of transgenic mice carrying the corresponding construct. The line drawings at the top of each image show the constructs, and the differences between the test and control constructs are circled. Open and shaded bars with standard-deviation tabs show the expression levels of γ-gene expression in the controls and the experiments. The statistical significance (p) between experiment and control is indicated on the upper right corner of each histogram. (A) Comparison of effects of the human –159 minimal promoter and the galago –157 minimal promoter on expression of the γ-globin gene in adult erythropoiesis. (B) Comparison of the human CAC/CAT box with the galago CAC/CAT box. (C) Comparison of the human TATA box with the galago TATA box. (D) Comparison the human CAC box with the galago CAC box. (E) Comparison of the human CAT box with galago CAT box. (F) Comparison the human γCAC box with the human εCAC box.
Table 2. Expression of the human γ-gene in transgenic mice carrying a μLCRAγ construct with the human -159γ or the galago -157γ promoter.
| Human γ, % of murine α-mRNA per copy
|
|||||
|---|---|---|---|---|---|
| Embryonic erythropoiesis
|
Definitive erythropoiesis
|
||||
| Line | Copy no. | Day 12 blood | Day 12 yolk sac | Day 12 fetal liver | Adult blood |
| LCR(-157g)Aγ | |||||
| F | 7 | 16.0 ± 0.7 | 27.6 ± 2.5 | 7.8 ± 1.1 | 2.3 ± 0.6 |
| G | 15 | 1.4 ± 0.1 | |||
| H | 19 | 11.0 ± 0.6 | 25.0 ± 5.1 | 6.6 ± 0.4 | 1.3 ± 0.3 |
| I | 4 | 14.2 ± 6.1 | 19.5 ± 6.3 | 10.8 ± 4.2 | 1.6 ± 0.1 |
| J | 14 | 14.5 ± 2.2 | 30.8 ± 6.3 | 9.9 ± 0.5 | 1.3 ± 0.3 |
| K | 4 | 14.5 ± 0.2 | 23.9 ± 1.9 | 6.3 ± 0.1 | 0.7 ± 0.1 |
| Mean | 14.0 ± 1.8 | 25.4 ± 4.2 | 8.3 ± 2.0 | 1.4 ± 0.5 | |
| μLCR(-159h)Aγ | |||||
| L | 1 | 14.4 ± 0.7 | 36.9 ± 2.7 | 20.5 ± 0.2 | 13.4 ± 1.0 |
| M | 53 | 3.7 ± 0.1 | 16.5 ± 8.4 | 9.9 ± 1.1 | 17.7 ± 3.2 |
| N | 61 | 5.0 ± 0.9 | 23.1 ± 5.0 | 10.5 ± 0.9 | 10.1 ± 0.8 |
| O | 23 | 13.1 ± 2.2 | 30.0 ± 8.4 | 15.2 ± 3.4 | 16.1 ± 8.7 |
| Mean | 9.0 ± 5.5 | 26.6 ± 8.8 | 14.0 ± 4.9 | 14.3 ± 3.3 | |
See Table 1 legend and footnote.
The TATA Box and the Core γ-Gene Promoter Are Not Involved in γ-Gene Silencing in Adult Erythropoiesis. The minimal γ-promoter consists of a core promoter including the TATA box and a proximal promoter containing the CACCC and CCAAT boxes. Each conventional box is required for full level of γ-gene expression. To maintain full strength of the promoter in the γ-gene recombinant constructs, we produced a chimeric human/galago minimal promoter by interchanging the corresponding cis-elements between the human and the galago promoters. To study the role of the galago core promoter in developmental regulation, a NaeI/NcoI fragment containing the TATA box and the 5′ untranslated region of the human minimal γ-promoter was substituted by the corresponding galago fragment, resulting in the construct μLCR(–159h)Aγ(TATAg) (Fig. 3C). In five transgenic lines carrying this construct, the substitution of the human TATA box by the galago TATA box maintained high-level expression of the human γ-gene in embryonic erythropoiesis (Table 3). Notably, in the adult blood, γ-gene expression was also maintained at a level similar to that of the control –159h Aγ mice (17.8 ± 3.4% vs. 14.3 ± 3.3%) (Table 3 and Fig. 3C). We conclude that the galago core promoter (the TATA box and the 5′ nontranslated region) does not play a major role in γ-gene silencing.
Table 3. Human γ-globin gene expression in transgenic mice carrying the construct μLCR (-159h)Aγ (TATAR) or μLCR(-157g)Aγ(TATAh).
| Human γ, % of murine α-mRNA per copy
|
|||||
|---|---|---|---|---|---|
| Embryonic erythropoiesis
|
Definitive erythropoiesis
|
||||
| Line | Copy no. | Day 12 blood | Day 12 yolk sac | Day 12 fetal liver | Adult blood |
| μLCR(-159h)Aγ(TATAR) | |||||
| P | 45 | 21.2 ± 4.0 | |||
| Q | 11 | 22.0 ± 1.3 | 44.8 ± 12.6 | 31.6 ± 5.9 | 15.5 ± 2.1 |
| R | 8 | 21.1 ± 2.2 | |||
| S | 6 | 21.8 ± 4.8 | 34.9 ± 3.6 | 17.0 ± 1.1 | 13.4 ± 1.7 |
| T | 25 | 18.5 ± 4.7 | 50.9 ± 13.1 | 12.7 ± 1.4 | 18.0 ± 6.5 |
| Mean | 20.8 ± 2.0 | 43.5 ± 8.1 | 20.4 ± 9.9 | 17.8 ± 3.4 | |
| μLCR(-157g)Aγ (TATAh) | |||||
| U | 28 | 16.4 ± 2.8 | 32.3 ± 5.4 | 13.8 ± 0.5 | 5.7 ± 0.7 |
| V | 9 | 27.9 ± 3.9 | 37.0 ± 6.7 | 18.5 ± 5.1 | 6.9 ± 1.6 |
| W | 2 | 16.2 | 17.3 | 10.4 | 4.7 ± 2.0 |
| X | 12 | 24.0 ± 3.3 | 38.0 ± 3.3 | 16.9 ± 3.7 | 5.9 ± 0.7 |
| Mean | 21.1 ± 6.0 | 31.2 ± 9.6 | 14.9 ± 3.6 | 5.8 ± 0.9 | |
| μLCR(-159h)Aγ | |||||
| Mean | 9.0 ± 5.5 | 26.6 ± 8.8 | 14.0 ± 4.9 | 14.3 ± 3.3 | |
See Table 1 legend and footnote.
The CACCC and CCAAT Box Region of the Galago γ-Gene Promoter Is Mainly Responsible for γ-Gene Down-Regulation in Adult Erythropoiesis. To study the role of the proximal promoter in the developmental regulation of the γ-gene, the human NaeI/NcoI fragment containing the TATA box and 5′ untranslated region replaced the corresponding fragment in the –157g minimal galago promoter, forming μLCR(–157g)Aγ(TATAh) (Fig. 3B). Four transgenic lines carrying this construct were established (Table 3). The replacement of the galago TATA box by the human TATA box did not impair the γ-promoter strength in embryonic erythropoiesis as shown by the similar levels of γ-gene expression in the μLCR(–157g)Aγ(TATAh) mice and the control mice (31.2 ± 9.6% vs. 26.6 ± 8.8%, P > 0.05). In contrast, the galago proximal promoter (CAC/CAT) caused a significant degree of down-regulation of γ-gene expression in the adult (5.8 ± 0.9% vs. 14.3 ± 3.3%, P < 0.01) (Fig. 3B). These results suggest that the galago proximal γ promoter plays a major role in developmental down-regulation of γ-gene expression.
The CACCC Box Is the Dominant Element in Developmental Regulation of the γ-Globin Gene. The CACCC and CCAAT boxes of the proximal γ-gene promoter are essential for activation of the γ-globin gene; the CCAAT box region has also being implicated in γ-gene silencing (17, 18). To delineate the roles of the two boxes in developmental regulation we swapped the galago and human CACCC and CCAAT boxes. When the galago CACCC box replaced the human counterpart [μLCR(–159h)Aγ(CACg)], γ-gene expression in the adult blood was reduced 4-fold (i.e., 4.4 ± 0.3% of murine α-mRNA, whereas the level of γ-gene expression was 14.3% in the control –159h Aγ mice) (Fig. 3D and Table 4). This replacement did not impair the γ-promoter strength as demonstrated by high-level γ-gene expression in embryonic erythropoiesis (Table 4).
Table 4. Human γ-globin gene expression in transgenic mice carrying the construct μLCR(-159h)Aγ(CACg) or μLCR(-159h)Aγ(CATg).
| Human γ, % of murine α-mRNA per copy
|
|||||
|---|---|---|---|---|---|
| Embryonic erythropoiesis
|
Definitive erythropoiesis
|
||||
| Line | Copy no. | Day 12 blood | Day 12 yolk sac | Day 12 fetal liver | Adult blood |
| μLCR(-159h)Aγ(CACg) | |||||
| AA | 23 | 17.9 ± 3.1 | 55.9 ± 5.0 | 7.4 ± 0.6 | 4.6 ± 0.4 |
| BB | 15 | 30.2 ± 2.9 | 41.4 ± 7.5 | 6.8 ± 0.9 | 4.6 ± 0.7 |
| CC | 4 | 24.1 ± 3.5 | 32.4 ± 3.8 | 8.2 ± 2.0 | 4.0 ± 0.3 |
| DD | 13 | 20.7 ± 0.7 | 33.9 ± 2.2 | 9.2 ± 0.4 | 4.3 ± 1.3 |
| Mean | 23.2 ± 5.3 | 40.9 ± 10.7 | 7.9 ± 1.0 | 4.4 ± 0.3 | |
| μLCR(-159h)Aγ(CATg) | |||||
| EE | 6 | 28.0 ± 7.0 | 30.4 ± 5.2 | 26.8 ± 0.1 | 11.3 ± 1.4 |
| DD | 18 | 23.4 ± 2.8 | 68.5 ± 21.0 | 17.9 ± 1.7 | 13.5 ± 0.5 |
| FF | 4 | 16.9 ± 1.7 | 29.8 ± 2.1 | 12.3 ± 2.6 | 6.7 ± 0.9 |
| Mean | 22.8 ± 5.6 | 42.9 ± 22.2 | 19.0 ± 7.3 | 10.5 ± 3.5 | |
| μLCR(-159h)Aγ | |||||
| Mean | 9.0 ± 5.5 | 26.6 ± 8.8 | 14.0 ± 4.9 | 14.3 ± 3.3 | |
See Table 1 legend and footnote.
When the galago CCAAT box replaced the human CCAAT box in the [μLCR(–159h)Aγ] (CATg) mice, γ-gene expression was maintained at a high level in the embryonic stage and no statistically significant decline occurred in adult cells (Fig. 3E).
These results suggest that the CACCC box is the main determinant of silencing of galago γ-gene expression in definitive erythropoiesis.
The Human ε-Globin Gene CACCC Box Directs Down-Regulation of the Human γ-Globin Gene. Because the galago CACCC box is largely responsible for the down-regulation of the human γ-globin gene in adult erythropoiesis, we postulated that the CACCC box of the human ε-globin gene would also be able to down-regulate the human γ-globin gene. To test this hypothesis we replaced the 18-bp γ-gene CACCC box region by the corresponding human ε-gene CACCC region in the context of the μLCR(–382h)Aγ construct. In all four established transgenic mouse lines, expression of the human γ-globin gene in the adult mice was low (Table 5 and Fig. 3F). Replacement of the γCACCC by εCACCC reduced γ-gene expression by 85% (i.e., from 16.5 ± 4.5% to 4.6 ± 1.2%) in the adult blood, whereas it did not affect the expression in the day 12 yolk sac (21.0 ± 7.4% vs. 26.0 ± 4.9%). These results indicate that the ε-gene CACCC box is able to down-regulate γ-globin gene expression even in the context of an HPFH-like γ-gene promoter.
Table 5. Human γ-globin gene expression in transgenic mice carrying the construct μLCR (-382h)Aγ (εCACh).
| Human γ, % of murine α mRNA per copy
|
|||||
|---|---|---|---|---|---|
| Embryonic erythropoiesis
|
Definitive erythropoiesis
|
||||
| Line | Copy no. | Day 12 blood | Day 12 yolk sac | Day 12 fetal liver | Adult blood |
| μLCR(-382h)Aγ(εCACh) | |||||
| GG | 1 | 15.5 ± 3.3 | 26.1 ± 0.9 | 16.5 ± 3.7 | 4.4 ± 0.9 |
| HH | 3 | 6.4 ± 1.2 | |||
| II | 1 | 19.7 ± 1.4 | 21.0 ± 3.2 | 13.9 ± 4.5 | 4.0 ± 0.8 |
| JJ | 4 | 16.7 ± 2.5 | 30.8 ± 6.2 | 10.7 ± 1.3 | 3.7 ± 0.7 |
| Mean | 17.3 ± 2.2 | 26.0 ± 4.9 | 13.7 ± 2.9 | 4.6 ± 1.2 | |
| μLCR(-382h)Aγ | |||||
| Mean | 10.4 ± 9.5 | 21.0 ± 7.4 | 11.8 ± 2.8 | 16.5 ± 4.5 | |
See Table 1 legend and footnote.
Discussion
Earlier studies in transgenic mice suggest that the key elements for developmental regulation of the human γ-gene are located within the gene or the immediate vicinity, in particular, in the promoter (2–5). In this report we demonstrated that the minimal promoter, in particular, the CACCC box is mostly responsible for developmental regulation of the galago γ-gene. We also showed that the CACCC box of the human ε-globin gene suppresses γ-gene expression in adult erythropoiesis. Together, these observations suggest that the CACCC box is involved in the developmental regulation of γ-gene expression in adult erythropoiesis.
The CACCC box is a common element in the proximal promoters of many housekeeping and lineage-specific genes. All mutations or deletions of this box impair expression of the affected genes, suggesting that the CACCC box functions as a transcriptionally positive element. Studies of Kruppel-like factors have shown that CACCC box-binding proteins do not always play such a role (19, 20). A prominent example is KLF3/BKLF, a member of the KLF family, which behaves primarily as a strong transcriptional repressor; suppression is achieved by recruiting a general corepressor, CtBP2 (19). It is still unknown what factor binds to the CACCC box of the human γ-globin gene in vivo. In the CACCC region 4-bp differences exist between the human and galago sequences; two of them are located 5′ to the CACCC core and another two are 3′ to the core. It is unknown whether the differences could result in differential binding of the KLF family members. Studies in transgenic mice confirmed that the γCACCC box is essential for γ-gene activation in vivo in the adult stage and the CACCC box might mediate the γ-gene reactivation induced by the HPFH –175 mutation (21). In this study we provide direct in vivo evidence that the galago γ- and human εCACCC boxes play a suppressive role in γ-gene expression in adult erythropoiesis. Because the human γ-gene, like the galago γ-gene, is silenced in the normal condition, we speculate that the human CACCC box also functions as a negative element in vivo, in addition to its transcriptionally positive function. This dual role of the CACCC box could reconcile all the findings related to γ-globin gene regulation: In adult erythropoiesis the γ-globin gene is silenced by suppressive chromatin, which is initiated by a repressor complex recruited by the γCACCC and other elements in the γ-gene promoter. In certain conditions, for instance, in HPFH patients, the repressor complex is suppressed, resulting in reestablishment of interaction between the LCR and the promoter, leading to γ-gene reactivation. In this model, the CACCC box is required both in recruitment of the repressor complex and in the LCR/promoter interaction. Such a dual role of the γCACCC box can explain why no naturally occurring CACCC mutation has been found despite intensive screening.
The γCCAAT box may also play a dual role in developmental regulation of the γ-globin gene. A positive role of the γCCAAT box has been demonstrated by various in vitro studies and confirmed by in vivo experiments in transgenic mice (22). A negative role of γCCAAT box is suggested in the –117 HPFH mutation (23), which resides next to the CCAAT box. The observation that the –117 HPFH mutation in the γ-promoter reduced GATA-1 binding suggested that GATA-1 might play a role as a repressor, but subsequent studies did not support this model (24). We have shown that the DR-1 sequence of the εCCAAT box region is involved in the silencing of the ε-globin gene in transgenic mice (17), and that this DR-1 site was able to bind with an orphan receptor, COUP-TFII (17). Recently, Tanabe et al. (18) provided evidence that a repressor complex, designated as DRED, which contains a nuclear receptor TR2/TR4 heterodimer, binds to the DR-1 site (18). The DR-1 binding site is also found in the γ-promoter, and overlaps with the γCCAAT box (17, 18). The role of the DR-1 element in γ-gene silencing remains to be defined.
In contrast to the CACCC and CCAAT boxes the galago TATA box does not appear to participate in the silencing of the γ-gene in adult erythropoiesis: in the context of the human minimal γ-gene promoter, the galago TATA box did not reduce γ-gene expression in the adult stage (Table 3). Conversely, the human TATA box impairs the silencing ability of the galago γ-promoter; thus, in the context of galago γ-gene, replacement of the galago TATA box by its human counterpart resulted in a 5-fold elevation of γ-gene expression in adult erythrocytes. We have previously reported that the human TATA box is essential for γ-gene expression in adult erythropoiesis, whereas it is not required for γ-gene expression in embryonic erythropoiesis (25). This element, therefore, is required for γ-gene expression in the fetal stage of definitive erythropoiesis when the bulk of fetal hemoglobin synthesis in human and other primates takes place. The continuation of γ-gene expression in the fetal stage of erythropoiesis, the so-called “fetal recruitment” of the γ-gene, presumably took place during the evolution of simian primates 35 to 50 million years ago. The difference in the behavior of the recombinant galago γ-genes carrying either the human or the galago TATA boxes we reported here raise the possibility that interactions of coregulators with this element may have contributed to the fetal recruitment of γ-gene expression.
Acknowledgments
We thank Dr. Morris Goodman for generous gifts of λ-phage containing the galago γ-globin gene, Miaohua Zhang for technical assistance, and Dr. Grainne Barkess for critical reading the manuscript. This work was supported by National Institutes of Health Grants DK45365, HL20899, and DK61805.
Abbreviations: LCR, locus control region; HPFH, hereditary persistence of fetal hemoglobin.
References
- 1.Stamatoyannopoulos, G. & Grosveld, F. (2000) in Molecular Basis of Blood Diseases, eds. Stamatoyannopoulos, G., Majerus, P. W., Perlmutter, R. M. & Varmus, H. (Saunders, Philadelphia), pp. 135–182.
- 2.Townes, T. M., Lingrel, J. B., Chen, H. Y., Brinster, R. L. & Palmiter, R. D. (1985) EMBO J. 4, 1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magram, J., Chada, K. & Costantini, F. (1985) Nature 315, 338–340. [DOI] [PubMed] [Google Scholar]
- 4.Chada, K., Magram, J. & Costantini, F. (1986) Nature 319, 685–689. [DOI] [PubMed] [Google Scholar]
- 5.Kollias, G., Wrighton, N., Hurst, J. & Grosveld, F. (1986) Cell 46, 89–94. [DOI] [PubMed] [Google Scholar]
- 6.Grosveld, F., van Assendelft, G. B., Greaves, D. R. & Kollias, G. (1987) Cell 51, 975–985. [DOI] [PubMed] [Google Scholar]
- 7.Enver, T., Raich, N., Ebens, A. J., Papayannopoulou, T., Costantini, F. & Stamatoyannopoulos, G. (1990) Nature 344, 309–313. [DOI] [PubMed] [Google Scholar]
- 8.Behringer, R. R., Ryan, T. M., Palmiter, R. D., Brinster, R. L. & Townes, T. M. (1990) Genes Dev. 4, 380–389. [DOI] [PubMed] [Google Scholar]
- 9.Dillon, N. & Grosveld, F. (1991) Nature 350, 252–254. [DOI] [PubMed] [Google Scholar]
- 10.Peterson, K. R., Li, Q. L., Clegg, C. H., Furukawa, T., Navas, P. A., Norton, E. J., Kimbrough, T. G. & Stamatoyannopoulos, G. (1995) Proc. Natl. Acad. Sci. USA 92, 5655–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enver, T., Ebens, A. J., Forrester, W. C. & Stamatoyannopoulos, G. (1989) Proc. Natl. Acad. Sci. USA 86, 7033–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagle, D. A., Koop, B. F., Goodman, M., Slightom, J. L., Hess, D. L. & Jones, R. T. (1988) J. Mol. Biol. 203, 439–455. [DOI] [PubMed] [Google Scholar]
- 13.TomHon, C., Zhu, W., Millinoff, D., Hayasaka, K., Slightom, J. L., Goodman, M. & Gumucio, D. L. (1997) J. Biol. Chem. 272, 14062–14066. [DOI] [PubMed] [Google Scholar]
- 14.Yu, T., Thomas, D. M., Zhu, W., Goodman, M. & Gumucio, D. L. (2002) Blood 99, 1082–1084. [DOI] [PubMed] [Google Scholar]
- 15.Stamatoyannopoulos, G., Josephson, B., Zhang, J. W. & Li, Q. (1993) Mol. Cell. Biol. 13, 7636–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Q. & Stamatoyannopoulos, J. A. (1994) Mol. Cell. Biol. 14, 6087–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipe, A., Li, Q., Deveaux, S., Godin, I., Romeo, P. H., Stamatoyannopoulos, G. & Mignotte, V. (1999) EMBO J. 18, 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanabe, O., Katsuoka, F., Campbell, A. D., Song, W., Yamamoto, M., Tanimoto, K. & Engel, J. D. (2002) EMBO J. 21, 3434–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieker, J. J. (2001) J. Biol. Chem. 276, 34355–34358. [DOI] [PubMed] [Google Scholar]
- 20.Philipsen, S. & Suske, G. (1999) Nucleic Acids Res. 27, 2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Q., Duan, Z. J. & Stamatoyannopoulos, G. (2001) EMBO J. 20, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan, Z., Stamatoyannopoulos, G. & Li, Q. (2001) Mol. Cell. Biol. 21, 3083–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry, M., Grosveld, F. & Dillon, N. (1992) Nature 358, 499–502. [DOI] [PubMed] [Google Scholar]
- 24.Ronchi, A., Berry, M., Raguz, S., Imam, A., Yannoutsos, N., Ottolenghi, S., Grosveld, F. & Dillon, N. (1996) EMBO J. 15, 143–149. [PMC free article] [PubMed] [Google Scholar]
- 25.Duan, Z. J., Fang, X., Rohde, A., Han, H., Stamatoyannopoulos, G. & Li, Q. (2002) Proc. Natl. Acad. Sci. USA 99, 5509–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]