Abstract
Epigenetics refers to heritable changes in patterns of gene expression that occur without alterations in DNA sequence. The epigenetic mechanisms involve covalent modifications of DNA and histones, which affect transcriptional activity of chromatin. Since chromatin states can be propagated through mitotic and meiotic divisions, epigenetic mechanisms are thought to provide heritable ‘cellular memory’. Here, we review selected examples of epigenetic memory in plants and briefly discuss underlying mechanisms.
Keywords: epialleles, epigenetics, memory, plants, transposons
Introducing epigenetics
The term ‘epigenetics’ combines two words ‘epigenesis’ and ‘genetics’ and was coined by Conrad H. Waddington in 1942. He defined epigenetics as “the branch of biology that studies the causal interaction between genes and their products, which brings the phenotype into being” (Waddington, 1942) and proposed the concept of the epigenetic landscape as a metaphor for cell differentiation (Waddington, 1957). At various points during the progression toward their final differentiated states, changes occur in cells according to genetic and/or environmental factors. For this process to occur, altered features of the cells must be memorized after each cell division. Epigenetics has since been redefined several times. Nowadays, it is commonly taken to mean the study of mitotically and/or meiotically heritable changes in patterns of gene expression that occur without alterations in DNA sequence. Current epigenetic studies are often focused on chemical modifications of chromatin and their roles in active transcription and transcriptional silencing. Chemical modifications of chromatin alter both DNA and histone proteins.
DNA methylation is a covalent modification of DNA, and although it is found across many genera, its crucial role in epigenetic regulation of transcription is best documented in plants and mammals. DNA hydroxymethylation is another DNA modification recently discovered in mammals. It is possible that this modification represents an intermediate of DNA demethylation, but it may also contribute to epigenetic regulation. Histone proteins are subjected to various covalent modifications, including acetylation, methylation, phosphorylation, ubiquitination, and sumoylation. In addition, incorporation of histone variants and relocation of nucleosomes can also affect chromatin structure and its function in transcriptional regulation.
Non-coding RNAs, including small RNAs, frequently influence the distribution patterns of epigenetic marks and can thus act in a sequence-specific manner to regulate gene expression at both transcriptional and post-transcriptional levels. In plants, certain small RNAs direct DNA methylation at their homologous regions in a process known as RNA-directed DNA methylation (RdDM).
It is well documented that the interplay of epigenetic marks determines particular chromatin states essential to the regulation of various biological processes. In plants, years of work aiming to understand the molecular mechanisms underlying paramutation, gene imprinting, suppression of transposons, and silencing of transgenic loci led to the discovery of epigenetic regulation that contribute to heritability: memorization as well as mitotic and meiotic transmission of particular transcriptional states.
In the first part of this review, we will briefly discuss examples of ‘epigenetic memory’ in the regulation of plant development—modifications that are reset at each generation allowing progeny to recapitulate developmental steps of their parents. In the second part, we provide selected examples of epigenetic contributions to transgenerational inheritance in plants, as well as illustrative examples of stable epialleles found in nature or induced experimentally. Finally, we address the somewhat controversial topic of environmentally induced transgenerational changes in epigenetic memory.
Mitotically heritable epigenetic memory—resetting marks between generations
Imprinting—memory of parental origin
Genomic imprinting is a phenomenon that leads to differential allelic expression depending on whether a gene was inherited through a female or male gamete. Genomic imprinting is well documented for seed plants and for mammals but is thought to have evolved independently (Feil & Berger, 2007). In both groups of organisms, imprinting occurs in embryo-nourishing tissues: the endosperm in plants and the placenta in mammals (Kohler & Weinhofer-Molisch, 2010).
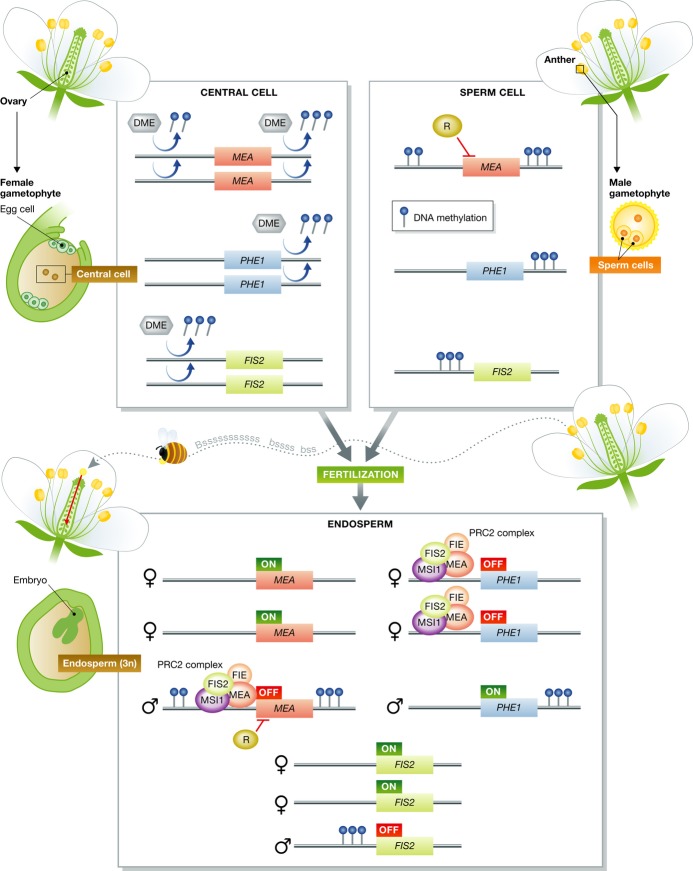
Double fertilization in flowering plants is a specific process involving multicellular male and female gametophytes, pollen grain and embryo sac, respectively. The pollen grain contains two sperm cells. One sperm cell fuses with an egg cell and a second fuses with the bi-nucleated central cell of the embryo sac, leading to development of the embryo and the triploid endosperm, respectively (Fig1). The endosperm, thought to be functionally analogous to the placenta in mammals, supports and nourishes the embryo during seed development and/or seed germination (Ingram, 2010).
Figure 1. Schematic illustration of parental imprinting.
In females, the central cell DME removes DNA methylation from maternally expressed genes, MEA and FIS2, and from the paternally expressed gene PHE1. DNA methylation at these loci is maintained in the male gametophyte. During fertilization, the central cell fuses with one sperm cell to form the endosperm. In endosperm, maternal alleles of MEA and FIS2 are expressed. The PRC2 complex including MEA and FIS2 binds to the promoter of the paternal allele of MEA and mediates silencing by catalyzing H3K27 tri-methylation. Another unknown repressor (R) may be required for repression of the paternal allele of MEA. The PRC2 complex mediates silencing of the maternal allele of PHE. In addition to the PRC2 complex, maternal removal of DNA methylation downstream of PHE gene is required for silencing of its maternal allele.
During gametogenesis, imprinted gene alleles are epigenetically silenced either maternally or paternally. The epigenetic memory of parental origin persists beyond fertilization and results in differential transcriptional activity of maternal and paternal alleles in the developing endosperm. Since the endosperm is a terminal tissue, imprinting features of specific genes cannot be transmitted to the next generation and are thus not reset.
In plants, two epigenetic marks of DNA methylation and histone methylation are involved in the regulation of imprinting. DNA demethylase DEMETER (DME), which has DNA glycosylase activity directed toward methylated cytosines, is present in the central cell and removes methylated cytosines from maternally expressed genes (MEGs) such as MEA, FIS2, and FWA, leading to transcriptional activation of their maternal alleles (Choi et al, 2002, 2004; Gehring et al, 2006; Morales-Ruiz et al, 2006). DNA methyltransferase MET1 also regulates maternally imprinted genes. In somatic tissues, DNA methylation is maintained by MET1; however, expression of MET1 is suppressed in the central cell during female gametogenesis, and this seems to contribute to DNA hypomethylation of MEGs (Jullien et al, 2006b, 2008).
Further factors regulating imprinting include the evolutionally conserved polycomb group proteins. Arabidopsis polycomb complex PRC2, consisting of MEA, FIE, FIS2, and MSI1, catalyzes H3K27 tri-methylation, and this repressive histone mark leads to the suppression of paternal alleles of MEGs or the maternal alleles of paternally expressed genes (PEGs) (Kohler et al, 2005; Baroux et al, 2006; Jullien et al, 2006a; Makarevich et al, 2006).
A certain subset of imprinted genes undergoes dual regulation by PRC2 and DME. For example, silencing of a maternal allele of PHE1 (PEG) involves hypomethylation of repeats located in the 3′ region of PHE1 as well as binding of PRC2 to the gene promoter (Makarevich et al, 2008). Recent genome-wide analysis has revealed antagonistic distributions of DNA methylation and H3K27 tri-methylation, and it was suggested that DNA methylation prevents PRC binding while its removal allows PRC2 to bind histones and catalyze H3K27 tri-methylation (Weinhofer et al, 2010).
In maize, certain imprinted genes such as MEE1 and FIE2 are differentially methylated in endosperm but not in gametes, illustrating that differential methylation patterns are established after fertilization (Gutierrez-Marcos et al, 2006; Jahnke & Scholten, 2009). In Arabidopsis, it was shown that the regulation of imprinted MEA expression by DME and MET1 may also occur indirectly (Wohrmann et al, 2012). These results suggest the existence of additional epigenetic signals besides methylation that contribute to establishing imprinting marks. The mechanisms involved in imprinting are summarized in Fig1.
Although imprinting is widely conserved among plant species, its biological significance is not clear. One hypothesis explaining its origin is that imprinting is a by-product of transposon (TEs) silencing. Indeed, in Arabidopsis, the majority of imprinted genes harbor TEs or repeated sequences in their flanking regions (Wolff et al, 2011). In the endosperm, the activity of DME combined with the absence of MET1 results in hypomethylation of TEs and biogenesis of TEs-derived small RNAs (Mosher et al, 2009). These small RNAs may relocate to the embryo reinforcing TE silencing there (Hsieh et al, 2009; Bauer & Fischer, 2011). Transcriptionally active TEs in the endosperm may also affect expression of neighboring genes. Therefore, imprinting observed in the endosperm could be linked to activation of TEs (Gehring et al, 2009; Hsieh et al, 2009; Zemach et al, 2010).
A hypothesis explaining the evolutionary maintenance of imprinting is that of parent conflict, which proposes that genomic imprinting evolved via competition between parents in the allocation of resources to their progeny. Several male individuals can contribute to the offspring of one female, and maximizing flow of resources to their own offspring is of paternal interest. In contrast, maternal resources are distributed equally to offspring. Therefore, PEGs would stimulate growth and thus increase seed size, whereas MEGs would limit growth (Haig & Westoby, 1989; Wilkins & Haig, 2003; Kohler & Weinhofer-Molisch, 2010). Indeed, several imprinted genes are found to be involved in endosperm development and in the control of seed size in Arabidopsis (Grossniklaus et al, 1998; Kiyosue et al, 1999; Kohler et al 2003), and nutrient uptake and allocation in maize (Costa et al, 2012; Xin et al, 2013).
Further evidence supporting the parent conflict theory is the observation that a 2:1 maternal to paternal genome ratio in the endosperm is required for proper seed development and that imbalanced parental genome dosage alters seed size. In Arabidopsis, increasing paternal genome dosage in the endosperm by pollination of a diploid plant with pollen derived from a tetraploid (2m: 2p) results in larger seeds. In contrast, increasing the maternal genome dosage by pollination of a tetraploid plant with haploid pollen (4m:1p) results in smaller seeds (Scott et al, 1998; Tiwari et al, 2010). A recent study showed that most small RNAs found in the developing endosperm are expressed from the maternal genome (Mosher et al, 2009), and levels of these siRNAs are responsive to parental genome dosage. It has also been suggested that maternal siRNAs mediate parental genome balance and gene expression during endosperm development (Lu et al, 2012).
Vernalization—memory of winter
Unlike the development of animals, in which most organs are formed during embryogenesis, the organogenesis in plants continues throughout the entire life span. There are mechanisms in plants that adjust form and flexibility in developmental timing according to the ambient environment. In particular, environmental control of the timing of developmental changes often requires a certain delay between the environmental trigger and the initiation of a differentiation process. Consequently, a prolonged memory of the trigger is needed. One such well-studied developmental process is the vernalization response, in which cold exposure of winter annual plants synchronizes flowering to the optimal season. Vernalized plants thus appear to propagate a ‘memory of winter’ during most of their vegetative development (Chouard, 1960).
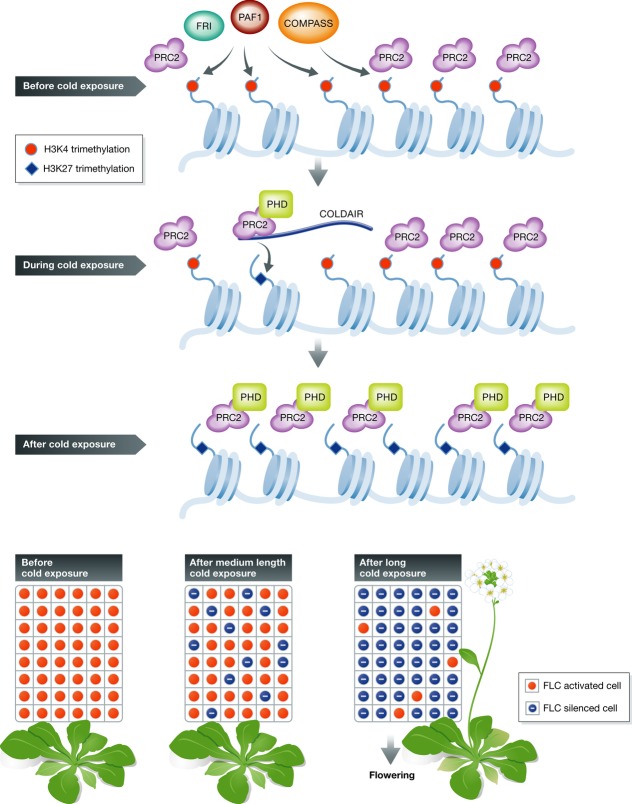
Molecular mechanisms of vernalization have mainly been studied in Arabidopsis where the flowering suppressor, FLC, plays a central role. FLC encodes a MADS box transcription factor that inhibits flowering in a dose-dependent manner (Michaels & Amasino, 1999; Sheldon et al, 1999). FLC is expressed throughout the early vegetative development of vernalization-sensitive Arabidopsis strains, prior to the exposure to prolonged cold. After a certain cold period, FLC is silenced and flowering can be initiated according to environmental cues characteristic of a particular season (temperature, day length, etc.). Remarkably, the chromatin properties of the FLC gene are modified dynamically depending on the environmental phases of plant growth to reflect states before cold exposure, during cold exposure, and after cold exposure (Michaels & Amasino, 2000; Kim et al, 2009).
Before cold exposure
The expression of FLC is reset at every generation. This means that the memory of parental vernalization is erased prior to vernalization of the progeny thus allowing de novo adjustment of the flowering time. FLC resetting is associated with its transcriptional reactivation during embryogenesis (Sheldon et al, 2008; Choi et al, 2009), and several factors are involved in FLC activation. First, the FRI complex acts as an activator of FLC by binding to the FLC promoter and contributing to induction of FLC transcription (Johanson et al, 2000). In addition, the PAF1 complex associates with RNA polymerase II and influences transcription elongation (Oh et al, 2004). EFS, a component of PAF1 complex, recruits FRI at the FLC locus, and both ERF and FRI are required for H3K4 tri-methylation and H3K36 dimethylation (Zhao et al, 2005; Xu & Shen, 2008; Ko et al, 2010). The COMPASS-like complex, including the Trithorax family proteins ATX1 and ATXR7, mediates H3K4 tri-methylation (Saleh et al, 2008; Tamada et al, 2009). PAF1 may coordinate these activities by recruiting COMPASS (Krogan et al, 2003) as such tight cooperation of similar complexes has been shown in yeast. The SWR1 complex, which is involved in H2A.Z deposition, is also required for full activation of FLC expression (Choi et al, 2007).
During cold exposure
Transcription of FLC is gradually silenced during prolonged cold treatment, and this is associated with PRC2-mediated H3K27 tri-methylation (Bastow et al, 2004). The PRC2 complex regulating FLC expression consists of VRN2, SWN, FIE, and MSI1 and thus differs from the imprinting complex described in the previous section (De Lucia et al, 2008). Although the core PRC2 associates with the FLC locus before cold exposure, PRC2 associates with plant homeodomain (PHD) proteins only during prolonged low ambient temperatures. This gives rise to the PRC2-PHD complex, which targets a specific nucleation region of the FLC locus, resulting in increased H3K27 tri-methylation (Sung & Amasino, 2004; Sung et al, 2006b; Greb et al, 2007; De Lucia et al, 2008).
Two long non-coding RNAs, COLDAIR and COOLAIR, also seem to be involved in vernalization. COLDAIR, transcribed from the first intron of FLC, accumulates during cold treatment and interacts physically with PRC2 (Heo & Sung, 2011). This suggests that COLDAIR acts as a scaffold to target PRC2 to the FLC locus, similar to the involvement of HOTAIR in PRC2-mediated silencing in humans (Zhao et al, 2008). COOLAIR, also induced in the cold period, is an antisense non-coding RNA relative to the FLC transcript that seems to enhance silencing of FLC (Swiezewski et al, 2009). Noticeably, regulation of the FLC locus is an important example of regulation of chromatin by long non-coding RNA.
After cold exposure
When warm temperatures return, FLC remains silent and this state is mitotically inherited due to the presence of PRC2-PHD over the entire region of FLC (De Lucia et al, 2008). As a result, H3K27 tri-methylation spreads to the whole region of FLC and this epigenetic silencing mark is stable during the rest of the plant's life cycle (Finnegan & Dennis, 2007; Angel et al, 2011). The stability of vernalization also depends on other factors, including VRN1 and LHP1; the latter is a homolog of HP1 in animals (Levy et al, 2002; Mylne et al, 2006; Sung et al, 2006a).
Importantly, the duration of the cold period is critical to the final stability of FLC silencing. Just how the duration of the cold period is registered in plants remains an open, fascinating question. VIN3, one of the PHD proteins associated with PRC2, may play a role. The expression of VIN3 is stimulated by cold, and this increase in transcript levels may be correlated with the duration of the cold treatment, apparently antiparallel to the decrease in FLC transcripts (Sung & Amasino, 2004; Greb et al, 2007). Thus, the increasing abundance of VIN3-PRC2 may act as a molecular measure of the cold period. However, the accumulation of VIN3 transcripts is only transient, diminishing rapidly after the cold period. This suggests that the initial memory of cold duration, possibly triggered only by VIN3, is converted to a more stable state by other mechanisms.
Notably, studies of vernalization at the level of single cells combining ChIP, FLC reporter gene, and mathematical modeling revealed that each cell can be switched autonomously between ‘active’ and ‘silenced’ states (Angel et al, 2011). At the end of the cold period, the accumulation of H3K27 tri-methylation at the nucleation region of FLC in a subset of random cells switches them into a stable silenced state. Importantly, the probability for a given cold-exposed cell to switch to a silenced state increases with the duration of the cold period. Therefore, the quantitative nature of vernalization is determined by a subpopulation of cells in which FLC is stably silenced (Angel et al, 2011; Song et al, 2012). An overview of FLC regulation is presented in Fig2.
Figure 2. FLC regulation.
FRI, PAF1, and COMPASS-like complexes are involved in activation/reset of FLC at every generation. During cold exposure, the PRC2-PHD complex and non-coding RNA COLDAIR are recruited at the nucleation region of FLC and catalyze H3K27 tri-methylation. After return to higher temperatures, PRC2-PHD associates across the entire region of FLC leading to cell-autonomous stable transcriptional silencing. After prolonged cold exposure, the number of cells in which FLC is stably silenced increases.
Acclimation—abiotic stress memory
Mechanisms of transcriptional epigenetic regulation are known to be involved in plant stress responses. For example, when rice seedlings are submerged, the levels of H3K4 methylation and H3 acetylation increase on the submergence-inducible genes ADH1 and PDC1 (Tsuji et al, 2006). In Arabidopsis, drought stress changes histone modifications at the drought stress-inducible loci RD29A, RD29B, RD20, and At2g20880 (Kim et al, 2008). The expression levels of HDA6 and HDA19, members of the histone deacetylase family (HDACs), increase during environmental stresses such as low temperature, wounding, or hormonal signals, suggesting that these HDACs regulate stress-associated target genes (Zhou et al, 2005).
Small RNAs also seem to play an important role in stress responses. For example, salt stress in Arabidopsis induces the production of siRNAs from overlapping gene pairs of P5CDH and SRO5 that in turn influence salt stress tolerance (Borsani et al, 2005).
There are several examples of stress affecting DNA methylation. In maize, cold stress induces hypomethylation of ZmMI1 in roots (Steward et al, 2002). White clover and industrial hemp treated with heavy metals display hypomethylation of specific loci in their roots (Aina et al, 2004). The biological significance of these changes in methylation is not clear, though, and since reduced levels of DNA methylation are only found in roots, they cannot be passed to the next generation.
In addition to the implication of epigenetic regulation in immediate stress responses, such mechanisms have also been suggested to be involved in long-term stress adaptation. This can be illustrated by the exposure of plants to long-term cold (2°C for 3 days), a treatment that increases future freezing tolerance. Such plant hardening has been defined as cold acclimation. Cold-treated Arabidopsis hda6 mutants are not only less tolerant to freezing than cold-treated wild-type plants but also resist cold acclimation, which suggests the involvement of HDA6-mediated chromatin modifications in the acclimation process (To et al, 2011).
Memory of pathogen attack—systemic acquired resistance
The first exposure of a plant to a pathogen can induce long-lasting, systemic immunity against subsequent pathogen attacks; this is now known as systemic acquired resistance (SAR) (Vlot et al, 2008). SAR involves the plant hormone salicylic acid (SA) (Loake & Grant, 2007) and the downstream signaling protein NPR1 (Durrant & Dong, 2004), which are both essential for SAR. During SAR, the transcription of SA-responsive genes is activated, including genes encoding antimicrobial pathogenesis-related proteins (PR) (Ryals et al, 1996). Elevated levels of SA induce changes in chromatin modification at these target genes. For example, the levels of H3 acetylation, H4 acetylation, and H3K4 methylation are increased at the PR-1 promoter (Butterbrodt et al, 2006). It is still not clear to which extent these modifications contribute to the stability of SAR in terms of enhanced memory of the initial pathogen attack. However, it has been suggested that histone modification and/or histone replacement by histone variants may prime pathogen responsive genes for rapid activation during subsequent pathogen attacks.
WRKY genes encode transcription factors that are also induced by pathogen infection or SA treatment (Asai et al, 2002; Dong et al, 2003). It has been shown that local pathogen infections induce changes in histone modifications at promoters of several WRKY genes and that this also occurs in leaves distant from the infection sites. Interestingly, although the levels of active histone marks such as H3 acetylation and H3K4 methylation increase, the genes remain silent. It has been postulated that these modifications are primed for amplified transcriptional responses during subsequent pathogen attacks, thus implicating histone modifications in possible mechanisms of memory in SAR (Jaskiewicz et al, 2011).
A further epigenetic mechanism that may contribute to memory in SAR involves histone variant H2A.Z. As one of the most conserved eukaryotic histone variants, H2A.Z is enriched at the transcription start sites of genes, and it has been suggested that its incorporation contributes to gene activation, transcriptional memory, heterochromatic silencing, and thermal sensing (Dhillon et al, 2006; Brickner et al, 2007; Zlatanova & Thakar, 2008; Kumar & Wigge, 2010; Light et al, 2010). In Arabidopsis mutants deficient in the SWR1 complex, which is required for H2A.Z deposition, a large number of genes induced in SAR are constitutively expressed (March-Diaz et al, 2008). Since deposition of H2A.Z is associated with transcriptional memory and rapid reactivation of genes, H2A.Z may be important for priming genes induced in SAR.
Meiotically heritable epigenetic memory—the formation of epialleles
In this section, we will consider examples where certain loci are converted to alternative and relatively stable epigenetic states that are transmitted between generations in the form of heritable epialleles. We also discuss epigenetic mechanisms possibly involved in epiallelic switching—using examples of experimentally induced epialleles—and address the question of environmentally triggered deposition of transgenerational epigenetic memory.
Experimentally induced epialleles
In plants, DNA methylation is an epigenetic mark for which meiotic inheritance has been clearly demonstrated. DNA methylation is restricted to cytosines and is found in plants in multiple sequence contents: CG, CHG, and CHH (H stands for A, C, or T), in contrast to mammals where DNA is found almost exclusively on CG sequences. Mechanisms maintaining CG methylation through the DNA replication cycle are well characterized in plants and mammals and involve similar DNA methyltransferases, MET1 and DNMT1, respectively. During replication, these enzymes recognize hemi-methylated DNA and add methylation to cytosines of the newly synthesized strand using the old, methylated strand as a guide. Consequently, CG methylation patterns are faithfully maintained throughout mitotic or meiotic cell divisions. However, if CG methylation patterns are altered, the aberrant methylation will also be propagated (Saze et al, 2003; Mathieu et al, 2007; Law & Jacobsen, 2010).
Non-CG methylation, a characteristic of plants, is maintained by the redundant activities of DNA methyltransferases CMT3 and DRM2, and other associated activities. CMT3, a plant-specific chromomethylase, catalyzes non-CG methylation in cooperation with histone modifications, especially H3K9 methylation. DRM2 is guided by siRNAs in a process of RdDM. In addition, the chromatin remodeling protein DDM1 is required as evidenced by ddm1 mutants where the levels of DNA methylation in all sequence contexts are decreased (Law & Jacobsen, 2010).
The maintenance of proper CG methylation patterns is important for plant development and is thus most faithfully inherited. met1 and ddm1 mutants have decreased levels of CG methylation and show severe developmental phenotypes, while mutants defective in non-CG methylation have only minor developmental alterations. Certain phenotypes in met1 or ddm1 mutants can be explained by the loss of DNA methylation at particular genes, a process that results in the generation of hypomethylated epiallelic variants. For example, the FWA gene that acts as a flowering repressor is normally transcriptionally silenced in the sporophyte by CG methylation of its promoter. In met1 or ddm1 mutants, CG methylation is lost and transcriptional activation of FWA results in a late flowering phenotype (Soppe et al, 2000). Interestingly, the hypomethylated state of FWA is stably maintained, and its normal methylation status cannot be regained even after MET1 or DDM1 are provided in backcrosses (Kankel et al, 2003). This can be explained by the loss of the methylation template in the promoter of the FWA gene.
Using these properties of MET1 and DDM1, two populations of epigenetic recombinant inbred lines (epiRILs) were constructed (Johannes et al, 2009; Reinders et al, 2009; Teixeira et al, 2009). Both epiRIL populations were initiated from F1 hybrids between isogenic wild type and met1 or ddm1 mutants. Genetically identical parents were highly divergent epigenetically due to the methylation deficiencies of the mutants. Individuals homozygous for wild-type allele (MET1 or DDM1) were selected in the F2 generation, and these plants were inbred for 7–8 generations by single-seed descent (where the ddm1-derived F1 hybrid was backcrossed to wild type before inbreeding). DNA methylation analyses performed after inbreeding demonstrated that hypomethylation of distinct chromosomal segments derived from the mutant backgrounds was stably inherited over many generations in the presence of MET1 or DDM1. However, re-methylated regions derived from the mutant backgrounds were also found in both epiRIL populations. These regions were associated with siRNAs, suggesting that re-methylation occurs through an RdDM pathway (Teixeira et al, 2009). Interestingly, various novel phenotypic traits were observed during the inbreeding process. Certain traits such as delayed flowering were stably inherited, but most traits were unstable, probably due to dynamic methylation changes during inbreeding. It remains unknown what properties determine the stability of DNA methylation at some loci but not others. This is an important question that needs clarification to allow the prediction of genes that can be epigenetically altered in a stable, heritable fashion and those that would rapidly return to their original epigenetic state.
Natural epialleles
Besides experimentally induced epialleles, there are several examples of naturally occurring stable epialleles. In toadflax (Linaria vulgaris), different flower shapes are found ranging from bilaterally symmetrical to radial forms. This phenotypic variability is caused by variable levels of methylation of the promoter of the CYCLOIDEA gene (Cubas et al, 1999).
The tomato Colorless non-ripening (Cnr) variant displays bright, immature patches on its fruits due to spontaneous hypermethylation at the CNR locus (Manning et al, 2006). In melon, DNA methylation spreading from a transposon induces transcriptional silencing of the CmWIP1 gene that controls sex determination and thus varying proportions of male and female flowers (Martin et al, 2009). A recent example of a natural epiallele was revealed by studies of genetic incompatibility between Arabidopsis accessions. The incompatibility was due to epigenetic characteristics of duplicated AtFOLT genes where a particular rearrangement of one AtFOLT locus promoted DNA methylation of the second copy through an RdDM pathway (Durand et al, 2012).
It is not clear whether environmental cues contributed to the establishment of these natural epialleles. However, the frequent observation of TE or TE-related sequences in the vicinity of genes forming natural epialleles suggests that transposon-derived cis elements could be involved in the acquisition of epiallelic properties for individual genes.
Transposons, environmental stress, and epigenetic variation
TEs are found in chromosomes of most organisms and often constitute a major component of the genome in multicellular eukaryotes. Most TEs are epigenetically silenced, but some TEs are transcriptionally activated in mutants defective in epigenetic regulation. In addition, transcription of TEs can be activated by stress, a process that occurs over a wide evolutionary range from bacteria to mammals (Capy et al, 2000).
Barbara McClintock was the first to observe that environmental stresses can activate movement of TEs, a finding that has been extensively supported in later work (McClintock, 1984; Wessler, 1996; Grandbastien, 1998). This ability of TEs to display such environmental sensing is illustrated by the following examples: Tnt1 and Tto1 are LTR-type retroelements in tobacco, and their transposition is induced by wounding or pathogen attack (Takeda et al, 2001; Perez-Hormaeche et al, 2008). The Bs1 LTR-type retroelement in maize was shown to transpose after virus infection (Mottinger et al, 1984; Johns et al, 1985). For ONSEN, an LTR-type retroelement in Arabidopsis, transcription is induced by heat stress, and ONSEN transposes in siRNA-defective mutants (Ito et al, 2011). All the above examples involve the most abundant TEs belonging to the class I retroelements that transpose by a ‘copy and paste’ mechanism. However, there are also a few examples of class II DNA transposons that transpose by a ‘cut and paste’ mechanism following stress exposure. For example, the frequency of excision of the Ac/Ds type transposon Tam3 is enhanced at low temperature in Antirrhinum majus (Harrison & Fincham, 1964; Carpenter et al, 1987).
Barbara McClintock postulated that activation of TEs reflects a response of the genome to a challenge (McClintock, 1984). Several examples of TEs playing a crucial role in gene regulation and genome evolution support this hypothesis (Slotkin & Martienssen, 2007; Fedoroff, 2012). It has been suggested that environmentally activated TEs create new genetic and epigenetic variability that, when under selection, could contribute to enhanced adaptive potential of plants subjected to stresses (Mirouze & Paszkowski, 2011; Bucher et al, 2012) (Fig3).
Figure 3. Environmentally induced genetic and epigenetic variations.
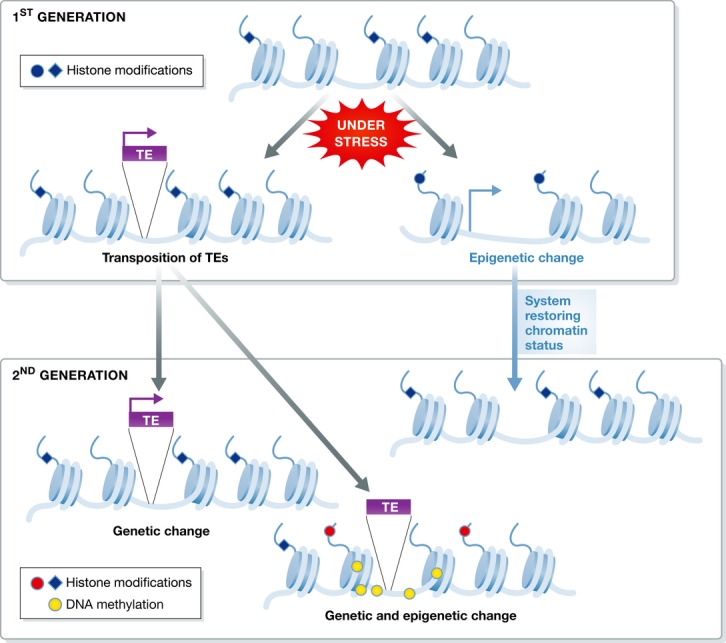
Stress induces activation of transposons and epigenetic changes at various silent genomic loci, including heterochromatic regions. Activated transposons may transpose and generate genetic variation. New insertions of transposons also generate epigenetic variation in the vicinity of the new insertions. In contrast, epigenetic changes are mostly transient due to restoration of the pre-stress chromatin status. Therefore, transgenerational transmission of stress-induced epigenetic changes is very restricted.
Recent studies have directly demonstrated that newly inserted TEs can indeed provide stress-responsive regulation to adjacent genes. In rice, it was shown that the active DNA transposon mPing preferentially inserts into 5′ flanking regions of genes and not into exons. Transcription of a subset of genes harboring an mPing insertion in the promoter region was found to be induced by cold or salt stress (Naito et al, 2009).
In Arabidopsis, new copies of ONSEN preferentially insert into genic regions rather than to the heterochromatic regions where the majority of TEs are located. It has been shown that the LTR of ONSEN has a heat-responsive element that is activated by transcriptional heat stress responses (Cavrak et al, 2014). Consequently, genes in the vicinity of or harboring newly inserted ONSEN copies become heat responsive (Ito et al, 2011). A further study showed that phenotypic variation in a particular Italian strain of blood oranges around Mount Etna is caused by the insertion of an LTR retrotransposon in the promoter of Ruby, a gene that encodes a transcriptional activator of anthocyanin biosynthesis. The LTR retrotransposon in the promoter confers cold responsiveness on the Ruby gene in fruits, thus determining the temperature-dependent coloration of blood oranges (Butelli et al, 2012).
Environmentally induced transgenerational epigenetic memory
The concept that adaptive traits can be acquired by an individual and inherited by its progeny was proposed by Jean-Baptiste Lamarck, but later gave way to the Darwinian theory of evolution. After the discovery of epigenetic mechanisms of inheritance and especially recent studies suggesting transgenerational inheritance of acquired traits in plants and animals, the previously abandoned Lamarckian theory has regained limited attention.
In Arabidopsis, it was demonstrated that UV-C radiation or introduction of the bacterial elicitor flagellin induces a higher frequency of somatic homologous recombination, and this ‘induced’ state is transmitted in a dominant manner as a newly acquired trait to the progeny (Molinier et al, 2006). A similar study performed in tobacco demonstrated that a tobacco mosaic virus (TMV)-induced systemic signal increases somatic recombination rates. The progeny of TMV-infected plants also showed a higher frequency of recombination (Boyko et al, 2007). Further studies showed that SAR can be transmitted to the next generation in tomato and Arabidopsis (Luna et al, 2012; Rasmann et al, 2012; Slaughter et al, 2012).
Although there are many more examples in plants suggesting inheritance of environmentally induced traits, the issue remains controversial (Boyko & Kovalchuk, 2011; Mirouze & Paszkowski, 2011; Paszkowski & Grossniklaus, 2011; Pecinka & Mittelsten Scheid, 2012). This is mainly due to the absence of defined molecular mechanisms that could account for such phenomena, although the involvement of epigenetic regulation has been repeatedly suggested.
The prospect that environmental stresses can lead to the emergence of transgenerationally heritable epigenetic traits in plants may be associated with negative consequences. Despite the very tempting possibility that such mechanisms could potentially contribute to adaptive advantage, it may also be the case that accumulation of epigenetic information reflecting the ‘stress memories’ of previous generations could impair responses to current environmental challenges. Moreover, bona fide examples of transgenerational transmission of environmentally induced traits are still quite scarce, which is surprising given the centuries of plant domestication and human driven selection for use in agriculture and horticulture. During much of this time, Lysenko (Gordin, 2012) was the only proponent of the inheritance of acquired traits. Therefore, it is conceivable that an as yet unknown mechanism hinders the inheritance of environmentally induced epigenetic traits (Fig3).
Recently, a forward genetic screen in Arabidopsis apparently revealed such a system. Two chromatin regulators DDM1 and MOM1 were found to act redundantly in preventing the transmission of stress-induced transcriptional changes to progeny of the stressed plants. In ddm1 mom1 double mutants, transcriptional signatures induced by stress were found in the subsequent generation (Iwasaki & Paszkowski, 2014). Thus, such DDM1- and MOM1-mediated or other mechanisms of chromatin resetting could prevent or act very restrictively on transgenerational transmission of environmentally induced epigenetic traits.
Acknowledgments
We thank Patrick King for editing of the manuscript and Ian Furner for critical reading. This work was supported by the Gatsby Charitable Foundation and the European Research Council.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aina R, Sgorbati S, Santagostino A, Labra M, Ghiani A, Citterio S. Specific hypomethylation of DNA is induced by heavy metals in white clover and industrial hemp. Physiol Plant. 2004;121:472–480. [Google Scholar]
- Angel A, Song J, Dean C, Howard M. A Polycomb-based switch underlying quantitative epigenetic memory. Nature. 2011;476:105–108. doi: 10.1038/nature10241. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Baroux C, Gagliardini V, Page DR, Grossniklaus U. Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev. 2006;20:1081–1086. doi: 10.1101/gad.378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- Bauer MJ, Fischer RL. Genome demethylation and imprinting in the endosperm. Curr Opin Plant Biol. 2011;14:162–167. doi: 10.1016/j.pbi.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Kathiria P, Zemp FJ, Yao Y, Pogribny I, Kovalchuk I. Transgenerational changes in the genome stability and methylation in pathogen-infected plants: (virus-induced plant genome instability) Nucleic Acids Res. 2007;35:1714–1725. doi: 10.1093/nar/gkm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Kovalchuk I. Genome instability and epigenetic modification–heritable responses to environmental stress? Curr Opin Plant Biol. 2011;14:260–266. doi: 10.1016/j.pbi.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Brickner DG, Cajigas I, Fondufe-Mittendorf Y, Ahmed S, Lee PC, Widom J, Brickner JH. H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol. 2007;5:e81. doi: 10.1371/journal.pbio.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher E, Reinders J, Mirouze M. Epigenetic control of transposon transcription and mobility in Arabidopsis. Curr Opin Plant Biol. 2012;15:503–510. doi: 10.1016/j.pbi.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Butelli E, Licciardello C, Zhang Y, Liu J, Mackay S, Bailey P, Reforgiato-Recupero G, Martin C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell. 2012;24:1242–1255. doi: 10.1105/tpc.111.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterbrodt T, Thurow C, Gatz C. Chromatin immunoprecipitation analysis of the tobacco PR-1a- and the truncated CaMV 35S promoter reveals differences in salicylic acid-dependent TGA factor binding and histone acetylation. Plant Mol Biol. 2006;61:665–674. doi: 10.1007/s11103-006-0039-2. [DOI] [PubMed] [Google Scholar]
- Capy P, Gasperi G, Biemont C, Bazin C. Stress and transposable elements: co-evolution or useful parasites? Heredity (Edinb) 2000;85(Pt 2):101–106. doi: 10.1046/j.1365-2540.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- Carpenter R, Martin C, Coen ES. Comparison of genetic behavior of the transposable element Tam3 at 2 unlinked pigment loci in Antirrhinum-Majus. Mol Gen Genet. 1987;207:82–89. [Google Scholar]
- Cavrak VV, Lettner N, Jamge S, Kosarewicz A, Bayer LM, Mittelsten Scheid O. How a retrotransposon exploits the plant's heat stress response for its activation. PLoS Genet. 2014;10:e1004115. doi: 10.1371/journal.pgen.1004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Hyun Y, Kang MJ, In Yun H, Yun JY, Lister C, Dean C, Amasino RM, Noh B, Noh YS, Choi Y. Resetting and regulation of Flowering Locus C expression during Arabidopsis reproductive development. Plant J. 2009;57:918–931. doi: 10.1111/j.1365-313X.2008.03776.x. [DOI] [PubMed] [Google Scholar]
- Choi K, Park C, Lee J, Oh M, Noh B, Lee I. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development. 2007;134:1931–1941. doi: 10.1242/dev.001891. [DOI] [PubMed] [Google Scholar]
- Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- Choi Y, Harada JJ, Goldberg RB, Fischer RL. An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene. Proc Natl Acad Sci USA. 2004;101:7481–7486. doi: 10.1073/pnas.0402328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard P. Vernalization and its relations to dormancy. Ann Rev Plant Physiol Plant Mol Biol. 1960;11:191–238. [Google Scholar]
- Costa LM, Yuan J, Rouster J, Paul W, Dickinson H, Gutierrez-Marcos JF. Maternal control of nutrient allocation in plant seeds by genomic imprinting. Curr Biol. 2012;22:160–165. doi: 10.1016/j.cub.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N, Oki M, Szyjka SJ, Aparicio OM, Kamakaka RT. H2A.Z functions to regulate progression through the cell cycle. Mol Cell Biol. 2006;26:489–501. doi: 10.1128/MCB.26.2.489-501.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- Durand S, Bouche N, Perez Strand E, Loudet O, Camilleri C. Rapid establishment of genetic incompatibility through natural epigenetic variation. Curr Biol. 2012;22:326–331. doi: 10.1016/j.cub.2011.12.054. [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- Fedoroff NV. Presidential address. Transposable elements, epigenetics, and genome evolution. Science. 2012;338:758–767. doi: 10.1126/science.338.6108.758. [DOI] [PubMed] [Google Scholar]
- Feil R, Berger F. Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 2007;23:192–199. doi: 10.1016/j.tig.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Finnegan EJ, Dennis ES. Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr Biol. 2007;17:1978–1983. doi: 10.1016/j.cub.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin MD. How lysenkoism became pseudoscience: dobzhansky to velikovsky. J Hist Biol. 2012;45:443–468. doi: 10.1007/s10739-011-9287-3. [DOI] [PubMed] [Google Scholar]
- Grandbastien MA. Activation of plant retrotransposons under stress conditions. Trends Plant Sci. 1998;3:181–187. [Google Scholar]
- Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol. 2007;17:73–78. doi: 10.1016/j.cub.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Costa LM, Dal Pra M, Scholten S, Kranz E, Perez P, Dickinson HG. Epigenetic asymmetry of imprinted genes in plant gametes. Nat Genet. 2006;38:876–878. doi: 10.1038/ng1828. [DOI] [PubMed] [Google Scholar]
- Haig D, Westoby M. Parent-specific gene-expression and the triploid endosperm. Am Nat. 1989;134:147–155. [Google Scholar]
- Harrison BJ, Fincham JRS. Instability at pal locus in Antirrhinum Majus. I. Effects of environment on frequencies of somatic and germinal mutation. Heredity. 1964;19:237–258. [Google Scholar]
- Heo JB, Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram GC. Family life at close quarters: communication and constraint in angiosperm seed development. Protoplasma. 2010;247:195–214. doi: 10.1007/s00709-010-0184-y. [DOI] [PubMed] [Google Scholar]
- Ito H, Gaubert H, Bucher E, Mirouze M, Vaillant I, Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature. 2011;472:115–119. doi: 10.1038/nature09861. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Paszkowski J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc Natl Acad Sci USA. 2014;111:8547–8552. doi: 10.1073/pnas.1402275111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke S, Scholten S. Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol. 2009;19:1677–1681. doi: 10.1016/j.cub.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz M, Conrath U, Peterhansel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12:50–55. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F, Porcher E, Teixeira FK, Saliba-Colombani V, Simon M, Agier N, Bulski A, Albuisson J, Heredia F, Audigier P, Bouchez D, Dillmann C, Guerche P, Hospital F, Colot V. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 2009;5:e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science. 2000;290:344–347. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- Johns MA, Mottinger J, Freeling M. A low copy number, copia-like transposon in maize. EMBO J. 1985;4:1093–1101. doi: 10.1002/j.1460-2075.1985.tb03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien PE, Katz A, Oliva M, Ohad N, Berger F. Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr Biol. 2006a;16:486–492. doi: 10.1016/j.cub.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Jullien PE, Kinoshita T, Ohad N, Berger F. Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell. 2006b;18:1360–1372. doi: 10.1105/tpc.106.041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008;6:e194. doi: 10.1371/journal.pbio.0060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankel MW, Ramsey DE, Stokes TL, Flowers SK, Haag JR, Jeddeloh JA, Riddle NC, Verbsky ML, Richards EJ. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics. 2003;163:1109–1122. doi: 10.1093/genetics/163.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol. 2009;25:277–299. doi: 10.1146/annurev.cellbio.042308.113411. [DOI] [PubMed] [Google Scholar]
- Kim JM, To TK, Ishida J, Morosawa T, Kawashima M, Matsui A, Toyoda T, Kimura H, Shinozaki K, Seki M. Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:1580–1588. doi: 10.1093/pcp/pcn133. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, Wells D, Katz A, Margossian L, Harada JJ, Goldberg RB, Fischer RL. Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Mitina I, Tamada Y, Hyun Y, Choi Y, Amasino RM, Noh B, Noh YS. Growth habit determination by the balance of histone methylation activities in Arabidopsis. EMBO J. 2010;29:3208–3215. doi: 10.1038/emboj.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U. The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 2003;17:1540–1553. doi: 10.1101/gad.257403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- Kohler C, Weinhofer-Molisch I. Mechanisms and evolution of genomic imprinting in plants. Heredity (Edinb) 2010;105:57–63. doi: 10.1038/hdy.2009.176. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, Dean K, Ryan OW, Golshani A, Johnston M, Greenblatt JF, Shilatifard A. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell. 2003;11:721–729. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy YY, Mesnage S, Mylne JS, Gendall AR, Dean C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science. 2002;297:243–246. doi: 10.1126/science.1072147. [DOI] [PubMed] [Google Scholar]
- Light WH, Brickner DG, Brand VR, Brickner JH. Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol Cell. 2010;40:112–125. doi: 10.1016/j.molcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G, Grant M. Salicylic acid in plant defence–the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhang C, Baulcombe DC, Chen ZJ. Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proc Natl Acad Sci USA. 2012;109:5529–5534. doi: 10.1073/pnas.1203094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Bruce TJ, Roberts MR, Flors V, Ton J. Next-generation systemic acquired resistance. Plant Physiol. 2012;158:844–853. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Kohler C. Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 2006;7:947–952. doi: 10.1038/sj.embor.7400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevich G, Villar CB, Erilova A, Kohler C. Mechanism of PHERES1 imprinting in Arabidopsis. J Cell Sci. 2008;121:906–912. doi: 10.1242/jcs.023077. [DOI] [PubMed] [Google Scholar]
- Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet. 2006;38:948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- March-Diaz R, Garcia-Dominguez M, Lozano-Juste J, Leon J, Florencio FJ, Reyes JC. Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J. 2008;53:475–487. doi: 10.1111/j.1365-313X.2007.03361.x. [DOI] [PubMed] [Google Scholar]
- Martin A, Troadec C, Boualem A, Rajab M, Fernandez R, Morin H, Pitrat M, Dogimont C, Bendahmane A. A transposon-induced epigenetic change leads to sex determination in melon. Nature. 2009;461:1135–1138. doi: 10.1038/nature08498. [DOI] [PubMed] [Google Scholar]
- Mathieu O, Reinders J, Caikovski M, Smathajitt C, Paszkowski J. Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell. 2007;130:851–862. doi: 10.1016/j.cell.2007.07.007. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Memories of winter: vernalization and the competence to flower. Plant, Cell Environ. 2000;23:1145–1153. [Google Scholar]
- Mirouze M, Paszkowski J. Epigenetic contribution to stress adaptation in plants. Curr Opin Plant Biol. 2011;14:267–274. doi: 10.1016/j.pbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Molinier J, Ries G, Zipfel C, Hohn B. Transgeneration memory of stress in plants. Nature. 2006;442:1046–1049. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marin MI, Martinez-Macias MI, Ariza RR, Roldan-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci USA. 2006;103:6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, Baulcombe DC. Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature. 2009;460:283–286. doi: 10.1038/nature08084. [DOI] [PubMed] [Google Scholar]
- Mottinger JP, Johns MA, Freeling M. Mutations of the Adh1 gene in maize following infection with barley stripe mosaic virus. Mol Gen Genet. 1984;195:367–369. doi: 10.1007/BF00332775. [DOI] [PubMed] [Google Scholar]
- Mylne JS, Barrett L, Tessadori F, Mesnage S, Johnson L, Bernatavichute YV, Jacobsen SE, Fransz P, Dean C. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc Natl Acad Sci USA. 2006;103:5012–5017. doi: 10.1073/pnas.0507427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K, Zhang F, Tsukiyama T, Saito H, Hancock CN, Richardson AO, Okumoto Y, Tanisaka T, Wessler SR. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature. 2009;461:1130–1134. doi: 10.1038/nature08479. [DOI] [PubMed] [Google Scholar]
- Oh S, Zhang H, Ludwig P, van Nocker S. A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell. 2004;16:2940–2953. doi: 10.1105/tpc.104.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski J, Grossniklaus U. Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr Opin Plant Biol. 2011;14:195–203. doi: 10.1016/j.pbi.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Pecinka A, Mittelsten Scheid O. Stress-induced chromatin changes: a critical view on their heritability. Plant Cell Physiol. 2012;53:801–808. doi: 10.1093/pcp/pcs044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Hormaeche J, Potet F, Beauclair L, Le Masson I, Courtial B, Bouche N, Lucas H. Invasion of the Arabidopsis genome by the tobacco retrotransposon Tnt1 is controlled by reversible transcriptional gene silencing. Plant Physiol. 2008;147:1264–1278. doi: 10.1104/pp.108.117846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012;158:854–863. doi: 10.1104/pp.111.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J, Wulff BB, Mirouze M, Mari-Ordonez A, Dapp M, Rozhon W, Bucher E, Theiler G, Paszkowski J. Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev. 2009;23:939–950. doi: 10.1101/gad.524609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Yilmaz M, Le O, Hou G, Sadder M, Al-Abdallat A, Xia Y, Lu G, Ladunga I, Avramova Z. The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell. 2008;20:568–579. doi: 10.1105/tpc.107.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze H, Mittelsten Scheid O, Paszkowski J. Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet. 2003;34:65–69. doi: 10.1038/ng1138. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Hills MJ, Lister C, Dean C, Dennis ES, Peacock WJ. Resetting of FLOWERING LOCUS C expression after epigenetic repression by vernalization. Proc Natl Acad Sci USA. 2008;105:2214–2219. doi: 10.1073/pnas.0711453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012;158:835–843. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- Song J, Angel A, Howard M, Dean C. Vernalization - a cold-induced epigenetic switch. J Cell Sci. 2012;125:3723–3731. doi: 10.1242/jcs.084764. [DOI] [PubMed] [Google Scholar]
- Soppe WJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, Koornneef M, Peeters AJ. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell. 2000;6:791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- Steward N, Ito M, Yamaguchi Y, Koizumi N, Sano H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J Biol Chem. 2002;277:37741–37746. doi: 10.1074/jbc.M204050200. [DOI] [PubMed] [Google Scholar]
- Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- Sung S, He Y, Eshoo TW, Tamada Y, Johnson L, Nakahigashi K, Goto K, Jacobsen SE, Amasino RM. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat Genet. 2006a;38:706–710. doi: 10.1038/ng1795. [DOI] [PubMed] [Google Scholar]
- Sung S, Schmitz RJ, Amasino RM. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 2006b;20:3244–3248. doi: 10.1101/gad.1493306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sugimoto K, Kakutani T, Hirochika H. Linear DNA intermediates of the Tto1 retrotransposon in Gag particles accumulated in stressed tobacco and Arabidopsis thaliana. Plant J. 2001;28:307–317. doi: 10.1046/j.1365-313x.2001.01151.x. [DOI] [PubMed] [Google Scholar]
- Tamada Y, Yun JY, Woo SC, Amasino RM. ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell. 2009;21:3257–3269. doi: 10.1105/tpc.109.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira FK, Heredia F, Sarazin A, Roudier F, Boccara M, Ciaudo C, Cruaud C, Poulain J, Berdasco M, Fraga MF, Voinnet O, Wincker P, Esteller M, Colot V. A role for RNAi in the selective correction of DNA methylation defects. Science. 2009;323:1600–1604. doi: 10.1126/science.1165313. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Spielman M, Schulz R, Oakey RJ, Kelsey G, Salazar A, Zhang K, Pennell R, Scott RJ. Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biol. 2010;10:72. doi: 10.1186/1471-2229-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To TK, Nakaminami K, Kim JM, Morosawa T, Ishida J, Tanaka M, Yokoyama S, Shinozaki K, Seki M. Arabidopsis HDA6 is required for freezing tolerance. Biochem Biophys Res Commun. 2011;406:414–419. doi: 10.1016/j.bbrc.2011.02.058. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Saika H, Tsutsumi N, Hirai A, Nakazono M. Dynamic and reversible changes in histone H3-Lys4 methylation and H3 acetylation occurring at submergence-inducible genes in rice. Plant Cell Physiol. 2006;47:995–1003. doi: 10.1093/pcp/pcj072. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Klessig DF, Park SW. Systemic acquired resistance: the elusive signal(s) Curr Opin Plant Biol. 2008;11:436–442. doi: 10.1016/j.pbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- Waddington CH. The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology. London: George Allen & Unwin; 1957. [Google Scholar]
- Weinhofer I, Hehenberger E, Roszak P, Hennig L, Kohler C. H3K27me3 profiling of the endosperm implies exclusion of polycomb group protein targeting by DNA methylation. PLoS Genet. 2010;6:e1001152. doi: 10.1371/journal.pgen.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler SR. Turned on by stress. Plant retrotransposons. Curr Biol. 1996;6:959–961. doi: 10.1016/s0960-9822(02)00638-3. [DOI] [PubMed] [Google Scholar]
- Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet. 2003;4:359–368. doi: 10.1038/nrg1062. [DOI] [PubMed] [Google Scholar]
- Wohrmann HJ, Gagliardini V, Raissig MT, Wehrle W, Arand J, Schmidt A, Tierling S, Page DR, Schob H, Walter J, Grossniklaus U. Identification of a DNA methylation-independent imprinting control region at the Arabidopsis MEDEA locus. Genes Dev. 2012;26:1837–1850. doi: 10.1101/gad.195123.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P, Weinhofer I, Seguin J, Roszak P, Beisel C, Donoghue MT, Spillane C, Nordborg M, Rehmsmeier M, Kohler C. High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis Endosperm. PLoS Genet. 2011;7:e1002126. doi: 10.1371/journal.pgen.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yang R, Li G, Chen H, Laurie J, Ma C, Wang D, Yao Y, Larkins BA, Sun Q, Yadegari R, Wang X, Ni Z. Dynamic expression of imprinted genes associates with maternally controlled nutrient allocation during maize endosperm development. Plant Cell. 2013;25:3212–3227. doi: 10.1105/tpc.113.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Shen WH. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Silva P, Rodrigues JA, Dotson B, Brooks MD, Zilberman D. Local DNA hypomethylation activates genes in rice endosperm. Proc Natl Acad Sci USA. 2010;107:18729–18734. doi: 10.1073/pnas.1009695107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Yu Y, Meyer D, Wu C, Shen WH. Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat Cell Biol. 2005;7:1256–1260. doi: 10.1038/ncb1329. [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhang L, Duan J, Miki B, Wu K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell. 2005;17:1196–1204. doi: 10.1105/tpc.104.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Thakar A. H2A.Z: view from the top. Structure. 2008;16:166–179. doi: 10.1016/j.str.2007.12.008. [DOI] [PubMed] [Google Scholar]